Abstract
We report a rare case of a 27-year-old woman with Takayasu arteritis (TAK) complicated by diffuse sclerosing osteomyelitis. She first presented with sclerosing osteomyelitis of the right mandible without evidence of arteritis in the carotid arteries. Eight months later, she complained of left neck pain, and imaging studies revealed the presence of arteritis in the left carotid artery. She was diagnosed with TAK, and immunosuppressive treatment was initiated, which was effective for both the arteritis and the osteomyelitis. Osteomyelitis is an important complication of TAK and bone scintigraphy is useful for its detection.
Keywords: chronic recurrent multifocal osteomyelitis, osteomyelitis, synovitis-acne-pustulosis-hyperostosis-osteitis syndrome, Takayasu arteritis
Introduction
Takayasu arteritis (TAK) is characterized by inflammation of the systemic large arteries (the aorta and its branches, coronary artery, and pulmonary artery), and results in stenosis, obstruction, and aneurysm formation by the affected arteries (1). Although a correlation with human leukocyte antigen (HLA)-B52 and B67 has been reported, much of its pathogenesis remains unclear (2). Importantly, TAK is sometimes complicated by other inflammatory conditions, including ulcerative colitis, Crohn's disease, hypertrophic pachymeningitis, and relapsing polychondritis (3-5). Sterile osteomyelitis is an inflammatory bone disease that presents with bone pain of insidious onset and which is also associated with other inflammatory disorders, such as palmoplantar pustulosis, psoriasis vulgaris, and inflammatory bowel disease. Although both TAK and sterile osteomyelitis have similar comorbidities, the relationship between these two conditions has been poorly investigated. Recently, Vettiyil et al. described an unusual association among chronic recurrent multifocal osteomyelitis (CRMO), pyoderma gangrenosum (PG), and TAK (6). CRMO represents 2-5% of the cases of osteomyelitis and is the term that is often used in pediatrics. Meanwhile, synovitis-acne-pustulosis-hyperostosis-osteitis (SAPHO) syndrome is also known to present with osteomyelitis as a primary clinical feature, and is used more frequently in the adult literature (although the definition of these two terms is uncertain) (7). We herein describe a rare case of TAK complicated by diffuse sclerosing osteomyelitis of the mandible, which developed 8 months before the onset of TAK.
Case Report
A 27-year-old woman was referred to our rheumatology department for the evaluation of large vessel vasculitis. Eight months before referral, she had complained of swelling and pain in the right lower jaw. At the time, imaging studies [computed tomography (CT) and contrast-enhanced by magnetic resonance imaging (MRI)] revealed an irregular increase in bone density and periosteal reaction in the right mandible (Fig. 1a and b). Tracer accumulation was observed in the right mandible as well as the sternoclavicular joints, the first rib, and shoulder joints, by bone scintigraphy (Fig. 2). The carotid arteries were normal at this point (Fig. 1c). The examination of a biopsy specimen from the right lower mandibular lesion revealed infiltration of neutrophils and lymphocytes without malignant cells or microorganisms. A tentative diagnosis of sterile osteomyelitis was made. Five months before referral, C-reactive protein elevation was observed (1.7 mg/dL), and the patient was treated with amoxicillin/clavulanate because infection was suspected. However, antibiotics did not improve her symptoms at all. She was considered to be suffering from