Abstract
Introduction
In the heart, pathways that transduce extracellular environmental cues (e.g. mechanical force, inflammatory stress) into electrical and/or chemical signals at the cellular level are critical for the organ-level response to chronic biomechanical/neurohumoral stress. Specifically, a diverse array of membrane-bound receptors and stretch-activated proteins converge on a network of intracellular signaling cascades that control gene expression, protein translation, degradation and/or regulation. These cellular reprogramming events ultimately lead to changes in cell excitability, growth, proliferation, and/or survival.
Areas covered
The actin/spectrin cytoskeleton has emerged as having important roles in not only providing structural support for organelle function but also in serving as a signaling “super highway,” linking signaling events at/near the membrane to distal cellular domains (e.g. nucleus, mitochondria). Furthermore, recent work suggests that the integrity of the actin/spectrin cytoskeleton is critical for canonical signaling of pathways involved in cellular response to stress. This review discusses these emerging roles for spectrin and consider implications for heart function and disease.
Expert Commentary
Despite growth in our understanding of the broader roles for spectrins in cardiac myocytes and other metazoan cells, there remain important unanswered questions, the answers to which may point the way to new therapies for human cardiac disease patients.
Keywords: ankyrin, arrhythmia (mechanisms), calmodulin dependent kinase II, heart failure, ion channels, spectrin
1.0 Spectrin structure and function
1.1 What are spectrins and why should we care?
The evolution of metazoans from unicellular ancestors required the emergence of cellular systems to support, among other functions, cell adhesion, long-range communication, defense, and membrane integrity in the face of high mechanical stress [1, 2]. Importantly, with multicellular animals arose de novo cellular pathways to exert spatiotemporal control over gene programs, often involving redeployment of ancestral genes [1]. Spectrins are cytoskeletal proteins originating with early metazoans that help resolve some of these unique challenges faced by multicellular animals [2].
First discovered as a key constituent of detergent-extracted erythrocytes, or “ghosts,” the spectrin molecule is a long, flexible chain ~200 nm in length formed as a heterotetramer (dimer of anti parallel heterodimers) of α- and β-subunits [2, 3]. The human spectrin family includes two α- and five β-spectrin subunits (expanded from one α- and two β-subunits in invertebrates) encoded by distinct genes with additional diversity through alternative splicing (Table 1). Spectrins are widely expressed in mammalian tissues with αII- and βII-spectrin as the predominant non-erythrocytic isoforms. A characteristic feature of both α- and β-spectrin structure is the presence of multiple triple-helical repeats (spectrin repeats), which confer flexibility to the spectrin molecule and facilitate protein-protein interaction including between α- and β-subunits themselves (involves coupling of incomplete helical repeats) [2]. Canonical α-spectrin consists of 20 spectrin repeats with a src homology domain (SH3) between repeat 9 and 10 and a C-terminal calmodulin-related domain. β-spectrin is comprised of a highly conserved N-terminal actin-binding region, followed by 17 spectrin repeats (except for βV-spectrin, which has 30 repeats) and a C-terminal domain with interesting and understudied signaling motifs including a pleckstrin homology domain. Repeat 15 in β-spectrin contains a highly conserved motif that facilitates interaction with ankyrins, cytoskeletal proteins that link membrane proteins to the spectrin-actin cytoskeleton [4]. An interaction between spectrin and protein 4.1 likely stabilizes spectrin-actin interaction [5, 6].
Table 1.
Characteristics, tissue expression and disease associations of spectrin isoforms
| Gene | Isomer | Size (amino acids, full length) |
Tissue Expression |
Associated diseases |
|---|---|---|---|---|
| SPTA1 | αI-spectrin | 2419 | Erythrocytes, neutrophils and lung alveolar lavage | spherocytosis, type 3 pyropoikilocytosis elliptocytosis-2 spta1-related spherocytosis hereditary spherocytosis hereditary elliptocytosis hypophosphatasia |
