Abstract
Background
How to speed the recovery of viable myocardium in chronic total occlusion (CTO) patients after revascularization is still an unsolved problem. Breviscapine is widely used in cardiovascular diseases. However, there has been no study focused on the effect of breviscapine on viable myocardium recovery and left ventricular remodeling after CTO revascularization.
Material/Methods
We propose to recruit 78 consecutive coronary artery disease (CAD) patients with CTO during a period of 12 months. They will be randomly assigned to receive either breviscapine (40 mg) or placebo in the following 12 months. Blood tests, electrocardiogram, and Major Adverse Cardiac Events (MACE) will be collected at baseline and the follow-up visits at 1, 3, 6, 9, and 12 months. Low-dose dobutamine MRI will be applied for the assessment of viable myocardium, microcirculation perfusion, and left ventricular remodeling, and the concentrations of angiogenic cytokine, vascular endothelial growth factor (VEGF), and basic fibroblast growth factor (bFGF) will be investigated at baseline and at 1- and 12-month follow-up. The recovery of viable myocardium after revascularization in CTO patients was the primary endpoint. Improvement of microcirculation perfusion, left ventricular remodeling, peripheral concentrations of VEGF and bFGF as well as MACE will be the secondary endpoints.
Results
Breviscapine treatment obviously improve the recovery of viable myocardium, myocardial microcirculation perfusion, and left ventricular remodeling after revascularization in CTO patients, and reduce the occurrence of MACE. We also will determine if breviscapine increases the peripheral blood angiogenic cytokine concentrations of VEGF and bFGF.
Conclusions
This study will aim to demonstrate the effect of breviscapine on the recovery of viable myocardium and left ventricular remodeling in CTO patients after revascularization.
MeSH Keywords: Angina, Stable; Cell Survival; Magnetic Resonance Imaging
Background
With socioeconomic development in China, the incidence of coronary artery disease (CAD) has steadily increased. Chronic totally occluded (CTO) patients account for about 15–30% of all CAD patients [1]. Left ventricular dysfunction induced by CTO has become one of the most common causes of cardiac mortality and mobility.
Recent studies have shown that stunned or hibernating myocardium can survive in the area where the blood supply is provided by collateral circulation in the CTO lesions [2,3]. This part of viable myocardium can return to robust condition after myocardial revascularization, which provides the possibility for improvement of cardiac function. However, the recovery of viable myocardial function and improvement of left ventricular function is a continuous process that takes several weeks or months, suggesting that simple reconstruction of the effective blood flow and pressure is not sufficient. Previous studies reported that myocardial angiogenesis [4], establishment of collateral circulation, recovery of endothelial function [5], and improvement of microcirculation are also involved. Therefore, how to speed the recovery of viable myocardium and improve left ventricular function is a key point in the CTO therapy.
Breviscapine is a flavonoid extracted from the whole plant Euphorbiaceae eucalyptus, which has anti-microbial, anti-inflammatory, antioxidant [6], free radical-eliminating, and antineoplastic effects. It was also reported that breviscapine can inhibit protein kinase C (PKC) activity [7–9] and promote the recovery of endothelial function and microcirculation, thus ameliorating myocardial ischemia-reperfusion injury [10–12] and delaying the reversal of left ventricular hypertrophy and myocardial fibrosis induced by pressure overload [8]. However, there has been no study focused on the effect of breviscapine on viable myocardium and left ventricular remodeling after CTO revascularization.
In the proposed study, we will investigate whether Breviscapine therapy can improve the recovery of viable myocardium and left ventricular remodeling after CTO revascularization. We will use low-dose dobutamine MRI imaging to assess the effect of breviscapine on the recovery of viable myocardium, myocardial microcirculation, and left ventricular remodeling after CTO revascularization. We also will examine whether Breviscapine treatment can increase the peripheral blood concentrations of VEGF and bFGF angiogenic cytokine. Th proposed study is intended to provide evidence for further clinical application of breviscapine in the recovery of cardiac function of CTO patients after revascularization.
Material and Methods
Study program
The proposed research will be designed as a prospective, single-center, randomized, double-blind, placebo-controlled study. CAD patients with at least 1 CTO lesion confirmed either by coronary CTA or coronary angiography (CAG) in subjects who are randomly allocated to receive either breviscapine (40 mg) or placebo therapy for 12 months. All patients will receive basic standard drug therapy (including antiplatelet drugs, statins, ACEI/ARB [angiotensin-converting enzyme inhibitors/angiotensin receptor blocker], and β receptor blockers) for the treatment of coronary heart disease. All participants will provide written informed consent. Automated randomization (randomization and medication ordering system, Glaxo Smith Kline) will be used for a randomly permuted block of 4 subjects in a 2: 2 ratio for breviscapine: placebo distribution in the different treatment groups. The primary endpoint will be the change of viable myocardium percentage assessed by MRI after 12-month treatment with breviscapine or placebo when compared with the baseline. The secondary endpoints will be incidence of MACE, including death, myocardial infarction, stroke, recurrent angina, re-hospitalization rate, change of viable myocardium from baseline to 1 month, improvement of myocardial microcirculation perfusion, left ventricular remodeling, and change of peripheral concentrations of VEGF and bFGF angiogenic cytokine at baseline and at 1 and 12 months. Details of the proposed study, including the enrollment, exclusion criteria, and follow-up visit plan, are listed in Table 1.
