Abstract
The use of microalgae in biotechnological processes has received much attention worldwide. This is primarily due to the fact that they are inexpensive to grow, requiring only sunlight and CO2, whilst lending themselves to a range of uses, such as to reduce CO2 levels, as fish feed, in biofuel production, for the generation of secondary metabolites of interest, and in bioremediation. These features mean that microalgae are excellent candidates for the implementation of a range of eco-friendly technologies. Here, we investigated the behavior and feasibility of the use of the microalgal strain Tetraselmis marina AC16-MESO against heavy metal contamination focused on potential use in bioremediation. The following key parameters were recorded: (i) the sedimentation efficiency, which reached 95.6% after five hours of decantation; (ii) the ion tolerance (Ca2+, Co2+, Cu2+, Fe3+, Mn2+ and Ni2+) at concentrations of 0.1, 1.0, 5.0, 10.0 and 20.0 mg*L−1 and (iii) ion removal efficiency (Cu2+, Fe3+ and Mn2+). Our results indicated a higher tolerance for iron and calcium (20 ± 1.10 mg*L−1; 100 ± 8.10 mg*L−1), partial to nickel, manganese and copper (4.4 ± 0.10 mg*L−1; 4.4 ± 0.15 mg*L−1; 5 ± 1.25 mg*L−1) and less for cobalt (0.1 ± 0.20 mg*L−1). Moreover, removal efficiency of 40–90% for Cu2+, 100% for Fe3+, and 20–50% for Mn2+ over a 72 hours period, for ion concentrations of 1.0 and 5.0 mg*L−1.
Keywords: Microalgae, Tetraselmis, Heavy metals, Bioremediation, Bioremediation
Introduction
Every day, industrial processes generate large amounts of contaminated water, which are discharged into the environment. Most pollution in these waters is due to heavy metals (HMs) such as copper, chromium, nickel, iron, cadmium, and arsenic (Doshi et al., 2008). Due to their non-biodegradability and hazardous characteristics, heavy metals pose a great threat to the health of the environment. Aquatic organisms acquire heavy metals directly from contaminated water or through the food chain. Prolonged exposure of soils to heavy metals may result in a marked decrease in soil enzyme activities (Irha, Slet & Petersell, 2003). It is therefore of utmost importance to remove HMs from the industrial wastewaters released into water courses and soil (Doshi et al., 2008).
Current conventional methods for the removal of metals from industrial wastewater include chemical precipitation, ion exchange and membrane purification (Chen et al., 2002). However, these conventional approaches are often ineffective or expensive, especially when the metals in solution are in the range of 1–100 mgL−1 (Kumar et al., 2015). In addition, some of these methods have the disadvantage of producing toxic sludge (Volesky, 2001; Ahalya, Ramachandra & Kanamadi, 2003; Ahluwalia & Goyal, 2007). The necessary treatment of this sludge requires large amounts of energy and chemical reagents (Ahalya, Ramachandra & Kanamadi, 2003). There is therefore great interest in the development of innovative, cost-effective, efficient and sustainable methods for the removal of toxic substances from wastewater and aquatic ecosystems. Recent years have seen the development of unconventional technologies for the prevention of HM contamination, the decrease of HM concentrations (e.g., by reducing the flow of HMs into water courses and soil), and for the removal of HMs from the contaminated milieu via remediation (Kumar et al., 2015). Among these techniques, bioremediation shows great potential to make an eco-friendly contribution to HM decontamination. In addition, it may facilitate at least a part recovery of certain metals of interest (Wilde & Benemann, 1993).
The advantages of microalgae for the biosorption of HMs have widely been recognized (Kumar et al., 2015). The effectiveness of marine algae in the remediation of metal ions has been demonstrated (Volesky, 1990), and microalgal biomass has been shown to capture different metals, with different species showing an affinity to different metal ions (Doshi et al., 2008). These algae are therefore a promising candidate for use as a biosorbent material for the low-cost removal of contaminants. Marine and freshwater algae have already been used in adsorption and elution of gold, silver and cobalt (Hamdy, 2000; Fujita, Kuzuno & Mamiya, 1992). Furthermore, the efficiency of certain algae in HMs removal has been reported to be greater than that of activated coal, natural zeolites and synthetic ion-exchange polymers (Volesky, 1992). The kinetics of HM removal by biologically active microalgae can be divided in two steps: an initial phase of physical adsorption to the cell surface, and a second, slow, phase called biosorption, which relies on intracellular transport and chelation (Folgar et al., 2009; Nourbakhsh et al., 1994). Adsorption hence relies on various processes, including ion exchange, metal ion chelation and micro-precipitation, all of which occur at the cell wall. Differences in these processes among microalgae result in different remediation efficiencies (Zhao et al., 2013).
On this background, the behavior and feasibility of Tetraselmis marina AC16-MESO was assessed directed at water treatment isolated off the coast of Antofagasta, Chile. Key parameters such as sedimentation efficiency, cell viability and HMs removal capacity was evaluated.
