Abstract
We report a new continuous spectrophotometric assay for human cystathionine beta-synthase (hCBS). This assay relies upon the finding that hCBS will take cysteamine in place of L-homocysteine, thereby producing thialysine. Thialysine is, in turn, decarboxylated by lysine decarboxylase, releasing CO2 that is monitored by the sequential action of phosphoenolpyruvate carboxylase and L-malate dehydrogenase. The decrease in absorbance at 340 nm is monitored as reduced nicotinamide adenine dinucleotide is consumed. Using this four-enzyme couple, we find that for L-serine and 5.6 ± 2.2 mM for cysteamine, with kcat = 1.3 ± 0.1 s−1 for the formation of thialysine by hCBS. For comparison purposes, the same hCBS reaction was monitored via a radioactive single time point assay using 14C-(C-1)-labeled L-serine and cysteamine as substrates, counting the thialysine product, following ion exchange chromatography. This assay yielded for L-serine and 6.6 ± 2.2 for cysteamine, with kcat = 2.5 ± 0.4 s−1. These numbers indicate that, although it possesses a shortened carbon chain and lacks a carboxyl group, cysteamine displays a catalytic efficiency (kcat/Km) with hCBS that is within an order of magnitude of that observed with its natural thiol cosubstrate, L-homocysteine.
Keywords: Cystathionine beta-synthase, Continuous assay, Cysteamine, Thialysine, Lysine decarboxylase, Phosphoenolpyruvate carboxylase, Malate dehydrogenase
Cystathionine beta-synthase (CBS;1 EC 4.2.1.22) is a pivotal enzyme in human sulfur metabolism [1,2]. The transposition of the γ-sulfur atom from essential dietary L-methionine to the β-carbon of L-cysteine is achieved through the sequential action of two PLP-dependent enzymes, CBS and cystathionine γ-lyase (CGL). CBS converts L-serine and L-homocysteine into (L,L)-cystathionine and water. It thereby also plays an important role in the regulation of L-homocysteine levels. Since elevated levels of total homocysteine correlate with cardiovascular disease [3–5], neural tube defects [6], and Alzheimer’s disease [7], there is considerable biomedical interest in this enzyme. Furthermore, it has recently been shown that when CBS is exposed to physiological concentrations of L-serine, L-cysteine, and L-homocysteine, 5% of the cystathionine formed is from L-cysteine [8]. This “side reaction” releases H2S in place of H2O. Observations such as this suggest another potential role for CBS in the brain, namely, biosynthesis of the neuromodulator H2S [9,10].
Human CBS (hCBS) is a modular enzyme, comprising an N-terminal heme domain and a C-terminal S-adenosylmethionine (SAM)-binding regulatory domain, both flanking a central catalytic core that carries the essential PLP cofactor [1,2]. In contrast to the mammalian enzyme, the yeast enzyme possesses neither a heme cofactor nor a SAM binding site. Full-length hCBS is a tetramer of 63 kDa subunits and tends to aggregate, hampering crystallization efforts. Limited proteolysis trims full-length hCBS to a catalytic core (ca. 45 kDa) that exists as a dimer, shows higher activity than full-length enzyme, and is not subject to SAM regulation [11,12]. The truncated version of the protein, lacking the C-terminal 143–145 amino acids, has been expressed and similarly results in an active dimer of 48 kDa subunits [13,14]. The truncated form of CBS is observed when liver cells or mice are exposed to proinflammatory agents and appears to be an adaptive response for increasing flux through the transsulfuration pathway in response to oxidative challenge [15]. Two X-ray crystal structures for hCBS have been reported, both for the truncated dimer [16,17]. The assays described herein are performed with the highly active hCBS-ΔC143 dimer.
Heretofore, the human CBS enzyme has typically been assayed by quantitating the formation of radioactively labeled [14C]cystathionine from labeled L-serine (Fig. 1), at a fixed time point, following separation of the two amino acids by ion exchange chromatography, much as originally described by Mudd et al. [13,18–20]. Variants of this assay, in which the labeled amino acids are separated by paper chromatography [21,22] or thin-layer chromatography [23], have also been reported. A complementary time point assay relies on the release of radiolabeled water from α-tritiated L-serine by CBS [23]. A colorimetric time point assay, based upon the serine sulfhydrylase activity of mammalian CBS, in vitro, has also been described [24].
Fig. 1.