Table 1.
Study plan.
| Activity | Visit 0 Screening |
Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 |
|---|---|---|---|---|---|---|
| Study month | 0 day | 1 m±7day | 3 m±7day | 6 m±7day | 9 m±7day | 12 m±7day |
| Informed consent | x | |||||
| Demography and medical history | x | |||||
| Inclusion/exclusion criteria | x | |||||
| Physical examination/Vital signs | x | x | x | x | x | x |
| 12-lead ECG | x | x | x | x | x | x |
| Concomitant medication | x | x | x | x | x | x |
| Complete blood count | x | x | x | x | x | x |
| ALT/AST/ALB/TB/DB | x | x | x | x | x | x |
| Scr/BUN | x | x | x | x | x | x |
| TC/LDL-C/HDL-C/Lp(a)/TG | x | x | x | x | x | x |
| Cardiac MRI | x | x | x | |||
| Serum VEGF/bFGF concentration |
x | x | x | |||
| Adverse events | x | x | x | x | x | x |
ECG – electrocardiography; ALT – alanine aminotransferase; AST – aspartate aminotransferase; Scr – serum creatinine; BUN – blood urea nitrogen; TC – total cholesterol; LDL-C – low-density lipoprotein cholesterol; HDL-C – low-density lipoprotein cholesterol; TG – total triglycerides; Lp(a) – lipoprotein a; MRI – magnetic resonance imagingl VEGF – vascular endothelial growth factor, bFGF – basic fibroblast growth factor.
This study will be funded by the Traditional Chinese Medical Science and Technology Plan Projects of Zhejiang Province. This trial protocol will be conducted in agreement with the ethics guidelines of the 1975 Declaration of Helsinki. Protocols and informed consent forms of the trial will be approved by the Institutional Ethics Committee of Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University, and informed consent forms will be approved by the Institutional Ethics Committee of Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University. Written informed consent will be obtained from all patients. This trial will be registered in the Chinese Clinical Trial Registry (ChiCTR-IPC-16010161).
Study objectives
The proposed study will aim to verify the assumption that breviscapine (40 mg, 3 time a day [13,14], for 12 months) significantly promotes the recovery of viable myocardium and improves left ventricular remodeling after CTO revascularization, and to explore the potential mechanism. This study will be conducted over a 2-year period, including an estimated screening and enrollment period of 12 months and follow-up visit period of 1 year. The main purpose will be to verify that Breviscapine can significantly promote the recovery of viable myocardium after CTO revascularization. The secondary objective will be to confirm that breviscapine can improve myocardial microcirculation perfusion and left ventricular remodeling, increase left ventricular function, and reduce MACE. The exploratory goal will be to establish the underlying mechanism among serum VEGF and bFGF, the viable myocardium, myocardial microcirculation perfusion, and left ventricular remodeling. Oral breviscapine therapy will be applied as an additional treatment on the basis of the standard drug therapy. Therefore, the fundamental efficacy and safety of the patients will be provided and ensured due to the standard drug therapy. In addition, Breviscapine’s adverse effects such as bleeding, hepatic or renal function impairment, and other AEs will be carefully observed and documented.
Inclusion and exclusion criteria
Seventy-eight CAD patients with at least 1 CTO lesion confirmed by CTA or CAG are expected to be included in Sir Run Run Shaw Hospital, School of Medicine, Zhejiang University. The enrollment period is anticipated to be finished before 2018. All eligible patients will be registered. More details of inclusion and exclusion criteria are listed in Table 2.
Table 2.
Inclusion and exclusion criteria.
| Inclusion criteria |
|---|
|
| Exclusion criteria |
|
CTA – computed tomography angiography; PCI – percutaneous coronary intervention; eGFR – estimated glomerular filtration rate; CABG – coronary artery bypass graft.
Exclusion criteria during the follow-up periods
Patients’ demand to withdrew from the study and withdrawal the inform consent.
Study protocol deviation.
Lost to follow-up due to patients.
Patients bearing high risk (e.g., hepatic dysfunction as transaminase levels ≥3×ULN or renal dysfunction as eGFR ≤30 ml/min/1.73 m2).