Materials and Methods
Microalgae
The following microalgae were used: Muriellopsis sp., Nannochloropsis gaditana and Tetraselmis marina AC16-MESO. Tetraselmis was isolated from the intertidal area of the San Jorge Bay, Antofagasta, specifically from the area known as the Beach of the Oil Companies (”Playa de las Petroleras”), and identified and preserved at the University of Antofagasta marine mesocosm facility (Mata et al. in press). Algae were grown in Erlenmeyer flasks of 2 liters until reaching sufficient biomass for analysis, in UMA5 medium (Riveros et al., 2018) (NaNO3 4.55*10−5 M; NaH2PO4*H2O 2.41*10−4 M; NaHCO3 1.99*10−3 M) at 20 °C and a continuous photosynthetic photon flux of 70 µmol m−2s−1(24 h light).
Sedimentation efficiency
In order to evaluate the potential use of Tetraselmis marina AC16-MESO in bioremediation, sedimentation efficiency was determined and subsequently compared it to that of the microalgae Muriellopsis sp. and Nannochloropsis gaditana, which are characterized by high and low sedimentation efficiencies (SE), respectively. Samples of the microalgal suspension were taken and diluted in a cuvette of polystyrene (Sartedt, Nümbrecht, Germany), the suspension was left to settle at 22 °C in the dark in a spectrophotometer (Pharo 300; Merck, Kenilworth, NJ, USA). During the settling period, turbidity of the sample was measured at 550 nm at the same height in the cuvette to determine the sedimentation activity.
Sedimentation efficiency (SE) was calculated according to Eq. (1) (Smith & Davis, 2012):
| (1) |
where A corresponds to the supernatant’s absorbance at 550 nm at time t (120 min), and A0 corresponds to the absorbance of the initial suspension culture.
Heavy metal assays
Stock solutions of each ion (Ca2+, Co2+, Cu2+, Fe3+, Mn2+ and Ni2+) were prepared at a concentration of 50 mg*L−1 in distilled water, using the following salts: calcium nitrate tetrahydrate (Ca(NO3)2*4H2O), Cobalt (II) chloride hexahydrate (CoCl2*6H2O), copper (II) sulfate pentahydrate (CuSO4*5H2O), iron (III) chloride hexahydrate (FeCl3*6H2O), manganese (II) chloride tetrahydrate (MnCl2*4H2O) and nickel (II) sulfate hexahydrate (NiSO4*6H2O). All the glass and plastic material was washed with a solution of 10% (v/v) hydrochloric acid for 12 h and rinsed with distilled water in order to remove any contamination prior to use.
Toxicity assay (EC50)
The effect of Ca2+, Co2+, Cu2+, Fe3+, Mn2+ and Ni2+ ions on the microalga Tetraselmis marina AC16-MESO was studied in an EC50 assay, which measures concentrations resulting in a 50% of maximal effect of the test organisms (Hu, Luo & Huang, 2014). All assays were carried out in 96-well plates with an initial inoculum of 2 ×105 cells*mL−1 in 200 µL of marine saline solution (7 mg*L−1 MgSO4*7H2O; 0.8 mg*L−1 KCl; 24 mg*L−1 NaCl) autoclaved at 121 °C. Increasing metal concentrations were added (0.001, 0.01, 0.1, 1, 5, 10; 20; 40; 80; 100 mg/L). Conditions were controlled throughout, with a temperature of 20 °C and continuous illumination at 70 µmol m−2s−1. The respective heavy metal treatments were applied in triplicate for 72 h. Cell toxicity was assessed by tracking the OD550 as a proxy for the number of cells. The linear relationship between microalgal density and OD550 is shown in Eq. (2) of Toxicity assay EC50. All the glass and plastic material was washed with a solution of 10% (v/v) hydrochloric acid for 12 h and rinsed with distilled water in order to remove any contamination prior to use.
Effect of metal ions on Tetraselmis marina AC16-MESO
For each metal, cellular viability was evaluated by tracking cell density at 0, 24, 48, 72 and 96 h at the following metal concentrations (in mgl*L−1): 0.0 (control), 0.1, 1.0, 5.0, 10.0, and 20.0. All assays were conducted in triplicate. The sample of precultivated microalgae was centrifuged at 6,000 rpm for 10 min, and the supernatant was discarded. The pelleted microalgal cells were washed twice with sterile Milli-Q water to remove impurities and re-suspended in sterile Milli-Q water for inoculation into the growth medium. Microalgae were added to flasks containing 250 mL modified f/2 medium prepared with artificial seawater, with absence of trace elements (Goldman & McCarthy, 1978), at a concentration of 2 ×105 cells*mL−1. Each metal was added separately to achieve the concentrations described above. Microalgal cultures were kept at 20 °C under continuous exposure to light (70 µmol m−2s−1) and constant aeration.
