Abstract
In mouse, retinoic acid (RA) is required for the early phase of body axis extension controlled by a population of neuromesodermal progenitors (NMPs) in the trunk called expanding-NMPs, but not for the later phase of body axis extension controlled by a population of NMPs in the tail called depleting-NMPs. Recent observations suggest that zebrafish utilize depleting-NMPs but not expanding-NMPs for body axis extension. In zebrafish, a role for RA in body axis extension was not supported by previous studies on aldh1a2 (raldh2) mutants lacking RA synthesis. Here, by treating zebrafish embryos with an RA synthesis inhibitor, we also found that body axis extension and somitogenesis was not perturbed, although loss of pectoral fin and cardiac edema were observed consistent with previous studies. The conclusion that zebrafish diverges from mouse in not requiring RA for body axis extension is consistent with zebrafish lacking early expanding-NMPs to generate the trunk. We suggest that RA control of body axis extension was added to higher vertebrates during evolution of expanding-NMPs.
Keywords: Body axis extension, somitogenesis, neuromesodermal progenitors, NMPs, retinoic acid
Graphical Abstract
1. Introduction
Retinoic acid (RA) functions as an essential signal required for several developmental processes in vertebrate embryos (Cunningham and Duester, 2015; Rhinn and Dolle, 2012). In mouse, studies on Aldh1a2 (Raldh2) knockout embryos lacking endogenous RA synthesis revealed that RA generated at E7.5–E8.5 is required to ensure normal body axis extension (somitogenesis), forelimb bud initiation, and heart anterorposterior patterning (Mic et al., 2002; Niederreither et al., 1999; Ryckebusch et al., 2008; Sirbu et al., 2008; Zhao et al., 2009). Interestingly, all three of these defects are associated with a loss of Fgf8 repression by RA, i.e. ectopic anterior expansion of the caudal Fgf8 expression domain that encroaches into the developing trunk were somitogenesis occurs (Sirbu and Duester, 2006; Vermot et al., 2005), and ectopic posterior expansion of the heart Fgf8 expression domain that encroaches into the forelimb field (Cunningham et al., 2013). In vivo mechanistic studies revealed that mouse Fgf8 contains an upstream RA response element that recruits nuclear receptor corepressors NCOR1/NCOR2, Polycomb Repressive Complex 2 (PRC2), and histone deacetylase 1 (HDAC1) in an RA-dependent fashion to repress caudal Fgf8 so that its expression does not encroach into the developing trunk during body axis extension (Kumar et al., 2016; Kumar and Duester, 2014). The mechanism through which RA repression limits the size of the heart Fgf8 expression domain remains unknown.
The role of RA in other vertebrate embryos has revealed some conserved functions. For instance, zebrafish aldh1a2 null mutants do not develop forelimbs (pectoral fins) similar to mouse (Begemann et al., 2001; Grandel et al., 2002). Pectoral fin outgrowth is rescued in zebrafish aldh1a2 mutants expressing a dominant-negative FGF receptor, demonstrating that RA antagonizes FGF similar to mouse (Cunningham et al., 2013). Also, zebrafish lacking RA synthesis exhibit heart anteroposterior patterning defects due to ectopic heart Fgf8 expression similar to mouse (Sorrell and Waxman, 2011). However, zebrafish aldh1a2 mutants exhibit normal body axis extension (Begemann et al., 2001) and caudal Fgf8 expression in zebrafish does not expand ectopically in the absence of RA (Sorrell and Waxman, 2011), thus potentially revealing a difference with mouse during body axis extension.
