Abstract
Prior research has indicated that as an important biomarker of chronic low-grade inflammation, high-sensitivity C-reactive protein (hs-CRP) can play important roles on the onset of metabolic syndrome and cardiovascular diseases (CVD). We conducted an integrative approach, which combines biological wet-lab experiments, statistical analysis, and semantics-oriented bioinformatics & computational analysis, to investigate the association among hs-CRP, body fat mass (FM) distribution, and other cardiometabolic risk factors in young healthy women. Research outcomes in this study resulted in two novel discoveries. Discovery 1: There are four primary determinants for hs-CRP, i.e., central/abdominal FM (a.k.a. trunk FM) accumulation, leptin, high density lipoprotein cholesterol (HDL-C), and plasminogen activator inhibitior-1 (PAI-1). Discovery 2: Chronic inflammation may involve in adipocyte-cytokine interaction underlying the metabolic derangement in healthy young women.
Keywords: C-reactive protein, adipocytokines, inflammation marker, microRNA, bio-ontology, semantic search
1. Introduction
Prior research [1] has demonstrated that chronic low-grade inflammation is one of the important characteristics of obesity-related metabolic syndrome. Adipose tissue is known [2, 3] to be a source of cytokines such as tumor necrosis factor-α (TNF-α) and interleukin-6 (IL-6), where the latter stimulates hepatocytes to produce a variety of acute phase reactants including C-reactive protein (CRP) [4]. As a representative biomarker of inflammation in the body, long-term elevation of CRP may have prognostic value in predicting individuals with increased risk of developing cardiovascular diseases (CVD) [5]. In fact, prospective studies [5–7] have discovered that CRP may add moderate forecasting power to predict the development of CVD in middle-aged or elderly persons who have already been frequently accompanied with typical CVD risk conditions such as dyslipidemia, insulin resistance, or cigarettes smoking. In particular, according to the study in [8], CRP was shown to be a strong risk predictor for myocardial infraction even 20 years after the initial blood samples were collected. However, little research to date has investigated the association between high-sensitivity CRP (hs-CRP) and obesity-related metabolic phenotypes (including atherosclerosis) in young people without classical CVD risk factors. Such association study is of particular importance for us to better prevent and intervene the onset of metabolic syndrome and CVD. Towards this end, we conducted an integrative study in young healthy women to investigate the association between hs-CRP and body fat mass (FM) distribution, as well as the association between hs-CRP and other cardiometabolic risk factors. Our approach effectively combines biological wet-lab experiments, statistical analysis, and semantics-oriented bioinformatics & computational analysis.
The rest of this paper is organized as follows. Section 2 describes in detail our methods and materials; Section 3 reports experimental results along with discussion; and finally, Section 4 concludes with future work.
2. Methods and materials
2.1 Overview of our integrative methods
Our methods consist of three steps.
Step I: Biological experiments
We recruited a cohort of healthy young women without conventional cardiovascular risk factors and then performed a series of wet-lab experiments on these subjects. Our experiments were organized into five categories: (1) anthropometry, body composition, and FM distribution; (2) insulin, glucose, and insulin resistance; (3) plasma lipids, lipoprotein, and Apo measurements; (4) inflammation markers, oxidative stress marker, and adipocytokines; and (5) arterial properties.
Step II: Statistical analysis
After obtaining the results from biological experiments, we conducted statistical analysis including univariate analysis and multiple regression analysis. These analysis results provided us with evidence to either support or discourage possible association among hs-CRP, FM distribution, and other cardiometabolic risk factors.
Step III: Semantics-oriented bioinformatics and computational analysis
We utilized an ontology-based analytical software tool to obtain a set of computationally putative microRNA (miR) molecules that may regulate hs-CRP. Besides, rich, additional data for each candidate miR were also provided to us through the software user interface. Such semantically federated knowledge further assisted us in better analyzing results from the first two steps.
2.2 Biological wet-lab experiment design
2.2.1 Subjects
A total of 308 female students at Mukogawa Women’s University (MWU) were recruited in this study. All subjects were Japanese and aged between 18 to 22 years old. We excluded any subjects with clinically diagnosed CVD, acute or chronic inflammatory diseases, endocrine diseases, hepatic diseases, and renal diseases. Subjects with hormonal contraception, regular cigarette smoking and alcohol drinking, and unusual dietary habits were also excluded. In addition, no subject was receiving any medications during the study period. Our study was approved by the Ethics Committees at MWU, and written informed consents were obtained from all participants.
2.2.2 Anthropometry, body composition, and FM distribution
Body weight, height, waist circumferences (WC) were measured following standard procedures, and body mass index (BMI) was then calculated. Dual-energy X-ray absorptiometry (DXA)-derived trunk FM was demonstrated [9] to have strong association with total abdominal adiposity measured by computer tomography (CT). Moreover, when combined with anthropometry, DXA offers a good alternative to CT for the prediction of abdominal adiposity. DXA was thus recommended for the early detection of central/abdominal obesity. In this study, DXA with a scanner (Hologic QDR-2000, Waltham, MA) was utilized to measure body FM distribution. A scanned image of the whole body was divided into six sub-regions: head, trunk, and upper/lower limbs on both sides. The dividing borders between those subregions were differentiated by a line underneath the chin, a line between the humerus head and the glenoid fossa, and a line at the femoral neck. The trunk region included the chest and abdomen, excluding the pelvis. The low body region included the entire hip, thigh, and leg. To better express regional fat deposition, we introduced four body fat ratio parameters denoted as %X FM, where X stands for Total, Trunk, Arm, or Lower-body, measuring the percentage of corresponding fat tissue weight over body weight. That is, %Total FM, %Trunk FM, %Arm FM, and %Lower-body FM were calculated as , , , and , respectively.
2.2.3 Insulin, glucose, and insulin resistance
Blood samples were obtained in the morning after 12-hr overnight fast. Insulin resistance determined by homeostasis model assessment (HOMA-IR) was calculated using fasting plasma glucose and insulin levels [10].
