Abstract
This paper deliberates the extraction, characterization and examination of potential application of soluble polysaccharides of palm kernel cake (PKC) as a prebiotic. The PKC was defatted and crude polysaccharide was obtained through water, citric acid or NaOH extraction. The physiochemical properties of the extracted polysaccharides viz. total carbohydrates, protein content, solubility rate, monosaccharides composition, structural information and thermal properties were also determined. The extracted soluble polysaccharides were further subjected to a digestibility test using artificial human gastric juice. Finally, their prebiotic potential on two probiotics, namely Lactobacillus plantarum ATCC 8014 and Lb. rhamnosus ATCC 53103 were evaluated in vitro. It was observed that PKC contained ash (5.2%), moisture (7.4%), carbohydrates (65.8%), protein (16.5%) and fat (5.1%). There were significant differences (P < 0.05) between the values of NaOH-extracted crude polysaccharides (8.73%) and that of water (3.03%) and citric acid (3.07%)-extracted polysaccharides. The extracted polysaccharides composed of mannose, galactose, glucose, arabinose, xylose and rhamanose, with highest percentage of mannose (62.49%) and galactose (25.42%) in SPCA. Total carbohydrate content in SCPW, SCPCA and SCPN are 57.11%, 56.94% and 50.95%, respectively. The polysaccharides from PKC in this study were found to be highly soluble (> 95%). Protein content in SCPW, SCPCA and SCPN are 0.72, 0.40 and 0.58, respectively, and the peaks which indicated the presence of protein were observed at approximately 1640 cm−1 (amide I). FTIR spectroscopy revealed that the polysaccharides extracts were linked to β and α-glycosidic bonds and thermal analysis using differential scanning calorimeter (DSC) showed the main degradation temperature of SP is about 121 to 125 °C. The SP were found to be highly resistance (> 96%) to hydrolysis when subjected to artificial human gastric juice. The prebiotics potentials of the polysaccharides on probiotics in vitro demonstrated an increase in proliferation of Lb. plantarum ATCC 8014 and Lb. rhamnosus ATCC 53103 with decrease in the pH of the medium and producing organic acids.All the above findings strongly indicated that polysaccharides extracted from PKC, an industrial waste, have a potential to be exploited as novel prebiotics.
Keywords: Prebiotic, Industrial waste, Extraction, Carbohydrates, Probiotic
Introduction
Consumers demand for food products which promote health and wellbeing is on the increase. Advances in nutritional research and developments in food technologies led to the formulation of novel food ingredients which in many ways reduced the risk towards chronic diseases. In the same context, health benefits derived from the consumption of prebiotics and probiotics have made these products in greater demand compared to that of conventional foods (Ares et al. 2009).
Prebiotics are selective non-digestible food substances which remain unchanged as they pass through the upper part of the gastrointestinal tract. They stimulate the growth and concurrently promote the activities of probiotics colonizing the colon (Wang 2009; Al-Sheraji et al. 2013). Prebiotics which have been used in the human diet are lactose, galacto-oligosaccharides, fructooligosaccharides, inulin and its hydrolysates, malto-oligosaccharides and resistant starch polysaccharides of plant origin such as cereals, sweet potatoes, chicory roots and soybean have gained attention as novel source of prebiotics (Al-Sheraji et al. 2013). Highly soluble non-digestible polysaccharides have been reported to confer a number of beneficial properties by increasing the population of probiotics as compared to commercial prebiotics (Al-Sheraji et al. 2012; Wang et al. 2015).
PKC is an agriculture by-product produced following the extraction of oil from the fruits of palm oil (Fig. 1). Being nutritionally rich, cultivation of probiotics on these by-products could be a solution to transform the inedible wastes into a commodity of high-economic potential. Disposal of PKC is a major problem in the palm oil industry and their economic utilization will be a positive step towards solving environmental pollution (Najwa et al. 2016). Malaysia, as a major palm oil-producing country, produces to the tune of 2.40 million metric tons of PKC in 2012 (Nuzul Amri 2013). Although an economic use of PKC has found its way into the production of animal feeds, particularly for large ruminants, a major portion of the by-product is still left unutilized.
Fig. 1.
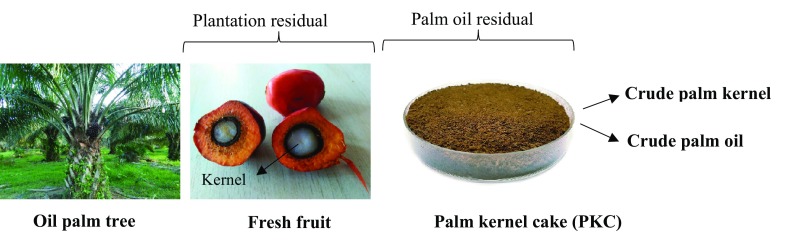
Oil palm tree, palm fruit and palm kernel cake
High consumers’ preference for natural products or ingredients had prompted the search for prebiotics from other natural sources which are economically viable and more importantly affordable from the consumers’ perspective. Therefore, this research focuses on (1) extraction of PKC’s non-digestible soluble polysaccharides using different extractant (water, citric acid and NaOH) (2) analysis of chemical compositions and functional properties of the polysaccharides (3) evaluation of PKC’s polysaccharide digestibility using artificial human gastric juice and (4) proliferation rate and acid production by two probiotic strains, Lactobacillus plantarum ATCC 8014 and Lb. rhamnosus ATCC 53103 when cultured on to these polysaccharides.
Materials and methods
Samples and supplies
PKC powder was obtained from a local palm oil-processing plant in Serdang, Selangor, Malaysia. Fructooligosaccharides (FOS, purity ≥ 90%, polymerization degree < 10), trifluoroacetic acid, bradford reagent, phenol, peptone water and α-amylase from human salivary (Type IX-A) were purchased from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). de Man, Rogosa and Sharpe (MRS), Luria–Bertani (LB) and anaerobic gas pack, glucose, galactose, mannose, arabinose, rhamnose and xylose were sourced from Merck (Darmstadt, Germany). Ethanol, petroleum ether, sulfuric acid, citric acid, sodium hydroxide, 3,5-dinitrosalicylic acid (DNS) and all other chemicals used in this study were of analytical grade.
Bacterial strains
Two lactic acid bacteria (LAB) strains, Lb. plantarum ATCC 8014 and Lb. rhamnosus ATCC 53103 were used in this study. The two LAB strains in MRS broth containing 10% glycerol were stored at − 80 °C until use. Prior to assays the bacteria were activated according to the procedures described by Abbasiliasi et al. (2012).
Chemical compositions of PKC
Chemical compositions of the PKC was analyzed according to the method of the Association of Official Analytical Chemists (AOAC 2005). Protein content was estimated using the Kjeldahl method (Havilah et al. 1977) with a conversion factor of 6.25. Crude fat content was determined using AOAC (2003) on an automated fat determination (AFD) system (FOSS Soxtec™ 2050, Hilleroed, Denmark) and calculated using Eq. (1):
| 1 |
where W1 is the sample (PKC) weight (g), W2 is the Extraction cup weight (g) and W3 is the Extraction cup weight (g)+ residue weight (g).
Total carbohydrate content was determined by calculating the cumulative percentage of moisture, ash, protein and fat from overall 100% using Eq. (2):
| 2 |
Extraction of soluble polysaccharides from PKC using three different solvents
To extract the crude polysaccharides from the PKC, three different solvents/extractant, namely water, citric acid and NaOH– were used. Before extraction, sample were first defatted with 90% (v/v) petroleum ether (C6H14) at the ratio of 1:5 (sample: solvent) at 60 °C for 5 h and dried at room temperature followed by treatment with 80% (v/v) ethanol (C2H6O) and re-dried. Sample was kept in room temperature till used.