Pregnant women or new-onset malignant tumor during the study periods.
All of above conditions will be documented in the case report form (CRF). Patients who cannot complete the follow-up will be scheduled an additional visit if possible.
Study procedures
Figure 1 shows the study protocol. Enrolled patients will be randomly assigned to receive either Breviscapine (40 mg) or placebo, 3 times a day for 12 months. Besides the Breviscapine or placebo, all other interventions strictly comply with the standard. Demography and medical history as well as a simple physical examination, including measurement of weight and vital signs (supine systolic and diastolic blood pressure, heart rate) will be collected at baseline visit and every follow-up visit; blood tests and a 12-lead electrocardiogram inspection analysis will also be done at baseline and each follow-up visit. Blood tests will include complete blood count (CBC), serum creatinine (Scr), blood urea nitrogen (BUN), alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum albumin (ALB), total bilirubin (TB), direct bilirubin (DB), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), lipoprotein a (LPa), triglyceride (TG). Cardiac low-dose dobutamine MRI will be done at baseline, 1 month, and 12 months follow-up to assess viable myocardium, microcirculation perfusion and left ventricular remodeling. Additionally, peripheral blood VEGF and bFGF cytokine concentrations will also tested at baseline, 1 month, and 12 months follow-up. All MACE and AEs will be recorded at each follow-up visit. We will evaluate all the AE and SAE and adjust the treatment plan in time if it is necessary. In case of a serious adverse event or severe reactions which patients cannot tolerate during the course of the trial, researchers can withdraw the medication according to the protocol of the study.
Figure 1.
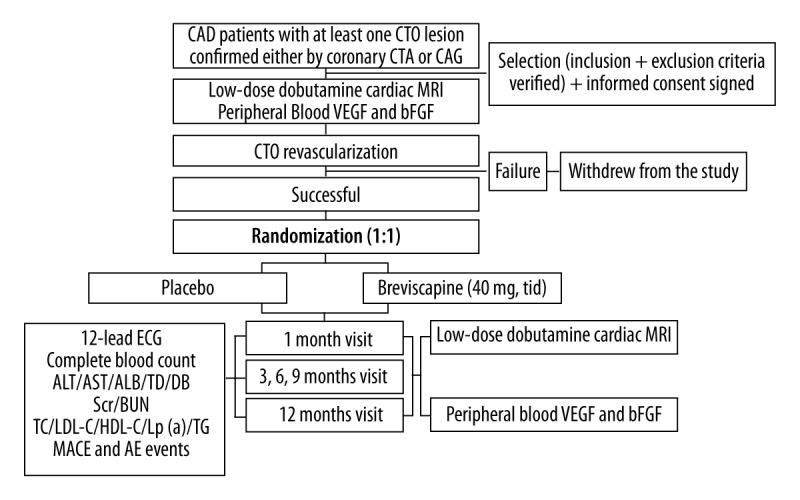
Flowchart outlining the trial protocol.
Determination of peripheral blood VEGF and bFGF cytokine concentrations
At baseline, 1 month, and 12 months follow-up visit, peripheral blood VEGF and bFGF cytokine concentrations will be assessed by ELISA assay following the manufacturer’s instructions (R & D System, Minneapolis, MN, USA).
Cardiac magnetic resonance imaging (MRI)
Aruges software will be used to select the appropriate time to clearly record the endocardium. Left ventricular remodeling parameters such as the thickness and thickening rate of each segment of left ventricular, left ventricular mass, left ventricular mass index, ejection fraction, end-diastolic volume, end-diastolic volume index, end-systolic volume, end-systolic volume index, cardiac output, cardiac index and myocardial wall function index will be measured and calculated [15,16].
Myocardial delayed scan will be analyzed according to 17-segment method [17]. The results of semi-quantitative analysis will be used to determine the viable myocardium and myocardial microcirculation. The proportion of left ventricular wall thickness in delayed enhancement will be divided into 4 grades: no delayed enhancement was defined as 1 point, 1% to 25% for 2 points, 25% to 75% for 3 points, and >75% for 4 points.
Sample determination and statistical analyses
The minimum necessary sample size in the trial will be established by the requirement to detect the smallest expected clinically meaningful treatment difference between Breviscapine treatment and placebo. According to the clinical literature and our previous basic research, we set the hypothesis that the improvement of viable myocardium treated by Breviscapine (40 mg, 3 times a day) is 20% when compared with the control group. In the case of 80% power, the sample size is calculated as 35 cases in each group. According to the 10% loss rate, the total sample size is 78 cases, 39 cases in each group. All analysis will be performed on an intention-to-treat basis.
