Abstract
Background
Functional Assessment of Cancer Therapy-Esophagus (FACT-E) is a health-related quality of life (HRQOL) instrument validated in patients with esophageal cancer. It is made up of both a general component and an esophageal cancer subscale (ECS). Our objective was to explore the relationship between baseline FACT-E, ECS and clinically determined T-stage in patients with stage II–IV cancer of the gastroesophageal junction or thoracic esophagus.
Methods
Data from four prospective studies in Canadian academic hospitals were combined. These were consecutive and eligible patients treated between 1996 and 2014 with clinical stage II–IV cancer of the gastroesophageal junction or thoracic esophagus. All patients completed pre-treatment FACT-E. Parametric (ANOVA) and non-parametric (Kruskal-Wallis) analyses were performed.
Results
Of the 135 patients that were deemed eligible, the T-stage distribution determined clinically was: 10 (7.4%) T1, 33 (24.4%) T2, 79 (58.5%) T3 and 13 (9.6%) T4. Parametric analysis showed no significant association between FACT-E & T-stage, although there was a trend towards significance (P=0.08). Non-parametric analysis showed a significant association between FACT-E and T-stage (P=0.05). Post-hoc tests identified that the most significant differences in FACT-E scores were between T1 and T3 patients. Both parametric (P=0.002) and non-parametric (P=0.003) analyses showed an association between ECS & T-stage. Post-hoc analyses showed significant differences in ECS scores between T1 and higher T-stages (P<0.01).
Conclusions
Patient-reported HRQOL scores appear to be significantly different in patients with clinical T1 esophageal cancer as compared to those with higher clinical T stages. Since distinguishing T1 from T2/T3 lesions is important in guiding the most appropriate treatment modality and since EUS appears to have difficulties reliably making such T-stage distinctions, FACT-E and ECS scores may be helpful as an adjunct to guide decision-making.
Keywords: Esophageal cancer, cancer staging, quality of life, surgery
Introduction
Esophageal cancer has a significant effect on health-related quality of life (HRQOL) (1). Poor HRQOL has been shown to be associated with poorer long-term survival in different cancers (2-6). Since esophageal cancer may initially present with significant HRQOL issues, it seems likely that pretreatment HRQOL may be an important prognostic tool. We have previously shown that higher pretreatment HRQOL measures were independently associated with better overall survival (7). We focused on the Functional Assessment of Cancer Therapy-Esophagus (FACT-E), which is a HRQOL instrument validated in esophageal cancer patients (7). It is comprised of a general component [Functional Assessment of Cancer Therapy General (FACT-G)] and an esophageal cancer subscale (ECS) (7). Lower scores reflect reduced HRQOL. We have also previously shown that the discriminative ability of pretreatment FACT-E and ECS was better than clinician-assigned performance status (ECOG—Eastern Cooperative Oncology Group) in predicting survival in patients with Stage II–III esophageal cancer (6). Furthermore, we found that ECS was a better discriminator for survival than FACT-E (6). We were interested in investigating the mechanism by which FACT-E and ECS predict survival. Since many of the questions used to derive the ECS deal with dysphagia, we hypothesized that the basis of survival prediction was that FACT-E (and especially ECS) was serving as a proxy for T-stage. We hypothesized this because different T-stages manifest with varying effects on HRQOL. It has also previously been shown that dysphagia grade has a positive correlation with EUS T-stage of esophageal cancer (8). Our objective was to explore the relationship between baseline FACT-E, ECS and clinically determined T-stage in patients with stage II–IV cancer of the gastroesophageal junction or thoracic esophagus.
Methods
Patient population
Data from 4 prospective, non-randomized studies in three large Canadian academic hospitals (Toronto General Hospital, Princess Margaret Cancer Centre, The Ottawa Hospital) were combined. These have been previously described (6,7). The current study and all four studies were approved by institutional ethics boards. Patients provided informed consent to the original studies and use of anonymized data. These studies included consecutive eligible patients with clinical stage II–IV cancer of the gastroesophageal junction or thoracic esophagus who received chemotherapy and concomitant radiation either as neoadjuvant therapy or as part of bimodality therapy without surgery. Staging was defined according to 7th edition of the AJCC TNM Staging system. Clinical T-stage was determined by a combination of endoscopic ultrasound (EUS) and computed tomography.
