Abstract
The precise regulation of fluid and energy homeostasis is essential for survival. It is well appreciated that ingestive behaviors are tightly regulated both by peripheral sensory inputs and central appetite signals. With recent neurogenetic technologies, considerable progress has been made in our understanding of basic taste qualities, the molecular/cellular basis of taste sensing, and the central circuits for thirst and hunger. In this review, we first highlight the functional similarities and differences between mammalian and invertebrate taste processing. We then discuss how central thirst and hunger signals interact with peripheral sensory signals to regulate ingestive behaviors. We finally indicate some of the directions for future research.
Keywords: taste, thirst, hunger, top-down regulation, sensory valence
Sensing internal and external nutrient factors
Animals continuously lose water and energy by various physiological processes such as sweating, urination, and basal metabolic activity [1–3]. To compensate for such losses, animals must ingest sufficient water and food from external sources at appropriate timing [1, 4]. The maintenance of this in-and-out balance represents a key homeostatic function for survival in all organisms. After several decades of studies, it is now evident that this homeostatic regulation is finely controlled at the entire organism level, including the peripheral sensory system, brain appetite circuits, the autonomic nervous system, and the endocrine system (see Box 1) [4–7]. Clarifying the interactions between each of the regulatory systems remains an active research area.
Text Box 1. Peripheral signals regulating appetite in mammals.
Peripheral signals originating from the oral cavity, oropharynx and gastrointestinal tract play an important role in the regulation of appetite [5]. For example, a number of circulating factors such as leptin and insulin act on the hypothalamus and the hindbrain to regulate feeding. The vagal afferent neurons (VANs) from the gut also convey enteric information to the NTS via the nodose ganglia [5]. A recent notable study showed that there are genetically-distinct subsets of vagal afferent neurons each responding to different aspects of nutrient ingestion. The stomach and intestine are innervated by GLP1R-expressing neurons that detect gastric distension and relay this information to the medial NTS. On the other hand, GPR65-expressing neurons detect nutrients in the intestinal villi, and synapse onto the NTS subcommisural zone[122]. The hindbrain has a number of receptors for feeding-related neuropeptides. The direct injection of GLP1[123] and leptin[124] into the NTS is known to suppress feeding. The PBN, one of the major downstream targets of the NTS, appears to integrate the taste, hormonal signals (e.g. GLP1 and leptin), and visceral malaise [108, 125–127]. Two studies have shown that the intragastric infusion of nutrients as well as hormones such as CCK, PYY and serotonin rapidly modulates interoceptive AgRP neurons [86, 87]. Thirst neurons are also rapidly modulated by oral temperature change and ingestion of fluid [65, 85]. Taken together, peripheral signals from different sites regulate appetite-related circuits at varying time-scales.
The initiation of consummatory behaviors relies heavily on two major sensory mechanisms, i.e., peripheral taste system [6, 8], and central interoceptive system [4]. This review will describe recent progress in peripheral and central nutrient sensing mechanisms, focusing on functional similarities between invertebrate and vertebrate systems. We will also discuss potential mechanisms by which central appetite circuits modulate sensory valence.
Vertebrate and invertebrate taste systems: functional similarities and dissimilarities
Sweet, umami, and bitter: taste qualities hardwired to attractive and aversive behaviors
Sweet and umami tastes are associated with sugars and L-amino acids respectively, both of which are palatable taste qualities for animals. Conversely, the aversive bitter taste is generally evoked by toxic chemicals that are hazardous to animals [6, 8]. The receptors, cells, and signaling cascades for these three taste qualities have been well-studied (for the relevant receptors of these and the other taste qualities, see Figure 1).
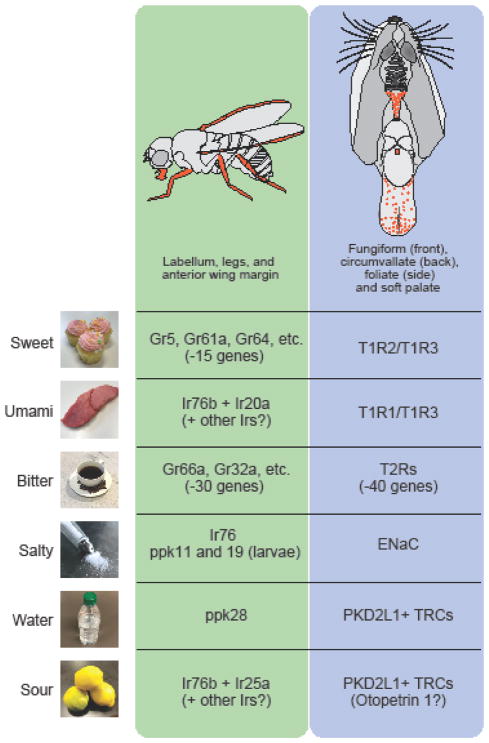
Figure 1. Taste detection in insects and mammals.
Taste organs in Drosophila melanogaster and mouse (top). In flies, taste stimuli are detected by gustatory receptor neurons (GRNs) in labella of the proboscis, legs, and wings (left, highlighted in orange). These taste organs express distinct but partially overlapping subsets of taste receptors. In mammals, taste buds are distributed in different regions of the tongue including fungiform (front), foliate (side), circumvallate (back) papilla, as well as soft palate (right, highlighted in orange). Most taste receptors are expressed in all papilla on the tongue, but functional ENaC is expressed only in fungiform or palate buds. Each basic taste quality is mediated by a unique subset of gustatory receptors (Grs), ionotoropic receptors (Irs) or ppk channels in flies. In mammals, taste receptors (T1Rs and T2Rs) and ion channels are responsible for basic taste detection. Vertebrates and invertebrates share similar cellular organization for taste detection in that different taste qualities are generally encoded by anatomically distinct neural populations.
In vertebrates, sweet and umami compounds are sensed by specific sets of G protein-coupled receptors (GPCRs), called T1Rs (see Glossary) [9–13]. A combination of T1R2 and T1R3 subunits detects a wide range of sugars, whereas T1R1 and T1R3 subunits function as the receptor for L-amino acids. Genetic studies support these findings. For instance, knocking out T1R2 gene selectively abolishes taste responses in the chorda tympani nerves as well as behavioral attraction toward sweet substances[12]. On the other hand, T1R3 KO animals show drastically reduced sensitivity to both sweet and umami [12]. These studies have demonstrated that T1R3 functions as a co-receptor for sweet and umami tastes.
Bitter taste is recognized by T2Rs that belong to another GPCR family [14–17]. Unlike T1Rs, individual bitter-sensing taste receptor cells (TRCs) express multiple T2Rs, each of which recognizes a unique set of bitter compounds. This multiplex receptor expression pattern allows animals to detect a wide variety of bitter compounds through a single type of taste receptor cells. While the functions of T1Rs and T2Rs are widely accepted, there are significant genetic variations across animal species. For instance, T1R2 is pseudogenized in cats, which may be causally linked to their inability to taste sweet stimuli [18]. Dolphins and whales have a premature stop codon in all T1R1s and some T2Rs resulting in a loss of functional taste receptors. [19, 20]. These genomic data suggest that taste receptor genes in each species have evolved to adapt to their specific environments.
At the cellular level, sweet (T1R2+3), umami (T1R1+3), and bitter taste receptors (T2Rs) are expressed in distinct TRC populations on the tongue [6, 21]. Because of this anatomical segregation, each taste quality is mediated by distinct types of TRCs. An elegant study employing synthetic ligand-receptor pair (RASSL) has demonstrated that the taste quality is encoded by the activity of TRCs, but not by taste compounds or receptor activity [12].
