Abstract
Aim:
Accumulating evidence suggests that orexin signalling is involved in the regulation of blood pressure and cardiovascular function. However, the underlying mechanisms are not clear. Here, we test the hypothesis that upregulated orexin A signalling in the paraventricular nucleus (PVN) increases sympathetic nerve activity (SNA) through stimulating expression of proinflammatory cytokines (PICs).
Methods:
In vivo sympathetic nerve recordings were performed to test the impact of PVN orexin signalling on sympathetic outflow in Sprague Dawley (SD) rats. Real-time PCR was carried out to assess effects of central administration of orexin A on PVN PICs expression in SD rats. To test whether orexin A-induced increases in PICs were exclusively mediated by orexin receptor 1 (OX1R), OX1R-expressing PC12 (PC12-OX1R) cells were incubated with different dose of orexin A, and then, PICs mRNA and immunoreactivity were measured.
Results:
Orexin A microinjection (25 pmol) into the PVN significantly increased splanchnic SNA (93.5%) and renal SNA (83.3%) in SD rats, and these increases were attenuated by OX1R antagonist SB408124. Intracerebroventricular injection of orexin A (0.2 nmol) into SD rats increased mRNA levels of PICs including IL-1-β (2.7-fold), IL-6 (1.7-fold) and TNF-α (1.5-fold), as well as Fra1 (1.6-fold) in the PVN. Orexin A treatment in PC12-OX1R cells resulted in a dose- and time-dependent increase in the expression of PICs and Fra1, a subunit of AP1 transcriptional factor. The increase in the PICs was blocked by AP1 blocker curcumin.
Conclusion:
Paraventricular nucleus orexin system activation is involved in SNA regulation maybe through triggering AP1-PICs pathway.
Keywords: orexin receptor, paraventricular nucleus, proinflammatory cytokines, sympathetic nerve activity
1 |. INTRODUCTION
Orexins (orexin A and orexin B) are neuropeptides traditionally known for their functions in regulating arousal, wakefulness and appetite. However, studies in recent decades suggest that orexins, specifically orexin A, are also involved in regulation of sympathetic nerve activity (SNA) and blood pressure.1–3 Both orexin A and orexin B are derived from the same neuropeptide precursor, the preproorexin, and are only produced by a small group of neurons primary located in lateral hypothalamus.4,5 The actions of the orexins are mediated by two G protein-coupled receptors, orexin 1 receptor (OX1R) and orexin 2 receptor (OX2R). OX1R has a higher affinity for orexin A over orexin B, while OX2R has similar affinities for both orexin peptides.4 Although cell bodies of orexin-producing neurons are primary localized in lateral hypothalamus, the projections of orexin-producing neurons extend throughout the brain and spinal cord, where orexin receptors are also expressed.6,7 Both orexin projections and orexin receptors have been found to be enriched in the brain cardiovascular relevant regions, and they are well positioned to participate in the regulation of blood pressure and cardiovascular function.2,8 In normal rats and mice, central administration of orexins or microinjection of orexins into the brain cardiovascular control regions increases blood pressure and SNA, and these effects are blocked by orexin receptor antagonists.2,3,9–12 In contrast, prepro-orexin gene knockout mice show a significant lower blood pressure compared to their wild controls.13 In addition, the upregulation of orexin signalling has been observed in several animal models of hypertension such as spontaneously hypertensive rats (SHR),14,15 stress-induced hypertensive rats,1 obesity-related hypertensive Zucker rats,16 Dahl salt-sensitive hypertensive rats17 and BPH/2J Schlager mice.18 Furthermore, antagonism of orexin receptors significantly lowers blood pressure in some hypertensive models such as SHR and Dahl salt-sensitive hypertensive rat.15,17 Taken together, these evidences suggest that upregulation of orexin signalling may play important roles in the development and establishment of hypertension. However, the molecular mechanisms underlying the effects of orexin signalling on blood pressure and SNA control are not well determined.
The central nervous system plays a critical role in the development and maintenance of hypertension. A centrally mediated increase in SNA occurs in both primary human hypertension and animal models of hypertension. The neurons controlling the sympathetic branches of the autonomic nervous system are found in only a few brain areas, including the paraventricular nucleus (PVN) in the hypothalamus.19 The PVN neurons receive input from visceral and sensory nerves via mono- and polysynaptic connections from the nucleus of the solitary tract and integrate this with the input from the limbic system, circumventricular organs and other hypothalamus structures. The PVN neurons will then control the sympathetic outflow by sending projections directly to the spinal cord or the other major sympathetic regulatory brain area, the rostral ventrolateral medulla (RVLM).20 Activation of the PVN neurons can lead to sympathetic discharge. The activity of PVN sympathetic output is modulated by many factors such as glutamate, angiotensin II and proinflammatory cytokines (PICs).21–23 PICs are well known as the neuronal activity modulators.22 Excessive production of PICs in both the periphery and the brain has been demonstrated to contribute to hypertension.24 OX1R has been found to be expressed in the PVN where orexin-producing neurons also send projections, and its expression is increased in the hypertensive rats,16,17 indicating that PVN orexin signalling may be involved in SNA control. Taken together, we hypothesize that upregulation of orexin signalling in the PVN may stimulate PVN PICs expression and results in excessive production of PICs which in turn increases sympathetic outflow and results in high blood pressure. In this study, we aim to test the role of PVN orexin signalling in the control of SNA as well as PICs expression using both in vivo and in vitro study.
2 |. RESULTS
2.1 |. OX1R is expressed in RVLM-projecting PVN neurons
To determine whether the PVN orexin signalling is involved in SNA control, we first investigated whether orexin receptors are expressed on RVLM-projecting PVN neurons. To label PVN neurons projecting to the RVLM, three adult male SD rats were microinjected with rhodamine-containing microspheres (retrograde tracer) into the RVLM. The RVLM tracer microinjection is at a specific site where L-glutamate microinjection elicits a maximum increase in renal SNA (RSNA) and blood pressure. The rats were killed 10 days following retrograde tracer injection, and the brain coronal sections containing the PVN were cut and subjected to immunostaining using either OX1R or OX2R primary antibody. The immunoreactivities of OX1R and OX2R were observed under a fluorescent microscope. The results showed that OX1Rs (green) were enriched in the PVN in the brain coronal sections around the bregma −1.6 mm, and they are colocalized with retrograde labelled PVN-RVLM neurons (red, tracer) (Figure 1). In contrast, OX2Rs were little expressed in typical PVN region and there were not any neurons on which OX2Rs were colocalized with tracer in the brain coronal sections around the bregma −1.6 mm. Instead, the OX2Rs were highly expressed on the neurons in the submedius thalamic nucleus and mediodorsal thalamic nucleus in the brain coronal sections around the bregma −2.1 mm (Figure 2).
FIGURE 1.
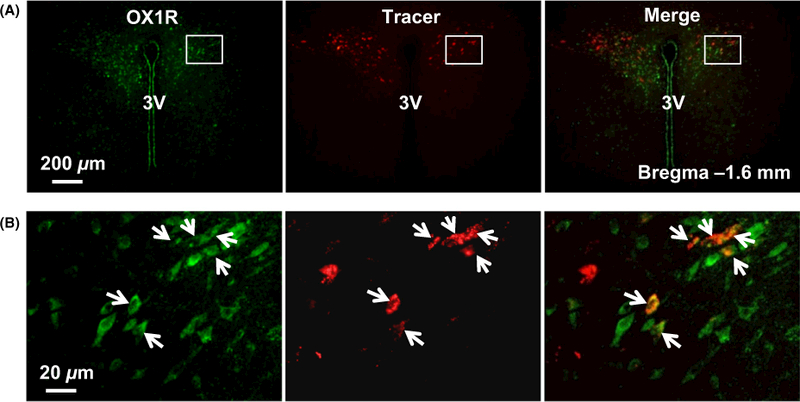
OX1R is expressed in RVLM-projecting PVN neurons. (A) A representative micrograph showing immunoreactivity of OX1R (green), tracer labelled RVLM-projecting PVN neurons (red) and merged micrograph of OX1R and tracer in the PVN. (B) Higher magnification micrograph for the area boxed in image (A). The arrows show the OX1R-expressing neurons which have axons projecting to the RVLM. The brain section is taken from about bregma −1.6 mm. 3V, the third ventricle
FIGURE 2.