Standard radioisotopic time point assay for measuring hCBS activity. Chromatographic separation of the radiolabeled cystathionine product from the unreacted labeled serine substrate is required.
Although the 14C radioisotopic time point assay is by far the most common hCBS assay employed, the tedious nature of this assay is widely acknowledged [2]. This has served to motivate efforts to develop continuous assays for CBS. In elegant work, Aitken and Kirsch [25,26] have recently described the first two such assays for yeast CBS (yCBS). The forward reaction is monitored by coupling with two enzymes, namely recombinant cystathionine beta-lyase and lactate dehydrogenase (commercially available). The reverse reaction can be monitored by capturing the homocysteine formed with Ellman’s reagent. However, this assay is less convenient, because the reverse reaction is thermodynamically highly unfavorable (ΔGrev ~ +8.5 kcal/mol).
Herein, a complementary coupled continuous assay for hCBS is described (Fig. 2). The assay takes advantages of two key observations: (i) cysteamine can be substituted for L-homocysteine as the thiol cosubstrate in the hCBS reaction and (ii) thialysine, the product of that β-replacement reaction, is an alternative substrate for lysine decarboxylase (LDC). While there were hints in the literature that CBS and LDC could employ such unnatural substrates [27,28], these activities were not well established. Decarboxylation of L-thialysine would release CO2 which could presumably be captured in a Claisen-type condensation mediated by PEPC. This would yield oxaloacetate, itself a substrate for malate dehydrogenase (MDH), which permits convenient monitoring of the reaction at 340 nm, associated with consumption of NADH. Note that the MDH step is particularly well suited to acting as the final step in this coupled enzyme assay as, even at pH 8.0, Keq for this reaction is approximately 104.1 [29], indicating that oxaloacetate reduction by NADH is exergonic by some 5.6 kcal mol−1 at room temperature.
Fig. 2.
Continuous coupled assay for hCBS. Substitution of cysteamine for the usual thiol substrate L-homocysteine results in the formation of a new CBS product, thialysine. Decarboxylation of thialysine by LDC releases CO2 that is captured by PEPC, giving oxaloacetate. Oxaloacetate, in turn, is reduced by NADH through the action of MDH. The consumption of NADH with time is conveniently monitored in a UV/vis spectrophotometer.
Materials and methods
Materials
14C-(C-1)-labeled L-serine was purchased from American Radioactive. L-Serine, (D,L)-homocysteine, cysteamine, pyridoxal 5-phosphate, phosophoenolpyruvate (PEP), EDTA, Tris, Bis–Tris, and NADH were from Sigma–Aldrich. Dowex 50 W × 8 cation exchange resin (100–200 mesh, hydrogen form) and Econo-columns were purchased from Bio-Rad. The Ecolite scintillation cocktail from ICN Biomedicals was used.
Enzymes
L-Malate dehydrogenase (porcine heart) was from Sigma [Nominally: 470 U/mg protein (reported by Sigma using NADH (0.14 mM), oxaloacetate (2.5 mM) in KPO4 buffer (50 mM, pH 7.5) at 25 °C); Found: 210 U/mg protein, wherein one unit oxidizes 1 μmol NADH (0.4 mM) per minute in the presence of oxaloacetate (3 mM) at pH 8.0 (200 mM Tris–HCl) and 37 °C]. Phosphoenopyruvate carboxylase (PEPC, Zea mays) was from Calbiochem [Nominally: 7.4 U/mg protein (reported by Calbiochem using PEP (2 mM), MgSO4 (18 mM), NaHCO3 (100 mM), NADH (0.15 mM), and MDH (50 U/mL) at pH 8.0 (50 mM Tris–HCl) at 30 °C); Found: 2.1 U/mg protein, wherein one unit catalyzes the formation of 1 μmol of oxaloacetate from PEP (2 mM), MgCl2 (1 mM), NaHCO3 (10 mM) at pH 8.0 (200 mM Tris–HCl) at 37 °C. Oxaloacetate formation was followed by its reduction with NADH (0.4 mM), as catalyzed by MDH (2.1 U/0.1 mL)]. Lysine decarboxylase from Hafnia alvei was purified by a modification of the published procedure [30,31]. From 84 g of wet cells, 3.6 mg of protein (homogeneous by SDS–PAGE) was obtained with a specific activity of 115 U/mg of protein. LDC activity was measured by a modification of the method of Palcic [32,33]. One LDC unit decarboxylates 1 μmol of L-lysine (2 mM) per minute at pH 8.0 (200 mM Tris–HCl). Protein concentration was determined by the Lowry method [34]. The recombinant dimer hCBS-ΔC143 was expressed as a GST fusion protein in Escherichia coli and purified according to the procedure by Taoka et al. [13,20]. The purified dimer displayed a specific activity of 10 U/mg, wherein one CBS unit catalyzes the formation of 1 μmol of (L,L)-cystathionine per minute, from L-serine (30 mM) and (D,L)-homocysteine (60 mM) at pH 8.0 (200 mM Tris–HCl) and 37 °C.