Studies in invertebrate species, mainly in Drosophila melanogaster, have revealed a similar coding logic of the taste system between vertebrates and invertebrates (Figure 1) [22, 23]. Taste receptors in insects are expressed in the proboscis, an organ equivalent to the tongue in mammals, as well as in multiple body parts including the wings and legs[24, 25]. In individual sensilla, generally one to four gustatory receptor neurons (GRNs) are housed, each specialized to detect one basic taste quality, just like mammals. The insect taste receptors belong to the gustatory receptor (Gr) and ionotoropic receptor (Ir) families [26, 27]. Sweet and bitter tastes are mainly detected by distinct sets of Grs expressed in sweet and bitter-sensing neurons [6, 23]. Interestingly, multiple Grs are expressed by sugar- and bitter-sensing neurons, and individual Grs recognize different sugars and bitter compounds. For instance, sweet receptors Gr5a and Gr64a are co-expressed in a subset of gustatory neurons that are distinct from Gr66a-expressing bitter-sensing neurons[28]. Gain-of-function studies for individual neural populations have shown that the stimulation of Gr5+ sweet neurons induces appetitive behaviors whereas activation of Gr66a+ bitter neurons drives avoidance [29, 30]. These results suggest that like in the mammalian taste system, the sweet (attractive) and bitter (aversive) tastes in invertebrates are hardwired to anatomically distinct neurons. A recent study employing behavioral and optical imaging has shown that a combination of Ir76b and Ir20a is involved in amino acid-sensing [31]. Intriguingly, Ir76b alone has been indicated as a salt taste receptor, as discussed below [32].
Salt and water tastes for body fluid homeostasis
Salt and water tastes play essential roles for body fluid homeostasis by providing the ability to detect external sodium and water. These two taste qualities are fundamentally different from other tastes like sweet, umami, and bitter in that the valence of salt and water alters based on internal state. For example, sodium strongly attracts salt-seeking (i.e. sodium deprived) animals but the same stimuli have little attractive effect on sodium-satiated animals [33, 34]. Recent studies have unveiled the sensing mechanisms of salt and water tastes in both mammals and insects (Figure 1).
Salt taste
There are two important characteristics of salt taste. First, behavioral preference to salt is a bell-shaped curve depending on its concentration[35]. Second, salt attraction is specific towards sodium salts while aversion is induced by any salts [36]. Generally, moderate concentrations of sodium (around 200 mM) are most appetitive to animals, while higher salt concentrations (over 400 mM for monovalent salts) drive aversive behavior under salt-satiated conditions [34]. These two-opposing behavioral responses are mediated by molecularly and anatomically distinct pathways in the taste system. In mice, the epithelial sodium channel (ENaC) functions as the low salt receptor [33, 37], and knocking out of the gene encoding the ENaCα subunit abolishes behavioral attraction and taste nerve response to low salt without affecting high salt aversive responses [33]. Functional ENaC is expressed in a unique set of TRCs that are distinct from the ones for other taste qualities. Interestingly, high salt does not activate its own taste population, but rather it appears to co-opt other taste pathways in mice [34]. In addition to the attractive ENaC pathway, higher concentrations of salt recruit additional pathways including bitter and acid (sour)-sensing TRCs. Consequently, salt preference is regulated by attractive (ENaC) and aversive (mainly bitter) signals depending on salt concentration.
In Drosophila, recent studies have suggested that Ir76b is involved in sodium attraction by forming a Na+ leak channel[32]. In addition, members of the ENaC family, ppk11 and ppk19, contribute to attractive salt responses in larvae [38]. However, it is still elusive how these putative channels functionally interact with each other in salt sensitive GRNs. Analogous to the mammalian system, bitter GRNs have been shown to respond to high concentration of salts[23].
Water taste
Water is one of the well-established taste qualities in insects [39–41]. In fruit flies, water taste is mediated by a specific subset of GRNs expressing ppk28, a member of the Deg/ENaC family [41, 42]. Functional analyses in cell culture system has revealed that ppk28 functions as a hypo-osmolality sensor [41]. While flies lacking ppk28 exhibit reduced water consumption, they retain normal water-seeking ability under thirsty conditions using hygro-sensation (vapor detection) [43].These data suggested that the taste system has a critical function for water detection, but can be compensated by additional water detection mechanisms.
Compared to insects, much less is known on how water is sensed by the mammalian taste system. Electrophysiological studies since the 1940s have demonstrated that pure water can stimulate taste nerves in various vertebrate species such as frogs, cats, and dogs [44]. However, the question of whether vertebrates can indeed sense water as an independent taste quality has not been resolved. Some key issues in this context are that 1) water does not evoke a unique taste sensation in humans, and 2) no dedicated cells and molecules have been found. With recent advances in genetic tools, our group revisited this question in mice and asked whether water is sensed by a specific type of TRCs [45]. Unexpectedly, the application of pure water on the tongue selectively stimulated acid-sensing taste cells expressing PKD2L1, a member of the TRP channel family. Moreover, optogenetic stimulation of PKD2L1-expressing TRCs triggered attractive licking behavior in thirsty mice, indicating that this population, at least in part, carries the information of external water. However, the molecular mechanisms of water detection in PKD2L1-expressing TRCs are still unclear. In addition, how water and acid (sour) tastes are encoded by the same TRC population needs to be addressed in the future.
Sour taste for sensing acidity
Sour is a unique taste quality evoked by protons in various acidic compounds. At the cellular level, acids are sensed by PKD2L1-expressing TRCs in mammals (Figure 1) [46, 47]. Silencing or ablating this population eliminates acid responses in taste nerves [45, 46]. By contrast, there are still a number of unsolved conundrums at the behavioral and molecular levels. First, eliminating acid taste responses does not affect acid aversion [45, 48]. These observations may indicate that “sour” perception or aversion may be a combination of taste and non-taste signals, for example via the trigeminal system [49]. Second, various candidate acid sensors have been proposed in the past decades such as ASIC, HCN and PKD2L1/1L3 [47, 50–52]. However, gene knockout studies did not support the idea that these molecules are the main acid sensor in taste buds. Recently, an acid-sensitive potassium channel (KIR2.1) and a H+ selective ion channel (Otopetrin 1) have been shown to mediate acid responses in PKD2L1-expressing TRCs [53, 54]. Whether these channels are involved in behavioral aversion to acids remains to be tested.
In Drosophila, a subset of GRNs (sour GRNs) that express Ir76b and Ir25a mediate acid sensing [55]. In addition, low pH also affects the activity of bitter and sweet gustatory neurons [56], suggesting complex sour-sensing mechanisms in flies. It is notable that IR76b appears to have diverse functions in multiple taste qualities including salt (Ir76b [32]), sour (Ir76b+25a [55]), and amino acid (Ir76b+20a [31]). A caveat is that other IRs or channels are likely involved in processing of each of the tastes. For instance, ectopic expression of Ir20a in salt-sensitive (Ir76b+) cells is not sufficient to confer amino acid sensitivity[31], indicating that additional components may be required to form functional taste receptors or channels in GRNs.
Taken together, vertebrate and invertebrate species appear to employ analogous taste sensing strategies despite the molecular diversity of taste receptors. They have similar sets of basic taste qualities: bitter and sweet/umami for sensing the hedonic value of food; salt and water for body fluid homeostasis; and sour for detecting external acidity. Individual taste qualities are generally encoded by anatomically segregated cell populations in taste organs. It would be interesting to elucidate how the molecularly dissimilar taste receptors/channels have evolved to achieve similar functions across species.
Central mechanisms for sensing internal water and energy balance
The main function of the taste system is to detect environmental information and send it to the central nervous system. However, peripheral sensory information is intensely modulated by internal body state. Recent studies have pinpointed neurons that control appetite by sensing internal fluid and energy balance. These central interoceptive neurons are uniquely located outside the blood brain barrier (BBB), and send their information to downstream circuits to regulate ingestive behaviors. The neural basis of appetite regulation has been discussed by a number of recent reviews[1, 4, 7, 57, 58]. Here we will briefly describe current understanding of neurons and circuits for appetite regulation (see also Box 2)
Text Box 2. Neural circuits involved in sodium appetite.