HRQOL instrument
The FACT-G is a general HRQOL instrument that has been validated for use in any cancer; it consists of 28 questions covering the domains of physical well-being, functional well-being, social well-being and emotional well-being using a 5-point Likert scale (9). A disease-specific module, the ECS was validated and added to the FACT-G to result in the FACT-E for esophageal cancer (9). The ECS consists of 17 items addressing eating, swallowing, enjoyment of food, voice, dry mouth, appetite, cough, choking, and pain; each of these is evaluated using a 5-point Likert scale to generate a summary score of esophageal-specific concerns (9). Higher scores denote better quality of life. All patients completed FACT-E at baseline; surveys were self-administered at the time of the first consultation with the thoracic surgeon or medical or radiation oncologist prior to initiation of any therapies.
Statistical analysis
For univariate analysis, normally-distributed continuous data were reported as means with standard deviations and analyzed using independent sample t-tests. Data that were not normally-distributed were reported as medians with interquartile ranges and analyzed using the Mann-Whitney U test. Fisher’s exact tests were used for univariate analysis of categorical data. The primary comparison was between clinical T-stage and (I) FACT-E and (II) ECS. Parametric analysis was performed with ANOVA, with subsequent use of post-hoc Tukey’s b tests to differentiate between different T-stages. Non-parametric analysis was performed with Kruskal-Wallis test, with subsequent use of post-hoc pairwise comparisons to differentiate between different T-stages. A 2-sided alpha of 0.05 was used for all tests of significance. Statistical analyses were performed using the PASW/SPSS statistical package (version 23; Armonk, NY: IBM Corp.).
Results
There were 135 patients with complete clinical staging that were eligible for this study. These were consecutive patients treated between 1996 and 2014 with clinical stage II–IV cancer of the gastroesophageal junction or thoracic esophagus who completed pre-treatment FACT-E. The completion rate of FACT-E at baseline was 100%. Table 1 shows the baseline characteristics. Mean age was 61.0±11.0 years. The majority of patients (68.1%, n=92) had adenocarcinoma. Figure 1 illustrates the T-stage distribution which was: 10 (7.4%) T1, 33 (24.4%) T2, 79 (58.5%) T3 and 13 (9.6%) T4. This was determined clinically through a combination of EUS and computed tomography.
Table 1. Baseline characteristics.
| Characteristic | Total (N=135) |
|---|---|
| Mean age (standard deviation) | 61.0 (11.0) |
| Adenocarcinoma, n (%) | 92 (68.1) |
| ECOG status, n (%) | |
| 0 | 31 (23.0) |
| 1 | 44 (32.6) |
| 2 | 2 (1.5) |
| Missing | 58 (43.0) |
| Clinical T-stage, n (%) | |
| 1 | 10 (7.4) |
| 2 | 33 (24.4) |
| 3 | 79 (58.5) |
| 4 | 13 (9.6) |
| Clinical N-stage, n (%) | |
| 0 | 73 (54.1) |
| 1 | 61 (45.2) |
| 2 | 1 (0.7) |
Figure 1.
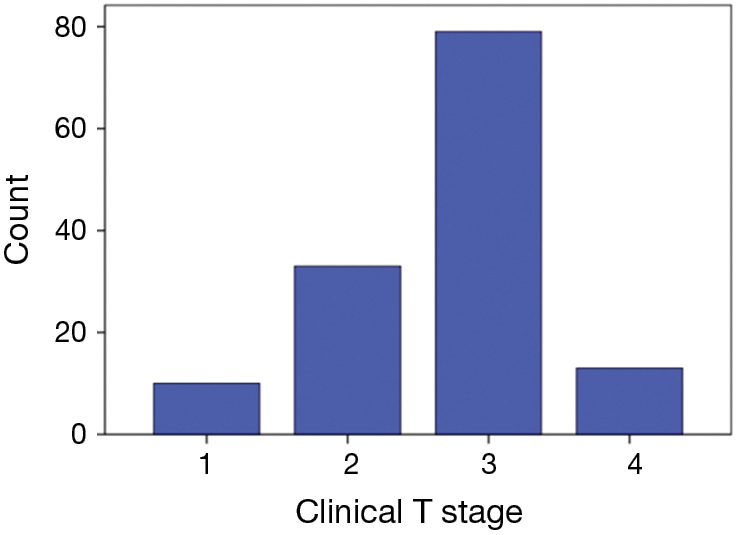
Distribution of clinical T-stage. FACT-E, Functional Assessment of Cancer Therapy-Esophagus.
