Abstract
To investigate the association of telomere length with trajectories of general cognitive abilities, we used data on 5955 participants from the Sex Differences in Health and Aging Study and the Swedish Adoption/Twin Study of Aging in Sweden, and the Mayo Clinic Study of Aging and the Health and Retirement Study in the US. Telomere length was measured at baseline, while general cognitive ability was assessed repeatedly up to seven occasions. Latent growth curve models were used to examine the associations. One standard deviation increase of telomere length was associated with 0.021 unit increase (95% confidence interval [CI]: 0.001, 0.042) of standardized mean general cognitive ability. After controlling for sex, the point estimate remained similar (0.019) with a wider confidence interval (95% CI: −0.002, 0.039). The association was attenuated with adjustment for educational attainment (0.009, 95%CI: −0.009, 0.028). No strong evidence was observed for the association of telomere length and decline in general cognitive ability. Longer telomere length was associated with higher general cognitive ability levels in the age-adjusted models, but not in the models including all covariates, nor with cognitive decline.
Keywords: Telomere, Cognition, Biological Aging, Cognitive Aging
1. Introduction
Telomeres are repetitive nucleotide sequences at the end of the chromosomes, protecting them from degradation. Because of its protective properties against cellular senescence, telomere length (TL) has been postulated as a biomarker of ageing in humans. Short TL has been associated with increased risk for mortality (Bakaysa et al., 2007; Mons et al., 2017), cardiovascular diseases (Hammadah et al., 2017; Zhan et al., 2017), some cancers (Barthel et al., 2017; Telomeres Mendelian Randomization Collaboration et al., 2017) and neurodegenerative disorders (Honig et al., 2012; Zhan et al., 2015). Several studies also reported a significant association of TL with cognitive performance (Harris et al., 2006; Harris et al., 2016; Hägg et al., 2017; Martin-Ruiz et al., 2006; Valdes et al., 2010; Yaffe et al., 2011); however, the results were not consistent. Moreover, most published studies used cross-sectional data or repeated measurements with only a few different time points available for cognition. Longitudinal studies with several repeated measurements of cognition are largely lacking. In this study, we hypothesized that longer baseline telomere length would be associated with better average level of general cognitive ability and slower cognitive decline in four prospective cohorts from Sweden and the US.
2. Methods
2.1 Study Population
The Sex Differences in Health and Aging (GENDER) is a population-based cohort study of unlike-sexed twins born between 1906 and 1925 in Sweden (Gold et al., 2002). Four hundred and ninety eight individuals of European ancestry participated in the first in-person testing including cognitive tests, health examination, and blood sample collection from 1995. In-person testing follow-up was conducted up to three times on a four-year interval during the years 1995 to 2005 with an average of 5.6 years (standard deviation [SD]: 2.0 years) follow up. In total, four hundred participants had at least one cognitive test and 404 participants had TL assessed. The combined data for TL and general cognitive ability were available for 327 participants.
The Swedish Adoption/Twin Study of Aging (SATSA) was a population-based study initiated in 1984 to study twin pairs reared apart or reared together, and 859 individuals of European ancestry participated in at least one wave of in-person testing (Finkel and Pedersen, 2004; Pedersen et al., 1991). In the present study, 632 participants had at least one cognitive assessment during the third, fifth, sixth, eighth, and ninth in-person testing, and 638 participants had telomere length measured. In total, 566 participants had both TL and general cognitive ability assessed. Follow-up was conducted up to 5 times during years 1992 to 2012 with an average of 10.5 years (SD: 5.0 years) follow-up.
The Mayo Clinic Study of Aging (MCSA) is a prospective population-based study using a stratified random sampling design that began in 2004 in Minnesota, US (Roberts et al., 2008). The study population consisted of participants aged 50 years and above. In this study, 1267 participants had TL assessed and 1225 participants had at least one cognitive measurement. The present analyses included 1205 participants primarily of European ancestry with available data on both TL and at least one cognitive testing. Participants were followed up at 15-monthly intervals for up to seven times from the year 2008 to 2017 with an average of 3.9 years (SD: 1.6 years) follow-up.