Categorical variables will be expressed in numerical values with their respective percentages and compared using the chi-squared test at baseline. Continuous variables will be expressed as mean ±SD and compared using the t test. Significance of primary and secondary endpoints will be evaluated with the paired-samples t test. A p value <0.05 indicates statistical significance. All data will be analyzed with SPSS software package, version 22.0 (SPSS Inc., Chicago, IL, USA).
Discussion
Recent studies have found viable myocardium in the CTO lesion where the blood supply is provided by the collateral circulation [2,3]. In 258 patients with chronic ischemic LV dysfunction, recovery of global LV function [defined as improvement ≥5% in LV ejection fraction (EF)] after revascularization was observed in 39% of patients [18]. Studies have shown that patients with viable myocardium have a better prognosis after revascularization; however, patients with non-viable myocardium have worse outcomes with higher perioperative morbidity and mortality subsequent to revascularization [19]. In patients with viable myocardium, an improvement of LVEF was observed (37–45% on average), while patients without viability did not improve in LVEF [20]. More importantly, the presence of viable myocardium has been proved to be related with long-term outcome. In 17 available retrospective prognostic studies, outcome was better in patients with viable myocardium who were revascularized compared with those without viable myocardium [21]. Thus, CTO patients with viable myocardium could benefit from revisualization, the extent of viable tissue predicted the magnitude of improvement of LVEF after revascularization. However, the recovery of myocardial function and the improvement of left ventricular function is a long and sustained process. In addition to effective revascularization, it may also involve blood reconstruction of myocardial neovascularization [4], collateral circulation, endothelial function recovery [5], microcirculation improvement, and other factors.
Currently, the imaging methods to identify viable myocardium include positron emission computed tomography (PET), single-photon emission tomography (SPECT), dobutamine stress echocardiography (DSE), myocardial contrast echocardiography (MCE), and magnetic resonance imaging (MRI). MRI has some advantages, such as high spatial resolution, no radiation, no violation, higher repeatability of three- dimensional images measurement. It allows for high-resolution assessment of the area at risk, myocardial necrosis, and microvascular damage (microvascular obstruction). Moreover, assessment of the occurrence and extent of reperfusion injury as well as the regional LV function (functional imaging) can be performed with MRI in a manner similar to echocardiography. Cine-MRI can directly measure the volume of heart cavity, as well as the thickness and the motion of ventricular wall, which provides objective and reproducible indicators for left ventricular remodeling [22–26]. Recent studies have proved that MRI can accurately assess the perfusion of myocardial microcirculation [27,28] and endothelial dysfunction [29]. Additionally, contrast-enhanced MRI can characterize myocardial ischemic injury, and has the ability to distinguish viable from nonviable zones [30]. Thus, the use of MRI imaging has unparalleled advantages over other methods in assessing viable myocardium, microcirculation perfusion, and ventricular remodeling in CTO patients.
Late gadolinium enhancement by MRI combined with low-dose dobutamine is the best technique for determining viable myocardium. The major advantages of dobutamine MRI over dobutamine stress echocardiography are the image quality and the possibility of quantifying the extent and severity of the wall motion abnormalities [31,32]. Similar to low-dose dobutamine echocardiography, lower dosages of dobutamine (10 μg/kg/min) MRI are mainly used to detect contractile reserve (and hence viability) in dysfunctional myocardium. The approach of low-dose dobutamine (10 μg/kg/min) MRI has been applied successfully in patients with chronic coronary artery disease [33,34]. Baer et al. [35] compared dobutamine MRI with FDG PET and dobutamine echocardiography and demonstrated excellent agreement among these 3 approaches. Subsequently, these authors showed that dobutamine MRI could adequately predict improvement of regional LV function after revascularization; moreover, the diagnostic accuracy of dobutamine MRI was superior to that of resting MRI (using EDWT ≥5.5 mm as a „viability marker”). Therefore, in the current study, we will choose low-dose dobutamine MRI to assess viable myocardium and cardiac function because it has the advantages of non-invasiveness, no radiation, no violation, higher repeatability, and image quality.
With the modernization of Chinese traditional medicine and technology, an increasing number of Chinese herbal medicines are used clinically. Breviscapine is a total flavonoid extracted from Erigeron breviscapus (Vant) Hand-Mazz. Studies have shown that Breviscapine can dilate capillaries, reduce vascular resistance, inhibit platelet aggregation, scavenge free radicals, and improve microcirculation [36–39]. A meta-analysis suggests that Breviscapine plus standard therapy achieved a superior therapeutic effect compared to standard Western medicine therapy for improving angina pectoris symptoms [40]. Meanwhile, severe adverse effects have rarely been reported for Breviscapine (including headache, palpitation, and fatigue).
