Abstract
Hematopoietic progenitor choice between multipotency and differentiation is tightly regulated by intrinsic factors and extrinsic signals from the surrounding microenvironment. The Drosophila melanogaster hematopoietic lymph gland has emerged as a powerful tool to investigate mechanisms that regulate hematopoietic progenitor choice in vivo. The lymph gland contains progenitor cells, which share key characteristics with mammalian hematopoietic progenitors such as quiescence, multipotency and niche-dependence. The lymph gland is zonally arranged, with progenitors located in medullary zone, differentiating cells in the cortical zone, and the stem cell niche or Posterior Signaling Center (PSC) residing at the base of the medullary zone (MZ). This arrangement facilitates investigations into how signaling from the microenvironment controls progenitor choice. The Drosophila Friend of GATA transcriptional regulator, U-shaped, is a conserved hematopoietic regulator. To identify additional novel intrinsic and extrinsic regulators that interface with U-shaped to control hematopoiesis, we conducted an in vivo screen for factors that genetically interact with u-shaped. Smoothened, a downstream effector of Hedgehog signaling, was one of the factors identified in the screen. Here we report our studies that characterized the relationship between Smoothened and U-shaped. We showed that the PSC and Hedgehog signaling are required for U-shaped expression and that U-shaped is an important intrinsic progenitor regulator. These observations identify a potential link between the progenitor regulatory machinery and extrinsic signals from the PSC. Furthermore, we showed that both Hedgehog signaling and the PSC are required to maintain a subpopulation of progenitors. This led to a delineation of PSC-dependent versus PSC-independent progenitors and provided further evidence that the MZ progenitor population is heterogeneous. Overall, we have identified a connection between a conserved hematopoietic master regulator and a putative stem cell niche, which adds to our understanding of how signals from the microenvironment regulate progenitor multipotency.
Introduction
Hematopoiesis, the life-long production of blood cells from multipotent progenitors, is tightly regulated by intrinsic factors and extrinsic signals from the surrounding microenvironment. A greater appreciation of the mechanisms that control progenitor choice between multipotency and differentiation will increase our understanding of tissue homeostasis and regeneration and how dysregulation of these processes leads to disease.
Drosophila melanogaster has emerged as a powerful model system to investigate mechanisms that drive hematopoietic progenitor choice in vivo because key aspects of hematopoiesis are conserved across taxa1–7 Drosophila has a rudimentary blood system, with three blood cells that develop from a common progenitor and carry out functions associated with the vertebrate myeloid lineages8–18. Plasmatocytes are operational macrophages; crystal cells are named for their crystalline inclusion bodies; and lamellocytes, which are rarely observed under steady-state conditions, differentiate in response to wasp parasitization and other forms of stress including increased levels of reactive oxygen species (ROS) and nutritional depravation18–39. Moreover, the common hematopoietic progenitor or prohemocyte shares key characteristics with mammalian hematopoietic progenitors including quiescence, multipotency and niche-dependence18,25,40,40–65. Similar to vertebrates, Drosophila hematopoiesis occurs at multiple sites throughout the life cycle of the organism. During the larval stage, there are two hematopoietic sites: hematopoietic hubs located along the lateral side of the animal beneath the cuticle; and the lymph gland, a bilateral organ that flanks the insect heart15,18,22,28,30,32,40,41,44,50,65–76.
The lymph gland consists of a pair of primary lobes followed by a series of secondary lobes. The primary lobes are divided into three well defined regions or zones. Prohemocytes reside in the inner region, known as the medullary zone (MZ). Differentiating cells are located at the periphery in the cortical zone (CZ). At the base of the primary lobe is the Posterior Signaling Center (PSC), a small group of cells which produce a variety of signals that control blood cell differentiation in the MZ and Cz40,41,50. This zonal arrangement enables one to determinate the origin of regulators that control hematopoiesis and assess how the interaction between prohemocytes, differentiating cells and the microenvironment control prohemocyte choice between multipotency and differentiation20,40–42,50,59,60,63,64,77,78. The PSC has been shown to be essential for lamellocyte differentiation in response to wasp parasitization 21,43,79. Additionally, the PSC was thought to function as a stem cell niche that maintains the population of undifferentiated prohemocytes during steady-state conditions24,40,41,64,80,81.
Earlier work suggested that Hedgehog (Hh) signaling from the PSC was one of the pathways required to maintain the prohemocyte population 8,41,45,48,63,64,78,82. Hh signaling is a conserved pathway that regulates cell proliferation, migration and differentiation during development and organ formation across taxa. The pathway is activated when the Hh ligand binds to the transmembrane receptor, Patched (Ptc). This suppresses Ptc activity, which activates the G-protein-like receptor, Smoothened (Smo). In Drosophila, activated Smo inhibits the proteolysis of Cubitus interruptus (Ci), the Drosophila homolog of the mammalian Gli proteins, and full length Ci translocates to the nucleus to regulate gene expression83–85
More recently the role of the PSC as a stem cell niche operating through various signaling components has been called into question by work showing that genetic ablation of the PSC had no effect on prohemocyte number43. On the other hand, the results from this and another study demonstrated that Early B-cell Factor, Collier (Col) is an intrinsic prohemocyte factor that is required to maintain prohemocytes in a multipotent state43,58. These studies suggest that prohemocyte maintenance is controlled by intrinsic regulators that act independently of the PSC. We previously identified another factor that maintains prohemocyte multipotency, the Friend of GATA (FOG) homolog, U-shaped (Ush)86. In this study, we show that Ush acts as an intrinsic prohemocyte regulator. FOG proteins bind GATA factors to activate or repress GATA transcriptional activity. GATA:FOG complexes are master regulators that control the differentiation of selected blood cell types across taxa87–97.
To identify additional novel regulators that interface with Ush to control hematopoiesis, we conducted an in vivo screen for genes that genetically interact with ush. Using this approach, we identified Smo as a factor that acts with Ush to block lamellocyte differentiation. Here we report our studies that characterized the relationship between Smo and Ush. In the process, we demonstrated that the PSC and Hh signaling are required for Ush expression, thereby linking this important intrinsic prohemocyte regulator to extrinsic signals from the PSC. Furthermore, using four different prohemocyte markers (Odd-skipped, E-cadherin, DomeMESO and Collier), we also provide compelling evidence for the notion that the PSC and Hh signaling are required to maintain a subpopulation of prohemocytes during steady-state hematopoiesis. We showed that loss of Hh signaling or PSC ablation results in a significant reduction in prohemocytes marked with Odd-skipped (Odd), E-cadherin or DomeMESO, but not those marked with Collier (Col). Importantly, we showed that the Col-positive population is also Oddpositive, whereas the Odd-positive population consists of both Col-positive and Col-negative cells. Furthermore, the Odd-postive/Col-negative cells appeared to be largely PSC-dependent, while Col-positive cells appeared to be largely PSC-independent. However, PSC ablation also produced a small population of Col-positive cells with substantially reduced levels of Odd expression; a cell type that was not observed in control lymph glands. This suggests that the PSC maintains optimal levels of Odd expression in a subpopulation of Col-positive prohemocytes. Thus, our findings support the notion that the PSC functions as a hematopoietic niche during steady-state hematopoiesis. Overall, we have identified a connection between a conserved hematopoietic master regulator and a putative stem cell niche, which adds to our understanding of how signals from the microenvironment regulate progenitor multipotency.
Materials and Methods
Fly strains
The w1118 strain served as the control stock for these studies. The following strains were generous gifts from colleagues: Tep4-Gal4, hhF4f;Antp-Gal4/TM6B Tb , Col-Gal4/CyO GFP, hhF4f-GFP; Pcol85/CyO GFP and MSNF9mo-DsRed (MSN-C) from T. Tokusumi and R. A. Schulz (University of Notre Dame); UAS-GFP;DomeMESO-GFP,Antp-Gal4,/TM6B Tb from S. Govind (City College of New York); Domeless-Gal4 from M. Crozatier (University Paul Sabatier); w p{w+, Dome-MESO}BN1 from M. P. Zeidler (University of Sheffield) and J. C. Hombria (Universidad Pablo de Olavide); UAS-hh from Xiaoyan Zheng (The George Washington University). The following strains were obtained from the Bloomington Stock Center: smo3 b1 pr1/CyO, w*;P{UAS-smo.5A}2, w1118; P{UAS-rpr.C}14 , y1 v1; P{ TRiP.JF01804} (UAS-hhRNAi), y1 v1; P{ TRiP.JF01715} (UAS-ciRNAi), w*; P{ UAS-GFP-ptc.WT}2, w*; P{ UAS-GFP-ptc.WT}3, the 2L Deficiency Kit (DK2L) and y1 sc* v1; P{ TRiP.HMS00492}/TM3, Sb1 (UAS-hhRNAi), which was the alternate strain used in these studies to confirm the results obtained with UAS-hhRNAi. UAS-u-shapedRNAi transformant number 104102 was obtained from the Vienna Drosophila RNAi Center. The following stocks have been previously described: y w67c23; ushvx22/CyO y+, y w67c23; ushR24/CyO y+, y w67c23; UAS-ush 86,98. The yw; ushvx22, MSN-C/CyO y+ was created using standard recombination procedures.
Screen for factors that genetically interact with ush
The yw; ushvx22, MSN-C/CyO y+ stock enabled us to rapidly screen for genes that genetically interact with ush to block lamellocyte differentiation. The MSN-C lamellocyte marker99 was used to identify larvae with an increase in circulating lamellocytes using fluorescence microscopy. MSN-C is also constitutively active in larval muscle and serves as a marker for larvae that carry the ushvx22, MSN-C chromosome. The yw; ushvx22, MSN-C/CyO y+ stock was crossed to each of the 100 large multi-gene deficiencies that map to Chromosome 2L, which produced ush/Df(2L) trans-heterozygotes. However, we could not score the deficiency that uncovers ush, as ush homozygotes are embryonic lethal and ush maps to between 21D1 and 21E2 on chromosome 2L. Scoring was carried out on late third-instar wandering larvae, which were cultured at 23°C. Larvae were placed on a slide with a drop of PBS and observed under fluorescent microscopy using a Zeiss Axioplan microscope.
Gene expression analyses
Gene expression analyses were conducted using lymph glands from mid-third instar larvae (collected 96 to 114 hours after egg laying). As indicated in specific experiments, gene expression analyses were also conducted using lymph glands from late-third instar larvae (collected 120 to 144 hours after egg laying). All control and experimental samples were age-matched and cultured on standard media at 23°C. The UAS/Gal4 binary system100 was used to express transgenes in a tissue-restricted manner and in these experiments, larvae were shifted to 25°C 48 hours after egg laying. Where possible, for each UAS/Gal4 combination we tested two independent PSC or MZ drivers to show that knockdown or over expression of the transgene in question produced the same phenotype. However, with UAS-ush or UAS-rpr only the Col-Gal4 driver was used to express PSC because Antp-Gal4 driven ush or rpr animals die before gene expression can be assessed. Likewise, the Dome-Gal4 driven UAS-smoDN animals die before hematopoiesis can be assessed, so the Tep-Gal4 driver was used to express UAS-smoDN in the MZ. Controls for these experiments included the Gal4 drivers crossed to w1118 mates. Variability across experiments was inevitable, including lymph gland size, due to the fact that these studies were conducted over a considerable period of time by different co-authors. Thus, each experiment was run with an age matched control and we sampled at least 18 primary lymph gland lobes consisting of at least 9 control and 9 experimental samples.