Other studies demonstrated that maternal aldh1a2 mRNA in zebrafish embryos prevents complete loss of RA synthesis during the early stages of embryogenesis (Alexa et al., 2009). In those studies, aldh1a2 mutants exhibited a pancreas defect, but treatment with the Aldh1a2 inhibitor 4-diethylaminobenzaldehyde (DEAB) reduced RA activity more than aldh1a2 mutants and a more severe pancreas defect was observed; the focus of this paper was pancreas development, but body axis extension still appeared relatively normal although somite staining was not reported (Alexa et al., 2009). With the use of an aldh1a2 morpholino, it was reported that zebrafish requires RA for somite bilateral symmetry (Kawakami et al., 2005) similar to mouse (Sirbu and Duester, 2006; Vermot et al., 2005) and chick (Vermot and Pourquié, 2005), although the reported effect in zebrafish was relatively mild and there was no effect on somite size or body axis length; as this result contrasts with studies on aldh1a2 mutant zebrafish which do not exhibit somite left-right asymmetry, it is possible that the morpholino caused a non-specific laterality side-effect. The zebrafish T/Bra homolog ntl was reported to activate caudal expression of an RA-degrading enzyme cyp26a1 (Martin and Kimelman, 2010) similar to mouse where Fgf8 has been found to activate caudal Cyp26a1 to ensure that RA generated in the trunk does not interfere with outgrowth during body axis extension (Wahl et al., 2007). However, the presence of caudal cyp26a1 expression does not necessarily mean that RA is required for zebrafish body axis extension, just that RA diffusing from the posterior trunk (where it is needed for spinal cord development) disrupts some aspect of caudal outgrowth. Thus, most evidence suggests that zebrafish does not require RA for body axis extension, but some doubt may remain.
Recent studies on body axis extension in mouse and zebrafish embryos revealed that bipotential neuromesodermal progenitors (NMPs) serve as the source of both spinal cord neural progenitors and somitic mesodermal progenitors (Henrique et al., 2015; Kimelman, 2016; Kondoh and Takemoto, 2012; Wilson et al., 2009). During body axis extension, differentiation of NMPs to either neuroectodermal or presomitic mesodermal progeny is coordinated to enable generation of a spinal cord surrounded by somites to form the posterior body axis (Martin and Kimelman, 2009; Nowotschin et al., 2012; Tzouanacou et al., 2009). RA is required for balanced NMP differentiation in amniote embryos. Studies on mouse Aldh1a2−/− embryos showed that loss of RA activity resulted in reduced appearance of the spinal cord lineage and increased appearance of the presomitic mesoderm lineage associated with segmentation defects leading to small somites (Cunningham et al., 2015). RA-deficient quail embryos also exhibit delayed appearance of spinal cord neural progenitors and small somites (Diez del Corral et al., 2003). Loss of RA in mouse or quail results in ectopic expression of caudal Fgf8 that expands into the developing trunk (Cunningham et al., 2015; Diez del Corral et al., 2003; Sirbu and Duester, 2006; Vermot et al., 2005; Vermot and Pourquié, 2005), leading to the hypothesis that a major conserved role of RA is antagonism of caudal FGF signaling to control body axis extension in amniotes (Cunningham and Duester, 2015; Henrique et al., 2015).
A recent comparison of NMPs in various vertebrate embryos suggested the existence of two different types of NMPs, with mouse utilizing both types but zebrafish utilizing just one type (Steventon and Martinez Arias, 2017). According to this hypothesis, mouse utilizes a population of early NMPs called expanding-NMPs that generate somites/spinal cord in the trunk, then a later population of NMPs in the tailbud called depleting-NMPs that generate somites/spinal cord in the tail until these NMPs are completely depleted and body axis extension ends. In contrast, due to differences in gastrulation with mouse, zebrafish generate both head structures and trunk somites/spinal cord during gastrulation convergence and extension, following by utilization of depleting-NMPs in the tailbud to generate tail somites/spinal cord. Thus, zebrafish lack the expanding-NMP population (Steventon and Martinez Arias, 2017). Intriguingly, studies on mouse Aldh1a2−/− embryos demonstrated that RA is required for caudal Fgf8 repression in the developing trunk (somites 1–25) but not afterward during formation of tail somites (Cunningham et al., 2011). Thus, during mouse body axis extension, RA function correlates with expanding-NMPs but not depleting-NMPs.