Basic research has revealed that Breviscapine can inhibit protein kinase C (PKC) activity [7–9] and increase the mitochondrial function of myocardium, consequently ameliorating the myocardial ischemia-reperfusion injury [10–12]. Our previous study also suggested that Breviscapine significantly inhibited high glucose-induced PKC signaling pathway activation, thereby attenuating cardiac hypertrophy, regulating calmodulin, and then enhancing myocardial contractility [7,41]. In addition, we also found that Breviscapine could inhibit ERK (extracellular signal-regulated kinase) signaling pathways, resulting in downregulation of high glucose-induced smooth muscle cell proliferation and migration [42]. Therefore, a series of basic research results have shown that Breviscapine can improve endothelial function, microcirculation perfusion, and cardiac function. However, no has study focused on the effect of Breviscapine on viable myocardium and left ventricular remodeling after CTO revascularization.
Vascular endothelial growth factor (VEGF) is a signal protein produced by cells that stimulates vasculogenesis and angiogenesis [43,44], which has the function of creating new blood vessels (collateral circulation) to bypass blocked vessels. Basic fibroblast growth factor (bFGF) is a member of the fibroblast growth factor family [45], which presents in basement membranes and in the subendothelial extracellular matrix of blood vessels [46,47]. bFGF is also a major mediator of vascular angiogenesis, having the function of mediating the formation of new blood vessels and playing a central role in the development of coronary collaterals [48]. Clinical studies have shown that patients with myocardial ischemia and infarction have elevated levels of VEGF mRNA in myocardial tissues, potentially as an important cardiac response to hypoxia [49,50]. Through their proven angiogenic effect, the administration of VEGF and bFGF have been explored as an indicator of increased collateralization in patients with end-stage coronary heart disease.
Limitations
First, though the proposed study is designed as a prospective, randomized, double-blind, placebo-controlled study, it will be a single-center study, which may cause the baseline drift of the selected patients. At the same time, the study sample size will be relatively small.
Second, there is a relatively high rate of in-stent restenosis (ISR) in CTO lesions when compared with non-CTO lesions [51]. According to several studies, CTO lesions have a 1.4- to 5-fold higher rate of ISR than standard coronary lesions [52], which may present as unstable angina (16–66%) or myocardial infarction (1–20%) [51]. Therefore, the incidence of ISR occurs in the 1-year follow-up may lead to myocardial ischemia, which will affect the results of the study, causing biased results, as the myocardial ischemia induced by stent restenosis will influence the treatment efficacy of Breviscapine.
Third, CTO patients are more common in the presence of other non-significantly-narrowed coronary arteries. In the Canadian CTO Registry [53], multi-vessel CAD (>50% diameter stenosis) was present in three-fourths of patients with CTO. Those non-CTO lesions are more likely to progress gradually compared with lesions not combined with CTO. Thus, the original existence of non-CTO lesions that may advance at the time of 1-year follow-up will also lead to results bias.
Fourth, some patients with CTO lesions may not be able to tolerate dobutamine stress MRI before revascularization or during subsequent follow-up visits for various reasons; for example, those with severe cardiac insufficiency cannot tolerate the load test or contraindication to dobutamine, which results in selection bias.
Conclusions
The proposed study will be the first to investigate the influence of Breviscapine on the recovery of viable myocardial in CTO patients after revascularization. We expect that the study results will confirm whether Breviscapine treatment can significantly promote the recovery of viable myocardium and improve left ventricular remodeling, as well as microcirculation after CTO revascularization.
Footnotes
Conflict of interest
None.