The cell density of the microalgal suspension was tracked by daily optical density (OD) measurements at 550 nm (Zhou et al., 2012). Measurements were carried out in 96-well plates using a microplate reader (GloMax Multi Detection System; Promega, Madison, WI, USA). The linear relationship between microalgal density and OD550 is given by Eq. (2).
| (2) |
Heavy metal removal capacity
To evaluate the metal removal capacity, they selected the ions whit best results for cellular growth and toxicity assay (Cu+2, Mn+2 and Fe+3). microalgae were added to one liter of UMA 5 medium prepared with artificial seawater with absence of trace elements (Goldman & McCarthy, 1978) at a concentration of 2 ×105 cells*mL−1. Each metal was added at final concentrations of 1 and 5 mg*L−1. Metal ion concentrations in the culture medium were checked after 72 h using a colorimetric kit (Spectroqant® Merck) and a spectrophotometer (Pharo 300; Merck, Kenilworth, NJ, USA). 50 mL samples were taken from the culture, and after leaving the microalgae to settle, the remaining metal ion concentration in the supernatant was measured. Pure medium was used as a blank. All assays were performed in triplicate. Lighting and temperature remained constant throughout at 72 µmol m−2s−1 and 20 ± 1 °C, respectively.
Statistical analyses
All assays were performed in triplicate. EC50, effect of metal ions on cell density and HM removal assays were evaluated using a Bonferroni-corrected by one way-ANOVA at a statistical significance threshold of p ≤ 0.05, using the software GraphPad Prism version 5.01.
Results
Sedimentation efficiency
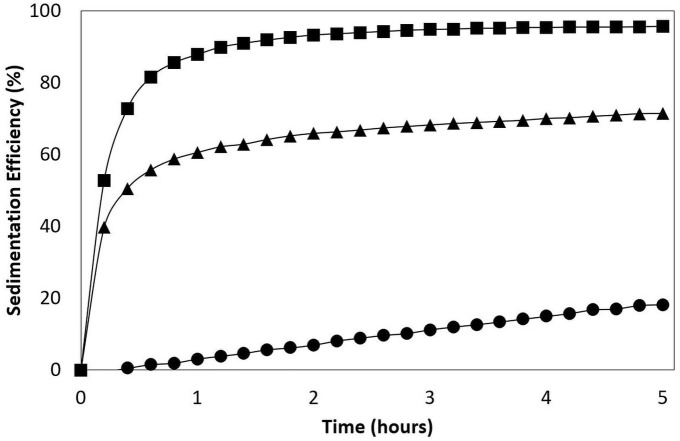
Different, microalgae were studied; the freshwater microalgae Muriellopsis sp.; marines microalgae such as T. AC16-MESO and N. gaditana. The SE of the flocculating microalgae were higher than those of non-flocculant microalgae. For example, T. AC16-MESO reached 95,6%, above Muriellopsis sp. (71,3%), and substantially over that of N. gaditana (18,2%), measured at 5 h (Fig. 1).
Figure 1. Sedimentation efficiency (SE).
Tetraselmis marina AC16-MESO (■) and the reference microalgaes Muriellopsis sp. (▴) and Nannochloropsis gaditana (• ). LTIT 1.
Acute toxicity (EC50)
In Fig. 2 shows the curves of EC50 obtained for each of the ions and concentrations tested. Co2+ ion was the lowest concentration tested, reaching a value of 0.1 ± 0.20 mg*L−1after 72 h. On the other hand, Ni2+; Mn2+ and Cu2+ ions were better tolerated, with EC50 values of 4.4 ± 0.10 mg*L−1; 4.4 ± 0.15 mg*L−1 and 5 ± 1.25 mg*L−1. By contrast, EC50 values for Fe3+ and Ca2+ ions reached higher tolerance, reaching values of 20 ± 1.10 mg*L−1 and 100 ± 8.10 mg*L−1.
Figure 2. Half maximal effective concentration (EC50.).
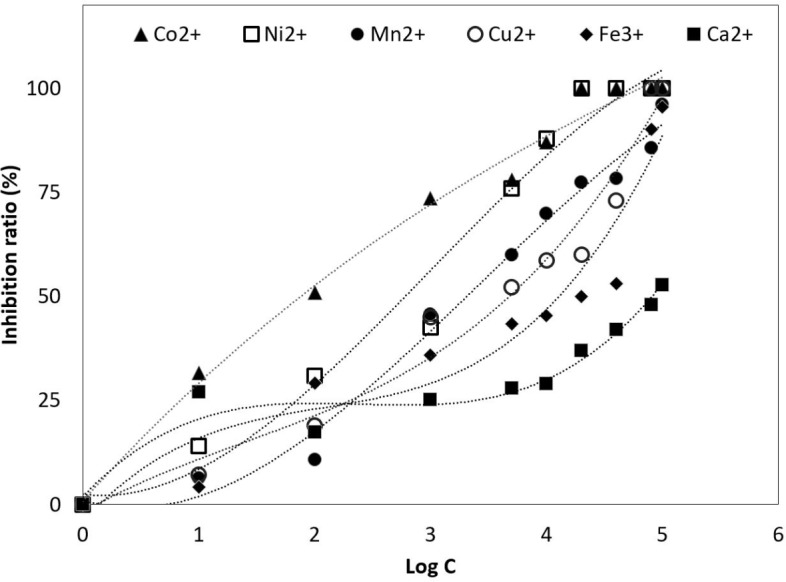
Tetraselmis marina AC16-MESO at different concentration of metal ions. (▴) Co2+; (□) Ni+2; (•) Mn2+; () Cu2+; (♦) Fe3+ and (■) Ca2+. The y-axis shows the inhibition ratio (%) for each ion at concentrations of 0.0 (control), 0.001, 0.01, 0.1, 1, 5, 10, 20, 40, 80 and 100 mg*L−1. The EC50 was determined for each ion.