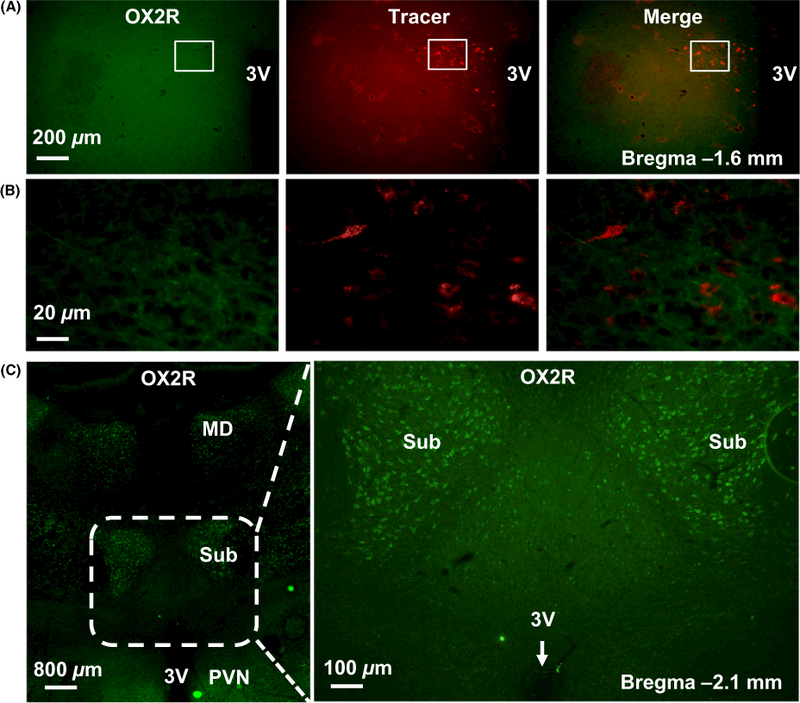
OX2R is not expressed in RVLM-projecting PVN neurons. (A) Representative immunofluorescence micrographs showing no OX2R (green) expressed on the RVLM-projecting PVN neurons labelled with retrograde tracer (red). (B) High magnification for the area boxed in images (A). The brain coronal sections in (A) were taken from about bregma −1.6 mm. (C) Representative images showing the expression of OX2R (green) in brain coronal section around Bregma −2.1 mm. Left: low-magnification image. Right: high-magnification image shows the area which was boxed with dashed line in left panel. The OX2R expression is enriched in the submedius thalamic nucleus and mediodorsal thalamic nucleus. PVN, paraventricular nucleus; 3V, the third ventricle; Sub, submedius thalamic nucleus; MD, mediodorsal thalamic nucleus
2.2 |. Microinjection of orexin A into the PVN induces an increase in the splanchnic SNA (SSNA) and RSNA in Sprague Dawley (SD) rats
The above study showed that OX1R is expressed in the PVN pre-sympathetic neurons indicating that the PVN OX1R may be involved in regulation of the sympathetic outflow control and blood pressure. In this study, we test the impact of increasing availability of the PVN OX1R ligand orexin A on SNA and blood pressure in normal SD rats. Anesthetized male adult SD rats received orexin A (25 pmol) injection bilaterally into the PVN, their SSNA, RSNA, mean arterial pressure (MAP) and heart rate (HR) were recorded as we described in the Methods. The results showed that PVN orexin A microinjection significantly increased SSNA (93.5 ± 11.8%, n = 5) and RSNA (83.3 ± 7.4%, n = 5) in SD rats. However, no significant changes were observed in MAP (ΔMAP = 7.0 ± 2.1 mmHg, t = 0.821, P = .435) and HR (ΔHR = 7.2 ± 4.9 beats/min, t = 0.261, P = .800) in response to PVN orexin A microinjection. The increases in SNA outflow were effectively attenuated by PVN pre-injection of OX1R antagonist SB408124 (SSNA: 27.2 ± 3.6%; RSNA: 22.6 ± 2.1%; P < .01) (Figure 3A). Figure 3B,C shows representative traces for the SSNA, RSNA and MAP in response to bilateral PVN microinjection of DMSO + Orexin A (25 pmol) and SB408124 (300 pmol) + orexin A (25 pmol) respectively. Figure 4 shows a representative microinjection site of the same volume of Chicago blue dye (2% in saline) in the PVN suggesting that orexin A was injected accurately into the PVN. These results indicate that increasing orexin A peptide in the PVN promotes SNA outflow by increasing SSNA and RSNA, and this effect is primary mediated by OX1R.
FIGURE 3.
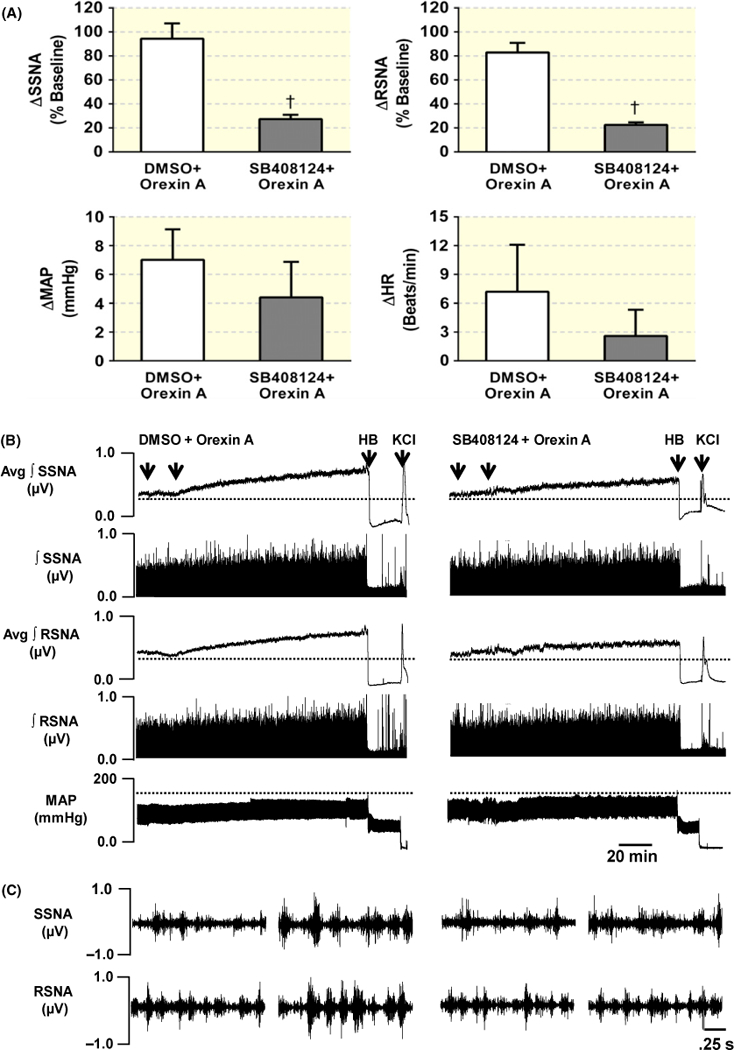
Activation of PVN OX1R receptors increases sympathetic nerve activity (SNA). (A) Summary data show changes in SSNA, RSNA, MAP and HR in response to PVN bilateral microinjections of DMSO + orexin A (n = 5) or SB408124 + orexin A (n = 5) in SD rats. (B) Representative traces showing SSNA, RSNA and MAP responses to bilateral microinjection of DMSO + orexin A (25 pmol) (left), or SB408124 (300 pmol) + orexin A (25 pmol) (right) into PVN in two individual SD rats respectively. (C) Left: 2.5-second specimen traces of SSNA (top) and RSNA (bottom) before and after orexin A microinjection into the PVN in rat receiving DMSO + orexin A microinjection. (C) Right: 2.5-second specimen traces of SSNA (top) and RSNA (bottom) before and after injection of orexin A into PVN in rat receiving SB408124 + orexin A injection. SSNA, splanchnic SNA; RSNA, renal SNA; MAP, mean arterial blood pressure; HR, heart rate; HB, hexamethonium bromide; †P < .01 compared with DMSO + orexin A microinjection group; Avg, average; ∫, integrated
FIGURE 4.
