Abstract
A 70-year-old woman with hepatitis C cirrhosis underwent balloon-occluded retrograde transvenous obliteration for hepatic encephalopathy due to spleno-renal shunt. Because the shunt was thick, long, and winding, we used a coaxial and double interruption system, which enables the effective occlusion of the drainage route, and shape-memory coils, which are more physically stable than conventional metallic coils because they form three-dimensional loops. The patient was successfully treated with the combined usage of these devices, resulting in a normal serum ammonia level. Thereafter, the patient was treated with direct-acting antivirals, and a sustained virological response was achieved.
Keywords: portosystemic shunt, hepatic encephalopathy, balloon-occluded retrograde transvenous obliteration, coaxial and double interruption system, coil with shape-memory function
Introduction
Balloon-occluded retrograde transvenous obliteration (B-RTO) is established as the standard treatment for gastric varices (GV) and hepatic encephalopathy associated with porto-systemic shunt. However, in some cases, complete occlusion is difficult to achieve by conventional B-RTO because the shunt is large in diameter, long, or markedly winding.
We herein report a case of hepatic encephalopathy due to spleno-renal shunt (SRS) that could not be controlled with medical treatment.
The insertion of catheters has been shown to be difficult in several cases with thick, long, and winding shunts on three-dimensional computed tomography (3D-CT). Therefore, B-RTO was attempted using a coaxial and double interruption system (CANDIS™Ⓡ; Medikit, Tokyo, Japan). However, the shunt was too large in diameter to be occluded completely with a balloon and treated with sclerosing agents. Therefore, we attempted re-treatment using TargetⓇ XL 360 shape-memory coils (Stryker Japan, Tokyo, Japan). The TargetⓇ XL 360 coils are equipped with a shape-memory function and are more physically stable than conventional metallic coils because they form 3D loops. The combined use of CANDIS™Ⓡ and TargetⓇ XL 360 led to successful shunt occlusion. After treatment, the patient was completely free from hepatic encephalopathy, with a serum ammonia level within the normal range. These results suggest that B-RTO using a combination of CANDIS™Ⓡ and TargetⓇ XL 360 is effective in complicated shunt cases that are difficult to treat using conventional B-RTO.
Case Report
The patient was a 70-year-old woman with liver cirrhosis associated with hepatitis C virus (HCV) (genotype 1b) infection. She had a CP score of 6 and a CP classification of A. She had been treated with ursodeoxycholic acid, a branched-chain amino acid (BCAA)-enriched elemental diet, and lactitol hydrate. BCAA and lactitol hydrate treatment was discontinued in order to initiate DAA therapy, but the patient was admitted to the hospital two months later because of disturbance of consciousness. A physical examination on admission revealed the following: height, 156 cm; body weight, 54 kg; body temperature, 36.8°C; pulse, 78 bpm; blood pressure, 102/54 mmHg; chest, no abnormal sounds on auscultation; abdomen, soft and flat; liver, not palpable; palmar erythema, present; and flapping tremor, positive. Laboratory testing on admission revealed the following: alanine aminotransferase, 83 IU/L (normal range, 6-35 IU/L); platelet count, 9.4×104 g/dL (normal range, 15-35×104 g/dL); total bilirubin, 1.80 mg/dL (normal range, 0.3-1.2); ammonia, 159 μg/dL (normal range, 20-80); indocyanine green retention rate at 15 minutes of 39.2% (normal range, <10); HCV genotype, 1b; and HCV viral load, 6.3 log IU/L (high titer). The patient had a slightly worse CP score of 7 (CP classification of B) on admission (Table).
Table.
Laboratory Data on Admission.