diffuse sclerosing osteomyelitis as tracer accumulation was observed from the body to the ramus of the right mandible (Fig. 2). Three months before her referral, she complained of left neck pain, blurred vision, and headache. Because antibiotic treatment was not sufficient to relieve her symptoms, CT was again performed to evaluate the neck lesion. Unexpectedly, CT showed circumferential wall thickening of the left common carotid artery, with intense enhancement. She was then thought to have large vessel vasculitis and was referred to our department. On admission, she had swelling and pain of the right mandible bone. A physical examination revealed the following findings: blood pressure, 153/63 mmHg (without any difference between the upper limbs); pulse, 96/min; body temperature, 36.6℃; and oxygen saturation on room air, 98%. A cardiovascular examination revealed normal findings, her lungs were clear to auscultation, and the findings of an abdominal examination were completely unremarkable, with no skin lesions noted. A neck examination revealed a left neck bruit as well as tenderness and a tuberous swelling. Laboratory tests revealed an elevated white blood cell count, erythrocyte sedimentation rate, and C-reactive protein level (inflammatory markers). Although the patient was negative for antinuclear antibodies (ANA), anti SS-A antibodies were detected. We did not investigate further because she did not complain of sicca symptoms. Tests for other autoantibodies were negative. Neither syphilis nor chlamydia infection were detected. She was serotyped as HLA-B40 and HLA-B44. Carotid ultrasonography revealed irregular wall thickening and stenosis (inner diameter of the stenosis site was 3.4 mm) of the left common carotid artery. A double ring pattern of wall thickening was seen on contrast CT (Fig. 3a and b), and MRI showed strong enhancement around the left carotid artery, with irregular stenosis (Fig. 3c). Positron emission tomography showed mild uptake in the left common carotid artery (Fig. 3d). Echocardiography revealed neither valvular disease nor cardiac dysfunction. A diagnosis of TAK was made as the patient satisfied the diagnostic criteria established by the JCS Joint Working Group (8). Prednisolone (50 mg) treatment was started. Since significant angiostenosis was observed, thromboprophylaxis with acetylsalicylic acid (100 mg/day) was also initiated. At one week after the initiation of corticosteroids, there was improvement in the fever and neck pain, and the patient's C-reactive protein levels had normalized. On day 14 of admission, reexamination of the carotid artery by ultrasonography revealed an increase in blood flow from 142 cm/s to 201 cm/s. Repeat MRI showed improvement of the enhancement in the left carotid artery (Fig. 4). She was discharged on the 22nd day after admission. The prednisolone dose had been tapered to 12 mg/day, without relapse. Importantly, the swelling and pain of the right mandible bone had also been alleviated (Fig. 4c).
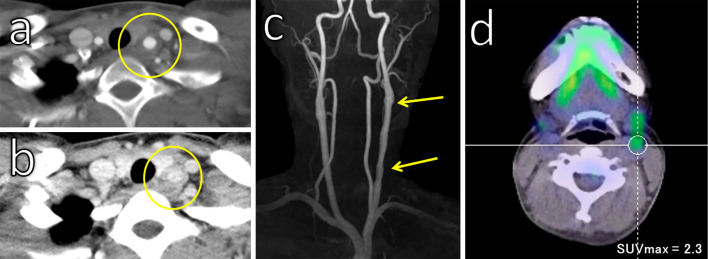
Figure 1.
Imaging studies at the onset of osteomyelitis. Computed tomography showed an irregular increase in bone density and a periosteal reaction in the right mandible bone (a). Magnetic resonance imaging revealed contrast enhancement in the right mandible bone (b). The carotid artery was normal at this point (c).
Figure 2.
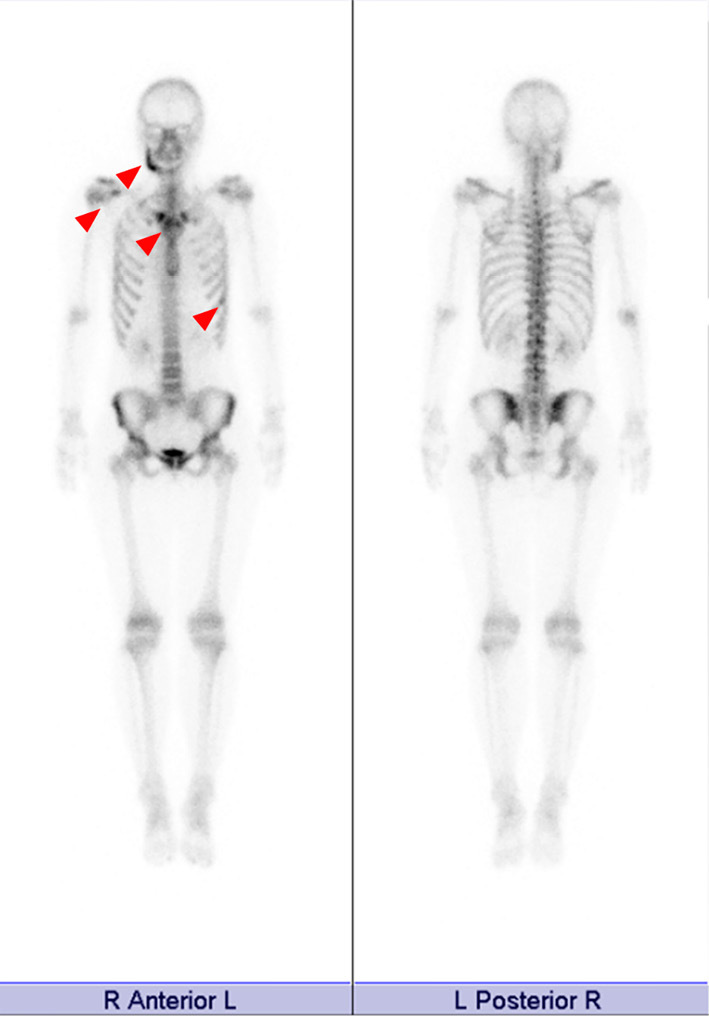
The bone scintigraphy findings. Bone scintigraphy showed tracer accumulation in the right mandible bone as well as the sternoclavicular joints, first rib, and shoulder joints (arrows).