A representative image showing PVN injection site. PVN, paraventricular nucleus; 3V, the third ventricle
2.3 |. Central administration of orexin A increases mRNA levels of PICs and promotes expression of Fra1 in the PVN of SD rats
In this study, we aimed to investigate whether central administration of orexin A promotes PICs expression in the PVN. Orexin A (0.2 nmol in 4 μL) was intracerebroventricularly infused into the lateral ventricle of male SD rats. Three hours following intracerebroventricular (ICV) infusion, rats were killed and their brains were removed and PVN tissues were punched out and subjected to real-time PCR to assess the mRNA levels of genes of interest using Taqman primers and probes. The results showed that central administration of orexin A significantly increased mRNA levels of PICs including IL-1-β (2.7-fold; n = 6; P < .05), IL-6 (1.7-fold; n = 6; P < .05) and TNF-α (1.5-fold; n = 6; P < .05), as well as Fra1 (1.6-fold; n = 6; P < .05), a chronic neuronal activation marker and a subunit of transcription regulator AP1 (Figure 5).
FIGURE 5.
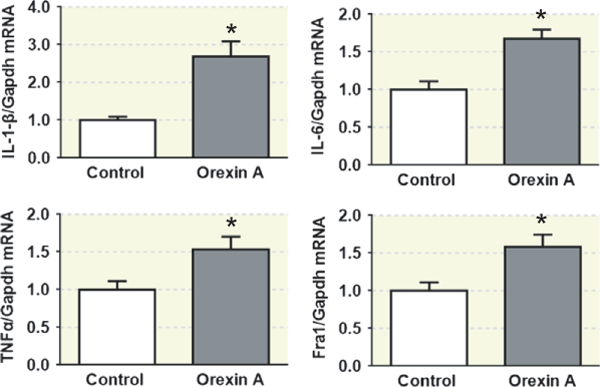
Central administration of orexin A stimulates the expression of proinflammatory cytokines (PICs) in the PVN of SD rats. Real-time PCR analysis of paraventricular nucleus (PVN) PICs mRNA expression levels at 3 hours following acute intracerebroventricular (ICV) infusion of vehicle control (0.9% saline) or orexin A (0.2 nmol) using male adult SD rats. ICV injection of orexin A increases PVN IL-1-β, IL-6, TNF-α and Fra1 mRNA expression in SD rats. The mRNA level in the control sample was assigned to be arbitrary unit (a.u). *P < .05 vs vehicle control; n = 6 each group
The experiment described above indicated that central administration of orexin A increased PVN Fra1 mRNA transcript. In this experiment, we further determined whether OX1R and Fra1 are colocalized in the PVN, and whether OX1R activation can stimulate Fra1 protein expression in the PVN. Co-immunostaining of OX1R and Fra1 was performed using brain coronal sections containing the PVN from ICV orexin A infusion rats. Brain sections from ICV saline (0.9% NaCI) infusion rats were used as controls. The results showed that OX1R and Fra1 are colocalized in the PVN neurons and ICV infusion of orexin A increased PVN Fra1 immunoreactivity (Figure 6).
FIGURE 6.
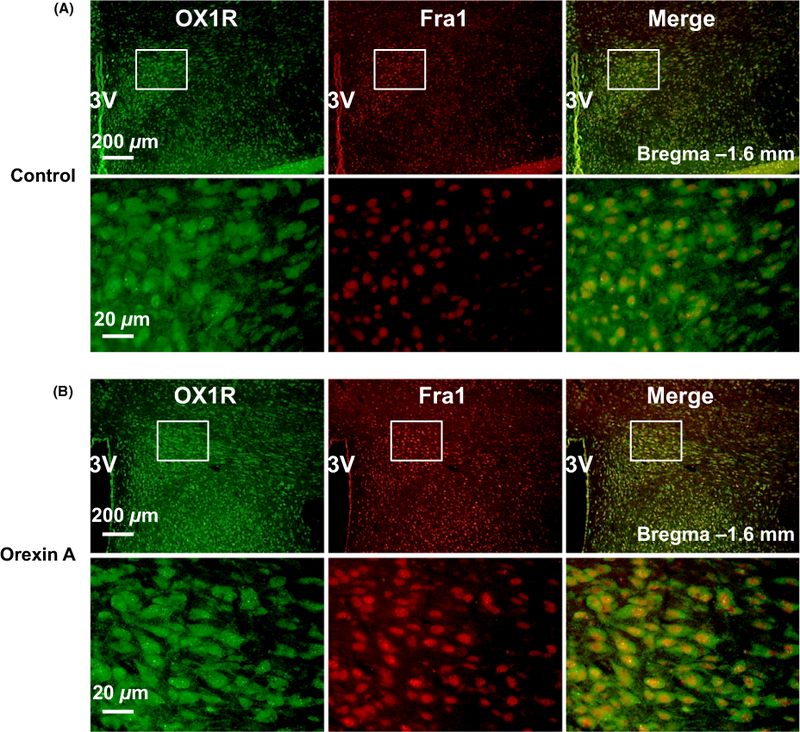
Colocalization of OX1R and Fra1 in the paraventricular nucleus (PVN). (A) a representative micrograph showing immunoreactivity of OX1R (green), Fra1 (red) and merged image in the PVN of vehicle control rat; (B) a representative micrograph showing immunoreactivity of OX1R (green), Fra1(red) and merged image in the PVN of central administration of orexin A SD rat. The brain coronal sections were taken from bregma −1.6 mm. 3V, the third ventricle
2.4 |. Orexin A treatment results in an increase in the expression of PICs and Fra1 in OX1R-expressing PC12 cells
The data in Figure 3 show that orexin A microinjection into the PVN increases SNA, and this increase is attenuated by the OX1R antagonist, which suggests that orexin A-OX1R signalling triggers an increase in the SNA. The result in Figures 5 and 6 indicates that central administration of orexin A promotes PICs and Fra1 expressions in the PVN. In this study, we want to determine whether orexin A promotes PICs expression through activation of OX1R. We used a neurone-like PC12 cell line that artificially expresses human OX1R (PC12-OX1R) to carry out this study. PC12 is a cell line derived from rat adrenal pheochromocytoma and does not express any orexin receptors. PC12-OX1R cell line is a gift from Dr. Kukkonen.25 PC12-OX1R cells were incubated with or without different doses of orexin A for 6 hours, and then, cells were collected for real-time PCR to test mRNA levels of PICs. The data showed that orexin A treatment increases mRNA levels of PICs including IL-6 and TNF-α, as well as Fra1 in a dose-dependent manner, and the maximum increases occurred in 100 nmol L−1 of orexin A-treated cells (Figure 7A). We further incubated the PC12-OX1R cells with 100 nmol L−1 of orexin A for the following times: 0, 3, 6, 24 hours. The cells were then collected for real-time PCR to assess PIC mRNA levels. The results presented that orexin A (100 nmol L−1) treatment caused time-dependent increases in the mRNA levels of the PICs and Fra1, with maximum increases of IL-6 and TNF-α at 6 hours and Fra1 at 3 hours, following orexin A incubation (Figure 7B). We then co-incubated PC12-OX1R cells with orexin A (100 nmol L−1) together with or without the AP1 blocker curcumin (50 μmol L−1) for 6 hours. Real-time PCR was performed to evaluate mRNA levels of the PICs and Fra1. Consistently, the data showed significantly higher mRNA levels for IL-6 (3.8-fold), TNF-α (3.7-fold) and Fra1 (7.4-fold) in orexin A-treated cells compared to vehicle control cells. These stimulatory effects on the PICs were almost completely blocked by curcumin (Figure 7C), but the increase in Fra1 was not attenuated by curcumin. In contrast, Fra1 mRNA expression became even higher in curcumin plus orexin A-treated samples compared to orexin A alone treated samples (Figure 7C).