pH Profiles of reporting enzymes
MDH
The pH profile of MDH was obtained via the continuous UV assay described above but using a 100 mM Tris/100 mM Bis–Tris–HCl buffer across the pH range (6.5–8.5) tested. Percentage activity vs pH was plotted.
PEPC
The pH profile was carried out using the coupled UV assay described above, except that 100 mM Tris/100 mM Bis–Tris–HCl buffer was used across the pH range (6–8.5) tested.
LDC
For the pH profile of LDC the postworkup dye-based time point assay of Phan et al. [35] was used. LDC (0.06 U) was incubated with L-lysine (4 mM) at 37 °C in 100 mM Tris/100 mM Bis–Tris–HCl at the specified pH. The reaction was quenched after 0, 2, 4, 6, 8, and 10 min with 1 M K2CO3 and then heated for 5 min at 40 °C with 2,4,6-trinitrobenzenesulfonic acid. The bis–sulfonamide adduct so formed was extracted with toluene, and its absorption was measured at 340 nm. Relative LDC activity was assessed by comparing the slopes of linear fits of the absorbance vs time data thereby obtained across the pH range studied.
CBS
The pH profile was obtained from a typical radioactive time point (single time point at 30 min) assay (vide infra) with the unnatural substrate cysteamine (30 mM) and L-serine (30 mM) in 100 mM Tris/100 mM Bis–Tris–HCl at 37 °C across the pH range studied. Following the standard separation procedure, the concentration of thialysine formed (measured via liquid scintillation counting) was determined and plotted against pH.
Titration experiments for the individual reporting enzymes
These assays were monitored using a Shimadzu UV-2401PC UV/visible spectrophotometer, outfitted with a quartz multimicrocell (features 16 parallel microwells of 100 μL each). Titration experiments were performed with 30 mM L-serine, 30 mM cysteamine, 1 mM MgCl2, 2 mM PEP, and 0.1 mM PLP and CBS (2.8 μg) at pH 8.0 (200 mM Tris–HCl) and 37 °C (water-jacketed cell holder/circulating water bath for temperature control, 100 μL final volume). The following reporting enzyme concentrations were sampled: LDC (0, 0.15, 0.30, 0.45 to 0.60 thialysine U), PEPC (0, 0.04, 0.08, 0.16, 0.20, 0.28, 0.45, 0.60 U), and MDH (0, 0.5, 1.0, 2.11 U). [Note: The zero point here is not actually zero, as we have found that the commercial PEPC (product No. 524810, lot No. B51536) contains MDH (i.e., 0.45 PEPC U contain 0.03 MDH U)].
Continuous coupled spectrophotometric assay for CBS
The assay was carried out in a 100-μL well of the multimicrocell containing 2 mM PEP, 0.1 mM PLP, 0.6 mM NADH, 1 mM MgCl2, 30 mM L-serine and CBS (2.8 μg/mL), with coupling enzymes LDC (0.52 U = 0.31 thialysine U), PEPC (0.45 U), and MDH (2.11 U) in 200 mM Tris buffer, pH 8.0, at 37 °C. The reaction was initiated by the addition of 30 mM cysteamine. Note: The buffer itself (comprising approximately 40% of the final cuvette volume) was purged with Ar for 15 min prior to use to remove exogenous CO2. The multimicrocell was sealed with parafilm, following addition of cysteamine. A control well, lacking CBS, was run in parallel. The decrease of absorbance at 340 nm was recorded. Rates were routinely corrected for background CO2 (typically <10 mAbs/min). [Note that while this procedure works well for the removal of background CO2 from buffered solutions prepared in the laboratory, biological samples might contain appreciable amounts of bicarbonate. In such cases, it may be necessary to purge the sample in the presence of carbonic anhydrase to drive the bicarbonate to gaseous CO2, which would then be removed by the infused Ar stream.]