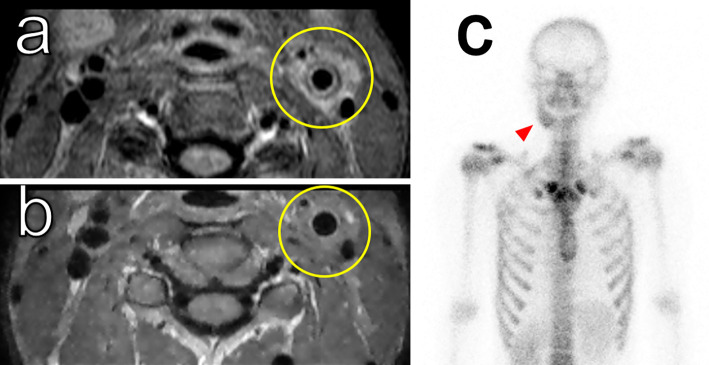
Figure 3.
Imaging of arteritis in the left carotid artery. A double ring pattern of wall thickening was observed on contrast computed tomography (a: arterial phase; b: venous phase). Magnetic resonance imaging revealed irregular narrowing of the left carotid artery (c). Positron emission tomography showed mild uptake in the left common carotid artery (d).
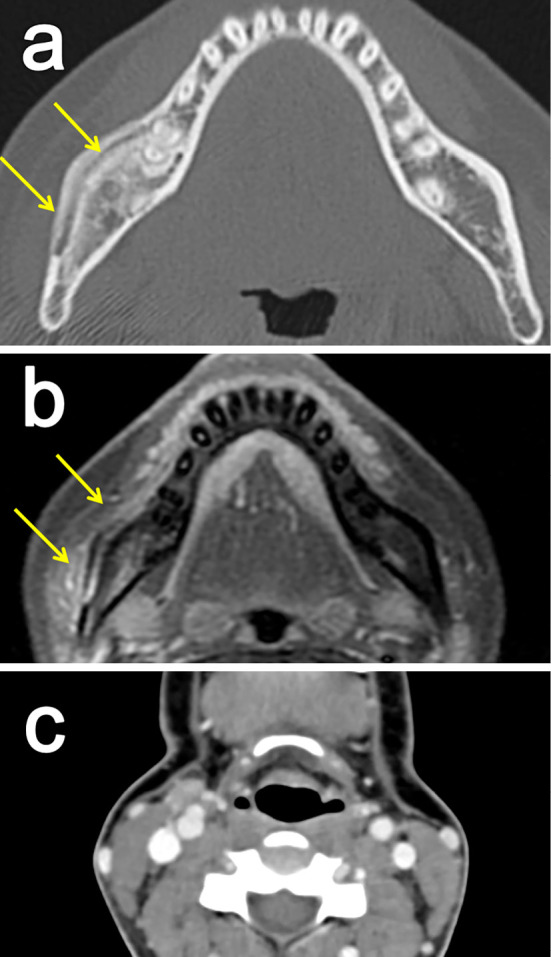
Figure 4.