2. Loss of RA does not perturb zebrafish body axis extension or somitogenesis
Previous studies on zebrafish aldh1a2 mutants have not reported defects in body axis extension (Begemann et al., 2001). As zebrafish embryos possess maternal aldh1a2 mRNA, the zebrafish aldh1a2 mutant may show less severe defects than mouse Aldh1a2−/− embryos (Alexa et al., 2009). However, treatment of zebrafish embryos with DEAB to inhibit Aldh1a2 enzyme activity eliminates RA synthesis and results in defects similar to mouse Aldh1a2−/− embryos (Alexa et al., 2009); also, as the loss of pectoral fin and heart defects observed in aldh1a2 mutant zebrafish are phenocopied in DEAB-treated zebrafish, DEAB treatment has been demonstrated to be a reliable method of eliminating RA activity in zebrafish (Alexa et al., 2009; Gibert et al., 2006; Sorrell and Waxman, 2011).
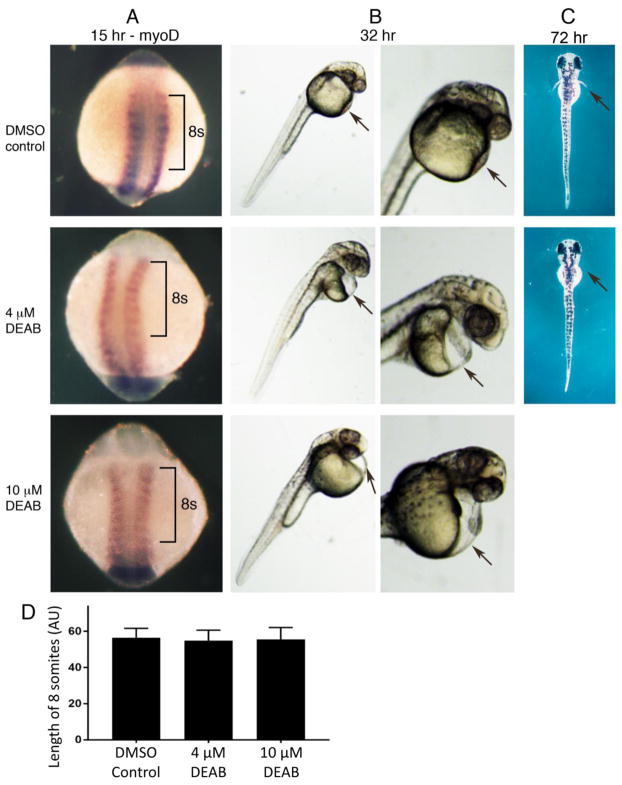
Here, we treated zebrafish embryos from 5 hours post-fertilization (hpf), which is prior to somitogenesis that begins at 10 hpf, until 15 hpf (10–12 somite stage) with solvent control, 4 μM DEAB, or 10 μM DEAB. Embryos were examined at 15 hpf for myoD expression which marks somites. We found that all three groups of fish had similar size somites with no significant difference in body length along the anteroposterior axis over an 8-somite stretch, and no defects in left-right bilateral symmetry; solvent control (n = 62), 4 μM DEAB (n = 108), and 10 μM DEAB (n = 101) (Fig. 1A, D);. In contrast, previous studies on mouse Aldh1a2 knockout embryos reported that somite size is greatly reduced such that the anteroposterior axis along the trunk is shortened to approximately half its normal length (Cunningham et al., 2015; Mic et al., 2002; Niederreither et al., 1999).
Fig. 1.