Effect of metal ions on cell density of Tetraselmis marina AC16-MESO
The lowest concentrations of Co2+ ions significantly decreased cell density values below those of the control. After 96 h, cell densities were substantially lower than in the control, by as much as 322,759 (53%) and 548,874 (90%) cells*mL−1 at concentrations of 0.1 and 20.0 mg*L- 1, respectively (Fig. 3A). Likewise, cultivation with Ni2+ at any concentration led to a significant decrease in cell densities compared to the control (Fig. 3B).
Figure 3. Effect of different concentrations of metal ions on the growth of the microalga Tetraselmis marina AC16-MESO.
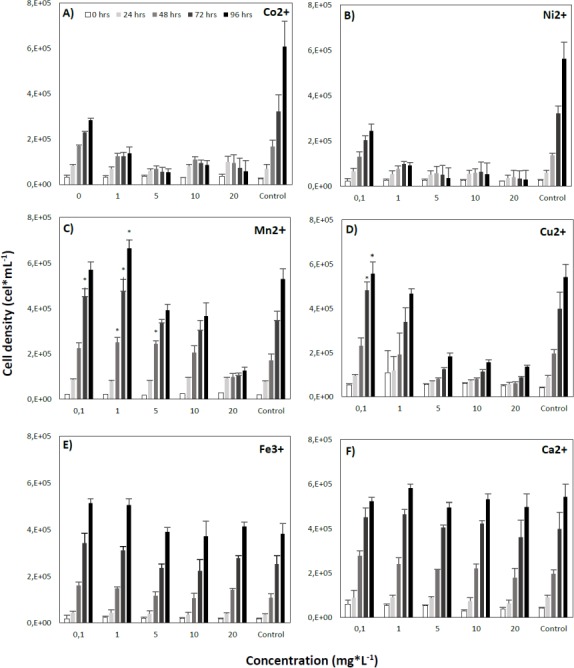
(A) Co2+; (B) Ni+2; (C) Mn2+; (D) Cu 2+; (E) Fe3+ and (F) Ca2+. For each metal, the microalga was cultured at ion concentrations of 0.0 (control), 0.1, 1.0, 5.0, 10.0 and 20.0 mg*L−1, and cellular densities were measured at 0, 24, 48, 72 and 96 h. Asterisks indicate significant differences between metal treated and control cultures at each time of measurement, at a 95% confidence level after a Bonferroni correction for n = 3, at p < 0.05. All assays were carried out in triplicate.
On the other hand, even at low concentrations of Mn2+, a significant increase in cell density over control values was observed. After 72 h at a concentration of 0.1 mg*L−1, cell densities exceeded those of the control by 105,008 (30%) cells*mL- 1, and after 48, 72 and 96 h at a concentration of 1 mg*L−1, control cell densities were exceeded by 79,515 (46%), 130,029 (38%) and 133,611 (25%) cells*mL−1, respectively. Higher concentrations led to a decrease of cell density relative to the control (Fig. 3C).
By contrast, in the presence of Cu2+, the microalgal suspension reached significantly higher cell densities than the control. Control cell densities were exceeded by 83,794 (21%) and 14.212 (3%) cells*mL−1 at a concentration of 0.1 mg*L−1 after 72 and 96 h of cultivation, respectively. However, at higher Cu2+ concentrations, a decrease of cell density was observed, and after 96 h of cultivation, final cell densities were 359,280 (66%), 384,935 (71%) and 406,170 (75%) cells*mL−1 below those of the control, at concentrations of 5.0, 10.0 and 20.0 mg*L−1, respectively (Fig. 3D).
Fe3+ also led to a significant increase in cell density compared to the control, and at a concentration of 0.1 mg*L−1, cell density values exceeded those of the control by 51,691 (47%), 89,402 (35%) and 132,243 (34%) cells*mL−1 after 48, 72 and 96 h, respectively. At a concentration of 1.0 mg*L- 1, cell density values exceeded those of the control by 57,191 (23%) and 124,663 (33%) cells*mL- 1 after 72 and 96 h, respectively. At the remaining concentrations, no significant differences in cell density compared to the control were seen (Fig. 3E). Finally, Ca2+ did not result in a significant variation of microalgal cell density over the sampling period, nor there were any significant differences in cell density with respect to the control at the different concentrations employed (Fig. 3F).
Heavy metal removal
Finally, the capacity of Tetraselmis marina AC16-MESO to remove those ions who had given the best results in the cell density assays while resulting least toxic in EC50 assays over 72 h. The efficiency of Cu2+, Fe3+ and Mn2+ removal at concentrations of 1.0 and 5.0 mg*L−1 was tested. For Cu2+, the removal percentage was 42.9% at 1.0 mg*L−1 and 92% at 5.0 mg*L−1 (Fig. 4A). For Fe3+, it was 100%, both at 1.0 and 5.0 mg*L−1 (Fig. 4B). Finally, for Mn2+, the removal percentage was 50.4% at 1.0 mg*L−1 and 23.4% at 5.0 mg*L−1 (Fig. 4C).
