Abstract
Forty years ago, non-steroidal anti-inflammatory drugs were first reported to decrease systemic prostaglandin levels and promote ductus arteriosus (DA) closure. And yet, prolonged patency of the DA (PDA) remains a significant clinical problem, complicated by imperfect therapies and wide variations in treatment strategy. There are few pharmacology-based tools available for treating PDA (indomethacin, ibuprofen, acetaminophen), or for maintaining DA patency (PGE1) as is needed to facilitate corrective surgery for ductus-dependent congenital heart defects. Unfortunately, all of these treatments are inefficient and are associated with concerning adverse effects. This review highlights novel potential DA drug targets that may expand our therapeutic repertoire beyond the prostaglandin pathway.
Introduction
The ductus arteriosus (DA) is a muscular artery that connects the fetal aorta and pulmonary artery, providing an integral shunt of the fetal circulation, essential for maintaining fetal wellbeing. In utero, the DA diverts approximately 90% of the right ventricular output away from the high-resistance pulmonary circulation and into the systemic umbilical-placental circulation where gas exchange can occur with maternal blood1. Postnatally, the DA normally closes within 72 hours after birth to facilitate perfusion of the newly-inflated lungs. Failure to close results in a persistent left-to-right shunt termed patent ductus arteriosus (PDA). PDA affects up to 80% of premature infants2 and accounts for 5–10% of all congenital heart defects in term infants3. Hemodynamically significant PDA is associated with significant morbidities including neurodevelopmental impairment, intraventricular hemorrhage, pulmonary hemorrhage, respiratory distress syndrome, bronchopulmonary dysplasia, necrotizing enterocolitis (NEC), spontaneous intestinal perforation, and congestive heart failure4. On the other hand, in ductus-dependent heart lesions, which account for 20–25% of all congenital heart defects, it is necessary that patency of the neonatal DA be maintained in order to preserve life-saving pulmonary or systemic circulation prior to corrective surgery5.
The fetal DA has intrinsic tone and requires dilating factors to maintain its patency in utero. Nitric oxide (NO) and prostaglandin E2 (PGE2) induce vessel relaxation by activating cGMP/PKG and cAMP/PKA signaling respectively. While NO and PGE2 are typically considered primary mediators of DA dilation, other factors, including potassium (K+) channels, adenosine, and atrial naturetic peptide, also play a role6–9. At birth, the DA constricts in response to the acute increase in oxygen (O2) tension coupled with a loss of vasodilators. O2 may act through several different pathways to exert its contractile effects on the DA4, including cytochrome P450-mediated induction of endothelin 110 and reactive oxygen species-inhibition of voltage-gated potassium (K+) channels (Kv1.5 and Kv2.1)11. O2 may also inhibit ATP-sensitive K+ (KATP) channels, resulting in membrane depolarization, activation of voltage dependent Ca2+ channels and increased intracellular Ca2+ accumulation12.
Current Therapies
There are few pharmacology-based tools currently available for modulating DA tone13–15. Introduced as a PDA therapy in the mid 1970s16–18, the nonsteroidal anti-inflammatory drug (NSAID), indomethacin, facilitates DA closure by inhibiting cyclooxygenase COX-1 (PTGS1) and COX-2 (PTGS2) enzymes that are required for PGE2 synthesis. A potent vasoconstrictor, indomethacin became the PDA drug of choice for many years, despite its association with renal dysfunction, platelet abnormalities, spontaneous intestinal perforation, and NEC19–21. In the mid 1990s, ibuprofen emerged as an alternative to indomethacin, demonstrating equal efficacy, but with a decreased risk of NEC22–24. However, use of either of these non-selective NSAIDs is still not ideal given their propensity to induce generalized vasoconstriction20, 21, 25. In addition, indomethacin and ibuprofen are ineffective in approximately 30% of patients26, exposing such infants to increased risk of renal dysfunction, spontaneous intestinal perforation, and NEC with no therapeutic benefit. Recently, acetaminophen has been touted as the optimal choice for PDA management, because it is less likely to cause peripheral vasoconstriction and can be administered to infants with contraindications to NSAIDs27. Like the others, acetaminophen is a COX inhibitor, although it may not be as effective at blocking PGE2 synthesis in immature vessels, potentially limiting its effectiveness in premature infants28.
The ability of PGE1 and PGE2 to relax the ex vivo fetal lamb DA, first demonstrated in 1973 by Coceani and Olley29, led to initiation of several clinical trials to evaluate the efficacy of PGE1 in maintenance of DA patency in infants with ductus-dependent congenital heart defects30–32. Today, PGE1 is still the only pharmacology-based therapy used to keep the DA open, despite its association with peripheral vasodilation, apnea, fever, and other physiologic disruptions13. Prolonged treatment produces even more severe side effects, including cortical hyperostosis, gastric-outlet obstruction, and pseudo-Bartter syndrome33. In addition, while PDA therapies can be administered orally or intravenously, PGE1-mediated DA dilation requires continuous intravenous infusion, another significant limitation. Despite the inefficient nature of these current tools, relatively little effort has been expended over the last four decades to identify novel targets for pharmacology-based DA manipulation.