Immunofluorescence
The dissection and fixation of larval lymph glands were performed as previously described 86. Rabbit anti-Odd was a generous gift from J. Skeath (Washington University School of Medicine)101, and used at a 1:4,000 dilution. Mouse anti-Nimrod (PI) and mouse anti-Attila (LI)12 were generous gifts from I. Ando (Biological Research Center of the Hungarian Academy of Sciences) and used at a 1:50 dilution. Rabbit anti-prophenoloxidase A1 (anti-ProPO) was a generous gift from F. C. Kafatos (EMBL)102 and used at a 1:100 dilution. Mouse anti-Collier (Col) antibody was a generous gift from M. Crozatier (University Paul Sabatier)24 and was used at a 1:150 dilution. Rabbit anti-U-shaped was used at a 1:4,000 dilution86. Note, we occasionally observed reduced expression in some MZ cells compared to presumptive CZ cells. This could reflect downregulation of Ush to permit MZ cell differentiation, or alternatively, cases where the antibody did not fully penetrate the compacted cellular arrangement of the MZ.
Mouse anti-β-galactosidase was obtained from Promega and used at a 1:2,000 dilution. Rabbit anti-GFP was obtained from Invitrogen and used at a 1:4,000 dilution. Rat anti-E-cadherin and mouse anti-Smo, anti-Antennapedia (Antp), and anti-GFP, were obtained from the Developmental Studies Hybridoma Bank and used at a concentration of 10 pg/ml. Alexafluor-555, or −488-conjugated secondary antibodies directed against rabbit, rat or mouse (Invitrogen) were used at a 1:2,000 dilution.
Fluorescence was captured, analyzed and recorded using Olympus confocal microscopy or Zeiss Axioplan optics. Different biological endpoints were measured including densitometric mean values, the percentage of specific cell types (percentage marker-positive cells) and the area comprising the MZ marker expression domain. Densitometric mean values were measured to determine whether the expression level of the protein in question was significantly different in response to an altered genotype. The percentage of marker-positive cells was measured to determine whether the number of a specific cell type was significantly increased or decreased by an altered genotype. The area comprising the MZ was measured to determine if a specific MZ marker expression domain changed in response to an altered genotype. This latter method was used primarily in cases where counting the number of MZ marker–positive cells is not feasible, such as E-cadherin positive cells. The relative expression was determined from the densitometric mean values calculated for fluorescent antibody staining using Zeiss Axiovision or ImageJ software as previously described 47,103,104. The size of the MZ zone was determined by measuring the area of Odd- or E-cadherin-expressing cells and statistical significance was evaluated using the Student’s t-test. Blood cell counts were determined using ImageJ and the percentage was determined by dividing the number of cells expressing a particular protein and by the number of Dapiexpressing nuclei. The statistical significance was evaluated using the Student’s t-test. In our hands, control lymph glands have an average of 1 lamellocyte per lymph gland lobe. However, lamellocytes can form large aggregates making it difficult to obtain accurate cell counts. For this reason, we scored primary lymph gland lobes positive for increased lamellocyte differentiation when aggregates were greater than 300 μm2 or more than 5 individual lamellocytes were visible, as previously described 47 When comparing 2 samples, statistical significance was evaluated using increased differentiation as a categorical variable for experimental and control samples in 2×2 contingency tables and P values were calculated using Fisher’s Exact test. When comparing 3 samples, 2×3 contingency tables were used and P values were calculated using Chi-square test.
Results
Smo maintains Ush protein expression and genetically interacts with ush to block lamellocyte differentiation
A number of GATA:FOG co-regulators have been identified; however, the intrinsic and extrinsic signaling networks that interact with GATA:FOG complexes to control hematopoiesis are largely unknown105. To begin to identify novel intrinsic and extrinsic FOG interacting factors, we conducted an in vivo screen for genes that genetically interact with the Drosophila FOG homolog, ush, to block lamellocyte differentiation. To accomplish this goal, we constructed theyw; ushvx22, MSN-C/CyO y+ stock. MSN-C is a marker for lamellocytes99, which was used to rapidly identify larvae with an increase in circulating lamellocytes. The screen was conducted by crossing the yw; ushvx22, MSN-C/CyO y+ stock to each of the 100 large multi-gene deficiencies that map to Chromosome 2L, producing ush/Df(2L) trans-heterozygotes. However, we could not score the deficiency that uncovers ush, as ush homozygotes are embryonic lethal and ush maps to between 21D1 and 21E2 on chromosome 2L. In our hands, increased lamellocyte differentiation in ush hypomorphic (ushvx22/r24) populations ranges from 70% to 100% penetrance (Gao and Fossett unpublished). To minimize false positives, we a priori set a minimum 40% penetrance level for lamellocyte differentiation in trans-heterozygotes. While this is less than that observed for ush hypomorphs, it was significantly greater than the 6.7% (2 out of 30) observed for negative controls.
Of the 100 deficiencies tested, 21 scored positive in the first pass. We re-tested 9 and confirmed that these genetically interact with ush to block lamellocyte differentiation (Baldeosingh and Fossett, unpublished observations). We then tested individual genes that map to the 9 positive deficiencies. One of the deficiencies (Df(2L)ED19) uncovered the gene that encodes Smo. We then showed that smo genetically interacts with ush to block lamellocyte differentiation in the lymph gland. This was accomplished by demonstrating that ush/smo double heterozygotes exhibited a significant increase in the number of lymph gland lobes with increased lamellocyte differentiation compared to animals that were singularly heterozygous for either ush or smo (Figure 1, A–D).
Figure 1. Smo acts with Ush to block lamellocyte differentiation and is required to maintain Ush expression levels.
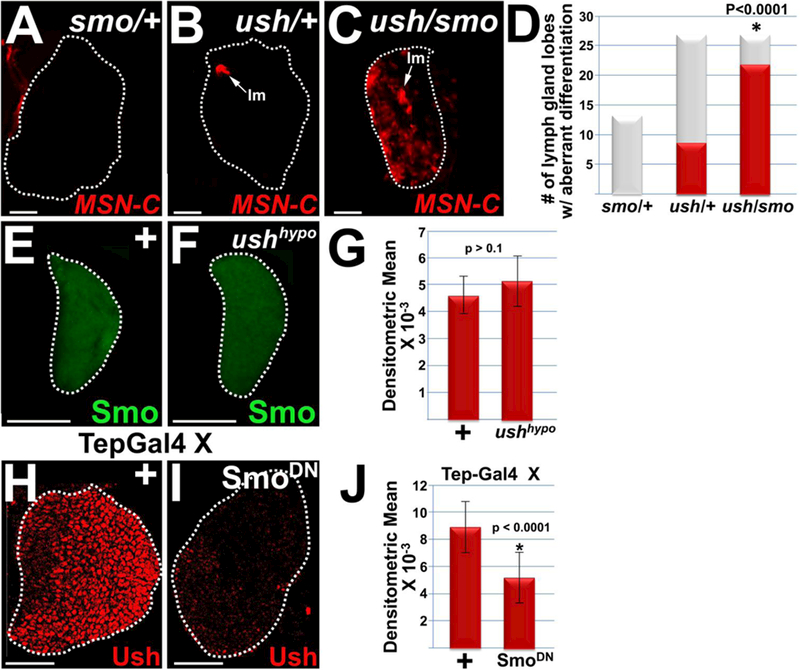
(A–D) Smo acts with Ush to block lamellocyte (lm) differentiation. Lymph glands from (C) ush/smo double heterozgyotes had significantly increased numbers of lymph gland lobes with lamellocyte differentiation compared to either (A) smo heterozygotes or (B) ush heterozygotes. Lamellocytes were detected using the MSN-C reporter and are marked with arrows. (D) Histogram showing the results of the statistical analyses. Chi-square test; P value is as shown; smo (n=214); ush and ush/smo (n= 26). (E–G) Loss of Ush function has no effect on Smo expression. Smo expression in (F) ushvx22/r24 {ush hypomorphs) was assessed and compared to (E) wild-type controls (+). (G) Histogram showing the results of the statistical analyses. Student’s t-test; error bars show standard deviation; P values are as shown; control and ush hypomorphs (n = 15). (H–J) Loss of Smo function (SmoDN) significantly reduced Ush levels compared to controls (+). Tep-Gal4 females were crossed to control (+) males or males that carry UAS-smoDN. (J) Histogram showing the results of the statistical analyses. Student’s t-test; error bars show standard deviation; P values are as shown; control and SmoDN (n=18). White dotted lines delineate the entire lymph gland. Scale bars: panels A–C, 100 pm; remaining panels, 50 μm. Panels A–D, late-third instar larvae; panels E–J, mid-third instar larvae.
Next, we conducted epistatic analyses to determine the relationship between Smo and Ush during hematopoiesis. Previous work by Tokusumi et al. showed that Ush acts with the GATA factor, Serpent (Srp) to repress Hh expression63. Given that Smo is a downstream effector of Hh signaling, we first tested the possibility that Ush also regulates Smo expression. However, loss of Ush function had no effect on the level of Smo expression (Figure 1, E–G). We then tested if Smo regulated Ush expression. Using the Tep-Gal4 driver to express a dominant negative form of Smo (SmoDN) in prohemocytes, we showed that loss of Smo function resulted in a statistically significant reduction in Ush expression levels (Figure 1, H–J). Thus, Smo is required for Ush expression, however Ush is not required for Smo expression.
Hh signaling maintains Ush expression levels
Previous studies have suggested that Hh signaling from the PSC maintains prohemocytes 41,63.
However, the results of two recent studies have indicated that the prohemocyte population is not regulated by Hh signaling 43,58, and indeed, does not require any information from the PSC, as ablation of these cells has no effect on the number of DomeMESO-positive or Col-positive prohemocytes43. Ush is expressed in prohemocytes86 and these conflicting reports prompted us to ask if Smo regulates Ush expression through a non-canonical signaling pathway, or alternatively, if Ush expression is indeed maintained by Hh signaling from the PSC. To distinguish between these possibilities, we tested if altering the expression of several Hh signaling pathway members reduced Ush expression levels.
We observed that knockdown of Hh in the PSC resulted in a significant reduction in Ush expression levels (Figure 2 A,B,I). Knockdown of Hh was achieved using Antp-Gal4 to drive UAS-hhRNAi in the PSC. Ptc is a negative regulator of Hh signaling and Tep-Gal4 driven over-expression of Ptc in prohemocytes also resulted in a significant reduction in the level of Ush expression (Figure 2 C,D,I). Ci is a downstream effector of Hh signaling. Using Tep-Gal4, we expressed UAS-ciRNAi in prohemocytes and observed that knockdown of Ci significantly reduced the level of Ush expression (Figure 2 E,F,I). We confirmed these results using additional UAS/Gal4 strains. We used another UAS-hhRNAi allele to knockdown Hh in the PSC. We also used alternate Gal4 drivers including Col-Gal4 and Dome-Gal4, to express UAS-transgenes in the PSC and MZ, respectively. Under these conditions we again showed that disruption of Hh signaling significantly reduced Ush expression (Supplementary Figure 1).
Figure 2. Hedgehog signaling maintains Ush expression.