Sodium appetite is modulated by the “synergy” of two hormones, angiotensin II (ATII) and aldosterone [128]. The detection of sodium depletion and the regulation of sodium appetite is mediated by two main brain sites: the LT (mainly ATII-related) and the NTS (mainly aldosterone-related). In the NTS, multiple studies have demonstrated that 11β-hydroxysteroid dehydrogenase 2 (HSD2)-expressing neurons are activated under sodium-depleted conditions, and artificial stimulation of this population promotes sodium intake[129, 130]. A recent study has raised the possibility that HSD2 neurons require concurrent angiotensin signals to fully drive sodium intake[130]. In addition to the NTS, the LT has also been suggested to contribute to sodium appetite via a subset of Agtr1a-expressing neurons in the SFO. Knocking out Agtr1a in the SFO, and optogenetic inhibition of SFO glutamatergic neurons that project to the ventral lateral bed nucleus of the stria terminalis (BNST) suppress sodium appetite[131]. A caveat is that sodium appetite in these studies required additional motivational drives such as thirst, suggesting that there are more factors/circuits to be discovered. Interestingly, both NTS and SFO neurons that promote sodium appetite project to the BNST[129, 131]. Identification of specific neurons and circuitry in the BNST underlying sodium appetite should be a focus for future investigation.
Sensing water balance and regulating thirst
The lamina terminalis (LT) in the forebrain is the main brain structure that monitors internal water balance by detecting blood tonicity and dipsogenic hormones such as angiotensin (ANGII) [1, 7, 59, 60]. This region contains three nuclei: the subfornical organ (SFO), vascular organ of lamina terminalis (OVLT), and median preoptic nucleus (MnPO), where the former two structures lack the normal BBB. It has been shown that stimulation of excitatory neurons in the SFO expressing neuronal NO synthase (nNOS) and a transcription factor, ETV1, rapidly drives drinking (within few seconds), while stimulation of the GABAergic population specifically suppressed thirst [61, 62]. More recently, additional genetic markers for thirst neurons in individual LT nuclei have been found [63, 64]. Our group has also shown that the excitatory neurons within the LT form a hierarchical neural architecture, with the MnPO being its final output[65]. At the molecular level, changes in blood osmolality and ANGII are known stimulators of SFOnNOS and OVLT neurons. It has been demonstrated that an ANGII receptor, Agtr1a is highly enriched in the LT, likely mediating ANGII-induced drinking [1, 62, 64]. By contrast, the molecular basis of osmotic/sodium sensing in the LT remains unsolved. Multiple ion channels have been proposed as candidate osmolality sensors including TRPV1 and TRPV4 [66, 67]. For example, OVLT neurons in TRPV1 KO mice exhibit compromised responses to hypertonic stimuli in acute brain slice preparation. A study in TRPV1/TRPV4 double knockout animals however has shown normal water intake and neural activity (measured by c-Fos expression) in the LT following a hyperosmotic challenge in vivo [68]. These findings indicate the existence of redundant mechanisms for osmolality sensing in the LT, which may compensate for the absence of TRPV1/TRPV4 channels.
Sensing energy balance and hunger regulation
Two distinct neural populations in the arcuate nucleus (Arc) play critical roles in regulating energy balance and feeding behavior: one population expressing Agouti-related Protein (AgRP) and another population expressing Proopiomelanocortin (POMC)-derived peptide [4]. A recent study demonstrated that a majority of AgRP neurons but not POMC neurons are located outside the BBB and exposed to the bloodstream [69], showing that the AgRP population is the primary sensor of internal energy state in the Arc. Both ablation studies and optogenetic/chemogenetic analyses have established that the activity of AgRP neurons is necessary and sufficient to orchestrate normal eating behavior [70–73]. AgRP neurons sense various hunger-related blood-borne factors. One of such factors is ghrelin that is known as a hunger-inducing hormone secreted from the stomach when it is empty [74, 75]. Under hungry conditions, this peptide activates AgRP neurons through the ghrelin receptor, GHS-R, driving animals to eating behavior [75–77]. Recent studies demonstrated that many other factors such as insulin, leptin, and glucose affect the activity of AgRP and POMC neurons [78–81].
Anticipatory nature of hunger and thirst
Classical models of homeostasis posited a passive feedback loop: internal energy/water deficit drives ingestive behavior, and the behavior ceases when internal state recovers. In addition to this classical scheme, recent studies have shed light on a number of “active” feed-forward signals driven by peripheral sensory cues highlighting their anticipatory nature (Figure 2a) [61, 65, 82–85]. AgRP neurons rapidly decrease their activity both in response to nutrient ingestion[86, 87] and during anticipation of food reward in hungry mice[61, 82], while POMC neurons show an increased activity during ingestive behavior (Figure 2b) [82, 88]. Specific GABAergic neurons in the dorsomedial hypothalamic nucleus contribute to the rapid inhibition of AgRP neurons[89]. Somewhat analogously, thirst neurons in the LT and vasopressin neurons in the supraoptic nucleus are suppressed with drinking onset under thirsty conditions (Figure 2c) [65, 84, 85, 90]. The former population is causally linked to drinking behavior, while the latter population is involved in thirst-associated vasopressin release (direct involvement in drinking has not been tested). We recently found that drinking action itself stimulates a specific inhibitory population of the MnPO, marked by Glucagon-like peptide-1 Receptor (GLP1R), which in turn sends monosynaptic inhibition to thirst driving SFOnNOS neurons[65]. This neural circuit appears to mediate drinking-induced rapid thirst alleviation prior to the systemic fluid recovery. These rapid feedforward signals are proposed to help animals match their intake to the homeostatic need on a real-time basis.
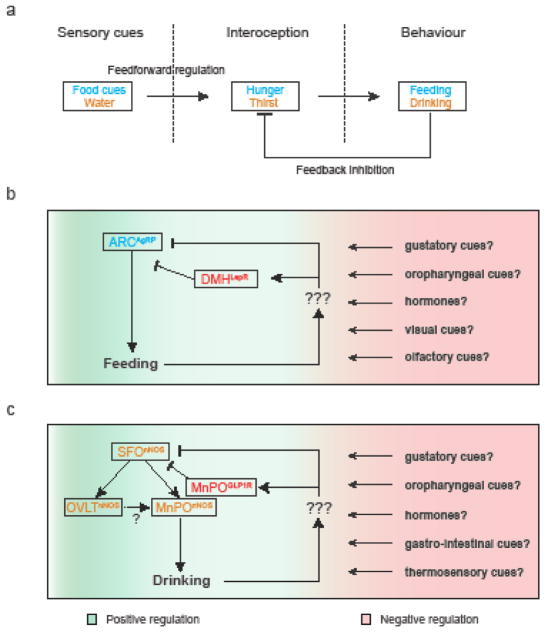
Figure 2. Anticipatory nature of hunger and thirst regulation.
a) A schematic of feedforward-feedback regulation of thirst and hunger. Sensory cues and food ingestion (hunger), or liquid drinking (for thirst) directly modulate the interoceptive circuits. Feedback and feedforward signals help optimize the amount and timing of ingestion on a real-time basis.
b) Hunger interoceptive neurons in the arcuate nucleus (AgRP neurons) detect energy deficits and drive feeding. A number of peripheral signals modulate the activity of AgRP neurons. Leptin receptor-expressing neurons in the DMH are the only known neurons underlying this feedforward regulation[89].
c) The excitatory neurons of the lamina terminalis (composed of the SFO, MnPO and OVLT), marked by nNOS, form a hierarchical circuit to process thirst. Thirst interoceptive neurons (SFOnNOS and OVLTnNOS) respond to deviations in body fluid balance and convey this information to MnPOnNOS neurons. SFOnNOS neurons are also rapidly modulated upon water intake. The inhibitory MnPOGLP1R neurons are activated by drinking (gulping) action, which monosynaptically inhibit SFOnNOS neurons of the SFO[65].