Improvement on imaging studies. (a, b) Magnetic resonance imaging revealed a reduction in the contrast enhancement in the left carotid artery (a: before treatment; b: after treatment). Bone scintigraphy showed an improvement of the tracer accumulation in the right mandible bone (c).
Discussion
To date, the co-existence of TAK and osteomyelitis has been reported in six cases (Table). To retrieve data regarding osteomyelitis and TAK, we performed a search of the PubMed database using the term “Takayasu arteritis AND osteomyelitis OR SAPHO” (9). The references of the retrieved articles were also reviewed for this purpose. Three case reports (6,10,11) were found between the years 1995-2017. Among these reports, one case of TAK was complicated by CRMO (6). The other case reports presented in Table (12-14) were found from the reference list of the report by Ferguson et al. (7). In addition to the present case, we identified an additional patient in our institution who initially presented osteomyelitis and who developed TAK 12 years later (Table, case 4). At the time of writing, we are treating 136 patients with TAK (15), and the prevalence of osteomyelitis in adult TAK patients is 1.47%.
Table.
The Clinical Characteristics of Patients with Takayasu Arteritis Complicated by Osteomyelitis.
| Case | Age | Sex | Complications | Order of onset | Interval between osteomyelitis and TAK | Bones involved | Treatment | Response to treatment | Ref |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 years and 9 months | Male | PG | PG+osteomyelitis →TAK |
3 years | Ninth and tenth ribs Left tibia | High-dose prednisolone for three months | Clinical improvement | (10) |
| 2 | 10 years | Female | PG | CRMO →TAK→PG |
2 years | Right mandible | Prednisolone (1 mg/kg/day) Mycophenolate mofetil Surgical intervention |
Clinical improvement | (6) |
| 3 | 15-16 years (detail unknown) | Female | CRMO→TAK | 6 years | Multifocal | Sulfasalazine Methotrexate Azathioprine Glucocorticoid therapy | No response | (14) | |
| 4 | 20 years | Female | HT, DM | Osteomyelitis →TAK |
12 years | Right limb | Prednisolone Tocilizumab Tacrolimus | Clinical improvement | |
| 5 | 21 years | Female | TAK →osteomyelitis |
7 years | Clavicles Left Scapula | Treated conservatively (detail unknown) | Unchanged | (12) | |
| 6 | 27 years | Female | Osteomyelitis →TAK |
8 months | Right mandible | Prednisolone | Clinical improvement | Present case | |
| 7 | 35 years | Female | TAK +osteomyelitis |
Uncertain | Left ulna, radius Right tibia | Prednisolone (1 mg/kg/day) for two weeks. |
Clinical improvement | (13) | |
| 8 | 49 years | Female | AR | TAK +osteomyelitis |
Uncertain | Sternum Clavicles | Prednisolone (0.5 mg/kg/day) for three weeks. |
Clinical improvement | (11) |
AR: aortic regurgitation, CRMO: chronic recurrent multifocal osteomyelitis, DM: diabetes mellitus, HT: hypertension, PG: pyoderma gangrenosum, TAK: Takayasu arteritis
Three cases were reported in children and three in adults (Table). CRMO mainly occurs in pediatric patients. CRMO is an idiopathic non-pyrogenic auto-inflammatory bone disorder involving multiple sites, with clinical progression persisting for more than 6 months, which may have episodes of remission and exacerbation over the long term. It is predominantly found in female patients; the average age at the disease onset is 8-11 years (16). CRMO is known to be associated with other inflammatory diseases, such as palmoplantar pustulosis, psoriasis vulgaris, and inflammatory bowel disease. Interestingly, two cases were further complicated by PG (Table. cases 1 and 3), suggesting that CRMO, TAK, and PG are manifestations of one syndrome, especially in children. There are no reports of adult patients with TAK complicated by both SAPHO and PG. However, the combinations of PG and TAK are described in adults. Ujiie et al. reviewed 35 PG cases of TAK, which revealed that this association occurred particularly in young females (17). The median time interval between the two diseases was 3 years and PG preceded TAK in 22 of 35 cases (18-22).