FIGURE 7.
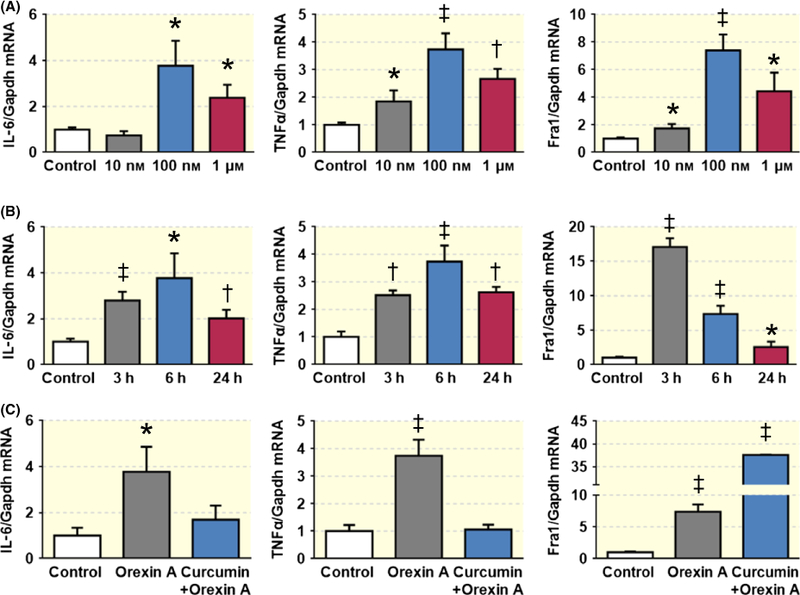
Orexin A treatment increases mRNA levels of PICs and Fra1 in PC12-OX1R cells. (A) Incubation of PC12-OX1R cells with orexin A for 6 hours resulted in a dose-dependent increase in mRNA levels of IL-6, TNF-α and Fra1 with maximum increase occurring in 100 nmol/L orexin A treatment. (B) Orexin A (100 nmol/L) stimulation caused time-dependent increase in mRNA level of IL-6, TNF-α and Fra1 in PC12-OX1R cells. (c) AP1 blocker curcumin (50 μmol L−1) attenuates the increase in the mRNA levels of PICs induced by orexin A. *P < .05, †P < .01, ††P < .001 vs Control
We then selected to test the effects of orexin A on protein expression of TNF-α and Fra1 using immunocytochemistry. PC12-OX1R cells were incubated with orexin A (100 nmol L−1) for 6 hours; then, the cell medium was removed and cells were subjected to immunostaining. The results showed that orexin A treatment significantly increased the immunoreactivity of TNF-α (Figure 8) and Fra1 protein (Figure 9).
FIGURE 8.
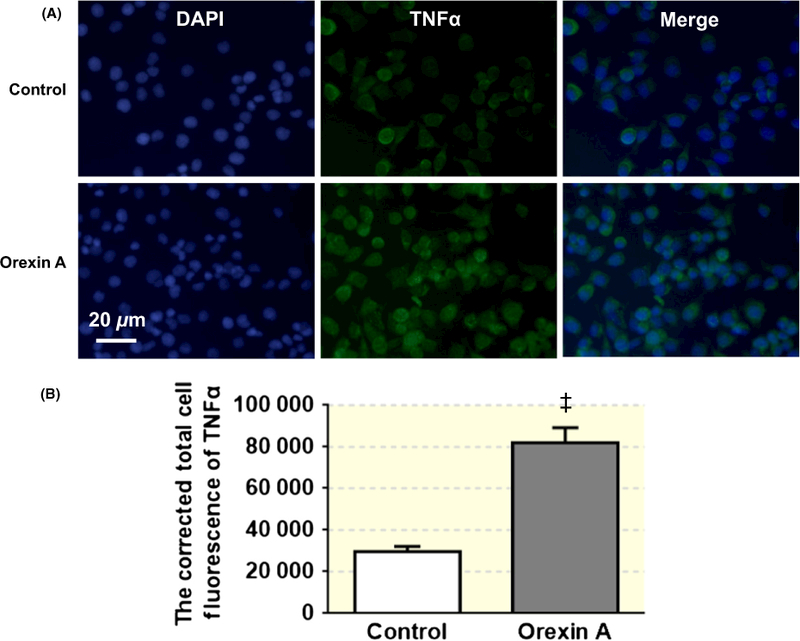
Orexin A (100 nmol L−1) treatment enhances immunoreactivity of TNF-α in PC12-OX1R cells. (A) The pictures show the DAPI staining (blue) and TNF-α staining (green) from cells in control and orexin A treatment group respectively. (B) The bar graph showing the summary data of the TNF-α expression in cells from control and orexin A treatment group. ††P < .001 vs Control
FIGURE 9.
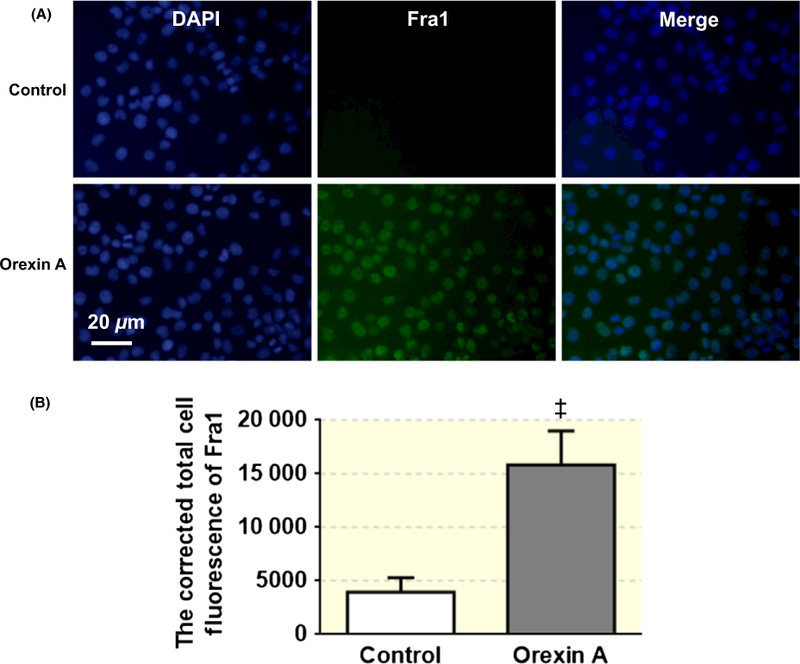
Orexin A (100 nmol L−1) treatment increases immunoreactivity of Fra1 in PC12-OX1R cells. (A) The pictures show the DAPI staining (blue) and Fra1 staining (green) from cells in control and orexin A treatment group respectively. (B) The bar graph showing the summary data of statistical analysis of the Fra1 expression in cells from control and orexin A treatment group. ††P < .001 vs Control
Finally, to confirm that orexin A stimulates PICs expression and this stimulation effect is mediated by OX1R, we incubated PC12, OX2R-expressing PC12 (PC12-OX2R) cells with or without orexin A (100 nmol L−1) for 6 hours and then collected cells and performed real-time PCR to assess mRNA levels of PICs. The results showed that orexin A treatment did not show significant alteration in PICs including IL-6 and TNF-α, as well as Fra1, in PC12. The mRNA levels of IL-6 and Fra1 were slightly increased in PC12-OX2R cells in response to orexin A treatment, but the increase did not reach significance (Figure 10).
FIGURE 10.