| SPTAN1 | αII-spectrin | 2472 | Brain, spinal cord, cardiac muscle, retina, liver | epileptic encephalopathy, early infantile, 5 west syndrome infantile epileptic encephalopathy quadriplegia neonatal lupus erythematosus ohtahara syndrome spastic quadriplegia |
| SPTB | βI-spectrin | 2137 | Skeletal muscle, heart, neutrophils | sptb-related spherocytosis elliptocytosis 3 pyropoikilocytosis hereditary elliptocytosis gnathomiasis otopalatodigital syndrome hereditary spherocytosis |
| SPTBN1 | βII-spectrin | 2364 | Brain, spinal cord, heart, liver | Williams-Neuren syndrome, neurofibromatosis type 2 |
| SPTBN2 | βIII-spectrin | 2390 | Brain, salivary glands, retina and cervix | spinocerebellar ataxia 5 spinocerebellar ataxia, autosomal recessive 14 spectrin-associated autosomal recessive cerebellar ataxia spinocerebellar ataxia ataxia |
| SPTBN4 | βIV-spectrin | 2564 | Brain, heart, lung, retina and pancreas | Cardiac arrhythmia, myopathy |
| SPTBN5 | βV-spectrin | 3674 | Adipocytes, platelets and breast tissue | Macular disorders |
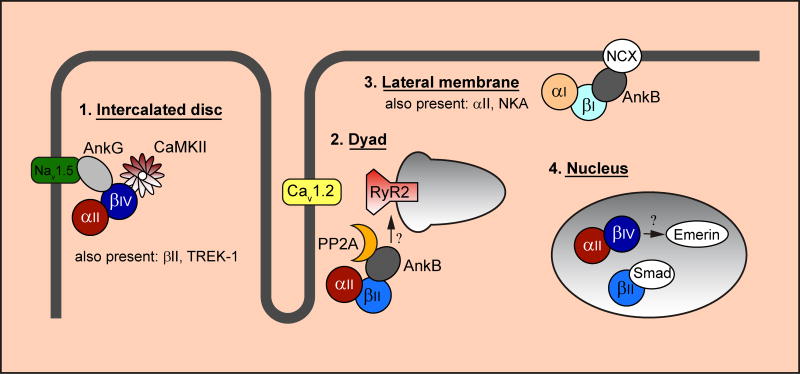
Expression of αI-, βI-and βIV-spectrins, in addition to αII- and βII-spectrins, has been reported in cardiomyocytes with distinct localization patterns [5, 7–9] that is affected by alternative splicing [10]. αII- and βII–spectrin are the predominant isoforms found at the Z-line and sarcoplasmic reticulum (SR) membranes. In contrast, αI- and αII- spectrin along with βI-spectrin account for major spectrin components at the lateral membrane. Finally, αII-, βII-, βIV-spectrin are principal family members localized to intercalated disc (Figure 1) [5, 7, 11–14]. The localization of spectrin isoforms to myocyte membrane domains important for cell-cell communication (e.g. intercalated disc) and contraction (e.g. Z-lines, SR membranes) suggests important roles in regulating both electrical and mechanical cardiac function.
Figure 1. Spectrin isoforms organize macromolecular complexes at distinct membrane domains to regulate myocyte function.
αI-, αII-, βI-, βII- and βIV-spectrin are all expressed in cardiac myocytes with differential localization to key cellular domains (e.g. intercalated disc, cardiac dyad, lateral membrane and nucleus). αII/βIV-spectrin complexes with Nav1.5 (via ankyrin-G) and CaMKII at the intercalated disc (βII-spectrin and TREK-1 are also expressed in this region, not depicted). At the cardiac dyad, αII/βII-spectrin targets protein phosphatase 2A (PP2A, via ankyrin-B) and regulates expression/localization of sarcoplasmic reticulum ryanodine receptor Ca2+ release channels (RyR2). αI- and βI-spectrin are found exclusively at the lateral membrane where they likely control membrane targeting of Na+/Ca2+ exchanger (NCX via ankyrin-B) and Na+/K+ ATPase (not depicted). Several spectrin isoforms, including αII-, βII- and βIV-spectrin, are found in the nucleus where they are implicated in transcriptional regulation (e.g. by shuttling of Smad proteins into the nucleus) and in nucleoskeleton support via emerin.
1.2 Established roles for spectin in membrane stability and ion channel targeting
Spectrin serves as a principal component of the molecular scaffolding linking the plasma membrane and associated proteins to the actin cytoskeleton in numerous tissue types [2, 5, 15]. In both erythrocytes and nucleated cell types, the spectrin-based cytoskeleton is critical in maintaining normal cell morphology, plasma membrane stability, and mechanical properties. A prototypical example of this canonical function is found in the erythrocyte where spectrin forms a polygonal network with actin that links to membrane proteins via ankyrin to support the lipid bilayer [2]. In neurons, super resolution microscopy has revealed a distinct periodic pattern of ring-like actin structures interconnected by spectrin tetramers aligned along the axon shaft [16]. Spectrin isoforms are found not only at the submembrane but also in the nucleus where they contribute to nucleoskeleton flexibility and chromosome stability [17, 18]. Interestingly, in this regard, studies point to an important role for nuclear αII-spectrin as a scaffold for repair of DNA interstrand cross-links [18]. Given its central role in formation of the erythrocyte membrane cytoskeleton, it is not surprising that defects in spectrin lead to erythrocyte membrane fragility, which ultimately may manifest as elliptocytosis and anemia [19]. Similarly, epithelial cells deficient in either βII-spectrin or ankyrin-G fail to maintain normal basolateral and apical membrane area, converting cells from columnar to squamous cell morphology [20].