Source of support: This study was supported by the Traditional Chinese Medical Science and Technology Plan Projects of Zhejiang Province (grants 2017ZQ018 and LQ16H020001)
References
- 1.George S, Cockburn J, Clayton TC, et al. Long-term follow-up of elective chronic total coronary occlusion angioplasty: Analysis from the U.K. Central Cardiac Audit Database. J Am Coll Cardiol. 2014;64(3):235–243. doi: 10.1016/j.jacc.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 2.Petronio AS, Baglini R, Limbruno U, et al. Coronary collateral circulation behaviour and myocardial viability in chronic total occlusion treated with coronary angioplasty. Eur Heart J. 1998;19(11):1681–87. doi: 10.1053/euhj.1998.1154. [DOI] [PubMed] [Google Scholar]
- 3.Nii H, Wagatsuma K, Kabuki T, et al. Significance of percutaneous transluminal coronary intervention for chronic total occlusions assessed as non-viable by myocardial scintigraphy. J Cardiol. 2007;50(6):363–70. [PubMed] [Google Scholar]
- 4.Finn AV, Kolodgie FD, Nakano M, Virmani R. The differences between neovascularization of chronic total occlusion and intraplaque angiogenesis. JACC Cardiovasc Imaging. 2010;3(8):806–10. doi: 10.1016/j.jcmg.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 5.Brugaletta S, Martin-Yuste V, Masotti M, et al. Endothelial function in coronary chronic total occlusions: need for rigorous methodology. J Am Coll Cardiol. 2012;60(9):871–72. doi: 10.1016/j.jacc.2012.03.073. [DOI] [PubMed] [Google Scholar]
- 6.Wang M, Xie C, Cai RL, et al. Studies on antioxidant activities of Breviscapine in the cell-free system. Am J Chin Med. 2008;36(6):1199–207. doi: 10.1142/S0192415X08006521. [DOI] [PubMed] [Google Scholar]
- 7.Wang M, Zhang WB, Zhu JH, et al. Breviscapine ameliorates hypertrophy of cardiomyocytes induced by high glucose in diabetic rats via the PKC signaling pathway. Acta Pharmacol Sin. 2009;30(8):1081–91. doi: 10.1038/aps.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yan L, Huang H, Tang QZ, et al. Breviscapine protects against cardiac hypertrophy through blocking PKC-alpha-dependent signaling. J Cell Biochem. 2010;109(6):1158–71. doi: 10.1002/jcb.22495. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Qi F, Wu W. Effect of intervention in the diacylglycerol protein kinase C signaling pathway on JNK1 expression and its downstream signaling in diabetic cardiomyopathy. Mol Med Rep. 2014;9(3):979–84. doi: 10.3892/mmr.2014.1904. [DOI] [PubMed] [Google Scholar]
- 10.Jia JH, Chen KP, Chen SX, et al. Breviscapine, a traditional Chinese medicine, alleviates myocardial ischaemia reperfusion injury in diabetic rats. Acta Cardiol. 2008;63(6):757–62. doi: 10.2143/AC.63.6.2033394. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Ji SY, Liu SZ, et al. Cardioprotective effect of Breviscapine: Inhibition of apoptosis in H9c2 cardiomyocytes via the PI3K/Akt/eNOS pathway following simulated ischemia/reperfusion injury. Pharmazie. 2015;70(9):593–97. [PubMed] [Google Scholar]
- 12.Guo C, Zhu Y, Weng Y, et al. Therapeutic time window and underlying therapeutic mechanism of Breviscapine injection against cerebral ischemia/reperfusion injury in rats. J Ethnopharmacol. 2014;151(1):660–66. doi: 10.1016/j.jep.2013.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Zhou X, Gao YY, Hu JY, et al. Effect of Breviscapine on CYP3A metabolic activity in healthy volunteers. Eur J Clin Pharmacol. 2018;74(1):37–44. doi: 10.1007/s00228-017-2346-8. [DOI] [PubMed] [Google Scholar]
- 14.Gao J, Chen G, He H, et al. Therapeutic effects of breviscapine in cardiovascular diseases: A review. Front Pharmacol. 2017;8:289. doi: 10.3389/fphar.2017.00289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dendale PA, Franken PR, Waldman GJ, et al. Low-dosage dobutamine magnetic resonance imaging as an alternative to echocardiography in the detection of viable myocardium after acute infarction. Am Heart J. 1995;130(1):134–40. doi: 10.1016/0002-8703(95)90248-1. [DOI] [PubMed] [Google Scholar]
- 16.Sayad DE, Willett DL, Hundley G, et al. Dobutamine magnetic resonance imaging with myocardial tagging quantitatively predicts improvement in regional function after revascularization. Am J Cardiol. 1998;82(9):1149–51. doi: 10.1016/s0002-9149(98)00579-7. [DOI] [PubMed] [Google Scholar]
- 17.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–42. doi: 10.1161/hc0402.102975. [DOI] [PubMed] [Google Scholar]