AgRP, Agouti Related Peptide; LepR, Leptin Receptor; nNOS, neuronal Nitric Oxide Synthase; GLP1r, Glucagon-like peptide 1 receptor; Arc, Arcuate Nucleus; DMH, Dorsomedial Hypothalamic Nucleus; SFO, Subfornical Organ; OVLT, Vascular Organ of Lamina Terminalis; MnPO, Median Preoptic Nucleus
Potential neural mechanisms of top-down control of sensory valence
Putative pathways from interoceptive neurons to the cortex
According to the incentive motivation theory, the valence of sensory stimuli is highly dependent on the internal state[91–93]. However, the neural mechanisms underlying such internal-state-dependent valence shifts are still largely unclear and remains an active research area (see Box 3). In this section, we summarize evidence on neural pathways that process appetite and sensory signals, and describe potential mechanisms of top-down control of the representation and valence of sensory stimuli.
Text Box 3. The valence encoded by appetite circuits.
The behavioral definition of positive and negative valence is the animal’s willingness to work for access to a specific stimulus. Recent studies have begun to uncover the valence encoded by central appetite circuits. Context and state modulation appear to be crucial with regards to valence encoding. For instance, animals will work to receive stimulation of AgRP neurons (i.e. self optogenetic stimulation of AgRP neurons) when food is available, and will continue doing so even if the food is taken away[132]. On the contrary, in the absence of food, animals will avoid stimulation of AgRP neurons[61] or will fail to learn to self-stimulate during training[132]. The lateral hypothalamus (LH) appears to be another node involved in valence encoding related to appetite. Excitatory and inhibitory neurons of the LH have orthogonal effects on feeding and motivation. Stimulation of LH excitatory neurons inhibits feeding and drives aversion[133], whereas LH inhibitory stimulation is rewarding and induces feeding[134, 135]. A thorough review by Rossi and Stuber[58] covers these in detail. Thirst is negatively reinforcing and the stimulation of thirst neurons in the LT seems to encode negative valence [61, 64, 90]. The MnPO dissociates the behavioral, cardiovascular and affective outputs of the LT with photostimulation of the excitatory projections to the PVH and LH driving aversion[64].
Thirst
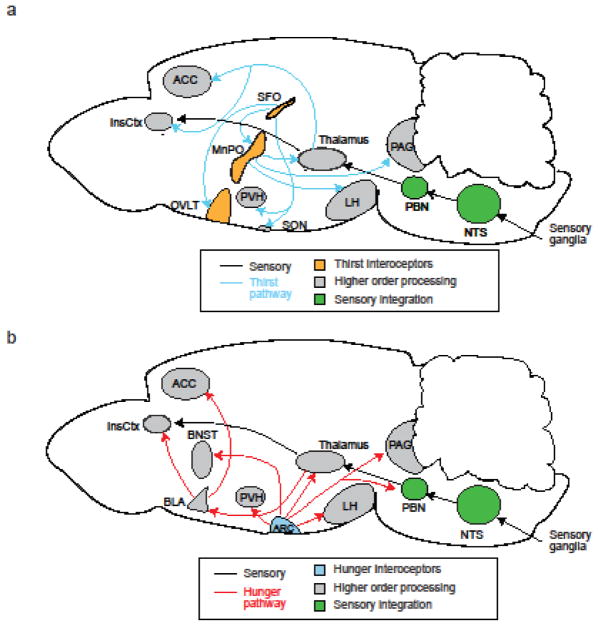
Anatomical tracing from the LT in rodents has revealed that the LT sends information to the insular (InsCtx) and cingulate cortex via the mid-thalamus [94]. Reciprocal connections also exist between the mid-thalamus and the cortical sites, forming a thalamocortical loop that modulates viscerosensory reflexes and behavior [95]. Consistently, optogenetic stimulation of the excitatory projection from the MnPO to the paraventricular thalamic nucleus (PVT) elicited robust water intake [64, 90]. These results suggest that the thalamus may serve as a key relay point of osmosensory signals from the LT to the cortex. In rhesus monkeys, the electrical stimulation of the anterior-mid cingulate cortex (ACC) elicited time-locked water intake [96]. Neuroimaging (fMRI and PET) studies in humans have also revealed a strong correlation between the subjective perception of thirst and the cortical activity (ACC, posterior cingulate cortex, and InsCtx) [97–100]. Collectively, studies from rodents to primates indicate potential information flow: the LT detects deviations from the homeostatic set-point, which is relayed to higher cortical areas through the mid-thalamic nuclei (Interoceptive LT → Thalamus → ACC/InsCtx), where the subjective feeling of thirst is likely encoded (Figure 3).
Figure 3. Neural pathways for sensory and interoceptive processing of thirst and hunger signals.
Schematics showing the sensory and interoceptive pathways in the mammalian brain. The NTS and PBN are potential sites that integrate peripheral and visceral signals. a) Thirst: Black arrows indicate sensory ascending pathways, while blue arrows show thirst-related circuits. b) Hunger: Red arrows show hunger-related circuits.
ACC, Anterior Cingulate Cortex; BLA, Basolateral Amygdala; BNST, Bed Nucleus of the Stria Terminalis; InsCtx, Insular Cortex; OVLT, Vascular Organ of Lamina Terminalis; SON, Supraoptic Nucleus; PVH, Paraventricular Hypothalamic Nucleus; Arc, Arcuate Nucleus; LH, Lateral Hypothalamus; PAG, Periaqueductal Gray; SFO, Subfornical Organ; MnPO, Median Preoptic Nucleus; PBN, Parabrachial Nucleus; NTS, Nucleus Tractus Solitarius
Hunger
Human fMRI studies have demonstrated that various brain areas including the prefrontal cortex, thalamus, and InsCtx are activated in response to food-associated cues under hungry conditions [101]. These functional data are supported by anatomical studies in mice using virus tracing from AgRP neurons in the Arc[102]. Among afferent projections from AgRP neurons, inputs to the bed nucleus of the stria terminalis, paraventricular hypothalamic nucleus, lateral hypothalamus (LHA), and PVT are individually sufficient to drive voracious feeding [103]. This study, therefore, suggested a model where feeding behavior is regulated by a parallel-circuit architecture in the brain. Further genetically-defined circuit mapping has revealed that the information from AgRP neurons is transmitted to the InsCtx via the PVT and basolateral amygdala(BLA) (AgRP → PVT → BLA → InsCtx, Figure 3) [104]. Taken together, studies in the thirst and hunger circuits point to a model where the thalamus plays a pivotal role in transmitting information from brain interoceptive neurons to the cortex [1, 4]. It would be interesting to explore whether separate neural substrates in the thalamus process distinct appetites.
Modulation of sensory valence by appetite circuits
The valence of sensory stimuli such as visual and taste cues is modulated by internal state [45, 105, 106]. Among these cues, taste is a particularly important one for animals to assess the palatability of a substance. All taste signals are relayed via sensory ganglia to the rostral and lateral nucleus tractus solitaris (NTS) [6, 107]. The lateral parabrachial nucleus (PBN) receives input from the NTS[108] and relays it to the ventroposteromedial nucleus of the thalamus, from where it is conveyed onto higher cortical structures like ACC/InsCtx [6, 107, 109, 110]. As mentioned above, the ACC/InsCtx also receive indirect inputs from interoceptive neurons of the Arc and the LT. In addition, these regions integrate inputs from reward-related areas such as BLA, LHA, and the ventral tegmental area [111]. Therefore, ACC/InsCtx are best suited to integrate peripheral taste, central interoceptive, and reward signals [112]. Consistently, recent studies have shown that the neural representation of food-associated cues in the InsCtx dynamically changes under sated and food-deprived conditions [4, 104, 105]. A key next step would be to dissect the neural mechanisms underlying internal-state-dependent plasticity of sensory representation at the cortical level.