Although SAPHO syndrome represents osteomyelitis in adults, no skin lesions, a common symptom of SAPHO (7), were observed in this case. However, bone scintigraphy showed uptake in the sternoclavicular joint, which is sometimes affected in SAPHO syndrome. Furthermore, it has been recognized that sclerosing osteomyelitis of the mandible is a local manifestation of SAPHO syndrome (23). Although there are no validated diagnostic criteria, the inclusion and exclusion criteria formulated by Benhamou et al. (24) are usually applied (25). According to these criteria, this patient was diagnosed with SAPHO syndrome.
The bone lesions involved differ among cases (Table). The most commonly affected bones included the mandible, clavicle, rib, and tibia; the sternum was affected in one case (case 8). Although our patient only showed right mandible pain, bone scintigraphy showed lesions in other areas including sternoclavicular joints, the first rib, and shoulder joints. This indicates the usefulness of bone scintigraphy in determining the subclinical bone lesions in such patients. According to the reported cases - including our own-osteomyelitis precedes TAK by 8 months to 6 years in five out of six cases. However, it is very difficult to define the precise onset of TAK, and there has been no evidence that TAK was actually absent when patients presented with osteomyelitis. Since there was no thickening of the carotid artery wall at the onset of osteomyelitis in our case (Fig. 1b), this is valuable evidence that osteomyelitis definitely occurred prior to TAK.
She had pain of the right mandible bone on admission. Although she had been treated with antibiotics including minocycline and clarithromycin, no significant improvement was observed for eight months. At first, we suspected an infection as the cause of sclerosing osteomyelitis. However, we could not find an apparent site of infection and treatments with antibiotics did not improve her symptoms. After steroid therapy, the symptoms of our patient showed dramatic improvement. Surprisingly, in addition to the resolution of her TAK-related symptoms, her osteomyelitis improved. The treatment of diffuse sclerosing osteomyelitis is challenging, and corticosteroids have been reported to be effective in the chronic stage to some degree (26). Bisphosphonate infusion has recently been reported as an alternative treatment (27). The treatments in other cases included corticosteroids or immunosuppressants such as mycophenolate mofetil, which also resulted in a marked clinical response (Table). Antibiotics, on the other hand, were not effective.
The manifestation of lower jaw pain has been reported in TAK, and ischemia was considered to be one of its causes (28). In a case reported by McConachie et al. (12), bone lesions were located in the clavicles and scapula where the arteritis activity was high. They therefore proposed that ischemic hypoxia due to arterial stenosis was responsible for the skeletal abnormality. However, the locations of the bone lesions in other cases, including our own were not related to the artery lesions, suggesting that osteomyelitis was caused by active inflammation rather than hypoxia. Cell-mediated immunity by T cells is considered to play a key role in the pathophysiology of TAK, which is supported by its relationship with specific HLA. In particular, Terao et al. revealed that the IL12B region was significantly associated with TAK, and indicated that IL12B played a fundamental role in the pathophysiology of TAK in combination with HLA-B*52:01 (29).
Macrophages are one of the major effector cells in TAK (30,31) and osteoclasts play central roles in sclerosing osteomylelitis (32). Granuloma is one of the characteristics of TAK and these are formed by multinucleated macrophages and giant cells (33). These cells release growth factors and cytokines, which lead to endothelial activation and dysfunction in patients with vasculitis (34). Meanwhile, osteoclast-mediated bone resorption is considered to be a primary disease process that is followed by new bone formation in diffuse sclerosing osteomyelitis (35). The underlying pathomechanisms of TAK and sclerosing osteomyelitis are uncertain, and this rare association suggests that common abnormalities promote the progression of both diseases.
In conclusion, osteomyelitis is an important complication of TAK and bone scintigraphy is useful for its detection. This association suggests a common pathophysiology, and further studies would be helpful in improving the understanding of the mechanisms involved in the onset of TAK.
Written informed consent for publication of this case report was obtained from the patient.
The authors state that they have no Conflict of Interest (COI).
Financial Support
This work was supported by JSPS KAKENHI Grant Number 16H06642 for TS and a research grant from the Kanae Foundation for TS.