Towards Specific DA-targeted Therapies
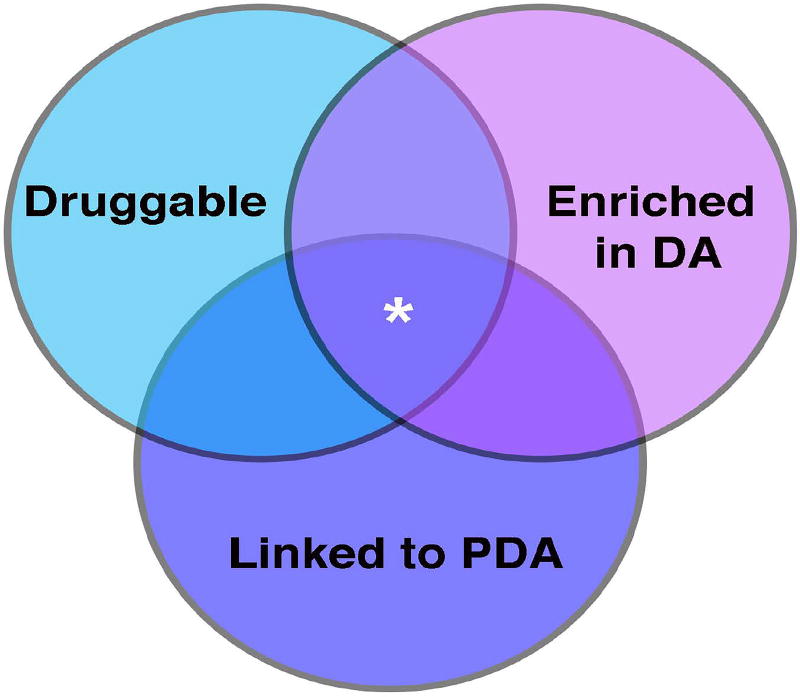
Lack of progress towards novel DA drug development is not entirely unexpected given the low probability of success for drug development programs in general. A recent study34, the largest of its kind, reports that from 2005–2015 only 8.5 % of all industry-sponsored drug development programs ended in FDA approval. Moreover, the rate at which new drugs are validated and brought to market is staggeringly slow, with the average drug spending 8.14 years in clinical trials34. However, drugs with the highest likelihood of success target proteins with these key characteristics: druggability, enrichment in the tissue of interest, and demonstrated relevance to disease35, 36 (Figure 1). Targets with some precedent for binding a drug-like molecule are considered part of the “druggable genome”37. Most modern drugs act on receptors, enzymes, and ion channels/transporters. However, as the pharmaceutical industry devises new ways to target moieties such as protein-protein interactions and nucleic acids, the druggable genome will expand to include non-traditional targets. Accordingly, there will be room for expansion of the potential targets for DA therapy, beyond the PGE-pathway.
Figure 1. Characteristics of ideal drug targets.
DA drug targets with the highest likelihood of receiving FDA approval are those that are druggable, enriched in the DA, and have an established link to PDA (denoted by asterisk in the central, overlapping region of the diagram)
Traditional and non-traditional potential DA drug target candidates listed in Table 1, include novel targets identified as enriched in the DA, using array and RNA-seq analyses to identify the DA’s unique transcriptional identity (reviewed by Yarboro et al, this issue)38–48. In the majority of these studies, the DA was compared to the ascending aorta, a natural choice given their close proximity and the shared neural crest origin of each vessel’s smooth muscle cells49. While these analyses are helpful, looking at DA-specific targets should be expanded to include a comparison to renal, mesenteric, and cerebral vessels, given the potential that novel drugs could, as do all current DA drugs, have adverse effects on these vascular beds50. While it is unlikely that any candidate will be exclusively expressed in the DA, those that are significantly enriched lend themselves to the possibility of targeting them with a relatively low “Goldilocks” dose of drug – one that would have just the right effect on the DA, but would be insufficient to act on other tissues given low expression of the target in those tissues26.
Table 1.
Representative List of Potential DA Drug Targets
| Receptors | Enzyme | Channel/Transporter | Non-traditional Targets | |
|---|---|---|---|---|
| Enriched in the DA |
|
|
|
|
| Mouse models |
|
|
|
|
| Human Syndromes |
|
|
|
|
| SNPs |
|
|
|
|
Candidates are classified as traditional (receptor, enzyme, ion channel or transporter) or non-traditional drug targets and are organized by protein category and type of evidence linking them to PDA (mouse model, human syndrome, or non-syndromic SNP)
Additionally, several transcriptome studies analyzed changes in gene expression in preterm vs term-gestation vessels. This is important to consider given that PDA most often occurs in the setting of prematurity and that immature DAs are less responsive to indomethacin and often lack the smooth muscle machinery to maintain DA closure even if initial constriction is achieved51. Therefore, targeting pathways involved in fetal DA maturation may be key to developing therapies with a higher success rate in preterm infants. For instance, fetal DAs are significantly less responsive to oxygen-induced constriction than term-gestation vessels52–54. Therefore, novel PDA therapies that can compensate for a diminished response to O2 by promoting endothelin signaling or blocking K+ channels might be more effective at closing premature vessels.