Figure 4. Removal efficiency of MPS.
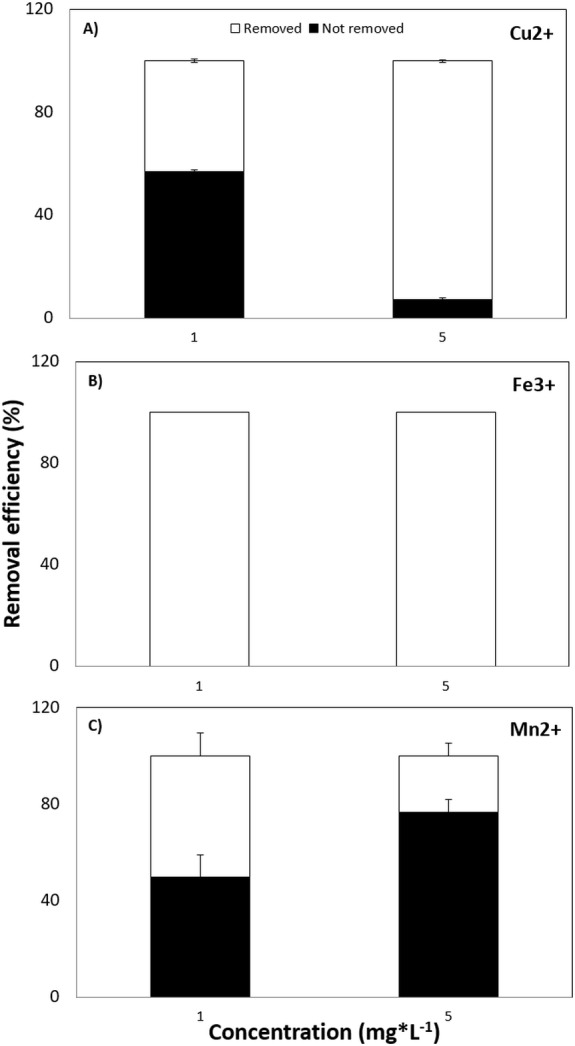
(A) Cu2+, (B) Fe3+ and (C) Mn2+ by Tetraselmis marina AC16-MESO. The y-axis shows the removal efficiency (%) at 72 h, at ion concentrations of 1.0 and 5.0 mg*L−1.
Discussion
Sedimentation efficiency
Microalgae are considered a workable alternative for the remediation of heavy-metal contaminated environments. However, the collection of biomass from microalgal cultures represents a significant hurdle for the economically viable development of this process (Alam et al., 2015). Harvesting in commercial microalgae production plants in generally done by centrifugation. Different studies showed a contribution of the costs for harvesting to more than 30% of the total cost in case of algal production in open ponds (Zittelli et al., 2006). The microalga used here, Tetraselmis marina AC16-MESO, is autoflocculating; its biomass could therefore efficiently be harvested via sedimentation at a very minor cost. Compared to two microalgal reference strains, one with a high sedimentation efficiency, the other with a low one, Tetraselmis marina AC16-MESO showed a good capacity sedimentation efficiency of 95,6% for 5 h, in contrast to Muriellopsis sp. (71,3%) and Nannochloropsis gaditana (18,2%). The separation of Tetraselmis marina biomass via decantation is therefore practicable and could be achieved in a relatively short time, and at low cost.
Effect of heavy metal ions on the growth of Tetraselmis marina AC16-MESO
Co2+ and Ni2+ both had an inhibitory effect on the cellular growth of Tetraselmis marina AC16-MESO at all concentrations tested here. By contrast, low concentrations of Co2+ (0.1 and 0.5 mg*L−1) have been found to boost the cellular growth of the chlorophyte microalga Monoraphidium minutum by 8 to 13% over 240 h, while Co2+ concentrations of 0.5 and 1.5 mg*L−1 increased the cellular growth of Nytzchia perminuta by 5 to 9% (El-Sheekh et al., 2003). For Ni2+, on the other hand, the literature is consistent with the findings of the present study. The cellular growth of Ankistrodesmus falcatus over 96 h has been found to be inhibited by Ni2+ concentrations of 30, 60, and 120 µg*L−1 (Martínez-Ruiz & Martínez-Jerónimo, 2015). These concentrations are substantially below those applied in the present study. Meanwhile, a negative effect was also found in the lowest tested concentration, 0.1 mg*L−1. These findings show that, compared to the other ions, Ni2+ has a considerable negative effect on cellular viability.
On the other hand, Manganese is an essential cofactor in photosynthesis; its deficiency leads to the inhibition of photosystem II (Yang et al., 2015). At micro-concentrations, Mn2+ is fundamental to the optimum growth of Dunaliella tertiolecta (Chen et al., 2011). Yang et al. (2015) found a 6% increase of Chlorella minutissima biomass compared to a control after 168 h at a Mn2+ concentration of 213 mg*L−1. Here, increased cell density for Tetraselmis marina AC16-MESO at concentrations much below those applied by Yang et al. (2015), reaching a 30% increase with respect to the control after 72 h at a concentration of 0.1 mg*L−1, and a 38% increase at a concentration of 1.0 mg*L−1. Nevertheless, Concentrations above 10.0 mg*L−1 had a significant negative effect on microalgal growth.
