Abstract
Background.
Prophylactic ganciclovir (GCV) is used in high-risk renal transplant patients to prevent acute cytomegalovirus (CMV) disease, but its impact on inflammation within the allograft itself remains undefined.
Methods.
To study the effect of GCV prophylaxis on allograft inflammation, murine CMV (MCMV)-infected allo-grafts were analyzed in a murine donor positive/recipient negative allogeneic renal transplantation model by flow cytometry and immunofluorescent staining.
Results.
By flow cytometry, CD45+ leukocyte infiltrates were more abundant in MCMV-infected allografts at 14 days posttransplant compared with uninfected grafts (P<0.01) and decreased in the presence of GCV (P<0.05). CD11c+ dendritic cells, Gr-1+ myeloid cells, CD204+ macrophages, and CD49b+ natural killer cells were reduced in GCV-treated allografts compared with MCMV-infected grafts without GCV treatment (P<0.05). However, GCV failed to reduce these cell types to levels found in MCMV-uninfected allografts. By day 7 after cessation of GCV prophylaxis, dendritic cells, macrophages, and natural killer cells increased in number and became statistically indistinguishable from numbers of cells found in MCMV-infected allografts without GCV. GCV treatment did not affect the numbers of CD4+, CD8+, or CD19+/B220+ lymphocytes infiltrating the allografts. Infiltrates were confirmed histologically by immunofluorescent staining for CD3+ and CD11b+ cells.
Conclusions.
In this model, MCMV-infected allografts developed significantly greater innate and adaptive leukocytic infiltrates compared with uninfected grafts. GCV attenuated the MCMV-associated innate leukocyte infiltrates in infected allo-grafts but not the lymphocytic infiltrates. The attenuated innate response was limited to the period of GCV prophylaxis.
Keywords: Cytomegalovirus, Kidney, Transplantation, Ganciclovir
Cytomegalovirus (CMV) infection has been associated with adverse effects in renal allograft recipients, attributed either to direct consequences of viral replication (CMV disease and febrile syndromes) or to indirect effects of virus-host interactions (acute rejection, allograft loss, and immune dysregulation) (1–5). Seroepidemiologic studies have associated human CMV (HCMV)-positive donor or recipient serostatus with inferior graft outcome compared with HCMV-seronegative transplantations (6–10). HCMV antigens have been identified more often in biopsies from rejecting organs compared with those without rejection, supporting an association between expression of viral gene products and rejection (11–13). The combination of CMV and acute rejection has also been associated with more severe vascular changes in 6-month protocol renal allograft biopsies (14).
Ganciclovir (GCV) prophylaxis is used in some transplant centers to prevent acute HCMV disease in high-risk patients (15, 16). The impact of GCV prophylaxis on allograft function is controversial, with some studies suggesting a benefit and other studies finding no difference in long-term outcome between those with and without GCV prophylaxis (10, 15, 17–20). GCV prevents viral replication by inhibiting the viral DNA polymerase, so it is possible that GCV might reduce the local antiviral immunologic response by limiting intragraft viral replication. Because human transplant recipients have multifactorial causes for allograft outcome, this study uses a murine renal transplantation model with murine CMV (MCMV) infection to test the hypothesis that GCV prophylaxis might reduce intragraft inflammation in CMV-infected renal allografts.
RESULTS
MCMV Replication Is Inhibited by GCV
Animals receiving MCMV-infected allografts were divided into groups with and without GCV prophylaxis. No animals developed clinical acute MCMV disease. At day 14 posttransplant, MCMV DNA was detectable by quantitative polymerase chain reaction (PCR) at 103 to 104 copies/mL blood in MCMV-infected recipients without GCV but was not detectable (nd) in blood of GCV-treated animals, showing that GCV inhibited viral replication at the administered dose (Fig. 1A).
FIGURE 1.
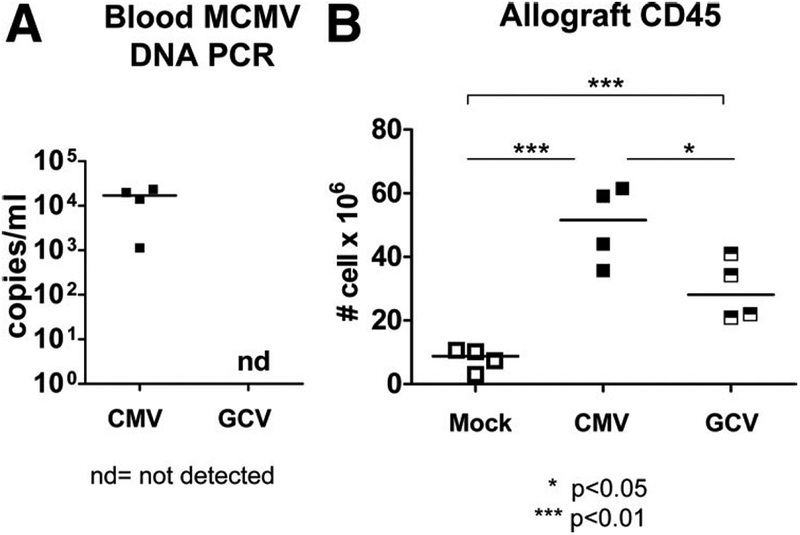
Ganciclovir (GCV) treatment terminates murine cytomegalovirus (MCMV) replication and is associated with reduction of CD45+ leukocyte infiltrates into the allograft. (A) DNA was extracted from whole blood of CMV-infected animals with and without GCV treatment at 14 days posttransplant, quantitative DNA PCR performed using primers, probe and standards specific for MCMV immediate-early 1, and results depicted as copies per mL blood. MCMV DNA was consistently detectable in blood from CMV-infected transplant recipients without GCV (CMV, closed squares) but was not detectable (nd) in animals treated with GCV (GCV). (B) CD45+ leukocytes were quantitated by flow cytometry from allografts of uninfected (Mock, open squares), CMV-infected (CMV, closed squares), and CMV-infected, GCV-treated (GCV, hatched squares) animals, and depicted as cells per organ. CMV-infected allografts showed greater CD45+ infiltrates compared with Mock allografts (P<0.01). CD45+ leukocytes in the GCV allografts were decreased compared with CMV allografts (P<0.05) but were still greater (P<0.01) than numbers found in Mock allografts.