2.2.4 Plasma lipids, lipoprotein, and Apo measurements
Serum lipids, including triglycerides (TG), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), and free fatty acid (FFA), were measured with an autoanalyzer (AU5232, Olympus, Tokyo, Japan). Apolipoprotein A-1 (ApoA1) and apolipoprotein B-100 (ApoB) were measured by an Olympus autoanalyzer (AU600, Mitsubishi Chemicals, Tokyo, Japan). Low-density lipoprotein cholesterol (LDL-C) was determined using the Friedewald formula [11]. Small density LDL-C (sd-LDL) was measured with a precipitation method described in [12]. Remnant-like particle-cholesterol (RLP-C) was measured by an immunoaffinity separation method (RLP-C assay, Otsuka, Japan). Lipoprotein lipase (LPL) was determined by enzyme-linked immunosorbent assay using an assay system (Dai-ichi Pure Chemicals, Tokyo, Japan).
2.2.5 Inflammation markers, oxidative stress marker, and adipocytokines
Hs-CRP was measured by an immunoturbidometric assay with the use of reagents and calibrators from Dade Behring Marbura GmbH (Marburg, Germany; interassay CV < 5.0%, CV: coefficients of variance). TNF-α was measured by immunoassays (R&D Systems, Inc., Minneapolis, MN; interassay CV = 6.0%). PAI-1 was measured by an ELISA method (Mitsubishi Chemicals; interassay CV = 8.1 %). For the purpose of statistical analysis, serum concentrations of hs-CRP and TNF-α below the limit of detection were assigned a value of 0.05mg/L and 0.50pg/mL (the lowest limit of detection), respectively. Systemic oxidative stress was evaluated by urinary creatinine-indexed 8-epi-prostagland in F-2α (8-epi-PG2α), a validated biomarker of oxidative stress [13]. Urinary 8-epi-PGF2α was measured in the first-voided morning urine sample with an enzyme-liked immunosorbent assay (8-Isoprostane EIA kit, Cayman, Ann Arbor, MI). Intra- and inter- assay CV were 7.5% and 9.2%, respectively. Urinary 8-epi-PG2α was indexed to creatinine as pictograms per millimole creatitine. Adiponectin was assayed by a sandwich enzyme-linked immunosorbent assay (Otsuka Pharmaceutical Co. Ltd., Tokushima City, Japan). Intra- and inter- assay CV were 3.3% and 7.5%, respectively. Leptin was assessed by an RIA kit (LINCO research, St. Charles, MO; interassay CV = 4.9%).
2.2.6 Arterial properties
Arterial stiffness was indicated by cardio-ankle vascular index (CAVI) measured by VaSera device (VS-1000, Fukuda Denshi, Tokyo, Japan). CAVI is a recently developed index that reflects stiffness of the aorta, femoral, and tibial artery [14]. CAVI involves the measurement of pulse valve velocity (PWV) but the effects of blood pressure are minimized. CAVI was thus proved to be a reliable screening tool for atherosclerosis [14]. Because carotid intima-media thickness (IMT) was clinically used as an indicator of generalized atherosclerosis [15], arterial thickness was evaluated by carotid artery IMT measured with an ultrasonic device (SDU-1100, Shimadzu, Tokyo, Japan). The maximal IMT was assessed at the far wall as the distance between the lumen-intima interface and media-adventitia interface. The maximal IMT of two measurements conducted at each of the four segments vessels was recorded on both sides and then averaged for both sides. The mean IMT (Avg IMT) and maximal IMT (Max IMT) of the four IMT values were used for analysis.
2.3 Statistical analysis
All data were presented as mean ± standard deviation (SD). Due to deviation from normal distribution, hs-CRP was logarithmic transformed for analysis. (1) First of all, univariate correlations between hs-CRP and regional FM distribution, as well as between hs-CRP and other metabolic parameters, were evaluated with Pearson correlation coefficients. (2) Secondly, stepwise multiple regression analysis was performed to further identify the most significant variables contributing to the variation of hs-CRP. (3) Thirdly, all variables with statistically significant association with hs-CRP in univariate analysis were entered into the model simultaneously, excluding those variables having the least significant P value. (4) Finally, all variables with P ≤ 0.05 remained in the model. All statistical analyses were performed with SPSS system 15.0 (SPSS Inc., Chicago, IL).
2.4 Semantics-oriented bioinformatics and computational analysis
We utilized OmniSearch [16, 17], a semantic search and analysis software tool, to obtain a set of computationally putative miRs that may regulate hs-CRP. These regulating miRs were semantically integrated from various miR target prediction and validation databases (i.e., miRDB [18], TargetScan[19], miRanda [20], and miRTarBase [21]). Besides, OmniSearch also provided us with a rich set of additional data for each candidate miR, including Gene Ontology (GO) annotations, PubMed publications, non-coding RNA (ncRNA) sequences, relevant MeSH terms, and involved pathways. We utilized semantically federated knowledge retrieved from OmniSearch to further facilitate the analysis of results from biological experiments and statistical analysis.
3. Results and discussion
3.1 Wet-lab experimental results along with statistical analysis
Tables 1, 2, and 3 demonstrate our experimental results, exhibiting anthropometric, biochemical, and arterial variables along with their unadjusted Pearson correlation coefficients with hs-CRP, whose value ranged from 0.05 to 2.53 mg/L. “P” in Tables 1 through 4 refers to p-value.
Table 1.
Anthropometric, DXA parameters, and their correlation with hs-CRP
| Variables | Mean ± SD | r |
|---|---|---|
| Age (year) | 20.6 ± 1.2 | 0.017 |
| BMI (kg/m2) | 20.4 ± 2.3 | 0.198† |
| WC (cm) | 70.3 ± 5.6 | 0.208† |
| Total FM (kg) | 14.33 ± 4.40 | 0.267† |
| %Total FM (%) | 27.8 ± 5.5 | 0.265† |
| Trunk FM (kg) | 6.96 ± 2.46 | 0.287† |
| %Trunk FM (%) | 28.6 ± 6.6 | 0.278† |
| Arm FM (kg) | 1.21 ± 0.57 | 0.222† |
| %Arm FM (%) | 25.2 ± 8.2 | 0.198† |
| Lower-body FM (kg) | 5.57 ± 1.52 | 0.205† |
| %Lower-body FM (%) | 31.0 ± 5.2 | 0.218† |
P < 0.001
Table 2.