The Health and Retirement Study (HRS) is a nationally representative longitudinal survey of more than 37000 individuals over the age of 50 years in 23000 households in the US. The survey, which has been fielded every two years since 1992, was established to provide a national resource for data on the changing health and economic circumstances associated with aging at both individual and population levels. Details of HRS were described elsewhere (Sonnega et al., 2014). Cognitive ability was assessed up to four times in 20819 participants during years 2008 to 2014 with an average of 5.0 years (SD: 1.5 years) follow-up. A subset of participants (n=5808) had TL assessed. In the present study, we included 3857 participants of European ancestry who had data available on both TL and general cognitive ability.
Informed consent was obtained from all participants in each cohort. This study was approved by the Regional Ethics Board in Stockholm (2014/1757-31/2, 2017/353-32).
2.2 Telomere Length Assessment
Telomere length was measured using a quantitative PCR based technique by comparing telomere sequence copy number in each participant’s sample (T) to a single-copy gene copy number (S). The resulting T/S ratio is proportional to the average length of telomere. TL was measured in peripheral blood leukocytes in GENDER, SATSA, and MCSA and in saliva in HRS. The details of the measurement procedures can be found in the supplementary file.
2.3 General Cognitive Ability
In GENDER and SATSA, a general cognitive ability score based on performance of all cognitive tests was derived through the extraction of the first principal component analysis of Synonyms, Block design, Thurstone Picture Memory, and Symbol Digit tests, excluding any prevalent dementia cases (Pedersen et al., 1992; Reynolds et al., 2005). The principal component analysis scoring coefficients were applied from the baseline wave to the subsequent waves with subtests z-transformed to their respective baseline means and standard deviations so that intra-individual change could be assessed. For MCSA, a global cognitive z-score was calculated using the z-score-transformed means of the four cognitive domain z-scores for memory, language, executive function, and visuospatial skills domains (Mielke et al., 2017). For HRS, a total cognitive score was constructed from immediate and delayed word recall, serials 7, backwards counting from 20, and object naming and then z-transformed (Fisher et al., 2017).
2.4 Educational Attainment
Educational attainment was the self-reported highest education. In GENDER, it was classified as less than elementary school, elementary school, more than elementary school, vocational school, high school, and university. In SATSA, education attainment was classified as elementary school, vocational school, high school, and university or higher. Educational attainment was defined as the number of years in school in MCSA, while it was defined as the highest degree of education that was classified as no degree, general education development, high school diploma, two year college degree, four year college degree, Master degree, professional degree (Ph.D., M.D., or J.D.), and degree unknown/some college in HRS.
2.5 Statistical Analysis
For ease in comparison across studies, we standardized both TL and longitudinal general cognitive ability scores to the mean of 0 and standard deviation of 1 across all waves in all cohorts. We also centered educational attainment in each cohort. We used latent growth curve models to examine the association of TL with mean general cognitive ability levels and trajectories. Attained age was used as the time scale and centered at age 73 years based on the age ranges of the four cohorts. We performed analyses for three models. Model one included a random intercept (to allow different participants to have different intercepts) and a fixed slope for attained age. Model two included an additional adjustment for sex. Model three was fitted with a random intercept and a random slope (to allow different participants to have different slopes) for attained age with adjustment for sex. Analyses also accounted for the correlation within twin-pairs by modeling individuals nested in twin pairs as random effects in GENDER and SATSA. Study specific estimates were meta-analyzed using inverse variance weighted method. Fixed effects models were used if no significant heterogeneity was observed, otherwise random effects models applied to estimate the pooled effect sizes (Lumley, 2012). We also performed additional analysis by adjusting for educational attainment. The association of TL with general cognitive ability decline was assessed by including an interaction term between TL and centered age in years. The details of our model specification are in the supplementary file. All statistical analyses were performed using SAS 9.4 (Cary, NC) and R 3.3.