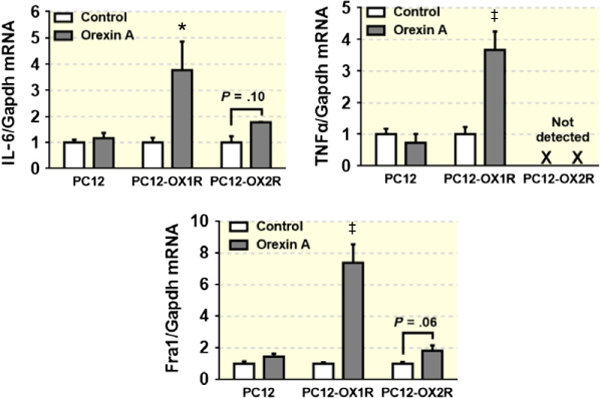
Orexin A (100 nmol L−1) treatment for 6 hours increases mRNA level of IL-6, TNF-α and Fra1 in PC12-OX1R, but not in PC12-OX2R and PC12 cells. *P < .05, ††P < .001 vs Control
3 |. DISCUSSION
The present study reports two novel findings: (i) the activation of PVN OX1R contributes to sympathoexcitation in normal SD rats, and (ii) orexin A-OX1R signalling contributes to neuronal activation and may involve neuronal AP1-PICs signalling. Orexins are neuropeptides associated with multiple physiological functions including sleep/arousal, energy homeostasis, endocrine and visceral functions. Orexin A levels in the cerebrospinal fluid (CSF) are high during the active, waking period in rats and fall to approximately half their peak levels during sleep.26 Dysregulation of orexin signalling leads to many pathological states. For example, loss of orexin function results in the sleep disorder narcolepsy, and increased CSF orexin levels are found in patients with increased anxiety and panic associated symptoms and in rats at a stress state.27,28 Recent studies indicate that brain orexin system is also involved in the regulation of blood pressure and SNA, and its hyperactivity in the brain is associated with hypertension.1,14–17 Thus, the objectives of this study are to investigate whether the PVN orexin signalling is involved in regulating SNA. If it does, what is the potential signalling pathway mediating this regulation?
Our immunostaining studies showed that OX1R is expressed in the RVLM-projecting PVN neurons indicating the potential role of OX1R in SNA outflow control. Surprisingly, there is no OX2R in the RVLM-projecting PVN neurons although weak OX2R immunofluorescence is observed, and real-time PCR demonstrated OX2R mRNA presence in the PVN.17 Instead, OX2R is highly expressed in submedius thalamic nucleus and mediodorsal thalamic nucleus. To be consistent with immunohistochemical results, microinjection of orexin A into the PVN elicited a significant increase in SSNA and RSNA. The increases in SSNA and RSNA were effectively blocked (>60%) by premicroinjection of SB408124, an antagonist of OX1R, suggesting that OX1R mediates orexin A-induced sympathoexcitation in the PVN. In this in vivo study, we did not observe significant increase in blood pressure in response to PVN orexin A microinjection although SSNA and RSNA were elevated. While the previous studies demonstrated that central administration of orexin elevated blood pressure.2,3 The discrepancy could be explained by the following possibilities: the previous studies investigate the impact of orexin A on blood pressure in conscious rats in which orexin acts whole brain, while we focus on the PVN in anesthetized rats, a potential limitation in considering the effect of orexin signalling on both SNA and blood pressure because anaesthesia may inhibit the activity of PVN neurons to alter cardiovascular responses. Previous studies have shown differential responses in blood pressure and sympathetic outflow in response to PVN stimulation between conscious and anesthetized rats.29–31 Either electrical or chemical stimulation of the PVN in anesthetized rats evoked a depressor response accompanied with a decrease in sympathetic outflow,30,31 while in conscious rats, electrical stimulation evoked increases in both blood pressure and SNA.29 Therefore, anaesthesia could potentially underestimate the role of orexin signalling in the PVN in regulating SNA and blood pressure. The importance of this study is that activation of OX1R in the PVN plays a role in the regulation of sympathetic outflow. Another possibility could be that the overall increase in sympathetic outflow induced by activation of OX1R in the PVN may not be sufficient to elevate blood pressure in physiological conditions, although both SSNA and RSNA were increased up to ~90%. This may be due to the low level of endogenous OX1R expression in the PVN of normotensive rat. The OX1R expression may be increased in the PVN of hypertensive rats compared to that in normotensive animals. Therefore, it would be expected that microinjection of orexin A into the PVN of hypertensive animals could induce sympathoexcitation and presser responses in a greater degree. Whether upregulation of orexin receptors’ expression in hypertensive animals can enhance sympathoexcitation and induce a pressor response remains to be studied in the future.
Next, we determined what signal pathway is potentially involved in mediating the sympathoexcitation evoked by activation of OX1R in the PVN. It is well known that PICs are neuronal activity modulators. Excessive productions of PICs such as IL-1-β and IL-6 can stimulate neurotransmitter glutamate secretion and therefore increase SNA.32,33 We therefore investigated whether central administration of orexin A upregulates expression of PICs in the PVN in normal SD rats. The results showed that ICV injection of orexin A significantly increased the mRNA levels of PICs including IL-1-β, IL-6 and TNF-α in the PVN. In addition to promoting PICs mRNA expression, ICV injection of orexin A also upregulated expression of Fra1, a chronic neuronal activation marker, in the PVN. Furthermore, immunostaining demonstrated that OX1R and Fra1 are colocalized in the PVN neurons implying that OX1R activation may trigger downstream signalling through stimulating Fra1.
To confirm the stimulation effect of orexin A on PICs and identify whether the orexin A-induced stimulation effect on PICs is meditated by OX1R, a neuro-like OX1R-expressing PC12 cell line was used to test the impact of orexin A on the expression of PICs and Fra1. PC12 cell is a cell line derived from a pheochromocytoma of the rat adrenal medulla. When given neuronal growth factor (NGF) in culture medium, PC12 cells cease to proliferate and undergo terminal differentiation into a neuronal phenotype.34 PC12 cells do not express any orexin system components. Using PC12-OX1R cells enable us to identify whether orexin A-induced effects are exclusively mediated by OX1R. Our results showed that orexin A treatment induces increases in mRNA levels of PICs including IL-6 and TNF-α, as well as Fra1, in a time- and dose-dependent manner. The maximum increase occurs at 6 hours in PICs and 3 hours in Fra1, following orexin A (100 nmol L−1) incubation. To be consistent with this finding, our further study showed that orexin A treatment also increase protein expression of TNF-α and Fra1 in the PC12-OX1R cells.
AP-1 is a transcription regulator that controls transcription of multiple genes through binding with the consensus sequence in or near their promoter regions. There are AP-1 binding sites in the promoter regions of PICs.35 This evidence, coupled with the observations that (i) OX1R and Fra1 are colocalized in the PVN neurons and (ii) the maximum increase of Fra1 occurred earlier than PICs (3 hours in Fra1 and 6 hours in PICs) in response to orexin A stimulation, suggests that increased AP1 may promote transcriptions of PICs. Pre-incubation of PC12-OX1R cells with curcumin, an AP1 inhibitor, attenuated the increases in mRNA levels of IL-6 and TNF-α, confirming our hypothesis that the increase in PIC expression is mediated via activation of transcription factor AP1. Intriguingly, curcumin amplified the mRNA transcription of Fra1, which might be a compensatory mechanism for AP1 inhibition.
Although orexin A upregulates expression of Fra1, a chronic neuronal activation marker, in both in vivo and in vitro experiments, differences occurred in PIC expression in response to orexin A stimulation between in vivo and in vitro studies. Central administration of orexin A increased mRNA expression of IL-1-β, IL-6 and TNF-α in the PVN. While in vitro study showed that orexin A treatment only increased mRNA levels of TNF-α and IL-6, IL-1-β was undetectable in both control and orexin A-treated PC12-OX1R cells. This discrepancy could be explained by the following possibilities: (i) IL-1β may not be expressed in PC12 cells (ii) cell communications: the PVN contains different type of cells including neurons, astrocytes and microglia. All these three types of cells can produce cytokines.36 For example, many studies have demonstrated that microglia can secrete PICs and be involved in regulation of blood pressure and SNA.37–40 These different types of cells may have communications and interactions in response to orexin A administration. Binding of orexin A with OX1R in neurons may subsequently signal and change the behaviour of their adjacent cells such as microglial cells and stimulate their IL-1-β expression. This communication therefore altered the outcome of gene expression pattern. PC12-OX1R is only one type of cell and does not have similar communications like that of brain tissues.