Glucose, lipid metabolic characteristics, and their correlation with hs-CRP
| Variables | Mean ± SD | r |
|---|---|---|
| Fasting plasma glucose (mmol/L) | 4.61 ± 0.39 | 0.066 |
| Fasting insulin (μU/mL) | 6.2 ± 3.5 | −0.020 |
| HbA1c (%) | 4.8 ± 0.2 | 0.080 |
| HOMA-IR | 1.27 ± 0.88 | 0.050 |
| TG (mmol/L) | 0.66 ± 0.38 | 0.108 |
| TC (mmol/L) | 4.7 ± 0.72 | −0.085 |
| HDL-C (mmol/L) | 1.93 ± 0.35 | −0.246† |
| Non-HDL-C (mmol/L) | 2.78 ± 0.66 | 0.037 |
| LDL-C (mmol/L) | 2.47 ± 0.62 | 0.008 |
| sd-LDL (mg/dL) | 11.1 ± 5 | 0.222§ |
| ApoA1 (mg/dL) | 165 ± 20 | −0.218† |
| ApoB (mg/dL) | 70 ± 15 | 0.012 |
| RLP-C (mg/dL) | 3.04 ± 2.46 | 0.143* |
| FFA (mEq/L) | 0.56 ± 0.22 | 0.080 |
| LPL (ng/mL) | 72 ± 18 | 0.014 |
P < 0.05;
P < 0.01;
P < 0.001.
Table 3.
Adipocytokines, inflammation markers, oxidative stress marker, blood pressure, arterial properties, and their correlation with hs-CRP
| Variables | Mean ± SD | r |
|---|---|---|
| Leptin (ng/mL) | 8.6 ± 3.9 | 0.210† |
| Adiponectin (μg/mL) | 11.5 ± 4.3 | −0.139§ |
| Leukocyte count (/μL) | 5973 ± 1561 | 0.177§ |
| PAI-1 (ng/mL) | 21.1 ± 12.9 | 0.217† |
| TNF-α (pg/mL) | 0.68 ± 0.48 | −0.003 |
| 8-epi-PGF2α (pg/mg.creatinine) | 328.7 ± 106.2 | −0.007 |
| Systolic blood pressure (mmHg) | 106 ± 10 | −0.083 |
| Diastolic blood pressure (mmHg) | 61 ± 8 | 0.150† |
| CAVI | 5.78 ± 0.78 | −0.077 |
| Avg IMT (mm) | 0.41 ± 0.06 | −0.122 |
| Max IMT (mm) | 0.43 ± 0.06 | −0.086 |
P < 0.01;
P < 0.001.
Table 4.
Multiple stepwise regression analysis for hs-CRP as a dependent variable
| Independent variables | B | SE | P | R2 change (%) |
|---|---|---|---|---|
| Trunk FM | 0.032 | 0.014 | 0.022 | 8.0 |
| HDL-C | −0.255 | 0.073 | 0.001 | 10.2 |
| Leptin | 0.009 | 0.007 | 0.018 | 12.2 |
| PAI-1 | 0.006 | 0.002 | 0.002 | 13.9 |
We further explain results in Tables 1, 2, and 3 as follows.
Table 1 exhibits results for indirect anthropometric variables and their correlation with hs-CRP. BMI (r = 0.198, P < 0.001) and WC (r = 0.208, P < 0.001) showed significant positive correlation with hs-CRP. All DXA-derived regional FM variables were significantly and positively correlated with hs-CRP, with the coefficient values ranged from 0.198 to 0.287 (P < 0.001). Among anthropometric variables, indices of central obesity, i.e., Trunk FM (r = 0.287, P < 0.001), %Trunk FM (r = 0.278, P < 0.001), and WC (r = 0.208, P < 0.001) exhibited strong association with hs-CRP. Both indirect adiposity measures (BMI and WC) and direct measures (DXA-derived FM parameters) showed strong association with hs-CRP. Although BMI and WC represented useful markers of hs-CRP, DXA-derived indices exhibited better, independent power in predicting hs-CRP. In particular, Table 1 demonstrates that indices of central/abdominal adiposity displayed more intensive correlation with hs-CRP. These findings are consistent with previous reports [22–24] that abdominal adipose tissue measured by CT had the highest correlation with hs-CRP in both men [22] and women [23, 24]. In addition, various experimental data [2, 4, 25] have provided evidence that adipocyte is a major source of TNF-α [2] and may stimulate the expression of IL-6 [25], which was known as a major regulator of production and secretion of hs-CRP in the hepatocyte [4]. Therefore, our results in this paper suggest that expanded abdominal FM could be largely responsible, although not exclusively, for both the dysregulation of cytokines and a state of low chronic inflammation even in young healthy women without exposing to classical CVD risk factors.
Table 2 exhibits results for glucose, lipid metabolic characteristics, and their correlation with hs-CRP. We discovered that hs-CRP was reversely correlated with HDL-C (r = −0.246, P < 0.001) and ApoA1 (r = −0.218, P < 0.001) while positively correlated with sd-LDL (r = 0.222, P < 0.01) and RLP-C (r = 0.143, P < 0.05). No association was found between hs-CRP and HbA1c, TG, TC, non-HDL-C, LDL-C, FFA, LPL, fasting glucose, fasting insulin, ApoB, and HOMA-IR. These findings agree with previously reported observations in human beings [26, 27] that HDL-C and ApoA1 levels are decreased during acute and chronic inflammation, thus suggesting an anti-inflammatory role of HDL-C in inhibiting atherogenic effects of CRP in healthy young women.