| Peripheral blood | Biochemistry | Tumor marker | ||||||||
| WBC | 3,700 | /mm3 | T-Bil | 1.8 | mg/dL | AFP | 2.5 | ng/mL | ||
| RBC | 446×104 | /μL | D-Bil | 0.79 | mg/dL | PIVKA II | 50 | mAU/mL | ||
| Hb | 13.7 | g/dL | AST | 132 | U/L | |||||
| Ht | 39 | % | ALT | 83 | IU/L | Hepatic virus | ||||
| Plt | 9.4×104 | /μL | LDH | 322 | IU/L | HBs Ag | (-) | |||
| ALP | 516 | IU/L | HBs Ab | (-) | ||||||
| γ-GTP | 17 | IU/L | HBe Ag | (-) | ||||||
| Coagulation | BUN | 15.5 | mg/dL | HBe Ab | (-) | |||||
| PT | 14.6 | s | Cre | 0.68 | mg/dL | HCV RNA (TaqMan) | 6.5 | Log IU/mL | ||
| INR | 1.16 | TP | 7.1 | g/dL | HCV genotype | 1b | ||||
| PT% | 77 | % | Alb | 3 | g/dL | |||||
| APTT | 34.7 | s | Na | 140 | mEq/dL | |||||
| K | 4.5 | mEq/dL | ICG15 | 39.20 | % | |||||
| Cl | 108 | mEq/dL | ||||||||
| CRP | 1.1 | mg/dL | ||||||||
| NH3 | 86 | μg/dL | ||||||||
WBC: white blood cell count, RBC: red blood cell count, Hb: hemoglobin, Plt: platelet, PT: prothrombin time, INR : international normalized ratio, APTT: activated partial thromboplastin time, T-Bil: total bilirubin, D-Bil: direct bilirubin, AST: aspartate aminotransferase, ALT: alanine aminotransferase, LDH: lactate dehydrogenase, ALP: alkaline phosphatase, γ-GTP: gamma-glutamyl transpeptidase, BUN: blood urea nitrogen, Cre: creatinine, TP: total protein, Alb: albumin, Na: natrium, K: karium, Cl: crawl, Glu: fasting glucose, CRP: C-reactive protein, NH3: annmonia, AFP: alphafetoprotein, PIVKA II: protein induced by Vitamin K absence or antagonists-II, HBsAg: hepatic B surface antigen, HBsAb: hepatic B surface antibody, HBeAg: hepatic B envelope antigen, HBeAb: hepatic B envelope antibody, HCV-RNA: hepatic C virus-ribonucleic acid, ICG: indocyanine green
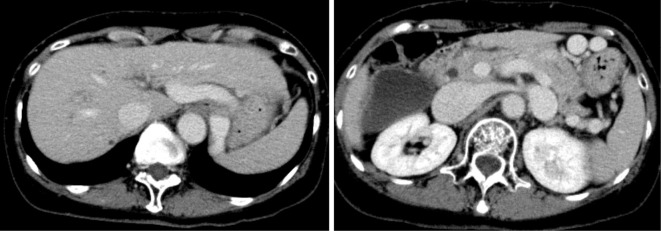
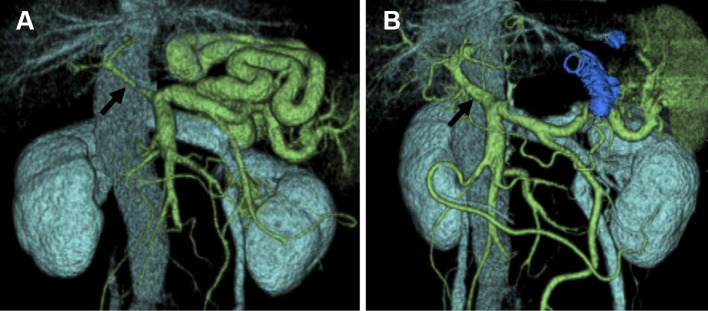
CT revealed a liver cirrhosis pattern with splenomegaly and complicated SRS but no hepatic tumors or ascites (Fig. 1). A 3D-CT scan showed a thick, long, and winding shunt that was supplied by the splenic vein and drained into the left renal vein. The main tract of the portal vein was extremely narrow, suggesting a decreased hepatopetal blood flow (arrow; Fig. 2A). Occlusion of the shunt was expected to improve hepatic encephalopathy. However, because this complicated shunt was thick, long, and winding, treatment with a conventional device was expected to be difficult. We therefore attempted B-RTO using CANDIS™Ⓡ equipped with a guiding balloon catheter and a 5-Fr balloon catheter, as this enabled the insertion of the catheter deep into the shunt.
Figure 1.
A contrast enhancement-computed tomography scan showing a liver cirrhosis pattern with splenomegaly and a complicated spleno-renal shunt but no hepatic tumors or ascites.
Figure 2.
(A) Three-dimensional computed tomography performed before balloon-occluded retrograde transvenous obliteration clearly showing a thick, long, and winding shunt supplied by the splenic vein and draining into the left renal vein. The main tract of the portal vein is markedly narrow (arrow). (B) Three-dimensional computed tomography one week after balloon-occluded retrograde transvenous obliteration showing complete occlusion of the spleno-renal shunt and an increase in the diameter of the main tract of the portal vein, suggesting an increased blood flow into the liver (arrow).