Radioactive time point assays for CBS
The reaction mixture, containing 60 mM (D,L)-homocysteine (or 30 mM cysteamine), 30 mM 14C-1 labeled L-serine (specific activity typically 20,000 cpm/μmol), 112 μg/mL CBS, 2.5 mM EDTA, and 0.1 mM PLP in 100 mM Tris–HCl buffer (0.5 mL; pH 8.0) was incubated at 37 °C for 30 min. The reaction was then terminated by addition of 0.5 mL 10% trichloroacetic acid. Half of the reaction mixture (0.5 mL) was loaded onto an Econo-column packed with Dowex 50 W × 8 cationic exchange resin (2 g dry resin per column), and the column was washed with 20 mL dd water. Then, unreacted L-serine was eluted with 40 mL 0.4 N HCl. After washing again with 20 mL water, the product was eluted with 4 × 4 mL of 4 N NH4OH. Each basic fraction (4 mL) was diluted with scintillation cocktail (Ecolite, 15 mL) and counted (Packard Model 1900 CA Tricarb scintillation counter), after standing overnight. The control reaction, lacking CBS, was run with every set of assays. When the concentration of radiolabeled L-serine was varied (i.e., for Km determinations), separate controls were run for each serine concentration. However, in cases where the thiol (cysteamine or homocysteine) concentration was varied, a single control experiment sufficed, as tests indicated an invariant background count in the “second elution peak” regardless of (cold) thiol concentration.
Results and discussion
To assess the feasibility of the coupled CBS assay depicted in Fig. 2, a pH/rate profile of each enzyme in the couple was determined. Where possible, a single enzyme assay was used here to insure that the pH effects observed reflect properties of the enzyme being interrogated. Single-enzyme assays were employed for CBS, LDC, and MDH, whereas PEPC was coupled with MDH. In all cases, a mixed Tris (pKa = 8.1)/Bis–Tris (pKa = 6.5) buffer was chosen to build-in buffering capacity across the pH range under study, without the need to alter buffer composition. The results are presented in Fig. 3 and indicate a relatively broad pH optimum for LDC, stretching from pH 6 to 8. The other three enzymes display peak activity at pH 8 ± 0.5. Given that most reported kinetic data for CBS are at pH ≥8, these results are promising.
Fig. 3.

pH profile of hCBS and the reporting enzymes for the coupled assay.
It was thus decided to fix the pH at 8.0 for this coupled spectrophotometric assay and to titrate the three reporting enzymes up to levels where they would not be partially rate-limiting. In Fig. 4, the response of the overall rate to changes in the individual reporting enzyme levels is plotted. It is worth noting that this coupled assay can be conveniently run in a commercial multimicrocell (Shimadzu) that features 16 parallel 100-μL miniwells. Beginning with MDH (Fig. 4A) and working backward toward LDC (Fig. 4C), the reporting enzymes were brought up to kinetically saturating levels, one at a time.
Fig. 4.

Titration of the reporting enzymes: (A) variation of added MDH concentration with LDC (0.52 U) and PEPC fixed (0.45 U). Note that a substantial rate is seen with no added MDH, as commercial PEPC (Calbiochem) already contains MDH activity (approximately 0.03 U per 0.45 U PEPC). (B) Variation of PEPC concentration with LDC (0.52 U) and MDH (2.1 U) fixed. (C) Variation of LDC concentration with PEPC (0.45 U) and MDH (2.1 U) fixed. All assays are performed in 100 μL microwells containing 2.8 μg hCBS at pH 8 and 37 °C, as described under Materials and methods. Rates are plotted as absolute values, as one observes the decrease in absorbance at 340 nm as NADH is consumed in the MDH step.
Having established conditions under which the coupled assay is dependent on the activity of hCBS, we next evaluated the dependence of the assay on the concentration of hCBS itself (Fig. 5A). Particularly noteworthy here is the continuous nature of this assay, in sharp contrast to the single product concentration vs time information that is gleaned from the radioisotopic assay normally employed for hCBS. In Fig. 5B, the slopes of each of the primary traces are replotted as a function of the concentration of hCBS. A linear dependence of observed rate upon hCBS concentration is observed.
Fig. 5.