Insights from Animal Studies
Animal models of PDA have been informative regarding disease pathways that could be therapeutically targeted4, 55. Interestingly, PDA is the most common cardiac anomaly in dogs. Studies show a sex-linked genetic predisposition, with certain breeds and female dogs being at higher risk55–57. A similar sex-linked predisposition has been reported in humans58 although this is not supported by more recent analyses59. Similarly, Brown Norway rats have a high prevalence of PDA, making them a popular experimental tool for identifying gene alterations in the setting of disease45. Finally, mouse models of PDA highlight the role of specific genes in DA development and function. Some of the latter have impaired smooth muscle development (Tfap2b−/−, Myocd−/−, Myh1−/−), while others have impaired prostaglandin signaling (Ptgs1/2−/−, Ptger4−/−, Slco2a1−/−, Hpdg−/−), which may seem counterintuitive given the primary role of PGE2 in DA dilation. However, these animals suggest an additional role for prostaglandin signaling in maturing the contractile program of the ductus60. Still other animal models lack various genes that are key in development pathways, including Notch (Notch2/3−/−, Jag1−/−), BMP (BMP9/10−/−), chromatin remodeling (Brg1−/−), platelet homeostasis (Nfe2−/−, Itga2b−/−), or elastin and collagen crosslinking (Lox−/−)61. These animals not only give molecular insight into disease-causing mechanisms, they also provide established in vivo platforms for pre-clinical validation of second-generation PDA drugs.
Insights from Human Studies
Results of animal studies are not always faithful predictors of patient outcomes62, highlighting the need for human genetic studies63. A representative set of the more than 100 single-gene mutation syndromes featuring PDA that have now been identified55, 64, 65 is listed in Table 1. Many of these syndromes also feature complicated congenital heart defects, making it difficult to parse out whether PDA is a primary lesion or the result of other structural and hemodynamic anomalies. Therefore, identifying gene variants associated with non-syndromic PDA may be more informative for sporadically occurring PDA, which makes up the vast majority (90%) of all PDA cases66. Single nucleotide polymorphisms (SNPs) associated with PDA26 or response to indomethacin67 have also been reported (Table 1), although it has been difficult to confirm these findings in replication cohorts68, 69.
Proof of Concept
Interestingly, targets of current PDA therapeutics fulfill all three of the key characteristics of successful drug targets illustrated in Figure 1. Indomethacin, ibuprofen, and acetaminophen all inhibit COX enzymes encoded by PTGS1 and PTGS2 genes. These enzymes catalyze PGE2 synthesis, which subsequently regulates DA tone by binding to the DA-enriched receptor, PTGER4. Mice lacking these enzymes or the receptor have a lethal PDA phenotype44, 70–73, demonstrating relevance of these targets to this disease. Therefore, it may be reasonable to assume that novel DA drug targets with the best chance of having clinical success will conform to a similar pattern. As one example, KATP channels possess all three key characteristics listed above. These channels are hetero-octameric complexes of pore-forming inward rectifier K+ channel subunits (Kir6.1 or Kir6.2) and regulatory sulfonylurea receptor subunits (SUR1, SUR2A, or SUR2B). Channel isoforms have distinct pharmacological and physiological characteristics and are expressed in a tissue-specific manner (Table 2), making them potentially ideal targets for DA-selective therapies74.
Table 2.
Isoform-specific expression of KATP channel subunits
| Subunits | Tissue |
|---|---|
| Kir6.1/SUR2B | Vascular Smooth Muscle and Endothelium |
| KIR6.1/SUR2B | Visceral Smooth Muscle |
| Kir6.2/SUR2A | Skeletal Muscle |
| Kir6.1/SUR2 | Cardiac Conduction System |
| Kir6.2/SUR1,2 | Cardiomyocytes |
| Kir6.2/SUR1 | Pancreatic β Cells, Brain |
A regulatory link between KATP channels and DA tone is becoming increasingly apparent. Vascular KATP channels, formed of the Kir6.1/SUR2B isoform combination, are developmentally regulated and significantly enriched in the DA compared to other vessels38–42, 45, 48. Animal studies have shown that the DA constricts in response to glibenclamide12, 75, a non-specific KATP channel inhibitor commonly used to treat diabetes. Conversely, fetal or neonatal exposure to diazoxide, a KATP channel activator, inhibited DA constriction in rats76, and was reported to cause DA reopening in hypoglycemic infants77. Most notably, 50% of patients with Cantu syndrome, caused by activating mutations in Kir6.1 or SUR2B, have a clinically significant PDA that is often resistant to indomethacin therapy, thus requiring surgical ligation to achieve closure78.
Capitalizing on the druggability and therapeutic potential of KATP channels, several groups, including our own, are working to develop subunit-specific activators and inhibitors79, 80. Using a thallium flux-based high-throughput screen, we identified a very potent novel KATP channel agonist (VU063), with preferential activity towards the pancreatic/neuronal channel isoform (Kir6.2/SUR1)74, 79. We performed pressure myography assays on mouse DAs as previously described41, 81 to test the specificity and vasoactive potential of this compound (Figure 2). As expected, the SUR2-preferring KATP channel activator, pinacidil, dilated the DA in a dose-dependent manner, but VU063 had no effect on DA tone. Thus, while VU063 would, just like diazoxide, be conducive for treating hyperinsulinemia, unlike diazoxide it would not induce off-target vascular effects. Along these lines, it is likely that future studies will identify small molecule antagonists/agonists that preferentially target the vascular channel isoform (Kir6.1/SUR2B), which will allow for a more specific way to modulate DA tone.