Loss of RA synthesis does not perturb zebrafish body axis extension. (A) Embryos treated with DMSO (control), 4 μM DEAB, or 10 μM DEAB from 5–15 hpf, followed by analysis of myoD expression. Brackets mark an 8 somite region in each embryo that is unchanged in length along the anteroposterior axis following DEAB treatment used to inhibit RA synthesis. (B) Embryos treated as described above from 5–15 hpf followed by a return to normal growth medium until 32 hpf. Arrows point to the heart region showing cardiac edema in embryos treated with DEAB. (C) Shown are embryos treated with DMSO (control) or 4 μM DEAB from 9.5–15 hpf followed by a return to normal growth medium until 72 hpf when it can be observed that DEAB treatment results in failure of pectoral fin outgrowth (arrows). (D) Comparison of body axis length along an 8-somite region in embryos at 15 hpf across the indicated treatment conditions; data are expressed as mean ± SD. For all comparisons, p > 0.05 (not significant difference) using one-way ANOVA non-parametric test (DMSO control, n = 62; 4 μM DEAB n = 108; 10 μM DEAB n = 101; each are biological replicates). AU, arbitrary units.
Some embryos treated from 5–15 hpf were returned to normal growth medium at 15 hpf and allowed to grow until 32 hpf. At 32 hpf we noticed that all embryos treated from 5–15 hpf with 4 μM DEAB or 10 μM DEAB exhibited cardiac edema; solvent control (n = 49), 4 μM DEAB (n = 71), and 10 μM DEAB (n = 66) (Fig. 1B). As DEAB inhibition of RA synthesis has well-documented effects on heart development including cardiac edema (Sorrell and Waxman, 2011), it is clear that our DEAB treatment was effective. Also, to verify that our DEAB treatment has an effect on pectoral fin outgrowth as previously reported, zebrafish embryos were treated with 4 μM DEAB from 9.5 hpf (bud stage) to 15 hpf (10–12 somites), then returned to normal growth medium until 72 hpf as previously reported (Gibert et al., 2006). All embryos treated with 4 μM DEAB were found to lack pectoral fins (n = 30) whereas all solvent controls had normal pectoral fins (n = 30) (Fig. 1C). Altogether, our studies confirm the view that zebrafish does not require RA for body axis extension.
3. Discussion
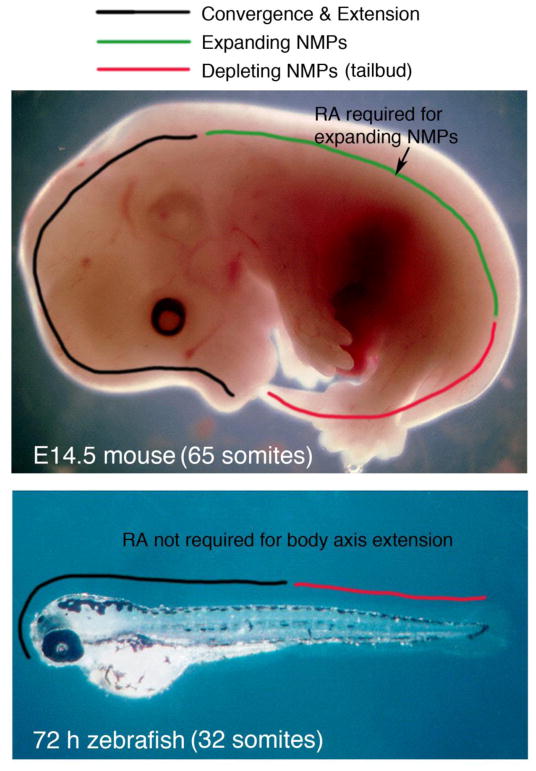
Formation of the vertebrate body axis begins with generation of the head during gastrulation convergence and extension movements, followed by body axis extension in which NMPs generate a major portion of the posterior body axis, i.e. spinal cord and surrounding somites. Recent studies suggest that mouse and zebrafish body axis extension occurs differently in that mouse utilizes a population of NMPs called expanding-NMPs that extend the trunk body axis immediately after the head has formed, whereas the zebrafish trunk is formed by continued gastrulation convergence and extension movements after the head forms; following formation of trunk spinal cord and somites, mouse and zebrafish both use another population of NMPs called depleting-NMPs to generate the tail spinal cord and somites (Steventon and Martinez Arias, 2017). Previous studies have also demonstrated that the RA requirement for mouse body axis extension is limited to control of the trunk somites and not required for tail somites (Cunningham et al., 2011). Together with our studies here confirming that zebrafish does not require RA for body axis extension and somitogenesis, we propose a model for body axis extension that supports two different populations of NMPs in which RA controls the expanding-NMP population but not the depleting-NMP population (Fig. 2).