GCV Reduces Intragraft MCMV-Induced CD45+ Leukocyte Infiltrates
Animals receiving MCMV-infected allografts were divided into groups with and without GCV prophylaxis. Recipients of uninfected allografts served as controls. At day 14 posttransplant, CD45+ leukocytes in allograft kidneys were quantitated by flow cytometry (fluorescence-activated cell sorting [FACS], Fig. 1B). Uninfected allografts (Mock, open squares) contained detectable CD45+ infiltrates (7.803±1.739×106 cells), attributable to the suppressed, but not absent, alloimmune response in these cyclosporine-treated animals. MCMV-infected allografts (CMV, closed squares) exhibited a robust CD45+ infiltrate (50.08±6.154×106 cells), which was significantly greater than those found in Mock allografts (P<0.01). The induction of CD45+ leukocyte infiltrates in the CMV animals was greater than the allo-immune response observed in Mock animals and therefore may be attributed to MCMV infection of the allograft. CMV transplants with GCV prophylaxis (GCV, hatched squares) had reduced CD45+ infiltrates (29.53±4.869×106 cells) into the allografts compared with CMV animals without GCV treatment (P<0.05) but remained greater than those found in Mock allografts (P<0.01). This result suggests that GCV reduced the MCMV-associated CD45+ leukocyte infiltrates but not to the level observed in MCMV-uninfected allografts.
MCMV Induces Innate Leukocyte Infiltrates Which Are Attenuated by GCV
To characterize the CD45+ leukocytes observed in MCMV-infected allografts, CD45+ cells were stained for CD11b+, CD11c+, Gr-1+, CD204+, and CD49b+ leukocyte subsets for FACS analysis (Fig. 2). Similar to the CD45+ stainings, the CD11b+ infiltrates (Fig. 2A) were greater in CMV allografts (26.38±2.692×105 cells) compared with Mock allografts (2.329±0.1404×105 cells, P<0.01), and these infiltrates diminished in the presence of GCV (10.35±2.784×105 cells, P<0.01) but again remained greater than those found in Mock allografts (P<0.05).
FIGURE 2.
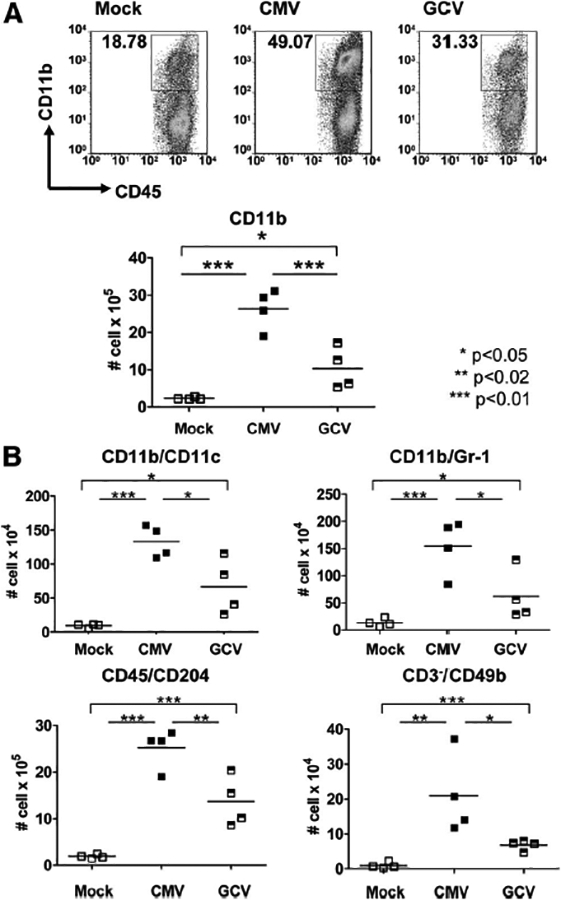
Innate inflammatory infiltrates are induced in murine cytomegalovirus (MCMV)-infected allografts and reduced with ganciclovir (GCV) treatment. (A) Cellular infiltrates from allografts of uninfected (Mock, open squares), CMV-infected (CMV, closed squares), and CMV infected, GCV-treated (GCV, hatched squares) animals were stained for CD45+/CD11b+ cells, evaluated by flow cytometry, and depicted as cells per organ. CD11b+ infiltrates were greater in the CMV grafts compared with Mock grafts (P<0.01) and were reduced in GCV grafts compared with CMV grafts (P<0.01). CD11b+ infiltrates were greater in the GCV grafts compared with Mock (P<0.05). (B) Allo-grafts were analyzed for CD11b+/CD11c+, CD11b+/Gr-1+, CD45+/CD204+, and CD3-/CD49b+ cells by flow cytometry (cells per organ). CMV grafts had greater infiltrates of all innate subsets compared with Mock grafts (P<0.01 for all except CD49b+, P<0.02). GCV grafts had lower numbers of all innate subsets compared with CMV grafts (P<0.05 for all except CD204+, P<0.02). GCV grafts contained greater numbers of CD11c+ and Gr-1+ cells (P<0.05) as well as CD204+ and CD49b+ cells (P<0.01) compared with Mock grafts.
Analyses for dendritic cells (CD11b+/CD11c+), myeloid cells (CD11b+/Gr-1+), macrophages (CD45+/CD204+), and natural killer (NK) cells (CD3-/CD49b+) showed similar results (Fig. 2B), with CMV grafts containing greater numbers of these cells than Mock grafts (P<0.02 to P<0.01) and GCV grafts containing fewer of these cells than CMV grafts (P<0.05 to P<0.02) but still more than Mock grafts (P<0.05 to P<0.01). These results indicate that MCMV infection induced infiltration of all subsets of phagocytic and antigen-presenting leukocytes into MCMV-infected allografts compared with uninfected grafts and that GCV attenuated the numbers of these cell types but not to levels found in Mock allografts.
GCV Does Not Modulate MCMV-Induced Lymphocytic Infiltrates
Next, the CD45+ cells were stained for CD8+ and CD4+ lymphocytes (Fig. 3A) and for CD19+/B220+ lymphocytes (Fig. 3B) and analyzed by FACS. Compared with Mock allografts, CMV allografts again demonstrated significantly increased CD8+ (143.3±22.52×104 vs. 30.66±9.856×104 cells) and CD4+ (60.61±9.614×104 vs. 15.26±5.068×104 cells) cells infiltrating the allograft (P<0.01). However, these cellular infiltrates persisted in the presence of GCV (CD8+, 142.8±26.54×104 cells; CD4+, 36.88±10.26×104 cells) compared with CMV grafts (P>0.05, ns). Similar results were found (Fig. 3B) for the CD19+/B220+ cells (Mock, 9.176±3.703×104 cells; CMV, 34.65±5.906×104 cells; GCV, 28.83±6.893×104 cells). These results suggest that in this model, MCMV induced an adaptive immune response detectable in the allograft, even when viral replication was inhibited by GCV.