3. Results
Characteristics of the study participants in each cohort are presented in Table 1 and the number of participants in each follow-up wave are shown in Supplementary Table 1. The mean ages at the first measurement occasion of the total 5955 participants (women: 54.7%) ranged from 67.8 to 75.0 years old across the four cohorts, while average follow-up duration spanned from 3.9 to 10.5 years.
Table 1.
Basic characteristics of study participants in each cohort
| Variables | GENDER (n=327) | SATSA (n=566) | MCSA (n=1205) | HRS (n=3857) |
|---|---|---|---|---|
| Age(years, SD) | 75.0 (2.9) | 67.8 (9.1) | 72.4 (9.8) | 73.7 (7.5) |
| Women (%) | 159 (48.5) | 328 (57.9) | 562 (46.6) | 2211 (57.3) |
| Average follow-up (years, SD) | 5.6 (2.0) | 10.5 (5.0) | 3.9 (1.6) | 5.0 (1.5) |
| Depression (%) | 49 (15.0) | 184 (32.5) | - | 649 (16.8) |
| Coronary heart disease (%) | 20 (6.1) | 31 (5.48) | 343 (28.5) | 1193 (30.9) |
SD: standard deviation
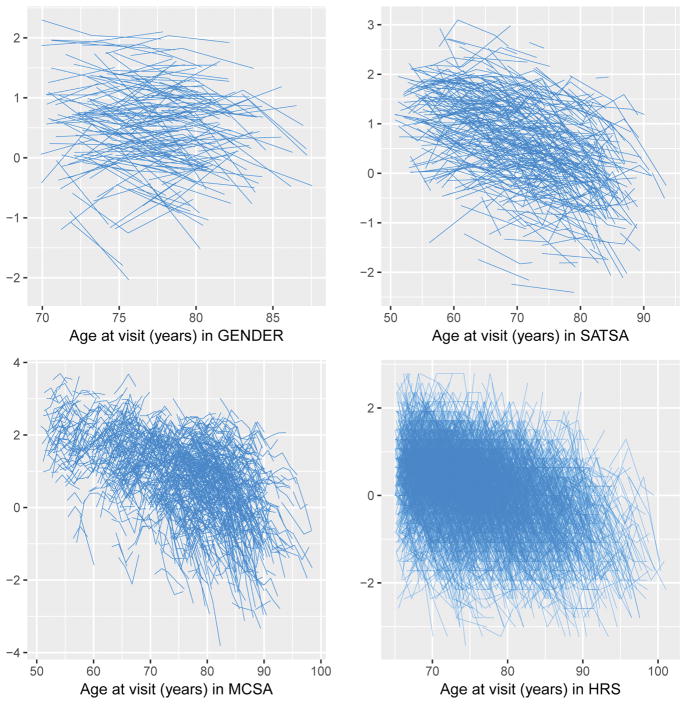
Figure 1 describes the cognitive trajectory in each of these cohorts. General cognitive ability declined with increasing age over time. Latent growth curve models were fitted to examine general cognitive ability and its association with TL (Table 2). In the first and random intercept model, controlling for attained age, longer TL was associated with a higher mean level of general cognitive ability at age 73 (β: 0.021, 95% confidence interval [CI]: 0.001, 0.042 P-value=0.043) in the meta-analysis. Additional adjustment for sex achieved the similar point estimate with slightly wider CI (−0.002, 0.039, P-value=0.079). Then a random intercept and random slope model was fitted and showed a similar result (β: 0.018, 95%CI: −0.003, 0.039, P-value=0.091). In the sensitivity analyses, educational attainment was further added to the models (Table 3). The effect size of the association of TL with general cognitive ability was then attenuated (β: 0.009, 95%CI: −0.009, 0.028, P-value=0.327) with this adjustment. Further adjustment of coronary heart diseases and depression yielded similar results. Additional analyses by study type (twin-study or not) were presented in Supplementary Table 2. We did not find a significant association of TL with the decline of general cognitive ability (Table 4) over time (β: 0.002, 95%CI: −0.0002, 0.004, P-value=0.073).
