Abstract
Recently it has been observed in preclinical models that that radiation enhances the recruitment of circulating tumor cells to primary tumors, and results in tumor regrowth after treatment. This process may have implications for clinical radiotherapy, which improves control of a number of tumor types but which, despite continued dose escalation and aggressive fractionation, is unable to fully prevent local recurrences. By irradiating a single tumor within an animal bearing multiple lesions, we observed an increase in tumor cell migration to irradiated and unirradiated sites, suggesting a systemic component to this process. Previous work has identified the cytokine GM-CSF, produced by tumor cells following irradiation, as a key effector of this process. We evaluated the ability of systemic injections of a PEGylated form of GM-CSF to stimulate tumor cell migration. While increases in invasion and migration were observed for tumor cells in a transwell assay, we found that daily injections of PEG-GM-CSF to tumor-bearing animals did not increase migration of cells to tumors, despite the anticipated changes in circulating levels of granulocytes and monocytes produced by this treatment. Combination of PEG-GM-CSF treatment with radiation also did not increase tumor cell migration. These findings suggest that clinical use of GM-CSF to treat neutropenia in cancer patients will not have negative effects on the aggressiveness of residual cancer cells. However, further work is needed to characterize the mechanism by which GM-CSF facilitates systemic recruitment of trafficking tumor cells to tumors.
Keywords: Radiation therapy, Cancer, Metastasis, GM-CSF
Introduction
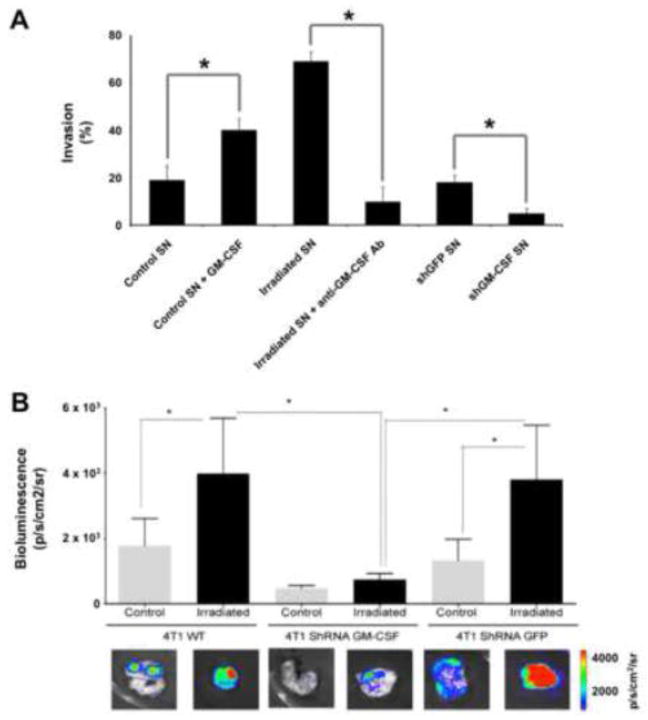
Radiation therapy is used in the management of up to 70% of all cancer patients. However, historically it is limited in the treatment of patients with metastatic disease, due to concerns over the cumulative dose to normal tissues and the resulting toxicity when delivering radiation to sites spread over a large anatomic area. While there are over a century of radiobiological studies characterizing the direct effects of radiation on tumors in terms of metrics ranging from clonogenic survival to change in radiologic volume, the systemic effects of radiation, including its influence on occult disseminated tumor cells, remain unclear. A number of diverging observations have been made of the effects of radiation on tumor cell migration and metastasis, ranging from stimulation of local invasion to inhibition of tumor growth through activation of systemic immunity [1]. Recently, we have reported that irradiation of tumors can induce the recruitment of tumor cells migrating from distant sites through radiation-induced stimulation of the cytokine Granulocyte-macrophage colony-stimulating factor (GM-CSF) [2], as shown in Figure 1. Radiation can induce the expression and secretion of GM-CSF by both normal fibroblasts and tumor cells [2, 3], which promotes invasion and migration of cancer cells in vitro (Figure 1A), a finding that has been reported by other groups [4, 5]. Using a two-tumor “donor-recipient” murine model system in which an unlabeled recipient tumor is irradiated and then measured for the influx of luciferase-labeled cells from the donor tumor, the importance of GM-CSF for radiation-induced tumor cell migration was observed in vivo as well (Figure 1B). This finding suggests that regrowth of tumors following radiotherapy may not be solely due to a failure to kill a critical number of tumor cells within the irradiated volume, but also potentially due to an influx of untreated cancer cells that were induced to return to the primary tumor by the therapy itself.