Interaction between different motivational drives
Based on the availability of resources, environmental conditions, and internal state, animals need to choose a particular behavior over others, a principle known as “singleness of action” [113]. How different motivational drives interact to give rise to a single behavioral output remains unsolved [114]. Recent studies have focused on two distinct appetites, thirst and hunger, to tackle this question. In flies, genetically-defined four interoceptive neurons in the subesophageal zone are activated under hunger state and inhibited under thirst state [115]. Interestingly, stimulation of this neural population promoted sugar consumption, and suppressed water consumption. Thus, these neurons represent a key neural substrate for processing the motivational competition between eating and drinking. In mice, equivalent neural substrates have not yet been identified. However, activation of AgRP neurons has been shown to suppress competing drives including thirst, pain, fear, and territory marking [116–119]. Interestingly, projections from AgRP neurons to the PBN mediate the suppression of inflammatory pain, providing a neural basis for competing motivational drives between hunger and pain[116]. Whether the similar logic applies to thirst neurons in the LT remains unknown. Besides the PBN, AgRP neurons [103], MnPO neurons [63, 65, 90], and aggression-related neurons in the ventromedial hypothalamus [120] all have dense projections to the periaqueductal gray (PAG). Since this brain region processes both ascending and descending sensory information [121], the PAG may also be involved in the integration of multiple drives. Some key questions remain, however, including (1) which neurons receive distinct motivational signals, and (2) how the PBN/PAG integrates and processes these inputs. Future work employing cell-type-specific imaging/manipulation should help unravel the neural logic for processing competing motivational drives.
Concluding remarks
The main function of the peripheral sensory system is to create an internal representation of the external environment. Taste is a key modality for assessing nutrient and regulating appetite. Although taste qualities and their receptors are still being discovered, and many of the specific receptors vary among species, there is a striking similarity in the overall cellular logic of tastes across organisms. Central processing of taste information is currently being explored in both vertebrates and invertebrates. These studies continue to reveal similarities across various species in the coding logic of taste in the brain.
Hunger and thirst are primordial and innate drives, and impairments in these functions have significant impact on the organism’s overall functioning. Pathological conditions involving appetite dysregulation include obesity, anorexia, and polydipsia. Using contemporary neural manipulation and mapping tools, recent studies have shown that brain appetite circuits are regulated by internal state as well as by real-time ingestive behaviors such as eating and drinking. One of the important next goals for the field would be to unveil in greater detail the neural pathways that integrate sensory and enteric signals with brain appetite circuits (see Outstanding Questions).
Outstanding Questions.
How are taste signals encoded and processed at the periphery and in higher brain areas? How does the valence of tastes change under different conditions, for instance varying degrees of depletion?
Acid-sensing taste cells may contribute to water taste detection. How are acid and water detected and perceived by the taste system?
How do various nodes of the hunger and thirst circuits interact to produce specific motivational drives? Are there dedicated cortical circuits for the processing of distinct appetites?
What are the functional roles for each of the AgRP projection fields in hunger regulation?
Feeding and drinking are intrinsically rewarding under deprived states. How does the reward circuitry modulate the hunger/thirst circuits to regulate consumption?
What are the neural substrates underlying the feed-forward regulation of hunger and thirst? What is the physiological role of this regulation?
What are the genetic identities of circuits in higher brain centers for integrating peripheral sensory signals and internal state information? How do peripheral taste signals shape appetite?
Which neural circuits are critical for processing competing motivational drives?
Sensory valence is influenced by appetite signals originating from interoceptive neurons in the brain. Accumulating evidence suggests that the thalamus and cortex are potential areas that process peripheral and central signals to control sensory valence of food and water. A ripe area of future research would be to dissect micro- and macro-circuits underlying internal-state-dependent valence shifts.
Highlights.
Vertebrates and invertebrates employ similar cellular logic for taste detection. Cells and receptors for most individual taste qualities have been discovered, but mechanisms of sour taste are not yet fully understood
Interoceptive neurons for hunger and thirst receive extensive modulation both by internal state and peripheral sensory cues
The valence of sensory stimuli is modulated by internal body environment. Recent studies began to dissect the underlying neural circuits, which involve the thalamus, the amygdala, and the InsCtx.
Multiple motivational drives are processed in the brain, resulting in the selection of the final behavioral path. Emerging anatomical evidence indicates potential sites of this interaction, including (but not limited to) the Parabrachial Nucleus (PBN), and the Periaqueductal Gray (PAG).
Acknowledgments
We thank the Oka lab members and A. Dahanukar for valuable comments. Y.O. is supported by the Searle Scholars Program, the Mallinckrodt Foundation, the McKnight Foundation and the Klingenstein-Simons Foundation, and NIH U01 (U01 NS099717).
GLOSSARY
- T1Rs
A family of three G-protein-coupled receptors that mediate sweet and umami tastes in mammals. Different combinations of T1Rs sense sugars and L-amino acids.
- Acid-sensing ion channels (ASICs)
ASICs are voltage insensitive ion channels that sense extracellular protons. They are involved in sensing the acidity in the extracellular environment.
- Hyperpolarization-activated cyclic-nucleotide-gated (HCN) channels
HCN channels are nonselective proton channels. HCN4, a member of HCN family has been suggested as a sour taste receptor.
- Gustatory receptors neurons (GRNs)
The neurons that express genes encoding gustatory receptors (GRs) in Drosophila melanogaster. These GRs are responsible for chemosensation, mainly taste.
- Ionotoropic receptors (IRs)
Glutamate ionotoropic receptors, among their various functions in the nervous system, are expressed in olfactory neurons and GRNs that contribute to odorant and taste sensing. They have been shown to respond to amines, organic acids and other environmental cues.
- Degenerin/Epithelial sodium channel family (Deg/ENaC)
The Deg/ENaC gene family represents a set of ion channels that are amiloride-sensitive. The subunits of this family mediate the responses to mammalian salt taste and invertebrate water taste.
- PKD2L1
A polycystic-kidney-disease-like channel. A genetic marker for mammalian acid-sensing taste receptor cells. This channel is not required for acid responses in these taste cells.
- TRP
A large family of cation channels that mediate various noxious stimuli and temperature. Some members such as TRPM5 function as a key transduction channel.
- Agouti-related peptide (AgRP) neurons
A specific neural population in the arcuate nucleus (Arc) located in the ventral hypothalamus that is crucial for the control of feeding behavior.
- Proopiomelanocortin (POMC) neurons
POMC neurons in the arcuate nucleus (Arc) are another important population for feeding control. In contrast to AgRP neurons, the stimulation of POMC neurons suppresses feeding behavior.
- Lamina Terminalis (LT)
LT is a brain region located in the forebrain and responsible for detecting and controlling body fluid homeostasis. Three nuclei located in the LT are the subfornical organ (SFO), the vascular organ of lamina terminalis (OVLT), and the median preoptic nucleus (MnPO). Two of these nuclei, SFO and OVLT, are circumventricular organs that lack the normal blood-brain-barrier (BBB) and are in contact with blood circulation. Deviations from the body fluid balance are detected by these two nuclei. The third LT nucleus, MnPO, has been shown to be the integration center for thirst regulation and fluid intake.