To summarize, the current study showed that in anesthetized SD rats, PVN orexin A injection induced an increase in sympathetic outflow and blockage of OX1R attenuated the increase in SNA, indicating OX1R acute activation promotes sympathetic activity. The in vitro study using PC12-OX1R cells showed orexin A stimulation triggers AP1-PICs signalling. These data combined with the importance of PICs in regulating SNA and blood pressure suggest that upregulation of PVN orexin A-OX1R signalling may contribute to the development of hypertension by promoting sympathetic tone through AP1-PIC signalling.
4 |. MATERIALS AND METHODS
4.1 |. Animals
SD rats used in this study were purchased from Charles River Laboratories Inc. Rats were given ad libitum access to water and food and maintained at a room temperature of 23 ± 2°C, humidity of 55 ± 10% and on a 12-hour light/dark cycle. The rats were used for brain ICV infusion, brain mRNA measurements, immunohistochemistry and SNA recordings. The protocols of all the rat experiments were approved by the Michigan Technological University Institutional Animal care and Use Committee prior to conducting this research.
4.2 |. PVN-RVLM neurons labelling
Paraventricular nucleus neurons were labelled with retrograde tracer from the RVLM as previously described.41 Briefly, three male adult SD rats (350–400 g) were anesthetized with isoflurane (3% in O2) and placed in a stereotaxic frame. The cerebellum was exposed through a small burr hole. A glass micropipette was lowered into RVLM region (coordinates: −12.0 mm caudal to bregma, 1.8 mm lateral to midline and 8.9 mm below the dura). We chose this coordinate because maximum increase in RSNA and arterial blood pressure has been observed in response to L-glutamate (0.1 nmol in 50 nL) injection in this site based on the previous study.42 A pneumatic pump (Dagan Corporation, PMI-200) was used to inject rhodamine-containing microspheres (50 nL; Thermo Fisher Scientific, Waltham, MA, USA) into the RVLM. Rats were then received subcutaneous injection of penicillin G (30 000 units) and meloxicam (1 mg kg−1) for consecutive 3 days post-surgical care. Ten days following microinjection, rats were deeply anesthetized and received perfusion with 4% paraformaldehyde (PFA), and their brains were removed and kept in 4% PFA (in PBS) overnight; the rat brains were then stored in 30% sucrose solution until the brains sink to the bottom of the container. Then, brain coronal sections containing the PVN were cut and undergone immunohistochemistry to observe the OX1R and OX2R expressions as described below.
4.3 |. Experiment preparation for the in vivo sympathetic nerve recordings
Animal surgery was performed according to the previously described protocol.43 Briefly, rats were anesthetized with an intraperitoneal injection containing a mixture of α-chloralose (80 mg kg−1) and urethane (800 mg kg−1). The level of anaesthesia was assessed before surgery by lack of the pedal withdrawal reflex. The rats were first given a surgery of tracheal cannulation for easy of connection to the ventilator during the nerves recordings. Animals were instrumented with an arterial catheter inserted into the aorta via route through the left femoral artery. The catheter was connected to a pressure transducer to measure arterial blood pressure. Another catheter was placed in the left femoral vein to administer drugs. HR was obtained from the R-wave of the electrocardiogram (lead I). Rats were paralysed with gallamine triethiodide (25 mg·kg−1·h−1, i.v.) after artificially ventilating with oxygen-enriched room air using a ventilator (Kent Scientific Corporation). Supplemental doses of anaesthesia equal to 10% of the initial dose were given when needed. End-tidal CO2 pressure was continuously monitored using the End-Tidal CO2 Analyzer (CapStar-100; CWE Inc., Ardmore, PA, USA) and maintained at normal ranges (35–40 mmHg) by adjusting the O2 input pressure to the inhalation air. Body temperature was maintained at 37°C with a water-circulating pad. All animals were allowed to stabilize at least 2 hours following surgery.
4.4 |. PVN microinjection
Animals were divided into two groups, one group received vehicle control DMSO (100 nL per side) plus orexin A (25 pmol, 100 nL per side) and another group received OX1R antagonist SB408124 (300 pmol, 100 nL per side) plus orexin A (25 pmol, 100 nL per side) microinjection bilaterally into the PVN. PVN microinjections were performed as we described previously.43 Briefly, after the catheters were implanted into the left femoral artery and vein as we described above, the animals were placed in a stereotaxic head frame. The rat skull was levelled between bregma and lambda. A small hole on the skull was made by the drill to expose the dura. After more than 2 hours stabilizing for the signal of SSNA and RSNA, a single-barrelled glass microinjector pipette was lowered vertically into the PVN. The single-barrelled glass pipette contained either vehicle control, DMSO or orexin A, an agonist of OX1R and OX2R, or SB408124, an antagonist of the OX1R. The following coordinates were used for the microinjection: 1.4–1.6 mm caudal to bregma, 0.5 mm lateral to midline and 7.0–7.2 mm ventral to dura. The SB408124 was dissolved in DMSO, and the orexin A was dissolved in 0.9% saline. DMSO (or SB408124) and orexin A were microinjected into the PVN by an interval of 20 minutes with a pneumatic pump (PMI-200, Dagan Corporation). The micropipette was withdrawn between injections, and bilateral injections were separated by about 8 minutes. The response of the nerves to the PVN microinjection was recorded for more than 2 hours. At the end of each experiment, Chicago blue dye (2% in saline, 100 nL) was injected into the PVN to mark the injection sites. While the rats were still anesthetized, decapitation was performed to obtain their brains. The brain was removed and then fixed in 4% PFA. The hypothalamus including the PVN area was sliced in coronal sections, and microinjection sites were visualized under light microscopy. Rats with injection site(s) that were not inside the PVN were excluded from the data analysis.
4.5 |. In vivo sympathetic nerve recordings
Sympathetic nerve activity recording was carried out as described previously.43 With the use of a left flank incision, a left renal nerve and splanchnic sympathetic nerve bundle was isolated from the surrounding tissue. The nerve bundles were mounted on separate silver wire electrode (0.127 mm OD; A-M Systems, Sequim, WA, USA) and covered with a silicon-based impression material (Kwik-Sil, WPI) to insulate the recording nerves from body fluids. The recorded signal was input to an alternating current amplifier (P511; Grass Technologies, West Warwick, RI, USA). The signal was processed with rectify, integration (10 seconds time constant) and sampled at a frequency of 5000 Hz using a 1401 Micro3 hardware and Spike 2 software (version 7.04; Cambridge Electronic Design, Cambridge, UK). The background noise was determined by a bolus injection of hexamethonium bromide (30 mg kg−1 i.v.), a ganglionic blocker. The anesthetized rat was killed by administration of 0.5 mL 10% KCl (i.v.) at the end of the experiment.
4.6 |. ICV injection of orexin A
The ICV injection was carried out using the same protocol as our previous study.17 In brief, 10-week-old male adult SD rats were anesthetized with isoflurane (3% in O2). The rat was placed in the stereotaxic apparatus; the skull was kept in the same level between the bregma and lambda. Orexin A or control saline was infused into the lateral ventricle using the following coordinates: 0.8–0.9 mm caudal to the bregma; 1.4–1.8 mm lateral to the midline; and 3.2–3.8 mm below the dura surface. Each rat received only one injection. We separate the rats into two groups (n = 9 per group), one group received vehicle control (0.9% saline in 4 μL), another group received orexin A (0.2 nmol dissolved in 0.9% saline, 4 μL) injected into the lateral ventricle with a 10 μL NanoFil syringe connected to minipump (Micro 4, WPI Sarasota, FL USA). The injection rate was 1 μL min−1. After injection, the rats were returned to a clean cage; they became consciousness and move freely in a couple of minutes. Three hours following ICV injection, rats were killed. Three rats from each group were transcardially perfused with 4% PFA; then, their brains were removed and used for OX1R and Fra1 co-immunostaining. The remaining six rats from each group were used for PVN tissue punch and subjected to real-time PCR to assess the genes of interest in the PVN.