Riiza Hanaoka and Tsuyoshi Shirai contributed equally to this work.
Acknowledgement
The authors thank the staff of the Department of Hematology & Rheumatology, Tohoku University for their helpful discussions.
References
- 1.de Sauza AW, de Carvalho JF. Diagnostic and classification criteria of Takayasu arteritis. J Autoimmun 48-49: 79-83, 2014. [DOI] [PubMed] [Google Scholar]
- 2.Terao C, Yoshifuji H, Ohmura K, et al. Association of Takayasu arteritis with HLA-B 67:01 and two amino acids in HLA-B protein. Rheumatology 52: 1769-74, 2013. [DOI] [PubMed] [Google Scholar]
- 3.Ohta Y, Ohya Y, Fujii K, et al. Inflammatory disease associated with Takayasu artiritis. Angiology 54: 339-344, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Wattamwar PR, Doshi SA, Thomas B. Hypertrophic pachymeningitis in a patient with Takayasu arteritis: one more association? Ann Indian Acad Neurol 15: 56-59, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kobak S. Relapsing polychondritis-associated Takayasu's arteritis. Folia Med 51: 49-52, 2009. [PubMed] [Google Scholar]
- 6.Vettiyil G, Punnen A, Kumar S. An unusual association of chronic recurrent multifocal osteomyelitis, pyoderma gangrenosum, and Takayasu's arteritis. J Rheumatol 44: 127-128, 2017. [DOI] [PubMed] [Google Scholar]
- 7.Ferguson PJ, Sandu M. Current understanding of the pathogenesis and management of chronic recurrent multifocal osteomyelitis. Curr Rheumatol Rep 14: 130-141, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.JCS Joint Working Group.. Guideline for management of vasculitis syndrome (JCS 2008). Japanese Circulation Society. Circ J 75: 474-503, 2011. [DOI] [PubMed] [Google Scholar]
- 9.Gasparyan AY, Ayvazyan L, Blackmore H, et al. Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31: 1409-1417, 2011. [DOI] [PubMed] [Google Scholar]
- 10.Dagan O, Barak Y, Metzker A. Pyoderma gangrenosum and sterile multifocal osteomyelitis preceding the appearance of Takayasu arteritis. Pediatr Dermatol 12: 39-42, 1995. [DOI] [PubMed] [Google Scholar]
- 11.Hoshino A, Sawada T, Matsuda M, Miyagawa S, Nakamura T, Matsubara H. A case of Takayasu's arteritis and aortic regurgitation, which presented much difficulty in the diagnosing process because of complicated osteomyelitis and non-typical manifestations. J Cardiol 54: 148-152, 2009. [DOI] [PubMed] [Google Scholar]
- 12.McConachie NS, Morley KD, Jones MC. Periosteal new bone formation in Takayasu arteritis. Clin Radiol 50: 578-580, 1995. [DOI] [PubMed] [Google Scholar]
- 13.Kim JE, Kolh EM, Kim DK. Takyasu's arteritis presenting with focal periostitis affecting two limbs. Int J Cardiol 67: 267-270, 1998. [DOI] [PubMed] [Google Scholar]
- 14.Job-Deslandre C, Krebs S, Kahan A. Chronic recurrent multifocal osteomyelitis: five-year outcomes in 14 pediatric cases. Joint Bone Spine 68: 245-251, 2001. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe R, Ishii T, Nakamura K, et al. Ulcerative colitis is not a rare complication of Takayasu arteritis. Mod Rheumatol 24: 372-373, 2014. [DOI] [PubMed] [Google Scholar]
- 16.Queiroz RM, Rocha PHP, Lauar LZ, et al. Chronic recurrent multifocal osteomyelitis exhibiting predominance of periosteal reaction. Rev Assoc Med Bras 63: 303-306, 2017. [DOI] [PubMed] [Google Scholar]
- 17.Ujiie H, et al. Pyoderma gangrenosum associated with Takayasu's arteritis. Clin Exp Dermatol 29: 357-359, 2004. [DOI] [PubMed] [Google Scholar]