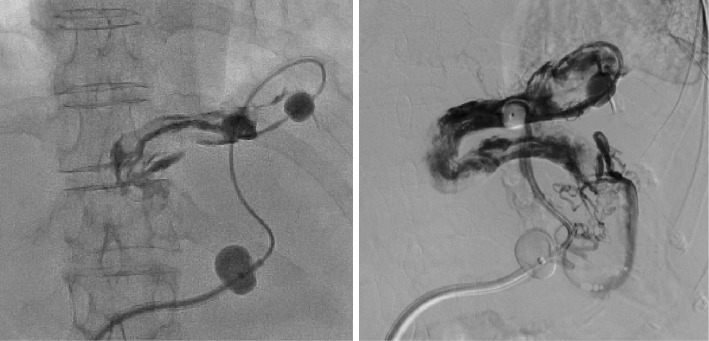
Although it was difficult to insert the catheter, we were able to steer it to a certain point. However, because the diameter of shunt was larger than the diameter of the 5-Fr balloon catheter (φ10 mm), balloon occlusion was unsuccessful. We inflated the balloon (φ20 mm) after the guiding catheter was fixed at the optimal position and performed B-RTO using a sclerosing agent [20 mL; 5% ethanolamine oleate iopamidol (EOI)] after stasis of the contrast medium was observed (Fig. 3). Stasis of the EOI was observed by radiography immediately after completing the procedure. After treatment, both the sheath and catheter were left in place overnight, and venography was performed with the guiding balloon catheter the next morning. Venography revealed the uneven visualization of the shunt, suggesting insufficient embolization (Fig. 4). This indicated that although stasis of the EOI had been temporarily achieved, complete occlusion could not be achieved due to the large vascular diameter.
Figure 3.
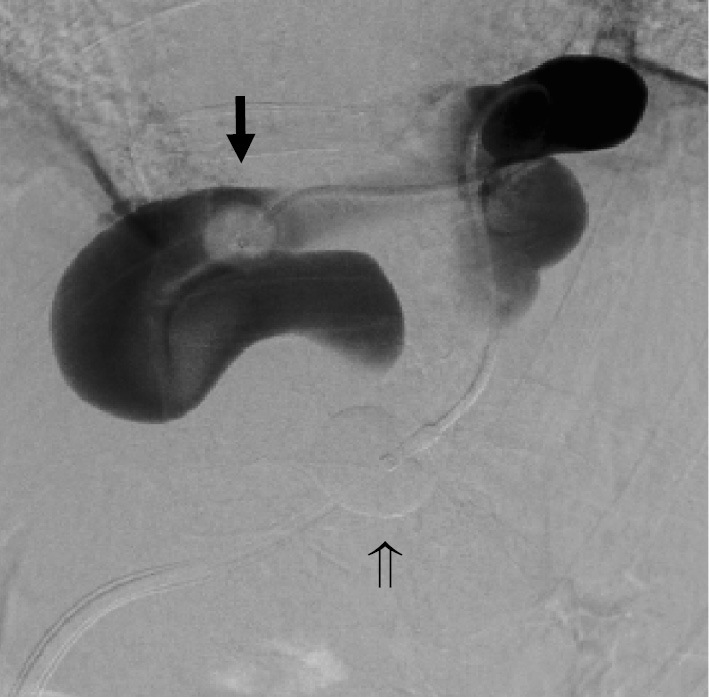
Balloon-occluded retrograde transvenous obliteration was attempted using a coaxial and double interruption system. Cytography was performed by inserting the catheter into the shunt. Complete balloon occlusion was not achieved with a 5-Fr balloon catheter (arrow ➡). The φ20 mm balloon was inflated when the guiding balloon catheter was fixed at the optimum position (arrow ⇒), following which stasis of the contrast medium was observed. We then performed balloon-occluded retrograde transvenous obliteration using a sclerosing agent.
Figure 4.
Venography with the guiding balloon catheter showing uneven visualization of the shunt, suggesting insufficient embolization.