- Polydipsia
An appetite-related disorder characterized by excessive feeling of thirst independent of the homeostatic need. While polydipsia is mainly caused by a kidney dysfunction, other cases are associated with psychiatric disorders or unknown neural dysfunction.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gizowski C, Bourque CW. The neural basis of homeostatic and anticipatory thirst. Nature Reviews Nephrology. 2017 doi: 10.1038/nrneph.2017.149. nrneph. 2017.2149. [DOI] [PubMed] [Google Scholar]
- 2.Saper CB, et al. The need to feed: homeostatic and hedonic control of eating. Neuron. 2002;36:199–211. doi: 10.1016/s0896-6273(02)00969-8. [DOI] [PubMed] [Google Scholar]
- 3.Weigle DS. Appetite and the regulation of body composition. The FASEB Journal. 1994;8:302–310. doi: 10.1096/fasebj.8.3.8143936. [DOI] [PubMed] [Google Scholar]
- 4.Andermann ML, Lowell BB. Toward a wiring diagram understanding of appetite control. Neuron. 2017;95:757–778. doi: 10.1016/j.neuron.2017.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim K-S, et al. Signalling from the periphery to the brain that regulates energy homeostasis. Nature Reviews Neuroscience. 2018 doi: 10.1038/nrn.2018.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yarmolinsky DA, et al. Common sense about taste: from mammals to insects. Cell. 2009;139:234–244. doi: 10.1016/j.cell.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zimmerman CA, et al. Neural circuits underlying thirst and fluid homeostasis. Nature Reviews Neuroscience. 2017;18:459–469. doi: 10.1038/nrn.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roper SD, Chaudhari N. Taste buds: cells, signals and synapses. Nat Rev Neurosci. 2017;18:485–497. doi: 10.1038/nrn.2017.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson G, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 10.Nelson G, et al. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 11.Li X, et al. Human receptors for sweet and umami taste. Proceedings of the National Academy of Sciences. 2002;99:4692–4696. doi: 10.1073/pnas.072090199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao GQ, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115:255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- 13.Matsunami H, et al. A family of candidate taste receptors in human and mouse. Nature. 2000;404:601–604. doi: 10.1038/35007072. [DOI] [PubMed] [Google Scholar]
- 14.Pronin AN, et al. Identification of ligands for two human bitter T2R receptors. Chemical Senses. 2004;29:583–593. doi: 10.1093/chemse/bjh064. [DOI] [PubMed] [Google Scholar]
- 15.Pronin AN, et al. Specific alleles of bitter receptor genes influence human sensitivity to the bitterness of aloin and saccharin. Current Biology. 2007;17:1403–1408. doi: 10.1016/j.cub.2007.07.046. [DOI] [PubMed] [Google Scholar]
- 16.Mueller KL, et al. The receptors and coding logic for bitter taste. Nature. 2005;434:225–229. doi: 10.1038/nature03352. [DOI] [PubMed] [Google Scholar]
- 17.Chandrashekar J, et al. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 18.Li X, et al. Pseudogenization of a sweet-receptor gene accounts for cats’ indifference toward sugar. PLoS genetics. 2005;1:e3. doi: 10.1371/journal.pgen.0010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang P, et al. Major taste loss in carnivorous mammals. Proceedings of the National Academy of Sciences. 2012;109:4956–4961. doi: 10.1073/pnas.1118360109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng P, et al. Massive losses of taste receptor genes in toothed and baleen whales. Genome biology and evolution. 2014;6:1254–1265. doi: 10.1093/gbe/evu095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chandrashekar J, et al. The receptors and cells for mammalian taste. Nature. 2006;444:288–294. doi: 10.1038/nature05401. [DOI] [PubMed] [Google Scholar]
- 22.Hallem EA, et al. Insect odor and taste receptors. Annu Rev Entomol. 2006;51:113–135. doi: 10.1146/annurev.ento.51.051705.113646. [DOI] [PubMed] [Google Scholar]
- 23.Scott K. Taste recognition: food for thought. Neuron. 2005;48:455–464. doi: 10.1016/j.neuron.2005.10.015. [DOI] [PubMed] [Google Scholar]
- 24.Freeman EG, Dahanukar A. Molecular neurobiology of Drosophila taste. Current opinion in neurobiology. 2015;34:140–148. doi: 10.1016/j.conb.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joseph RM, Carlson JR. Drosophila chemoreceptors: a molecular interface between the chemical world and the brain. Trends in Genetics. 2015;31:683–695. doi: 10.1016/j.tig.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Benton R, et al. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rytz R, et al. Ionotropic receptors (IRs): chemosensory ionotropic glutamate receptors in Drosophila and beyond. Insect Biochem Mol Biol. 2013;43:888–897. doi: 10.1016/j.ibmb.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 28.Dahanukar A, et al. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56:503–516. doi: 10.1016/j.neuron.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inagaki HK, et al. Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship. Nature methods. 2014;11:325–332. doi: 10.1038/nmeth.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marella S, et al. Imaging taste responses in the fly brain reveals a functional map of taste category and behavior. Neuron. 2006;49:285–295. doi: 10.1016/j.neuron.2005.11.037. [DOI] [PubMed] [Google Scholar]
- 31.Ganguly A, et al. A molecular and cellular context-dependent role for Ir76b in detection of amino acid taste. Cell reports. 2017;18:737–750. doi: 10.1016/j.celrep.2016.12.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang YV, et al. The molecular basis for attractive salt-taste coding in Drosophila. Science. 2013;340:1334–1338. doi: 10.1126/science.1234133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chandrashekar J, et al. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oka Y, et al. High salt recruits aversive taste pathways. Nature. 2013;494:472–475. doi: 10.1038/nature11905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duncan C. Salt preferences of birds and mammals. Physiological Zoology. 1962;35:120–132. [Google Scholar]
- 36.Geerling JC, Loewy AD. Central regulation of sodium appetite. Exp Physiol. 2008;93:177–209. doi: 10.1113/expphysiol.2007.039891. [DOI] [PubMed] [Google Scholar]
- 37.Shigemura N, et al. Amiloride-sensitive NaCl taste responses are associated with genetic variation of ENaC α-subunit in mice. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2008;294:R66–R75. doi: 10.1152/ajpregu.00420.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu L, et al. Contribution of Drosophila DEG/ENaC genes to salt taste. Neuron. 2003;39:133–146. doi: 10.1016/s0896-6273(03)00394-5. [DOI] [PubMed] [Google Scholar]
- 39.Wolbarsht ML. Water Taste in Phormia. Science (New York, NY) 1957;125:1248–1248. doi: 10.1126/science.125.3260.1248. [DOI] [PubMed] [Google Scholar]
- 40.Evans DR, Mellon D. Electrophysiological studies of a water receptor associated with the taste sensilla of the blowfly. The Journal of general physiology. 1962;45:487–500. doi: 10.1085/jgp.45.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cameron P, et al. The molecular basis for water taste in Drosophila. Nature. 2010;465:91–95. doi: 10.1038/nature09011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen Z, et al. The amiloride-sensitive epithelial Na+ channel PPK28 is essential for Drosophila gustatory water reception. Journal of Neuroscience. 2010;30:6247–6252. doi: 10.1523/JNEUROSCI.0627-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin S, et al. Neural correlates of water reward in thirsty Drosophila. Nature neuroscience. 2014;17:1536–1542. doi: 10.1038/nn.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zotterman Y. Species differences in the water taste. Acta Physiologica. 1956;37:60–70. doi: 10.1111/j.1748-1716.1956.tb01342.x. [DOI] [PubMed] [Google Scholar]
- 45.Zocchi D, et al. The cellular mechanism for water detection in the mammalian taste system. Nature neuroscience. 2017 doi: 10.1038/nn.4575. [DOI] [PubMed] [Google Scholar]
- 46.Huang AL, et al. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishimaru Y, et al. Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proceedings of the National Academy of Sciences. 2006;103:12569–12574. doi: 10.1073/pnas.0602702103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finger TE, et al. ATP signaling is crucial for communication from taste buds to gustatory nerves. Science. 2005;310:1495–1499. doi: 10.1126/science.1118435. [DOI] [PubMed] [Google Scholar]
- 49.Julius D. TRP channels and pain. Annu Rev Cell Dev Biol. 2013;29:355–384. doi: 10.1146/annurev-cellbio-101011-155833. [DOI] [PubMed] [Google Scholar]