Fig. 2.
Model comparing mechanisms of body axis extension in zebrafish and mouse. Mouse and zebrafish body axis extension occurs differently in that mouse utilizes expanding-NMPs that extend the trunk body axis (spinal cord and somites), whereas the zebrafish trunk is formed by continued gastrulation convergence and extension movements after the head forms; both mouse and zebrafish utilize depleting-NMPs to generate the tail spinal cord and somites (Steventon and Martinez Arias, 2017). Our studies here show that zebrafish body axis extension does not require RA, whereas previous studies demonstrated that the mouse RA requirement for body axis extension is limited to the trunk somites (Cunningham et al., 2011), suggesting that RA controls expanding-NMPs but not depleting-NMPs.
In mouse, RA repression of caudal Fgf8 is required for proper differentiation of NMPs to ensure balanced production of neural and somitic tissues during body axis extension (Cunningham et al., 2015), but this role is limited to trunk NMPs as tail NMPs do not require RA to repress caudal Fgf8 (Cunningham et al., 2011). Similar to mouse tail NMPs, zebrafish tail NMPs do not require RA to repress caudal fgf8 as loss of RA does not result in anterior expansion of caudal fgf8 expression (Sorrell and Waxman, 2011). Whether RA plays other roles in NMP biology in addition to differentiation is less convincing. For instance, RA does not appear to be needed for maintenance of NMPs as mouse and zebrafish tail NMPs do not require RA. There exists convincing evidence that RA is not required for establishment of NMPs as mouse Aldh1a2−/− embryos, that completely lack RA activity when NMPs are first established at E8.0, still produce at least 20 somites (albeit small) and spinal cord (albeit with altered differentiation) (Cunningham et al., 2015). In contrast, loss of T/Bra or Cdx genes does result in a failure of NMP establishment with no body axis extension beyond the hindbrain (Amin et al., 2016). However, a recent report examining mouse embryonic stem cell-derived NMPs in vitro suggested that RA may play a role in NMP establishment, and it was proposed that the presence of NMPs in mouse Aldh1a2−/− embryos may be due to maternal RA reaching the embryo via extraembryonic tissues (Gouti et al., 2017). However, this proposed mechanism is not supported by in vivo studies showing that several RA-degrading enzymes (Cyp26a1, Cyp26b1, and Cyp26c1) are expressed in mouse extraembryonic tissues beginning at E6, thus preventing maternal RA from entering the embryo proper and limiting the source of embryonic RA to embryonic cells that synthesize endogenous RA (Uehara et al., 2009). Thus, the most parsimonious model for RA action during vertebrate body axis extension is one in which RA repression of caudal Fgf8 controls differentiation of expanding-NMPs in vertebrate embryos that possess this type of NMP including mouse, chick, and perhaps other amniotes.
In fish, RA control of pectoral fin (forelimb) initiation and heart anteroposterior patterning demonstrates that RA signaling evolved early to control some developmental pathways common to all vertebrates, but not other pathways such as body axis extension. With our observations here and those reported previously (Steventon and Martinez Arias, 2017) we suggest that RA control of body axis extension was added to higher vertebrates during evolution, coincident with evolution of trunk expanding-NMPs that so far have been reported only in amniotes.