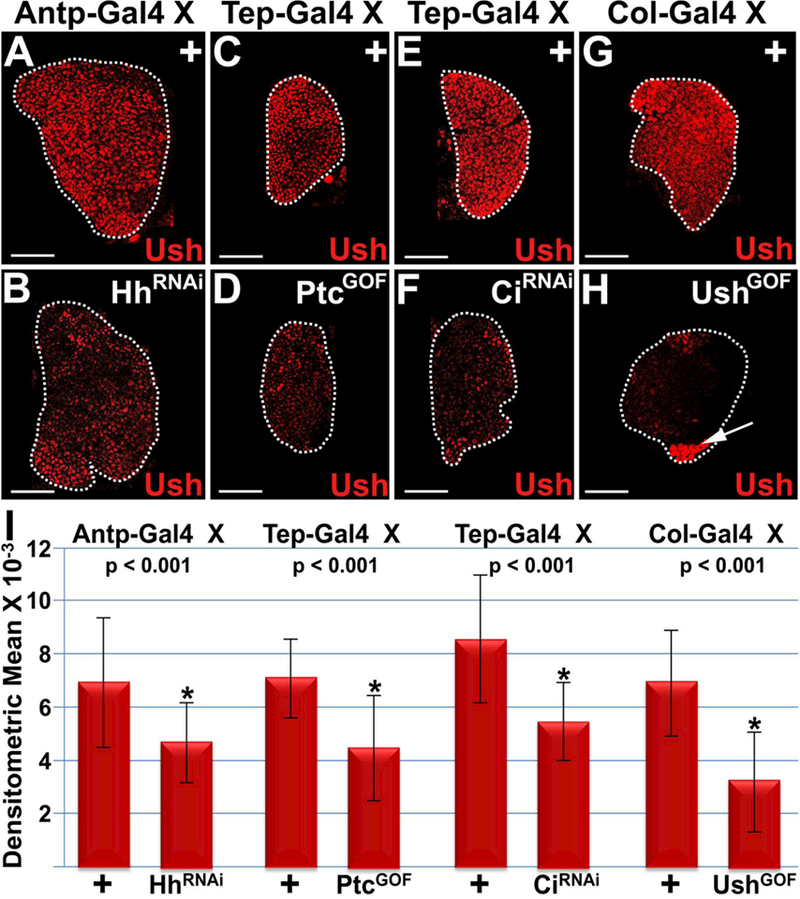
Hh signaling was disrupted by (A,B) knocking down Hh expression in the PSC (Antp>HhRNAi), (C,D) over-expressing Ptc in the MZ (Tep>PtcGOF), (E,F) knocking down Ci in the MZ (Tep>CiRNAi) or (G,H) mis-expressing Ush in the PSC (Col>UshGOF). This resulted in a significant reduction in Ush expression levels. Arrow marks ectopically expressed Ush in the PSC. (I) Histogram showing that the level of Ush expression is significantly reduced in lymph glands with disrupted Hh signaling. Student’s t-test; error bars show standard deviation; P values are as shown; control and HhRNAi (n=20); control and PtcGOF (n=20); control and CiRNAi (n=24); control and UshGOF (n=22). White dotted lines delineate the entire lymph gland. Scale bars: 50 μm. Mid-third instar larvae.
hh gene expression in the PSC is upregulated by Srp 63. In contrast, Ush is not expressed in the PSC50,86 and mis-expressing Ush in this tissue blocks hh enhancer activity63, presumably by binding Srp and converting it from an activator to a repressor. We confirmed that mis-expressing Ush blocks hh enhancer activity, but does not reduce the number of PSC cells (Supplementary Figure 2). Furthermore, we also showed that mis-expressing Ush in the PSC significantly reduced endogenous Ush expression (Figure 2 G,H,I). These results are consistent with the hypothesis that loss of canonical Hh signaling results in loss of Ush expression. Moreover, we consistently observed that disruption of Hh signaling in either the PSC or MZ resulted in a significant reduction in Ush expression throughout the lymph gland, including in the CZ (Figures 1 and 2 and Supplementary Figure 2). Perhaps precocious activation of the differentiation pathway sets up a reinforcing feedback loop that further represses Ush expression in both the MZ and the differentiating cells of the CZ. In support of this hypothesis, we have previously shown that Ush expression is downregulated in a subset of plasmatocytes, crystal cells and lamellocytes86.
Hh signaling is required to block hemocyte differentiation
The observation that Hh signaling maintains Ush expression is in line with previous studies showing that loss of either ush expression or Hh signaling results in increased numbers of crystal cells and plasmatocytes41,58,63,86. Previous studies achieved loss of Hh signaling using either a hh temperature sensitive (ts) allele or by knocking down the hh gene activator, Srp, in the PSC 41,58,63. Thus, knockdown of Hh in the PSC using Antp-Gal4 to express UAS-hhRNAi should phenocopy the results obtained with the hh ts allele and Srp knockdown, that is increased numbers of plasmatocytes and crystal cells. Indeed, we observed that lymph glands with Antp-Gal4 driven UAS-hhRNAi had a statistically significant increase in the numbers of crystal cells (Figure 3 A–C) and plasmatocytes (Figure 3 E–F) compared to controls. Together, these studies indicate that Hh signals from the PSC limit the differentiation of crystal cells and plasmatocytes.
Figure 3. Hedgehog signaling blocks blood cell differentiation.
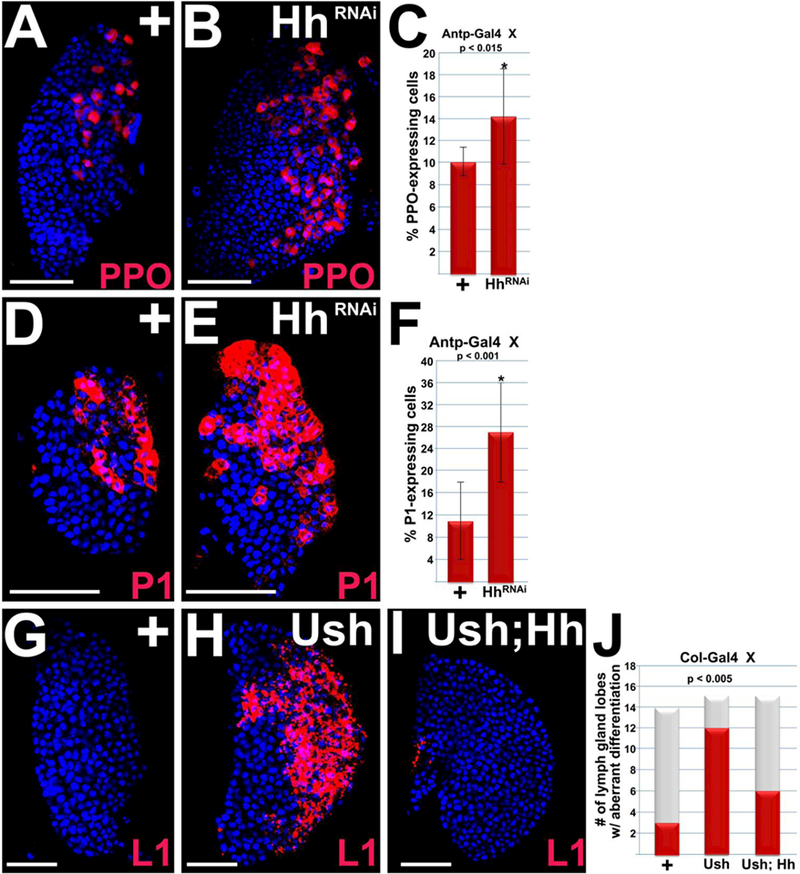
(A–F) Hh signaling was disrupted by using Antp-Gal44 to drive UAS-hhRNAi in the PSC. This resulted in a significant increase in the number of crystal cells and plasmatocytes in mid-third instar larvae. (A,B) Crystal cells are marked with ProPO (PPO) and (D,E) plasmatocytes are marked with PI. (C,F) Histograms showing the results of the statistical analyses. Student’s t-test; error bars show standard deviation; P values are as shown; (C) control and HhRNAi (n=10); (F) control and HhRNAi (n=12). (G–J) Col-Gal4 was used to express UAS-ush or co-express UAS-ush;UAS-hh in the PSC of later third instar larvae. (G,H) Mis-expressing Ush resulted in a significant increase in lamellocyte differentiation, (G–I) which was significantly repressed by co-expressing Ush and Hh. (G–I) Lamellocytes are marked with LI. (J) Histogram showing the results of the statistical analyses. Chi-square test; P value is as shown; Col-Gal4/+ (n=14); Col>Ush (n= 15); Col>Ush;Hh (n=15). Lymph glands are counterstained with Dapi. Scale bars: 50 μm.
Ush is required to block lamellocyte differentiation 86,106. Furthermore, our new findings show that ush genetically interacts with smo to block lamellocyte differentiation. These observations suggested that Hh signaling blocks lamellocyte differentiation. To test this hypothesis, we disrupted Hh signaling by mis-expressing Ush in the PSC. Under these conditions, we observed a significant increase in the number of lymph gland lobes with increased lamellocyte differentiation (Figure 3 G,H,J). Furthermore, co-expressing Hh and Ush in the PSC rescued the Ush mis-expression phenotype, significantly reducing the number of lymph gland lobes exhibiting lamellocyte differentiation compared to mis-expressing Ush alone (Figure 3 G–J). Collectively, these data provide strong support for the hypothesis that Hh signaling blocks lamellocyte differentiation, which is also consistent with a previous study that showed loss of Smo function in the MZ also increased the number of lamellocytes107.
Ush functions in the MZ to maintain Odd-positive and E-cadherin-positive prohemocytes
Previous studies have identified Odd and E-cadherin as prohemocyte markers50,103,104. Our work has shown that over-expressing Ush in the MZ increased the number of Odd-positive prohemocytes, whereas systemic loss of Ush function results in the loss of Odd-positive prohemoctyes and a significant reduction in the E-cadherin expression domain47,86,103,104. Collectively, these observations support the hypothesis that Ush functions in the MZ to maintain the prohemocyte population. To provide additional support for this hypothesis, we used the Tep-Gal4 driver to express UAS-ushRNAi in the MZ and observed a significant reduction in both the percentage of Odd-positive prohemocytes and the E-cadherin expression domain (Figure 4). We also used an alternate MZ-restricted driver, Dome-Gal4, to knockdown Ush and showed that under these conditions the percentage of Odd-positive prohemocytes was again significantly reduced (Supplementary Figure 3). Thus, these data show that Ush functions in the MZ to maintain a prohemocyte population.
Figure 4. Ush is required to maintain the number of Odd-positive prohemocytes and the level of E-cadherin expression.
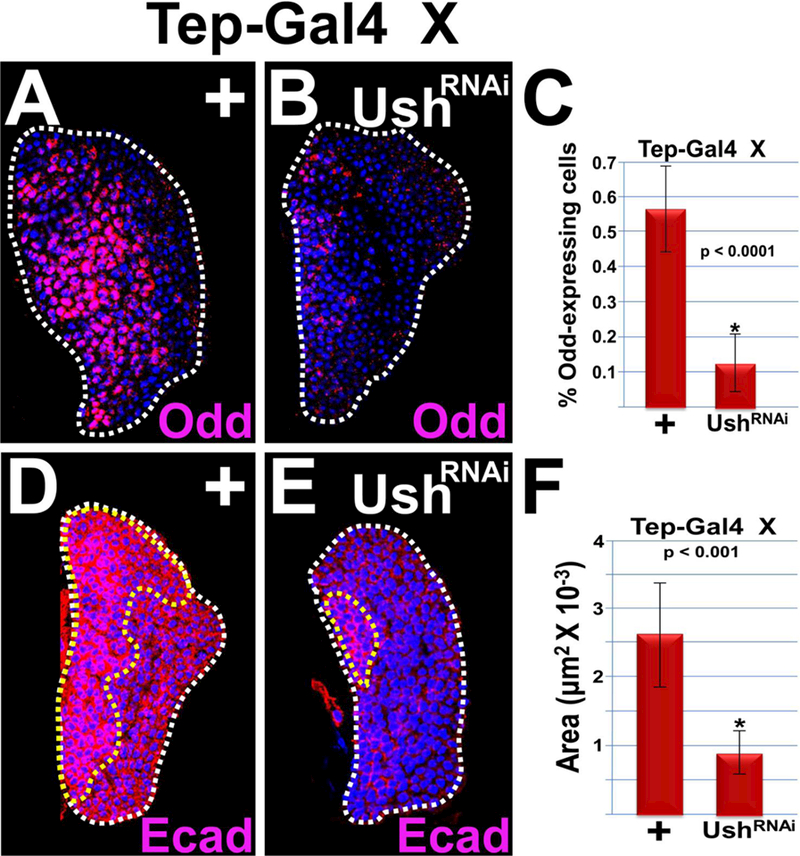
Knockdown of Ush expression in the MZ (Tep>UshRNAi) significantly reduced (A–C) the percentage of Odd-positive prohemocytes and (D–F) the E-cadherin (Ecad) expression domain compared to controls. (C,F) Histograms showing the results of the statistical analyses. Student’s t-test; error bars show standard deviation; P values are as shown; (C) control and Ush knockdown (n=12); (F) control and Ush knockdown (n=10). Lymph glands are counterstained with Dapi. White dotted lines delineate the entire lymph gland; yellow dotted line delineates the Ecad-positive prohemocyte pool. Scale bars: 50 μm. Mid-third instar larvae.