- 50.Horio N, et al. Sour taste responses in mice lacking PKD channels. PLoS One. 2011;6:e20007. doi: 10.1371/journal.pone.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Richter TA, et al. Acid-sensing ion channel-2 is not necessary for sour taste in mice. J Neurosci. 2004;24:4088–4091. doi: 10.1523/JNEUROSCI.0653-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevens DR, et al. Hyperpolarization-activated channels HCN1 and HCN4 mediate responses to sour stimuli. Nature. 2001;413:631–635. doi: 10.1038/35098087. [DOI] [PubMed] [Google Scholar]
- 53.Tu YH, et al. An evolutionarily conserved gene family encodes proton-selective ion channels. Science. 2018;359:1047–1050. doi: 10.1126/science.aao3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye W, et al. The K+ channel KIR2. 1 functions in tandem with proton influx to mediate sour taste transduction. Proceedings of the National Academy of Sciences. 2016;113:E229–E238. doi: 10.1073/pnas.1514282112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen Y, Amrein H. Ionotropic Receptors Mediate Drosophila Oviposition Preference through Sour Gustatory Receptor Neurons. Current Biology. 2017;27:2741–2750. e2744. doi: 10.1016/j.cub.2017.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Charlu S, et al. Acid sensing by sweet and bitter taste neurons in Drosophila melanogaster. Nat Commun. 2013;4:2042. doi: 10.1038/ncomms3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sternson SM, Eiselt AK. Three Pillars for the Neural Control of Appetite. Annual review of physiology. 2017;79:401–423. doi: 10.1146/annurev-physiol-021115-104948. [DOI] [PubMed] [Google Scholar]
- 58.Rossi MA, Stuber GD. Overlapping Brain Circuits for Homeostatic and Hedonic Feeding. Cell metabolism. 2017 doi: 10.1016/j.cmet.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bourque CW. Central mechanisms of osmosensation and systemic osmoregulation. Nature Reviews Neuroscience. 2008;9:519–531. doi: 10.1038/nrn2400. [DOI] [PubMed] [Google Scholar]
- 60.McKinley M, et al. The median preoptic nucleus: front and centre for the regulation of body fluid, sodium, temperature, sleep and cardiovascular homeostasis. Acta Physiologica. 2015;214:8–32. doi: 10.1111/apha.12487. [DOI] [PubMed] [Google Scholar]
- 61.Betley JN, et al. Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature. 2015;521:180–185. doi: 10.1038/nature14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Oka Y, et al. Thirst driving and suppressing signals encoded by distinct neural populations in the brain. Nature. 2015;520:349–352. doi: 10.1038/nature14108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abbott SB, et al. Reciprocal control of drinking behavior by median preoptic neurons in mice. Journal of Neuroscience. 2016;36:8228–8237. doi: 10.1523/JNEUROSCI.1244-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leib DE, et al. The Forebrain Thirst Circuit Drives Drinking through Negative Reinforcement. Neuron. 2017;96:1272–1281. e1274. doi: 10.1016/j.neuron.2017.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Augustine V, et al. Hierarchical neural architecture underlying thirst regulation. Nature. 2018 doi: 10.1038/nature25488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ciura S, Bourque CW. Transient receptor potential vanilloid 1 is required for intrinsic osmoreception in organum vasculosum lamina terminalis neurons and for normal thirst responses to systemic hyperosmolality. Journal of Neuroscience. 2006;26:9069–9075. doi: 10.1523/JNEUROSCI.0877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liedtke W, Friedman JM. Abnormal osmotic regulation in trpv4−/−mice. Proceedings of the National Academy of Sciences. 2003;100:13698–13703. doi: 10.1073/pnas.1735416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kinsman B, et al. Osmoregulatory thirst in mice lacking the transient receptor potential vanilloid type 1 (TRPV1) and/or type 4 (TRPV4) receptor. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2014;307:R1092–R1100. doi: 10.1152/ajpregu.00102.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yulyaningsih E, et al. Acute Lesioning and Rapid Repair of Hypothalamic Neurons outside the Blood-Brain Barrier. Cell reports. 2017;19:2257–2271. doi: 10.1016/j.celrep.2017.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Aponte Y, et al. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature neuroscience. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Atasoy D, et al. Deconstruction of a neural circuit for hunger. Nature. 2012;488:172–177. doi: 10.1038/nature11270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Krashes MJ, et al. Rapid versus delayed stimulation of feeding by the endogenously released AgRP neuron mediators GABA, NPY, and AgRP. Cell metabolism. 2013;18:588–595. doi: 10.1016/j.cmet.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luquet S, et al. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 74.Kojima M, et al. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 75.Nakazato M, et al. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194–198. doi: 10.1038/35051587. [DOI] [PubMed] [Google Scholar]
- 76.Andrews ZB, et al. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cowley MA, et al. The distribution and mechanism of action of ghrelin in the CNS demonstrates a novel hypothalamic circuit regulating energy homeostasis. Neuron. 2003;37:649–661. doi: 10.1016/s0896-6273(03)00063-1. [DOI] [PubMed] [Google Scholar]
- 78.Könner AC, et al. Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell metabolism. 2007;5:438–449. doi: 10.1016/j.cmet.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 79.van den Top M, et al. Orexigen-sensitive NPY/AgRP pacemaker neurons in the hypothalamic arcuate nucleus. Nature neuroscience. 2004;7:493. doi: 10.1038/nn1226. [DOI] [PubMed] [Google Scholar]
- 80.Belgardt BF, et al. Hormone and glucose signalling in POMC and AgRP neurons. The Journal of physiology. 2009;587:5305–5314. doi: 10.1113/jphysiol.2009.179192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Blouet C, Schwartz GJ. Hypothalamic nutrient sensing in the control of energy homeostasis. Behavioural brain research. 2010;209:1–12. doi: 10.1016/j.bbr.2009.12.024. [DOI] [PubMed] [Google Scholar]
- 82.Chen Y, et al. Sensory detection of food rapidly modulates arcuate feeding circuits. Cell. 2015;160:829–841. doi: 10.1016/j.cell.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gizowski C, et al. Clock-driven vasopressin neurotransmission mediates anticipatory thirst prior to sleep. Nature. 2016;537:685–688. doi: 10.1038/nature19756. [DOI] [PubMed] [Google Scholar]
- 84.Mandelblat-Cerf Y, et al. Bidirectional anticipation of future osmotic challenges by vasopressin neurons. Neuron. 2017;93:57–65. doi: 10.1016/j.neuron.2016.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zimmerman CA, et al. Thirst neurons anticipate the homeostatic consequences of eating and drinking. Nature. 2016;537:680. doi: 10.1038/nature18950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Beutler LR, et al. Dynamics of Gut-Brain Communication Underlying Hunger. Neuron. 2017;96:461–475. e465. doi: 10.1016/j.neuron.2017.09.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Su Z, et al. Nutritive, Post-ingestive Signals Are the Primary Regulators of AgRP Neuron Activity. Cell reports. 2017;21:2724–2736. doi: 10.1016/j.celrep.2017.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mandelblat-Cerf Y, et al. Arcuate hypothalamic AgRP and putative POMC neurons show opposite changes in spiking across multiple timescales. Elife. 2015;4:e07122. doi: 10.7554/eLife.07122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garfield AS, et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nature neuroscience. 2016 doi: 10.1038/nn.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Allen WE, et al. Thirst-associated preoptic neurons encode an aversive motivational drive. Science. 2017;357:1149–1155. doi: 10.1126/science.aan6747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Berridge KC. Motivation concepts in behavioral neuroscience. Physiology & behavior. 2004;81:179–209. doi: 10.1016/j.physbeh.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 92.Olds J. Self-stimulation of the brain: Its use to study local effects of hunger, sex, and drugs. Science. 1958;127:315–324. doi: 10.1126/science.127.3294.315. [DOI] [PubMed] [Google Scholar]
- 93.Toates FM. Motivational systems. CUP Archive 1986 [Google Scholar]
- 94.Hollis JH, et al. The trajectory of sensory pathways from the lamina terminalis to the insular and cingulate cortex: a neuroanatomical framework for the generation of thirst. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2008;294:R1390–R1401. doi: 10.1152/ajpregu.00869.2007. [DOI] [PubMed] [Google Scholar]