Figure 1.
General cognitive trajectory in all cohorts
Table 2.
Association between telomere length and mean general cognitive ability, β(95% CI)
| Study | Model 1 | P value | Model 2 | P value | Model 3 | P value |
|---|---|---|---|---|---|---|
| GENDER | 0.042 (−0.042, 0.126) | 0.324 | 0.035 (−0.048, 0.119) | 0.405 | 0.034 (−0.049, 0.118) | 0.423 |
| SATSA | 0.032 (−0.023, 0.088) | 0.252 | 0.029 (−0.026, 0.085) | 0.305 | 0.039 (−0.017, 0.094) | 0.176 |
| MCSA | 0.007 (−0.045, 0.059) | 0.803 | 0.004 (−0.048, 0.055) | 0.890 | −0.009 (−0.061, 0.043) | 0.741 |
| HRS | 0.021 (−0.005, 0.047) | 0.119 | 0.018 (−0.008, 0.044) | 0.165 | 0.019 (−0.007, 0.045) | 0.162 |
| Meta-analysis | 0.021 (0.001, 0.042) | 0.043 | 0.019 (−0.002, 0.039) | 0.079 | 0.018 (−0.003, 0.039) | 0.091 |
Model 1 was adjusted for attained age, Model 2 was further adjusted for sex, Model 3 was adjusted for sex using random intercept and slope model, detailed model specification is in the supplementary file.
Table 3.
Additional adjustment for educational attainment, β(95% CI)
| Study | Model | P value |
|---|---|---|
| GENDER | 0.022 (−0.055, 0.099) | 0.571 |
| SATSA | 0.015 (−0.038, 0.067) | 0.580 |
| MCSA | 0.008 (−0.040, 0.055) | 0.748 |
| HRS | 0.008 (−0.016, 0.031) | 0.519 |
| Meta-analysis | 0.009 (−0.009, 0.028) | 0.327 |
Table 4.
Association between telomere length and decline of general cognitive ability, β(95% CI)
| Study | Model | P value |
|---|---|---|
| GENDER | 0.004 (−0.008, 0.015) | 0.531 |
| SATSA | 0.002 (−0.001, 0.006) | 0.164 |
| MCSA | 0.006 (0.0005, 0.011) | 0.032 |
| HRS | −0.0002 (−0.003, 0.003) | 0.920 |
| Meta-analysis | 0.002 (−0.0002, 0.004) | 0.073 |
Model specification is in the supplementary file.
4. Discussion
In this longitudinal study involving more than 5000 participants from two Swedish and two US cohorts, we found longer TL to be associated with higher levels of general cognitive ability in the age-adjusted models, but not in the models when other covariates were adjusted, nor with cognitive decline. The magnitude of the association, however, was small and attenuated after controlling for other covariates.
To our knowledge, this is the largest longitudinal study of general cognitive ability, measuring up to seven occasions, and its association with TL. A recent meta-analysis based on European cross-sectional studies found that the genetic risk score of TL was associated with better cognitive performance, while the observational association of longer TL per se with general cognitive performance did not reach a statistical significance level (Hägg et al., 2017) after controlling for multiple covariates. Our present results, largely agree with this finding, in particular the estimated effect sizes are almost identical. Similar results were also reported in earlier studies where cognitive performance was assessed using Mini-Mental State Examination (MMSE) scores during two-year follow-up in non-demented stroke survivors (Martin-Ruiz et al., 2006), the Cambridge Neuropsychological Test Automated Battery in a cross-sectional survey of UK twins sample (Valdes et al., 2010), the composite score from six cognitive tests in ten-year follow-up of nurses (Devore et al., 2011), and the modified MMSE or NeuroTrax battery in other prospective studies (Cohen-Manheim et al., 2016; Yaffe et al., 2011). However, other studies did not observe a significant association with cognitive decline (Harris et al., 2016) or specific cognitive domains (Hägg et al., 2017; Mather et al., 2010; Yaffe et al., 2011). The discrepancy of results among studies could be attributed to sample size, study population, TL assessment method, or cognitive tests battery. It is worth noting that the magnitude of the associations of TL with cognitive ability was very small in most of these previous studies, which is also the case in our present analysis. In terms of the better-known units of IQ, where IQ scores follow a normal distribution with mean 100 and SD 15, one SD T/S ratio decrease of TL approximately corresponds to a 0.3 point decline in IQ. Based on one previous study, we estimated one SD T/S was approximately equal to 1000 base pairs of TL (Codd et al., 2013). Thus, the magnitude of the association between TL and general cognitive ability is indeed quite small. The predictive value of TL for cognitive aging may be limited.