Hh signaling is required to maintain a subpopulation of prohemocytes
Given that Hh signaling is required to maintain Ush expression levels and that Ush functions in the MZ to maintain the Odd-positive prohemocyte pool, we then tested if Hh signaling is also required to maintain the Odd-positive prohemocyte population. Knockdown of Hh in the PSC using Antp-Gal4 driven UAS-hhRNAi resulted in a statistically significant reduction in the percentage of Odd-positive prohemocytes (Figure 5 A,B,G). This result was confirmed using the Col-Gal4 driver to express an alternate UAS-h1fNAl allele in the PSC (Supplementary Figure 4 A–C). Likewise, disrupting Hh signaling by mis-expressing Ush in the PSC significantly reduced the percentage of Odd-positive prohemocytes (Figure 5 C,D,G). Knockdown of Ci in the MZ also significantly reduced the percentage of Odd–positive prohemocytes (Figure 5 E–G). Moreover, the Odd expression domain was significantly reduced in lymph glands with Tep-Gal4 driving UAS-SmoDN compared to controls (Supplementary Figure 4 D–F). Thus, loss of canonical Hh signaling led to a significant reduction in Odd-positive prohemocytes.
Figure 5. Hedgehog signaling maintains the Odd-positive and DomeMESO-positive but not Col-positive prohemocyte population.
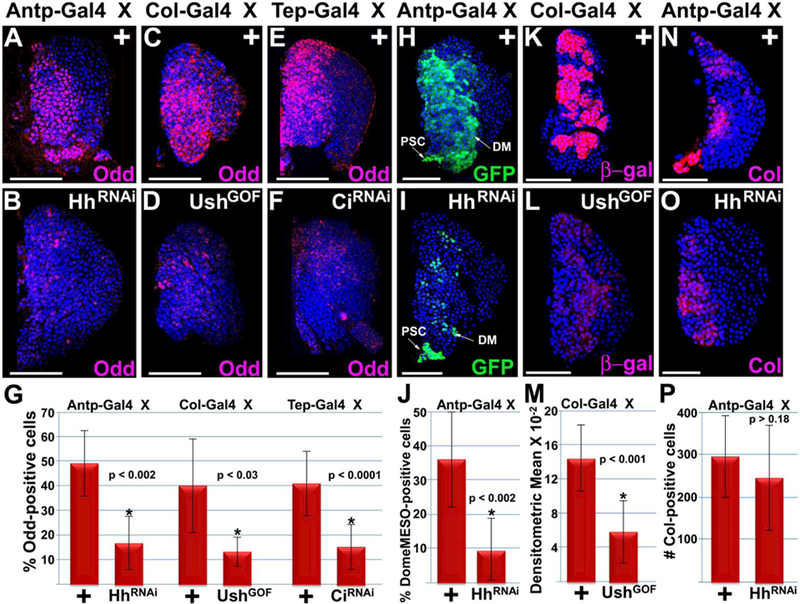
(A–G) Disrupted Hh signaling significantly reduced the percentage of Odd-positive prohemocytes compared to controls. Hh signaling was disrupted by (A–D) reducing Hh expression in the PSC (Antp>HhRNAi or Col>UshGOF) or (E,F) loss of Ci function in the MZ (Tep>CiRNAi). (H–J) Knockdown of Hh expression in the PSC resulted in a significant reduction in the number of DomeMESO-GFP expressing prohemocytes. (K–M) Disrupting Hh signaling by mis-expressing Ush in the PSC (Col>UshGOF) significantly reduced DomeMESO-βgal expression levels. (N–P) Knockdown of Hh expression in the PSC did not significantly reduce the number of Col-positive prohemocytes. (G,J,M,P) Histograms show the results of the statistical analyses. Student’s t-test; error bars show standard deviation; P values are as shown; (G) control and HhRNAi (n=11), control and UshGOF (n=10), control and CiRNAi (n=10); (J) control and HhRNAi (n=11); (M) control and UshGOF (n=12); (P) control and HhRNAi (n=11). Mid-third instar lymph glands are counterstained with Dapi. Scale bars: 50 μm.
Our findings that Hh signaling maintains Odd-positive prohemocytes is in contrast to a recent study showing that the PSC is not required to maintain prohemocytes marked with either DomeMESO or Col 43. We considered that the Odd-positive prohemocyte population may be distinct from that of the DomeMESO- and Col-positive population. To test this hypothesis, we first determined if Odd, DomeMESO, and Col are expressed in different prohemocyte populations. To compare the Odd and DomeMESO expression domains, we used two different fly strains carrying two different versions of the DomeMESO prohemocyte marker: 1) the cytoplasmic marker, DomeMESO-β-galactosidase (DomeMESO-βgal) and 2) the nuclear marker, DomeMESO-GFP. Lymph glands from each of these two strains were analyzed using immuno-fluorescence to determine if Odd was co-expressed with β-gal and/or GFP. We observed that the Odd expression domain largely overlapped that of both DomeMESO reporters (Supplementary Figure 5 A-A’”, B-B”‘). However, we did observe a small number of cells with either predominately Odd expression (Oddhigh7DomeMES0low) or predominately DomeMESO expression (Oddlow/DomeMESOHigh). Compare the insets from Supplementary Figure 5A” with A”‘ and Supplementary Figure 5 B”withB”’.
Next, we compared the Odd and Col expression domains by testing if Odd was co-expressed with Col in lymph glands from w1118 larve (Supplementary Figure 5 C-C’”). Co-localization studies showed that the Col-positive population was also Odd-positive, but the Odd-positive population was heterogeneous with both Col-positive and Col-negative cells. Indeed, all of the Col-positive cells appeared to express high levels of Odd (ColHigh/OddHigh); however, none of the Col-positive cells appeared to have low levels of Odd expresion (ColHigh/Oddlow). On the other hand, Col expression was not detected in approximately 36% of the Odd-positive cells (Colneg/OddHigh). Compare the insets from Supplementary Figure 5C’, C” and C”\ Thus, the Odd-positive population includes a majority (−64%) of Col-positive cells (ColHigh/OddHigh) with a considerable minority (−36%) of Col-negative cells (Colneg/OddHigh). Furthermore, approximately 30% of the Colneg/OddHigh minority (−11% of the total Odd-positive population) was located in a region that may include the CZ or perhaps the border region between the MZ and CZ. Compare the panels from Supplementary Figure 5 C’, C” and C”’ Overall, the results from these co-localization analyses are consistent with an earlier study that demonstrated heterogeneity within the prohemocyte population108.
We then tested the effect of loss of Hh signaling on the population of DomeMESO-positive and Col-positive cells. When we knocked down Hh in the PSC using Antp-Gal4 {UAS-GFP;DomeMESO-GFP,Antp-Gal4) to drive UAS-hhRNAi, we observed a significant reduction in the number of DomeMESO-GFP expressing cells (Figure 5, H–J). We also observed GFP expression in the PSC, which resulted from Antp-Gal4 driving UAS-GFP in addition to lIAS-hhRXAl. We confirmed that DomeMESO positive cells were maintained by Hh signaling by showing that when Col-Gal4 was used to drive an alternate UAS-hhRNAi construct in the PSC, βgal expression was significantly reduced (Supplementary Figure 4 G–I). We also disrupted Hh signaling by over-expressing Ush in the PSC and observed that the level of DomeMESO-βgal was significantly reduced (Figure 5 K–M). Conversely, knock down of Hh did not reduce the number of Col-positive cells (Figure 5 N–P), which is consistent with previous findings that show Hh signaling from the PSC is not required to maintain the Col-positive prohemocyte population43,58. Collectively, these results suggest that Hh signaling is required to maintain Odd-positive and DomeMESO-positive prohemocytes, but not Col-positive prohemocytes.
The PSC is required to maintain a subpopulation of prohemocytes
Previous studies have shown that ablation of the PSC has no effect on Col-positive prohemocytes43. Consistent with this observation, our current studies and those from another laboratory58 show that loss of Hh signaling from the PSC has no effect on the Col-positive prohemocyte population. In contrast, here we showed that Odd-positive and DomeMESO-positive prohemocytes are reduced when Hh signaling is disrupted. Together, these observations suggest that the MZ contains both PSC-dependent and PSC-independent prohemocytes. To test this hypothesis, we determined if ablating the PSC would alter the expression of Ush and the prohemocyte markers, Odd, E-cadherin, DomeMESO and Col. We used Col-Gal4 driven UAS-reaper (rpr) to ablate the PSC according to the method of Benmimoun et al. 43. This resulted in a complete loss of Antp marked PSC cells (Supplementary Figure 6) confirming that the PSC was indeed ablated. Under these conditions, we observed that Ush expression was significantly reduced (Figure 6 A–C). Likewise, the number of Odd-positive cells and the E-cadherin expression domain was also significantly reduced (Figure 6 D–I). We also observed a significant reduction in DomeMESO-positive cells in lymph glands with the PSC ablated (Figure 6 J–L). Conversely, PSC ablation did not significantly reduce the percentage of Col-positive prohemocytes, consistent with the results reported by Benmimoun et al. 43. Collectively, our findings support the hypothesis that the MZ contains both PSC-dependent and PSC-independent prohemocytes.
Our new data indicate that the PSC is required to maintain the full complement of Odd-positive, but not Col-positive, prohemocytes. Given that the Odd-positive population includes both Col-positive and Col-negative prohemocytes (Figure 7 A-A”’; Supplementary Figure 5C-C’”), we asked whether the population of ColHigh/OddHigh prohemocytes was PSC-independent, while the Colneg/OddHigh population was PSC-dependent. To address this question, we assayed for Odd and Col co-expression in lymph glands with PSC ablation. Under these conditions, we again observed a statistically significant reduction in the percentage of Odd-positive cells, but no significant change in the percentage of Col-positive cells (Figure 7). Furthermore, the number of Odd-positive cells that remained after PSC ablation was not statistically different from the number of Col-positive cells in either control or PSC ablated lymph glands (Figure 7C). These data suggest that PSC ablation reduces the number of Colneg/OddHigh positive prohemocytes, whereas Col-positive prohemocytes are resistant to the effects of PSC ablation. This supports the hypothesis that Colneg/OddHigh prohemocytes are largely PSC-dependent, while Col-positive prohemocytes appeared to be largely PSC-independent. However, some of the Col-positive cells exhibited substantially reduced levels of Odd expression (ColHigh/Oddlow; compare insets in Figure 7 B’-B”’) in PSC ablated lymph glands. This ColHigh/Oddlow cell type was not observed in either w1118 or Col-Gal4/+ controls (Figure 7 A’-A’”; Supplementary Figure 5C’-C’”). This suggests that the PSC maintains optimal levels of Odd expression in a subset of Col-positive prohemocytes. We observed that Odd was also co-expressed with Col in the PSC (Figure 7 A-A’). However, the level of Odd expression appears to be similar in the MZ and PSC, whereas Col expression is clearly greater in the PSC than in the MZ.
Figure 6. The PSC maintains a subpopulation of prohemocytes.