Extraction of defatted PKC polysaccharides using hot water
Extraction was carried out according to method of Chen et al. (2008) and Azmi et al. (2012) with some modifications. Briefly, 10 g of the defatted sample was extracted using 200 mL of hot distilled water (1:20w/v) at 80 °C for 1 h and the supernatant filtered through a four-layer cheese cloth. This procedure was repeated twice to remove any remaining polysaccharides. All extracts were mixed and the mixture was then concentrated in a rotary evaporator (at 175 MPa and 60 °C) to 1/5 of its original volume. The mixture was centrifuged at 12,857 g for 15 min at 4 °C, filtered using Whatmann No.1 filter paper and dialyzed against distilled water using dialysis tube (Cut off MWCO- 12-14000 Daltons) for 48 h. This is followed by adding four times the original volume of 95% (v/v) ethanol to precipitate the polysaccharides. The mixtures were stirred for 15 min and stored at 4 °C for 4 h. The water-soluble polysaccharides was then centrifuged at 12,857 g for 10 min at 4 °C and the pellet washed twice with distilled water and oven-dried at 50 °C overnight. The water-soluble polysaccharides obtained were stored at room temperature for further analysis.
Extraction of defatted PKC polysaccharides using citric acid
Extraction was carried out as described by Gannasin et al. (2015) with some modifications. 10 g of defatted samples was extracted with 200 mL of 1% of citric acid (C6H8O7) at pH 2.3 at 60 °C for 1 h (the ratio of sample to citric acid was 1:20 (w/v). The mixture was then centrifuged at 12,857 g for 10 min at 4 °C and filtered through a four-layer cheese cloth. The procedure was repeated twice. The mixed extracts were concentrated using a rotary evaporator (at 175 MPa and 60 °C), filtered and dialyzed using a dialysis tube (Cut off MWCO- 12-14000 Daltons) against distilled water at 4 °C for 48 h. The samples were precipitated with 95% ethanol (ratio of sample to ethanol: 1:4) and allowed to stand for 4 h at 4 °C. The water soluble polysaccharides were then centrifuged at 12,857 g for 10 min at 4 °C and the pellet washed twice with distilled water and oven-dried at 50 °C overnight. The water-soluble polysaccharides obtained were stored at room temperature until analysed.
Extraction of defatted PKC polysaccharides using NaOH
Extraction was carried out using the same protocol as that of water extraction. 2% NaOH was used as the extracting solvent and the mixtures were concentrated at low temperature of 40 °C.
Percentage yield of soluble polysaccharides were calculated using Eq. (3):
| 3 |
where W1 is the weight of raw sample and W2 is the weight of polysaccharide after extraction.
Determination of total carbohydrate and protein contents and solubility rate of PKC crude polysaccharides
Determination of total carbohydrate content of PKC crude soluble polysaccharides
Total carbohydrate content of PKC crude polysaccharides, an indication of raw polysaccharides was determined using phenol sulfuric acid method as described by Dubois et al. (1956). Two millilitres of the sample solution (0.25 mg of sample/mL of water) was mixed with 1 mL of 5% (v/v) phenol and topped up with 5 mL of concentrated sulfuric acid (98%). The mixture was vortexed and incubated for 30 min at 25 °C and absorbance was read at 490 nm using UV–VIS spectrophotometer (Hitachi U 2810, Tokyo, Japan). Total carbohydrate of polysaccharides was calculated by referring to a glucose standard curve in mg/mL.
Determination of protein content of PKC crude soluble polysaccharides
Bradford reagent (Sigma-Aldrich, St. Louis, MO, USA) with Bovine serum albumin (Sigma-Aldrich, St. Louis, MO, USA) as a standard protein was used to determine total protein concentration (Bradford 1976). A total of 20 µL of the sample was added to 200 µL of Bradford reagent in a microtiter plate and incubated at 37 °C for 10 min. Absorbance was measured at 590 nm.
Determination of solubility rate of PKC crude polysaccharides
The solubility rate of PKC crude polysaccharides was determined as described by Azmi et al. (2012). 5% (w/v) crude polysaccharide solution was dissolved in distilled water and incubated at 100 °C for 5 min. The mixture was then cooled and filtered through a pre-weighed ashless filter paper (Wathman No. 1). The latter was then transferred into a previously weighed crucible and dried in an oven overnight at 105 °C. The rate of solubility of polysaccharides was calculated based on the difference in the weight of the ashless filter paper before and after drying. Solubility rate percentage was computed using Eq. (4):
| 4 |
where W1 is the weight of filter paper before drying and W2 is the weight of filter paper after drying.
Monosaccharide composition of PKC crude soluble polysaccharides
Monosaccharide composition of crude polysaccharides was determined according to the method described by Qiao et al. (2010) with minor modifications. Briefly, 5 mg of crude polysaccharides was dissolved in 4 mL of 2M trifluoroacetic acid (TFA); the mixture was hydrolyzed at 120 °C for 2 h followed by concentrating it to about 1/5 of its original volume by a rotary evaporator (at 175 MPa and 60 °C). The hydrolyzed products were then derivatized by adding 10 mg of hydroxyl ammonium chloride (HONH2·HCl), 5 mg of inositol (C6H12O6) (Sigma-Aldrich, St. Louis, MO, USA) as internal reference and 0.6 mL of pyridine (C5H5N) (Sigma-Aldrich, St. Louis, MO, USA). The mixture was incubated in a water bath at 90 °C for 30 min. Following cooling to room temperature, 1 mL of acetic anhydride (C4H6O3) (Sigma-Aldrich, St. Louis, MO, USA) was added, and re-incubated at 90 °C for 30 min.
The mixture was analyzed in GC (GC-6890N, Agilent, California, US) with a flame ionization detector and a HP-5 capillary column (30m × 0.32mm × 0.25 m) (Agilent, California, US). The operational conditions of the GC were as follows: flow rate of N2, H2 and air was 25 (mL/min), 30 (mL/min) and 400 (mL/min), respectively. The temperatures of detector and inlet were set at 270 °C and 240 °C, respectively and the oven temperature program was set from 120 °C (standing for 3 min) up to 200 °C (standing for 4 min) at a rate of 3 °C/min. per change. Standard monosaccharides such as mannose, glucose, galactose, rhamnose, arabinose and xylose were also derivatized and analyzed as references.
Determination of functional groups and glycosidic linkages of PKC crude soluble polysaccharide
Fourier transform infrared (FTIR) spectrophotometer (Nicolet, USA) was used to determine the structural characteristic of PKC crude soluble polysaccharides of different solvent extracts. Analysis was carried out in the spectral region of 4000–280 cm−1. β-glucan from Berley (Sigma-Aldrich, St. Louis, MO, USA) was used as the standard reference.
Determination of the thermal properties of PKC crude soluble polysaccharides
Thermal properties of soluble PKC crude polysaccharide of each solvent extract was determined using differential scanning calorimeter (DSC) (Mettler Toledo Star System, Columbus, USA) based on the method described by Jia et al. (2015). Briefly, 5 to 6 mg of samples was placed into an aluminum pans and hermetically sealed and allowed to stand for 1 h at room temperature. This was followed by heating the sample at a temperature range of 0–350 °C under nitrogen atmosphere at a heating rate of 10 °C per min. The parameters of onset (To), peak (Tp), end set (Te) and enthalpy change (ΔH) were recorded.
Determination of prebiotic potential of PKC crude soluble polysaccharides
Effect of artificial human gastric juice on hydrolysis of PKC crude soluble polysaccharides
The effect of artificial human gastric juice on hydrolysis of PKC crude soluble polysaccharides were determined by calculating the degree of hydrolysis of the samples when subjected to artificial human gastric juice according to Wichienchot et al. (2010). Artificial human gastric juice was prepared as follows: 8.25 g of Na2HPO4·H2O, 14.35 g of Na2HPO4, 8 g of NaCl, 0.2 g of KCl, 0.1 g of CaCl2·2 H2O and 0.18 g of MgCl2·6 H2O were dissolved in distilled water to make a 1,000 mL solution. The solution was graded/adjusted to five different pH (1, 2, 3, 4 and 5) using 5M hydrochloric acid (HCl). Fructooligosaccharides (FOS) was used as positive control. 0.05 gm of FOS and soluble crude polysaccharides of water extract (SCPW) and citric acid extract (SCPCA) were dissolved with 5 mL solution of pH 1, 2, 3, 4 and 5 to make their final concentration to 1.0% (w/v). This was followed by incubation of the samples at 37(± 1 °C) for 6 h. The reducing sugar and total sugar of the mixtures were measured at 0, 1, 2, 4 and 6 h of incubation. Hydrolysis percentage of samples were calculated based on the Eq. (5):
| 5 |
where reducing sugar released is the difference between its final and initial content.