FIGURE 3.
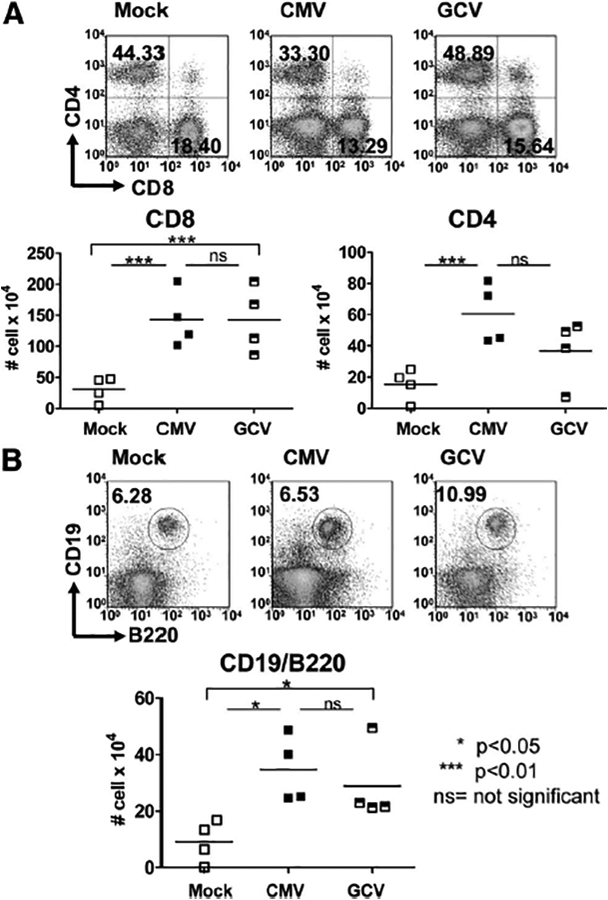
Adaptive inflammatory infiltrates are induced in murine cytomegalovirus (MCMV)-infected allo-grafts but are not affected by ganciclovir (GCV) treatment. (A) Cells from uninfected (Mock, open squares), CMV-infected (CMV, closed squares), and CMV-infected, GCV-treated (GCV, hatched squares) allografts were stained for CD45+/CD8+ and CD45+/CD4+ surface markers, evaluated by flow cytometry and shown as cells per organ. CMV grafts showed induction of CD8+ and CD4+ infiltrates (P<0.01) compared with Mock grafts. No difference was observed in these T lymphocyte subsets between the CMV-infected allografts with and without GCV treatment (P>0.05, ns). (B) Cells from allografts of Mock, CMV, and GCV animals were stained for CD19+/B220+ markers and evaluated by flow cytometry. CMV-infected grafts showed greater CD19+/B220+ lymphocyte infiltrates (P<0.05) compared with Mock grafts, which was sustained in the GCV grafts compared with CMV grafts (P>0.05, ns) and remained elevated in the GCV grafts compared with the Mock grafts (P<0.05).
CMV-Associated Infiltrates Are Modulated by GCV in Histology and Immunofluorescent Staining
To confirm FACS results, allograft microscopy was performed using hematoxylin-eosin staining or with anti-CD3 or anti-CD11b antibodies followed by fluorophore-conjugated secondary antibodies. Consistent with FACS, CMV-infected allografts demonstrated greater mononuclear infiltrates compared with Mock allografts by light microscopy (Fig. 4, top row) and greater immunostaining for CD3+ cells (middle row) and CD11b+ cells (bottom row) in CMV compared with Mock tissues. GCV-treated allografts showed less intense mononuclear infiltrates compared with untreated CMV-infected grafts, accompanied by reduced CD11b+ immunostaining but no reduction in CD3+ immunostaining (Fig. 4, right column), consistent with FACS results.
FIGURE 4.
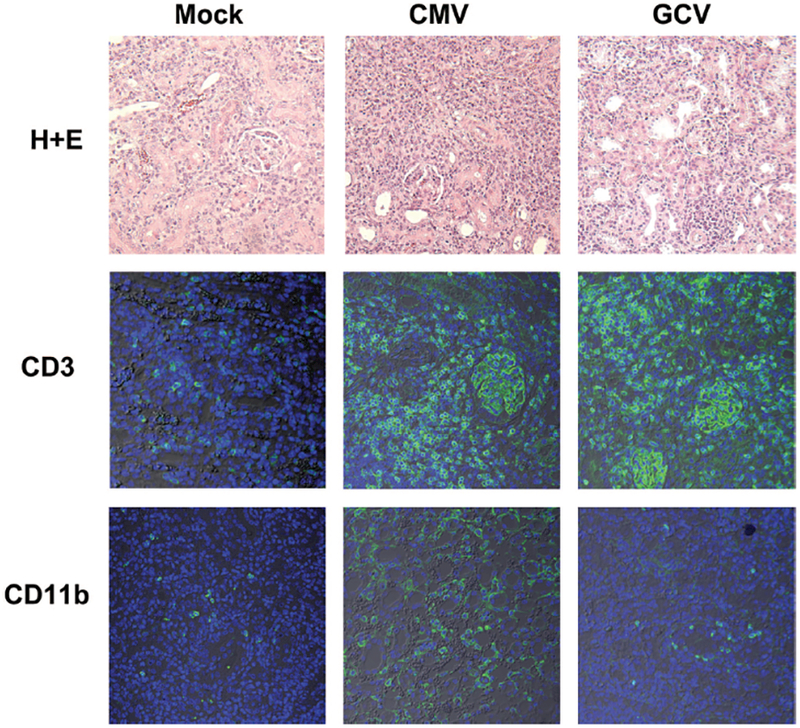
Ganciclovir (GCV) attenuates CD11b+ immunostaining in cytomegalovirus (CMV)-infected allo-grafts. Paraffin-embedded allografts from Mock (left column), CMV (middle column) and GCV-treated (right column) animals were stained by hematoxylin-eosin for light microscopy (top row), or immunostained with primary antibodies for CD3+ cells (middle row), or for CD11b+ cells (bottom row) followed by AlexaFluor488-conjugated secondary antibodies (green) and nuclear counterstain (blue) for confocal microscopy. Compared with Mock grafts, CMV-infected allografts showed induction of mononuclear infiltrates that included both CD3+ and CD11b+ cells. GCV treatment reduced the mononuclear and CD11b+ infiltrates but not the CD3+ infiltrates.