Through its interaction with ankyrin family proteins, spectrins not only confer membrane stability but also play key roles in localization of ion channels, transporters and exchangers to membrane domains important for cell function. Classic examples of this function are found in the requirement of spectrin for membrane targeting of anion exchanger band 3 in erythrocytes [21, 22] and Na+/K+ ATPase in epithelial cells [23–25]. In neurons, βIV-spectrin is highly enriched with ankyrin-G and voltage-gated ion channels at axon initial segments (AISs) and nodes of Ranvier [26–31] while βII-spectrin is found primarily at paranodal regions with ankyrin-B [32]. Defects in βIV-spectrin or ankryin-G result in loss of normal voltage-gated Na+ (Nav) channel clustering, abnormal cell membrane excitability and neurological dysfunction (e.g. ataxia) in mice [26, 33–35]. Similarly, spectrin and ankyrin have been shown to be important for organization of ion channels at several membrane domains in cardiac myocytes including the cardiac dyad and intercalated disc (Figure 1). Namely, βII-spectrin is enriched with ankyrin-B at the cardiac dyad, a micro domain integral to cardiomyocyte excitation-contraction coupling [8, 36–38]. Loss of βII-spectrin or ankyrin-B results in abnormal targeting of Ca2+ cycling proteins (e.g. Na+/Ca2+ exchanger, ryanodine receptor SR Ca2+ release channels), aberrant Ca2+ cycling and arrhythmias [8, 37, 39]. In contrast, βIV-spectrin is highly localized with ankyrin-G at the cardiac intercalated disc, a specialized membrane domain important for electrical and mechanical cell-to-cell coupling [7, 40–42]. While it is clear that ankyrin-G is required for proper localization of βIV-spectrin and intercalated disc proteins, including Nav1.5 (primary cardiac Nav), the role of βIV-spectrin in ion channel targeting is less established [12, 40–43]. It is likely that βIV-spectrin, in fact, plays a more prominent role in regulation of Nav1.5 rather than targeting (discussed in more detail below). However, βIV-spectrin has been linked to membrane targeting of other cardiac ion channels, specifically, the two-pore K+ channel, TREK-1 [12, 44]. TREK-1 channels encoded by KCNK2, belong to the two-pore-domain background potassium channel protein family (K2P) and are expressed in nervous and cardiovascular systems where they regulate cell membrane excitability and participate in transduction of a variety of environmental stimuli [45, 46]. Interestingly, loss of βIV-spectrin or TREK-1 has been shown to produce arrhythmia in mice characterized by pronounced sinus node dysfunction in response to acute adrenergic stimulation [12, 44]. Interestingly, several groups have identified involvement of spectrin in post-golgi targeting and long range transport of ion channel cargo to the membrane, although its specific role remains controversial [2, 23, 47, 48]. In a similar vein, exciting work has identified a spectrin-based complex involving Mena, VASP and αII-spectrin for regulating actin dynamics [49].
1.3 Emerging roles for spectrins in control of signaling pathways
While the ways in which spectrins provide metazoans with increased membrane support and organization are relatively established, only recently has the field begun to appreciate alternative roles for spectrins in coordinating signaling and gene programs. Studies on mice lacking βII-spectrin (also known as embryonic liver fodrin, ELF) provide an excellent example of how spectrin might have evolved to mediate sophisticated cell signaling pathways [50]. Smad proteins are a group of transcription factors important for mediating TGF-β signaling that evolved with early metazoans [1]. βII-spectrin associates with Smad family members to regulate TGF-β-dependent signaling and βII-spectrin-deficient mice display systemic developmental defects including aberrant cardiac development [50]. More recently, studies have shown that analogous to this βII-spectrin/TGF-β/Smad signaling pathway,βIV-spectrin coordinates spatial and temporal organization of Ca2+/calmodulin kinase II (CaMKII) signaling in cardiomyocytes and neurons [7]. CaMKII is a multifunctional serine/threonine protein kinase with broad tissue distribution and a single ancestral gene, which likely evolved at a point in metazoan evolution just prior to spectrin (found in unicellular eukaryote and metazoan ancestor choanoflagellate, but not plants or yeast) [51–54]. βIV-spectrin organizes a macromolecular signaling complex with ankyrin-G to regulate CaMKII-dependent phosphorylation of the cardiac voltage-gated Na+ channel, Nav1.5 [7, 41, 55, 56]. This spectrin-based complex is important for CaMKII-dependent regulation of cardiac cell membrane excitability and cardiac function in response to both acute and chronic adrenergic stress [7, 55, 56]. Interestingly, spectrins via their association with ankyrins likely also regulate the negative axis of CaMKII-dependent signaling via targeting of protein phosphatase 2A [57–59].
2.0 Roles for spectrin in disease
2.1 Association between spectrin dysfunction and disease in multiple organ systems
Given the many ways that spectrins support metazoan cell function, the close link between spectrin dysfunction and human disease is not surprising. Mutations in spectrins and ankryins have been identified as the underlying cause of forms of hereditary sperocytosis and hemolytic anemia, as well as spinocerebellar ataxia in mice and humans [15, 19, 35]. In a similar vein, genome-wide association studies have uncovered ANK3 (encodes for spectrin-associated AnkG) as a susceptibility locus for human bipolar disorder [60] while mutations in ANK3 have been linked to broad spectrum neurological disorders including autism [61, 62]. More recently, it has been reported that a homozygous nonsense mutation in SPTBN4 (encoding βIV-spectrin) is a novel candidate disease gene for congenital myopathy, neuropathy, and deafness in a consanguineous Kurdish family [63].