Figure 1.
Radiation stimulates migration and invasion of tumor cells. (A) 4T1 cells cultured in vitro migrate preferentially toward supernatant (SN) from 4T1 cells spiked with recombinant GM-CSF. SN harvested from 4T1 cells irradiated to a dose of 20 Gy also stimulates migration, while inhibition of GM-CSF through shRNA or neutralizing antibodies blocks it. (B) Irradiation of 4T1 tumors in mice promotes recruitment of metastatic luciferase-labeled 4T1 cells. This process is inhibited when GM-CSF is genetically inhibited in the irradiated tumors. * denotes P < 0.05. Adapted from [2].
While these previous studies demonstrated a critical role for GM-CSF in this process, the precise molecular and cellular mechanism of this process has not been fully defined. Radiotherapy of tumors produces an elevation in circulating levels of GM-CSF, however it is unclear whether this systemic protein results in an overall increase in metastasis, or whether it is targeted to the site of irradiation that is producing GM-CSF. If the migration is targeted, it is not apparent how this functions in terms of the classical concentration gradient model of chemotaxis. Furthermore, the use of GM-CSF in the clinic to overcome chemotherapy-induced neutropenia raises concerns that this could enhance tumor cell migration and recurrence following radiotherapy [6]. Studies of GM-CSF for the management of radiotherapy-induced toxicities in cancer patients have not included tumor control as an endpoint [7], leaving this question unanswered. GM-CSF has also been combined with radiation for the treatment of patients with metastatic cancers, in order to enhance the immunostimulatory aspects of radiotherapy in order to enhance tumor control [8]. This cytokine therefore appears to have multifaceted effects in the context of tumor treatment, whose composite effect is as yet unclear.
In this study, we investigated the mechanism by which radiation induces tumor cell migration, focusing on the role of GM-CSF in this process. Using variants of the two-tumor murine model, we evaluated whether radiation facilitates this process in a systemic fashion. We then focused on the ability of exogenous GM-CSF administration to stimulate this process. This assessed both any potential negative aspects of this clinical neutropenia therapy as well as its role in radiation-induced tumor cell homing.
Materials and Methods
Cell lines
Highly metastatic mouse 4T1 mammary carcinoma cells were grown according to ATCC specifications (American Type Culture Collection (ATCC), Manassas, VA). Wild type cells (4T1) were stably transduced with a retrovirus encoding both firefly luciferase (FLuc) and green fluorescent protein (GFP), producing labelled cells referred to as 4T1-luc. In addition, wild type 4T1 cells were stably transduced with viruses produced from mouse shRNA clones targeting the GM-CSF gene (TRCN0000054618, TRCN0000054619, TRCN0000054620, TRCN0000054621, TRCN0000054622, Thermo Scientific, Phoenix), producing cells referred to as 4T1-shGM-CSF. A vector encoding an shRNA targeting GFP was used as a control (4T1-shGFP).
GM-CSF
Recombinant murine GM-CSF was purchased from Kingfisher Biotech Inc. (St. Paul, MN). In addition, a modified version of this protein conjugated to polyethylene glycol (PEG) chains to increase its half-life in blood [9] was obtained as a generous gift from Bolder Biotechnology (Boulder, CO). A sandwich enzyme-linked immunosorbent assay (ELISA) kit specific to murine GM-CSF was obtained from R&D Systems (Minneapolis, MN) and performed according to the manufacturer’s specifications.
Invasion assays
106 cells were plated in a 10 cm dish and were irradiated to different doses using a cesium source. Media from irradiated cells was collected at different time points after radiation and used as conditioned media in a transwell migration assay (BD Biosciences, San Jose, CA). 105 unirradiated cells were placed in the upper chamber of the transwell setup. Migration of these cells toward the conditioned media was measured 24 hours later by counting cells in the transwell membrane. Subsequent experiments included the addition of 0.125 g/L PEG-GM-CSF to conditioned media prior to introduction to the transwell setup.
Animal models
All animal experiments were done according to a protocol approved by the Institutional Animal Care and Use Committee. 7 week old nude female mice (Charles River Laboratories, Wilmington, MA) were inoculated with 5×104 4T1 cells suspended in 50 μL of PBS in contralateral mammary glands. Tumor volumes were monitored by caliper measurement three times a week. Once tumors reached a diameter of 7 mm, one of the two tumors was focally irradiated using a 225 kVp cabinet x-ray system filtered with 0.5 mm Cu (Kimtron Inc., Oxford, CT) and a 3.2 mm thick lead shield with a 1 cm aperture placed over the target tumor. Immediately after irradiation, 105 4T1-luc cells were injected intravenously through the tail vein. A group of tumor-bearing mice were left unirradiated as a control. Ten days later, animals were injected 100μg/g D-luciferin and sacrificed 10 minutes later. Tumors were excised and imaged ex vivo using an IVIS-200 bioluminescence imaging system (Perkin Elmer, Waltham, MA). Bioluminescence images were quantified using Living Image 4.3.1 software.