Table 3 exhibits results for adipocytokines, inflammation markers, oxidative stress marker, blood pressure, and arterial properties, as well as their correlation with hs-CRP. Our results indicate that hs-CRP was negatively correlated with adiponectin (r = −0.139, P < 0.01) while positively correlated with leptin (r = 0.210, P < 0.001), PAI-1 (r = 0.217, P < 0.001), and leukocyte count (r = 0.177, P < 0.01). No correlation was found with either TNF-α or urinary 8-epi-PGF2α. Also, hs-CRP was correlated with diastolic blood pressure (dBP), but not with systolic blood pressure (sBP), CAVI, and IMT. These results suggest that hs-CRP-leptin interaction might have already existed in non-smokers before the onset of insulin resistance and atherosclerosis. Note that compared with results reported in previous studies [28–35], there are some conflicting results from the present study. To be more specific, correlation was found between hs-CRP and markers of oxidative stress [28] as well as between hs-CRP and arterial stiffness and thickness indices (PWV [29–31] and IMT [32–35]), whereas we did not find these correlations. We speculate that the homogeneity of our sample may have contributed to such different findings. As detailed in Section 2.2.1, the subjects in the present study included a healthy cohort without exposing to tobacco smoking, which is one of the most important confounders affecting relationships between hs-CRP and CVD risk factors [36]. Additionally, our study population had no signs of either insulin resistance (mean HOMA-IR < 1.6) or atherosclerosis (mean IMT < 0.5mm). The relatively narrow value ranges of these variables may not reflect their subtle associations with hs-CRP. Furthermore, most of the studies reported in [28–35] included both smokers and non-smokers but without adjusting for parameters of adiposity; therefore, it is also possible that correlation between hs-CRP and aforementioned variables is in fact not causal; rather, largely explained by the concomitant variation in confounders such as central adiposity and cigarette smoking.
Additionally, we conducted an analysis using the multiple stepwise regression models. Hs-CRP was treated as a dependent variable, and those variables significantly correlating with hs-CRP (in Tables 1, 2, and 3) were treated as covariates. We identified the following four variables that were independent determinants for hs-CRP: trunk FM, HDL-C, leptin, and PAI-1. As exhibited in Table 4, these four variables together explained 13.9% variance of hs-CRP. In addition to central adiposity and leptin, it is interesting to discover that PAI-1 was an important correlator of hs-CRP in healthy young non-smokers. Recent in-vitro studies [37–41] provided evidence to support the relationship between hs-CRP and PAI-1, where hs-CRP was found to stimulate PAI-1 gene expression in human endothelial cells [37, 38]. Besides, hs-CRP was shown to: (1) increase the expression of cell adhesion molecules, chemokines, and endothelin-1; (2) decrease endothelial nitrogen (NO) synthase (eNOS) expression and activity; and (3) augment monocyte-endothelial cell adhesion [39–41]. These findings further support our hypothesis in the present study that hs-CRP may play an important role in promoting the inflammatory component of atherosclerosis through PAI-1 activation. Note that one of the limitations of this study is, the nature of cross-sectional study cannot directly imply causation.
3.2 Bioinformatics and computational analysis results
The OmniSearch software provided with us a user-friendly, convenient interface that enables side-by-side comparison among results from numerous miR target prediction and validation databases, as well as the federated knowledge integrated from other relevant data sources. Search results on CRP from the OmniSearch interface are demonstrated in Figure 1. Our bioinformatics and computational analysis was focused on the returned miR list and relevant GO annotations (shown in Figure 2). The semantically confederated knowledge in OmniSearch further helped us to better analyze results from biological experiments and statistical analysis, explained in detail as follows.
Figure 1.
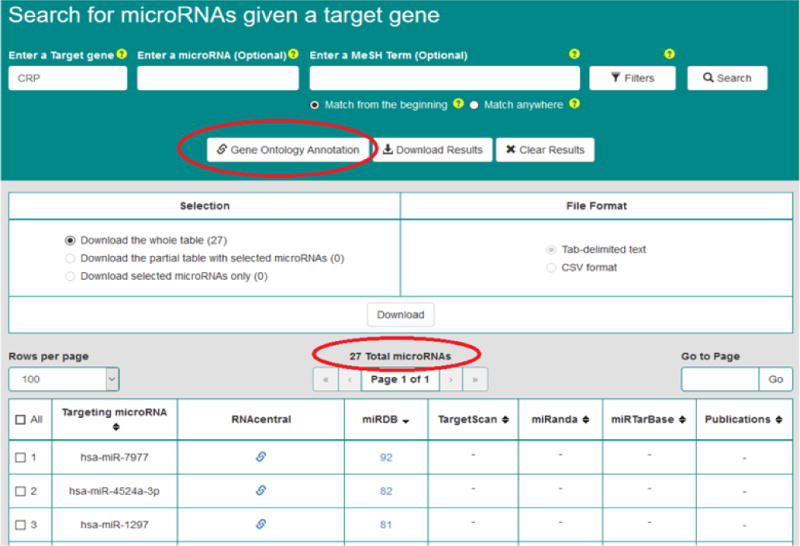
The semantic search results on CRP’s putative regulating miRs.
Figure 2.
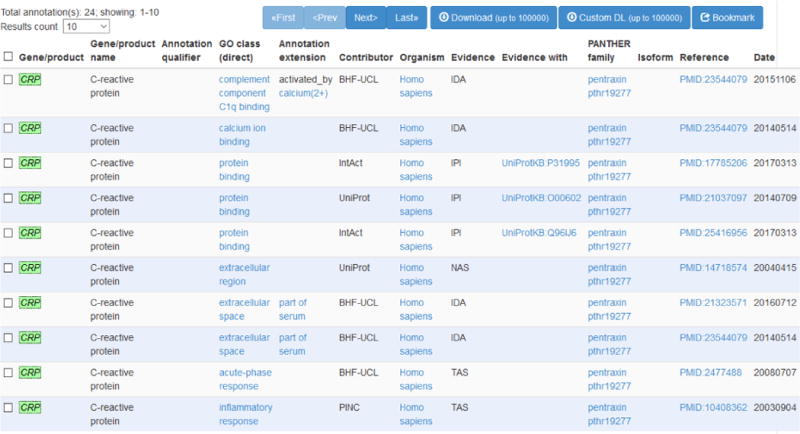
The GO annotations on CRP.
3.2.1 Findings from computationally putative CRP-regulating miRs
Prior research [42–46] has indicated that miR∷mRNA regulatory interactions have great potential in human disease including metabolic syndrome and CVD. If we can well understand such regulation mechanisms, it will significantly help researchers to obtain an enhanced molecular understanding of both diseases. Therefore, we utilized the OmniSearch tool to conduct a semantic search on CRP-regulating miRs. Table 5 exhibits a list of 23 miRs that were computationally predicted to regulate hs-CRP.