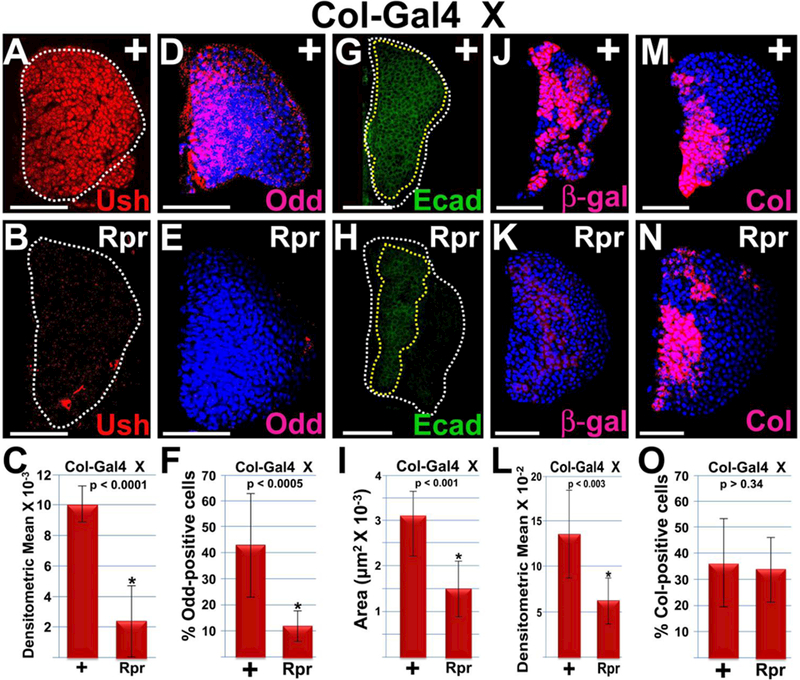
Ablation of the PSC (Col>Rpr) results in a significant reduction in (A–C) the level of Ush expression, (D–F) the percentage of Odd-positive prohemocytes, (G–I) the E-cadherin (Ecad) expression domain, (I–?) the percentage of DomeMESO-GFP-positive prohemocytes, but not (M–O) the percentage of Col-positive cells. (C,F,I,L,0) Histograms show the results of the statistical analyses. Student’s t-test; error bars show standard deviation; P values are as shown; (C) control and Rpr (n=16); (F) control and Rpr (n=10); (I) control and Rpr (n=16); (L) control and Rpr (n=12); (O) control and Rpr (n=10). (D,E,J,K,M,N) Lymph glands are counterstained with Dapi. White dotted lines delineate the entire lymph gland; yellow dotted line delineates the Ecad-positive prohemocyte pool. Scale bars: 50 μm. Mid-third instar larvae.
Discussion
The original hypothesis that the PSC functions as a niche from which multiple pathways, including Hh, signal to the MZ to maintain prohemocytes in multipotent state was based on evaluating the hematopoietic function of Col 41. Col is expressed in the PSC and systemic loss of Col function resulted in loss of the prohemocyte population 41. However, more recent work has shown that Col also functions in MZ and loss of Col in this zone, rather than in the PSC, leads to loss of prohemocytes 43,58. The role of the PSC in maintaining prohemocyte multipotency was further challenged by work showing that genetic ablation of the PSC had no effect on prohemocyte number 43. This also cast doubt on whether Hh, or any other signaling pathway that originates from the PSC, maintains the prohemocyte population. Furthermore, the conclusion that Hh signaling maintains the prohemocyte population was based on studies showing that loss of Hh signaling led to increased numbers of terminally differentiated plasmatocytes and crystal cells rather than direct assessment of prohemocyte marker expression41. Loss of Hh signaling does lead to a reduction in the MZ marker, Ptc63. However, the ptc gene is upregulated by Hh signaling109, leaving unanswered the question of whether Hh signaling maintains the prohemocyte population.
Our new findings provide compelling evidence that the PSC and Hh signaling are required to maintain a subpopulation of prohemocytes during steady-state hematopoiesis. In this study, we directly assessed the prohemocyte population using four different prohemocyte markers, Odd, E-cadherin, DomeMESO and Col. We showed that loss of Hh signaling or PSC ablation results in a significant reduction in prohemocytes marked with Odd and DomeMESO and a significant reduction in the level of DomeMESO and E-cadherin expression. However, consistent with previously published work, we did not see a statistically significant reduction in the number of Col-expressing cells under either condition43,58. Importantly, we showed that the Odd-positive prohemocyte population consists of both Col-positive (Colhigh/Oddhigh) and Col-negative (Colneg/Oddhigh) cells. Furthermore, Colneg/Oddhigh cells appear to be largely PSC-dependent, while Colhigh/Oddhigh prohemocytes appear to be largely PSC-independent. Nevertheless, PSC ablation also produced some Col-positive cells with substantially reduced levels of Odd expression (Colhigh/Oddlow); a cell type not observed in control lymph glands. This suggests that the PSC maintains optimal levels of Odd expression in some of the Col-positive prohemocytes. Furthermore, the possibility remains that loss of the PSC can affect the transcriptome in a subpopulation of Col-positive prohemocytes by reducing the expression level of the transcription factor, Odd. Overall, our findings support the notion that the PSC functions as a hematopoietic niche during steady-state hematopoiesis.
We speculate that PSC-dependent and PSC-independent prohemocytes might fill two different roles during hematopoiesis. Col-positive, PSC-independent prohemocytes may be resistant to extrinsic signals and regulated primarily by intrinsic signals. This strategy would maintain a pool of undifferentiated cells that are capable of self-renewal and are protected against progenitor exhaustion during steady state hematopoiesis. On the other hand, PSC-dependent prohemocytes would be extrinsically regulated and poised to respond to normal differentiation signals. These new findings may serve as a foundation for studies designed to delve deeper into the underlying mechanisms by which prohemocytes are maintained in a multipotent state. Ultimately, such studies may provide insights into how human hematopoietic stem cells (HSCs) can be generated and maintained indefinitely ex vivo. In this regard, recent reports showed that ex vivo reprogramming of mammalian tissues requires as yet uncharacterized extracellular cues in order to obtain fully functional HSCs110–112.
A recent report demonstrated that Dpp signaling from the PSC is required during the early first instar to maintain a transient Notch-positive prohemocyte precursor. Loss of Dpp signaling during the first larval instar reduced the number of prohemocyte precursors, leading to a dramatic reduction in lymph gland size by the third larval instar45. In contrast, the method of Benmimoun et al. achieves maximum PSC ablation later in development, during the second larval instar43. Under these conditions, PSC ablation did not affect the size of the third instar lymph gland. This supports the hypothesis of Dey et al.45, which states that Dpp signaling from the PSC is required in first larval instar to maintain Notch-positive prohemocyte precursors; however, Dpp is dispensable for lymph gland growth from the second larval instar onward. Furthermore, loss of Dpp did not alter the relative number of CZ and MZ cells45. Thus, Dpp controls early precursor cell number but does not regulate cell fate. In contrast, we showed that Hh is required to maintain MZ Odd-positive prohemocytes and limit CZ cells, including plasmatocytes, crystal cells and lamellocytes. Thus, Hh signaling promotes prohemocyte multipotency while limiting differentiation. This is reminiscent of the role of aberrant Hh signaling in hematopoietic malignancies. In this case, Hh signaling from adjacent stromal or tumor cells are thought to maintain cancer stem cells by upregulating pluripotency factors113.
Our findings show that DomeMESO-positive prohemocytes were reduced in response to disrupted Hh signaling or PSC ablation, which is in contrast to previous reports43,58. Nevertheless, our results and conclusions are supported by the following aspects of our experimental approach. First, we obtained the same results using two different DomeMESO strains and with multiple methods designed to disrupt Hh signaling, including PSC ablation. Additionally, our methods are comparable to the ones used in those previous studies43,58 given that we, like they, observed that the number of Col-positive cells were unchanged in response to Hh disruption or PSC ablation. While we have no explanation for the differences obtained with the DomeMESO marker across laboratories, we have no doubt that continued characterization of the lymph gland system will uncover the reasons for these inter-lab differences, which may be of considerable importance in understanding the mechanism of progenitor multipotency.
Notably, there is a paucity of information about how extrinsic signaling pathways interface with GATA:FOG complexes during mammalian hematopoiesis105. However, studies in Drosophila have identified new interactions between signaling pathways and the GATA:FOG (Srp:Ush) complex. For example, a previous study showed that Ush acts with Srp to block hh gene expression63. This finding, coupled with our new data, points to a potential negative feedback loop in which Hh signaling from the PSC upregulates Ush in the MZ, which then binds Srp to limit hh expression. Notably, abnormal Hh signaling promotes tumor development and hematological malignancies 83,114 and Hh pathway members, such as Smo, have become anti-neoplastic therapeutic targets. In contrast, GATA transcription factors are typically poor drug targets115. Thus, disorders driven by interactions between GATA and Hh signaling could potentially be treated by targeting Hh pathway members, such as Smo. Considering that PSC ablation also leads to loss of Ush expression, the possibility remains that additional extrinsic signaling pathways also regulate Ush expression. For example, we recently showed that over–expression of the NFkB homolog, Dorsal, in the PSC reduced Ush expression in the MZ and CZ 47. Thus, our in vivo screen that identified Smo, and by extension Hh signaling, as Ush regulators may identify additional conserved signaling pathways that interface with GATA:FOG complexes to control mammalian hematopoiesis.
In conclusion, our findings support the original hypothesis that the PSC functions as a hematopoietic niche 40,41, thereby affirming the use of this larval signaling tissue as a tool to investigate the mechanisms by which the microenvironment maintains progenitors in a multipotent state. Notably, we have delineated PSC-dependent and PSC-independent subpopulations of prohemocytes. Further characterization of these functional subpopulations may increase our knowledge of how hematopoietic progenitors can be regulated by either intrinsic or extrinsic mechanisms. Toward this goal, our findings describe a connection between a major a signal transduction pathway and a hematopoietic master regulator that maintains a prohemocyte subpopulation, illustrating how extrinsic signals interface with the intrinsic progenitor regulatory machinery to regulate hematopoiesis.
Supplementary Material
Additional Gal4 drivers and an alternate UAS-hhRNAi strain were used to confirm that Hh signaling maintains Ush expression. Hh signaling was disrupted by (A,B) knocking down Hh expression in the PSC using an alternate UAS-hhRNAi strain driven by Col-Gal4, (C,D) over-expressing Ptc in the MZ using Dome-Gal4 driven UAS-ptc or (E,F) knocking down Ci in the MZ using Dome-Gal4 driven UAS-ciRXAl. This resulted in a significant reduction in Ush expression levels. (G) Histogram shows the results of the statistical analyses. Student’s t-test; error bars show standard deviation; P values are as shown; control and HhRNAi (n=10); control and PtcGOF (n=15); control and CiRNAi (n=15). White dotted lines delineate the entire lymph gland. Scale bars: 50 μm. Mid-third instar larvae.
Col-Gal4 females were crossed to control or UAS-ush males (Col>Ush). (A,B) hh enhancer activity is abolished in Col>Ush lymph glands. All control lymph glands showed hh enhancer activity (n=12), while none of the lymph glands with Ush mis-expressed in the PSC showed hh enhancer activity (n=12). (C–E) PSC cells are marked with Antp. (E) Histograms show the results of the statistical analyses. Student’s t-test; error bars show standard deviation; P values are as shown; control and UshGOF, (n=9). Scale bars: 50 μm. Mid-third instar larvae.
Knockdown of Ush expression in the MZ (Dome>UshRNAi) significantly reduced the percentage of Oddpositive prohemocytes compared to controls. (C) Histograms show the results of the statistical analyses. Student’s t-test; error bars show standard deviation; P values are as shown; control and UshRNAi (n=10). Scale bars: 50 μιη. Mid-third instar larvae.
Additional reagents, including Col-Gal4 driven expression of an alternate UAS-hhRNAi construct and Tep-Gal4 driven UAS-smoDN were used to confirm that Hh signaling maintains Odd-positive prohemocytes and the level of Odd and DomeMESO expression. (A–C) Knockdown of Hh in the PSC using Col-Gal4 to drive expression of an alternate UAS-hhRNAi construct produced a statistically significant reduction in the percentage of Odd-positive prohemocytes. (D–F) Loss of Smo function (SmoDN) significantly reduced the size of the Odd-positive expression domains compared to controls. White dotted lines delineate the entire lymph gland; yellow dotted lines delineate the Odd-expression domain. (G–I) Knockdown of Hh in the PSC using Col-Gal4 to drive expression of an alternate UAS-hhRNAi construct produced a statistically significant reduction in the expression of DomeMESO-βgal. (C,F,I) Histograms show the results of the statistical analyses. Student’s t-test; error bars show standard deviation; P values are as shown; (C,I) control and HhRNAi (n=10); (F) control and SmoDN (n=18). Scale bars: 50 μm; mid-third instar larvae.