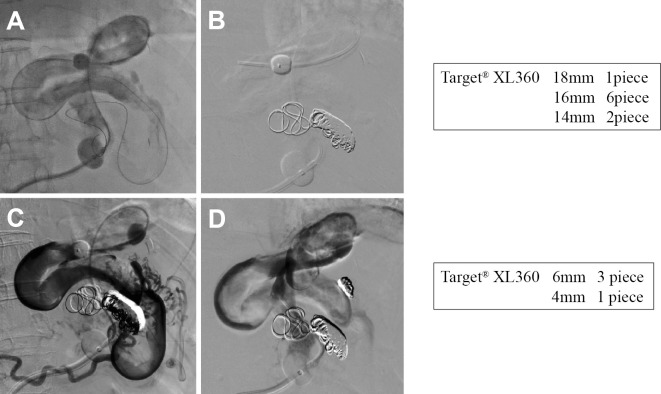
The use of additional EOI to achieve complete occlusion was expected to require an extremely large amount of EOI, and even then, complete occlusion would not have been guaranteed. Therefore, we performed additional B-RTO treatment using metallic coils as an embolization agent instead of a sclerosing agent. The 5-Fr balloon catheter was again steered to a point close to the supplying vessel (Fig. 5A), and a TargetⓇ XL 360 coil with a shape-memory function was used for embolization. Shape-memory coils were selected for two reasons: they have size specifications that enable their use in larger vessels than conventional detachable metallic coils, and they have an anchoring ability that is superior to and safer than that of conventional detachable metallic coils because they form 3D loops that fit well in the intravascular space. Nine relatively large coils of 14-18 mm diameter and 40-50 cm length were used (Fig. 5B), followed by 4 small coils of 4-6 mm diameter for the occlusion of the collateral vessels (Fig. 5C), thereby completing the B-RTO procedure (Fig. 5D).
Figure 5.
(A) The 5-Fr balloon catheter was again inserted close to the supplying vessel. (B) Embolization was performed with Target® XL 360 coils, which have a shape-memory function and are more physically stable than conventional metallic coils because they form three-dimensional loops. We inserted a total of nine coils, including one 14-mm coil, six 16-mm coils, and two 18-mm coils. (C) Visualization of the collaterals after coiling. (D) Balloon-occluded retrograde transvenous obliteration was completed by adding three 6-mm coils and one 4-mm coil for the collaterals.
After confirming the absence of blood removal from the guiding balloon catheter, both the sheath and catheter were removed next morning. There was no hematoma at the puncture site, and no other complications, including abdominal pain and pyrexia, occurred. The patient was discharged two days after treatment.
One week after treatment, 3D-CT revealed complete obstruction of the SRS and an enlarged diameter of the main tract of the portal vein, suggesting an increased blood flow into the liver (arrow; Fig. 2B). After treatment, her hepatic encephalopathy was completely ameliorated, and her serum ammonia level was within the normal range. No complications such as hepatocellular carcinoma, ascites, or varices occurred, and the total bilirubin, prothrombin time, and serum albumin levels improved to their within normal ranges. In addition, the indocyanine green retention rate at 15 minutes improved from 39.2% to 16.5%.
At 6 months after B-RTO, the patient had a CP score of 6 with a CP classification of A, and DAAs (ledipasvir/sofosbuvir) were introduced after confirming that the patient had not developed hepatic encephalopathy despite the discontinuation of BCAA and lactitol hydrate. At six months after starting treatment with DAAs, a sustained virological response was achieved, and the patient showed no signs of hepatocellular carcinoma. Although there were concerns regarding the potential for complications associated with portal hypertension secondary to the occlusion of a large porto-systemic shunt, esophagogastroduodenoscopy before B-RTO and at 12 months after B-RTO showed no esophageal varices (EV) or GV. In addition, the patient did not develop ascites after the procedure.
Discussion
The main causes of hepatic coma include (1) congenital urea cycle abnormalities, (2) hepatic insufficiency secondary to extensive loss of hepatic cells due to liver cirrhosis or acute liver failure, and (3) so-called recurrent porto-systemic shunts. Portal hypertension, which is mainly associated with liver cirrhosis, causes a persistent increase in vascular resistance in the intrahepatic portal vein and increases the hepatopetal blood flow. The consequent development of hepatofugal porto-systemic shunts results in the transfer of hepatic coma-inducing agents (mainly ammonia) present in the portal vein from the systemic circulation into the brain, causing repeated episodes of hepatic coma. This condition, termed portal-systemic encephalopathy, was proposed by Sherlock et al. in 1954 (1).
Because the presence of a shunt results in recurrent episodes of encephalopathy, successful treatment requires the obliteration or occlusion of the shunt, mainly through surgery and interventional radiology (IVR). As IVR can be performed under local anesthesia without a skin incision, it has little effect on the liver function and is a low-invasion procedure. Therefore, IVR is considered a first-line treatment modality for hepatic coma due to porto-systemic shunt.