Subsequent experiments added daily intravenous injections of either formulation buffer or 20 pg PEG-GM-CSF to mice beginning the day after radiation treatment. In a pilot experiment, blood samples were collected via retro orbital bleeds from alternating eyes in three groups of mice at 0, 6, 12, 24, 48, and 120 hours after a single injection of PEG-GM-CSF in order to measure its blood half-life. Tumor experiments included the collection of blood through retro orbital bleeds at weekly intervals in order to measure complete blood counts.
Results
Stimulation of tumor cell recruitment by radiation is a systemic process
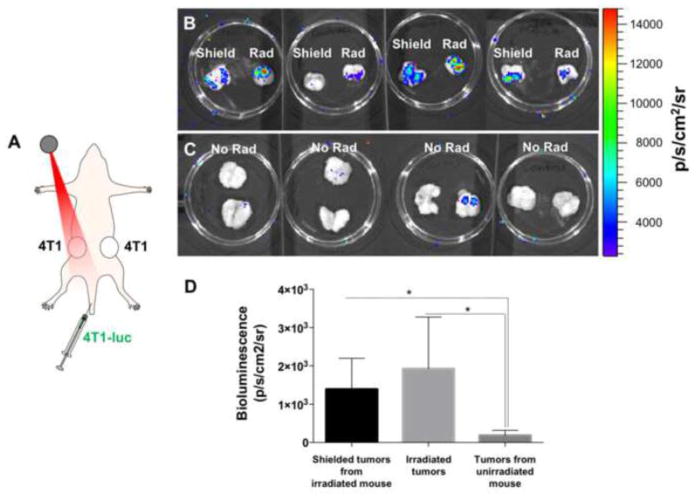
Previous work had suggested that recruitment of migrating tumor cells to irradiated tumors functions through the secreted cytokine GM-CSF, which is observed to be elevated in blood plasma in mice within a week of single fraction radiation treatment [2]. In order to further understand the mechanism behind this process, we modified the one donor-one recipient model used previously in favor of a system in which two unlabeled recipient 4T1 tumors were implanted in contralateral mammary fat pads in the same mice, while donor 4T1-luc cells were delivered intravenously (Figure 2A). One of the two recipient tumors was irradiated to a dose of 20 Gy, these tumors exhibited the greatest ex vivo bioluminescence signal when excised 10 days after treatment (Figure 2B). However, the contralateral tumors in these mice, which were shielded during irradiation and received approximately 5% of dose to the target tumor (1 Gy), also demonstrated significantly greater bioluminescence relative to tumors excised from mice that received no radiation (Figure 2C). There was no statistically significant difference in bioluminescence between the irradiated and shielded tumors. This observation suggests that the recruitment of trafficking tumor cells to tumors after radiotherapy is facilitated by a systemic response instead of, or in addition to, a direct local response to radiation in the irradiated tumor.
Figure 2.
Radiation promotes recruitment of migrating tumor cells in a systemic manner. (A) A donor-recipient system was created in which two unlabeled 4T1 tumors were grown in contralateral mammary fat pads of nude mice. One was focally irradiated to a dose of 20 Gy, immediately after which luciferase-expressing 4T1 cells were injected intravenously through the tail vein. (B) Bioluminescence images of irradiated recipient tumors (Rad), as well as the contralateral tumors from the irradiated mice (Shield). (C) Ex vivo bioluminescence images of recipient tumors harvested from untreated mice 10 days post-treatment. (D) Quantification of the bioluminescence signals seen in (B) and (C). * denotes P < 0.05.
