Abstract
Patients with clinically amyopathic dermatomyositis (CADM), a subset of dermatomyositis characterized by a lack of muscle involvement, frequently develop rapidly progressive and treatment-resistant interstitial lung disease. We report the case of a 49-year-old man who was diagnosed with CADM. He developed interstitial pneumonia, which did not respond to combination therapy with methylprednisolone pulse therapy, cyclophosphamide, and cyclosporine. We therefore attempted plasma exchange. After 7 courses of therapeutic plasma exchange, the interstitial pneumonia gradually improved. This case suggests that plasma exchange might be an effective therapeutic option for patients with progressive interstitial lung disease in steroid- and immunosuppressive therapy-refractive CADM.
Keywords: clinically amyopathic dermatomyositis, interstitial lung disease, plasma exchange
Introduction
Dermatomyositis is a connective tissue disease that results from an autoimmune reaction, primarily against the muscle and skin (1). Several autoantibodies associated with dermatomyositis and corresponding to its distinct clinical involvement and prognosis have been identified (2). Anti-melanoma differentiation-associated gene 5 (MDA-5) antibody is a specific antibody associated with clinically amyopathic dermatomyositis (CADM), a subtype of dermatomyositis characterized by typical cutaneous manifestations without myopathy (3). CADM patients with anti-MDA-5 antibodies frequently develop rapidly progressive interstitial lung disease (ILD). CADM-associated ILD (CADM-ILD) is characterized by resistance to combined immunosuppressive therapy, consisting of high-dose corticosteroids and immunosuppressive agents.
We herein report the case of a 49-year-old man who was diagnosed with CADM. He developed rapidly progressive interstitial pneumonia that was resistant to immunosuppressive therapy but was successfully treated with plasma exchange.
Case Report
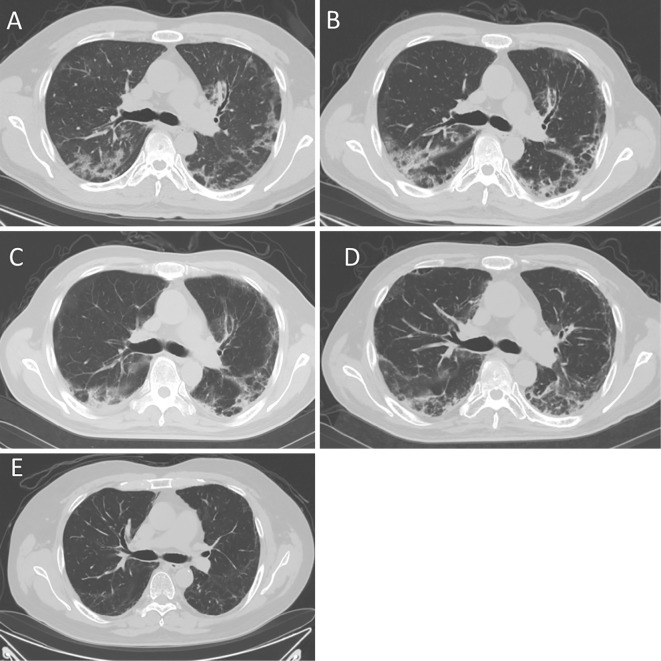
A 49-year-old man was referred to our hospital because of suspected dermatomyositis. He complained of fever, joint pain, and skin rash, which had persisted for two months. A physical examination revealed erythema over the metacarpophalangeal (MCP) joints of both hands (Gottron papules) and the face. Some of the erythema was accompanied by ulceration. Although the skin rash was typical of dermatomyositis, none of the muscular manifestations (i.e., myalgia and muscle weakness) were present. A laboratory analysis of the patient's serum revealed the following findings: creatine phosphokinase (CK), 414 IU/L; lactate dehydrogenase (LDH), 635 IU/L; C-reactive protein (CRP), 4.2 mg/dL; and ferritin, 2,912 ng/mL. The patient's serum was negative for antinuclear antibody (ANA) and anti-aminoacyl-tRNA sythetase (ARS) antibody. Although he had no respiratory symptoms on admission to the hospital, his respiratory condition worsened after admission. He developed dyspnea at one week after admission. An arterial blood gas analysis revealed that his partial pressure of arterial oxygen (PaO2) level was 61 Torr. His serum Krebs von den Lungen (KL)-6 level was 749 IU/mL, and computed tomography (CT) revealed reticular shadows and patchy ground-glass opacities in both lungs (Fig. 1A). A pathological examination of the erythema on his hand revealed findings that were consistent with dermatomyositis. Thus, CADM was diagnosed based on the typical skin rash with poor muscle symptoms and rapidly progressive interstitial pneumonia.