By contrast, Low concentrations of copper, iron and manganese even accelerated its cellular growth. At concentrations between 0.2 and 0.5 mg*L−1, Cu2+ has been reported to enhance the growth of the microalgae Chlorella pyrenoidosa and Scenedesmus obliquus (Zhou et al., 2012). Here, the Cu2+ had a positive effect on the growth of Tetraselmis marina AC16-MESO at a concentration of 0.1 mg*L−1 was observed, leading to a 21% increase in cell density compared to the control over a period of 72 h. Likewise, a 14% increase in cell density has been reported for cultures of Isochrysis galbana at 0.6 mg*L-1 Fe3+ over 408 h (Liu & Wang, 2014). Here, a Fe3+ concentration of 0.1 mg*L−1 increased Tetraselmis marina AC16-MESO cell density by 35% over 72 h compared to the control. A Fe3+ concentration of 1.0 mg*L−1 led to a 23% increase.
Finally, Ca2+ has a central role in many processes related to plant development and growth (Hepler, 2005). At a concentration of 6.4 mg*L−1, it has been reported to boost the increase in cellular density of Chlorella vulgaris over 432 h by 20% compared to a control, as well that of Scenedesmus obliquus by 25% compared to a control, (Gorain, Bagchi & Mallick, 2013). By contrast, Ca2+ was not found to have any effect on the cellular growth of Tetraselmis marina AC16-MESO at any of the concentrations tested. However, the period of culture was shorter (96 h).
Heavy metal removal
The maximal removal efficiency was 90% for Cu2+, 100% for Fe+3and 50% for Mn2+, all at 72 h. A copper removal efficiency of close to 100% has been described for the microalgae Chlorella pyrenoidosa and Scenedesmus obliquus (Zhou et al., 2012). While the removal of iron and copper reached a maximum during the initial adsorption phase (at 72 h), the efficiency of Mn2+ removal was only 50% in the same period. In this context, it is worth mentioning that no significant HM removal has been reported beyond between 96 and 120 h, indicating that at that point, the microalgal cells may have reached saturation with HMs (Alam et al., 2015). In line with this, the removal of copper ions has shown to reach a maximum in the first days of culture, with only an insignificant increase happening after that (Zhou et al., 2012). This change of efficiency over time can be explained by the complexation of metal ions by functional groups at the cell surface and the increasing competition between ions, as the availability of free complexation sites decreases within the biomass (Kumar et al., 2015).
Conclusion
The efficiency of HM removal by microalgae depends on the microalgal species, the properties and concentration of the metal ion, and the period of culture. We found the microalga Tetraselmis marina AC16-MESO to tolerate, and to be capable of the removal of high concentrations of metal ions. In addition, it was capable of removing these metals at a high rate, within a relatively short time and with a high sedimentation efficiency. These characteristics make of Tetraselmis marina AC16-MESO a promising candidate for use in bioremediation and a model for the use of microalgae in the bioremediation of water contaminated with copper, iron and manganese.
Supplemental Information
Tetraselmis marina AC16-MESO (■) and the reference microalgaes Muriellopsis sp. (▴) and Nannochloropsis gaditana (•).
Tetraselmis marina AC16-MESO at different concentration of metal ions. () Co2+; (■) Ni+2; (•) Mn2+; () Cu2+; (♦) Fe3+ and (■) Ca2+. The y-axis shows the inhibition ratio (%) for each ion at concentrations of 0.0 (control), 0.001, 0.01, 0.1, 1, 5, 10, 20, 40, 80 and 100 mg*L−1. The EC50 was determined for each ion.
Effect of different concentrations of metal ions on the growth of the microalga Tetraselmis marina AC16-MESO. (A) Ca2+; (B) Co2+; (C) Cu2+; (D) Fe3+; (E) Mn2+; and (F) Ni2+. For each metal, the microalga was cultured at ion concentrations of 0.0 (control), 0.1, 1.0, 5.0, 10.0 and 20.0 mg*L−1, and cellular densities were measured at 0, 24, 48, 72 and 96 h. Asterisks indicate significant differences between metal treated and control cultures at each time of measurement, at a 95% confidence level after a Bonferroni correction for n = 3, at pλτ0.05. All assays were carried out in triplicate.
Efficiency of (A) Cu2+; (B) Fe3+ and (C) Mn2+ removal by Tetraselmis marina AC16-MESO. The y-axis shows the removal efficiency (%) at 72 h, at ion concentrations of 1.0 mg*L−1 and 5.0 mg*L−1.
Funding Statement
This work was supported by “Talleres de redacción de artículos científicos”, Dirección de Gestión de la Investigación, Universidad de Antofagasta. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Henry Cameron conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, authored or reviewed drafts of the paper, approved the final draft.
Maria Teresa Mata conceived and designed the experiments, analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Carlos Riquelme analyzed the data, contributed reagents/materials/analysis tools, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.