2.2 Spectrin dysfunction in cardiac arrhythmia and disease
Defects in spectrin-based pathways have been linked to cardiac arrhythmia and disease. Interestingly, spectrin dysfunction has been shown to alter both electrical and mechanical function suggesting that spectrin may be a therapeutic node for effectively treating both arrhythmias and underlying substrate in disease.αI-spectrin deficiency in mice results in a dilated cardiomyopathy, although the presence of severe anemia complicates the phenotype in these animals [5, 64]. Global deletion of βII-spectrin in mice is embryonic lethal, with profound developemental defects in heart and other organs [50]. In particular, βII-spectrin deficiency leads to a dramatic loss or disorganization of dystrophin, F-actin, α-smooth muscle actin, and tropomyosin, contributing to compromised myocyte contractile function. At the same time, loss of βII-spectrin promotes aberrant expression/activity of myocardial transcription factors Nkx2.5, GATA4, and MEF2c, together with pronounced defects in TGF-β/Smad signaling (addressed above), which likely contributes to loss of the normal myocardial trabeculated pattern and severe thinning of the compact layer by E14.5. Cardiac specific βII-spectrin deletion in mice, although not embryonic lethal, results in pronounced arrhythmia and increased mortality/remodeling in response to chronic pressure overload [8]. Human ANK2 variants (encoding ankyrin-B) result in a broad spectrum arrhythmia syndrome recapitulated by mice deficient in ankyrin-B [37, 65, 66]. Recently, a novel human ANK2 variant associated with increased susceptibility to arrhythmias was shown to alter binding to βII-spectrin [67].
Beyond rare, inherited disorders, mounting data support a role for dysfunction in spectrin-based pathways in common forms of cardiac disease. Recent studies have identified downregulation of both spectrin and ankyrin family proteins in animal models and human patients with common forms of acquired cardiac disease [12, 67–69]. Specifically, significant decreases in the levels of βII-spectrin, βIV-spectrin, and ankyrin-B have been reported in human failing hearts [12, 67], which are also observed in animal models of heart failure and myocardial infarction [67, 69]. Similar changes in βII-spectrin,βIV-spectrin, and ankyrin-B expression have been observed in atria of human AF patients and canine model of sinus node dysfunction [44, 66, 67]. In general, chronic stress conditions appear to promote decreased spectrin/ankyrin levels at the protein but not transcript levels, indicating abnormal post-translational processing, linked to elevations in the Ca2+ activated protease, calpain [67, 68], a phenomenon also observed in tramautic brain injury [70]. Interestingly, αII-spectrin breakdown products have been suggested as biomarker for brain injury during open heart surgery in neonates with congenital heart disease [71]. Together, these data indicate the highly conserved loss of spectrin across species in response to adverse cardiac remodeling from either pressure overload and/or ischemic stress, suggesting a potentially prominent role in contributing to and driving disease remodeling.
2.3 Molecular mechanisms linking spectrin dysfunction to disease
The molecular mechanisms underlying maladaptive remodeling and arrhythmias in animals with spectrin dysfunction remain unclear, due in part to the lack of appropriate animal models (e.g. cardiac-specific spectrin knockout models like for βII-spectrin). Several possible mechanisms have been touched on already in this review and include defects in ion channel localization/activity and/or aberrant cell signaling, perhaps converging on pathways important for myocyte survival and fibrosis. For example, in the case of cardiac-specific βII-spectrin deletion, there is loss of normal Ca2+ homeostasis due in part to a precise defect in the expression and localization of SR ryanodine receptor Ca2+ release channels (without global changes in t-tubule structure or Cav1.2), giving rise to increased frequency of spontaneous Ca2+ waves and arrhythmogenic after depolarizations [8]. In addition to the pro-arrhythmic contributions from these changes, the resulting defect in Ca2+ handling may drive myocyte loss, hypertrophy, and an accelerated decline in contractile performance through Ca2+-dependent signaling pathways (e.g. CaMKII, calcineurin). At the same time, it is possible that defects in βII-spectrin may disrupt intracellular signaling and gene expression programs directly through its role in coordinating cell signaling networks (e.g. TGF-β/SMAD signaling [50], as discussed in the previous section). In a similar vein, the link between βIV-spectrin and CaMKII signaling provides a potential mechanism underlying pathogenesis induced by spectrin dysfunction. Aberrant CaMKII activity/expression is a common finding across a broad spectrum of cardiac disease states and has been linked to not only altered ion channel activity but also changes in transcriptional, inflammatory, apoptotic and fibrotic pathways [52]. It is interesting to consider the possibility that given its role its association with CaMKII, βIV-spectrin may dually regulate electrical and mechanical function in cardiac myocytes.
3. Expert commentary
Although we have learned a great deal about spectrin since its discovery almost 50 years ago, there remain a host of important unanswered questions, especially related to its role in cardiac physiology and disease. As addressed in this review, spectrin satisfies a number of unique metazoan needs from membrane support to long range signaling, which begs the question: Why has the metazoan (more specifically, cardiac) cell evolved to impart both structural and signaling functions to a single protein family (also observed with integrins, catenins, etc.)? Similarly, how has a single class of proteins evolved to participate in so many different cellular processes? A compelling theory in this regard is that spectrin family members evolved with metazoans to “uncouple” cellular reprogramming from environmental cues, effectively subjugating the individual cell to the needs of the organism [1]. It is interesting to consider the corollary then that loss of an essential support for this uncoupling would effectively cause cells to revert to a “unicellular” ancestral state to the detriment of the organism. Aside from these larger more philosophical questions, further investigation is required to address the more basic but no less important questions related to the roles of spectrin isoforms in controlling cardiac myocyte function, consequences at the organ and organismal level, and ultimately novel ways for repairing the spectrin-based cytoskeleton in the setting of disease. For example, while the structure and function of elaborate spectrin/actin networks have been mapped out in erythrocytes, neurons and other cells [16, 20, 31, 72], we lack the same level of detailed information in cardiomyocytes. At the same time, intriguing gaps remain in our understanding of the broader roles for spectrin aside from membrane support and ion channel targeting. For instance, how is spectrin able to link distal signals to the nucleus and ultimately altered gene expression? Aside from chromosome repair, are spectrins able to alter cell gene programs via interplay with signaling molecules (e.g CaMKII) and/or transcription factors (e.g. Smads) and what are the consequences for cardiac disease?