- 18.Francés C, Boisnic S, Blétry O, et al. Cutaneous manifestations of Takayasu arteritis. A retrospective study of 80 cases. Dermatologica 181: 266-272, 1990. [DOI] [PubMed] [Google Scholar]
- 19.Fearfield LA, Ross JR, Farrell AM, et al. Pyoderma gangrenosum associated with Takayasu's arteritis responding to cyclosporin. Br J Dermatol 141: 339-343, 1999. [DOI] [PubMed] [Google Scholar]
- 20.Shinkai H, Mori Y, Nishioka G. Pyoderma gangrenosum associated with aortitis syndrome. Rinsho Derma (Tokyo) 12: 773-785, 1970. (in Japanese). [Google Scholar]
- 21.Sekine K, Nishiyama K, Morooka M, et al. Aortitis syndrome associated with pyoderma gangrenosum. Naika (Tokyo) 46: 1041-1045, 1980. (in Japanese). [Google Scholar]
- 22.Kubota Y, Imayama S, Nakamura K, et al. Five cases of pyoderma gangrenosum. Nishinihon J Dematol 57: 709-720, 1995. (in Japanese). [Google Scholar]
- 23.Marí A, Morla A, Melero M, et al. Diffuse sclerosing osteomyelitis (DSO) of the mandible in SAPHO syndrome: a novel approach with anti-TNF therapy. Systematic review. J Craniomaxillofac 42: 1990-1996, 2014. [DOI] [PubMed] [Google Scholar]
- 24.Benhamou CL, Chamot AM, Kahn MF. Synovitis-acne-pustulosis hyperostosis-osteomyelitis syndrome (SAPHO): a new syndrome among the spondyloarthropathies? Clin Exp Rheumatol 6: 109-112, 1988. [PubMed] [Google Scholar]
- 25.Nguyen MT, Borchers A, Selmi C, et al. The SAPHO syndrome. Semin Arthritis Rheum 42: 254-265, 2012. [DOI] [PubMed] [Google Scholar]
- 26.Jacobsson S. Diffuse sclerosing osteomyelitis of the mandible. Int J Oral Surg 13: 363-385, 1984. [DOI] [PubMed] [Google Scholar]
- 27.Otto S, Troeltzsch M, Burian E, et al. Ibandronate treatment of diffuse sclerosing osteomyelitis of the mandible: pain relief and insight into pathogenesis. J Craniomaxillofac Surg 43: 1837-1842, 2015. [DOI] [PubMed] [Google Scholar]
- 28.Phulambrikar T, Kode M, Shrivastava M, et al. Takayasu's arteritis--report of a case with masquerading jaw pain. Oral Surg Oral Med Oral Pathol Oral Radiol 118: 16-21, 2014. [DOI] [PubMed] [Google Scholar]
- 29.Terao C, Yoshifuji H, Kimura A, et al. Two susceptibility loci to Takayasu arteritis reveal a synergistic role of the IL12B and HLA-B regions in a Japanese population. Am J Hum Genet 93: 289-297, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnaud L, Haroche J, Mathian A, et al. Pathogenesis of Takayasu's arteritis: a 2011 update. Autoimmun Rev 11: 61-67, 2011. [DOI] [PubMed] [Google Scholar]
- 31.Shirai T, Hilhorst M, Harrison DG, et al. Macrophages in vascular inflammation--from atherosclerosis to vasculitis. Autoimmunity 48: 139-151, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helming L, Gordon S. Molecular mediators of macrophage fusion. Trends Cell Biol 19: 514-522, 2009. [DOI] [PubMed] [Google Scholar]
- 33.Hilhorst M, Shirai T, Berry G, et al. T cell-macrophage interactions and granuloma formation in vasculitis. Front Immunol 5: 432, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fenyo IM, Gafencu AV. The involvement of the monocytes/macrophages in chronic inflammation associated with atherosclerosis. Immunobiology 218: 1376-1384, 2013. [DOI] [PubMed] [Google Scholar]
- 35.Montonen M, Li TF, Lukinmaa PL, et al. RANKL and cathepsin K in diffuse sclerosing osteomyelitis of the mandible. J Oral Pathol Med 35: 620-625, 2006. [DOI] [PubMed] [Google Scholar]