Effect of α-amylase on hydrolysis of PKC crude soluble polysaccharides
The effect of α-amylase on hydrolysis of PKC crude soluble polysaccharides was determined by using 20 mM of sodium phosphate buffer (NaPO4) which was adjusted to four different pH of 5, 6, 7 and 8. Control prebiotic (FOS), SCPW and SCPCA were dissolved in 20 mM of sodium phosphate buffer to make a concentration of 1% (w/v). Non-digestibility activities of the samples were determined by α-amylase according to the method of Al-Sheraji et al. (2012). Briefly, 2 unit/mL of the 5 mL enzyme solution was prepared in 6.7 mM NaCl solution and topped up with 5 mL of the each dissolved samples. The mixture was incubated at 37 ± 1 °C for 6 h. 2 mL of sample from each pH was tested at 0, 1, 2, 4 and 6 h to determine the reducing and total sugar content of the mixtures. Percentage hydrolysis of the samples was calculated using Eq. (5) above.
Proliferation and acidifying activity of probiotics on PKC crude soluble polysaccharides in vitro
Two LAB strains, Lb. plantarum ATCC 8014 and Lb. rhamnosus ATCC 53103 were used as probiotics to investigate the proliferative effect of the polysaccharides. Carbohydrate-free MRS supplemented with 0.05% (m/v) L-cysteine was used as basal culture medium (Wang et al. 2015). FOS was used as positive control while the basal medium devoid of any carbon source was used as blank. 0.1, 0.07, and 0.05 g of FOS and the crude soluble extracted polysaccharides were each dissolved in 10 mL of the basal medium to give a final concentration of 0.5, 0.7 and 1.0% (w/v) and sterilized by autoclaving at 121 °C for 15 min. Each tube was then inoculated with 1 × 106 CFU/mL of LAB strains and incubated at 37 °C for 48 h anaerobically. Bacterial count and pH of the medium were determined after 48 h using plate counting method and pH meter (Mettler Toledo, USA), respectively.
Enumeration process was performed by serial dilution of 1 mL of the culture to 9 mL of buffered Andrade peptone water (Bio-Chemika, India). 100 µL of the diluted sample was spread on the plate of MRS agar followed by incubation at 37 °C for 24 h under anaerobic conditions. Bacteria counts were expressed in colony-forming units per milliliter (CFU/mL). The increase of bacterial numbers between 0, 24 and 48 h was calculated according to the Eq. (6).
| 6 |
where A is the bacterial number at 0 h (CFU/mL) and B is the bacterial number after incubation for 48 h (CFU/mL).
Statistical analysis
Each experiment was repeated thrice and the results were reported as ± standard deviation (SD) of triplicate independent extractions. Data obtained were analyzed using Minitab software (version 16.0, Minitab Inc., State Collage, Pennsylvania, USA). Results were analyzed by analysis of variance (ANOVA) and Tukey’s HSD significant test. All statistics were based on a confidence level of 95% and P < 0.05 were considered statistically significant.
Results and discussion
Chemical compositions of PKC
The chemical compositions of PKC are as shown in Table 1. The percentage of ash in PKC (5.2%) which is an indication of mineral content was within the range of the fruits and vegetables as reported by Hussain et al. (2013). However, the ash content in PKC reported by Nuzul Amri (2013) and Alimon et al. (2004) was less than 3.5% (< 3.5%) while that reported by Dairo and Fasuyi (2007) was higher (8.6%). The moisture content of PKC in this study was 7.4% which is higher compared to a previous study by Nuzul Amri (2013). The percentage of moisture depends on the water-holding capacity, water retention and swelling capacity (Najwa et al. 2016). The protein content was 16.5% which was within the range of that of fruits and vegetables (2.70–24.9%). Although the major component of PKC is carbohydrate (65.8%), the amount of carbohydrate in PKC in this study was found to be lower compared to that of fruits and vegetables. Most fruits showed higher carbohydrate content (> 72.3%) compared to vegetables with the exception of apples which contained only 25.8% carbohydrate (Najwa et al. 2016). The crude fat of PKC is 5.1% which was within the range of fruits and vegetables (0.5–10.9%) (Grigelmo-Miguel and Martin-Belloso 1998). Overall these observed variations in composition values might have resulted from geographic, climatic and seasonal variations.
Table 1.
Chemical compositions of PKC
| No. | Parameters | Percentage (%) |
|---|---|---|
| 1 | Carbohydrate | 65.8 ± 0.43 |
| 2 | Protein | 16.5 ± 0.11 |
| 3 | Fat | 5.1 ± 0.05 |
| 4 | Moisture | 7.4 ± 0.42 |
| 5 | Ash | 5.2 ± 0.08 |
Data are presented as means ± SD from triplicate data
Effect of solvents on the yield of PKC soluble crude polysaccharides
Defatting of samples prior to polysaccharides extraction with a polar and non-polar solvents could help the release of polysaccharides from the plants cell wall (Azmi et al. 2012; Ballesteros et al. 2015). Effect of solvents on the yield of PKC crude soluble polysaccharides are as shown in Table 2. Highest PKC crude soluble polysaccharides was obtained by NaOH extraction (8.73%), followed by citric acid (3.07%) and hot water (3.0%) extraction.
Table 2.
Effects of solvent on the yield percentage of crude soluble polysaccharide of PKC
| Types of solvents | pH | PKC soluble crude polysaccharide yield (%) |
|---|---|---|
| Hot water | 7 | 3.07 ± 0.20b |
| 1% Citric acid | 2.5 | 3.03 ± 0.31b |
| 1% NaOH | 12 | 8.73 ± 0.40a |
Data were expressed as means ± SD of triplicate determination
Means within each column with different small letter superscripts are significantly different (P < 0.05)
The pH of the extractant could significantly influence the extraction yield and activity of polysaccharides as reported by Gan and Latiff, (2010). The extraction yield of crude polysaccharides in this study increased with increased pH (from acidic to alkaline). This is relevant to the structure of polysaccharide and the isoelectric point of protein (Tan et al. 2011). Due to the solubility of sugar in water or other organic solvents, solvent plays an important role in the extraction process and should be chosen in view of the organic compound of interest. Furthermore, the efficiency of extraction is dependent on many factors which include solid/liquid ratio, solvent, temperature, extraction time and variety of palms used (Ballesteros et al. 2015). Such parameters could be optimized which, however, is not the objective of the present study.
Determination of total carbohydrate and protein contents and solubility rate of PKC crude polysaccharides
Total carbohydrate and protein contents and solubility rate of PKC crude polysaccharides are as shown in Table 3. Highest percentage of total carbohydrate content of PKC soluble polysaccharides were 57.11% for SPW followed by 56.94% for SPCA and 50.95% for SPN. There was significant difference (P < 0.05) between total carbohydrate content of SCPN and each of SCPW and SCPCA. Thetsrimuang et al. (2011) reported that highest yield of crude polysaccharides is related with the lowest content of total carbohydrate which concur with the values obtained in this study.
Table 3.
Percentage of total carbohydrate and protein contents and solubility rate of PKC crude soluble polysaccharides
| Soluble polysaccharide of PKC | Chemical parameters | ||
|---|---|---|---|
| Total carbohydrates (%) | Protein (%) |
Solubility rate (%) |
|
| SPW | 57.11 ± 0.18a | 0.72 ± 0.01a | 97.66 ± 0.08b |
| SPCA | 56.94 ± 0.03a | 0.40 ± 0.04c | 99.91 ± 0.04a |
| SPN | 50. 95 ± 0.37b | 0.58 ± 0.03b | 96.74 ± 0.15b |
Data are presented as means ± SD from triplicate determination
Means with small letters superscript among solvent extract down the column are significantly different from each other (P < 0.05)
Protein content in all extracts of PKC crude polysaccharides are low (< 1). However, there are significant differences (P < 0.05) between the protein content of the each three extracts of SP with each other. The highest protein content was 0.72% in SPW followed by 0.58% in SCPN and 0.40% in SCPCA. Azmi et al.(2012) reported that low percentage of total protein content in the polysaccharides indicated proteins have been denatured in the soluble polysaccharides during extraction by heating at 60 °C. However, the effect of extraction duration, number of extraction step, ratio of sample to extractant and centrifugal speed on protein yield should be considered (Kain et al. 2009).