Figure 2 illustrates the distribution of FACT-E according to Clinical T-stage. Mean FACT-E scores stratified by T-stage were not significantly different: T1 =81.7±18.0, T2 =78.1±19.0, T3 =75.3±16.3, T4 =78.4±21.0 (P=0.65). There were inconsistent findings between parametric and non-parametric analysis. Parametric (ANOVA) analysis showed no significant association between FACT-E & T-stage, although there was a trend towards significance (P=0.08). Non-parametric (Kruskal-Wallis) analysis showed a significant association between FACT-E and T-stage (P=0.05). Post-hoc tests identified that the most significant differences in FACT-E scores were between T1 and T3 patients.
Figure 2.
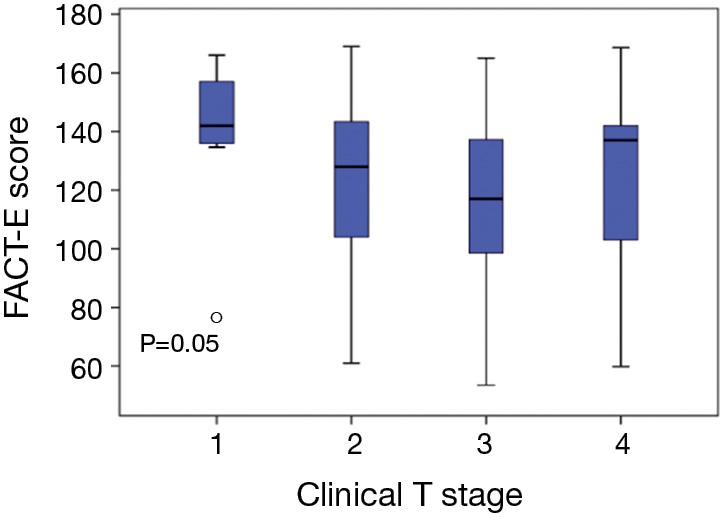
Distribution of pre-treatment FACT-E scores according to clinical T-stage. ○, outlier (>3 standard deviations from mean).FACT-E, Functional Assessment of Cancer Therapy-Esophagus; ECS, esophageal cancer subscale.
Figure 3 illustrates the distribution of ECS according to Clinical T-stage. Mean ECS scores stratified by T-stage were significantly different: T1 =58.7±9.1, T2 =45.6±12.3, T3 =42.3±12.6, T4 =44.5±15.4 (P=0.002). Both parametric (P=0.002) and non-parametric (P=0.003) analyses showed an association between ECS & T-stage. Post-hoc analyses showed significant differences in ECS scores between T1 and higher T-stages (P<0.01).
Figure 3.
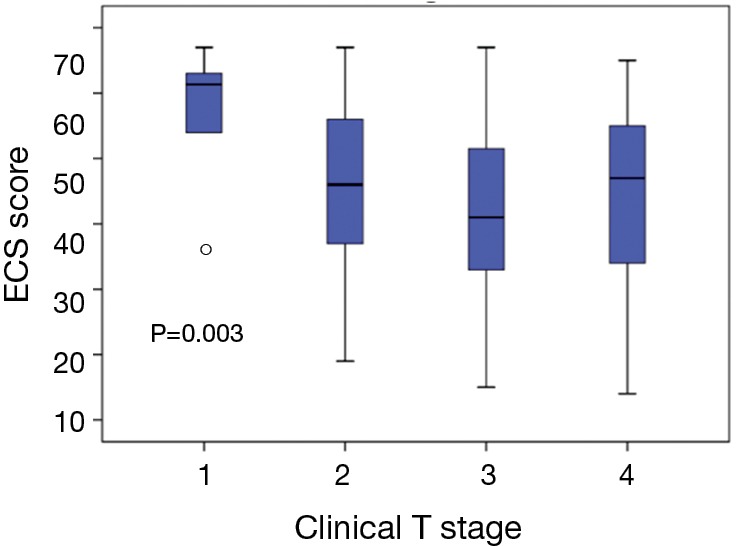
Distribution of pre-treatment ECS scores according to clinical T-stage. ○, outlier (>3 standard deviations from mean).ECS, esophageal cancer subscale.