4.7 |. PC12 cells and cell culturing
PC12, PC12-OX1R and PC12-OX2R were kindly provided by Professor Jyrki Kukkonen from the University of Helsinki (Helsinki, Finland). PC12 cells were grown in DMEM supplement with 10% horse serum (HS), 5% FBS and 0.1% NGF. PC12-OX1R and PC12-OX2R cells were grown in DMEM supplement with 10% HS, 5% FBS, 0.1% NGF and 0.1% G418. All cells were cultured at 37°C with 5% CO2.
4.8 |. Measurement of mRNA levels of genes of interest
Real-time PCR was performed to measure mRNA levels of genes of interest in PVN tissues and cultured cells. For PVN tissues, rats were killed, their brains were removed, and PVN tissues were punched out as we described previously.44 The PVN tissues were kept in lysis buffer and either stored in −80°C freezer until used or directly subjected to RNA isolation. For cultured cells, cells were incubated with different concentration of orexin A for differing time, and then, culture media was removed and cell lysates were collected with lysis buffer and used for RNA isolation.
RNA was isolated using RNeasy Plus Mini Kit (Qiagen, Germantown, MD, USA) following the manufacture’s instruction. 200~500 ng RNA was reverse-transcribed into cDNA in a 10 μL reaction system using iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). mRNA levels were analysed by quantitative real-time PCR using primers and probes specific for genes of interest including IL-1-β, IL-6, TNF-α and Fra1. Taqman primers and probes for IL-1-β (Rn00580432_m1), IL-6 (Rn01410330_m1), TNF-α (Rn99999017_m1) and Fra1 (Rn 00564119_m1) were purchased from Applied Biosystems (Foster City, CA). Data were normalized to GAPDH (Rn01775763_g1) mRNA.
4.9 |. Immunoreactivity assessment of OX1R, OX2R, TNF-α and Fra1
Immunostaining of PVN OX1R and OX2R was performed with the following protocols: brain coronal sections (25 μm) containing the PVN were first washed in PBS three times for 10 minutes each. The brain sections were then incubated with either rabbit anti-OX1R antibody (1:500 dilution) or rabbit anti-OX2R antibody (1:1000 dilution) in PBS containing 0.5% Triton X-100 and 5% horse serum for 72 hours at 4°C. Afterwards, brain sections were washed with PBS three times for 10 minutes each. Then, they were incubated with secondary antibody Alexa Fluor 488 goat anti-rabbit IgG (1:000), overnight at 4°C. The sections were mounted in Vectashield mounting medium, and images were taken with a Leica DMIL microscope.
The protocol for co-immunostaining of OX1R and Fra1 in the PVN was essentially the same as described above except that the primary antibodies used in this experiment were a cocktail consisting of 1:500 dilution of rabbit anti-OX1R antibody and 1:200 dilution of mouse anti-Fra1 antibody, and the secondary antibodies used were a mixture of 1:1000 dilution of Alexa Fluor 488 goat anti-rabbit IgG and Alexa Fluor 594 donkey anti-mouse IgG.
Immunocytochemistry of Fra1 and TNF-α was performed using PC12-OX1R cells with the following protocols: the cells were rinsed with cold PBS and then fixed with 4% PFA for 1 minute; afterwards, the cells were washed with PBS containing 0.2% Triton X-100 two times for 10 minutes each. Then, cells were treated with 100% methanol (−20°C) for 1 minute and followed by washed with PBS three times for 10 minutes each. The cells were then incubated with either mouse anti-Fra1 antibody (1:200 dilution), or mouse anti-TNF-α antibody (1:400 dilution) in PBS containing 0.5% Triton X-100 and 5% horse serum for 12 hours at 4°C. Afterwards, cells were washed with PBS three times for 10 minutes each. Then, they were incubated with secondary antibody Alexa Fluor 488 goat anti-mouse IgG for 4 hours at room temperature. The immunoreactivity of Fra1 or TNF-α was observed under Leica DMIL microscope, and micrographs were taken.
4.10 |. Reagents and antibodies
Orexin A and curcumin were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). SB408124 was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Primary antibodies rabbit anti-OX1R and rabbit anti-OX2R were products of Alomone laboratories (Jerusalem, Israel); mouse anti-Fra1 antibody and mouse anti-TNFα antibody were from Santa Cruz Biotechnology. Secondary antibodies goat anti-rabbit IgG (H + L) Alexa Fluor® 488 and donkey anti-mouse IgG (H + L) Alexa Fluor® 594 were purchased from Thermo Fisher Scientific. Vectashield mounting medium was purchased from Vector Labs (Burlingame, CA, USA).
4.11 |. Data analysis
All data are expressed as mean ± SEM. SSNA and RSNA were determined as an average of the rectified and integrated signal. Baseline values of all recorded variables were obtained by averaging a 10-minute segment of data recorded immediately before PVN orexin A microinjection. SSNA, RSNA, MAP and HR responses to orexin A microinjection were obtained by averaging a 2-minute period centred on the maximal response. For determining the effect of microinjection of orexin A on the SNA, a per cent (%) change from baseline was calculated for each rat by the following formula:
where the noise value of background was determined after bolus injection of the ganglionic blocker hexamethonium (30 mg kg−1, i.v.). Both in vivo and in vitro data were analysed using GraphPad Prism 5.0 for Windows (GraphPad Software). Statistical significance was calculated by a two-tailed Student’s unpaired t test. Differences were considered statistical significance at a critical value of P < .05.
5 |. PHYSIOLOGICAL RELEVANCE
This study has investigated the physiological and molecular mechanism involved in the regulation of cardiovascular activity by the orexin signalling in the PVN of the hypothalamus.
ACKNOWLEDGEMENTS
All cell lines used in this study including PC12, PC12-OX1R and PC12-OX2R were gifts from Professor Jyrki Kukkonen at the University of Helsinki (Helsinki, Finland).
Funding information
This study was supported by NIH R15HL129213 (Shan), NIH R15HL122952 (Chen) and Michigan Technological University Research Excellence Fund (Shan) and Portage Health Foundation Research Excellence Fund (Shan). Yuanyuan Fan was supported by an Exchange Scholarship from China Scholarship Council of the Ministry of Education of China (File No. 201606280270).
Footnotes
CONFLICT OF INTEREST
There are no conflicts of interests.