The soluble polysaccharides from PKC in this study were found to be highly soluble (> 95%). However, highest solubility rate was observed in SCPCA and there is no significant difference (P > 0.05) between the solubility rate of SCPW and SCPN. The solubility rate of the polysaccharides extracted from plant sources is greatly influenced by the galactose ratio, since galactose chains helps in extending the macromolecule of the mannan and allow water into the space which limit the insolubility nature of polysaccharides (Aspinal 1970; McCleary 1988; Kusakabe et al. 1990). Polysaccharides contain many hydroxyl groups, easily form hydrogen bonds and easily soluble in water (Yanhua et al. 2014). It was reported that inter- and intra-molecular hydrogen bond could affect to the solubility rates of polysaccharide in water (Huang and Zhang 2009).
Monosaccharide composition of PKC crude soluble polysaccharides
Table 4 shows the monosaccharide composition of PKC soluble crude polysaccharides (PKCSCP) which comprised of six monosaccharides - mannose, galactose, glucose, arabinose, rhamnose and xylose—with different concentration fractions. The monosaccharide’s composition clearly shows that mannose is the predominant sugar in the SCP.
Table 4.
Monosaccharide composition of PKC soluble crude polysaccharides
| Monosaccharide compositions | Percentage degree of soluble crude polysaccharide from different solvents | ||
|---|---|---|---|
| SPW (%) |
SPCA (%) |
SPN (%) |
|
| Mannose | 45.05 | 62.49 | 38.86 |
| Galactose | 9.46 | 25.42 | 15.53 |
| Glucose | 11.19 | 9.51 | 12.96 |
| Arabinose | 5.68 | 0.21 | 0.52 |
| Rhamnose | 0.96 | 1.43 | 1.7 |
| Xylose | 2.48 | 0.93 | 0.96 |
| Total | 74.83 | 100 | 70.52 |
PKC extracted using 1: water (SPW), 2: citric acid (SPCA) and 3: NaOH (SPN)
It was reported that polysaccharides with high content of mannose (52–81.9%) and galactose (14.5–39.2%) is a galactomannan in nature (Cerqueira et al. 2011). The mannose (62.49) and glucose (25.42) content of SCPCA in this study is in agreement with the galactomannan form Gleditsia triacanthos and Adenanthera pavoria reported by Cerqueira et al. (2011).
Jahromi et al. (2016) reported that the major monosaccharides of PKC are mannose. Furthermore, the study of Mohd-Jaffar and Jarvis (1992) revealed that the carbohydrate in the cell wall components of PKC contained mannan, cellulose and xylan. It was reported that PKC also contains glucose and galactose and xylose with different concentration which is dependent on many factors. Previous study showed that the yield and composition of soluble polysaccharides are strongly dependent on the extraction conditions such as temperature and time (Graham et al. 1988). For several polysaccharides there is generally no sharp distinction between soluble (or extractable) and insoluble fractions. The ratio between the two is dependent on conditions used (e.g., physical treatment, enzymatic treatment, temperature and time) in the solubilisation procedure.
Previous studies on PKC polysaccharides have been reported to contain a high percentage of mannose (35 to 56%) but low percentage of galactose (ranging between 12 to 20%) which has been claimed to be galactomannan (Dusterhoft et al. 1991; Knudsen 1997). Galactomannans are polysaccharides which comprises of 1-4-linked β-D-mannosyl residue with an α-D-galactose side chain (Santos et al. 2015) which are incorporated into numerous products in the food and pharmaceutical industries.
Determination of functional groups and glycosidic linkages of PKC crude soluble polysaccharides
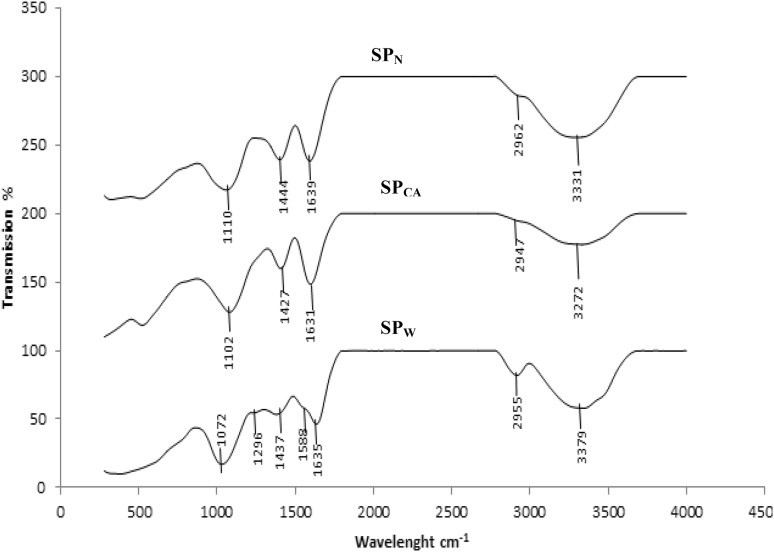
To elucidate the structure of crude polysaccharides in the PKC, FTIR analysis was performed and the results are as shown in Fig. 2. Generally in FTIR spectra, broad peaks in the range of 950–1200 cm−1 indicate the presence of polysaccharides as a major component in a given extract (Xu et al. 2009). The wave length values within this range allows the identification of major chemical groups in polysaccharides which revealed the position and intensity of the bands that were specific for each polysaccharide (Synytsya et al. 2009).
Fig. 2.
FTIR spectra of soluble polysaccharides from PKC extracted using 1: water (SCPW), 2: citric acid (SCPCA) and 3: NaOH (SCPN)
There were similarities in some of the absorption pattern of the three different polysaccharides of different solvent extract. Strong absorption was observed at 3279.62, 3272.35 and 3331.21 cm−1 corresponding to the hydroxyl vibration modes of O–H stretching band which showed the existence of intra- or and inter- molecular interactions between the polysaccharide chains as reported by Jia et al. (2015). The band in the region of 2918.08 cm−1 in SCPW was attributed to C–H stretching vibration which was regarded as characteristic absorption of polysaccharides. Absorption at 1635.08, 1631 and 1639.09 cm−1 spectrums in all samples under studied was due to the stretching vibration of carbonyl group. The weak band near 1588.86 cm−1 in SPW indicated the bending vibration of N–H group of amide II assigned for the presence of protein. Amide II band which is due to bending vibrations of N–H groups and is used for estimation of protein content which is weak in PKC crude polysaccharides. Therefore, the low-protein content in PKC crude polysaccharides confirmed the above mentioned finding.
The bands in the region of 1464, 1427 and 1444 cm−1 is attributed to COO-symmetric stretch of carboxyl group as reported by Gannasin et al. (2015). The bands at 1072, 1128 and 1110 cm−1 were due to the mannopyranose ring which showed the presence of mannose (Capek et al. 2000). The weak band at 932, 873 and 883 cm−1 in all samples corresponded to the β-glycosidc linkages as described by Chen et al. (2008) and Synytsya et al. (2009). The absorption bands near 1385, 1047, 1026 and 883 cm−1 showed the presence of β-glycosidc bonds while the bands around 1078 and 843 were assigned to α-glycosidic bonds (Capek et al. 2000). The bands around 1200–1000 cm−1 is due to C–OH bonds which is an indication of the presence of oligosaccharides such as mannose and galactose (Baseri and Baker 2011).
Determination of the thermal properties of PKC crude soluble polysaccharides
Structural and functional group differences in polysaccharide influence the thermal behavior and affect the transition temperature (Bothara and Singh 2012). The thermal properties of a given extracted polysaccharides compound provide a clue about the thermal stability of the compound as function of temperature and time which is an important factor for its various applications in foods and pharmaceutical industries (Iqbal et al. 2011). Thermal characteristic of given polysaccharide depends on the mannose to galactose ratio of polysaccharides since high mannose or low galactose contents could increase enthalpy values (Cerqueira et al. 2011; Jia et al. 2015).