Finally, it is important to note that in order to answer these and other pressing questions related to spectrin biology, we must overcome considerable technical challenges in dissecting the multiple aspects of pleiotropic protein function. Conventional biomedical science relies almost exclusively on large system perturbations (e.g. total gene knockout/overexpression) to elucidate function at the molecular level. While such studies have generated important insight across disciplines, their binary “all-or-nothing” nature potentially obfuscates a finer level of detail essential for accurate assessment of the system as a whole. It will be important, going forward, to establish novel paradigms for “molecular sensitivity analysis” to study the more nuanced aspects of the actin/spectrin signaling network discussed here.
4. Five-year view
In light of the recent developments in our understanding of spectrin and other cytoskeleton proteins, it is exciting to consider how our view will continue to evolve over the next five years. First, technological advances (e.g. high throughput CRISPR gene editing, “big data” science) will allow us to develop more nuanced views of protein function over the traditional “all-or-nothing” knockout/overexpression approach. Second, due in part to improved technology, we will develop a more complete view of how specific extracellular stimuli (chronic and acute) lead to very different cell-level responses (adaptive vs. maladaptive) and how cytoskeletal proteins like spectrin help transduce these signals. Finally, we hope that these advances will lead to a more complete grasp of how to manipulate cytoskeletal components to protect cell membrane integrity in the face of chronic adverse conditions without launching a cellular reprogramming that ultimately compromises organ function.
Key issues.
Spectrins are cytoskeletal proteins originating with early metazoans that help resolve some of the unique challenges faced by multicellular animals.
Spectrins provide metazoans with increased membrane support and organization, but also help coordinate signaling and gene programs.
Spectrin dysfunction is associated with multiple human diseases, including hereditary forms of spherocytosis, hemolytic anemia, neuropathy and myopathy.
Defects in spectrin have been shown to alter both electrical and mechanical cardiac function suggesting that spectrin may be a therapeutic node for effectively treating both arrhythmias and underlying substrate in cardiac disease.
Acknowledgments
Funding
The authors have support from NIH [grant numbers HL114893, HL134824, and HL135096 to TJH] James S. McDonnell Foundation [to TJH]; Saving tiny Hearts Society [to TJH]; American Heart Association [SU and AGS]; and a TriFit Challenge grant from Ross Heart Hospital and Davis Heart and Lung Research Institute.
Footnotes
Declaration of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
- 1.Sebe-Pedros A, Degnan BM, Ruiz-Trillo I. The origin of Metazoa: a unicellular perspective. Nat Rev Genet. 2017;18:498–512. doi: 10.1038/nrg.2017.21. [DOI] [PubMed] [Google Scholar]
- 2.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–92. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 3**.Marchesi VT, Steers E., Jr Selective solubilization of a protein component of the red cell membrane. Science. 1968;159:203–4. doi: 10.1126/science.159.3811.203. of considerable importance. This paper describes first report of spectrins as integral components of cytoskeleton from erythrhocyte "ghosts". [DOI] [PubMed] [Google Scholar]
- 4.Hashemi SM, Hund TJ, Mohler PJ. Cardiac ankyrins in health and disease. J Mol Cell Cardiol. 2009 doi: 10.1016/j.yjmcc.2009.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baines AJ, Pinder JC. The spectrin-associated cytoskeleton in mammalian heart. Front Biosci. 2005;10:3020–33. doi: 10.2741/1759. [DOI] [PubMed] [Google Scholar]
- 6.Stagg MA, Carter E, Sohrabi N, et al. Cytoskeletal Protein 4.1R Affects Repolarization and Regulates Calcium Handling in the Heart. Circ Res. 2008 doi: 10.1161/CIRCRESAHA.108.176461. [DOI] [PubMed] [Google Scholar]
- 7*.Hund TJ, Koval OM, Li J, et al. A beta IV spectrin/CaMKII signaling complex is essential for membrane excitability in mice. J Clin Invest. 2010;120:3508–19. doi: 10.1172/JCI43621. of importance. This paper describes expression of βIV-spectrin in cardiac myocytes and its role in coordinating local signaling domain for Ca2+/calmodulin-dependent protein kinase II. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith SA, Sturm AC, Curran J, et al. Dysfunction in the beta II spectrin-dependent cytoskeleton underlies human arrhythmia. Circulation. 2015;131:695–708. doi: 10.1161/CIRCULATIONAHA.114.013708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flick MJ, Konieczny SF. The muscle regulatory and structural protein MLP is a cytoskeletal binding partner of beta I-spectrin. J Cell Sci. 2000;113(Pt 9):1553–64. doi: 10.1242/jcs.113.9.1553. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Y, Resneck WG, Lee PC, et al. Characterization and expression of a heart-selective alternatively spliced variant of alpha II-spectrin, cardi +, during development in rat. J Mol Cell Cardiol. 2010;48:1050–9. doi: 10.1016/j.yjmcc.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bennett PM, Baines AJ, Lecomte MC, et al. Not just a plasma membrane protein: in cardiac muscle cells alpha-II spectrin also shows a close association with myofibrils. J Muscle Res Cell Motil. 2004;25:119–26. doi: 10.1023/b:jure.0000035892.77399.51. [DOI] [PubMed] [Google Scholar]