(A-A”‘) Lymph gland from DomeMESO-βgal larvae assayed for β-galactosidase (β-gal) and Odd expression. (A,A’) Lymph gland showing β-gal and Odd expression, (A) counterstained with Dapi. (A’) Inset showing a few cells with predominantly Odd or β-gal expression. (A”) Lymph gland showing Odd expression only, with the inset showing cells with high (red arrow) or low (green arrow) Odd expression. (A’”) Lymph gland showing β-gal expression only, with the inset showing cells with high (green arrow) or low (red arrow) β-gal expression. (B-B’”) Lymph gland from DomeMESO-GFP larvae assayed for GFP and Odd expression. (B,B’) Lymph gland showing GFP and Odd expression, (B) counterstained with Dapi. (B’) Inset showing a few cells with predominantly Odd or GFP expression. (B”) Lymph gland showing Odd expression only, with the inset showing cells with high (red arrow) or low (green arrow) Odd expression. (B’”) Lymph gland showing GFP expression only, with the inset showing cells with high (green arrow) or low (red arrow) GFP expression. (C-C’”) Lymph gland from w1118 larvae assayed for Col and Odd expression. (C,C’) Lymph gland showing Col and Odd expression, (C) counterstained with Dapi, (C”) Col expression only and (C’”) Odd expression only. (C’-C’”) Arrows mark Odd-positive cells located in or near the presumptive CZ and insets show cells within the presumptive MZ that express only Odd. Col expression was not detected in approximately 36% (0.36 + 0.18; n=12) of the Odd-positive cells (Colneg/OddHigh). Approximately 30% (0.30 + 0.19; n=12) of this Colneg/OddHigh minority (~11% of the total Odd-positive population) may be located in the CZ. White dotted lines delineate the entire lymph gland. Scale bars: 50 μm. Mid-third instar larvae.
PSC cells are marked with Antp. (A) Shows the complement of PSC cells (green arrow) in control lymph glands (+). All lymph glands assessed (n=14) showed similar expression patterns. This is in contrast to (B) in which Antp-expressing cells were not detected (white arrow) in any of the lymph glands (n=14) with Col-Gal4 driven UAS-rpr expression (Col>Rpr). White dotted lines delineate the entire lymph gland. Scale bars: 50 μm. Mid-third instar larvae.
Figure 7. The PSC is required to maintain the complement of Odd-positive, but not Col-positive prohemocytes.
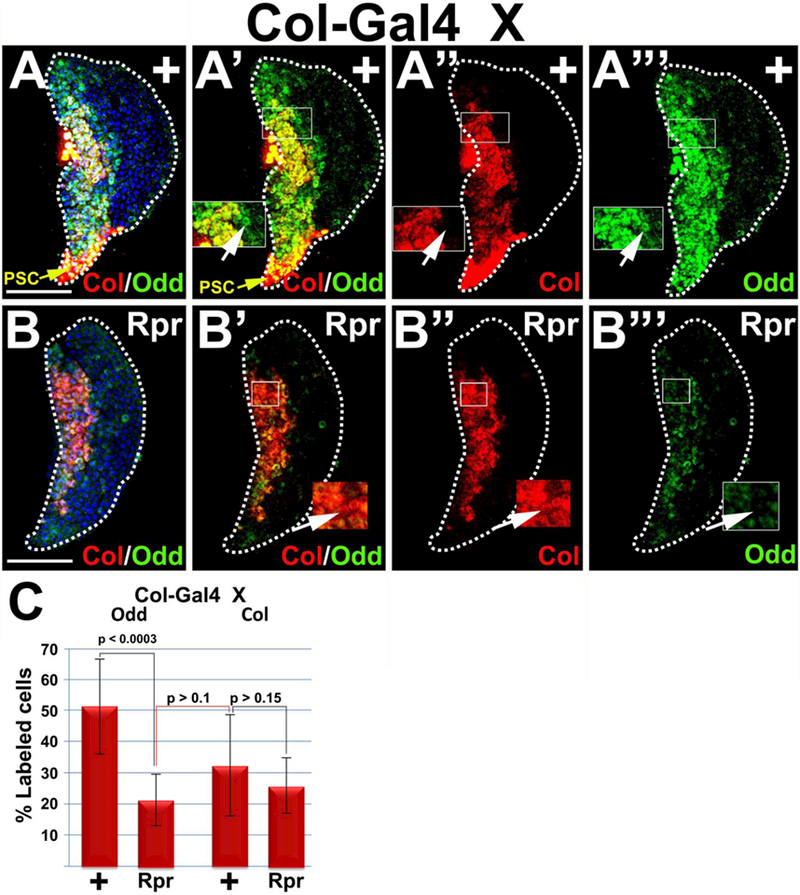
Ablation of the PSC (Col>Rpr) results in a significant reduction in the percentage of Odd-positive prohemocytes, but not the percentage of Col-positive cells. (A-A’”) Controls and (B-B’”) Col>Rpr. A,A’,B,B’) Lymph glands showing Col and Odd expression, (A, A’) PSC marked with yellow arrows, (A,B) counterstained with Dapi, (A”,B”) lymph glands showing only Col expression, (A’”, B’”) lymph glands showing only Odd expression. (A’-A’”) Insets showing cells that express Odd but not Col, marked with arrow. (B’-B’”) Insets showing cells with predominantly Col expression but with reduced Odd expression, marked with arrow. (C) Histogram showing that Odd-positive prohemocytes are significantly reduced in response to PSC ablation; whereas Col-positive prohemocytes are not significantly reduced in response to PSC ablation. Black connecting lines compare percentage of Odd control and experimental samples or Col control and experimental samples. Red connecting line compares percentage of Odd-positive cells in PSC ablated lymph glands with Col-positive cells in control lymph glands. Student’s t-test; error bars show standard deviation; P values are as shown; control and Rpr (n=10). White dotted lines delineate the entire lymph gland. Scale bars: 50 μm. Mid-third instar larvae.
Highlights:
The PSC and Hedgehog signaling are required for U-shaped expression
The progenitor population contains PSC-dependent and PSC-independent prohemocytes
Hedgehog signaling blocks lamellocyte differentiation
Revealed a link between intrinsic progenitor regulation and the extrinsic niche
Acknowledgements
This work was supported by Public Health Service grant DK072229 from the National Institutes of Health. We gratefully acknowledge our colleagues for providing fly strains and antibodies. T. Tokusumi and R. A. Schulz provided Tep4-Gal4, hhF4f;Antp-Gal4/TM6B Tb , Col-Gal4/CyO GFP and MSNF9mo-DsRed(MSN-C), S. Govind provided UAS-GFP;DomeMESO-GFP,Antp-Gal4,/TM6B Tb, from M. Crozatier provided Domeless-Gal4 and anti-Collier, Xiaoyan Zheng provided UAS-hh, M. P. Zeidler and J. C. Hombria provided, w p{w+, Dome-MESO}BN1, J. Skeath provided anti-Odd- skipped, I. Ando provided anti-Nimrod (P1) and mouse anti-Attila (L1), F. C. Kafatos provided anti prophenoloxidase A1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (1).Crozatier M, Vincent A. Drosophila: a model for studying genetic and molecular aspects of haematopoiesis and associated leukaemias. Dis Model Mech. 2011;4:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Gold KS, Bruckner K. Macrophages and cellular immunity in Drosophila melanogaster. Semin Immunol. 2015;27:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Letoumeau M, Lapraz F, Sharma A et al. Drosophila hematopoiesis under normal conditions and in response to immune stress. FEBS Lett 2016;590:4034–4051. [DOI] [PubMed] [Google Scholar]
- (4).Makhijani K, Bruckner K. Of blood cells and the nervous system: hematopoiesis in the Drosophila larva. Fly (Austin ). 2012;6:254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Parsons B, Foley E. Cellular immune defenses of Drosophila melanogaster. Dev Comp Immunol. 2016;58:95–101. [DOI] [PubMed] [Google Scholar]
- (6).Wang L, Kounatidis I, Ligoxygakis P. Drosophila as a model to study the role of blood cells in inflammation, innate immunity and cancer. Front Cell Infect Microbiol. 2014;3:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Fossett N Signal transduction pathways, intrinsic regulators, and the control of cell fate choice. Biochim Biophys Acta. 2013;1830:2375–2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Crozatier M, Meister M. Drosophila haematopoiesis. Cell Microbiol. 2007;9:1117–1126. [DOI] [PubMed] [Google Scholar]
- (9).Evans IR, Wood W. Drosophila blood cell chemotaxis. Curr Opin Cell Biol. 2014;30:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Hartenstein V Blood cells and blood cell development in the animal kingdom. Annu Rev Cell Dev Biol. 2006;22:677–712. [DOI] [PubMed] [Google Scholar]
- (11).Honti V, Csordas G, Kurucz E, Markus R, Ando I. The cell-mediated immunity of Drosophila melanogaster: Hemocyte lineages, immune compartments, microanatomy and regulation. Dev Comp Immunol. 2014;42:47–56. [DOI] [PubMed] [Google Scholar]
- (12).Kurucz E, Vaczi B, Markus R et al. Definition of Drosophila hemocyte subsets by cell-type specific antigens. Acta Biol Hung. 2007;58 Suppl:95–111. [DOI] [PubMed] [Google Scholar]
- (13).Lavine MD, Strand MR. Insect hemocytes and their role in immunity. Insect Biochem Mol Biol. 2002;32:1295–1309. [DOI] [PubMed] [Google Scholar]
- (14).Makhijani K, Alexander B, Rao D et al. Regulation of Drosophila hematopoietic sites by Activin-beta from active sensory neurons. Nat Commun. 2017;8:15990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Markus R, Laurinyecz B, Kurucz E et al. Sessile hemocytes as a hematopoietic compartment in Drosophila melanogaster. Proc Natl Acad Sci U S A. 2009;106:4805–4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Meister M, Lagueux M. Drosophila blood cells. Cell Microbiol. 2003;5:573–580. [DOI] [PubMed] [Google Scholar]
- (17).Dearolf CR. Fruit fly “leukemia”. Biochim Biophys Acta. 1998;1377:M13-M23. [DOI] [PubMed] [Google Scholar]
- (18).Lanot R, Zachary D, Holder F, Meister M. Postembryonic hematopoiesis in Drosophila. Dev Biol 2001;230:243–257. [DOI] [PubMed] [Google Scholar]
- (19).Rizki TM, Rizki RM. Lamellocyte differentiation in Drosophila larvae parasitized by Leptopilina. Dev Comp Immunol. 1992;16:103–110. [DOI] [PubMed] [Google Scholar]
- (20).Owusu-Ansah E, Banerjee U. Reactive oxygen species prime Drosophila haematopoietic progenitors for differentiation. Nature. 2009;461:537–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Gueguen G, Kalamarz ME, Ramroop J, Uribe J, Govind S. Polydnaviral ankyrin proteins aid parasitic wasp survival by coordinate and selective inhibition of hematopoietic and immune NF-kappa B signaling in insect hosts. PLoS Pathog. 2013;9:e1003580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Sorrentino RP, Carton Y, Govind S. Cellular immune response to parasite infection in the Drosophila lymph gland is developmentally regulated. Dev Biol. 2002;243:65–80. [DOI] [PubMed] [Google Scholar]
- (23).Babcock DT, Brock AR, Fish GS et al. Circulating blood cells function as a surveillance system for damaged tissue in Drosophila larvae. Proc Natl Acad Sci U S A. 2008;105:10017–10022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Crozatier M, Ubeda JM, Vincent A, Meister M. Cellular immune response to parasitization in Drosophila requires the EBF orthologue collier. PLoS Biol. 2004;2:E196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Dragojlovic-Munther M, Martinez-Agosto JA. Multifaceted roles of PTEN and TSC orchestrate growth and differentiation of Drosophila blood progenitors. Development. 2012;139:3752–3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Frandsen JL, Gunn B, Muratoglu S, Fossett N, Newfeld SJ. Salmonella pathogenesis reveals that BMP signaling regulates blood cell homeostasis and immune responses in Drosophila. Proc Natl Acad Sci U S A. 2008;105:14952–14957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Gold KS, Bruckner K. Macrophages and cellular immunity in Drosophila melanogaster. Semin Immunol. 2015;27:357–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Krzemien J, Crozatier M, Vincent A. Ontogeny of the Drosophila larval hematopoietic organ, hemocyte homeostasis and the dedicated cellular immune response to parasitism. Int J Dev Biol. 2010;54:1117–1125. [DOI] [PubMed] [Google Scholar]
- (29).Lebestky T, Chang T, Hartenstein V, Banerjee U. Specification of Drosophila hematopoietic lineage by conserved transcription factors. Science. 2000;288:146–149. [DOI] [PubMed] [Google Scholar]
- (30).Lebestky T, Jung SH, Banerjee U. A Serrate-expressing signaling center controls Drosophila hematopoiesis. Genes Dev. 2003;17:348–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Louradour I, Sharma A, Morin-Poulard I et al. Reactive oxygen species-dependent Toll/NF-kappaB activation in the Drosophila hematopoietic niche confers resistance to wasp parasitism. Elife. 2017;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Markus R, Kurucz E, Rus F, Ando I. Sterile wounding is a minimal and sufficient trigger for a cellular immune response in Drosophila melanogaster. Immunol Lett. 2005;101:108–111. [DOI] [PubMed] [Google Scholar]
- (33).Ratheesh A, Belyaeva V, Siekhaus DE. Drosophila immune cell migration and adhesion during embryonic development and larval immune responses. Curr Opin Cell Biol. 2015;36:71–79. [DOI] [PubMed] [Google Scholar]