Figure 1.
The computed tomography (CT) findings before and after the treatments. CT showed ground-glass opacities in both lungs before treatment (A). Interstitial pneumonia progressed despite combination therapy with immunosuppressive agents (B). Immediately after 7 courses of plasma exchange, there was no obvious change in the ground-glass opacities (C). At 2 months after plasma exchange, CT showed a slight improvement in the interstitial pneumonia (D). At 10 months after plasma exchange, most of the ground-glass opacities had disappeared (E).
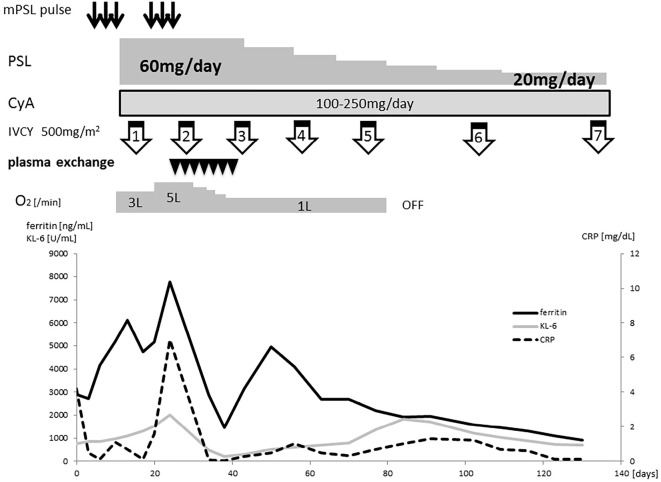
Immunosuppressive therapy with intravenous methylprednisolone (1,000 mg/day for 3 days) was started, followed by oral prednisolone [PSL; 60 mg/day (1 mg/kg/day)], cyclosporine (the dose was adjusted to maintain a blood trough level within the range of 150-200 ng/mL), and intravenous cyclophosphamide (IVCY; 500 mg/m2). However, despite this treatment, the patient's respiratory condition continued to worsen. At three weeks after the start of treatment, he required oxygen at a flow rate of 5 L/min, and his serum KL-6 and ferritin levels had increased to 2,005 IU/mL and 7,780 ng/mL, respectively. Chest CT showed progressive interstitial pneumonia (Fig. 1B). Thus, we attempted to perform therapeutic plasma exchange, with the replacement of fresh frozen plasma, to treat interstitial immunosuppressive therapy-resistant pneumonia. After 7 courses of plasma exchange, the patient's serum KL-6 and ferritin levels decreased, and the ground-glass opacities on CT and his oxygen demands gradually improved (Fig. 1C, D and E). Two months later, oxygen was tapered off. Intravenous cyclophosphamide was administered 7 times, and the PSL dose was tapered to 20 mg/day.
Subsequently, a serological analysis identified anti-MDA5 antibodies, which supported the diagnosis of CADM. The patient's clinical course is summarized in Fig. 2.
Figure 2.
The clinical course of the patient. The serum KL-6 and ferritin levels continued to increase during the combination therapy with methylprednisolone pulse therapy, cyclophosphamide, and cyclosporine. They gradually decreased after the 7th course of plasma exchange.
Discussion
The presence of the anti-MDA5 antibodies in patients with CADM-especially in Asia-is significantly associated with rapidly progressive ILD, which frequently shows a poor response to treatment (4-6). Anti-MDA5 antibodies were reported to be detected in 10-20% of dermatomyositis patients, and 60-100% of anti-MDA5 antibody-positive CADM patients were reported to develop ILD (7).
High levels of serum ferritin are related to the ILD activity of anti-MDA5 antibody-positive CADM (8,9). Hyperferritinemia, especially at levels of >1,500 ng/mL, is predictive of a poor prognosis; our patient could be said to have been affected by this condition. Anti-MDA5 antibody-positive CADM patients also have cutaneous features. It is noteworthy that skin ulcers-which were observed in our case-are more frequently observed in anti-MDA5 antibody-positive patients (10,11). Thus, in addition to respiratory symptoms, hyperferritinemia or cutaneous manifestations (including skin ulcers) should alert physicians to the possibility of CADM.
Combination immunosuppressive therapy consisting of high-dose corticosteroids, cyclosporine, and IVCY has been reported to be effective for CADM-ILD (12). However, the prognosis is still poor, with a mortality rate of >50% (13,14).