(A) Response of the coupled UV assay to hCBS concentration. Shown are the primary data for NADH oxidation with time, under typical assay conditions [30 mM L-serine, 30 mM cysteamine, 1 mM MgCl2, 2 mM PEP, 0.1 mM PLP, PEPC (0.45 U), LDC (0.52 U), and MDH (2.1 U) in 200 mM Tris buffer, pH 8.0] with increasing hCBS concentration [rate increases in the order: 0 (control; open triangles), 0.7 (filled circles), 1.4 (open squares), 2.1 (filled triangles), 2.8 (open diamonds), and 3.5 μg (filled diamonds); all in a 100-μL final volume]. (B) Data replotted as rate vs [CBS].
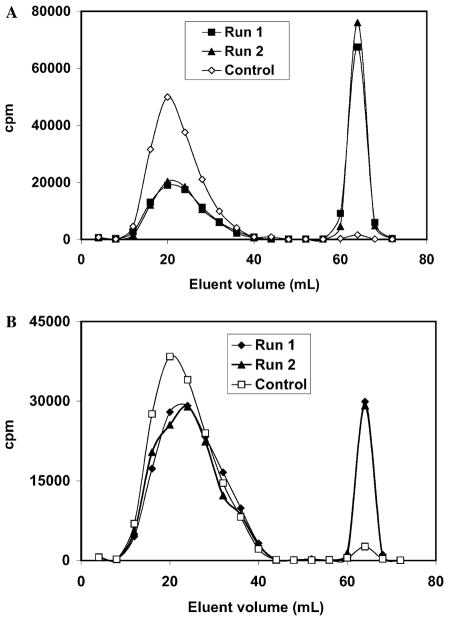
Since the new assay employs an unnatural cosubstrate, cysteamine, the efficacy of the latter was tested in a single time point radiometric assay, analogous to the standard Mudd assay with homocysteine [18]. The ion exchange column profile (Fig. 6A) reveals baseline separation for the natural radiolabeled amino acid substrate (L-serine) and product ((L,L)-cystathionine) under the conditions of this assay. Since L-thialysine is more basic than (L,L)-cystathionine, it is also readily separated from L-serine (Fig. 6B).
Fig. 6.
Dowex 50 column profiles for the separation of radiolabeled L-serine (first peak) from radiolabeled hCBS product (second peak). In (A) 30 mM L-homocysteine was used as thiol cosubstrate providing (L,L)-cystathionine as product. On the other hand, (B) shows the result of substituting cysteamine for L-homocysteine, yielding L-thialysine as the hCBS product. In each case, the assay was run in duplicate, and a control containing all components but hCBS was run. The reactions were run at 37 °C for 30 min, processed, chromatographed, and counted as described under Materials and methods.
Next, Michaelis–Menten kinetic analyses for both the natural (L-serine/homocysteine) substrate pair and the unnatural (L-serine/cysteamine) pair were conducted, using this single time point assay. The catalytic efficiencies and individual kinetic constants thereby obtained are collected in Table 1. The corresponding kinetic parameters from the literature (for homocysteine) and from the continuous UV assay reported herein (for cysteamine) are also included for comparison. The values that we obtain for Km(L-Ser), Km(L-homocysteine), kcat, and the catalytic efficiency (kcat/Km) for hCBS (entry 3) fall within the range of the values previously reported by others (entries 1 and 2). For the new thiol cosubstrate, cysteamine, we obtain a kcat that is 3- to 6-fold lower than that observed with homocysteine (entries 4 and 5). The Km values obtained for cysteamine are 1.5- to 1.8-fold higher than that seen for homocysteine. However, the Km value observed for L-serine is 2-to 4-fold lower with the unnatural second substrate than with homocysteine itself. Taken together, these data indicate that cysteamine is a useful unnatural substrate for CBS, with a catalytic efficiency (kcat/Km) that either is comparable to that seen with homocysteine (if one uses the L-serine Km values) or is within an order of magnitude of it (if one uses the thiol cosubstrate Km values).
Table 1.