Figure 2.
Isolated mouse DAs were mounted in microvessel perfusion chambers for use in pressure myography assays. Vessels were treated with increasing concentrations of pinacidil or VU063. Changes in lumen diameter were measured and plotted as a percent change from the initial lumen diameter reading under baseline (bl) conditions. Pinacidil dilated the DA, while VU063 had no effect.
Perspective and Prospects
In the 45 years since the first reports of indomethacin-induced DA closure16–18, 29, 82, 83, relatively little headway has been made in developing novel approaches for therapeutic modulation of DA tone. As a consequence, there has been little progress in establishing a standard of care over the subsequent decades, leading to significant variability in treatment strategies amongst neonatal care centers and even between providers in a single care center84, 85. As we learn more about the DA, it is becoming clear that PDA is a deceptively complex condition that will likely benefit more from personalized medicine rather than a one-pathway-fits all approach to therapeutics. In lieu of redundant retrospective case analyses, a greater emphasis should be placed on multidisciplinary efforts to identify DA-modulating pathways outside of the prostaglandin signaling cascade and to develop specific pharmacological modifiers of those targets.
Moving towards this goal, increased access to human tissue repositories86 coupled with the ability to perform large-scale GWAS and other genome-wide studies will uncover new PDA-associated genes. Furthermore, these studies will provide important pharmacogenetic information that could be used at the bedside to prospectively determine the likelihood of an individual patient responding to indomethacin, thereby shielding non-responders from unnecessary exposure87. Moreover, emerging technologies including the generation of tissue-engineered ductus vessels88 and the creation of immortalized human DA cell lines for use in high-throughput small molecule screens will provide novel tools to identify and validate second generation DA drugs, setting the stage for rapid advances towards novel DA therapeutic development.
Acknowledgments
Supported by: This work was supported by R21HL132805 and AHA15SDG25280015 awarded to ELS and R35HL140024 awarded to CGN.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: We have no Conflicts of Interest to report.
References
- 1.Rudolph AM. Fetal and neonatal pulmonary circulation. Annu Rev Physiol. 1979;41:383–395. doi: 10.1146/annurev.ph.41.030179.002123. [DOI] [PubMed] [Google Scholar]
- 2.Koch J, Hensley G, Roy L, Brown S, Ramaciotti C, Rosenfeld CR. Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics. 2006;117:1113–1121. doi: 10.1542/peds.2005-1528. [DOI] [PubMed] [Google Scholar]
- 3.Dice JE, Bhatia J. Patent ductus arteriosus: an overview. J Pediatr Pharmacol Ther. 2007;12:138–146. doi: 10.5863/1551-6776-12.3.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stoller JZ, Demauro SB, Dagle JM, Reese J. Current Perspectives on Pathobiology of the Ductus Arteriosus. J Clin Exp Cardiolog. 2012;8 doi: 10.4172/2155-9880.S8-001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider M, Zartner P, Sidiropoulos A, Konertz W, Hausdorf G. Stent implantation of the arterial duct in newborns with duct-dependent circulation. Eur Heart J. 1998;19:1401–1409. doi: 10.1053/euhj.1998.0977. [DOI] [PubMed] [Google Scholar]
- 6.Akaike TMS. Role of Ion Channels in Ductus Arteriosus Closure. Human Genetics & Embryology. 2014;3 [Google Scholar]
- 7.Mentzer RM, Jr, Ely SW, Lasley RD, Mainwaring RD, Wright EM, Jr, Berne RM. Hormonal role of adenosine in maintaining patency of the ductus arteriosus in fetal lambs. Ann Surg. 1985;202:223–230. doi: 10.1097/00000658-198508000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toyoshima K, Momma K, Imamura S, Nakanishi T. In vivo dilatation of the postnatal ductus arteriosus by atrial natriuretic peptide in the rat. Neonatology. 2007;92:139–144. doi: 10.1159/000101526. [DOI] [PubMed] [Google Scholar]
- 9.Andersson S, Tikkanen I, Pesonen E, Meretoja O, Hynynen M, Fyhrquist F. Atrial natriuretic peptide in patent ductus arteriosus. Pediatr Res. 1987;21:396–398. doi: 10.1203/00006450-198704000-00015. [DOI] [PubMed] [Google Scholar]
- 10.Coceani F, Kelsey L, Seidlitz E, Korzekwa K. Inhibition of the contraction of the ductus arteriosus to oxygen by 1-aminobenzotriazole, a mechanism-based inactivator of cytochrome P450. Br J Pharmacol. 1996;117:1586–1592. doi: 10.1111/j.1476-5381.1996.tb15325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Michelakis ED, Rebeyka I, Wu X, et al. O2 sensing in the human ductus arteriosus: regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ Res. 2002;91:478–486. doi: 10.1161/01.res.0000035057.63303.d1. [DOI] [PubMed] [Google Scholar]