Intragraft Innate Leukocyte Infiltrates Increase After Discontinuing GCV
Next, a cohort of animals receiving MCMV-infected transplants were treated with GCV prophylaxis for 14 days, followed by discontinuation of GCV for 7 days (“postGCV” group). This treatment mimics the use of GCV prophylaxis in human renal transplant patients who are treated with antivirals for a standard period of time to prevent acute CMV disease, followed by empiric antiviral discontinuation. Quan-titative DNA PCR showed detectable viral loads in allografts before and after, but not during, GCV prophylaxis (Fig. 5A). Because the innate leukocyte infiltrates were attenuated during GCV prophylaxis, these cell types were also examined (Fig. 5B). The numbers of CD11c+, CD204+, and CD49b+ leukocytes increased after GCV discontinuation and became similar to CMV-infected allografts not treated with GCV (P>0.05, ns). This result indicates that attenuation of these intragraft infiltrates by GCV was limited to the time of anti-viral usage.
FIGURE 5.
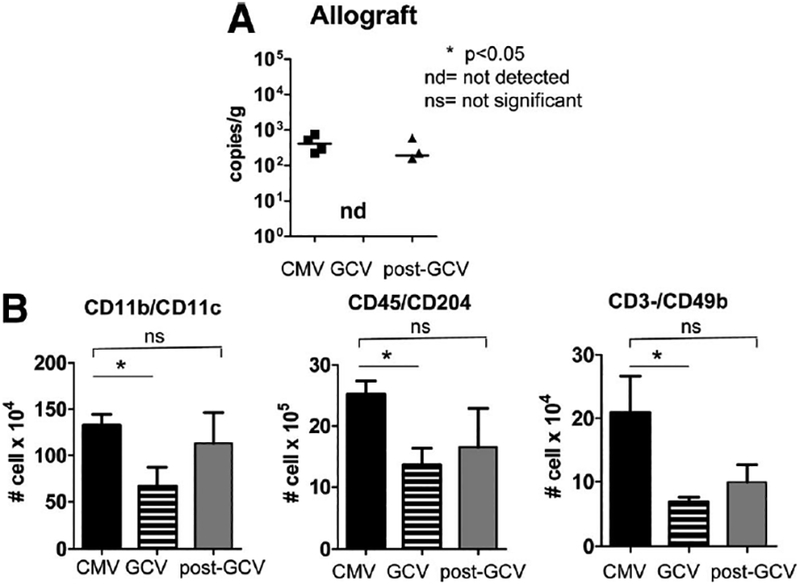
Viral loads rise and innate leukocyte infiltrates reaccumulate in allografts after cessation of ganciclovir (GCV). (A) DNA was extracted from allografts of animals that were cytomegalovirus (CMV) infected (CMV, closed squares), GCV treated (GCV, nd), or treated with GCV which was subsequently discontinued for 7 days (post-GCV, closed triangles). Quantitative DNA PCR for murine CMV (MCMV) immediate-early 1 was performed and results depicted as copies/gram tissue. MCMV DNA was detectable in CMV allografts, became undetectable in GCV grafts, and became detectable again after discontinuation of GCV. (B) Leukocytes from CMV, GCV, and post-GCV allografts were analyzed by flow cytometry for CD11c+, CD204+, and CD49b+ cells. CD11b+, CD204+, and CD49b+ leukocytes were lower in GCV grafts compared with CMV grafts (P<0.05), but after cessation of GCV, numbers of these leukocytes increased (post-GCV) to similar numbers as the CMV grafts without GCV treatment (P>0.05, ns).
GCV Reduces Intragraft Gr-1+ Cells But Does Not Induce Global Neutropenia
Allograft infiltrates from post-GCV animals were also stained for Gr-1+ leukocytes and analyzed by flow cytometry (Fig. 6A). The numbers of Gr-1+ cells decreased in GCV-treated animals and remained low after discontinuing GCV (P<0.01, CMV vs. post-GCV), unlike the other phagocytic leukocyte subsets that increased in the post-GCV allografts. Because systemic neutropenia is a known toxicity of GCV, spleens from Mock, CMV, GCV, and post-GCV were analyzed for CD45+/Gr-1+ cells by flow cytometry to determine whether the decreased number of myeloid cells in the allo-grafts of GCV-treated animals was due to systemic neutropenia. The number of Gr-1+ cells in the spleen (Fig. 6B) were statistically similar in all animals both with and without GCV treatment (P>0.05, ns), indicating that the decrease in Gr-1+ cells observed in the GCV and post-GCV allografts was not due to systemic neutropenia from GCV toxicity.
FIGURE 6.