4. Materials and methods
4.1. Generation of zebrafish embryos and treatment protocol
Zebrafish embryos were obtained from standard matings of wild-type fish. All zebrafish studies conformed to the regulatory standards adopted by the Institutional Animal Care and Use Committee at the Sanford Burnham Prebys Medical Discovery Institute which approved this study under Animal Welfare Assurance Number A3053-01 (permit #15-104).
Zebrafish embryos were treated with 4-diethylaminobenzaldehyde (DEAB) to inhibit RA synthesis as previously described with a few modifications (Alexa et al., 2009; Gibert et al., 2006; Sorrell and Waxman, 2011). DEAB (Sigma Chemical Co., inc.) was dissolved in dimethylsulfoxide (DMS0) and 1000x stock solutions were stored at −20C in single-use tubes for no more than one month. For studies on body axis extension, embryos were treated from 5 hpf to 15 hpf with growth medium containing 0.1% DMSO control solvent, 4 μM DEAB, or 10 μM DEAB, after which embryos were collected for whole-mount in situ hybridization or placed in normal growth medium until 32 hpf. For analysis of pectoral fins, embryos were treated from 9.5 hpf to 15 hpf with growth medium containing 0.1% DMSO control solvent or 4 μM DEAB, after which embryos were placed in normal growth medium until 72 hpf.
4.2. Gene expression analysis
Embryos were fixed in paraformaldehyde at 4°C overnight, dehydrated into methanol, and stored at −20°C. Detection of mRNA was performed by whole mount in situ hybridization as previously described (Thisse and Thisse, 2008).
4.3. Measurements and statistical analysis
ImageJ software (https://imagej.net) (Schneider et al., 2012) was used to measure body axis length along an 8-somite region at 15 hpf, with each specimen photographed at the same magnification. Statistical analysis was performed using one-way ANOVA (non-parametric test) to compare across all treatment conditions with data presented as mean ± standard deviation (SD) and with p > 0.05 indicating non-significance.
Highlights.
In mouse, but not zebrafish, body axis extension requires retinoic acid (RA) signaling.
Mouse requires RA to control expanding-NMPs in trunk but not depleting-NMPs in tail.
Zebrafish lacks expanding-NMPs in trunk but does possess depleting-NMPs in tail.
Lack of expanding-NMPs in zebrafish is consistent with RA-free body axis extension.
Acknowledgments
This work was funded by National Institutes of Health grant R01 AR067731 (G.D.).
Footnotes
AUTHOR CONTRIBUTIONS
M.B. and G.D. designed the study, analyzed the data and wrote the paper. M.B., T.J.C., and J.J.L., performed the experiments. J.J.L. and P.D.S.D. provided zebrafish for the studies. All authors discussed the results and commented on the manuscript.
DECLARATION OF INTERESTS
The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexa K, Choe SK, Hirsch N, Etheridge L, Laver E, Sagerstrom CG. Maternal and zygotic aldh1a2 activity is required for pancreas development in zebrafish. PLOS ONE. 2009;4:e8261. doi: 10.1371/journal.pone.0008261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin S, Neijts R, Simmini S, van Rooijen C, Tan SC, Kester L, van Oudenaarden A, Creyghton MP, Deschamps J. Cdx and T Brachyury Co-activate Growth Signaling in the Embryonic Axial Progenitor Niche. Cell Reports. 2016;17:3165–3177. doi: 10.1016/j.celrep.2016.11.069. [DOI] [PubMed] [Google Scholar]
- Begemann G, Schilling TF, Rauch GJ, Geisler R, Ingham PW. The zebrafish neckless mutation reveals a requirement for raldh2 in mesodermal signals that pattern the hindbrain. Development. 2001;128:3081–3094. doi: 10.1242/dev.128.16.3081. [DOI] [PubMed] [Google Scholar]