- (34).RIZKI MT, Rizki RM. Functional significance of the crystal cells in the larva of Drosophila melanogaster. J Biophys Biochem Cytol. 1959;5:235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Rizki TM, Rizki RM. Parasitoid-induced cellular immune deficiency in Drosophila. Ann N Y Acad Sci. 1994;712:178–194. [DOI] [PubMed] [Google Scholar]
- (36).Sampson CJ, Valanne S, Fauvarque MO et al. The RhoGEF Zizimin-related acts in the Drosophila cellular immune response via the Rho GTPases Rac2 and Cdc42. Dev Comp Immunol. 2012;38:160–168. [DOI] [PubMed] [Google Scholar]
- (37).Shim J, Mukherjee T, Banerjee U. Direct sensing of systemic and nutritional signals by haematopoietic progenitors in Drosophila. Nat Cell Biol. 2012;14:394–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Ulvila J, Vanha-aho LM, Ramet M. Drosophila phagocytosis - still many unknowns under the surface. APMIS. 2011;119:651–662. [DOI] [PubMed] [Google Scholar]
- (39).Vlisidou I, Wood W. Drosophila blood cells and their role in immune responses. FEBS J. 2015;282:1368–1382. [DOI] [PubMed] [Google Scholar]
- (40).Krzemien J, Dubois L, Makki R et al. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007;446:325–328. [DOI] [PubMed] [Google Scholar]
- (41).Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, Banerjee U. A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446:320–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Benmimoun B, Polesello C, Waltzer L, Haenlin M. Dual role for Insulin/TOR signaling in the control of hematopoietic progenitor maintenance in Drosophila. Development. 2012;139:1713–1717. [DOI] [PubMed] [Google Scholar]
- (43).Benmimoun B, Polesello C, Haenlin M, Waltzer L. The EBF transcription factor Collier directly promotes Drosophila blood cell progenitor maintenance independently of the niche. Proc Natl Acad Sci U S A. 2015;112:9052–9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Chiu H, Ring BC, Sorrentino RP et al. dUbc9 negatively regulates the Toll-NF-kappa B pathways in larval hematopoiesis and drosomycin activation in Drosophila. Dev Biol. 2005;288:60–72. [DOI] [PubMed] [Google Scholar]
- (45).Dey NS, Ramesh P, Chugh M, Mandal S, Mandal L. Dpp dependent Hematopoietic stem cells give rise to Hh dependent blood progenitors in larval lymph gland of Drosophila. Elife. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Ferguson GB, Martinez-Agosto JA. The TEAD family transcription factor Scalloped regulates blood progenitor maintenance and proliferation in Drosophila through PDGF/VEGFR receptor (Pvr) signaling. Dev Biol 2017;425:21–32. [DOI] [PubMed] [Google Scholar]
- (47).Gao H, Baldeosingh R, Wu X, Fossett N. The Friend of GATA Transcriptional Co-Regulator, U-Shaped, Is a Downstream Antagonist of Dorsal-Driven Prohemocyte Differentiation in Drosophila. PLoS One. 2016;11:e0155372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Grigorian M, Liu T, Banerjee U, Hartenstein V. The proteoglycan Trol controls the architecture of the extracellular matrix and balances proliferation and differentiation of blood progenitors in the Drosophila lymph gland. Dev Biol 2013;384:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Grigorian M, DeBruhl H, Lipsick JS. The role of variant histone H2AV in Drosophila melanogaster larval hematopoiesis. Development. 2017;144:1441–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Jung SH, Evans CJ, Uemura C, Banerjee U. The Drosophila lymph gland as a developmental model of hematopoiesis. Development. 2005;132:2521–2533. [DOI] [PubMed] [Google Scholar]
- (51).Khadilkar RJ, Rodrigues D, Mote RD et al. ARF1-GTP regulates Asrij to provide endocytic control of Drosophila blood cell homeostasis. Proc Natl Acad Sci U S A. 2014;111:4898–4903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Kulkarni V, Khadilkar RJ, Magadi SS, Inamdar MS. Asrij maintains the stem cell niche and controls differentiation during Drosophila lymph gland hematopoiesis. PLoS One. 2011;6:e27667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Lam V, Tokusumi T, Tokusumi Y, Schulz RA. bantam miRNA is important for Drosophila blood cell homeostasis and a regulator of proliferation in the hematopoietic progenitor niche. Biochem Biophys Res Commun. 2014;453:467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Mandal L, Banerjee U, Hartenstein V. Evidence for a fruit fly hemangioblast and similarities between lymph-gland hematopoiesis in fruit fly and mammal aorta-gonadal-mesonephros mesoderm. Nat Genet. 2004;36:1019–1023. [DOI] [PubMed] [Google Scholar]
- (55).Minakhina S, Druzhinina M, Steward R. Zfrp8, the Drosophila ortholog of PDCD2, functions in lymph gland development and controls cell proliferation. Development. 2007;134:2387–2396. [DOI] [PubMed] [Google Scholar]
- (56).Mondal BC, Shim J, Evans CJ, Banerjee U. Pvr expression regulators in equilibrium signal control and maintenance of Drosophila blood progenitors. Elife. 2014;3:e03626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Morin-Poulard I, Sharma A, Louradour I et al. Vascular control of the Drosophila haematopoietic microenvironment by Slit/Robo signalling. Nat Commun. 2016;7:11634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).Oyallon J, Vanzo N, Krzemien J et al. Two Independent Functions of Collier/Early B Cell Factor in the Control of Drosophila Blood Cell Homeostasis. PLoS One. 2016;11:e0148978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (59).Sinenko SA, Mandal L, Martinez-Agosto JA, Banerjee U. Dual role of wingless signaling in stem-like hematopoietic precursor maintenance in Drosophila. Dev Cell. 2009;16:756–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Sinenko SA, Shim J, Banerjee U. Oxidative stress in the haematopoietic niche regulates the cellular immune response in Drosophila. EMBO Rep. 2012;13:83–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (61).Small C, Ramroop J, Otazo M et al. An unexpected link between notch signaling and ROS in restricting the differentiation of hematopoietic progenitors in Drosophila. Genetics. 2014;197:471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Tokusumi T, Tokusumi Y, Hopkins DW et al. Germ line differentiation factor Bag of Marbles is a regulator of hematopoietic progenitor maintenance during Drosophila hematopoiesis. Development. 2011;138:3879–3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Tokusumi Y, Tokusumi T, Stoller-Conrad J, Schulz RA. Serpent, suppressor of hairless and U-shaped are crucial regulators of hedgehog niche expression and prohemocyte maintenance during Drosophila larval hematopoiesis. Development. 2010;137:3561–3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Tokusumi Y, Tokusumi T, Shoue DA, Schulz RA. Gene regulatory networks controlling hematopoietic progenitor niche cell production and differentiation in the Drosophila lymph gland. PLoS One. 2012;7:e41604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Yu S, Luo F, Jin LH. The Drosophila lymph gland is an ideal model for studying hematopoiesis. Dev Comp Immunol. 2017. [DOI] [PubMed] [Google Scholar]
- (66).Leitao AB, Sucena E. Drosophila sessile hemocyte clusters are true hematopoietic tissues that regulate larval blood cell differentiation. Elife. 2015;4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (67).Petraki S, Alexander B, Bruckner K. Assaying Blood Cell Populations of the Drosophila melanogaster Larva. J Vis Exp. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Small C, Paddibhatla I, Rajwani R, Govind S. An introduction to parasitic wasps of Drosophila and the antiparasite immune response. J Vis Exp. 2012;e3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Williams MJ, Wiklund ML, Wikman S, Hultmark D. Rac1 signalling in the Drosophila larval cellular immune response. J Cell Sci. 2006;119:2015–2024. [DOI] [PubMed] [Google Scholar]
- (70).Williams MJ, Habayeb MS, Hultmark D. Reciprocal regulation of Rac1 and Rho1 in Drosophila circulating immune surveillance cells. J Cell Sci. 2007;120:502–511. [DOI] [PubMed] [Google Scholar]
- (71).Makhijani K, Alexander B, Tanaka T, Rulifson E, Bruckner K. The peripheral nervous system supports blood cell homing and survival in the Drosophila larva. Development. 2011;138:5379–5391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Evans CJ, Hartenstein V, Banerjee U. Thicker than blood: conserved mechanisms in Drosophila and vertebrate hematopoiesis. Dev Cell. 2003;5:673–690. [DOI] [PubMed] [Google Scholar]
- (73).Holz A, Bossinger B, Strasser T, Janning W, Klapper R. The two origins of hemocytes in Drosophila. Development. 2003;130:4955–4962. [DOI] [PubMed] [Google Scholar]
- (74).Honti V, Csordas G, Markus R et al. Cell lineage tracing reveals the plasticity of the hemocyte lineages and of the hematopoietic compartments in Drosophila melanogaster. Mol Immunol. 2010;47:1997–2004. [DOI] [PubMed] [Google Scholar]
- (75).Martinez-Agosto JA, Mikkola HK, Hartenstein V, Banerjee U. The hematopoietic stem cell and its niche: a comparative view. Genes Dev. 2007;21:3044–3060. [DOI] [PubMed] [Google Scholar]
- (76).Qiu P, Pan PC, Govind S. A role for the Drosophila Toll/Cactus pathway in larval hematopoiesis. Development. 1998;125:1909–1920. [DOI] [PubMed] [Google Scholar]
- (77).Gao H, Wu X, Simon L, Fossett N. Antioxidants maintain e-cadherin levels to limit Drosophila prohemocyte differentiation. PLoS One. 2014;9:e107768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Mondal BC, Mukherjee T, Mandal L et al. Interaction between differentiating cell- and niche-derived signals in hematopoietic progenitor maintenance. Cell. 2011;147:1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Crozatier M, Ubeda JM, Vincent A, Meister M. Cellular immune response to parasitization in Drosophila requires the EBF orthologue collier. PLoS Biol. 2004;2:E196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Minakhina S, Steward R. Hematopoietic stem cells in Drosophila. Development. 2010;137:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Pennetier D, Oyallon J, Morin-Poulard I et al. Size control of the Drosophila hematopoietic niche by bone morphogenetic protein signaling reveals parallels with mammals. Proc Natl Acad Sci U S A. 2012;109:3389–3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Tokusumi T, Tokusumi Y, Schulz RA. The mir-7 and bag of marbles genes regulate Hedgehog pathway signaling in blood cell progenitors in Drosophila larval lymph glands. Genesis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Ingham PW, McMahon AP. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 2001;15:3059–3087. [DOI] [PubMed] [Google Scholar]
- (84).Lim Y, Matsui W. Hedgehog signaling in hematopoiesis. Crit Rev Eukaryot Gene Expr. 2010;20:129–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Lum L, Beachy PA. The Hedgehog response network: sensors, switches, and routers. Science. 2004;304:1755–1759. [DOI] [PubMed] [Google Scholar]