Differential scanning calorimetry (DSC) was used to measure the occurrence of exothermal or endothermal changes in soluble crude polysaccharides of PKC with an increase in temperature (Table 5). Polysaccharides of PKC obtained from different extraction methods show different onset temperature or initial endothermic phase. The initial endothermic phase was related to the presence of impurities in the sample and the vaporization of water (indicating the presence of hydrophilic groups), which occurs over a range of temperature. Similarly, endothermic peaks with differences in the enthalpy changes observed for these three extracts of PKC. The endothermic peak recorded for soluble polysaccharide extracted from PKC for SCPW, SCPCA and SCPN were 122, 121 and 125 °C with enthalpy values at 81, 90 and 147 J/g, respectively. The endothermic peak could be attributed to the disruption of hydrogen bonded network of water and polymer chains in polysaccharides as reported by Bothara and Singh, (2012). SCPN has the highest enthalpy values (147.44J/g). The high enthalpy value could be associated with the samples crystalline nature, high mannose contents and low galactose contents as reported by Cerqueri et al. (2011).
Table 5.
Thermal properties of PKC soluble crude polysaccharides
| Samples | Onset temperature (oC) |
End temperature (oC) |
Peaks (oC) |
Enthalpy change (J/g) |
|---|---|---|---|---|
| SCPW | 97.80 | 157.16 | 122.57 | 81.42 |
| SCPCA | 105.84 | 139.64 | 121.35 | 90.81 |
| SCPN | 98.68 | 147.44 | 125.23 | 147.44 |
Determination of prebiotic potential of PKC crude soluble polysaccharides
Effect of artificial human gastric juice on hydrolysis of SCPW and SCPCA
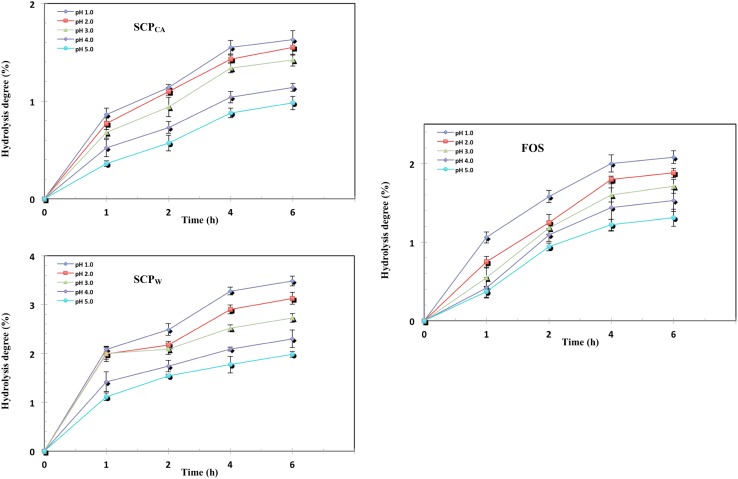
One of the criteria for determining prebiotic potential of a given polysaccharide is its ability to resist digestion by the digestive enzymes in an acidic environment. It is expected that, the potential prebiotic would be able to reach the intestine and undergo fermentation by the colonic probiotics (Gibson et al. 2004). Foods are usually retained in the stomach for about 2 h at pH 2–4, within the gastric juice environment before reaching the intestine (Wichienchot et al. 2010). The degree of hydrolysis (non-digestibility) of PCK crude polysaccharides as a function of time were carried out after incubation with artificial human gastric juice and the results are as shown in Fig. 3.
Fig. 3.
Effect of artificial human gastric juice on hydrolysis of: a SCPCA; b SCPW compared with c FOS as control
The degree of hydrolysis was found to decrease with increased pH (from1 to 5) of the artificial human gastric juice. The reason for this was probably due to the glycosidic bond being more easily ruptured at low pH which resulted in partial hydrolysis of the polysaccharides. For instance, after 4–6 h of incubation at pH 1, the percentage of hydrolysis for FOS, SCPW and SCPCA were 2.07, 3.46 and 1.59%, respectively. Among these three tested samples, SCPCA has the highest resistance (98%) to gastric juice. Different factors such as monosaccharide compositions, rings and linkages might affect the degree of hydrolysis of polysaccharides when subjected to human gastric juice as reported by Wang et al. (2015). SCPCA has higher amount of mannan-oligosaccharides (MOS) which made it more resistant to enzyme digestion compared to the other two SCP.
Incubation time also affected the degree of hydrolysis as a longer incubation duration was conducive for more polysaccharides to be degraded to mono- and di- saccharides in acidic conditions (Wang et al. 2015). Hydrolysis of the polysaccharides increased with increase in incubation time from 1 to 4 h, and remained constants from 4 to 6 h. This shows that after 4 h of incubation, carbohydrate digestion by the digestive enzymes had ceased.
The high degree of hydrolysis at pH 1 and 2 with increase in incubation time in all tested sample could be due to the glycosidic bonding being broken down more easily to mono- and di- saccharides at lower pH as reported by Wang et al. (2015). This could be attributed to the structural linkages of SCP being composed of β-(1–4)-D-mannopyranose with (1–6)-α-D-galactosepyranose side chain as confirmed by the FTIR.
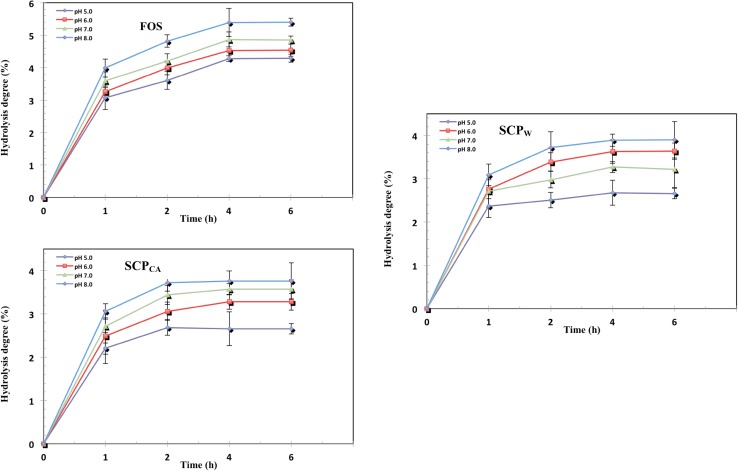
Effect of α-amylase on hydrolysis of SCPW and SCPCA
Degree of hydrolysis of SCPW and SCPCA determined as function of time after incubation with α-amylase at pH 5, 6, 7 and 8 for 1, 2, 4, and 6 h at 37 °C as seen in Fig. 4. The degree of hydrolysis increased with increase in pH from 5 to 8, indicating that the hydrolysis of SCPW and SCPCA were affected by pH. At pH 5, 6, 7 and 8 after 4 h of incubation, the degree of hydrolysis for SCPW were 1.89, 2.09, 2.46 and 2.78% and that of SCPCA were 1.68, 2.05, 2.26 and 2.68%, respectively. FOS has the highest degree (5.28%) of hydrolysis as compared to SCPW and SCPCA after 4 h of incubation. SCPW and SCPCA have higher enzymatic resistances (~ 97.5%) compared to FOS (~ 95%).
Fig. 4.
Effect of α-amylase on hydrolysis of SCPW and SCPCA in comparison with FOS
Food ingredient can be considered a prebiotic, it must not be hydrolyzed in the upper part of the gastrointestinal tract (Du et al. 2011). The degree of hydrolysis increased with increase in incubation time from 1 to 4 h and remained stable or nearly stable at 4 to 6 h. The higher resistance of SCPW, SCPCA to α-amylase compared to FOS could be due to their structural features which being linked together by β-glycosidic linkages similar to that of the well-known prebiotics FOS which is linked by β- 1,2-glycosidic linkages and are non-digestible by mammalian enzymes (Mussatto and Manchilha 2007).