- Cunningham TJ, Brade T, Sandell LL, Lewandoski M, Trainor PA, Colas A, Mercola M, Duester G. Retinoic Acid Activity in Undifferentiated Neural Progenitors Is Sufficient to Fulfill Its Role in Restricting Fgf8 Expression for Somitogenesis. PLOS ONE. 2015;10:e0137894. doi: 10.1371/journal.pone.0137894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TJ, Duester G. Mechanisms of retinoic acid signalling and its roles in organ and limb development. Nature Rev Mol Cell Bio. 2015;16:110–123. doi: 10.1038/nrm3932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TJ, Zhao X, Duester G. Uncoupling of retinoic acid signaling from tailbud development before termination of body axis extension. Genesis. 2011;49:776–783. doi: 10.1002/dvg.20763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham TJ, Zhao X, Sandell LL, Evans SM, Trainor PA, Duester G. Antagonism between retinoic acid and fibroblast growth factor signaling during limb development. Cell Reports. 2013;3:1503–1511. doi: 10.1016/j.celrep.2013.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez del Corral R, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- Gibert Y, Gajewski A, Meyer A, Begemann G. Induction and prepatterning of the zebrafish pectoral fin bud requires axial retinoic acid signaling. Development. 2006;133:2649–2659. doi: 10.1242/dev.02438. [DOI] [PubMed] [Google Scholar]
- Gouti M, Delile J, Stamataki D, Wymeersch FJ, Huang Y, Kleinjung J, Wilson V, Briscoe J. A Gene Regulatory Network Balances Neural and Mesoderm Specification during Vertebrate Trunk Development. Dev Cell. 2017;41:243–261. doi: 10.1016/j.devcel.2017.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandel H, Lun K, Rauch GJ, Rhinn M, Piotrowski T, Houart C, Sordino P, Küchler AM, Schulte-Merker S, Geisler R, Holder N, Wilson SW, Brand M. Retinoic acid signalling in the zebrafish embryo is necessary during pre-segmentation stages to pattern the anterior-posterior axis of the CNS and to induce a pectoral fin bud. Development. 2002;129:2851–2865. doi: 10.1242/dev.129.12.2851. [DOI] [PubMed] [Google Scholar]
- Henrique D, Abranches E, Verrier L, Storey KG. Neuromesodermal progenitors and the making of the spinal cord. Development. 2015;142:2864–2875. doi: 10.1242/dev.119768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Raya A, Raya RM, Rodriguez-Esteban C, Izpisúa-Belmonte JC. Retinoic acid signalling links left-right asymmetric patterning and bilaterally symmetric somitogenesis in the zebrafish embryo. Nature. 2005;435:165–171. doi: 10.1038/nature03512. [DOI] [PubMed] [Google Scholar]
- Kimelman D. Tales of Tails (and Trunks): Forming the Posterior Body in Vertebrate Embryos. Curr Top Dev Biol. 2016;116:517–536. doi: 10.1016/bs.ctdb.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondoh H, Takemoto T. Axial stem cells deriving both posterior neural and mesodermal tissues during gastrulation. Curr Opin Genet Dev. 2012;22:374–380. doi: 10.1016/j.gde.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Kumar S, Cunningham TJ, Duester G. Nuclear receptor corepressors Ncor1 and Ncor2 (Smrt) are required for retinoic acid-dependent repression of Fgf8 during somitogenesis. Dev Biol. 2016;418:204–215. doi: 10.1016/j.ydbio.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Duester G. Retinoic acid controls body axis extension by directly repressing Fgf8 transcription. Development. 2014;141:2972–2977. doi: 10.1242/dev.112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BL, Kimelman D. Wnt signaling and the evolution of embryonic posterior development. Curr Biol. 2009;19:R215–219. doi: 10.1016/j.cub.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BL, Kimelman D. Brachyury establishes the embryonic mesodermal progenitor niche. Genes Dev. 2010;24:2778–2783. doi: 10.1101/gad.1962910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, Haselbeck RJ, Cuenca AE, Duester G. Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development. 