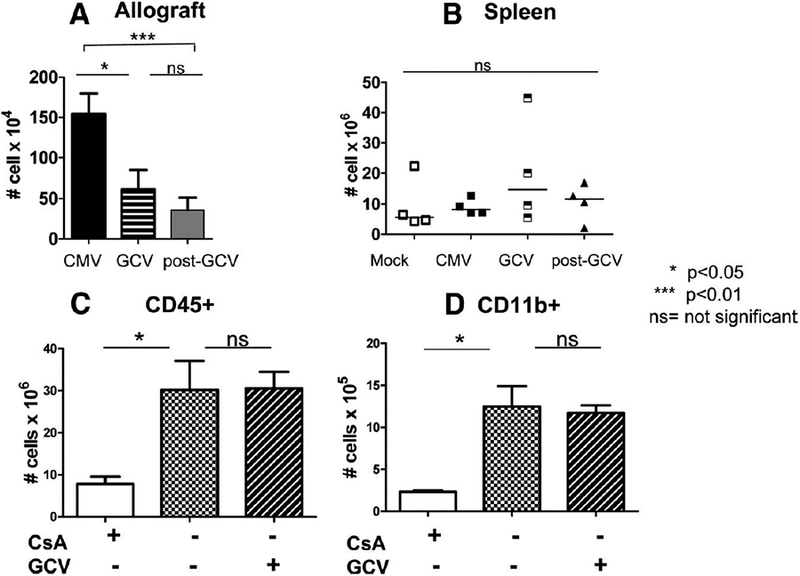
Ganciclovir (GCV) induces sustained reduction of Gr-1+ myeloid cells in allografts but not in spleen and does not influence the intragraft alloimmune response. (A) Gr-1+ cells were analyzed by flow cytometry in cytomegalovirus (CMV), GCV, and post-GCV allografts. Gr-1 cells decreased in GCV grafts compared with CMV grafts (P<0.05) and remained low after GCV was discontinued (GCV vs. post-GCV, P>0.05, ns). Post-GCV Gr-1+ numbers remained lower than those found in CMV grafts without GCV treatment (P<0.01). (B) Gr-1 cells were analyzed by flow cytometry in spleens from Mock, CMV, GCV, and post-GCV animals to determine whether GCV treatment induces neutropenia. Numbers of Gr-1+ cells were similar in spleens from animals with and without GCV treatment, indicating that GCV did not induce global neutropenia in the host animals. Mock animals were given cyclosporine (white bars), no treatment (checkerboard bars), or GCV without cyclosporine (diagonal hatched bars), and allografts were analyzed for CD45+ (C) and CD11b+ (D) infiltrates. Cyclosporine reduced the alloimmune-associated infiltrates compared with untreated grafts (P<0.05), but GCV did not reduce the alloimmune response in cyclosporine-untreated, CMV-negative animals compared with those not receiving GCV (ns).
GCV Does Not Reduce Infiltrates in Mock Allografts
Cohorts of Mock allograft recipients were treated with cyclosporine for 14 days, no cyclosporine, or GCV without cyclosporine (Fig. 6C,D). Cyclosporine-treated animals (white bars) had fewer intragraft CD45+ and CD11b+ infiltrates compared with animals not receiving cyclosporine (checkerboard bars), indicating that cyclosporine treatment decreased the alloimmune response in this model. Treatment with GCV (diagonal hatched bars) instead of cyclosporine had no effect on the CD45+ and CD11b+ infiltrates compared with untreated allografts, indicating that the dose of GCV used in these experiments did not reduce the alloimmune response itself. These results support that the GCV effect observed in CMV-infected allografts is specific to CMV and not a general immunosuppressive effect on the alloimmune response independent of CMV.
DISCUSSION
In this model, transplantation of an MCMV-infected kidney into a naïve recipient induced a marked CD45+ leukocyte infiltrate into the infected allografts compared with uninfected grafts and consisted of both the innate and adaptive immune effectors. GCV prophylaxis terminated MCMV replication and was associated with reduction of intragraft dendritic cells, myeloid cells, macrophages, and NK cells but not T and B lymphocytes in this model. Cessation of GCV prophylaxis permitted recrudescence of innate leukocyte infiltrates in the infected allograft to levels similar to those found in untreated MCMV-infected allografts.
The immunologic response to acute CMV infection has been characterized extensively. Macrophages are described in histology of clinical CMV disease (pneumonitis, hepatitis, and colitis) as well as in animal models of CMV-infected renal transplants and MCMV brain infection (21–23). Macrophages are a prominent infiltrating component during transplant rejection and are a criterion in the Banff classification of renal allograft rejection (24, 25). Macrophages may contribute to allograft damage by inflammatory cytokine production, lysis of infected and bystander parenchymal cells, and possibly by contributing to antigen presentation (26). In this model, macrophage infiltrates increased and decreased in association with viral replication, consistent with their functions in clearing viral infection, but could potentially also contribute to allograft injury.
In this model, intragraft Gr-1+ myeloid cells decreased both during and after GCV treatment. Direct effects of GCV on neutrophils during MCMV infection have not been described in other models. Neutrophilic infiltrates are included in the Banff criteria for acute rejection of human renal allo-grafts, and thus it is possible that attenuation of myeloid infiltrates during GCV treatment could also contribute to amelioration of acute rejection (24).
The immediate control of MCMV infection is generally attributed to NK cells. NK cell populations expand and contract with acute MCMV infection, and NK cell-deficient animals are unable to control initial viral replication (27, 28). In this model, numbers of intragraft NK cells increased and decreased in association with viral replication, consistent with their known role in acute viral clearance. In models of allo-graft rejection, NK cells have been shown to contribute to both allograft rejection and tolerance (29, 30). Although the primary role of NK cells in our model is likely the elimination of MCMV-infected cells, the effect of intragraft NK cell infiltration could be either detrimental or beneficial to the allo-graft itself and may deserve further study. Similarly, in renal transplantation, dendritic cells may play either a detrimental or tolerogenic role in allograft survival. Dendritic cells can produce reactive cytokines (tumor necrosis factor-α, inter-leukin-6, and interleukin-1β) and stimulate T effector cells but may also promote tolerance by induction of T regulatory cells (31, 32). The impact of GCV-mediated modulation of dendritic cells and their relationship to NK activation may also deserve further investigation.
Long-term control of MCMV infection requires T lymphocytes (33). In this model, the adaptive immune response to MCMV infection appeared intact even in the presence of cyclosporine, which is consistent with findings in rat renal transplantation, where rat CMV induced both macrophage infiltrates and lymphoid activation in the presence of triple immunosuppression (21, 22). In our model, use of a single immunosuppressant is less stringent than the more intense immunosuppression used clinically. The relatively recent infection of the donor kidneys in our model (12+ weeks) also differs from human renal transplants where the timing of initial donor kidney infection may be years or even decades before transplantation and thus might require a longer period to induce viral reactivation and the adaptive host response. Use of GCV prophylaxis did not seem to impact the priming of the adaptive response in this model, suggesting that the presence of nonreplicating virus in the allograft may still be sufficient to induce adaptive immunity. Consistent with this, Wilson et al. (34) showed that GCV-treated mice developed antibodies after MCMV infection which protected against subsequent lethal dose MCMV challenge, indicating that GCV itself does not necessarily abrogate the adaptive immune response. The contribution of this CMV-associated lymphoid response to activation of alloimmunity may be of further interest, as viral infections have been proposed to contribute to heterologous immunity in transplantation (35–37).