Table 5.
Putative regulating miRs retrieved from OmniSearch
| Targeting miR | miRDB prediction score | TargetScan prediction score | miRanda prediction score |
|---|---|---|---|
| hsa-miR-7977 | 92 | Not predicted | Not predicted |
| hsa-miR-4524a-3p | 82 | Not predicted | Not predicted |
| hsa-miR-6833-5p | 79 | Not predicted | Not predicted |
| hsa-miR-518c-5p | 77 | Not predicted | Not predicted |
| hsa-miR-4731-5p | 73 | Not predicted | Not predicted |
| hsa-miR-6499-3p | 71 | Not predicted | Not predicted |
| hsa-miR-4758-3p | 70 | Not predicted | Not predicted |
| hsa-miR-3688-5p | 68 | Not predicted | Not predicted |
| hsa-miR-939-3p | 56 | Not predicted | Not predicted |
| hsa-miR-6776-5p | 54 | Not predicted | Not predicted |
| hsa-miR-4530 | 51 | Not predicted | Not predicted |
| hsa-miR-448-3p | Not predicted | Not predicted | 0.1543 |
| hsa-miR-338-3p | Not predicted | Not predicted | 0.2513 |
| hsa-miR-199a-5p | Not predicted | Not predicted | 0.3134 |
| hsa-miR-199b-5p | Not predicted | Not predicted | 0.3134 |
| hsa-miR-491-5p | Not predicted | Not predicted | 0.6484 |
| hsa-miR-485-5p | Not predicted | 14 | Not predicted |
| hsa-miR-6884-5p | Not predicted | 14 | Not predicted |
| hsa-miR-499a-5p | Not predicted | 20 | Not predicted |
| hsa-miR-183-5p | Not predicted | 23 | Not predicted |
| hsa-miR-330-3p | Not predicted | 29 | Not predicted |
| hsa-miR-3681-3p | Not predicted | 29 | Not predicted |
| hsa-miR-128-3p | Not predicted | 32 | Not predicted |
On one hand, none of these miR∷hs-CRP regulation mechanisms have yet been biologically validated by wet-lab experiments; on the other hand, many of miRs in Table 5 were already validated to regulate respective target genes (other than hs-CRP) in either metabolic syndrome or CVD. For example, hsa-miR-128-3p and hsa-miR-330-3p were involved in metabolic syndrome and fatty tissue metabolism [47, 48]; and hsa-miR-448-3p, hsa-miR-338-3p, and hsa-miR-199a-5p were involved in CVD [49–51].
Authors in [47] presented an analysis of a set of miRs (including hsa-miR-128-3p) that were altered by high-fructose diet. Their research outcomes indicated that these miRs were assembled as a regulatory network to potentially target key genes in lipid, lipoprotein metabolism, and insulin signaling at multiple levels. Therefore, these miR∷mRNA interactions provided some important insights into the development of metabolic syndrome. Researchers in [48] investigated whether or not a sustained nuts-enriched diet can lead to changes in circulating miRs, in parallel to the dietary modification of fatty acids. Their experimental results demonstrated that hsa-miR-330-3p (along with hsa-miR-328, hsa-miR-221, and hsa-miR-125a-5p) was significantly decreased after the treatment of normocaloric diet enriched with polyunsaturated fatty acids (PUFAs). Down-regulation of hsa-miR-448-3p was reported in[49], which resulted in increased expression of Ncf1 gene and p47 (phox) protein. In addition, cellular oxidative stress subsequently triggered events that finally culminated in cardiac tissue damage and the development of cardiomyopathy. In [50], inhibition of hsa-miR-338-3p and hsa-miR-10b was validated to induce an increase of apoptosis as well as increased expression of apoptosis protease-activating factor-1 (Apaf-1) in HL-1 cardiomyocytes, thus contributing to cardiomyocyte damage and the development of heart failure. A list of miRs including hsa-miR-199a-5p was reported in [51] to have a role in the pathophysiology of heart failure by involved in pathways related to disease progression including fibrosis.
The analysis of our semantic search results on CRP-regulating miRs provided us with a suggested list of promising candidate miRs (Table 5) for further investigation and validation in our future research.
3.2.2 Findings from GO annotations on CRP, PAI-1, and IL-6
A total of 22 GO annotations were found to be related to human CRP, of which four, two, and two annotations were related to inflammation, lipid metabolism, and vascular function, respectively. Also, two annotations were discovered to implicate a close association between CRP and CVD. In particular, one annotation indicated [52] a possible mechanism, by which CRP involved in atherosclerosis, was mediated by the activation of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1), resulting in vasomotor dysfunction.
Analysis of GO annotations on PAI-1 led us to one study [53][33] noting PAI-1 as an important inflammatory mediator that may amplify the inflammation induced by cigarette smoke extraction (CSE) or lipopolysaccharides (LPS) through up-regulating IL-8 and leukotriene B4 (LTB4).
According to [54] contained in one GO annotation, IL-6 may stimulate vascular smooth muscle cells (SMC) into a pro-inflammatory state through up-regulating the expression of gp-130 and MCP-1, which suggested the role of IL-6 in mediating inflammation-induced atherosclerosis. Further, we pinpointed several GO annotations and found one paper [55] reporting that leptin induced the production of IL-6 and interleukin-8 (IL-8) in human osteoarthritic cartilage.
These findings from GO annotations either reinforce our discoveries reported in this study or provide us with additional clues regarding how to further explore genetic regulation mechanisms relevant to the role of hs-CRP in adipocyte-cytokine interaction and metabolic derangement. For example, (1) the mechanism for hs-CRP to interact with LOX-1 to be involved in atherosclerosis; (2) the possible association between hs-CRP and IL-6 in terms of mediating inflammation-induced atherosclerosis; and (3) the likely correlation among hs-CRP, leptin, IL-6, and IL-8 in regulating fatty tissue metabolism.