Lunderquist (2) first described the use of percutaneous transhepatic obliteration (PTO) for the treatment of EV in 1974, and subsequently, its use has been reported in Japan in many cases of not only varices but also hepatic coma (3,4). Kanagawa (5) first proposed the use of B-RTO for the treatment of GV in Japan in 1991. This technique was subsequently reported in many publications (6-8) and is now commonly used to treat hepatic coma (9).
In terms of the direction of blood flow of varices, IVR treatments for port-systemic shunts can be classified into retrograde approaches, including B-RTO and transjugular retrograde obliteration, and antegrade approaches, including PTO and transileocolic vein obliteration. In the PTO procedure, a representative antegrade approach, the embolization effects can be improved by inserting the microcatheter to a point close to the GV and injecting the sclerosing agents after reducing the blood flow with a microcoil (10). Challenges associated with the use of PTO include the relatively high invasiveness of the percutaneous transhepatic approach, its limited indication for cases with ascites, and complications associated with puncturing in cases where the narrow diameter of the portal vein caused by the growth of extrahepatic shunts results in a decrease in the hepatopetal blood flow. Therefore, B-RTO, a representative retrograde approach, is the treatment of choice for porto-systemic shunts. B-RTO is best indicated for cases such as those with gastro-renal shunts (GRS), wherein balloon occlusion of drainage routes can be successfully performed, and is the treatment of choice for hepatic coma as well as GV.
However, in many cases, the drainage route of the shunt is large in diameter and flows directly into the inferior vena cava, the shunt itself is extremely short or long, the shunt blood flow is extremely fast, or multiple drainage routes are present. Hirota et al. classified cases by the degree of growth of GRS collaterals and reported that grade 3 and 4 cases require deep insertion of the catheter into the shunt, beyond the drainage route (6). Because the SRS in the present case was thick, long, and winding, it would be difficult to insert a conventional Selecon MP catheter (Thermo Clinical Suppli, Tokyo, Japan) equipped with a single balloon deeply beyond the shunt; therefore, B-RTO was attempted with CANDIS™Ⓡ. Consequently, we were able to insert the catheter to a point close to the supplying route of the shunt.
The advantage of CANDIS™Ⓡ is that the guiding balloon catheter acts as a guide to improve the operability of the 5-Fr balloon catheter. This enables occlusion in the vicinity of varices beyond collaterals and effective occlusion of the drainage route to prevent leakage of sclerosing agents into the collaterals. Thus, the grade of the condition according to Hirota's classification can be reduced (6,11,12).
However, because the diameter of the 5-Fr balloon catheter is relatively small (φ10 mm), it cannot be used in cases where the minimum diameter of the shunt exceeds Ф10 mm. In the present case, 5% EOI was injected, but complete balloon occlusion was not achieved because the diameter of the shunt was larger than that of the balloon. Further forced addition of 5% EOI might have resulted in clotting in the shunt outflow into the systemic circulation when the balloon was deflated, leading to pulmonary embolism. We therefore changed the embolization agent to TargetⓇ XL 360 coils, which have a large diameter.
TargetⓇ XL 360 coils are equipped with a shape-memory function and are more physically stable than pushable micrometallic coils because they form 3D loops. Their additional merits include the availability of several devices with large diameters and the ability to pull back the catheter until it reaches the optimum placement location (13-15). We have alos used TargetⓇ XL360 coils for the occlusion of the splenic artery with a large diameter during partial splenic embolization and reported their usefulness with regard to their stability and therapeutic efficacy (13,14).
However, TargetⓇ XL360 coils are very expensive; therefore, we endeavor to use as few coils as necessary. Despite the confirmed usefulness of TargetⓇ XL360 coils for B-RTO in the present case, our institution's policy-based on the above factors-is to select a conventional sclerosing agent as the first line of treatment and then to use TargetⓇ XL360 coils in cases that are difficult to treat with the conventional agent. Furthermore, regarding the catheter system, the CANDIS™Ⓡ catheter is more expensive than a Selecon MP catheter. Given these associated expenses, we have made efforts recently to use coils as an anchor with the combined use of a sclerosing agent.