Summary of kinetic parameters for C-terminal-truncated hCBS
| Km(L-Ser) (mM) | Km (L-Hcy) (mM) | Km cysteamine (mM) | SA (μmol h−1 mg−1) | kcat (s−1) | kcat/Kmf (mM−1s−1) | |
|---|---|---|---|---|---|---|
| (1) Refs. [1,13] | 18 ± 8 | 9.7 ± 4.3 | 750 ± 330 | 10 ± 4.4 | 0.6 | |
| (2) Ref. [11] | 2.7 | 0.5 | 6.7 | 2.6 | ||
| (3) Mudd assay (L-Hcy)a | 4.9 ± 0.6c | 3.7 ± 0.5e | 566 ± 20c | 7.5 ± 0.3c | 1.5 | |
| (4) Mudd assay (cysteamine)b | 2.2 ± 0.5d | 6.6 ± 2.2e | 188 ± 26d | 2.5 ± 0.4d | 1.1 | |
| (5) Continuous UV assay | 1.2 ± 0.2d | 5.6 ± 2.2e | 93 ± 4 | 1.3 ± 0.1d | 1.0 |
In this assay, CBS activity is monitored by counting of radioactive (L,L)-cystathionine, as derived from 14C-L-serine and L-homocysteine, as described.
In this assay, CBS activity is monitored by counting of radioactive L-thialysine, as derived from 14C-L-serine and cysteamine, as described under Materials and methods.
In these runs, the (D,L)-homocysteine concentration was fixed at 60 mM.
In these runs, the cysteamine concentration was fixed at 30 mM.
In these runs, the L-serine concentration was fixed at 30 mM.
Km for L-Ser (available for all assays) is used to compare the “catalytic efficiency” function across the table.
In summary, a continuous UV assay for hCBS is reported. Nearly all previous assays of hCBS have used the natural substrates L-serine and L-homocysteine. The former is used to generate a Michael acceptor in the active site, in the form of a PLP-bound amino acrylate intermediate. The latter acts as a thiol nucleophile to capture that electrophilic intermediate, in a Michael sense. We reasoned that cysteamine might serve as a homocysteine surrogate, being a comparable nucleophile and being only one methylene unit short of the natural substrate, though lacking the carboxyl group.
As is demonstrated by steady state kinetic analysis (see the data in Table 1), cysteamine does indeed serve as a very good “second substrate” for hCBS, thereby generating the targeted unnatural enzymatic product L-thialysine. This reaction can then be effectively linked to a three-enzyme couple for L-lysine decarboxylase activity [32,33], because L-thialysine, in turn, is an excellent substrate for LDC. This coupled hCBS assay affords several conveniences in comparison to the radioactive time point assay commonly employed heretofore to assess hCBS activity. Specifically, the need to quench the reaction, take aliquots, and do chromatography is obviated, and a much more continuous readout of product concentration with time is obtained.
Acknowledgments
The authors thank the American Heart Association (AHA GIA 0350404N) for support of this research. D.B.B. thanks the Alfred P. Sloan Foundation for a fellowship. M.K.M. acknowledges support from a University of Nebraska for a UCARE (Undergraduate Creative Activities & Research Experiences) Fellowship. We are grateful to Professor Ruma Banerjee for providing the hCBS-ΔC143 clone and for useful conversations. Kannan R. Karukurichi and Roberto de la Salud-Bea are thanked for LDC purification.
Footnotes
Abbreviations used: CBS, cystathionine beta-synthase; Hcy, homocysteine; PLP, pyridoxal phosphate; CGL, cystathionine gammalyase; Tris, tris(hydroxymethyl)aminomethane; LDC, lysine decarboxylase; PEP, phosphoenolpyruvate; PEPC, phosphenolpyruvate carboxylase; MDH, L-malate dehydrogenase; SAM, S-adenosylmethionine; SDS–PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis; Tris, tris(hydroxymethyl)aminomethane; Bis-Tris, bis(2-hydroxyethyl)imino-tris(hydroxymethyl)methane.