Exogenous GM-CSF stimulates tumor cell invasion in vitro
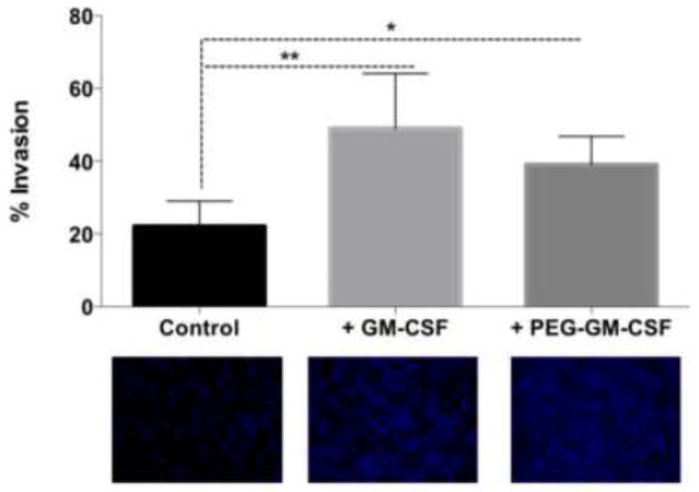
Based on previous observations that GM-CSF is required for radiation-induced recruitment of migrating tumor cells to irradiated tumors, and the apparent systemic nature of this stimulation as seen in Figure 2, we evaluated the effects of GM-CSF administration on tumor cell migration and invasion. While recombinant mouse GM-CSF had previously been evaluated in this regard, a PEGylated form of mouse GM-CSF with longer blood half-life has been synthesized and is of interest as a therapeutic in humans with applications in the treatment of cancer as well as acute radiation exposure[9]. We compared the ability of both forms of GM-CSF to stimulate invasion of 4T1 cells in an in vitro transwell assay. When added to supernatant from control, untreated 4T1 cells, both molecules increased the invasion of 4T1 cells (Figure 3). The magnitude of this increase corresponds to that seen in Figure 1. These findings demonstrate that GM-CSF, as well as PEG-GM-CSF, stimulates 4T1 cells to increase migration and invasion.
Figure 3.
GM-CSF as well as PEG-GM-CSF induce invasion of 4T1 cells in vitro. Conditioned media from 4T1 cells was used as an attractant in a transwell invasion assay. Either GM-CSF or PEG-GM-CSF was added to this media, and significant increases were obsereved in invasion, as visualized by DAPI staining of the transwell membrane. * denotes P < 0.05, ** denotes P < 0.01.
Exogenous GM-CSF increases blood cell counts but not tumor cell recruitment in vivo
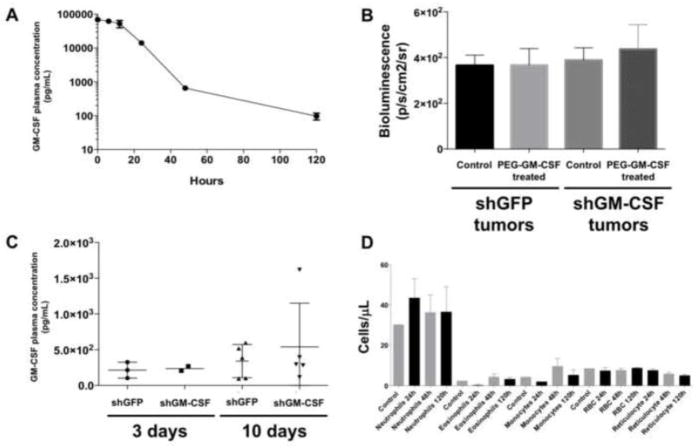
To assess whether systemic administration of GM-CSF increases tumor cell migration in vivo, in a manner that could be relevant for cancer patients treated with this cytokine to manage chemotherapy-induced neutropenia, we treated 4T1 tumor-bearing mice with PEG-GM-CSF. The pharmacokinetics of this form of the cytokine was first determined in mice through serial blood draws after delivery of a single intravenous dose of PEG-GM-CSF (Figure 4A). The half-life of PEG-GM-CSF in the blood of nude mice was measured to be approximately 19 hours, suggesting that daily dosing of mice in subsequent experiments could provide relatively stable circulating levels of the cytokine. A donor-recipient model consisting of a donor 4T1-luc tumor implanted in the mammary fat pad and a recipient 4T1 tumor in the contralateral fat pad was created. The recipient tumors in this experiment were stably transduced with an shRNA construct to suppress expression of either GFP (control) or GM-CSF, in order to evaluate the contribution of GM-CSF from the tumor itself. Inhibition of expression of the target was verified by western blot in vitro to be at least 75%. As seen in Figure 4B, in the absence of radiation, neither inhibition of tumoral GM-CSF by shRNA nor delivery of exogenous PEG-GM-CSF altered recruitment of donor 4T1-luc cells to recipient tumors. The presence of increased levels of GM-CSF in the blood of mice given PEG-GM-CSF was verified by ELISA, demonstrating increasing levels of the cytokine in contrast to it being undetectable at baseline (Figure 4C). Complete blood counts of plasma collected from mice at the end of the experimental period demonstrated significant increases in monocytes, neutrophils, and eosinophils within 120 hours of the initiation of PEG-GM-CSF treatment, relative to baseline levels (Figure 4D). As expected, PEG-GM-CSF had no effect on the levels of circulating red blood cells. Taken together, these data suggest that PEG-GM-CSF given to nude mice induces effects on the hematopoietic system consistent with the canonical role for this cytokine, but that it does not increase recruitment of circulating tumor cells to primary lesions.