References
- Ahalya, Ramachandra & Kanamadi (2003).Ahalya N, Ramachandra TV, Kanamadi RD. Biosorption of heavy metals. Research Journal of Chemical & Environmental Sciences. 2003;7(4):71–79. [Google Scholar]
- Ahluwalia & Goyal (2007).Ahluwalia SS, Goyal D. Microbial and plant derived biomass for removal of heavy metals from wastewater. Bioresource Technology. 2007;98(12):2243–2257. doi: 10.1016/j.biortech.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Alam et al. (2015).Alam MA, Wan C, Zhao XQ, Chen LJ, Chang JS, Bai FW. Enhanced removal of Zn 2+ or Cd 2+ by the flocculating Chlorella vulgaris JSC-7. Journal of Hazardous Materials. 2015;289:38–45. doi: 10.1016/j.jhazmat.2015.02.012. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2002).Chen JP, Hong L, Wu S, Wang L. Elucidation of interactions between metal ions and Ca alginate-based ion-exchange resin by spectroscopic analysis and modeling simulation. Langmuir. 2002;18(24):9413–9421. doi: 10.1021/la026060v. [DOI] [Google Scholar]
- Chen et al. (2011).Chen M, Tang H, Ma H, Holland TC, Ng KS, Salley SO. Effect of nutrients on growth and lipid accumulation in the green algae Dunaliella tertiolecta. Bioresource Technology. 2011;102(2):1649–1655. doi: 10.1016/j.biortech.2010.09.062. [DOI] [PubMed] [Google Scholar]
- Doshi et al. (2008).Doshi H, Seth C, Ray A, Kothari IL. Bioaccumulation of heavy metals by green algae. Current Microbiology. 2008;56(3):246–255. doi: 10.1007/s00284-007-9070-z. [DOI] [PubMed] [Google Scholar]
- El-Sheekh et al. (2003).El-Sheekh MM, El-Naggar AH, Osman MEH, El-Mazaly E. Effect of cobalt on growth, pigments and the photosynthetic electron transport in Monoraphidium minutum and Nitzchia perminuta. Brazilian Journal of Plant Physiology. 2003;15(3):159–166. doi: 10.1590/S1677-04202003000300005. [DOI] [Google Scholar]
- Folgar et al. (2009).Folgar S, Torres E, Pérez-Rama M, Cid A, Herrero C, Abalde J. Dunaliella salina as marine microalga highly tolerant to but a poor remover of cadmium. Journal of Hazardous Materials. 2009;165(1):486–493. doi: 10.1016/j.jhazmat.2008.10.010. [DOI] [PubMed] [Google Scholar]
- Fujita, Kuzuno & Mamiya (1992).Fujita T, Kuzuno E, Mamiya M. Adsorption of metal ions by river algae. Bunseki Kagaku. 1992;108:123–128. [Google Scholar]
- Goldman & McCarthy (1978).Goldman JC, McCarthy JJ. Steady state growth and ammonium uptake of a fast-growing marine diatom. Limnology and Oceanography. 1978;23(4):695–703. doi: 10.4319/lo.1978.23.4.0695. [DOI] [Google Scholar]
- Gorain, Bagchi & Mallick (2013).Gorain PC, Bagchi SK, Mallick N. Effects of calcium, magnesium and sodium chloride in enhancing lipid accumulation in two green microalgae. Environmental Technology. 2013;34(13–14):1887–1894. doi: 10.1080/09593330.2013.812668. [DOI] [PubMed] [Google Scholar]
- Hamdy (2000).Hamdy AA. Biosorption of heavy metals by marine algae. Current Microbiology. 2000;41(4):232–238. doi: 10.1007/s002840010126. [DOI] [PubMed] [Google Scholar]
- Hepler (2005).Hepler PK. Calcium: a central regulator of plant growth and development. The Plant Cell. 2005;17(8):2142–2155. doi: 10.1105/tpc.105.032508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Luo & Huang (2014).Hu C, Luo Q, Huang Q. Ecotoxicological effects of perfluorooctanoic acid on freshwater microalgae Chlamydomonas reinhardtii and Scenedesmus obliquus. Environmental Toxicology and Chemistry. 2014;33(5):1129–1134. doi: 10.1002/etc.2532. [DOI] [PubMed] [Google Scholar]
- Irha, Slet & Petersell (2003).Irha N, Slet J, Petersell V. Effect of heavy metals and PAH on soil assessed via dehydrogenase assay. Environment International. 2003;28(8):779–782. doi: 10.1016/S0160-4120(02)00124-1. [DOI] [PubMed] [Google Scholar]
- Kumar et al. (2015).Kumar KS, Dahms HU, Won EJ, Lee JS, Shin KH. Microalgae—a promising tool for heavy metal remediation. Ecotoxicology and Environmental Safety. 2015;113:329–352. doi: 10.1016/j.ecoenv.2014.12.019. [DOI] [PubMed] [Google Scholar]
- Liu & Wang (2014).Liu Z, Wang G. Effect of Fe 3+ on the growth and lipid content of Isochrysis galbana. Chinese Journal of Oceanology and Limnology. 2014;32:47–53. doi: 10.1007/s00343-014-3110-x. [DOI] [Google Scholar]