REFERENCES
- 1.Xiao F, Jiang M, Du D, et al. Orexin A regulates cardiovascular responses in stress-induced hypertensive rats. Neuropharmacology. 2013;67:16–24. [DOI] [PubMed] [Google Scholar]
- 2.Samson WK, Gosnell B, Chang JK, Resch ZT, Murphy TC. Cardiovascular regulatory actions of the hypocretins in brain. Brain Res. 1999;831:248–253. [DOI] [PubMed] [Google Scholar]
- 3.Shirasaka T, Nakazato M, Matsukura S, Takasaki M, Kannan H. Sympathetic and cardiovascular actions of orexins in conscious rats. Am J Physiol. 1999;277:R1780–R1785. [DOI] [PubMed] [Google Scholar]
- 4.Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1–696. [DOI] [PubMed] [Google Scholar]
- 5.Smart D, Jerman JC, Brough SJ, Neville WA, Jewitt F, Porter RA. The hypocretins are weak agonists at recombinant human orexin-1 and orexin-2 receptors. Br J Pharmacol. 2000;129:1289–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Peyron C, Tighe DK, van den Pol AN, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci. 1998;18:9996–10015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Date Y, Ueta Y, Yamashita H, et al. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc Natl Acad Sci USA. 1999;96:748–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Follwell MJ, Ferguson AV. Cellular mechanisms of orexin actions on paraventricular nucleus neurones in rat hypothalamus. J Physiol. 2002;545:855–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shahid IZ, Rahman AA, Pilowsky PM. Intrathecal orexin A increases sympathetic outflow and respiratory drive, enhances baroreflex sensitivity and blocks the somato-sympathetic reflex. Br J Pharmacol. 2011;162:961–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shahid IZ, Rahman AA, Pilowsky PM. Orexin A in rat rostral ventrolateral medulla is pressor, sympatho-excitatory, increases barosensitivity and attenuates the somato-sympathetic reflex. Br J Pharmacol. 2012;165:2292–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SJ, Pilowsky PM, Farnham MM. Intrathecal intermittent Orexin-A causes sympathetic long-term facilitation and sensitizes the peripheral chemoreceptor response to hypoxia in rats. J Pharmacol Exp Ther. 2016;358:492–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang W, Fukuda Y, Kuwaki T. Respiratory and cardiovascular actions of orexin-A in mice. Neurosci Lett. 2005;385:131–136. [DOI] [PubMed] [Google Scholar]
- 13.Kayaba Y, Nakamura A, Kasuya Y, et al. Attenuated defense response and low basal blood pressure in orexin knockout mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R581–R593. [DOI] [PubMed] [Google Scholar]
- 14.Lee YH, Dai YW, Huang SC, Li TL, Hwang LL. Blockade of central orexin 2 receptors reduces arterial pressure in spontaneously hypertensive rats. Exp Physiol. 2013;98:1145–1155. [DOI] [PubMed] [Google Scholar]
- 15.Li A, Hindmarch CC, Nattie EE, Paton JF. Antagonism of orexin receptors significantly lowers blood pressure in spontaneously hypertensive rats. J Physiol. 2013;591:4237–4248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou JJ, Yuan F, Zhang Y, Li DP. Upregulation of orexin receptor in paraventricular nucleus promotes sympathetic outflow in obese Zucker rats. Neuropharmacology. 2015;99:481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huber MJ, Fan Y, Jiang E, et al. Increased activity of the Orexin system in the paraventricular nucleus contributes to salt-sensitive hypertension. Am J Physiol Heart Circ Physiol. 2017. 10.1152/ajpheart.00822.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jackson KL, Nguyen-Hun T, Stevenson ER, Davern PJ, Carrive P, Head GA. The contribution of orexin to the neurogenic hypertension in Bph/2j mice. Hypertension. 2015;65:E27–E28. [DOI] [PubMed] [Google Scholar]
- 19.Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD. A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res. 1989;491:156–162. [DOI] [PubMed] [Google Scholar]
- 20.Ferguson AV, Latchford KJ, Samson WK. The paraventricular nucleus of the hypothalamus – a potential target for integrative treatment of autonomic dysfunction. Expert Opin Ther Targets. 2008;12:717–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osborn JW, Fink GD, Sved AF, Toney GM, Raizada MK. Circulating angiotensin II and dietary salt: converging signals for neurogenic hypertension. Curr Hypertens Rep. 2007;9:228–235. [DOI] [PubMed] [Google Scholar]
- 22.Shi P, Raizada MK, Sumners C. Brain cytokines as neuromodulators in cardiovascular control. Clin Exp Pharmacol Physiol. 2010;37:e52–e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ye ZY, Li DP, Pan HL. Regulation of hypothalamic presympathetic neurons and sympathetic outflow by group II metabotropic glutamate receptors in spontaneously hypertensive rats. Hypertension. 2013;62:255–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wei SG, Yu Y, Zhang ZH, Felder RB. Proinflammatory cytokines upregulate sympathoexcitatory mechanisms in the subfornical organ of the rat. Hypertension. 2015;65:1126–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Holmqvist T, Akerman KE, Kukkonen JP. Orexin signaling in recombinant neuron-like cells. FEBS Lett. 2002;526:11–14. [DOI] [PubMed] [Google Scholar]
- 26.Yoshida Y, Fujiki N, Nakajima T, et al. Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci. 2001;14:1075–1081. [DOI] [PubMed] [Google Scholar]
- 27.Johnson PL, Truitt W, Fitz SD, et al. A key role for orexin in panic anxiety. Nat Med. 2010;16:111–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martins PJ, D’Almeida V, Pedrazzoli M, Lin L, Mignot E, Tufik S. Increased hypocretin-1 (orexin-a) levels in cerebrospinal fluid of rats after short-term forced activity. Regul Pept. 2004;117:155–158. [DOI] [PubMed] [Google Scholar]
- 29.Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol. 1989;256:R1325–R1330. [DOI] [PubMed] [Google Scholar]
- 30.Kannan H, Niijima A, Yamashita H. Inhibition of renal sympathetic nerve activity by electrical stimulation of the hypothalamic paraventricular nucleus in anesthetized rats. J Auton Nerv Syst. 1987;21:83–86. [DOI] [PubMed] [Google Scholar]
- 31.Yamashita H, Kannan H, Kasai M, Osaka T. Decrease in blood pressure by stimulation of the rat hypothalamic paraventricular nucleus with L-glutamate or weak current. J Auton Nerv Syst. 1987;19:229–234. [DOI] [PubMed] [Google Scholar]
- 32.D’Arcangelo G, Tancredi V, Onofri F, D’Antuono M, Giovedi S, Benfenati F. Interleukin-6 inhibits neurotransmitter release and the spread of excitation in the rat cerebral cortex. Eur J Neuorsci. 2000;12:1241–1252. [DOI] [PubMed] [Google Scholar]
- 33.Mo ZL, Katafuchi T, Hori T. Effects of IL-1 beta on neuronal activities in the dorsal motor nucleus of the vagus in rat brain slices. Brain Res Bull. 1996;41:249–255. [DOI] [PubMed] [Google Scholar]
- 34.Greene LA. Nerve growth factor prevents the death and stimulates the neuronal differentiation of clonal PC12 pheochromocytoma cells in serum-free medium. J Cell Biol. 1978;78:747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luo Y, Zheng SG. Hall of fame among pro-inflammatory cytokines: interleukin-6 gene and its transcriptional regulation mechanisms. Front Immunol. 2016;7:604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sei Y, Vitkovic L, Yokoyama MM. Cytokines in the central nervous system: regulatory roles in neuronal function, cell death and repair. NeuroImmunoModulation. 1995;2:121–133. [DOI] [PubMed] [Google Scholar]
- 37.Bhandare AM, Mohammed S, Pilowsky PM, Farnham MM. Antagonism of PACAP or microglia function worsens the cardiovascular consequences of kainic-acid-induced seizures in rats. J Neurosci. 2015;35:2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhandare AM, Kapoor K, Pilowsky PM, Farnham MM. Seizure-induced sympathoexcitation is caused by activation of glutamatergic receptors in RVLM that also causes proarrhythmogenic changes mediated by PACAP and microglia in rats. J Neurosci. 2016;36:506–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapoor K, Bhandare AM, Farnham MM, Pilowsky PM. Alerted microglia and the sympathetic nervous system: a novel form of microglia in the development of hypertension. Respir Physiol Neurobiol. 2016;226:51–62. [DOI] [PubMed] [Google Scholar]
- 40.Kapoor K, Bhandare AM, Nedoboy PE, Mohammed S, Farnham MM, Pilowsky PM. Dynamic changes in the relationship of microglia to cardiovascular neurons in response to increases and decreases in blood pressure. Neuroscience. 2016;329:12–29. [DOI] [PubMed] [Google Scholar]
- 41.Chen QH, Andrade MA, Calderon AS, Toney GM. Hypertension induced by angiotensin II and a high salt diet involves reduced SK current and increased excitability of RVLM projecting PVN neurons. J Neurophysiol. 2010;104:2329–2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen QH, Toney GM. In vivo discharge properties of hypothalamic paraventricular nucleus neurons with axonal projections to the rostral ventrolateral medulla. J Neurophysiol. 2010;103: 4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huber MJ, Basu R, Cecchettini C, Cuadra AE, Chen QH, Shan Z. Activation of the (pro)renin receptor in the paraventricular nucleus increases sympathetic outflow in anesthetized rats. Am J Physiol Heart Circ Physiol. 2015;309:H880–H887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larson RA, Gui L, Huber MJ, et al. Sympathoexcitation in AngII-salt hypertension involves reduced SK channel function in the hypothalamic paraventricular nucleus. Am J Physiol Heart Circ Physiol. 2015;308:H1547–H1555. [DOI] [PMC free article] [PubMed] [Google Scholar]