Proliferation and acidifying activity of probiotics on SCPW and SCPCA in vitro
Results on the proliferation of probiotics on SCPW and SCPCA are presented in Table 6. The two tested LAB strains- Lb. plantarum ATCC 8014 and Lb. rhamnosus ATCC 53103 could utilize the tested polysaccharides as the proliferation of these two microorganisms in all conditions studied were higher compared to that of control. The proliferation of the two tested LAB strains increased with increased concentrations of SCP from 0 to 0.5%. The proliferation of the probiotics could be due to the solubility rate of the polysaccharides since good water soluble carbohydrates could be utilized readily, rapidly and completely by probiotics (Montagne et al. 2003). Moreover it has been reported that the monomeric compositions, polymerization degree and type of glycosidic linkages could affect the growth of probiotics (Hernandez–Hernandez et al. 2012). Molecular weight of polysaccharides is another important factor that determines the susceptibility of polysaccharides by probiotics as low-molecular weight polysaccharides and oligosaccharides were reported to be more readily absorbed by probiotics (Wichienchot et al. 2010; Wang et al. 2015).
Table 6.
Proliferation of probiotics (Lb. plantarum ATCC 8014 and Lb. rhamnosus ATCC 53103) on SCPW and SCPCA in vitro
| Prebiotics | Concentration (%) |
Lactobacillus plantarum
ATCC 8014 (CFU/mL) |
Lactobacillus rhamnosus
ATCC 53103 (CFU/mL) |
|---|---|---|---|
| Carbohydrate-free MRS (Blank control) |
0.0 | 1.01 ± 0.07Ae | 0.99 ± 0.12ABe |
| Carbohydrate-free MRS + FOS (Positive control) |
0.3 | 1.75 ± 0.21Cde | 1.66 ± 0.013CBc |
| 0.5 | 2.48 ± 0.09Ac | 2.32 ± 0.01ABcd | |
| 0.7 | 2.51 ± 0.05Ac | 2.50 ± 0.02Ac | |
| Carbohydrate-free MRS + SCPW | 0.3 | 1.96 ± 0.06Ccd | 1.77 ± 0.12Dde |
| 0.5 | 3.01 ± 0.07Abc | 2.28 ± 0.30BCcd | |
| 0.7 | 3.12 ± 0.09Ab | 2.48 ± 0.28Bc | |
| Carbohydrate-free MRS + SCPCA | 0.3 | 2.01 ± 0.14Dd | 1.82 ± 0.11DEd |
| 0.5 | 3.04 ± 0.09Bbc | 2.80 ± 0.07BCab | |
| 0.7 | 3.29 ± 0.12Aa | 2.92 ± 0.04BCa |
Data are presented as means ± SD from triplicate determination
Means with small letter superscript indicates significant differences down the column
Means with capital letter superscript indicates significant differences within the rows of each tested polysaccharides (P < 0.05)
Extracted soluble crude polysacharides using water (SCPW) and citric acid (SCPCA)
Proliferation of Lb. plantarum ATCC 8014 on SCPW and SCPCA enhanced by increased bacterial population from 1.01 to 3.12 and 3.29 CFU/mL from 0 to 0.7% concentration. There was no significant differences (P > 0.05) between the proliferation effect of SCPW and SCPCA on Lb. plantarum ATCC 8014 at 0.5 and 0.7% concentration. There was no significant difference (P > 0.05) between the bacteria count of Lb. rhamnosus in media supplemented with FOS (2.50 CFU/mL) and SCPW (2.48 CFU/mL) after 48 h of incubation. Growth in the media supplemented with SCPCA was highest among all tested prebiotics for both Lb. plantarum ATCC 8014 and Lb. rhamnosus ATCC 53103 in all concentrations. Growth of Lb. plantarum ATCC 8014 was higher than that of Lb. rhamnosus ATCC 53103 on all tested soluble polysaccharides which confirmed the report by Najwa et al. (2016) who claimed different probiotics species varies in the utilization of carbon sources.
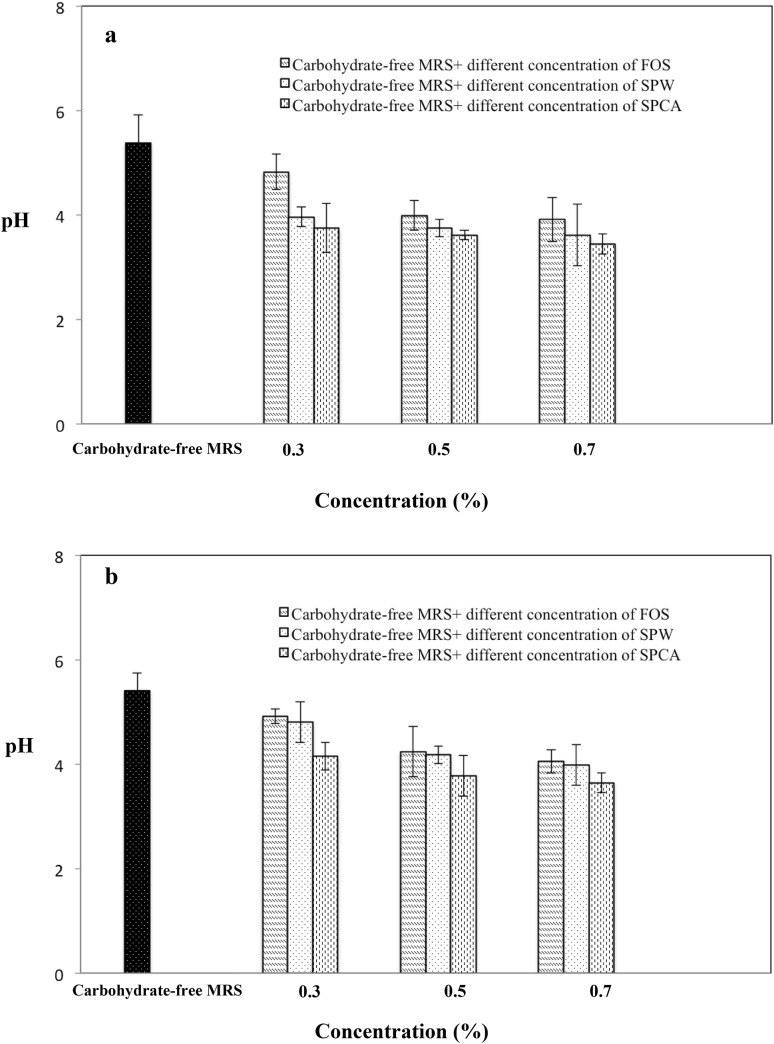
Figure 5 showed the acidifying activity of Lb. plantarum ATCC 8014 and Lb. rhamnosus ATCC 53103 cultured in carbohydrate-free media each supplemented with SCPW, SCPCA and FOS. Results showed that the pH of the media decreased to pH in the region 3 which confirmed the increased acidifying activity and growth of probiotic strains. However, there is no marked differences between the pH of the media at 0.5 and 0.7% concentration. The highest acidifying activity for both Lb. plantarum ATCC 8014 and Lb. rhamnosus ATCC 53103 were obtained with SCPCA at all three concentrations.
Fig. 5.
Effect of SCPW and SCPCA on acidifying activity of probiotics; a Lb. plantarum ATCC 8014 and b Lb. rhamnosus ATCC 53103 in vitro (after 48-h incubation at 37°C)
Results of this study indicated that type and concentration of prebiotics are important for the supporting effect of the prebiotics on the growth performance and acidifying activity of the probiotic bacterial strains. Results of this study on the supportive effect of prebiotics on the growth performance of probiotic bacterial strains are in agreement with the results of other studies (Saminathan et al. 2011; Goderska et al. 2008; Pennacchia et al. 2006). In general, as the concentration of prebiotics increases, positive effect of the prebiotics on the acidifying activity of the probiotic strains increases. Relatively higher acidifying activities were observed as the number of viable cell of the probiotic strains increased. However, results of various studies showed that ability of the probiotic bacteria to utilize prebiotics could be strain and/or substrate specific (Mumcu and Temiz 2014; Saarela et al. 2003; Najwa et al. 2016).