- Martínez-Ruiz & Martínez-Jerónimo (2015).Martínez-Ruiz EB, Martínez-Jerónimo F. Nickel has biochemical, physiological, and structural effects on the green microalga Ankistrodesmus falcatus: an integrative study. Aquatic Toxicology. 2015;169:27–36. doi: 10.1016/j.aquatox.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Nourbakhsh et al. (1994).Nourbakhsh M, Sag Y, Özer D, Aksu Z, Kutsal T, Caglar A. A comparative study of various biosorbents for removal of chromium (VI) ions from industrial waste waters. Process Biochemistry. 1994;29(1):1–5. doi: 10.1016/0032-9592(94)80052-9. [DOI] [Google Scholar]
- Riveros et al. (2018).Riveros K, Sepulveda C, Bazaes J, Marticorena P, Riquelme C, Acién G. Overall development of a bioprocess for the outdoor production of Nannochloropsis gaditana for aquaculture. Aquaculture Research. 2018;49(1):165–176. [Google Scholar]
- Smith & Davis (2012).Smith BT, Davis RH. Sedimentation of algae flocculated using naturally-available, magnesium-based flocculants. Algal Research. 2012;1(1):32–39. doi: 10.1016/j.algal.2011.12.002. [DOI] [Google Scholar]
- Volesky (1990).Volesky B. Removal and recovery of heavy metals by biosorption. In: Volesky B, editor. Biosorption of heavy metals. Boca Raton: CRC Press; 1990. pp. 7–44. [Google Scholar]
- Volesky (1992).Volesky B. Removal of heavy metals by biosorption. Harnessing Biotechnology, 21st Century. Int. Biotechnol. Symp. Expo; Washington, D.C.. 1992. pp. 420–426. [Google Scholar]
- Volesky (2001).Volesky B. Detoxification of metal-bearing effluents: biosorption for the next century. Hydrometallurgy. 2001;59(2):203–216. doi: 10.1016/S0304-386X(00)00160-2. [DOI] [Google Scholar]
- Wilde & Benemann (1993).Wilde EW, Benemann JR. Bioremoval of heavy metals by the use of microalgae. Biotechnology Advances. 1993;11(4):781–812. doi: 10.1016/0734-9750(93)90003-6. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2015).Yang J, Cao J, Xing G, Yuan H. Lipid production combined with biosorption and bioaccumulation of cadmium, copper, manganese and zinc by oleaginous microalgae Chlorella minutissima UTEX2341. Bioresource Technology. 2015;175:537–544. doi: 10.1016/j.biortech.2014.10.124. [DOI] [PubMed] [Google Scholar]
- Zhao et al. (2013).Zhao Y, Wang B, Liu C, Wu Y. Biosorption of trace metals from aqueous multimetal solutions by green microalgae. Chinese Journal of Geochemistry. 2013;32(4):385–391. doi: 10.1007/s11631-013-0646-y. [DOI] [Google Scholar]
- Zhou et al. (2012).Zhou GJ, Peng FQ, Zhang LJ, Ying GG. Biosorption of zinc and copper from aqueous solutions by two freshwater green microalgae Chlorella pyrenoidosa and Scenedesmus obliquus. Environmental Science and Pollution Research. 2012;19(7):2918–2929. doi: 10.1007/s11356-012-0800-9. [DOI] [PubMed] [Google Scholar]
- Zittelli et al. (2006).Zittelli GC, Rodolfi L, Biondi N, Tredici MR. Productivity and photosynthetic efficiency of outdoor cultures of Tetraselmis suecica in annular columns. Aquaculture. 2006;261:932–943. doi: 10.1016/j.aquaculture.2006.08.011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tetraselmis marina AC16-MESO (■) and the reference microalgaes Muriellopsis sp. (▴) and Nannochloropsis gaditana (•).
Tetraselmis marina AC16-MESO at different concentration of metal ions. () Co2+; (■) Ni+2; (•) Mn2+; () Cu2+; (♦) Fe3+ and (■) Ca2+. The y-axis shows the inhibition ratio (%) for each ion at concentrations of 0.0 (control), 0.001, 0.01, 0.1, 1, 5, 10, 20, 40, 80 and 100 mg*L−1. The EC50 was determined for each ion.
Effect of different concentrations of metal ions on the growth of the microalga Tetraselmis marina AC16-MESO. (A) Ca2+; (B) Co2+; (C) Cu2+; (D) Fe3+; (E) Mn2+; and (F) Ni2+. For each metal, the microalga was cultured at ion concentrations of 0.0 (control), 0.1, 1.0, 5.0, 10.0 and 20.0 mg*L−1, and cellular densities were measured at 0, 24, 48, 72 and 96 h. Asterisks indicate significant differences between metal treated and control cultures at each time of measurement, at a 95% confidence level after a Bonferroni correction for n = 3, at pλτ0.05. All assays were carried out in triplicate.
Efficiency of (A) Cu2+; (B) Fe3+ and (C) Mn2+ removal by Tetraselmis marina AC16-MESO. The y-axis shows the removal efficiency (%) at 72 h, at ion concentrations of 1.0 mg*L−1 and 5.0 mg*L−1.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data are provided in the Supplemental Files.