Figure 4.
Systemic administration of PEG-GM-CSF does not increase recruitment of migrating tumor cells to tumors. (A) Blood half life of GM-CSF after a single intravenous injection of 0.05 mg/kg of PEG-GM-CSF. (B) Ex vivo bioluminescence measured in shGFP or shGM-CSF tumors in mice treated with daily injections of 20 pg PEG-GM-CSF. (C) Plasma levels of GM-CSF measured 3 and 10 days after the initiation of treatment. (D) Counts for blood cells before (Control) and at specified times following the initiation of PEG-GM-CSF treatment.
Exogenous GM-CSF does not enhance tumor cell recruitment to irradiated tumors
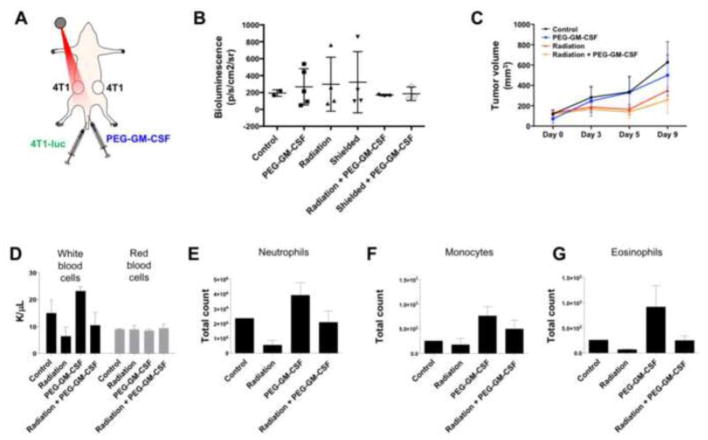
While the administration of GM-CSF was observed to have no effect on the migration of tumor cells by itself, we next investigated whether it could enhance the increase in tumor cell migration by radiation observed previously. A two recipient 4T1 tumor model was created in a manner identical to that used in Figure 2, with donor 4T1-luc cells being delivered via intravenous injection. As before, one of the recipient tumors was treated with radiation to a dose of 20 Gy, with some of the mice also receiving daily doses of PEG-GM-CSF beginning immediately after radiation treatment (Figure 5A). While radiation stimulated recruitment of tumor cells to target and shielded tumors and PEG-GM-CSF had no effect in the absence of radiation, as observed previously, we were surprised to find that combination of radiation and PEG-GM-CSF suppressed migration of tumor cells to recipient tumors (Figure 5B). Tumor response to treatment was quantified through periodic caliper measurements of tumor volume, revealing that radiation induced a significant growth delay in treated tumors, but that PEG-GM-CSF administration did not affect the volume of tumors when given by itself or in combination with radiation (Figure 5C). PEG-GM-CSF treatment again increased the levels of circulating neutrophils, monocytes, and eosinophils, as well as white blood cells (Figure 5D). Radiation decreased the levels of each of these hematopoietic cells, and combination of radiation and PEG-GM-CSF returned cell counts to levels roughly corresponding to those in untreated mice. Red blood cell counts were unaffected by PEG-GM-CSF. The results of Figures 4 and 5 demonstrate that delivery of exogenous GM-CSF is incapable of phenocopying the effects of radiation on recruitment of tumor cells, and furthermore that it is insufficient to enhance the stimulation of tumor cell migration by radiation when given in combination.
Figure 5.
Addition of PEG-GM-CSF to radiotherapy does not increase recruitment of migrating tumor cells. (A) The model system from Figure 2A was used, with the addition of daily intravenous injections of 20 pg PEG-GM-CSF beginning immediately after radiation treatment. (B) Ex vivo bioluminescence measured in tumors for the specified treatment groups. (C) Tumor volumes measured by calipers for treated tumors. Counts of white blood cells, red blood cells (D), neutrophils (E), monocytes (F), and eosinophils (G) measured 10 days after the initiation of treatment for the specified groups.