Educational attainment and cognitive performance are moderately correlated. The association between level of educational attainment and cognitive performance has been well studied. People with higher educational attainment generally perform better across a range of cognitive tests than their peers with less education (Lenehan et al., 2014; Wilson et al., 2009). Recent research also found higher educational attainment to be associated with longer TL (Adler et al., 2013). This motivates the hypothesis that educational attainment may underlie in part the association between TL and general cognitive ability. The analysis in the present study was in line with this hypothesis. When additionally adjusting for educational attainment, the association magnitude was attenuated and not statistically significant.
The strengths of this study include the longitudinal nature, with repeated measurements of general cognitive ability up to seven times in four Swedish and US cohorts. An observed significant association should not be due to the reverse causation in this study because TL was assessed before cognition, although early-life intelligence and cognitive changes have been shown to be predictive of TL in mid-later life (Rask et al., 2016; Schaefer et al., 2016). A further strength is that our combined analytic longitudinal study samples are the largest collection tested thus far and the age periods span from 50 to 100 years and beyond, which is representative of the underlying general aging population. A disadvantage of the study is the general cognitive ability was defined from different sets of cognitive tests among the four cohorts. Another limitation is that the assessment of TL was performed in different specimens and laboratories. TL was measured in peripheral blood leukocyte in GENDER, SATSA, and MCSA, and saliva in HRS. Comparisons of TL in blood and saliva are scarce. One study reported a good correlation between them in children (Mitchell et al., 2014) and 74% of the DNA in saliva derives from leukocytes (Cai et al., 2015). Thus, TL from saliva largely reflects the same biological mechanism as peripheral blood leukocyte TL.
In summary, by using four longitudinal cohorts with repeated measurements of cognition we found that TL was associated with the mean levels of general cognitive ability in the age-adjusted models, but not in the models when other covariates were adjusted, nor with cognitive decline; this association was very small and could be confounded by educational attainment.
Supplementary Material
Highlights.
Four longitudinal cohorts were used to examine the association between leukocyte telomere length and cognition.
General cognitive ability was measured repeatedly up to seven times.
Longer telomeres at baseline were associated with better general cognitive ability in age-adjusted models, but not with its decline.
Acknowledgments
The SATSA and GENDER studies are supported by NIH grants R01 AG04563, AG10175, AG028555, the MacArthur Foundation Research Network on Successful Aging, the Swedish Council for Working Life and Social Research (FAS/FORTE) (97:0147:1B, 2009-0795), the Swedish Research Council (825-2007-7460, 825-2009-6141). This study is supported by the Swedish Research Council (521-2013-8689, 2015-03255), FORTE (2013-2292), the Karolinska Institutet delfinansiering (KID) for doctoral student, the Loo & Hans Osterman Foundation, and the Foundation for Geriatric Diseases, the Magnus Bergwall Foundation, and the Strategic Research Program in Epidemiology at Karolinska Institutet. We thank Iiris Hovatta for telomere assessment.
The MCSA study is supported by the National Institute on Aging (U01 AG006786), the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR30582), the Mayo Foundation for Medical Education and Research, the Mayo Clinic Center for Individualized Medicine and was made possible by the Rochester Epidemiology Project (R01 AG034676). We thank Ruth Johnson for the lab work.