- 12.Waleh N, Reese J, Kajino H, et al. Oxygen-induced tension in the sheep ductus arteriosus: effects of gestation on potassium and calcium channel regulation. Pediatr Res. 2009;65:285–290. doi: 10.1203/PDR.0b013e31819746a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis AB, Freed MD, Heymann MA, Roehl SL, Kensey RC. Side effects of therapy with prostaglandin E1 in infants with critical congenital heart disease. Circulation. 1981;64:893–898. doi: 10.1161/01.cir.64.5.893. [DOI] [PubMed] [Google Scholar]
- 14.Coceani F. Therapeutic manipulation of the ductus arteriosus: current options and future prospects. Pol Arch Med Wewn. 2014;124:58–64. doi: 10.20452/pamw.2081. [DOI] [PubMed] [Google Scholar]
- 15.Olley PM, Coceani F. Prostaglandins and the ductus arteriosus. Annu Rev Med. 1981;32:375–385. doi: 10.1146/annurev.me.32.020181.002111. [DOI] [PubMed] [Google Scholar]
- 16.Coceani F, Olley PM, Bodach E. Lamb ductus arteriosus: effect of prostaglandin synthesis inhibitors on the muscle tone and the response to prostaglandin E2. Prostaglandins. 1975;9:299–308. doi: 10.1016/0090-6980(75)90034-9. [DOI] [PubMed] [Google Scholar]
- 17.Friedman WF, Hirschklau MJ, Printz MP, Pitlick PT, Kirkpatrick SE. Pharmacologic closure of patent ductus arteriosus in the premature infant. N Engl J Med. 1976;295:526–529. doi: 10.1056/NEJM197609022951003. [DOI] [PubMed] [Google Scholar]
- 18.Heymann MA, Rudolph AM, Silverman NH. Closure of the ductus arteriosus in premature infants by inhibition of prostaglandin synthesis. N Engl J Med. 1976;295:530–533. doi: 10.1056/NEJM197609022951004. [DOI] [PubMed] [Google Scholar]
- 19.Mehta S, Younoszai A, Pietz J, Achanti B. Pharmacological closure of the patent ductus arteriosus. Images Paediatr Cardiol. 2003;5:1–15. [PMC free article] [PubMed] [Google Scholar]
- 20.Christmann V, Liem KD, Semmekrot BA, van de Bor M. Changes in cerebral, renal and mesenteric blood flow velocity during continuous and bolus infusion of indomethacin. Acta Paediatr. 2002;91:440–446. doi: 10.1080/080352502317371698. [DOI] [PubMed] [Google Scholar]
- 21.Norton ME, Merrill J, Cooper BA, Kuller JA, Clyman RI. Neonatal complications after the administration of indomethacin for preterm labor. N Engl J Med. 1993;329:1602–1607. doi: 10.1056/NEJM199311253292202. [DOI] [PubMed] [Google Scholar]
- 22.Thomas RL, Parker GC, Van Overmeire B, Aranda JV. A meta-analysis of ibuprofen versus indomethacin for closure of patent ductus arteriosus. Eur J Pediatr. 2005;164:135–140. doi: 10.1007/s00431-004-1596-5. [DOI] [PubMed] [Google Scholar]
- 23.Van Overmeire B, Follens I, Hartmann S, Creten WL, Van Acker KJ. Treatment of patent ductus arteriosus with ibuprofen. Arch Dis Child Fetal Neonatal Ed. 1997;76:F179–184. doi: 10.1136/fn.76.3.f179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Overmeire B, Smets K, Lecoutere D, et al. A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med. 2000;343:674–681. doi: 10.1056/NEJM200009073431001. [DOI] [PubMed] [Google Scholar]
- 25.Reese J, Shelton EL, Slaughter JC, McNamara PJ. Prophylactic Indomethacin Revisited. J Pediatr. 2017;186:11–14. e11. doi: 10.1016/j.jpeds.2017.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis TRSE, Van Driest SL, Kannankeril PJ, Reese J. Genetics of the patent ductus arteriosus (PDA) and pharmacogenetics of PDA treatment. Seminars in Fetal and Neonatal Medicine. 2018 doi: 10.1016/j.siny.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hammerman C, Bin-Nun A, Markovitch E, Schimmel MS, Kaplan M, Fink D. Ductal closure with paracetamol: a surprising new approach to patent ductus arteriosus treatment. Pediatrics. 2011;128:e1618–1621. doi: 10.1542/peds.2011-0359. [DOI] [PubMed] [Google Scholar]
- 28.El-Khuffash A, Jain A, Corcoran D, et al. Efficacy of paracetamol on patent ductus arteriosus closure may be dose dependent: evidence from human and murine studies. Pediatr Res. 2014;76:238–244. doi: 10.1038/pr.2014.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coceani F, Olley PM. The response of the ductus arteriosus to prostaglandins. Can J Physiol Pharmacol. 1973;51:220–225. doi: 10.1139/y73-031. [DOI] [PubMed] [Google Scholar]
- 30.Elliott RB, Starling MB, Neutze JM. Medical manipulation of the ductus arteriosus. Lancet. 1975;1:140–142. doi: 10.1016/s0140-6736(75)91432-4. [DOI] [PubMed] [Google Scholar]