2002;129:2271–2282. doi: 10.1242/dev.129.9.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nature Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Nowotschin S, Ferrer-Vaquer A, Concepcion D, Papaioannou VE, Hadjantonakis AK. Interaction of Wnt3a, Msgn1 and Tbx6 in neural versus paraxial mesoderm lineage commitment and paraxial mesoderm differentiation in the mouse embryo. Dev Biol. 2012;367:1–14. doi: 10.1016/j.ydbio.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhinn M, Dolle P. Retinoic acid signalling during development. Development. 2012;139:843–858. doi: 10.1242/dev.065938. [DOI] [PubMed] [Google Scholar]
- Ryckebusch L, Wang Z, Bertrand N, Lin SC, Chi X, Schwartz R, Zaffran S, Niederreither K. Retinoic acid deficiency alters second heart field formation. Proc. Natl. Acad. Sci. USA. 2008;105:2913–2918. doi: 10.1073/pnas.0712344105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nature Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu IO, Duester G. Retinoic acid signaling in node ectoderm and posterior neural plate directs left-right patterning of somitic mesoderm. Nature Cell Biol. 2006;8:271–277. doi: 10.1038/ncb1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirbu IO, Zhao X, Duester G. Retinoic acid controls heart anteroposterior patterning by down-regulating Isl1 through the Fgf8 pathway. Dev Dyn. 2008;237:1627–1635. doi: 10.1002/dvdy.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorrell MR, Waxman JS. Restraint of Fgf8 signaling by retinoic acid signaling is required for proper heart and forelimb formation. Dev Biol. 2011;358:44–55. doi: 10.1016/j.ydbio.2011.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steventon B, Martinez Arias A. Evo-engineering and the cellular and molecular origins of the vertebrate spinal cord. Dev Biol. 2017;432:3–13. doi: 10.1016/j.ydbio.2017.01.021. [DOI] [PubMed] [Google Scholar]
- Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nature Protocols. 2008;3:59–69. doi: 10.1038/nprot.2007.514. [DOI] [PubMed] [Google Scholar]
- Tzouanacou E, Wegener A, Wymeersch FJ, Wilson V, Nicolas JF. Redefining the progression of lineage segregations during mammalian embryogenesis by clonal analysis. Dev Cell. 2009;17:365–376. doi: 10.1016/j.devcel.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Uehara M, Yashiro K, Takaoka K, Yamamoto M, Hamada H. Removal of maternal retinoic acid by embryonic CYP26 is required for correct Nodal expression during early embryonic patterning. Genes Dev. 2009;23:1689–1698. doi: 10.1101/gad.1776209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermot J, Llamas JG, Fraulob V, Niederreither K, Chambon P, Dollé P. Retinoic acid controls the bilateral symmetry of somite formation in the mouse embryo. Science. 2005;308:563–566. doi: 10.1126/science.1108363. [DOI] [PubMed] [Google Scholar]
- Vermot J, Pourquié O. Retinoic acid coordinates somitogenesis and left-right patterning in vertebrate embryos. Nature. 2005;435:215–220. doi: 10.1038/nature03488. [DOI] [PubMed] [Google Scholar]
- Wahl MB, Deng C, Lewandoski M, Pourquie O. FGF signaling acts upstream of the NOTCH and WNT signaling pathways to control segmentation clock oscillations in mouse somitogenesis. Development. 2007;134:4033–4041. doi: 10.1242/dev.009167. [DOI] [PubMed] [Google Scholar]
- Wilson V, Olivera-Martinez I, Storey KG. Stem cells, signals and vertebrate body axis extension. Development. 2009;136:1591–1604. doi: 10.1242/dev.021246. [DOI] [PubMed] [Google Scholar]
- Zhao X, Sirbu IO, Mic FA, Molotkova N, Molotkov A, Kumar S, Duester G. Retinoic acid promotes limb induction through effects on body axis extension but is unnecessary for limb patterning. Curr Biol. 2009;19:1050–1057. doi: 10.1016/j.cub.2009.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]