References
- 1.Banerjee R, Zou C-g. Redox regulation and reaction mechanism of human cystathionine-beta-synthase: a PLP-dependent hemesensor protein. Arch Biochem Biophys. 2005;433:144–156. doi: 10.1016/j.abb.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 2.Miles EW, Kraus JP. Cystathionine beta-synthase: structure, function, regulation, and location of homocystinuria-causing mutations. J Biol Chem. 2004;279:29871–29874. doi: 10.1074/jbc.R400005200. [DOI] [PubMed] [Google Scholar]
- 3.Maclean KN, Gaustadnes M, Oliveriusova J, Janosik M, Kraus E, Kozich V, Kery V, Skovby F, Ruediger N, Ingerslev J, Stabler SP, Allen RH, Kraus JP. High homocysteine and thrombosis without connective tissue disorders are associated with a novel class of cystathionine beta-synthase (CBS) mutations. Human Mutat. 2002;19:641–655. doi: 10.1002/humu.10089. [DOI] [PubMed] [Google Scholar]
- 4.Whincup PH, Refsum H, Perry IJ, Morris R, Walker M, Lennon L, Thomson A, Ueland PM, Ebrahim SB. Serum total homocysteine and coronary heart disease: prospective study in middle aged men. Heart (British Cardiac Society) 1999;82:448–454. doi: 10.1136/hrt.82.4.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke R, Lewington S, Donald A, Johnston C, Refsum H, Stratton I, Jacques P, Breteler MM, Holman R. Underestimation of the importance of homocysteine as a risk factor for cardiovascular disease in epidemiological studies. J Cardiovasc Risk. 2001;8:363–369. doi: 10.1177/174182670100800605. [DOI] [PubMed] [Google Scholar]
- 6.Mills JL, McPartlin JM, Kirke PN, Lee YJ, Conley MR, Weir DG, Scott JM. Homocysteine metabolism in pregnancies complicated by neural-tube defects. Lancet. 1995;345:149–151. doi: 10.1016/s0140-6736(95)90165-5. [DOI] [PubMed] [Google Scholar]
- 7.Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol. 1998;55:1449–1455. doi: 10.1001/archneur.55.11.1449. [DOI] [PubMed] [Google Scholar]
- 8.Chen X, Jhee KH, Kruger WD. Production of the neuromodulator H2S by cystathionine beta-synthase via the condensation of cysteine and homocysteine. J Biol Chem. 2004;279:52082–52086. doi: 10.1074/jbc.C400481200. [DOI] [PubMed] [Google Scholar]
- 9.Kimura H. Hydrogen sulfide is severely decreased in Alzheimer disease brains. Mol Neurobiol Alzheimer Dis Relat Disord. 2004:79–83. [Google Scholar]
- 10.Kimura H. Hydrogen sulfide as a neuromodulator. Mol Neurobiol. 2002;26:13–19. doi: 10.1385/MN:26:1:013. [DOI] [PubMed] [Google Scholar]
- 11.Kery V, Poneleit L, Kraus JP. Trypsin cleavage of human cystathionine beta-synthase into an evolutionarily conserved active core: structural and functional consequences. Arch Biochem Biophys. 1998;355:222–232. doi: 10.1006/abbi.1998.0723. [DOI] [PubMed] [Google Scholar]
- 12.Skovby F, Kraus JP, Rosenberg LE. Biosynthesis and proteolytic activation of cystathione beta-synthase in rat liver. J Biol Chem. 1984;259:588–593. [PubMed] [Google Scholar]
- 13.Taoka S, Widjaja L, Banerjee R. Assignment of enzymatic functions to specific regions of the PLP-dependent heme protein cystathionine beta-synthase. Biochemistry. 1999;38:13155–13161. doi: 10.1021/bi990865t. [DOI] [PubMed] [Google Scholar]
- 14.Shan X, Kruger WD. Correction of disease-causing CBS mutations in yeast. Nat Genet. 1998;19:91–93. doi: 10.1038/ng0598-91. [DOI] [PubMed] [Google Scholar]
- 15.Zou CG, Banerjee R. Tumor necrosis factor-alpha-induced targeted proteolysis of cystathionine beta-synthase modulates redox homeostasis. J Biol Chem. 2003;278:16802–16808. doi: 10.1074/jbc.M212376200. [DOI] [PubMed] [Google Scholar]