- 95.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 96.Robinson BW, Mishkin M. Alimentary responses to forebrain stimulation in monkeys. Experimental brain research. 1968;4:330–366. doi: 10.1007/BF00235700. [DOI] [PubMed] [Google Scholar]
- 97.Denton D, et al. Correlation of regional cerebral blood flow and change of plasma sodium concentration during genesis and satiation of thirst. Proceedings of the National Academy of Sciences. 1999;96:2532–2537. doi: 10.1073/pnas.96.5.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Denton D, et al. Neuroimaging of genesis and satiation of thirst and an interoceptor-driven theory of origins of primary consciousness. Proceedings of the National Academy of Sciences. 1999;96:5304–5309. doi: 10.1073/pnas.96.9.5304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Farrell MJ, et al. Cortical activation and lamina terminalis functional connectivity during thirst and drinking in humans. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2011;301:R623–R631. doi: 10.1152/ajpregu.00817.2010. [DOI] [PubMed] [Google Scholar]
- 100.Saker P, et al. Regional brain responses associated with drinking water during thirst and after its satiation. Proceedings of the National Academy of Sciences. 2014;111:5379–5384. doi: 10.1073/pnas.1403382111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fuhrer D, et al. Brain activity in hunger and satiety: an exploratory visually stimulated FMRI study. Obesity (Silver Spring) 2008;16:945–950. doi: 10.1038/oby.2008.33. [DOI] [PubMed] [Google Scholar]
- 102.Wang D, et al. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Frontiers in neuroanatomy. 2015;9:40. doi: 10.3389/fnana.2015.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Betley JN, et al. Parallel, redundant circuit organization for homeostatic control of feeding behavior. Cell. 2013;155:1337–1350. doi: 10.1016/j.cell.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Livneh Y, et al. Homeostatic circuits selectively gate food cue responses in insular cortex. Nature. 2017 doi: 10.1038/nature22375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Accolla R, Carleton A. Internal body state influences topographical plasticity of sensory representations in the rat gustatory cortex. Proc Natl Acad Sci U S A. 2008;105:4010–4015. doi: 10.1073/pnas.0708927105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Burgess CR, et al. Gating of visual processing by physiological need. Curr Opin Neurobiol. 2017;49:16–23. doi: 10.1016/j.conb.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Carleton A, et al. Coding in the mammalian gustatory system. Trends in neurosciences. 2010;33:326–334. doi: 10.1016/j.tins.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Palmiter RD. The Parabrachial Nucleus: CGRP Neurons Function as a General Alarm. Trends in Neurosciences. 2018;41:280–293. doi: 10.1016/j.tins.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tokita K, et al. Afferent connections of the parabrachial nucleus in C57BL/6J mice. Neuroscience. 2009;161:475–488. doi: 10.1016/j.neuroscience.2009.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Chen X, et al. A gustotopic map of taste qualities in the mammalian brain. Science. 2011;333:1262–1266. doi: 10.1126/science.1204076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shackman AJ, et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.de Araujo IE, Simon SA. The gustatory cortex and multisensory integration. Int J Obes (Lond) 2009;33(Suppl 2):S34–43. doi: 10.1038/ijo.2009.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sherrington C. The integrative action of the nervous system. CUP Archive 1910 [Google Scholar]
- 114.McFarland D, Sibly R. The behavioural final common path. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 1975;270:265–293. doi: 10.1098/rstb.1975.0009. [DOI] [PubMed] [Google Scholar]
- 115.Jourjine N, et al. Coupled sensing of hunger and thirst signals balances sugar and water consumption. Cell. 2016;166:855–866. doi: 10.1016/j.cell.2016.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Alhadeff Amber L, ZS, Hernandez Elen, Klima Michelle L, Phillips Sophie Z, Holland Ruby A, Guo Caiying, Hantman Adam W, De Jonghe Bart C, Nicholas Betley J. A Neural Circuit for the Suppression of Pain by a Competing Need State. Cell. 2018;173:140–152. doi: 10.1016/j.cell.2018.02.057. 4’Correspondence information about the author J. Nicholas Betley. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Burnett CJ, et al. Hunger-driven motivational state competition. Neuron. 2016;92:187–201. doi: 10.1016/j.neuron.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Jikomes N, et al. Preemptive stimulation of AgRP neurons in fed mice enables conditioned food seeking under threat. Current Biology. 2016;26:2500–2507. doi: 10.1016/j.cub.2016.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Padilla SL, et al. Agouti-related peptide neural circuits mediate adaptive behaviors in the starved state. Nature neuroscience. 2016;19:734–741. doi: 10.1038/nn.4274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Wang L, et al. Collateral pathways from the ventromedial hypothalamus mediate defensive behaviors. Neuron. 2015;85:1344–1358. doi: 10.1016/j.neuron.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Behbehani MM. Functional characteristics of the midbrain periaqueductal gray. Progress in neurobiology. 1995;46:575–605. doi: 10.1016/0301-0082(95)00009-k. [DOI] [PubMed] [Google Scholar]
- 122.Williams EK, et al. Sensory neurons that detect stretch and nutrients in the digestive system. Cell. 2016;166:209–221. doi: 10.1016/j.cell.2016.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hayes MR, et al. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell metabolism. 2011;13:320–330. doi: 10.1016/j.cmet.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Grill HJ, et al. Evidence that the caudal brainstem is a target for the inhibitory effect of leptin on food intake. Endocrinology. 2002;143:239–246. doi: 10.1210/endo.143.1.8589. [DOI] [PubMed] [Google Scholar]
- 125.Alhadeff AL, et al. Glucagon-like peptide-1 receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake and motivation to feed. Neuropsychopharmacology. 2014;39:2233. doi: 10.1038/npp.2014.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Alhadeff AL, et al. Leptin receptor signaling in the lateral parabrachial nucleus contributes to the control of food intake. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2014;307:R1338–R1344. doi: 10.1152/ajpregu.00329.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Carter ME, et al. Parabrachial calcitonin gene-related peptide neurons mediate conditioned taste aversion. J Neurosci. 2015;35:4582–4586. doi: 10.1523/JNEUROSCI.3729-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sakai RR, et al. Salt appetite is suppressed by interference with angiotensin II and aldosterone. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 1986;251:R762–R768. doi: 10.1152/ajpregu.1986.251.4.R762. [DOI] [PubMed] [Google Scholar]
- 129.Jarvie BC, Palmiter RD. HSD2 neurons in the hindbrain drive sodium appetite. Nature neuroscience. 2017;20:167. doi: 10.1038/nn.4451. [DOI] [PubMed] [Google Scholar]
- 130.Resch JM, et al. Aldosterone-Sensing Neurons in the NTS Exhibit State-Dependent Pacemaker Activity and Drive Sodium Appetite via Synergy with Angiotensin II Signaling. Neuron. 2017;96:190–206. e197. doi: 10.1016/j.neuron.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Matsuda T, et al. Distinct neural mechanisms for the control of thirst and salt appetite in the subfornical organ. Nature neuroscience. 2017;20:230. doi: 10.1038/nn.4463. [DOI] [PubMed] [Google Scholar]
- 132.Chen Y, et al. Hunger neurons drive feeding through a sustained, positive reinforcement signal. Elife. 2016:5. doi: 10.7554/eLife.18640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jennings JH, et al. The inhibitory circuit architecture of the lateral hypothalamus orchestrates feeding. Science. 2013;341:1517–1521. doi: 10.1126/science.1241812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Jennings JH, et al. Visualizing hypothalamic network dynamics for appetitive and consummatory behaviors. Cell. 2015;160:516–527. doi: 10.1016/j.cell.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Navarro M, et al. Lateral hypothalamus GABAergic neurons modulate consummatory behaviors regardless of the caloric content or biological relevance of the consumed stimuli. Neuropsychopharmacology. 2016;41:1505. doi: 10.1038/npp.2015.304. [DOI] [PMC free article] [PubMed] [Google Scholar]