- 31.Freed MD, Heymann MA, Lewis AB, Roehl SL, Kensey RC. Prostaglandin E1 infants with ductus arteriosus-dependent congenital heart disease. Circulation. 1981;64:899–905. doi: 10.1161/01.cir.64.5.899. [DOI] [PubMed] [Google Scholar]
- 32.Olley PM, Coceani F, Bodach E. E-type prostaglandins: a new emergency therapy for certain cyanotic congenital heart malformations. Circulation. 1976;53:728–731. doi: 10.1161/01.cir.53.4.728. [DOI] [PubMed] [Google Scholar]
- 33.Talosi G, Katona M, Turi S. Side-effects of long-term prostaglandin E(1) treatment in neonates. Pediatr Int. 2007;49:335–340. doi: 10.1111/j.1442-200X.2007.02380.x. [DOI] [PubMed] [Google Scholar]
- 34.Wong CH, Siah KW, Lo AW. Estimation of clinical trial success rates and related parameters. Biostatistics. 2018 doi: 10.1093/biostatistics/kxx069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Finan C, Gaulton A, Kruger FA, et al. The druggable genome and support for target identification and validation in drug development. Science translational medicine. 2017;9 doi: 10.1126/scitranslmed.aag1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gashaw I, Ellinghaus P, Sommer A, Asadullah K. What makes a good drug target? Drug discovery today. 2011;16:1037–1043. doi: 10.1016/j.drudis.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 37.Hopkins AL, Groom CR. The druggable genome. Nature reviews. Drug discovery. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- 38.Bokenkamp R, van Brempt R, van Munsteren JC, et al. Dlx1 and Rgs5 in the ductus arteriosus: vessel-specific genes identified by transcriptional profiling of laser-capture microdissected endothelial and smooth muscle cells. PLoS One. 2014;9:e86892. doi: 10.1371/journal.pone.0086892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa M, Barogi S, Socci ND, et al. Gene expression in ductus arteriosus and aorta: comparison of birth and oxygen effects. Physiol Genomics. 2006;25:250–262. doi: 10.1152/physiolgenomics.00231.2005. [DOI] [PubMed] [Google Scholar]
- 40.Jin MH, Yokoyama U, Sato Y, et al. DNA microarray profiling identified a new role of growth hormone in vascular remodeling of rat ductus arteriosus. J Physiol Sci. 2011;61:167–179. doi: 10.1007/s12576-011-0133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shelton EL, Ector G, Galindo CL, et al. Transcriptional profiling reveals ductus arteriosus-specific genes that regulate vascular tone. Physiol Genomics. 2014;46:457–466. doi: 10.1152/physiolgenomics.00171.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokoyama U, Sato Y, Akaike T, et al. Maternal vitamin A alters gene profiles and structural maturation of the rat ductus arteriosus. Physiol Genomics. 2007;31:139–157. doi: 10.1152/physiolgenomics.00007.2006. [DOI] [PubMed] [Google Scholar]
- 43.Goyal R, Goyal D, Longo LD, Clyman RI. Microarray gene expression analysis in ovine ductus arteriosus during fetal development and birth transition. Pediatr Res. 2016;80:610–618. doi: 10.1038/pr.2016.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gruzdev A, Nguyen M, Kovarova M, Koller BH. PGE2 through the EP4 receptor controls smooth muscle gene expression patterns in the ductus arteriosus critical for remodeling at birth. Prostaglandins Other Lipid Mediat. 2012;97:109–119. doi: 10.1016/j.prostaglandins.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsieh YT, Liu NM, Ohmori E, et al. Transcription profiles of the ductus arteriosus in Brown-Norway rats with irregular elastic fiber formation. Circ J. 2014;78:1224–1233. doi: 10.1253/circj.cj-13-1029. [DOI] [PubMed] [Google Scholar]
- 46.Mueller PP, Drynda A, Goltz D, Hoehn R, Hauser H, Peuster M. Common signatures for gene expression in postnatal patients with patent arterial ducts and stented arteries. Cardiol Young. 2009;19:352–359. doi: 10.1017/S1047951109004260. [DOI] [PubMed] [Google Scholar]
- 47.Parikh P, Bai H, Swartz MF, Alfieris GM, Dean DA. Identification of differentially regulated genes in human patent ductus arteriosus. Experimental biology and medicine. 2016;241:2112–2118. doi: 10.1177/1535370216661778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Waleh N, Barrette AM, Dagle JM, et al. Effects of Advancing Gestation and Non- Caucasian Race on Ductus Arteriosus Gene Expression. J Pediatr. 2015;167:1033–1041. e1032. doi: 10.1016/j.jpeds.2015.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfaltzgraff ER, Shelton EL, Galindo CL, et al. Embryonic domains of the aorta derived from diverse origins exhibit distinct properties that converge into a common phenotype in the adult. J Mol Cell Cardiol. 2014;69:88–96. doi: 10.1016/j.yjmcc.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clyman RI, Couto J, Murphy GM. Patent ductus arteriosus: are current neonatal treatment options better or worse than no treatment at all? Semin Perinatol. 2012;36:123–129. doi: 10.1053/j.semperi.2011.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Quinn D, Cooper B, Clyman RI. Factors associated with permanent closure of the ductus arteriosus: a role for prolonged indomethacin therapy. Pediatrics. 2002;110:e10. doi: 10.1542/peds.110.1.e10. [DOI] [PubMed] [Google Scholar]