Discussion
The ability of radiation to stimulate tumor metastasis through both production of viable circulating tumor cells in a primary tumor as well as creation of a favorable metastatic niche in distant tissues has been suggested by a number of studies [10, 11]. More recently, preclinical studies have suggested that radiation can stimulate the recruitment of tumor cells to irradiated tumors, which can result in tumor recurrence through a mechanism that is independent of the extent of cell kill produced by radiation in the treated tumor. The clinical significance of these findings is a topic of debate, given the long history of cancer radiotherapy and the excellent outcomes it achieves for many tumor types as demonstrated in a number of randomized trials [12]. Indeed, there is no clinical data suggesting that increasing radiation doses to tumors results in a corresponding increase in subsequent local recurrence, which would be the simple extrapolation of these findings. However, it is of note that in-field recurrences persist following radiation treatment for a number of tumor types, including breast [13], lung [14], head and neck [15], and brain [16], despite continuing advances in radiation oncology resulting in escalating doses and more aggressive fractionation schemes. This raises the question of whether recurrence mechanisms may exist that are independent of the intrinsic or microenvironment-mediated radioresistance of primary tumor cells.
Our observation of an increase in the recruitment of circulating tumor cells to irradiated tumors in mice offers such a mechanism [2]. Tumor cells outside the radiation field at the time of treatment may be induced to return through a mechanism relying on radiation-induced expression of GM-CSF by the treated tumor (Figure 1). We previously observed that tumors in which GM-CSF had been genetically inhibited by an shRNA exhibited an increased growth delay following radiation, relative to wild type, GM-CSF-expressing tumors. This was despite the fact that GM-CSF knockdown tumor cells displayed slightly increased radioresistance in vitro, suggesting that this change in tumor radiation response was not dependent on alteration of target cell radiosensitivity. In this study, we demonstrated that radiotherapy can enhance tumoral recruitment of circulating tumor cells throughout a treated organism, not solely within the volume that was irradiated (Figure 2). This suggests that the mechanism underpinning this phenomenon acts in a systemic manner, communicated throughout the animal. As GM-CSF is secreted into the circulation, it is a prime candidate for this role, although the precise microscopic mechanism of its action in this setting is unclear.
The association of GM-CSF with radiation response and tumor cell migration raises concerns over use of this cytokine in a therapeutic context. GM-CSF is a white blood cell growth factor secreted by a variety of immune and stromal cells that stimulates hematopoietic stem cells through JAK2 and STAT5 to produce granulocytes and monocytes. Pharmaceutical analogs of GM-CSF including sargramostim and molgramostim are used to reconstitute myeloid cells following bone marrow transplant in patients with lymphoma and leukemia, and also in the context of cancer to overcome chemotherapy-induced neutropenia, primarily in the setting of acute myeloid leukemia. Use of GM-CSF to manage neutropenia in patients with solid cancers such as breast is less common than recombinant granulocyte colony stimulating factor (G-CSF), a distinct cytokine with similar myeloid-stimulating properties. A number of efforts to develop tumor vaccines have utilized GM-CSF to achieve an immunostimulatory effect [17, 18], and more recently the ability of exogenous GM-CSF immunostimulation to produce an abscopal response when combined with radiotherapy in patients with metastatic disease has been studied [8]. GM-CSF has emerged as a primary medication to manage the hematological effects of acute radiation syndrome (ARS) [19, 20]. The cytokine has also been studied as a means of alleviating mucositis in head and neck cancer patients treated with radiation [7].
These pharmaceutical applications of GM-CSF demonstrate its ability as an exogenous therapeutic to modulate the hematological and immune systems, but its role in radiation response remains unclear. We have performed ELISA assays to measure GM-CSF in the plasma of breast cancer patients treated with fractionated radiotherapy, and have found that both the baseline levels of this cytokine as well as levels from 1–6 weeks after radiotherapy are below the threshold of detection. Previous observations of increases in circulating GM-CSF levels in mice after focal radiotherapy [2] may be due to the larger relative amount of tissue irradiated in these experimental animals, the larger fractional doses used in mice, the absence of frank tumor in these post-surgical patients, or some combination of these factors. The purpose of this study was to examine the effects of pharmacologic administration of GM-CSF on tumor cell migration, which was previously seen to depend on tumoral expression of GM-CSF in response to radiation. While addition of GM-CSF and PEG-GM-CSF to cell supernatant was sufficient to induce tumor cell migration in vitro (Figure 3), it did not stimulate recruitment of circulating tumor cells to tumors when given to tumor-bearing mice either alone (Figure 4) or in combination with radiotherapy (Figure 5), despite demonstration of increased circulating levels of the cytokine as well as hematologic changes consistent with its primary function. These results are encouraging clinically, as they alleviate fears that administration of GM-CSF to cancer patients to manage the side effects of cancer therapies may have unintended consequences on tumor control.