Studies reporting effects of GCV on MCMV infection have generally focused on viral replication rather than inflammation (38, 39). However, GCV treatment reduced both acute and chronic inflammation in a model of MCMV-induced myocarditis and reduced MCMV-induced interstitial pneumonitis (40, 41). These results support the plausibility of our finding that GCV decreased inflammation within MCMV-infected renal allografts. Although this study does not identify the mechanism(s) by which GCV could reduce innate infiltrates, potential reasons could include reduction of CMV antigen presentation to phagocytic cells, reduction of CMV-induced changes in infected host cells, or reduction in the number of intragraft infected host cells available for immune surveillance. In this study, the duration of GCV prophylaxis was relatively brief at 14 days, as compared with the 100 to 200 days currently used in clinical renal transplantation (16). It is possible that longer duration of prophylaxis in this model may have more pronounced or long-lasting effects on CMV-associated allograft inflammation by more complete suppression of reactivation and replication and could be studied using this model.
This work presents the first evidence that GCV may impact the intensity of CMV-associated leukocyte infiltrates into infected allografts. Attenuation of neutrophils and macrophages by GCV treatment could be beneficial to allograft function, whereas the impact of NK and dendritic cell reduction could be either beneficial or detrimental. Further work may assist in defining the role of these CMV-associated leukocytes on allograft injury and the impact of prophylactic antiviral treatment on allograft function.
MATERIALS AND METHODS
Virus and Animals
MCMV strain Smith with an open reading frame m157 deletion (MCMVSmithΔm157) was provided by Dr. Ulrich Koszinowski (Maz von Pettenkofer-Institute, Ludwig Maximilians-University, Munich, Germany). Virus stocks were propagated in tissue culture using murine embryonic fibroblasts, concentrated by centrifugation, and viral titers were quantitated by viral plaque assay on murine embryonic fibroblasts. Stocks were frozen in aliquots at −80°C and discarded after a single use.
Female BALB/cJ or C57BL/6J mice, aged 5 to 6 weeks, were purchased from Jackson Laboratory (Bar Harbor, ME) and maintained in Association for the Assessment and Accreditation of Laboratory Animal Care-approved animal facilities of the Animal Resources Program of the University of Alabama at Birmingham (Birmingham, AL) under pathogen-free conditions. Mouse maintenance and experimental protocols were approved by the University of Alabama at Birmingham Institutional Animal Care and Use Committee.
Renal Transplantation Surgery
For MCMV-infected transplants, donor Balb/cJ (H-2d) mice were infected by intraperitoneal injection with 104 plaque forming units of MCMV SmithΔ157 strain virus at least 12 weeks before renal transplantation, permitting establishment of persistent infection in the donor (D+) kidneys. Donors without MCMV infection (D–) served as controls. Allogeneic orthotopic kidney transplantation from donor BALB/cJ mice into recipient C57BL/6J (H-2b) mice (R–) was performed using vessel and bladder cuffs according to published techniques (42). The contralateral native kidney of the recipient was left intact, as life-sustaining transplantation was not required for these experiments. Some recipients were treated with cyclosporine (Novartis Pharmaceuticals Corp., East Hanover, NJ) at 10 mg/kg/day, subcutaneously once daily starting immediately postoperatively until time of killing (43). Some animals were treated with GCV prophylaxis (Roche Laboratories, Nutley, NJ) at 15 mg/kg/day, subcutaneously once daily starting immediately postoperatively for 14 days (39). For cyclosporine-treated animals, experimental groups were: (1) MCMV-uninfected control allografts (D–/R–, “Mock”); (2) MCMV-infected allografts (D+/R–, “CMV”); (3) MCMV-infected allografts treated with GCV for 14 days (D+/R–, “GCV”); and (4) MCMV-infected allografts treated with GCV for 14 days, followed by 7 days without GCV (D+/R–, “post-GCV”). A final group of Mock animals did not receive cyclosporine but received either GCV or no GCV to assess the effect of GCV without CMV infection.
MCMV Quantitative DNA PCR
MCMV SmithΔm157 was quantified from blood samples and organs as previously described (44). In brief, blood was collected in heparinized tubes at killing, intravascular spaces were perfused with at least 30 mL of ice-cold phosphate-buffered saline until organ pallor, and blood and organs were frozen at [H11002]80°C. Total DNA was isolated using the Qiagen QiaAMP DNA Kit according to the manufacturer’s protocol. Quantitative DNA PCR was performed by amplification of a fragment of the MCMV immediate-early 1 exon 4 and quantified by comparison to a standard curve generated by amplification of a plasmid encoding the MCMV immediate-early 1 exon 4 PCR product in serial dilution (44). Samples were run in triplicate using the described two-step amplification protocol on the ABI StepOnePlus Real-Time PCR cycler. Copy numbers were calculated per ml blood or gram tissue.
Flow Cytometric Analysis of Allograft Immune Infiltrates
Organs were perfused and harvested at day 14 to 21 posttransplant, followed by mechanical disruption, homogenization, and digestion of the tissue with 0.05 μg/mL collagenase for 30 min at 37°C. Propidium iodide was used as a counterstain to identify and exclude dead cells within the samples. After blocking Fc receptors with anti-mouse CD16/CD32 (clone 93; eBiosciences, San Diego, CA), flow cytometric analysis of single-cell suspensions was performed using a combination of the following monoclonal anti-mouse antibodies purchased from eBiosciences unless stated otherwise: fluorescein isothiocyanate (FITC)- or phycoerythrin-conjugated CD45 (30-F11), allophycocyanin-conjugated CD11b (M1/70), PE-conjugated CD11c (N418), FITC-conjugated Gr-1 (RB6–8C5), FITC-conjugated CD204 (2F8) (AbD Serotec, Oxford, UK), FITC-conjugated CD3e (145–2C11), APC-conjugated CD49b (DX5), PE-conjugated CD8α (53–6.7), APC-conjugated CD4 (GK1.5), and FITC-conjugated CD19 (1D3). Flow cytometry studies were performed using a dual-laser FACSCalibur and analyzed using FlowJo software (BD Biosciences, San Jose, CA). Results were quantitated as cell number per organ.