The HRS study is sponsored by the National Institute on Aging (grant number NIA U01AG009740, RC4AG039029, R25AG053227) and is conducted by the University of Michigan.
Appendix A. Supplementary Data
Supplementary data associated with this manuscript are attached.
Footnotes
Disclosure Statement
R.O. Roberts receives research funding from Roche and Biogen. M. Vassilaki receives research funding from Roche. R. C. Petersen is a consultant for Roche, Inc.; Merck, Inc.; Genentech, Inc.; Biogen, Inc. The other authors have no actual or potential conflicts of interest.
Verification
R.O. Roberts receives research funding from Roche and Biogen. M. Vassilaki receives research funding from Roche. R. C. Petersen is a consultant for Roche, Inc.; Merck, Inc.; Genentech, Inc.; Biogen, Inc. The other authors have no actual or potential conflicts of interest. The author’s institution has no contracts relating to this research through which it or any other organization may stand to gain financially now or in the future. There are no any other agreements of authors or their institutions that could be seen as involving a financial interest in this work.
Sources of financial support related to the manuscript being submitted are acknowledged in the manuscript.
The data contained in the manuscript being submitted have not been previously published, have not been submitted elsewhere and will not be submitted elsewhere while under consideration at Neurobiology of Aging.
Informed consent was obtained from all participants in each cohort. This study was approved by the Regional Ethics Board in Stockholm (2014/1757-31/2, 2017/353-32).
All authors have reviewed the contents of the manuscript being submitted, approve of its contents and validate the accuracy of the data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adler N, Pantell MS, O’Donovan A, Blackburn E, Cawthon R, Koster A, Opresko P, Newman A, Harris TB, Epel E. Educational attainment and late life telomere length in the Health, Aging and Body Composition Study. Brain Behav Immun. 2013;27(1):15–21. doi: 10.1016/j.bbi.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakaysa SL, Mucci LA, Slagboom PE, Boomsma DI, McClearn GE, Johansson B, Pedersen NL. Telomere length predicts survival independent of genetic influences. Aging Cell. 2007;6(6):769–774. doi: 10.1111/j.1474-9726.2007.00340.x. [DOI] [PubMed] [Google Scholar]
- Barthel FP, Wei W, Tang M, Martinez-Ledesma E, Hu X, Amin SB, Akdemir KC, Seth S, Song X, Wang Q, et al. Systematic analysis of telomere length and somatic alterations in 31 cancer types. Nat Genet. 2017;49(3):349–357. doi: 10.1038/ng.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai N, Chang S, Li Y, Li Q, Hu J, Liang J, Song L, Kretzschmar W, Gan X, Nicod J, et al. Molecular signatures of major depression. Curr Biol. 2015;25(9):1146–1156. doi: 10.1016/j.cub.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, Buxton JL, Hottenga JJ, Fischer K, Esko T, Surakka I, et al. Identification of seven loci affecting mean telomere length and their association with disease. Nat Genet. 2013;45(4):422–427. 427e421–422. doi: 10.1038/ng.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen-Manheim I, Doniger GM, Sinnreich R, Simon ES, Pinchas R, Aviv A, Kark JD. Increased attrition of leukocyte telomere length in young adults is associated with poorer cognitive function in midlife. Eur J Epidemiol. 2016;31(2):147–157. doi: 10.1007/s10654-015-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devore EE, Prescott J, De Vivo I, Grodstein F. Relative telomere length and cognitive decline in the Nurses’ Health Study. Neurosci Lett. 2011;492(1):15–18. doi: 10.1016/j.neulet.2011.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel D, Pedersen NL. Processing speed and longitudinal trajectories of change for cognitive abilities: The Swedish Adoption/Twin Study of Aging. Aging Neuropsychol C. 2004;11(2–3):325–345. [Google Scholar]