- 12.Hund TJ, Snyder JS, Wu X, et al. beta (IV)-Spectrin regulates TREK-1 membrane targeting in the heart. Cardiovasc Res. 2014;102:166–75. doi: 10.1093/cvr/cvu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayes NV, Scott C, Heerkens E, et al. Identification of a novel C-terminal variant of beta II spectrin: two isoforms of beta II spectrin have distinct intracellular locations and activities. J Cell Sci. 2000;113(Pt 11):2023–34. doi: 10.1242/jcs.113.11.2023. [DOI] [PubMed] [Google Scholar]
- 14.Hund TJ, Mohler PJ. Cardiac spectrins: alternative splicing encodes functional diversity. J Mol Cell Cardiol. 2010;48:1031–2. doi: 10.1016/j.yjmcc.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett V, Healy J. Organizing the fluid membrane bilayer: diseases linked to spectrin and ankyrin. Trends Mol Med. 2008;14:28–36. doi: 10.1016/j.molmed.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 16.Xu K, Zhong G, Zhuang X. Actin, spectrin, and associated proteins form a periodic cytoskeletal structure in axons. Science. 2013;339:452–6. doi: 10.1126/science.1232251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simon DN, Wilson KL. The nucleoskeleton as a genome-associated dynamic 'network of networks'. Nat Rev Mol Cell Biol. 2011;12:695–708. doi: 10.1038/nrm3207. [DOI] [PubMed] [Google Scholar]
- 18.Lambert MW. Nuclear alpha spectrin: Critical roles in DNA interstrand cross-link repair and genomic stability. Exp Biol Med (Maywood) 2016;241:1621–38. doi: 10.1177/1535370216662714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19**.Agre P, Casella JF, Zinkham WH, et al. Partial deficiency of erythrocyte spectrin in hereditary spherocytosis. Nature. 1985;314:380–3. doi: 10.1038/314380a0. of considerable importance. This study describes spectrin deficiency as principal defect undelying common human hereditary sperocytosis. [DOI] [PubMed] [Google Scholar]
- 20.Kizhatil K, Yoon W, Mohler PJ, et al. Ankyrin-G and beta2-spectrin collaborate in biogenesis of lateral membrane of human bronchial epithelial cells. The Journal of biological chemistry. 2007;282:2029–37. doi: 10.1074/jbc.M608921200. [DOI] [PubMed] [Google Scholar]
- 21**.Bennett V, Stenbuck PJ. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature. 1979;280:468–73. doi: 10.1038/280468a0. of considerable importance. This paper describes for the first time classical role for spectrin in membrane targeting of a membrane ion channel/transporter (via ankyrin). [DOI] [PubMed] [Google Scholar]
- 22.Drenckhahn D, Schluter K, Allen DP, et al. Colocalization of band 3 with ankyrin and spectrin at the basal membrane of intercalated cells in the rat kidney. Science. 1985;230:1287–9. doi: 10.1126/science.2933809. [DOI] [PubMed] [Google Scholar]
- 23.Devarajan P, Stabach PR, De Matteis MA, et al. Na, K-ATPase transport from endoplasmic reticulum to Golgi requires the Golgi spectrin-ankyrin G119 skeleton in Madin Darby canine kidney cells. Proc Natl Acad Sci U S A. 1997;94:10711–6. doi: 10.1073/pnas.94.20.10711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson WJ, Shore EM, Wang AZ, et al. Identification of a membrane-cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin-Darby canine kidney epithelial cells. J Cell Biol. 1990;110:349–57. doi: 10.1083/jcb.110.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nelson WJ, Veshnock PJ. Ankyrin binding to (Na+ + K+) ATPase and implications for the organization of membrane domains in polarized cells. Nature. 1987;328:533–6. doi: 10.1038/328533a0. [DOI] [PubMed] [Google Scholar]
- 26.Komada M, Soriano P. Beta IV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J Cell Biol. 2002;156:337–48. doi: 10.1083/jcb.200110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garrido JJ, Giraud P, Carlier E, et al. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science. 2003;300:2091–4. doi: 10.1126/science.1085167. [DOI] [PubMed] [Google Scholar]
- 28**.Berghs S, Aggujaro D, Dirkx R, Jr, et al. beta IV spectrin, a new spectrin localized at axon initial segments and nodes of ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151:985–1002. doi: 10.1083/jcb.151.5.985. of considerable importance. This paper identifies βIV-spectrin as a novel member of the β-spectrin family highly expressed in myelinated neurons at specific membrane domains. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kordeli E, Lambert S, Bennett V. Ankyrin G. A new ankyrin gene with neural-specific isoforms localized at the axonal initial segment and node of Ranvier. J Biol Chem. 1995;270:2352–9. doi: 10.1074/jbc.270.5.2352. [DOI] [PubMed] [Google Scholar]