The mechanism by which GM-CSF stimulates recruitment of tumor cells, particularly in a systemic manner such as that demonstrated in Figure 2, therefore remains unclear. It may be that systemic administration of GM-CSF at the doses employed here overwhelms the biological mechanism by which the cytokine leads to tumor cell migration. This mode of GM-CSF delivery also fails to duplicate the spatiotemporal dynamics of GM-CSF expression following focal radiotherapy, where it is produced and secreted specifically by irradiated tissues. Furthermore, while in vitro studies demonstrate that GM-CSF has a direct effect on tumor cells to stimulate invasion and migration (Figure 3), it is possible that the hematological changes induced by this cytokine may indirectly facilitate tumor cell recruitment in vivo. In particular, the ability of this protein to stimulate macrophages may create or enhance a tumor microenvironment conducive to trafficking tumor cells. Further study of the expression and activity of GM-CSF receptor in tumor cells as well as the cooperation between GM-CSF-stimulated granulocytes and monocytes and tumor cells is needed to fully understand the full biological and clinical consequences of upregulation of GM-CSF following radiation. Experiments utilizing positive and negative control cytokines that do and do not promote tumor cell migration, and comparison with the phenotype produced by endogenous and exogenous GM-CSF stimulation, is required to fully understand the specific effects of GM-CSF on radiation response.
It bears emphasizing that the clinical significance of these processes remains undetermined. Whether radiation stimulates pro-tumor migration, survival, and proliferation programs, and how to evaluate how these processes integrate with the direct and indirect antitumor effects of radiation to determine overall tumor response to treatment, has not been adequately studied in human cancer patients. This composite response likely depends on the specific radiation dose and fractionation scheme delivered [1], effects of radiation on other entities including immune cells [21] and blood vessels [22], and contributions of other components of multimodality treatments such as surgery [23]. Careful consideration of each of these parameters, including how they influence tumor cell response, tumor cell migration, and each other, is needed in order to critically assess whether radiation-induced tumor cell migration plays a role in the response of human tumors to radiotherapy. Fractionated radiation regimens may thwart the effects of radiation-induced tumor cell migration by killing recruited cells through subsequent radiation fractions. Recent studies examining the induction of immune responses following DNA damage have also shown a radiation dose and fractionation dependence [24, 25]. The interplay between all these processes will determine overall tumor response to radiation treatment.
Acknowledgments
This work was funded by the National Institutes of Health and the National Cancer Institute of the United States (R01 CA197136, P01 CA067166, S10 OD018208).
Funding:
This study was funded by the National Institutes of Health and the National Cancer Institute of the United States (R01 CA197136, P01 CA067166).
Footnotes
Compliance with Ethical Standards
Conflict of Interest:
M. Vilalta declares that she has no conflict of interest.
J. Brune declares that she has no conflict of interest.
M. Rafat declares that she has no conflict of interest.
L. Soto declares that he has no conflict of interest.
E.E. Graves declares that he has no conflict of interest.
Ethical approval:
All procedures performed in studies involving animal subjects were performed in accordance with the ethical standards of the institutional and national research committee, following approval of an animal protocol by the institutional animal care and use committee.