Discussion & conclusions
We have previously shown that in patients with stage II and III esophageal cancer being considered for therapy, higher pre-treatment FACT-E and ECS were independently associated with longer survival, even after controlling for age, stage, histology, and therapy received (7). We have also shown that pretreatment FACT-E and ECS, both patient-reported outcomes, have better discrimination for survival than does performance status (ECOG), which is a physician-assigned score/outcome (6). We hypothesized that this is because FACT-E, and more specifically, the ECS, is a proxy for T-stage.
In this study, we found that pre-treatment ECS and FACT-E scores appear to decrease as clinical T-stage increases from T1 to T3. A significant difference was seen in pre-treatment ECS scores between T1 patients compared to higher T-stage patients and thus pre-treatment ECS appears to differentiate between T1 and higher T stages. Furthermore, pre-treatment ECS appears to have narrow confidence intervals for distinguishing T1.
This is important because our current methods of distinguishing T1 from higher stages are suboptimal. Many staging modalities have been utilized for esophageal cancer, including chest CT, MRI, positron emission tomography (PET), and EUS (10). EUS has been shown to higher sensitivity and accuracy for locoregional node involvement than CT or PET (11). EUS has been shown to be good at evaluating T-stage (a pooled T1–T4 sensitivity of 81–90% and a pooled specificity of 99%), however it is still not optimal at differentiating between T1 and T2 stages (11). In general, EUS performs better at excluding T4 but does not perform as well at distinguishing other T-stages from each other (12-18). Patients with T1 tumours are eligible for organ-preserving esophageal resection [i.e., endoscopic submucosal dissection (ESD)] whereas T2 tumours are not (19). Thus, the ability to differentiate T1 from T2 pre-treatment has become of increasing importance.
ESD may also be used in the staging of esophageal cancer. Although it may not be reasonable to do ESD on every patient that we think may be T1 or T2, it is feasible to use ECS and/or FACT-E as an adjunct to guide in selection of patients that are good candidates for ESD (20). ESD allows resection of mucosa and submucosa, thereby allowing examination of resected tissue histopathologically to determine whether a lesion is truly T1 or T2 (21). Currently, ESD and organ-preserving esophageal cancer surgery are considered curative for T1a tumours, with only a 0.0–1.3% risk of metastases (22-24). It could also be argued as a definitive treatment for high-risk T1b patients, accepting that there is a high risk of occult lymph node metastases, even if complete resection is achieved. For most practice, it does not seem feasible to perform diagnostic ESD in all patients. Since EUS has lower sensitivity in the T1 to T2 range but appears to reliably distinguish T3/4 from lower stage disease, ECS has the potential to serve as a useful adjunct; in patients with EUS evidence of T1 or T2 tumours, ECS could potentially help differentiate T-stage and therefore help select patients for ESD. Furthermore, not all settings have EUS and thus ECS may be a viable and affordable alternative for selecting patients for organ-preserving therapy in healthcare settings without consistent access to EUS.
In conclusion, patient-reported HRQOL scores (FACT-E and ECS) appear to be significantly different in patients with clinical T1 esophageal cancer as compared to those with higher clinical T stages. Since distinguishing T1 from T2/T3 lesions is important in guiding the most appropriate treatment modality and since EUS appears to have difficulties reliably making such T-stage distinctions, FACT-E and ECS scores may be helpful as an adjunct to guide decision-making. This requires validation on a larger scale with prospective studies done in different settings.
Acknowledgements
None.