As discussed earlier, the novelty of our methodologies reported in this paper is the combination of biological wet-lab experiments, statistical analysis, and semantics-oriented bioinformatics & computational analysis. Without the integration of the OmniSearch software tool, it was still possible to retrieve similar findings (reported in Section 3.2.1 and Section 3.2.2) through conventional methods; however, it would have been less effective and less efficient. Note that the effectiveness and efficiency features of the OmniSearch tool were reported in our earlier publications ([16] for example).
4. Conclusions
Hs-CRP, an important biomarker of chronic low-grade inflammation, is able to significantly affect the onset of metabolic syndrome and CVD. To better prevent and intervene the development of these two diseases, there is an urgent need to investigate the association between hs-CRP and obesity-related metabolic phenotypes (including atherosclerosis) in young people without classical CVD risk factors. In this paper, we report our efforts to effectively combine biological experiments, statistical analysis, and semantics-oriented bioinformatics & computational analysis to conduct an integrative study in young healthy women. Through rigorously designed experiments, we obtained reproducible, promising results, indicating two novel discoveries in this study. The first discovery is, in young healthy women without conventional CVD risks, we found significant associations between plasma hs-CRP and indices of central/abdominal adiposity, leptin, PAI-1, and HDL-C. No association was identified between hs-CRP and any of insulin resistance, a systemic oxidative stress marker, and arterial stiffness & thickness indices. These results suggested that even in young female nonsmokers without hypertension, dyslipidemia, and insulin resistance, trunk fat accumulation is still a critical determinant for hs-CRP, along with three other important determinants, leptin, HDL-C, and PAI-1. The second discovery is, the reciprocal association between hs-CRP and leptin, PAI-1, and lipid-lipoprotein was also prominent, suggesting the role of chronic low-grade inflammation in adipocyte-cytokine interaction underlying the metabolic derangement before the initiation of metabolic syndrome and CVD.
In our future work, we plan to study the mechanisms by which central/abdominal adiposity leads to sustained systemic inflammation. In the planned study, we will recruit subjects from a broader cohort including male and old individuals with mild or moderate CVD risks. Additionally, we will also choose some candidate miRs reported in the current study for further investigation and biological validation.
Association of hs-CRP, FM, and cardiometabolic risk factors in young healthy women.
Primary determinants for hs-CRP: trunk FM accumulation, leptin, HDL-C, and PAI-1.
Chronic inflammation involved in adipocyte-cytokine interaction.
Acknowledgments
We are indebted to all the participants for their dedicated and conscientious collaboration. We also thank Mami Toyasaki and Rumi Fukada for their work on sampling and data collection. This study was supported by the Open Research Center Project for Private University: Matching Fund Subsidy for Private Universities, The Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan. In addition, research reported in this paper was partially supported by: (1) The National Natural Science Fund of China (NSFC), under Award Number 81660141. (2) Applied Basic Research of Yunnan Provincial Science and Technology Department, No. 2015FB023. (3) The National Cancer Institute (NCI) of the National Institutes of Health (NIH), under the Award Number U01CA180982. The views contained in this paper are solely the responsibility of the authors and do not represent the official views, either expressed or implied, of the MEXT, NSFC, NIH, and the Governments of Japan, China, and U.S.A.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that have no competing interests.
Authors’ Contributions
BW and JH designed the study, carried out the research, interpreted the results, and drafted the manuscript. TK and JH designed the study, analyzed the data, reviewed and revised the manuscript, and is responsible for the integrity of this work. LZ, MVK, FH, JB, KF, and TK assisted in study design, performed research, and reviewed the manuscript. All authors read and approved the final version of the manuscript.
References
- 1.Dandona P, Aljada A, Chaudhuri A, Mohanty P, Garg R. Metabolic syndrome: a comprehensive perspective based on interactions between obesity, diabetes, and inflammation. Circulation. 2005;111:1448–1454. doi: 10.1161/01.CIR.0000158483.13093.9D. [DOI] [PubMed] [Google Scholar]
- 2.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 4.Castell JV, Gomez-Lechon MJ, David M, Andus T, Geiger T, Trullenque R, et al. Interleukin-6 is the major regulator of acute phase protein synthesis in adult human hepatocytes. FEBS Lett. 1989;242:237–239. doi: 10.1016/0014-5793(89)80476-4. [DOI] [PubMed] [Google Scholar]
- 5.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 6.Folsom AR, Aleksic N, Catellier D, Juneja HS, Wu KK. C-reactive protein and incident coronary heart disease in the Atherosclerosis Risk In Communities (ARIC) study. Am Heart J. 2002;144:233–238. doi: 10.1067/mhj.2002.124054. [DOI] [PubMed] [Google Scholar]
- 7.Strandberg TE, Tilvis RS. C-reactive protein, cardiovascular risk factors, and mortality in a prospective study in the elderly. Arterioscler Thromb Vasc Biol. 2000;20:1057–1060. doi: 10.1161/01.atv.20.4.1057. [DOI] [PubMed] [Google Scholar]
- 8.Sakkinen P, Abbott RD, Curb JD, Rodriguez BL, Yano K, Tracy RP. C-reactive protein and myocardial infarction. J Clin Epidemiol. 2002;55:445–451. doi: 10.1016/s0895-4356(01)00502-9. [DOI] [PubMed] [Google Scholar]
- 9.Park YW, Heymsfield SB, Gallagher D. Are dual-energy X-ray absorptiometry regional estimates associated with visceral adipose tissue mass? Int J Obes Relat Metab Disord. 2002;26:978–983. doi: 10.1038/sj.ijo.0801982. [DOI] [PubMed] [Google Scholar]
- 10.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 11.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 12.Hirano T, Ito Y, Saegusa H, Yoshino G. A novel and simple method for quantification of small, dense LDL. J Lipid Res. 2003;44:2193–2201. doi: 10.1194/jlr.D300007-JLR200. [DOI] [PubMed] [Google Scholar]
- 13.Roberts LJ, 2nd, Morrow JD. The generation and actions of isoprostanes. Biochim Biophys Acta. 1997;1345:121–135. doi: 10.1016/s0005-2760(96)00162-2. [DOI] [PubMed] [Google Scholar]
- 14.Kadota K, Takamura N, Aoyagi K, Yamasaki H, Usa T, Nakazato M, et al. Availability of cardio-ankle vascular index (CAVI) as a screening tool for atherosclerosis. Circ J. 2008;72:304–308. doi: 10.1253/circj.72.304. [DOI] [PubMed] [Google Scholar]
- 15.Bots ML, de Jong PT, Hofman A, Grobbee DE. Left, right, near or far wall common carotid intima-media thickness measurements: associations with cardiovascular disease and lower extremity arterial atherosclerosis. J Clin Epidemiol. 1997;50:801–807. doi: 10.1016/s0895-4356(97)00059-0. [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Gutierrez F, Strachan HJ, Dou D, Huang W, Smith B, et al. OmniSearch: a semantic search system based on the Ontology for MIcroRNA Target (OMIT) for microRNA-target gene interaction data. J Biomed Semantics. 2016;7:25. doi: 10.1186/s13326-016-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.OmniSearch. [Online]. http://omnisearch.soc.southalabama.edu/ui/.