- (86).Gao H, Wu X, Fossett N. Upregulation of the Drosophila Friend of GATA gene U-shaped by JAK/STAT signaling maintains lymph gland prohemocyte potency. Mol Cell Biol. 2009;29:6086–6096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Amigo JD, Ackermann GE, Cope JJ et al. The role and regulation of friend of GATA-1 (FOG-1) during blood development in the zebrafish. Blood. 2009;114:4654–4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Cantor AB, Katz SG, Orkin SH. Distinct domains of the GATA-1 cofactor FOG-1 differentially influence erythroid versus megakaryocytic maturation. Mol Cell Biol. 2002;22:4268–4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Cantor AB, Iwasaki H, Arinobu Y et al. Antagonism of FOG-1 and GATA factors in fate choice for the mast cell lineage. J Exp Med. 2008;205:611–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Chang AN, Cantor AB, Fujiwara Y et al. GATA-factor dependence of the multitype zinc-finger protein FOG-1 for its essential role in megakaryopoiesis. Proc Natl Acad Sci U S A. 2002;99:9237–9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Fossett N, Tevosian SG, Gajewski K et al. The Friend of GATA proteins U-shaped, FOG-1, and FOG-2 function as negative regulators of blood, heart, and eye development in Drosophila. Proc Natl Acad Sci U S A. 2001;98:7342–7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Gao Z, Huang Z, Olivey HE et al. FOG-1-mediated recruitment of NuRD is required for cell lineage re-enforcement during haematopoiesis. EMBO J. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Kurata H, Lee HJ, McClanahan T et al. Friend of GATA is expressed in naive Th cells and functions as a repressor of GATA-3-mediated Th2 cell development. J Immunol. 2002;168:4538–4545. [DOI] [PubMed] [Google Scholar]
- (94).Muratoglu S, Hough B, Mon ST, Fossett N. The GATA factor Serpent cross-regulates lozenge and u-shaped expression during Drosophila blood cell development. Dev Biol. 2007;311:636–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Querfurth E, Schuster M, Kulessa H et al. Antagonism between C/EBPbeta and FOG in eosinophil lineage commitment of multipotent hematopoietic progenitors. Genes Dev. 2000;14:2515–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Tsang AP, Visvader JE, Turner CA et al. FOG, a multitype zinc finger protein, acts as a cofactor for transcription factor GATA-1 in erythroid and megakaryocytic differentiation. Cell. 1997;90:109–119. [DOI] [PubMed] [Google Scholar]
- (97).Tsang AP, Fujiwara Y, Hom DB, Orkin SH. Failure of megakaryopoiesis and arrested erythropoiesis in mice lacking the GATA-1 transcriptional cofactor FOG. Genes Dev. 1998;12:1176–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Fossett N, Zhang Q, Gajewski K et al. The multitype zinc-finger protein U-shaped functions in heart cell specification in the Drosophila embryo. Proc Natl Acad Sci U S A. 2000;97:7348–7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Tokusumi T, Sorrentino RP, Russell M et al. Characterization of a lamellocyte transcriptional enhancer located within the misshapen gene of Drosophila melanogaster. PLoS One 2009;4:e6429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. [DOI] [PubMed] [Google Scholar]
- (101).Ward EJ, Skeath JB. Characterization of a novel subset of cardiac cells and their progenitors in the Drosophila embryo. Development. 2000;127:4959–4969. [DOI] [PubMed] [Google Scholar]
- (102).Muller HM, Dimopoulos G, Blass C, Kafatos FC. A hemocyte-like cell line established from the malaria vector Anopheles gambiae expresses six prophenoloxidase genes. J Biol Chem. 1999;274:11727–11735. [DOI] [PubMed] [Google Scholar]
- (103).Gao H, Wu X, Fossett N. Odd-skipped maintains prohemocyte potency and blocks blood cell development in Drosophila. gensis, The Journal of Genetics and Development. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Gao H, Wu X, Fossett N. Drosophila E-cadherin functions in hematopoietic progenitors to maintain multipotency and block differentiation. PLoS One 2013;8:e74684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Bresnick EH, Katsumura KR, Lee HY, Johnson KD, Perkins AS. Master regulatory GATA transcription factors: mechanistic principles and emerging links to hematologic malignancies. Nucleic Acids Res. 2012;40:5819–5831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Sorrentino RP, Tokusumi T, Schulz RA. The Friend of GATA protein U-shaped functions as a hematopoietic tumor suppressor in Drosophila. Dev Biol. 2007;311:311–323. [DOI] [PubMed] [Google Scholar]
- (107).Giordani G, Barraco M, Giangrande A et al. The human Smoothened inhibitor PF-04449913 induces exit from quiescence and loss of multipotent Drosophila hematopoietic progenitor cells. Oncotarget. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Kalamarz M, Paddibhatla I, Nadar C, Govind S. Sumoylation is tumor-suppressive and confers proliferative quiescence to hematopoietic progenitors in Drosophila melanogaster larvae. Biology Open. 2012;1:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (109).Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the hedgehog/patched signaling pathway from flies to mice: induction of a mouse patched gene by Hedgehog. Genes Dev. 1996;10:301–312. [DOI] [PubMed] [Google Scholar]
- (110).Guibentif C, Gottgens B. Blood: Education for stem cells. Nature. 2017;545:415–417. [DOI] [PubMed] [Google Scholar]
- (111).Lis R, Karrasch CC, Poulos MG et al. Conversion of adult endothelium to immunocompetent haematopoietic stem cells. Nature. 2017;545:439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Sugimura R, Jha DK, Han A et al. Haematopoietic stem and progenitor cells from human pluripotent stem cells. Nature. 2017;545:432–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Cochrane CR, Szczepny A, Watkins DN, Cain JE. Hedgehog Signaling in the Maintenance of Cancer Stem Cells. Cancers (Basel). 2015;7:1554–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Xie J, Murone M, Luoh SM et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature. 1998;391:90–92. [DOI] [PubMed] [Google Scholar]
- (115).Zheng R, Blobel GA. GATA Transcription Factors and Cancer. Genes Cancer. 2010; 1:1178–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Gal4 drivers and an alternate UAS-hhRNAi strain were used to confirm that Hh signaling maintains Ush expression. Hh signaling was disrupted by (A,B) knocking down Hh expression in the PSC using an alternate UAS-hhRNAi strain driven by Col-Gal4, (C,D) over-expressing Ptc in the MZ using Dome-Gal4 driven UAS-ptc or (E,F) knocking down Ci in the MZ using Dome-Gal4 driven UAS-ciRXAl. This resulted in a significant reduction in Ush expression levels. (G) Histogram shows the results of the statistical analyses. Student’s t-test; error bars show standard deviation; P values are as shown; control and HhRNAi (n=10); control and PtcGOF (n=15); control and CiRNAi (n=15). White dotted lines delineate the entire lymph gland. Scale bars: 50 μm. Mid-third instar larvae.
Col-Gal4 females were crossed to control or UAS-ush males (Col>Ush). (A,B) hh enhancer activity is abolished in Col>Ush lymph glands. All control lymph glands showed hh enhancer activity (n=12), while none of the lymph glands with Ush mis-expressed in the PSC showed hh enhancer activity (n=12). (C–E) PSC cells are marked with Antp. (E) Histograms show the results of the statistical analyses. Student’s t-test; error bars show standard deviation; P values are as shown; control and UshGOF, (n=9). Scale bars: 50 μm. Mid-third instar larvae.
Knockdown of Ush expression in the MZ (Dome>UshRNAi) significantly reduced the percentage of Oddpositive prohemocytes compared to controls. (C) Histograms show the results of the statistical analyses. Student’s t-test; error bars show standard deviation; P values are as shown; control and UshRNAi (n=10). Scale bars: 50 μιη. Mid-third instar larvae.
Additional reagents, including Col-Gal4 driven expression of an alternate UAS-hhRNAi construct and Tep-Gal4 driven UAS-smoDN were used to confirm that Hh signaling maintains Odd-positive prohemocytes and the level of Odd and DomeMESO expression. (A–C) Knockdown of Hh in the PSC using Col-Gal4 to drive expression of an alternate UAS-hhRNAi construct produced a statistically significant reduction in the percentage of Odd-positive prohemocytes. (D–F) Loss of Smo function (SmoDN) significantly reduced the size of the Odd-positive expression domains compared to controls. White dotted lines delineate the entire lymph gland; yellow dotted lines delineate the Odd-expression domain. (G–I) Knockdown of Hh in the PSC using Col-Gal4 to drive expression of an alternate UAS-hhRNAi construct produced a statistically significant reduction in the expression of DomeMESO-βgal. (C,F,I) Histograms show the results of the statistical analyses. Student’s t-test; error bars show standard deviation; P values are as shown; (C,I) control and HhRNAi (n=10); (F) control and SmoDN (n=18). Scale bars: 50 μm; mid-third instar larvae.
(A-A”‘) Lymph gland from DomeMESO-βgal larvae assayed for β-galactosidase (β-gal) and Odd expression. (A,A’) Lymph gland showing β-gal and Odd expression, (A) counterstained with Dapi. (A’) Inset showing a few cells with predominantly Odd or β-gal expression. (A”) Lymph gland showing Odd expression only, with the inset showing cells with high (red arrow) or low (green arrow) Odd expression. (A’”) Lymph gland showing β-gal expression only, with the inset showing cells with high (green arrow) or low (red arrow) β-gal expression. (B-B’”) Lymph gland from DomeMESO-GFP larvae assayed for GFP and Odd expression. (B,B’) Lymph gland showing GFP and Odd expression, (B) counterstained with Dapi. (B’) Inset showing a few cells with predominantly Odd or GFP expression. (B”) Lymph gland showing Odd expression only, with the inset showing cells with high (red arrow) or low (green arrow) Odd expression. (B’”) Lymph gland showing GFP expression only, with the inset showing cells with high (green arrow) or low (red arrow) GFP expression. (C-C’”) Lymph gland from w1118 larvae assayed for Col and Odd expression. (C,C’) Lymph gland showing Col and Odd expression, (C) counterstained with Dapi, (C”) Col expression only and (C’”) Odd expression only. (C’-C’”) Arrows mark Odd-positive cells located in or near the presumptive CZ and insets show cells within the presumptive MZ that express only Odd. Col expression was not detected in approximately 36% (0.36 + 0.18; n=12) of the Odd-positive cells (Colneg/OddHigh). Approximately 30% (0.30 + 0.19; n=12) of this Colneg/OddHigh minority (~11% of the total Odd-positive population) may be located in the CZ. White dotted lines delineate the entire lymph gland. Scale bars: 50 μm. Mid-third instar larvae.
PSC cells are marked with Antp. (A) Shows the complement of PSC cells (green arrow) in control lymph glands (+). All lymph glands assessed (n=14) showed similar expression patterns. This is in contrast to (B) in which Antp-expressing cells were not detected (white arrow) in any of the lymph glands (n=14) with Col-Gal4 driven UAS-rpr expression (Col>Rpr). White dotted lines delineate the entire lymph gland. Scale bars: 50 μm. Mid-third instar larvae.