- 18.Schinkel AF, Poldermans D, Vanoverschelde JL, et al. Incidence of recovery of contractile function following revascularization in patients with ischemic left ventricular dysfunction. Am J Cardiol. 2004;93(1):14–17. doi: 10.1016/j.amjcard.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Ramos M, DePasquale E, Coplan NL. Assessment of myocardial viability: Review of the clinical significance. Rev Cardiovasc Med. 2008;9(4):225–31. [PubMed] [Google Scholar]
- 20.Schinkel AF, Bax JJ, Poldermans D, et al. Hibernating myocardium: Diagnosis and patient outcomes. Curr Probl Cardiol. 2007;32(7):375–410. doi: 10.1016/j.cpcardiol.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 21.Patel P, Ivanov A, Ramasubbu K. Myocardial viability and revascularization: Current understanding and future directions. Curr Atheroscler Rep. 2016;18(6):32. doi: 10.1007/s11883-016-0582-5. [DOI] [PubMed] [Google Scholar]
- 22.Goldfarb JW, Zhao W. Magnetic resonance imaging dynamic contrast enhancement (DCE) characteristics of healed myocardial infarction differ from viable myocardium. Magn Reson Imaging. 2014;32(10):1191–97. doi: 10.1016/j.mri.2014.08.023. [DOI] [PubMed] [Google Scholar]
- 23.Romero J, Lupercio F, Haramati LB, et al. Myocardial viability and microvascular obstruction: Role of cardiac magnetic resonance imaging. Cardiol Rev. 2014;22(5):246–52. doi: 10.1097/CRD.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 24.Schoos MM, Munthe-Fog L, Skjoedt MO, et al. Association between lectin complement pathway initiators, C-reactive protein and left ventricular remodeling in myocardial infarction – a magnetic resonance study. Mol Immunol. 2013;54(3–4):408–14. doi: 10.1016/j.molimm.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Zuern CS, Krumm P, Wurster T, et al. Reverse left ventricular remodeling after percutaneous mitral valve repair: Strain analysis by speckle tracking echocardiography and cardiac magnetic resonance imaging. Int J Cardiol. 2013;168(5):4983–85. doi: 10.1016/j.ijcard.2013.07.132. [DOI] [PubMed] [Google Scholar]
- 26.Merten C, Beurich HW, Zachow D, et al. Aortic regurgitation and left ventricular remodeling after transcatheter aortic valve implantation: A serial cardiac magnetic resonance imaging study. Circ Cardiovasc Interv. 2013;6(4):476–83. doi: 10.1161/CIRCINTERVENTIONS.112.000115. [DOI] [PubMed] [Google Scholar]
- 27.Salehi RM, Brix G, Laun FB, et al. Quantification of pulmonary microcirculation by dynamic contrast-enhanced magnetic resonance imaging: Comparison of four regularization methods. Magn Reson Med. 2013;69(1):188–99. doi: 10.1002/mrm.24220. [DOI] [PubMed] [Google Scholar]
- 28.Patel AR, Epstein FH, Kramer CM. Evaluation of the microcirculation: Advances in cardiac magnetic resonance perfusion imaging. J Nucl Cardiol. 2008;15(5):698–708. doi: 10.1016/j.nuclcard.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ichikawa Y, Kitagawa K, Kato S, et al. Altered coronary endothelial function in young smokers detected by magnetic resonance assessment of myocardial blood flow during the cold pressor test. Int J Cardiovasc Imaging. 2014;30(Suppl 1):73–80. doi: 10.1007/s10554-014-0387-y. [DOI] [PubMed] [Google Scholar]
- 30.Bax JJ, Delgado V. Detection of viable myocardium and scar tissue. Eur Heart J Cardiovasc Imaging. 2015;16(10):1062–64. doi: 10.1093/ehjci/jev200. [DOI] [PubMed] [Google Scholar]
- 31.Health Quality Ontario: Magnetic resonance imaging (MRI) for the assessment of myocardial viability: An evidence-based analysis. Ont Health Technol Assess Ser. 2010;10(15):1–45. [PMC free article] [PubMed] [Google Scholar]
- 32.Foley JR, Plein S, Greenwood JP. Assessment of stable coronary artery disease by cardiovascular magnetic resonance imaging: Current and emerging techniques. World J Cardiol. 2017;9(2):92–108. doi: 10.4330/wjc.v9.i2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baer FM, Theissen P, Schneider CA, et al. Dobutamine magnetic resonance imaging predicts contractile recovery of chronically dysfunctional myocardium after successful revascularization. J Am Coll Cardiol. 1998;31(5):1040–48. doi: 10.1016/s0735-1097(98)00032-1. [DOI] [PubMed] [Google Scholar]
- 34.Nagel E, Lehmkuhl HB, Bocksch W, et al. Noninvasive diagnosis of ischemia-induced wall motion abnormalities with the use of high dose dobutamine stress MRI. Comparison with dobutamine stress echocardiography. Circulation. 1999;99(6):763–70. doi: 10.1161/01.cir.99.6.763. [DOI] [PubMed] [Google Scholar]
- 35.Baer FM, Voth E, LaRosee K, et al. Comparison of dobutamine transesophageal echocardiography and dobutamine magnetic resonance imaging for detection of residual myocardial viability. Am J Cardiol. 1996;78(4):415–19. doi: 10.1016/s0002-9149(96)00329-3. [DOI] [PubMed] [Google Scholar]