- 18.miRDB. [Online]. http://mirdb.org/miRDB/.
- 19.TargetScan. [Online]. http://www.targetscan.org.
- 20.miRanda. [Online]. http://www.microrna.org
- 21.miRTarBase. [Online]. http://mirtarbase.mbc.nctu.edu.tw/.
- 22.Lemieux I, Pascot A, Prud’homme D, Almeras N, Bogaty P, Nadeau A, et al. Elevated C-reactive protein: another component of the atherothrombotic profile of abdominal obesity. Arterioscler Thromb Vasc Biol. 2001;21:961–967. doi: 10.1161/01.atv.21.6.961. [DOI] [PubMed] [Google Scholar]
- 23.Piche ME, Lemieux S, Weisnagel SJ, Corneau L, Nadeau A, Bergeron J. Relation of high-sensitivity C-reactive protein, interleukin-6, tumor necrosis factor-alpha, and fibrinogen to abdominal adipose tissue, blood pressure, and cholesterol and triglyceride levels in healthy postmenopausal women. Am J Cardiol. 2005;96:92–97. doi: 10.1016/j.amjcard.2005.02.051. [DOI] [PubMed] [Google Scholar]
- 24.Pannacciulli N, Cantatore FP, Minenna A, Bellacicco M, Giorgino R, De Pergola G. C-reactive protein is independently associated with total body fat, central fat, and insulin resistance in adult women. Int J Obes Relat Metab Disord. 2001;25:1416–1420. doi: 10.1038/sj.ijo.0801719. [DOI] [PubMed] [Google Scholar]
- 25.Stephens JM, Butts MD, Pekala PH. Regulation of transcription factor mRNA accumulation during 3T3-L1 preadipocyte differentiation by tumour necrosis factor-alpha. J Mol Endocrinol. 1992;9:61–72. doi: 10.1677/jme.0.0090061. [DOI] [PubMed] [Google Scholar]
- 26.Bausserman LL, Bernier DN, McAdam KP, Herbert PN. Serum amyloid A and high density lipoproteins during the acute phase response. Eur J Clin Invest. 1988;18:619–626. doi: 10.1111/j.1365-2362.1988.tb01277.x. [DOI] [PubMed] [Google Scholar]
- 27.Rossner S. Further studies on serum lipoproteins in connective tissue diseases. Atherosclerosis. 1978;31:93–99. doi: 10.1016/0021-9150(78)90041-2. [DOI] [PubMed] [Google Scholar]
- 28.Kelishadi R, Sharifi M, Khosravi A, Adeli K. Relationship between C-reactive protein and atherosclerotic risk factors and oxidative stress markers among young persons 10–18 years old. Clin Chem. 2007;53:456–464. doi: 10.1373/clinchem.2006.073668. [DOI] [PubMed] [Google Scholar]
- 29.Okamura T, Moriyama Y, Kadowaki T, Kanda H, Ueshima H. Non-invasive measurement of brachial-ankle pulse wave velocity is associated with serum C-reactive protein but not with alpha-tocopherol in Japanese middle-aged male workers. Hypertens Res. 2004;27:173–180. doi: 10.1291/hypres.27.173. [DOI] [PubMed] [Google Scholar]
- 30.Tomiyama H, Arai T, Koji Y, Yambe M, Hirayama Y, Yamamoto Y, et al. The relationship between high-sensitive C-reactive protein and pulse wave velocity in healthy Japanese men. Atherosclerosis. 2004;174:373–377. doi: 10.1016/j.atherosclerosis.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 31.Nagano M, Nakamura M, Sato K, Tanaka F, Segawa T, Hiramori K. Association between serum C-reactive protein levels and pulse wave velocity: a population-based cross-sectional study in a general population. Atherosclerosis. 2005;180:189–195. doi: 10.1016/j.atherosclerosis.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 32.Hulthe J, Wikstrand J, Fagerberg B. Relationship between C-reactive protein and intima-media thickness in the carotid and femoral arteries and to antibodies against oxidized low-density lipoprotein in healthy men: the Atherosclerosis and Insulin Resistance (AIR) study. Clin Sci (Lond) 2001;100:371–378. doi: 10.1042/cs1000371. [DOI] [PubMed] [Google Scholar]
- 33.Makita S, Nakamura M, Hiramori K. The association of C-reactive protein levels with carotid intima-media complex thickness and plaque formation in the general population. Stroke. 2005;36:2138–2142. doi: 10.1161/01.STR.0000181740.74005.ee. [DOI] [PubMed] [Google Scholar]
- 34.Lorenz MW, Karbstein P, Markus HS, Sitzer M. High-sensitivity C-reactive protein is not associated with carotid intima-media progression: the carotid atherosclerosis progression study. Stroke. 2007;38:1774–1779. doi: 10.1161/STROKEAHA.106.476135. [DOI] [PubMed] [Google Scholar]
- 35.Beauloye V, Zech F, Tran HT, Clapuyt P, Maes M, Brichard SM. Determinants of early atherosclerosis in obese children and adolescents. J Clin Endocrinol Metab. 2007;92:3025–3032. doi: 10.1210/jc.2007-0619. [DOI] [PubMed] [Google Scholar]
- 36.Tracy RP, Psaty BM, Macy E, Bovill EG, Cushman M, Cornell ES, et al. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscler Thromb Vasc Biol. 1997;17:2167–2176. doi: 10.1161/01.atv.17.10.2167. [DOI] [PubMed] [Google Scholar]
- 37.Chen C, Nan B, Lin P, Yao Q. C-reactive protein increases plasminogen activator inhibitor-1 expression in human endothelial cells. Thromb Res. 2008;122:125–133. doi: 10.1016/j.thromres.2007.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakakuki T, Ito M, Iwasaki H, Kureishi Y, Okamoto R, Moriki N, et al. Rho/Rho-kinase pathway contributes to C-reactive protein-induced plasminogen activator inhibitor-1 expression in endothelial cells. Arterioscler Thromb Vasc Biol. 2005;25:2088–2093. doi: 10.1161/01.ATV.0000183607.50230.9f. [DOI] [PubMed] [Google Scholar]