- Fisher GG, Hassan H, Faul JD, Rodgers WL, Weir DR. Health and Retirement Study Imputation of Cognitive Functioning Measures: 1992 – 2014 2017 [Google Scholar]
- Gold CH, Malmberg B, McClearn GE, Pedersen NL, Berg S. Gender and health: a study of older unlike-sex twins. J Gerontol B Psychol Sci Soc Sci. 2002;57(3):S168–176. doi: 10.1093/geronb/57.3.s168. [DOI] [PubMed] [Google Scholar]
- Hammadah M, Al Mheid I, Wilmot K, Ramadan R, Abdelhadi N, Alkhoder A, Obideen M, Pimple PM, Levantsevych O, Kelli HM, et al. Telomere Shortening, Regenerative Capacity, and Cardiovascular Outcomes. Circ Res. 2017;120(7):1130–1138. doi: 10.1161/CIRCRESAHA.116.309421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Deary IJ, MacIntyre A, Lamb KJ, Radhakrishnan K, Starr JM, Whalley LJ, Shiels PG. The association between telomere length, physical health, cognitive ageing, and mortality in non-demented older people. Neurosci Lett. 2006;406(3):260–264. doi: 10.1016/j.neulet.2006.07.055. [DOI] [PubMed] [Google Scholar]
- Harris SE, Marioni RE, Martin-Ruiz C, Pattie A, Gow AJ, Cox SR, Corley J, von Zglinicki T, Starr JM, Deary IJ. Longitudinal telomere length shortening and cognitive and physical decline in later life: The Lothian Birth Cohorts 1936 and 1921. Mech Ageing Dev. 2016;154:43–48. doi: 10.1016/j.mad.2016.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honig LS, Kang MS, Schupf N, Lee JH, Mayeux R. Association of shorter leukocyte telomere repeat length with dementia and mortality. Arch Neurol. 2012;69(10):1332–1339. doi: 10.1001/archneurol.2012.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägg S, Zhan Y, Karlsson R, Gerritsen L, Ploner A, van der Lee SJ, Broer L, Deelen J, Marioni RE, Wong A, et al. Short telomere length is associated with impaired cognitive performance in European ancestry cohorts. Transl Psychiatry. 2017;7(4):e1100. doi: 10.1038/tp.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenehan ME, Summers MJ, Saunders NL, Summers JJ, Vickers JC. Relationship between education and age-related cognitive decline: a review of recent research. Psychogeriatrics. 2014 doi: 10.1111/psyg.12083. [DOI] [PubMed] [Google Scholar]
- Lumley T. R package version 2.16. 2012. rmeta: Meta-analysis. p. [Google Scholar]
- Martin-Ruiz C, Dickinson HO, Keys B, Rowan E, Kenny RA, Von Zglinicki T. Telomere length predicts poststroke mortality, dementia, and cognitive decline. Ann Neurol. 2006;60(2):174–180. doi: 10.1002/ana.20869. [DOI] [PubMed] [Google Scholar]
- Mather KA, Jorm AF, Anstey KJ, Milburn PJ, Easteal S, Christensen H. Cognitive performance and leukocyte telomere length in two narrow age-range cohorts: a population study. BMC Geriatr. 2010;10:62. doi: 10.1186/1471-2318-10-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Hagen CE, Wennberg AMV, Airey DC, Savica R, Knopman DS, Machulda MM, Roberts RO, Jack CR, Jr, Petersen RC, et al. Association of Plasma Total Tau Level With Cognitive Decline and Risk of Mild Cognitive Impairment or Dementia in the Mayo Clinic Study on Aging. JAMA Neurol. 2017 doi: 10.1001/jamaneurol.2017.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell C, Hobcraft J, McLanahan SS, Siegel SR, Berg A, Brooks-Gunn J, Garfinkel I, Notterman D. Social disadvantage, genetic sensitivity, and children’s telomere length. Proc Natl Acad Sci U S A. 2014;111(16):5944–5949. doi: 10.1073/pnas.1404293111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mons U, Muezzinler A, Schottker B, Dieffenbach AK, Butterbach K, Schick M, Peasey A, De Vivo I, Trichopoulou A, Boffetta P, et al. Leukocyte Telomere Length and All-Cause, Cardiovascular Disease, and Cancer Mortality: Results From Individual-Participant-Data Meta-Analysis of 2 Large Prospective Cohort Studies. Am J Epidemiol. 