References
- 1.Vilalta M, Rafat M, Graves EE. Effects of radiation on metastasis and tumor cell migration. Cell Mol Life Sci. 2016;73:2999–3007. doi: 10.1007/s00018-016-2210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vilalta M, et al. Recruitment of Circulating Breast Cancer Cells Is Stimulated by Radiotherapy. Cell Reports. 2014;8:402–9. doi: 10.1016/j.celrep.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hachiya M, et al. Irradiation increases expression of GM-CSF in human fibroblasts by transcriptional and post-transcriptional regulation. Exp Cell Res. 1994;214:343–50. doi: 10.1006/excr.1994.1266. [DOI] [PubMed] [Google Scholar]
- 4.Uemura Y, et al. Effects of GM-CSF and M-CSF on tumor progression of lung cancer: roles of MEK1/ERK and AKT/PKB pathways. Int J Mol Med. 2006;18:365–73. [PubMed] [Google Scholar]
- 5.Gutschalk CM, et al. GM-CSF enhances tumor invasion by elevated MMP-2, -9, and -26 expression. Cancer Med. 2013;2:117–29. doi: 10.1002/cam4.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta HM, Malandra M, Corey SJ. G-CSF and GM-CSF in Neutropenia. J Immunol. 2015;195:1341–9. doi: 10.4049/jimmunol.1500861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ryu JK, et al. The impact of concurrent granulocyte macrophage-colony stimulating factor on radiation-induced mucositis in head and neck cancer patients: a double-blind placebo-controlled prospective phase III study by Radiation Therapy Oncology Group 9901. Int J Radiat Oncol Biol Phys. 2007;67:643–50. doi: 10.1016/j.ijrobp.2006.09.043. [DOI] [PubMed] [Google Scholar]
- 8.Golden EB, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol. 2015;16:795–803. doi: 10.1016/S1470-2045(15)00054-6. [DOI] [PubMed] [Google Scholar]
- 9.Plett PA, et al. PEGylated G-CSF (BBT-015), GM-CSF (BBT-007), AND IL-11 (BBT-059) Analogs Enhance Survival and Hematopoietic Cell Recovery in a Mouse Model of the Hematopoietic Syndrome of the Acute Radiation Syndrome. Health Phys. 2014;106:7–20. doi: 10.1097/HP.0b013e3182a4dd4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.von Essen CF. Radiation enhancement of metastasis: a review. Clin Exp Metastasis. 1991;9:77–104. doi: 10.1007/BF01756381. [DOI] [PubMed] [Google Scholar]
- 11.Martin OA, et al. Mobilization of viable tumor cells into the circulation during radiation therapy. Int J Radiat Oncol Biol Phys. 2014;88:395–403. doi: 10.1016/j.ijrobp.2013.10.033. [DOI] [PubMed] [Google Scholar]
- 12.McGale P, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383:2127–35. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowery AJ, et al. Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype. Breast Cancer Res Treat. 2012;133:831–41. doi: 10.1007/s10549-011-1891-6. [DOI] [PubMed] [Google Scholar]
- 14.Campeau MP, et al. Local control and survival following concomitant chemoradiotherapy in inoperable stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2009;74:1371–5. doi: 10.1016/j.ijrobp.2008.10.067. [DOI] [PubMed] [Google Scholar]
- 15.Garden AS, et al. Long-term results of concomitant boost radiation plus concurrent cisplatin for advanced head and neck carcinomas: a phase II trial of the radiation therapy oncology group (RTOG 99–14) Int J Radiat Oncol Biol Phys. 2008;71:1351–5. doi: 10.1016/j.ijrobp.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Barani IJ, Larson DA. Radiation therapy of glioblastoma. Cancer Treat Res. 2015;163:49–73. doi: 10.1007/978-3-319-12048-5_4. [DOI] [PubMed] [Google Scholar]
- 17.Dranoff G. GM-CSF-based cancer vaccines. Immunol Rev. 2002;188:147–54. doi: 10.1034/j.1600-065x.2002.18813.x. [DOI] [PubMed] [Google Scholar]
- 18.Harrington KJ, et al. Efficacy and safety of talimogene laherparepvec versus granulocyte-macrophage colony-stimulating factor in patients with stage IIIB/C and IVM1a melanoma: subanalysis of the Phase III OPTiM trial. Onco Targets Ther. 2016;9:7081–93. doi: 10.2147/OTT.S115245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waselenko JK, et al. Medical management of the acute radiation syndrome: recommendations of the Strategic National Stockpile Radiation Working Group. Ann Intern Med. 2004;140:1037–51. doi: 10.7326/0003-4819-140-12-200406150-00015. [DOI] [PubMed] [Google Scholar]
- 20.Gourmelon P, et al. European consensus on the medical management of acute radiation syndrome and analysis of the radiation accidents in Belgium and Senegal. Health Phys. 2010;98:825–32. doi: 10.1097/HP.0b013e3181ce64d4. [DOI] [PubMed] [Google Scholar]
- 21.Demaria S, Formenti SC. Role of T lymphocytes in tumor response to radiotherapy. Front Oncol. 2012;2:95. doi: 10.3389/fonc.2012.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brown JM. Vasculogenesis: a crucial player in the resistance of solid tumours to radiotherapy. Br J Radiol. 2014;87:20130686. doi: 10.1259/bjr.20130686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murthy SM, et al. The influence of surgical trauma on experimental metastasis. Cancer. 1989;64:2035–44. doi: 10.1002/1097-0142(19891115)64:10<2035::aid-cncr2820641012>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 24.Mavragani IV, et al. Complex DNA Damage: A Route to Radiation-Induced Genomic Instability and Carcinogenesis. Cancers (Basel) 2017;9 doi: 10.3390/cancers9070091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vanpouille-Box C, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun. 2017;8:15618. doi: 10.1038/ncomms15618. [DOI] [PMC free article] [PubMed] [Google Scholar]