Therapeutic plasma exchange is not an established therapy for aggressive ILD. There have been some reports about interstitial pneumonia related to autoimmune disease, including scleroderma and anti-synthetase syndrome, in which therapy-resistant interstitial pneumonia was successfully treated with plasma exchange (15,16). Recently, Silveira et al. reported a case of CADM-ILD that was successfully treated with therapeutic plasma exchange (17). Furthermore, some reports have pointed out the effectiveness of endotoxin adsorption therapy in the treatment of CADM with aggressive ILD, and it was noted that the anti-MDA5 antibody titer decreased after endotoxin adsorption therapy (18,19). The detailed mechanism of how therapeutic apheresis improves the activity of CADM-ILD remains unclear. However, because past studies indicating that the disease activity and prognosis of CADM-ILD are associated with the titers of anti-MDA5 antibodies and several inflammatory cytokines, including Interleukin-8, interferon-α and CX3CL1 (20-24), the removal of anti-MDA5 antibodies or inflammatory cytokines by therapeutic apheresis may exert a favorable influence on CADM-ILD.
Regarding the removal of the pathogenic factors associated with CADM-ILD, the difference between plasma exchange and endotoxin adsorption is not clear. However, we chose plasma exchange rather than endotoxin adsorption because it is possible that plasma exchange would remove a greater range of pathogenic factors. Actually, the patient in Silveria's case (cited above) was successfully treated with plasma exchange; in contrast, endotoxin adsorption had an insufficient therapeutic effect (17).
The present study is associated with some limitations. We did not analyze the anti-MDA 5 antibody titer after the plasma exchange. Thus, we could not evaluate the effect of removing anti-MDA 5 antibodies by plasma exchange. Furthermore, in our case, the concentrations of serum ferritin, KL-6 and CRP were observed to re-increase several weeks after the plasma exchange, and then subsequently decrease. However, despite these serum findings, the patient's respiratory condition and CT findings did not deteriorate. Although the reason for this phenomenon is not clear, it seems that the CADM-ILD activity was suppressed by plasma exchange in the acute-phase, and that it was gradually controlled by immunosuppressive therapy in the sub-acute-phase.
In conclusion, plasma exchange may be considered as a treatment option for immunosuppressive therapy-resistant CADM-ILD.
The authors state that they have no Conflict of Interest (COI).
Acknowledgement
We thank Dr. Flaminia Miyamasu, Medical English Communication Center for the critical reading of the manuscript.
References
- 1.Callen JP. Dermatomyositis. Lancet 355: 53-57, 2000. [DOI] [PubMed] [Google Scholar]
- 2.Bodoki L, Nagy-Vincze M, Griger Z, et al. Four dermatomyositis-specific autoantibodies-anti-TIF1γ, anti-NXP2, anti-SAE and anti-MDA5-in adult and juvenile patients with idiopathic inflammatory myopathies in a Hungarian cohort. Autoimmun Rev 13: 1211-1219, 2014. [DOI] [PubMed] [Google Scholar]
- 3.Nakashima R, Imura Y, Kobayashi S, et al. The RIG-I-like receptor IFIH1/MDA5 is a dermatomyositis-specific autoantigen identified by the anti-CADM-140 antibody. Rheumatology (Oxford) 49: 433-440, 2010. [DOI] [PubMed] [Google Scholar]
- 4.Sato S, Hirakata M, Kuwana M, et al. Autoantibodies to a 140-kd polypeptide, CADM-140, in Japanese patients with clinically amyopathic dermatomyositis. Arthritis Rheum 52: 1571-1576, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Sato S, Kuwana M. Clinically amyopathic dermatomyositis. Curr Opin Rheumatol 22: 639-643, 2010. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Cao M, Plana MN, et al. Utility of anti-melanoma differentiation-associated gene 5 antibody measurement in identifying patients with dermatomyositis and a high risk for developing rapidly progressive interstitial lung disease: a review of the literature and a meta-analysis. Arthritis Care Res (Hoboken) 65: 1316-1324, 2013. [DOI] [PubMed] [Google Scholar]