- 52.Dunn PM, Speidel BD. Use of oxygen to close patent ductus arteriosus in preterm infants. Lancet. 1973;2:333–334. doi: 10.1016/s0140-6736(73)90848-9. [DOI] [PubMed] [Google Scholar]
- 53.Noel S, Cassin S. Maturation of contractile response of ductus arteriosus to oxygen and drugs. Am J Physiol. 1976;231:240–243. doi: 10.1152/ajplegacy.1976.231.1.240. [DOI] [PubMed] [Google Scholar]
- 54.Reese J, O'Mara PW, Poole SD, et al. Regulation of the fetal mouse ductus arteriosus is dependent on interaction of nitric oxide and COX enzymes in the ductal wall. Prostaglandins Other Lipid Mediat. 2009;88:89–96. doi: 10.1016/j.prostaglandins.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bokenkamp R, DeRuiter MC, van Munsteren C, Gittenberger-de Groot AC. Insights into the pathogenesis and genetic background of patency of the ductus arteriosus. Neonatology. 2010;98:6–17. doi: 10.1159/000262481. [DOI] [PubMed] [Google Scholar]
- 56.Patterson DF, Pyle RL, Buchanan JW, Trautvetter E, Abt DA. Hereditary patent ductus arteriosus and its sequelae in the dog. Circ Res. 1971;29:1–13. doi: 10.1161/01.res.29.1.1. [DOI] [PubMed] [Google Scholar]
- 57.Broaddus K, Tillson M. Patent ductus arteriosus in dogs. Compend Contin Educ Vet. 2010;32:E3. [PubMed] [Google Scholar]
- 58.Schneider DJ. The patent ductus arteriosus in term infants, children, and adults. Semin Perinatol. 2012;36:146–153. doi: 10.1053/j.semperi.2011.09.025. [DOI] [PubMed] [Google Scholar]
- 59.Tripathi A, Black GB, Park YM, Jerrell JM. Prevalence and management of patent ductus arteriosus in a pediatric medicaid cohort. Clin Cardiol. 2013;36:502–506. doi: 10.1002/clc.22150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reese J, Waleh N, Poole SD, Brown N, Roman C, Clyman RI. Chronic in utero cyclooxygenase inhibition alters PGE2-regulated ductus arteriosus contractile pathways and prevents postnatal closure. Pediatr Res. 2009;66:155–161. doi: 10.1203/PDR.0b013e3181aa07eb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Staiculescu MC, Kim J, Mecham RP, Wagenseil JE. Mechanical behavior and matrisome gene expression in the aneurysm-prone thoracic aorta of newborn lysyl oxidase knockout mice. Am J Physiol Heart Circ Physiol. 2017;313:H446–H456. doi: 10.1152/ajpheart.00712.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bracken MB. Why animal studies are often poor predictors of human reactions to exposure. J R Soc Med. 2009;102:120–122. doi: 10.1258/jrsm.2008.08k033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Plenge RM, Scolnick EM, Altshuler D. Validating therapeutic targets through human genetics. Nature reviews. Drug discovery. 2013;12:581–594. doi: 10.1038/nrd4051. [DOI] [PubMed] [Google Scholar]
- 64.Hajj H, Dagle JM. Genetics of patent ductus arteriosus susceptibility and treatment. Semin Perinatol. 2012;36:98–104. doi: 10.1053/j.semperi.2011.09.019. [DOI] [PubMed] [Google Scholar]
- 65.Patel PM, Momany AM, Schaa KL, et al. Genetic Modifiers of Patent Ductus Arteriosus in Term Infants. J Pediatr. 2016;176:57–61. e51. doi: 10.1016/j.jpeds.2016.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mani A, Meraji SM, Houshyar R, et al. Finding genetic contributions to sporadic disease: a recessive locus at 12q24 commonly contributes to patent ductus arteriosus. Proc Natl Acad Sci U S A. 2002;99:15054–15059. doi: 10.1073/pnas.192582999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Smith CJ, Ryckman KK, Bahr TM, Dagle JM. Polymorphisms in CYP2C9 are associated with response to indomethacin among neonates with patent ductus arteriosus. Pediatr Res. 2017;82:776–780. doi: 10.1038/pr.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kawase K, Sugiura T, Nagaya Y, et al. Single nucleotide polymorphisms in AGTR1, TFAP2B, and TRAF1 are not associated with the incidence of patent ductus arteriosus in Japanese preterm infants. Pediatr Int. 2016;58:461–466. doi: 10.1111/ped.12861. [DOI] [PubMed] [Google Scholar]