- 39.Pasceri V, Cheng JS, Willerson JT, Yeh ET. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. 2001;103:2531–2534. doi: 10.1161/01.cir.103.21.2531. [DOI] [PubMed] [Google Scholar]
- 40.Pasceri V, Willerson JT, Yeh ET. Direct proinflammatory effect of C-reactive protein on human endothelial cells. Circulation. 2000;102:2165–2168. doi: 10.1161/01.cir.102.18.2165. [DOI] [PubMed] [Google Scholar]
- 41.Verma S, Wang CH, Li SH, Dumont AS, Fedak PW, Badiwala MV, et al. A self-fulfilling prophecy: C-reactive protein attenuates nitric oxide production and inhibits angiogenesis. Circulation. 2002;106:913–919. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 42.Chen C, Wang Y, Yang S, Li H, Zhao G, Wang F, et al. MiR-320a contributes to atherogenesis by augmenting multiple risk factors and down-regulating SRF. J Cell Mol Med. 2015;19:970–985. doi: 10.1111/jcmm.12483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang YT, Tsai PC, Liao YC, Hsu CY, Juo SH. Circulating microRNAs have a sex-specific association with metabolic syndrome. J Biomed Sci. 2013;20:72. doi: 10.1186/1423-0127-20-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao W, Zhao SP, Zhao YH. MicroRNA-143/-145 in Cardiovascular Diseases. Biomed Res Int. 2015;2015:531740. doi: 10.1155/2015/531740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen KC, Liao YC, Wang JY, Lin YC, Chen CH, Juo SH. Oxidized low-density lipoprotein is a common risk factor for cardiovascular diseases and gastroenterological cancers via epigenomical regulation of microRNA-210. Oncotarget. 2015;6:24105–24118. doi: 10.18632/oncotarget.4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kong X, Yu J, Bi J, Qi H, Di W, Wu L, et al. Glucocorticoids transcriptionally regulate miR-27b expression promoting body fat accumulation via suppressing the browning of white adipose tissue. Diabetes. 2015;64:393–404. doi: 10.2337/db14-0395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sud N, Zhang H, Pan K, Cheng X, Cui J, Su Q. Aberrant expression of microRNA induced by high-fructose diet: implications 131. in the pathogenesis of hyperlipidemia and hepatic insulin resistance. J Nutr Biochem. 2017;43:125. doi: 10.1016/j.jnutbio.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ortega FJ, Cardona-Alvarado MI, Mercader JM, Moreno-Navarrete JM, Moreno M, Sabater M, et al. Circulating profiling reveals the effect of a polyunsaturated fatty acid-enriched diet on common microRNAs. J Nutr Biochem. 2015;26:1095–1101. doi: 10.1016/j.jnutbio.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Kyrychenko S, Kyrychenko V, Badr MA, Ikeda Y, Sadoshima J, Shirokova N. Pivotal role of miR-448 in the development of ROS-induced cardiomyopathy. Cardiovasc Res. 2015;108:324–334. doi: 10.1093/cvr/cvv238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gallego I, Beaumont J, Lopez B, Ravassa S, Gomez-Doblas JJ, Moreno MU, et al. Potential role of microRNA-10b down-regulation in cardiomyocyte apoptosis in aortic stenosis patients. Clin Sci (Lond) 2016;130:2139–2149. doi: 10.1042/CS20160462. [DOI] [PubMed] [Google Scholar]
- 51.Marques FZ, Vizi D, Khammy O, Mariani JA, Kaye DM. The transcardiac gradient of cardio-microRNAs in the failing heart. Eur J Heart Fail. 2016;18:1000–1008. doi: 10.1002/ejhf.517. [DOI] [PubMed] [Google Scholar]
- 52.Hein TW, Qamirani E, Ren Y, Xu X, Thengchaisri N, Kuo L. Selective activation of lectin-like oxidized low-density lipoprotein receptor-1 mediates C-reactive protein-evoked endothelial vasodilator dysfunction in coronary arterioles. Circ Res. 2014;114:92–100. doi: 10.1161/CIRCRESAHA.114.301763. [DOI] [PubMed] [Google Scholar]
- 53.Xu X, Wang H, Wang Z, Xiao W. Plasminogen activator inhibitor-1 promotes inflammatory process induced by cigarette smoke extraction or lipopolysaccharides in alveolar epithelial cells. Exp Lung Res. 2009;35:795–805. doi: 10.3109/01902140902912519. [DOI] [PubMed] [Google Scholar]
- 54.Klouche M, Bhakdi S, Hemmes M, Rose-John S. Novel path to activation of vascular smooth muscle cells: up-regulation of gp130 creates an autocrine activation loop by IL-6 and its soluble receptor. J Immunol. 1999;163:4583–4589. [PubMed] [Google Scholar]
- 55.Vuolteenaho K, Koskinen A, Kukkonen M, Nieminen R, Paivarinta U, Moilanen T, et al. Leptin enhances synthesis of proinflammatory mediators in human osteoarthritic cartilage–mediator role of NO in leptin-induced PGE2, IL-6, and IL-8 production. Mediators Inflamm. 2009;2009:345838. doi: 10.1155/2009/345838. [DOI] [PMC free article] [PubMed] [Google Scholar]