- 16.Meier M, Janosik M, Kery V, Kraus JP, Burkhard P. Structure of human cystathionine beta-synthase: a unique pyridoxal 5′-phosphate-dependent heme protein. EMBO J. 2001;20:3910–3916. doi: 10.1093/emboj/20.15.3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taoka S, Lepore BW, Kabil O, Ojha S, Ringe D, Banerjee R. Human cystathionine beta-synthase is a heme sensor protein. Evidence that the redox sensor is heme and not the vicinal cysteines in the CXXC motif seen in the crystal structure of the truncated enzyme. Biochemistry. 2002;41:10454–10461. doi: 10.1021/bi026052d. [DOI] [PubMed] [Google Scholar]
- 18.Mudd SH, Finkelstein JD, Irreverre F, Laster L. Transsulfuration in mammals. Microassays and tissue distributions of three enzymes of the pathway. J Biol Chem. 1965;240:4382–4392. [PubMed] [Google Scholar]
- 19.Suda M, Nakagawa H, Kimura H. Cystathionine synthase. Methods Enzymol. 1971;17:454–458. [Google Scholar]
- 20.Taoka S, Ohja S, Shan X, Kruger WD, Banerjee R. Evidence for heme-mediated redox regulation of human cystathionine beta-synthase activity. J Biol Chem. 1998;273:25179–25184. doi: 10.1074/jbc.273.39.25179. [DOI] [PubMed] [Google Scholar]
- 21.Manowitz P, Racevskis J, Gilmour DG. A modified assay for cystathionine synthase. Clin Chim Acta. 1970;28:269–276. doi: 10.1016/0009-8981(70)90091-4. [DOI] [PubMed] [Google Scholar]
- 22.Kraus J, Packman S, Fowler B, Rosenberg LE. Purification and properties of cystathionine beta-synthase from human liver. Evidence for identical subunits. J Biol Chem. 1978;253:6523–6528. [PubMed] [Google Scholar]
- 23.Abeles RH, Borcsok E. Mechanism of action of cystathionine synthase. Arch Biochem Biophys. 1982;213:695–707. doi: 10.1016/0003-9861(82)90600-2. [DOI] [PubMed] [Google Scholar]
- 24.Stepien PP, Pieniazek NJ. Use of the L-serine sulfhydrylase assay for the estimation of cystathionine beta-synthase. Anal Biochem. 1973;54:294–299. doi: 10.1016/0003-2697(73)90278-9. [DOI] [PubMed] [Google Scholar]
- 25.Aitken SM, Kirsch JF. Kinetics of the yeast cystathionine beta-synthase forward and reverse reactions: continuous assays and the equilibrium constant for the reaction. Biochemistry. 2003;42:571–578. doi: 10.1021/bi026681n. [DOI] [PubMed] [Google Scholar]
- 26.Aitken SM, Kirsch JF. Role of active-site residues Thr81, Ser82, Thr85, Gln157, and Tyr158 in yeast cystathionine beta-synthase catalysis and reaction specificity. Biochemistry. 2004;43:1963–1971. doi: 10.1021/bi035496m. [DOI] [PubMed] [Google Scholar]
- 27.Braunstein AE, Goryachenkova EV. The pyridoxal phosphate-dependent enzymes exclusively catalyzing reactions of beta-replacement. Biochimie. 1976;58:5–17. doi: 10.1016/s0300-9084(76)80351-3. [DOI] [PubMed] [Google Scholar]
- 28.Blarzino C, De Marco C. Selenalysine as substrate of lysine decarboxylase. Ital J Biochem. 1977;26:444–450. [PubMed] [Google Scholar]
- 29.Nambiar KP, Stauffer DM, Kolodziej PA, Benner SA. A mechanistic basis for the stereoselectivity of enzymic transfer of hydrogen from nicotinamide cofactors. J Am Chem Soc. 1983;105:5886–5890. [Google Scholar]
- 30.Soda K, Moriguchi M. L-Lysine decarboxylase (Bacterium cadaveris) Methods Enzymol. 1971;17:677–681. [Google Scholar]
- 31.Soda K, Moriguchi M. Crystalline lysine decarboxylase. Biochem Biophys Res Commun. 1969;34:34–39. doi: 10.1016/0006-291x(69)90524-5. [DOI] [PubMed] [Google Scholar]
- 32.Scriven F, Wlasichuk KB, Palcic MM. A continual spectrophotometric assay for amino acid decarboxylases. Anal Biochem. 1988;170:367–371. doi: 10.1016/0003-2697(88)90644-6. [DOI] [PubMed] [Google Scholar]
- 33.Berkowitz DB, Jahng WJ, Pedersen ML. alpha-Vinyllysine and alpha-vinylarginine are time-dependent inhibitors of their cognate decarboxylases. Bioorg Med Chem Lett. 1996;6:2151–2156. doi: 10.1016/0960-894X(96)00366-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 35.Phan APH, Ngo TT, Lenhoff HM. Spectrophotometric assay for lysine decarboxylase. Anal Biochem. 1982;120:193–197. doi: 10.1016/0003-2697(82)90336-0. [DOI] [PubMed] [Google Scholar]