- 30*.Srinivasan Y, Elmer L, Davis J, et al. Ankyrin and spectrin associate with voltage-dependent sodium channels in brain. Nature. 1988;333:177–80. doi: 10.1038/333177a0. of importance. Among the first reports to identify a role for ankyrin/spectrin in membrane targeting of voltage-gated Na+ channels. [DOI] [PubMed] [Google Scholar]
- 31.Rasband MN. The axon initial segment and the maintenance of neuronal polarity. Nat Rev Neurosci. 2010;11:552–62. doi: 10.1038/nrn2852. [DOI] [PubMed] [Google Scholar]
- 32.Amor V, Zhang C, Vainshtein A, et al. The paranodal cytoskeleton clusters Na+ channels at nodes of Ranvier. Elife. 2017;6 doi: 10.7554/eLife.21392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou D, Lambert S, Malen PL, et al. Ankyrin G is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing. J Cell Biol. 1998;143:1295–304. doi: 10.1083/jcb.143.5.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uemoto Y, Suzuki S, Terada N, et al. Specific role of the truncated beta IV-spectrin Sigma6 in sodium channel clustering at axon initial segments and nodes of ranvier. J Biol Chem. 2007;282:6548–55. doi: 10.1074/jbc.M609223200. [DOI] [PubMed] [Google Scholar]
- 35.Ikeda Y, Dick KA, Weatherspoon MR, et al. Spectrin mutations cause spinocerebellar ataxia type 5. Nat Genet. 2006;38:184–90. doi: 10.1038/ng1728. [DOI] [PubMed] [Google Scholar]
- 36.Tuvia S, Buhusi M, Davis L, et al. Ankyrin-B is required for intracellular sorting of structurally diverse Ca2+ homeostasis proteins. J Cell Biol. 1999;147:995–1008. doi: 10.1083/jcb.147.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37**.Mohler PJ, Schott JJ, Gramolini AO, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003;421:634–9. doi: 10.1038/nature01335. of considerable importance. This paper for the first time links a defect in a non-ion channel encoding gene (spectrin-associated ankyrin-B) and cardiac long QT syndrome. [DOI] [PubMed] [Google Scholar]
- 38.Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR micro domain. PLoS Biol. 2005;3:e423. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Camors E, Mohler PJ, Bers DM, et al. Ankyrin-B reduction enhances Ca spark-mediated SR Ca release promoting cardiac myocyte arrhythmic activity. J Mol Cell Cardiol. 2012;52:1240–8. doi: 10.1016/j.yjmcc.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lowe JS, Palygin O, Bhasin N, et al. Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway. J Cell Biol. 2008;180:173–86. doi: 10.1083/jcb.200710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Makara MA, Curran J, Little SC, et al. Ankyrin-G coordinates intercalated disc signaling platform to regulate cardiac excitability in vivo. Circ Res. 2014;115:929–38. doi: 10.1161/CIRCRESAHA.115.305154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mohler PJ, Rivolta I, Napolitano C, et al. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:17533–8. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sato PY, Coombs W, Lin X, et al. Interactions between ankyrin-G, Plakophilin-2, and Connexin43 at the cardiac intercalated disc. Circ Res. 2011;109:193–201. doi: 10.1161/CIRCRESAHA.111.247023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Unudurthi SD, Wu X, Qian L, et al. Two-Pore K+ Channel TREK-1 Regulates Sino a trial Node Membrane Excitability. J Am Heart Assoc. 2016;5:e002865. doi: 10.1161/JAHA.115.002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Goonetilleke L, Quayle J. TREK-1 K (+) channels in the cardiovascular system: their significance and potential as a therapeutic target. Cardiovasc Ther. 2012;30:e23–9. doi: 10.1111/j.1755-5922.2010.00227.x. [DOI] [PubMed] [Google Scholar]
- 46.Honore E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–61. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- 47.Levine J, Willard M. Fodrin: axonally transported polypeptides associated with the internal periphery of many cells. J Cell Biol. 1981;90:631–42. doi: 10.1083/jcb.90.3.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sikorski AF, Sangerman J, Goodman SR, et al. Spectrin (beta SpII sigma1) is an essential component of synaptic transmission. Brain Res. 2000;852:161–6. doi: 10.1016/s0006-8993(99)02253-2. [DOI] [PubMed] [Google Scholar]
- 49.Benz PM, Merkel CJ, Offner K, et al. Mena/VASP and alpha II-Spectrin complexes regulate cytoplasmic actin networks in cardiomyocytes and protect from conduction abnormalities and dilated cardiomyopathy. Cell Commun Signal. 2013;11:56. doi: 10.1186/1478-811X-11-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50*.Tang Y, Katuri V, Dillner A, et al. Disruption of transforming growth factor-beta signaling in ELF beta-spectrin-deficient mice. Science. 2003;299:574–7. doi: 10.1126/science.1075994. of importance. This paper identifies a novel role for β-spectrin in control of cell signaling pathways important for development. [DOI] [PubMed] [Google Scholar]
- 51.Tombes RM, Faison MO, Turbeville JM. Organization and evolution of multifunctional Ca(2+)/CaM-dependent protein kinase genes. Gene. 2003;322:17–31. doi: 10.1016/j.gene.2003.08.023. [DOI] [PubMed] [Google Scholar]