2017:1–10. doi: 10.1093/aje/kww210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen NL, McClearn GE, Plomin R, Nesselroade JR, Berg S, DeFaire U. The Swedish Adoption Twin Study of Aging: an update. Acta Genet Med Gemellol (Roma) 1991;40(1):7–20. doi: 10.1017/s0001566000006681. [DOI] [PubMed] [Google Scholar]
- Pedersen NL, Plomin R, Nesselroade JR, McClearn GE. A Quantitative Genetic Analysis of Cognitive Abilities during the Second Half of the Life Span. Psychological Science. 1992;3(6):346–353. [Google Scholar]
- Rask L, Bendix L, Harbo M, Fagerlund B, Mortensen EL, Lauritzen MJ, Osler M. Cognitive Change during the Life Course and Leukocyte Telomere Length in Late Middle-Aged Men. Front Aging Neurosci. 2016;8:300. doi: 10.3389/fnagi.2016.00300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CA, Finkel D, McArdle JJ, Gatz M, Berg S, Pedersen NL. Quantitative genetic analysis of latent growth curve models of cognitive abilities in adulthood. Dev Psychol. 2005;41(1):3–16. doi: 10.1037/0012-1649.41.1.3. [DOI] [PubMed] [Google Scholar]
- Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer JD, Caspi A, Belsky DW, Harrington H, Houts R, Israel S, Levine ME, Sugden K, Williams B, Poulton R, et al. Early-Life Intelligence Predicts Midlife Biological Age. J Gerontol B Psychol Sci Soc Sci. 2016;71(6):968–977. doi: 10.1093/geronb/gbv035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnega A, Faul JD, Ofstedal MB, Langa KM, Phillips JW, Weir DR. Cohort Profile: the Health and Retirement Study (HRS) Int J Epidemiol. 2014;43(2):576–585. doi: 10.1093/ije/dyu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telomeres Mendelian Randomization Collaboration. Haycock PC, Burgess S, Nounu A, Zheng J, Okoli GN, Bowden J, Wade KH, Timpson NJ, Evans DM, et al. Association Between Telomere Length and Risk of Cancer and Non-Neoplastic Diseases: A Mendelian Randomization Study. JAMA Oncol. 2017;3(5):636–651. doi: 10.1001/jamaoncol.2016.5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdes AM, Deary IJ, Gardner J, Kimura M, Lu X, Spector TD, Aviv A, Cherkas LF. Leukocyte telomere length is associated with cognitive performance in healthy women. Neurobiol Aging. 2010;31(6):986–992. doi: 10.1016/j.neurobiolaging.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Hebert LE, Scherr PA, Barnes LL, Mendes de Leon CF, Evans DA. Educational attainment and cognitive decline in old age. Neurology. 2009;72(5):460–465. doi: 10.1212/01.wnl.0000341782.71418.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe K, Lindquist K, Kluse M, Cawthon R, Harris T, Hsueh WC, Simonsick EM, Kuller L, Li R, Ayonayon HN, et al. Telomere length and cognitive function in community-dwelling elders: findings from the Health ABC Study. Neurobiol Aging. 2011;32(11):2055–2060. doi: 10.1016/j.neurobiolaging.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Karlsson IK, Karlsson R, Tillander A, Reynolds CA, Pedersen NL, Hägg S. Exploring the Causal Pathway From Telomere Length to Coronary Heart Disease: A Network Mendelian Randomization Study. Circ Res. 2017;121(3):214–219. doi: 10.1161/CIRCRESAHA.116.310517. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Song C, Karlsson R, Tillander A, Reynolds CA, Pedersen NL, Hägg S. Telomere Length Shortening and Alzheimer Disease--A Mendelian Randomization Study. JAMA Neurol. 2015;72(10):1202–1203. doi: 10.1001/jamaneurol.2015.1513. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.