The reduction of pH during fermentation period is associated with the effect of the bacterial proliferation which implied that the bacteria were able to utilize the polysaccharides as their main carbon source and produce acid. These results further confirm that the tested soluble polysaccharides were metabolized by the tested probiotic bacteria to produce some short-chain fatty acids which led to the drop of pH in the media. Results from this study indicated that appropriate prebiotics should be selected for each probiotic strain for increased acidifying activities. It was reported that prebiotic effect of polysaccharides may be due to the structural features such as molecular weight, monosaccharide composition and chain conformation. However, the exact mechanism underlying the prebiotic effect exerted by polysaccharides is still not fully understood (He et al. 2015).
Conclusion
Results from this study revealed that soluble crude polysaccharides from PKC consist of β-glycosidic bands which made it non-digestible and resistant to artificial human gastric juice and α-amylase. Additionally, it stimulated the proliferation of the tested probiotics and increased their acidifying activity in vitro. Thus, the ability of PKC soluble crude polysaccharides to be metabolized by the two probiotics—Lb. plantarum ATCC 8014 and Lb. rhamnosus ATCC 53103—support its potential prebiotic activity. However, the ability of probiotics to utilize certain carbohydrates in vitro provide some indication of this ability by the probiotic strain under given conditions. An appropriate prebiotic substance should be selected for each probiotic bacterial strain for its viability and good growth and acidifying performance before the production of functional foods containing a combination of prebiotics and probiotics. In spite of all the above findings, the potential of soluble non-digestible polysaccharide extracted from PKC as a prebiotic is heavily dependent on in vivo studies.
Acknowledgements
The authors wish to specifically thank to Prof. Dr. Abdul Rahman Omar and Mrs. Abby Saleh, Laboratory of Vaccine and Therapeutics, Institute of Bioscience, University Putra Malaysia.
Funding
This study was financially supported under the Research University Grant Scheme (RUGS) (Project No: 05-02-12-2141RU) from Universiti Putra Malaysia, Serdang, Selangor.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no competing interests.
References
- Abbasiliasi S, Tan JS, Ibrahim TAT, Ramanan RN, Vakhshiteh F, Mustafa S, Ariff AB. Isolation of Pediococcus acidilactici Kp10 with ability to secrete bacteriocin-like inhibitory substance from milk products for applications in food industry. BMC Microbiol. 2012;12:260. doi: 10.1186/1471-2180-12-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alimon AR. The nutritive value of palm kernel cake for animal feed. Palm Oil Dev. 2004;40:12–16. [Google Scholar]
- Al-Sheraji SH, Ismail A, Yazid M, Mustafa S. Prebiotics as functional foods: a review. J Funct Foods. 2013;5:1542–1553. doi: 10.1016/j.jff.2013.08.009. [DOI] [Google Scholar]
- Al-Sheraji SH, Ismaila A, Manap MY, Mustafa S, Yusofa RM, Hassan FA. Fermentation and non-digestibility of Mangifera pajang fibrous pulp and its polysaccharides. J Funct Foods. 2012;4:933–940. doi: 10.1016/j.jff.2012.07.001. [DOI] [Google Scholar]
- AOAC (2003) Official methods of analysis vol 18th ed. AOAC, Washington
- AOAC (2005) Official methods of analysis vol 18th ed. AOAC, Washington
- Ares G, Gime ´nez A, Ga ´mbaro A. Consumer perceived healthiness and willingness to try functional milk desserts. Influence of ingredient, ingredient name and health claim. Food Qual Prefer. 2009;20:50–56. doi: 10.1016/j.foodqual.2008.07.002. [DOI] [Google Scholar]
- Aspinal GO. Polysaccharides. New York: Pergamon Press; 1970. [Google Scholar]
- Azmi AFMN, Mustafa S, Hashim DM, Manap YA. Prebiotic activity of polysaccharides extracted from Gigantochloa levis (Buluh beting) shoots. Molecules. 2012;17:1635–1651. doi: 10.3390/molecules17021635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros LF, Cerqueira MA, Teixeira JA, Mussatto SI. Characterization of polysaccharides extracted from spent coffee grounds by alkali pretreatment. Carbohyd Polym. 2015;127:347–354. doi: 10.1016/j.carbpol.2015.03.047. [DOI] [PubMed] [Google Scholar]
- Baseri MK, Baker S. Identification of cellular components of medical plants using FTIR. Roman J Biophys. 2011;21:277–284. [Google Scholar]
- Bothara SB, Singh S. Thermal studies on natural polysaccharide. J Trop Biomed. 2012;2:1031–1035. doi: 10.1016/S2221-1691(12)60356-6. [DOI] [Google Scholar]
- Capek P, Sasinkova V, Wellner N, Ebringerova A, Kac M. FT-IR study of plant cell wall model compounds: pectic. Polysacch Hemicellul. 2000;43:195–203. [Google Scholar]
- Cerqueira MA, Souza BWS, Simões J, Teixeira JA, Domingues MRM, Coimbra MA, Vicente AA. Structural and thermal characterization of galactomannans from non-conventional sources. Carbohyd Polym. 2011;83:179–185. doi: 10.1016/j.carbpol.2010.07.036. [DOI] [Google Scholar]
- Chen Y, Xie MY, Nie SP, Li C, Wang YX. Purification, composition analysis and antioxidant activity of a polysaccharide from the fruiting bodies of Ganoderma atrum. Food Chem. 2008;107:231–241. doi: 10.1016/j.foodchem.2007.08.021. [DOI] [Google Scholar]
- Dairo FAS, Fasuyi AO. Evaluation of fermented palm kernel meal and fermented copra meal proteins as substitute for soybean meal protein in laying hens diets. J Central Eur Agric. 2007;9:33–47. [Google Scholar]
- Du B, Song Y, Hu X, Liao X, Ni Y, Li Q. Oligosaccharides prepared by acid hydrolysis of polysaccharides from pumpkin (Cucurbita moschata) pulp and their prebiotic activities. Int J Food Sci Technol. 2011;46:982–987. doi: 10.1111/j.1365-2621.2011.02580.x. [DOI] [Google Scholar]
- Dubois M, Gilles KA, Hhamilton JKPAR, Smith F. Colorimetric method for determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- Dusterhoft EM, Voragen AGJ, Engels FM. Non starch polysaccharides from sunflower (Helianthus annuus) meal and palm kernel (Elaeis guineenis) meal preparation of cell wall material and extraction of polysaccharides fractions. J Sci Food Agric. 1991;55:411–422. doi: 10.1002/jsfa.2740550309. [DOI] [Google Scholar]
- Gan C-Y, Latiff AA. Optimisation of the solvent extraction of bioactive compounds from Parkia speciosa pod using response surface methodology. Food Chem. 2010;124:1277–1283. doi: 10.1016/j.foodchem.2010.07.074. [DOI] [Google Scholar]
- Gannasin SP, Adzahan NM, Hamzah MY, Mustafa S, Muhammad K. Physicochemical properties of tamarillo (Solanum betaceum Cav.) hydrocolloid fractions. Food Chem. 2015;182:292–301. doi: 10.1016/j.foodchem.2015.03.010. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Probert HM, Loo JV, Rastall RA, Roberfroid MB. Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutr Res Rev. 2004;17:259–275. doi: 10.1079/NRR200479. [DOI] [PubMed] [Google Scholar]
- Goderska K, Nowak J, Czarnecki Z. Comparison of the growth of Lactobacillus acidophilus and Bifidobacterium bifidum species in media supplemented with selected saccharides including prebiotics. ACTA Sci Pol Technol Aliment. 2008;7:5–20. [Google Scholar]