Histology and Immunofluorescent Staining
Allografts were perfused with saline to organ pallor and fixed with 4% neutral buffered formalin (Sigma, St. Louis, MO), followed by 70% ethanol and paraffin embedding. Sections were deparaffinized, rehydrated, and stained using hematoxylin-eosin according to standard procedures, and images collected by light microscopy at 200× magnification using ImagePro software (Media Cybernetics Inc., Bethesda, MD). For immunofluorescent staining, sections were rehydrated, antigen retrieval performed by boiling for 20 min in sodium citrate buffer at pH 6.0, blocked with 10% goat serum and 1% bovine serum albumin, incubated with primary antibody (rabbit anti-mouse CD3 [ab5690; Abcam, Cambridge, MA] at 1:100 dilution or rabbit anti-mouse CD11b [ab75476; Abcam] at 1:400 dilution) overnight at 4°C, followed by secondary antibody (goat anti-rabbit IgG-AlexaFluor-488 [Invitrogen, Carlsbad, CA]) for 1 hr at ambient temperature and Topro3 nuclear stain for 15 min. Images were collected under identical exposure times and identical gain for each fluorophore using confocal microscopy (Olympus Fluoview BX51, Center Valley, PA) at 200× magnification.
Statistical Analysis
All assays were analyzed using four animals for each experimental group. Groups were analyzed using the Student’s t test using Prism 5.0 software (GraphPad, San Diego, CA), accepting statistically significant differences at a P value of <0.05.
Acknowledgments
This work was supported by NIH 5K08AI059428-02 (M.S.), the Children’s Center for Research and Innovation of the Alabama Children’s Hospital Foundation (M.S.), the Kaul Pediatric Research Initiative of The Children’s Hospital of Alabama (M.S.); the UAB-UCSD O’Brien Core Center for Acute Kidney Injury Research, NIH NIDDK 1P30 DK079337 (J.F.G.); and the UAB Digestive Diseases Research Development Center grant P30 DK064400.
Footnotes
The authors declare no conflicts of interest.
This work was presented in part at the XXIII International Congress of The Transplantation Society, August 15–19, 2010, Vancouver, British Columbia, Canada; and at the 13th International CMV/ Betaherpes-virus Workshop, May 14–17, 2011, Nuremberg, Germany.
REFERENCES
- 1.Sagedal S, Nordal KP, Hartmann A, et al. The impact of cytomegalovirus infection and disease on rejection episodes in renal allograft recipients. Am J Transplant 2002; 2: 850. [DOI] [PubMed] [Google Scholar]
- 2.Tong CY, Bakran A, Peiris JS. The association of viral infection and chronic allograft nephropathy with graft dysfunction after renal transplantation. Transplantation 2002; 74: 576. [DOI] [PubMed] [Google Scholar]
- 3.Sagedal S, Hartmann A, Nordal KP, et al. Impact of early cytomegalo-virus infection and disease on long-term recipient and kidney graft survival. Kidney Int 2004; 66: 329. [DOI] [PubMed] [Google Scholar]
- 4.Sagedal S, Rollag H, Hartmann A. Cytomegalovirus infection in renal transplant recipients is associated with impaired survival irrespective of expected mortality risk. Clin Transplant 2007; 21: 309. [DOI] [PubMed] [Google Scholar]
- 5.Freeman RB Jr. The ‘Indirect’ Effects of Cytomegalovirus Infection. Am J Transplant 2009; 9: 2453. [DOI] [PubMed] [Google Scholar]
- 6.Rubin RH, Tolkoff-Rubin NE, Oliver D, et al. Multicenter seroepidemiologic study of the impact of cytomegalovirus infection on renal transplantation. Transplantation 1985; 40: 243. [DOI] [PubMed] [Google Scholar]
- 7.Schnitzler MA, Woodward RS, Brennan DC, et al. Impact of cytomegalovirus serology on graft survival in living related kidney transplantation: Implications for donor selection. Surgery 1997; 121: 563. [DOI] [PubMed] [Google Scholar]
- 8.Schnitzler MA, Woodward RS, Brennan DC, et al. The effects of cytomegalovirus serology on graft and recipient survival in cadaveric renal transplantation: Implications for organ allocation. Am J Kidney Dis 1997; 29: 428. [DOI] [PubMed] [Google Scholar]
- 9.Gerstenkorn C, Balupuri S, Mohamed MA, et al. The impact of cytomegalovirus serology for 7-year graft survival in cadaveric kidney transplantation—the Newcastle experience. Transpl Int 2000; 13(suppl 1): S372. [DOI] [PubMed] [Google Scholar]
- 10.Kuo HT, Ye X, Sampaio MS, et al. Cytomegalovirus serostatus pairing and deceased donor kidney transplant outcomes in adult recipients with antiviral prophylaxis. Transplantation 2010; 90: 1091. [DOI] [PubMed] [Google Scholar]
- 11.Markovic-Lipkovski J, Muller CA, Engler-Blum G, et al. Human cytomegalovirus in rejected kidney grafts; detection by polymerase chain reaction. Nephrol Dial Transplant 1992; 7: 865. [PubMed] [Google Scholar]
- 12.Holma K, Tornroth T, Gronhagen-Riska C, et al. Expression of the cytomegalovirus genome in kidney allografts during active and latent infection. Transpl Int 2000; 13: S363. [DOI] [PubMed] [Google Scholar]
- 13.Gerstenkorn C, Robertson H, Mohamed MA, et al. Detection of cytomegalovirus (CMV) antigens in kidney biopsies and transplant nephrectomies as a marker for renal graft dysfunction. Clin Chem Lab Med 2000; 38: 1201. [DOI] [PubMed] [Google Scholar]
- 14.Helantera I, Koskinen P, Tornroth T, et al. The impact of cytomegalo-virus infections and acute rejection episodes on the development of vascular changes in 6-month protocol biopsy specimens of cadaveric kidney allograft recipients. Transplantation 2003; 75: 1858. [DOI] [PubMed] [Google Scholar]
- 15.Reischig T, Jindra P, Hes O, et al. Valacyclovir prophylaxis versus preemptive valganciclovir therapy to prevent cytomegalovirus disease after renal transplantation. Am J Transplant 2008; 8: 69. [DOI] [PubMed] [Google Scholar]
- 16.Kotton CN, Kumar D, Caliendo AM, et al. International consensus guidelines on the management of cytomegalovirus in solid organ transplantation. Transplantation 2010; 89: 779. [DOI] [PubMed] [Google Scholar]