- 69.Dagle JM, Lepp NT, Cooper ME, et al. Determination of genetic predisposition to patent ductus arteriosus in preterm infants. Pediatrics. 2009;123:1116–1123. doi: 10.1542/peds.2008-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loftin CD, Trivedi DB, Tiano HF, et al. Failure of ductus arteriosus closure and remodeling in neonatal mice deficient in cyclooxygenase-1 and cyclooxygenase-2. Proc Natl Acad Sci U S A. 2001;98:1059–1064. doi: 10.1073/pnas.031573498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Reese J, Anderson JD, Brown N, Roman C, Clyman RI. Inhibition of cyclooxygenase isoforms in late- but not midgestation decreases contractility of the ductus arteriosus and prevents postnatal closure in mice. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1717–1723. doi: 10.1152/ajpregu.00259.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reese J, Paria BC, Brown N, Zhao X, Morrow JD, Dey SK. Coordinated regulation of fetal and maternal prostaglandins directs successful birth and postnatal adaptation in the mouse. Proc Natl Acad Sci U S A. 2000;97:9759–9764. doi: 10.1073/pnas.97.17.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Segi E, Sugimoto Y, Yamasaki A, et al. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem Biophys Res Commun. 1998;246:7–12. doi: 10.1006/bbrc.1998.8461. [DOI] [PubMed] [Google Scholar]
- 74.Kharade SV, Nichols C, Denton JS. The shifting landscape of KATP channelopathies and the need for 'sharper' therapeutics. Future Med Chem. 2016;8:789–802. doi: 10.4155/fmc-2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nakanishi T, Gu H, Hagiwara N, Momma K. Mechanisms of oxygen-induced contraction of ductus arteriosus isolated from the fetal rabbit. Circ Res. 1993;72:1218–1228. doi: 10.1161/01.res.72.6.1218. [DOI] [PubMed] [Google Scholar]
- 76.Toyoshima K, Momma K, Ishii T, Nakanishi T. Dilatation of the ductus arteriosus by diazoxide in fetal and neonatal rats. Pediatr Int. 2017;59:1246–1251. doi: 10.1111/ped.13424. [DOI] [PubMed] [Google Scholar]
- 77.Demirel F, Unal S, Cetin II, Esen I, Arasli A. Pulmonary hypertension and reopening of the ductus arteriosus in an infant treated with diazoxide. J Pediatr Endocrinol Metab. 2011;24:603–605. doi: 10.1515/jpem.2011.238. [DOI] [PubMed] [Google Scholar]
- 78.Grange DK, Nichols CG, Singh GK. Cantu Syndrome and Related Disorders. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews((R)) Seattle (WA): 1993. [PubMed] [Google Scholar]
- 79.Raphemot R, Swale DR, Dadi PK, et al. Direct activation of beta-cell KATP channels with a novel xanthine derivative. Mol Pharmacol. 2014;85:858–865. doi: 10.1124/mol.114.091884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dabrowski M, Ashcroft FM, Ashfield R, et al. The novel diazoxide analog 3-isopropylamino-7-methoxy-4H-1,2,4-benzothiadiazine 1,1-dioxide is a selective Kir6.2/SUR1 channel opener. Diabetes. 2002;51:1896–1906. doi: 10.2337/diabetes.51.6.1896. [DOI] [PubMed] [Google Scholar]
- 81.Hooper CW, Delaney C, Streeter T, et al. Selective serotonin reuptake inhibitor exposure constricts the mouse ductus arteriosus in utero. Am J Physiol Heart Circ Physiol. 2016;311:H572–581. doi: 10.1152/ajpheart.00822.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Starling MB, Elliott RB. The effects of prostaglandins, prostaglandin inhibitors, and oxygen on the closure of the ductus arteriosus, pulmonary arteries and umbilical vessels in vitro. Prostaglandins. 1974;8:187–203. doi: 10.1016/0090-6980(74)90042-2. [DOI] [PubMed] [Google Scholar]
- 83.Sharpe GL, Thalme B, Larsson KS. Studies on closure of the ductus arteriosus. XI. Ductal closure in utero by a prostaglandin synthetase inhibitor. Prostaglandins. 1974;8:363–368. doi: 10.1016/0090-6980(74)90110-5. [DOI] [PubMed] [Google Scholar]
- 84.Laughon M, Bose C, Clark R. Treatment strategies to prevent or close a patent ductus arteriosus in preterm infants and outcomes. J Perinatol. 2007;27:164–170. doi: 10.1038/sj.jp.7211662. [DOI] [PubMed] [Google Scholar]
- 85.Bose CL, Laughon MM. Patent ductus arteriosus: lack of evidence for common treatments. Arch Dis Child Fetal Neonatal Ed. 2007;92:F498–502. doi: 10.1136/adc.2005.092734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McGregor TL, Van Driest SL, Brothers KB, Bowton EA, Muglia LJ, Roden DM. Inclusion of pediatric samples in an opt-out biorepository linking DNA to de-identified medical records: pediatric BioVU. Clin Pharmacol Ther. 2013;93:204–211. doi: 10.1038/clpt.2012.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Carlson B. Vanderbilt pioneers bedside genetics. Biotechnol Healthc. 2012;9:31–32. [PMC free article] [PubMed] [Google Scholar]
- 88.Saito J, Yokoyama U, Nicho N, et al. Tissue-type plasminogen activator contributes to remodeling of the rat ductus arteriosus. PLoS One. 2018;13:e0190871. doi: 10.1371/journal.pone.0190871. [DOI] [PMC free article] [PubMed] [Google Scholar]