- Graham H, Gron Rydberg MB, Aman P. Extraction of soluble fiber. J Agric Food Chem. 1988;36:494–497. doi: 10.1021/jf00081a022. [DOI] [Google Scholar]
- Grigelmo-Miguel N, Martin-Belloso O. Characterization of dietary fiber from orange juice extraction. Food Res Int. 1998;35:355–361. doi: 10.1016/S0963-9969(98)00087-8. [DOI] [Google Scholar]
- Havilah ED, Morris WR, Woolnough J. A microcalorimetric method for determination of ammonia in Kjeldahl digests with a manual spectrophotometer. Lab Pract. 1977;26:545–547. [Google Scholar]
- He Z, Wang X, Li G, Zhao Y, Zhang J, Niu C, Zhang L, Zhang X, Ying D, Li S. Antioxidant activity of prebiotic ginseng polysaccharides combined with potential probiotic Lactobacillus plantarum C88. Int J Food Sci Technol. 2015;50:1673–1682. doi: 10.1111/ijfs.12824. [DOI] [Google Scholar]
- Hernandez-Hernandez O, Muthaiyan A, Moreno FJ, Montilla A, Sanz ML, Ricke SC. Effect of prebiotic carbohydrates on the growth and tolerance of Lactobacillus. Food Microbiol. 2012;30:355–361. doi: 10.1016/j.fm.2011.12.022. [DOI] [PubMed] [Google Scholar]
- Huang Z, Zhang L. Chemical structuresa of water-soluble polysaccharides from Rhizoma Panacis Japonici. Carbohyd Res. 2009;344:1136–1140. doi: 10.1016/j.carres.2009.02.014. [DOI] [PubMed] [Google Scholar]
- Hussain J, Rehman NU, Al-Harrasi A, Ali L, Khan AL, Albroumi MA. Essential oil composition and nutrient analysis of selected medicinal plants in sultanate of Oman. Asian Pac J Trop Diseases. 2013;3:421–428. doi: 10.1016/S2222-1808(13)60095-X. [DOI] [Google Scholar]
- Iqbal M, Akbar J, Saghir S, Karim A, Koschella A, Heinze T, Sher M. Thermal studies of plant carbohydrate polymer hydrogels. Carbohyd Polym. 2011;86:1775–1783. doi: 10.1016/j.carbpol.2011.07.020. [DOI] [Google Scholar]
- Jahromi MF, Liang B, Abdullah N, Meng Y. Extraction and characterization of oligosaccharides from palm kernel cake as prebiotic. J Bioresour. 2016;11:674–695. [Google Scholar]
- Jia X, Zhang C, Qiu J, Wang L, Bao J, Wang K, Zhang Y, Chen M, Wan J, Su H, Han J, He C. Purification, structural characterization and anticancer activity of the novel polysaccharides from Rhynchosia minima root. Carbohyd Polym. 2015;132:67–71. doi: 10.1016/j.carbpol.2015.05.059. [DOI] [PubMed] [Google Scholar]
- Kain RJ, Chen Z, Sonda TS, Abu-Kpawoh JC. Study on the Effect of Control Variables on the Extraction of Peanut Protein Isolates from Peanut Meal (Arachis hypogaea L.) Am J Food Technol. 2009;4:47–55. doi: 10.3923/ajft.2009.47.55. [DOI] [Google Scholar]
- Knudsen KEB. Carbohydrate and lignin contents of plant materials used in animal feeding. Anim Feed Sci Technol. 1997;67:319–338. doi: 10.1016/S0377-8401(97)00009-6. [DOI] [Google Scholar]
- Kusakabe I, Kaneko R, Tanaka N, Zamora AF, Fernandez WL, Murakami K. A simple method for elucidating structures of galactomanno-oligosaccharides by sequential actions of mannosidase and galactosidase. Agric Biol Chem. 1990;54:1081–1083. [PubMed] [Google Scholar]
- McCleary BV. Synthesis of β-D-mannopyranosides for the assay of β-Dmannosidase and exo-β-D-mannanase. Method Enzymol. 1988;160:515–518. doi: 10.1016/0076-6879(88)60161-3. [DOI] [Google Scholar]
- Mohd-Jaffar D, Jarvis M. Mannan of palm kernel. Phytochemistry. 1992;31:463–464. doi: 10.1016/0031-9422(92)90017-K. [DOI] [Google Scholar]
- Montagne L, Pluske JR, Hampson DJ. A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Anim Feed Sci Technol. 2003;108:95–117. doi: 10.1016/S0377-8401(03)00163-9. [DOI] [Google Scholar]
- Mumcu AS, Temiz A. Effects of prebiotics on growth and acidifying activity of probiotic bacteria. GIDA. 2014;39:71–77. [Google Scholar]
- Najwa NMN, Abbasiliasi S, Marika MN, Ariff A, Amid M, Lamasudin DU, Manap MY, Mustafa S. Defatted coconut residue crude polysaccharides as potential prebiotics: study of their effects on proliferation and acidifying activity of probiotics in vitro. J Food Sci Technol. 2016;54:1–10. doi: 10.1016/j.tifs.2016.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuzul Amri I. Characteristics of Malaysian palm kernel and its products. J Oil Palm Res. 2013;25:245–252. [Google Scholar]
- Pennacchia C, Vaughan EE, Villani F. Potential probiotic Lactobacillus strains from fermented sausages: further investigations on their probiotic properties. Meat Sci. 2006;73:90–101. doi: 10.1016/j.meatsci.2005.10.019. [DOI] [PubMed] [Google Scholar]
- Qiao D, Liu J, Ke C, Sun Y, Ye H, Zeng X. Structural characterization of polysaccharides from hyriopsis cumingii. Carbohyd Polym. 2010;82:1184–1190. doi: 10.1016/j.carbpol.2010.06.048. [DOI] [Google Scholar]
- Saarela M, Hallamaa K, Mattila-Sandholm T, Mättö J. The effect of lactose derivatives lactulose, lactitol and lactobionic acid on the functional and technological properties of potentially probiotic Lactobacillus strains. Int Dairy J. 2003;13:291–302. doi: 10.1016/S0958-6946(02)00158-9. [DOI] [Google Scholar]
- Saminathan M, Sieo CC, Kalavathy R, Abdullah N, Ho YW. Effect of prebiotic oligosaccharides on growth of Lactobacillus strains used as a probiotic for chickens. African J Microbiol Res. 2011;5:57–64. [Google Scholar]
- Synytsya A, Míčková K, Synytsya A, Jablonský I, Spěváček J, Erban V, Čopíková J. Glucans from fruit bodies of cultivated mushrooms Pleurotus ostreatus and Pleurotus eryngii: structure and potential prebiotic activity. Carbohyd Polym. 2009;76:548–556. doi: 10.1016/j.carbpol.2008.11.021. [DOI] [Google Scholar]
- Tan S, Xu Q, Luo Z, Liu Z, Yang H, Yang L. Inquiry of water-soluble polysaccharide extraction conditions from grapefruit skin. Engineering. 2011;3:1090–1094. doi: 10.4236/eng.2011.311135. [DOI] [Google Scholar]
- Thetsrimuang C, Khammuang S, Chiablaem K, Srisomsap C, Sarnthima R. Antioxidant properties and cytotoxicity of crude polysaccharides from Lentinus polychrous Lév. Food Chem. 2011;128:634–639. doi: 10.1016/j.foodchem.2011.03.077. [DOI] [Google Scholar]
- Wang Y. Prebiotics: Present and future in food science and technology. Food Res Int. 2009;42:8–12. doi: 10.1016/j.foodres.2008.09.001. [DOI] [Google Scholar]
- Wang X, Huang M, Yang F, Sun H, Zhou X, Guo Y, Zhang M. Rapeseed polysaccharides as prebiotics on growth and acidifying activity of probiotics in vitro. Carbohyd Polym. 2015;125:232–240. doi: 10.1016/j.carbpol.2015.02.040. [DOI] [PubMed] [Google Scholar]
- Wichienchot S, Jatupornpipat M, Rastall RA. Oligosaccharides of pitaya (dragon fruit) flesh and their prebiotic properties. Food Chem. 2010;120:850–857. doi: 10.1016/j.foodchem.2009.11.026. [DOI] [Google Scholar]
- Xu X, Chen P, Wang Y, Zhang L. Chain conformation and rheological behavior of an extracellular heteropolysaccharide Erwinia gum in aqueous solution. Carbohyd Res. 2009;344:113–119. doi: 10.1016/j.carres.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Yanhua W, Fuhua W, Zhaohan G, Mingxing P, Yanan Z, Ling PZ, Minhua D, Caiying Z, Zian L. Optimization of extraction process for polysaccharide in Salvia miltiorrhiza Bunge using response surface methodology. Open Biomed Eng J. 2014;8:153–159. doi: 10.2174/1874120701408010153. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]