- 36.Lin LL, Liu AJ, Liu JG, et al. Protective effects of scutellarin and Breviscapine on brain and heart ischemia in rats. J Cardiovasc Pharmacol. 2007;50(3):327–32. doi: 10.1097/FJC.0b013e3180cbd0e7. [DOI] [PubMed] [Google Scholar]
- 37.Yan L, Huang H, Tang QZ, et al. Breviscapine protects against cardiac hypertrophy through blocking PKC-alpha-dependent signaling. J Cell Biochem. 2010;109(6):1158–71. doi: 10.1002/jcb.22495. [DOI] [PubMed] [Google Scholar]
- 38.Wang M, Xie C, Cai RL, et al. Studies on antioxidant activities of Breviscapine in the cell-free system. Am J Chin Med. 2008;36(6):1199–207. doi: 10.1142/S0192415X08006521. [DOI] [PubMed] [Google Scholar]
- 39.Zhao S, Liu E, Wei K, et al. Interferon plus Chinese herbs are associated with higher sustained virological response than interferon alone in chronic Hepatitis C: A meta-analysis of randomised trials. Antiviral Res. 2011;89(2):156–64. doi: 10.1016/j.antiviral.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 40.Wang C, Li Y, Gao S, et al. Breviscapine injection improves the therapeutic effect of western medicine on angina pectoris patients. PLoS One. 2015;10(6):e0129969. doi: 10.1371/journal.pone.0129969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang M, Zhang WB, Zhu JH, et al. Breviscapine ameliorates cardiac dysfunction and regulates the myocardial Ca(2+)-cycling proteins in streptozotocin-induced diabetic rats. Acta Diabetol. 2010;47(Suppl 1):209–18. doi: 10.1007/s00592-009-0164-x. [DOI] [PubMed] [Google Scholar]
- 42.He M, Xue ZM, Li J, Zhou BQ. Breviscapine inhibits high glucose-induced proliferation and migration of cultured vascular smooth muscle cells of rats via suppressing the ERK1/2 MAPK signaling pathway. Acta Pharmacol Sin. 2012;33(5):606–14. doi: 10.1038/aps.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Golestani R, Jung JJ, Sadeghi MM. Molecular imaging of angiogenesis and vascular remodeling in cardiovascular pathology. J Clin Med. 2016;5(6) doi: 10.3390/jcm5060057. pii: E57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Palmer BF, Clegg DJ. Oxygen sensing and metabolic homeostasis. Mol Cell Endocrinol. 2014;397(1–2):51–58. doi: 10.1016/j.mce.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Ornitz DM, Itoh N. Fibroblast growth factors. Genome Biol. 2001;2(3):REVIEWS3005. doi: 10.1186/gb-2001-2-3-reviews3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.House SL, Bolte C, Zhou M, et al. Cardiac-specific overexpression of fibroblast growth factor-2 protects against myocardial dysfunction and infarction in a murine model of low-flow ischemia. Circulation. 2003;108(25):3140–48. doi: 10.1161/01.CIR.0000105723.91637.1C. [DOI] [PubMed] [Google Scholar]
- 47.Amoah V, Wrigley B, Holroyd E, et al. Vascular endothelial growth factor and hypoxia-inducible factor-1α gene polymorphisms and coronary collateral formation in patients with coronary chronic total occlusions. SAGE Open Med. 2016;4:1–7. doi: 10.1177/2050312116654403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toyota E, Warltier DC, Brock T, et al. Vascular endothelial growth factor is required for coronary collateral growth in the rat. Circulation. 2005;112(14):2108–13. doi: 10.1161/CIRCULATIONAHA.104.526954. [DOI] [PubMed] [Google Scholar]
- 49.Lee SH, Wolf PL, Escudero R, et al. Early expression of angiogenesis factors in acute myocardial ischemia and infarction. N Engl J Med. 2000;342(9):626–33. doi: 10.1056/NEJM200003023420904. [DOI] [PubMed] [Google Scholar]
- 50.El-Gendi H, Violaris AG, Foale R, et al. Endogenous, local, vascular endothelial growth factor production in patients with chronic total coronary artery occlusions: Further evidence for its role in angiogenesis. Heart. 2002;87(2):158–59. doi: 10.1136/heart.87.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dangas GD, Claessen BE, Caixeta A, et al. In-stent restenosis in the drug-eluting stent era. J Am Coll Cardiol. 2010;56(23):1897–907. doi: 10.1016/j.jacc.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 52.Valenti R, Vergara R, Migliorini A, et al. Predictors of reocclusion after successful drug-eluting stent-supported percutaneous coronary intervention of chronic total occlusion. J Am Coll Cardiol. 2013;61(5):545–50. doi: 10.1016/j.jacc.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 53.Fefer P, Knudtson ML, Cheema AN, et al. Current perspectives on coronary chronic total occlusions: The Canadian Multicenter Chronic Total Occlusions Registry. J Am Coll Cardiol. 2012;59( 11):991–97. doi: 10.1016/j.jacc.2011.12.007. [DOI] [PubMed] [Google Scholar]