- 52.Swaminathan PD, Purohit A, Hund TJ, et al. Calmodulin-dependent protein kinase II: linking heart failure and arrhythmias. Circ Res. 2012;110:1661–77. doi: 10.1161/CIRCRESAHA.111.243956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braun AP, Schulman H. The multifunctional calcium/calmodulin-dependent protein kinase: from form to function. Annual Review of Physiology. 1995;57:417–45. doi: 10.1146/annurev.ph.57.030195.002221. [DOI] [PubMed] [Google Scholar]
- 54.Burkhardt P, Sprecher SG. Evolutionary origin of synapses and neurons - Bridging the gap. Bio essays. 2017 doi: 10.1002/bies.201700024. [DOI] [PubMed] [Google Scholar]
- 55.Glynn P, Musa H, Wu X, et al. Voltage-Gated Sodium Channel Phosphorylation at Ser571 Regulates Late Current, Arrhythmia, and Cardiac Function In Vivo. Circulation. 2015;132:567–77. doi: 10.1161/CIRCULATIONAHA.114.015218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Koval OM, Snyder JS, Wolf RM, et al. Ca2+/calmodulin-dependent protein kinase II-based regulation of voltage-gated Na+ channel in cardiac disease. Circulation. 2012;126:2084–94. doi: 10.1161/CIRCULATIONAHA.112.105320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhasin N, Cunha SR, Mduannayake M, et al. Molecular basis for PP2A regulatory subunit B56 alpha targeting in cardiomyocytes. Am J Physiol Heart Circ Physiol. 2007;293:H109–19. doi: 10.1152/ajpheart.00059.2007. [DOI] [PubMed] [Google Scholar]
- 58.DeGrande S, Nixon D, Koval O, et al. CaMKII inhibition rescues proarrhythmic phenotypes in the model of human ankyrin-B syndrome. Heart Rhythm. 2012;9:2034–41. doi: 10.1016/j.hrthm.2012.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Little SC, Curran J, Makara MA, et al. Protein phosphatase 2A regulatory subunit B56alpha limits phosphatase activity in the heart. Sci Signal. 2015;8:ra72. doi: 10.1126/scisignal.aaa5876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferreira MA, O'Donovan MC, Meng YA, et al. Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet. 2008 doi: 10.1038/ng.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kloth K, Denecke J, Hempel M, et al. First de novo ANK3 nonsense mutation in a boy with intellectual disability, speech impairment and autistic features. Eur J Med Genet. 2017;60:494–8. doi: 10.1016/j.ejmg.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 62.Shi L, Zhang X, Golhar R, et al. Whole-genome sequencing in an autism multiplex family. Mol Autism. 2013;4:8. doi: 10.1186/2040-2392-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Knierim E, Gill E, Seifert F, et al. A recessive mutation in beta-IV-spectrin (SPTBN4) associates with congenital myopathy, neuropathy, and central deafness. Hum Genet. 2017;136:903–10. doi: 10.1007/s00439-017-1814-7. [DOI] [PubMed] [Google Scholar]
- 64.Kaysser TM, Wandersee NJ, Bronson RT, et al. Thrombosis and secondary hemochromatosis play major roles in the pathogenesis of jaundiced and spherocytic mice, murine models for hereditary spherocytosis. Blood. 1997;90:4610–9. [PubMed] [Google Scholar]
- 65.Le Scouarnec S, Bhasin N, Vieyres C, et al. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci U S A. 2008;105:15617–22. doi: 10.1073/pnas.0805500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cunha SR, Hund TJ, Hashemi S, et al. Defects in ankyrin-based membrane protein targeting pathways underlie a trial fibrillation. Circulation. 2011;124:1212–22. doi: 10.1161/CIRCULATIONAHA.111.023986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith SA, Hughes LD, Kline CF, et al. Dysfunction of the beta2-spectrin-based pathway in human heart failure. Am J Physiol Heart Circ Physiol. 2016;310:H1583–91. doi: 10.1152/ajpheart.00875.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kashef F, Li J, Wright P, et al. Ankyrin-B protein in heart failure: identification of a new component of metazoan cardioprotection. J Biol Chem. 2012;287:30268–81. doi: 10.1074/jbc.M112.368415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hund TJ, Wright PJ, Dun W, et al. Regulation of the ankyrin-B-based targeting pathway following myocardial infarction. Cardiovasc Res. 2009;81:742–9. doi: 10.1093/cvr/cvn348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kobeissy FH, Liu MC, Yang Z, et al. Degradation of beta II-Spectrin Protein by Calpain-2 and Caspase-3 Under Neurotoxic and Traumatic Brain Injury Conditions. Mol Neurobiol. 2015;52:696–709. doi: 10.1007/s12035-014-8898-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jain P, Spaeder MC, Donofrio MT, et al. Detection of alpha II-spectrin breakdown products in the serum of neonates with congenital heart disease*. Pediatr Crit Care Med. 2014;15:229–35. doi: 10.1097/PCC.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bennett M, Farnell L, Gibson W. The probability of quantal secretion within an array of calcium channels of an active zone. BIOPHYSICAL JOURNAL. 2000;78:2222–40. doi: 10.1016/S0006-3495(00)76770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]