The selection criteria for combined treatment with CANDIS™Ⓡ and TargetⓇ XL 360 at our institution include 1) a thick, long, and winding porto-systemic shunt, 2) big GVs, 3) a thick inferior subphrenic vein, 4) a complicated spleno-renal and mesenteric venous shunt, and 5) a grade of ≥4 according to Hirota's classification (6)
Conclusion
Our findings suggest that B-RTO using a combination of CANDIS™Ⓡ and TargetⓇ XL 360 is useful for cases of complicated shunts in which conventional B-RTO is difficult. However, because both devices are expensive, the cost effectiveness needs to be improved by using sclerosing agents in combination with coils.
The authors state that they have no Conflict of Interest (COI).
References
- 1.Sherlock S, Summerskill WHJ, White LP, et al. . Portal systemic encephalopathy: neurological complications of liver disease. Lancet ii: 453-455, 1954. [PubMed] [Google Scholar]
- 2.Lunderquist A, Vang J. Transhepatic catheterization and obliteration of the coronary vein in patients with portal hypertension and esophageal varices. New Eng J Med 26: 646-649, 1974. [DOI] [PubMed] [Google Scholar]
- 3.Tajiri T, Onda M, Yamashita K, et al. . Interventional radiology for portal hypertrnsion. PTO. TIO. Nihon Geka Gakkai Zasshi (J Jpn Surg Soc) 97: 70-77, 1996. [PubMed] [Google Scholar]
- 4.Ishikawa T, Imai M, Ko M, et al. . Percutaneous transhepatic obliteration and percutaneous transhepatic sclerotherapy for intractable hepatic encephalopathy and gastric varices improves the hepatic function reserve. Biomed Rep 6: 99-102, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanagawa H, Mima S, Kouyama H, et al. . Treatment of gastric fundal varices by balloon-occluded retrograde transvenous obliteration. J Gastroenterol Hepatol 11: 51-58, 1996. [DOI] [PubMed] [Google Scholar]
- 6.Hirota S, Matsumoto S, Tomita M, et al. . Retrograde transvenous obliteration of gastric varices. Radiolody 211: 349-356, 1999. [DOI] [PubMed] [Google Scholar]
- 7.Chikamori F, Shibuya S, Takase Y, et al. . Transjugular retrograde obliteration for gastric varices. Abdom Imaging 21: 299-303, 1996. [DOI] [PubMed] [Google Scholar]
- 8.Koito K, Namieno T, Nagakawa T, et al. . Balloon-occluded retrograde transvenous obliteration for gastric varices with gastrorenal or gastrocaval collaterals. AJR Am J Roentgenol 167: 1317-1320, 1996. [DOI] [PubMed] [Google Scholar]
- 9.Ibukuro K, Sugihara T, Tanaka R, et al. . Balloon-occluded retrograde transvenous obliteration for a direct shunt between the inferior mesenteric vein and the inferior vena cava in a patient with hepatic encephalopathy. J Vasc Interv Radiol 18: 121-125, 2007. [DOI] [PubMed] [Google Scholar]
- 10.Ninoi T, Nakamura K, Kaminou T, et al. . TIPS versus transcatheter sclerotherapy for gastric varices. AJR Am J Roentgenol 183: 369-376, 2004. [DOI] [PubMed] [Google Scholar]
- 11.Tanoue S, Kiyosue H, Matsumoto S, et al. . Development of a new coaxial balloon catheter system for balloon-occluded retrograde transvenous obliteration (B-RTO). Cardiovasc Intervent Radiol 29: 991-996, 2006. [DOI] [PubMed] [Google Scholar]
- 12.Kiyosue H, Mori H, Matsumoto S, Yamada Y, Hori Y, Okino Y. Transcatheter obliteration of gastric varices: Part 2. Strategy and techniques based on hemodynamic features. Radiographics 23: 921-937, 2003. [DOI] [PubMed] [Google Scholar]
- 13.Matsuoka S, Ishii T, Miyazawa S, et al. . Utility of partial splenic embolization for hypersplenism using Guglielmi detachable coils. Hepato-gastroenterology 62: 683-687, 2015. [PubMed] [Google Scholar]
- 14.Matsuoka S, Kamimura S, Mizutani T, et al. . Hypersplenism treated by partial splenic embolization using Guglielmi detachable coils. Intern Med 54: 2179-2183, 2015. [DOI] [PubMed] [Google Scholar]
- 15.Munawar M, Siswanto BB, Harimurti GM, et al. . Transcatheter closure of coronary artery fistula using Guglielmi detachable coil. J Geriatr Cardiol 9: 11-16, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]