- 17.Hodson EM, Jones CA, Webster AC, et al. Antiviral medications to prevent cytomegalovirus disease and early death in recipients of solid-organ transplants: A systematic review of randomised controlled trials. Lancet 2005; 365: 2105. [DOI] [PubMed] [Google Scholar]
- 18.Reischig T, Jindra P, Mares J, et al. Valacyclovir for cytomegalovirus prophylaxis reduces the risk of acute renal allograft rejection. Transplantation 2005; 79: 317. [DOI] [PubMed] [Google Scholar]
- 19.Li L, Chaudhuri A, Weintraub LA, et al. Subclinical cytomegalovirus and Epstein-Barr virus viremia are associated with adverse outcomes in pediatric renal transplantation. Pediatr Transplant 2007; 11: 187. [DOI] [PubMed] [Google Scholar]
- 20.Kliem V, Fricke L, Wollbrink T, et al. Improvement in long-term renal graft survival due to CMV prophylaxis with oral ganciclovir: Results of a randomized clinical trial. Am J Transplant 2008; 8: 975. [DOI] [PubMed] [Google Scholar]
- 21.Lautenschlager I, Soots A, Krogerus L, et al. CMV increases inflammation and accelerates chronic rejection in rat kidney allografts. Transplant Proc 1997; 29:802. [DOI] [PubMed] [Google Scholar]
- 22.Lautenschlager I, Soots A, Krogerus L, et al. Effect of cytomegalovirus on an experimental model of chronic renal allograft rejection under triple-drug treatment in the rat. Transplantation 1997; 64: 391. [DOI] [PubMed] [Google Scholar]
- 23.Kosugi I, Kawasaki H, Arai Y, et al. Innate immune responses to cytomegalovirus infection in the developing mouse brain and their evasion by virus-infected neurons. Am J Pathol 2002; 161: 919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Racusen LC, Colvin RB, Solez K, et al. Antibody-mediated rejection criteria: An addition to the Banff ‘97 classification of renal allograft rejection. Am J Transplant 2003; 3: 708. [DOI] [PubMed] [Google Scholar]
- 25.Sis B, Mengel M, Haas M, et al. Banff ‘09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 2010; 10: 464. [DOI] [PubMed] [Google Scholar]
- 26.Wyburn KR, Jose MD, Wu H, et al. The role of macrophages in allograft rejection. Transplantation 2005; 80: 1641. [DOI] [PubMed] [Google Scholar]
- 27.Bukowski JF, Woda BA, Welsh RM. Pathogenesis of murine cytomegalo-virus infection in natural killer cell-depleted mice. J Virol 1984; 52: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robbins SH, Tessmer MS, Mikayama T, et al. Expansion and contraction of the NK cell compartment in response to murine cytomegalovirus infection. J Immunol 2004; 173: 259. [DOI] [PubMed] [Google Scholar]
- 29.Yu G, Xu X, Vu MD, et al. NK cells promote transplant tolerance by killing donor antigen-presenting cells. J Exp Med 2006; 203: 1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroemer A, Xiao X, Degauque N, et al. The innate NK cells, allograft rejection, and a key role for IL-15. J Immunol 2008; 180: 7818. [DOI] [PubMed] [Google Scholar]
- 31.Paterson HM, Murphy TJ, Purcell EJ, et al. Injury primes the innate immune system for enhanced Toll-like receptor reactivity. J Immunol 2003; 171: 1473. [DOI] [PubMed] [Google Scholar]
- 32.Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol 2007; 7: 610. [DOI] [PubMed] [Google Scholar]
- 33.Crough T, Khanna R. Immunobiology of human cytomegalovirus: From bench to bedside. Clin Microbiol Rev 2009; 22: 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilson EJ, Medearis DN Jr, Hansen LA, et al. 9-(1–3-Dihydroxy-2-propoxymethyl)guanine prevents death but not immunity in murine cytomegalovirus-infected normal and immunosuppressed BALB/c mice. Antimicrob Agents Chemother 1987; 31: 1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burrows SR, Silins SL, Khanna R, et al. Cross-reactive memory T cells for Epstein-Barr virus augment the alloresponse to common human leukocyte antigens: Degenerate recognition of major histocompatibility complex-bound peptide by T cells and its role in alloreactivity. Eur J Immunol 1997; 27: 1726. [DOI] [PubMed] [Google Scholar]
- 36.Han Lee ED, Kemball CC, Wang J, et al. A mouse model for polyomavirus-associated nephropathy of kidney transplants. Am J Transplant 2006; 6: 913. [DOI] [PubMed] [Google Scholar]
- 37.Brehm MA, Daniels KA, Priyadharshini B, et al. Allografts Stimulate Cross-Reactive Virus-Specific Memory CD8 T Cells with Private Specificity. Am J Transplant 2010; 10: 1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reefschlaeger J, Bender W, Hallenberger S, et al. Novel non-nucleoside inhibitors of cytomegaloviruses (BAY 38 – 4766): In vitro and in vivo antiviral activity and mechanism of action. J Antimicrob Chemother 2001; 48: 757. [DOI] [PubMed] [Google Scholar]
- 39.Kern ER, Bidanset DJ, Hartline CB, et al. Oral activity of a methylenecyclopropane analog, cyclopropavir, in animal models for cytomegalovirus infections. Antimicrob Agents Chemother 2004; 48: 4745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shanley JD, Morningstar J, Jordan MC. Inhibition of murine cytomegalovirus lung infection and interstitial pneumonitis by acyclovir and 9-(1,3-dihydroxy-2-propoxymethyl)guanine. Antimicrob Agents Chemother 1985; 28: 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lenzo JC, Shellam GR, Lawson CM. Ganciclovir and cidofovir treatment of cytomegalovirus-induced myocarditis in mice. Antimicrob Agents Chemother 2001; 45: 1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Z, Schlachta C, Duff J, et al. Improved techniques for kidney transplantation in mice. Microsurgery 1995; 16: 103. [DOI] [PubMed] [Google Scholar]
- 43.Andoh TF, Gardner MP, Bennett WM. Protective effects of dietary L-arginine supplementation on chronic cyclosporine nephrotoxicity. Transplantation 1997; 64: 1236. [DOI] [PubMed] [Google Scholar]
- 44.Bantug GR, Cekinovic D, Bradford R, et al. CD8+ T lymphocytes control murine cytomegalovirus replication in the central nervous system of newborn animals. J Immunol 2008; 181: 2111. [DOI] [PMC free article] [PubMed] [Google Scholar]