- 7.Chaisson NF, Paik J, Orbai AM, et al. A novel dermato-pulmonary syndrome associated with MDA-5 antibodies: report of 2 cases and review of the literature. Medicine (Baltimore) 91: 220-228, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gono T, Kawaguchi Y, Satoh T, et al. Clinical manifestation and prognostic factor in anti-melanoma differentiation-associated gene 5 antibody-associated interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford) 49: 1713-1719, 2010. [DOI] [PubMed] [Google Scholar]
- 9.Gono T, Kawaguchi Y, Hara M, et al. Increased ferritin predicts development and severity of acute interstitial lung disease as a complication of dermatomyositis. Rheumatology (Oxford) 49: 1354-1360, 2010. [DOI] [PubMed] [Google Scholar]
- 10.Narang NS, Casciola-Rosen L, Li S, Chung L, Fiorentio DF. Cutaneous ulceration in dermatomyositis: association with anti-melanoma differentiation-associated gene 5 antibodies and interstitial lung disease. Arthritis Care Res (Hoboken) 67: 667-672, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fiorentino D, Chung L, Zwerner J, Rosen A, Casciola-Rosen L. The mucocutaneous and systemic phenotype of dermatomyositis patients with antibodies to MDA5 (CADM-140): a retrospective study. J Am Acad Dermatol 65: 25-34, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakashima R, Hosono Y, Mimori T. Clinical significance and new detection system of autoantibodies in myositis with interstitial lung disease. Lupus 25: 925-933, 2016. [DOI] [PubMed] [Google Scholar]
- 13.Ideura G, Hanaoka M, Koizumi T, et al. Interstitial lung disease associated with amyopathic dermatomyositis: review of 18 cases. Respir Med 101: 1406-1411, 2007. [DOI] [PubMed] [Google Scholar]
- 14.Ye S, Chen XX, Lu XY, et al. Adult clinically amyopathic dermatomyositis with rapid progressive interstitial lung disease: a retrospective cohort study. Clin Rheumatol 26: 1647-1654, 2007. [DOI] [PubMed] [Google Scholar]
- 15.Omotoso BA, Ogden MI, Balogun RA. Therapeutic plasma exchange in antisynthetase syndrome with severe interstitial lung disease. J Clin Apher 30: 375-379, 2015. [DOI] [PubMed] [Google Scholar]
- 16.Tamura K, Akiyama J, Oono K, Kadowaki S, Shimada T. A successful therapy with plasma exchange for interstitial pneumonia of progressive systemic sclerosis. Intern Med 31: 649-654, 1992. [DOI] [PubMed] [Google Scholar]
- 17.Silveira MG, Selva-O'Callaghan A, Ramos-Terrades N, et al. Anti-MDA5 dermatomyositis and progressive interstitial pneumonia. QJM 109: 49-50, 2016. [DOI] [PubMed] [Google Scholar]
- 18.Teruya A, Kawamura K, Ichikado K, et al. Successful polymyxin B hemoperfusion treatment associated with serial reduction of serum anti-CADM-140/MDA5 antibody levels in rapidly progressive interstitial lung disease with amyopathic dermatomyositis. Chest 144: 1934-1936, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Ichiyasu H, Horio Y, Tsumura S, et al. Favorable outcome with hemoperfusion of polymyxin B-immobilized fiber column for rapidly progressive interstitial pneumonia associated with clinically amyopathic dermatomyositis: report of three cases. Mod Rheumatol 24: 361-365, 2014. [DOI] [PubMed] [Google Scholar]
- 20.Sato S, Kuwana M, Fujita T, Suzuki Y. Anti-CADM-140/MDA5 autoantibody titer correlates with disease activity and predicts disease outcome in patients with dermatomyositis and rapidly progressive interstitial lung disease. Mod Rheumatol 23: 496-502, 2013. [DOI] [PubMed] [Google Scholar]
- 21.Gono T, Sato S, Kawaguchi Y, et al. Anti-MDA5 antibody, ferritin and IL-18 are useful for the evaluation of response to treatment in interstitial lung disease with anti-MDA5 antibody-positive dermatomyositis. Rheumatology (Oxford) 51: 1563-1570, 2012. [DOI] [PubMed] [Google Scholar]
- 22.Horai Y, Koga T, Fujikawa K, et al. Serum interferon-α is a useful biomarker in patients with anti-melanoma differentiation-associated gene 5 (MDA5) antibody-positive dermatomyositis. Mod Rheumatol 25: 85-89, 2015. [DOI] [PubMed] [Google Scholar]
- 23.Zou J, Chen J, Yan Q, Guo Q, Bao C. Serum IL8 and mRNA level of CD11b in circulating neutrophils are increased in clinically amyopathic dermatomyositis with active interstitial lung disease. Clin Rheumatol 35: 117-125, 2016. [DOI] [PubMed] [Google Scholar]
- 24.Takada T, Aoki A, Asakawa K, et al. Serum cytokine profiles of patients with interstitial lung disease associated with anti-CADM-140/MDA5 antibody positive amyopathic dermatomyositis. Respir Med 109: 1174-1180, 2015. [DOI] [PubMed] [Google Scholar]