Ethical Statement: The current study and all four studies were approved by institutional ethics boards. Patients provided informed consent to the original studies and use of anonymized data.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Darling GE. Quality of life in patients with esophageal cancer. Thorac Surg Clin 2013;23:569-75. 10.1016/j.thorsurg.2013.07.011 [DOI] [PubMed] [Google Scholar]
- 2.Safieddine N, Xu W, Quadri SM, et al. Health-related quality of life in esophageal cancer: effect of neoadjuvant chemoradiotherapy followed by surgical intervention. J Thorac Cardiovasc Surg 2009;137:36-42. 10.1016/j.jtcvs.2008.09.049 [DOI] [PubMed] [Google Scholar]
- 3.Blazeby JM, Brookes ST, Alderson D. The prognostic value of quality of life scores during treatment for oesophageal cancer. Gut 2001;49:227-30. 10.1136/gut.49.2.227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maisey NR, Norman A, Watson M, et al. Baseline quality of life predicts survival in patients with advanced colorectal cancer. Eur J Cancer 2002;38:1351-7. 10.1016/S0959-8049(02)00098-9 [DOI] [PubMed] [Google Scholar]
- 5.Djarv T, Lagergren P. Six-month postoperative quality of life predicts long-term survival after oesophageal cancer surgery. Eur J Cancer 2011;47:530-5. 10.1016/j.ejca.2010.10.014 [DOI] [PubMed] [Google Scholar]
- 6.Kidane B, Sulman J, Xu W, et al. Pretreatment quality-of-life score is a better discriminator of oesophageal cancer survival than performance status. Eur J Cardiothorac Surg 2017;51:148-54. 10.1093/ejcts/ezw264 [DOI] [PubMed] [Google Scholar]
- 7.Kidane B, Sulman J, Xu W, et al. Baseline measure of health-related quality of life (Functional Assessment of Cancer Therapy-Esophagus) is associated with overall survival in patients with esophageal cancer. J Thorac Cardiovasc Surg 2016;151:1571-80. 10.1016/j.jtcvs.2016.01.052 [DOI] [PubMed] [Google Scholar]
- 8.Fang TC, Oh YS, Szabo A, et al. Utility of dysphagia grade in predicting endoscopic ultrasound T-stage of non-metastatic esophageal cancer. Dis Esophagus 2016;29:642-8. 10.1111/dote.12394 [DOI] [PubMed] [Google Scholar]
- 9.Darling G, Eton DT, Sulman J, et al. Validation of the functional assessment of cancer therapy esophageal cancer subscale. Cancer 2006;107:854-63. 10.1002/cncr.22055 [DOI] [PubMed] [Google Scholar]
- 10.Daly JM, Karnell LH, Menck HR. National Cancer Data Base report on esophageal carcinoma. Cancer 1996;78:1820-8. [DOI] [PubMed] [Google Scholar]
- 11.Puli SR, Reddy JB, Bechtold ML, et al. Staging accuracy of esophageal cancer by endoscopic ultrasound: a meta-analysis and systematic review. World J Gastroenterol 2008;14:1479-90. 10.3748/wjg.14.1479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fujishiro M, Yahagi N, Kakushima N, et al. Endoscopic submucosal dissection of esophageal squamous cell neoplasms. Clin Gastroenterol Hepatol 2006;4:688-94. 10.1016/j.cgh.2006.03.024 [DOI] [PubMed] [Google Scholar]
- 13.Shimoyama S, Imamura K, Takeshita Y, et al. The useful combination of a higher frequency miniprobe and endoscopic submucosal dissection for the treatment of T1 esophageal cancer. Surg Endosc 2006;20:434-8. 10.1007/s00464-005-0144-3 [DOI] [PubMed] [Google Scholar]
- 14.Oyama T, Tomori A, Hotta K, et al. Endoscopic submucosal dissection of early esophageal cancer. Clin Gastroenterol Hepatol 2005;3:S67-70. 10.1016/S1542-3565(05)00291-0 [DOI] [PubMed] [Google Scholar]
- 15.Ciocirlan M, Lapalus MG, Hervieu V, et al. Endoscopic mucosal resection for squamous premalignant and early malignant lesions of the esophagus. Endoscopy 2007;39:24-9. 10.1055/s-2006-945182 [DOI] [PubMed] [Google Scholar]
- 16.Noguchi H, Naomoto Y, Kondo H, et al. Evaluation of endoscopic mucosal resection for superficial esophageal carcinoma. Surg Laparosc Endosc Percutan Tech 2000;10:343-50. 10.1097/00129689-200012000-00001 [DOI] [PubMed] [Google Scholar]
- 17.Higuchi K, Tanabe S, Koizumi W, et al. Expansion of the indications for endoscopic mucosal resection in patients with superficial esophageal carcinoma. Endoscopy 2007;39:36-40. 10.1055/s-2006-945148 [DOI] [PubMed] [Google Scholar]
- 18.Shimada H, Makuuchi H. Endoscopic treatment for esophageal cancer. Kyobu Geka 2006;59:768-75. [PubMed] [Google Scholar]
- 19.Sgourakis G, Gockel I, Lang H. Endoscopic and surgical resection of T1a/T1b esophageal neoplasms: a systematic review. World J Gastroenterol 2013;19:1424-37. 10.3748/wjg.v19.i9.1424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah PM, Gerdes H. Endoscopic options for early stage esophageal cancer. J Gastrointest Oncol 2015;6:20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishihara R, Yamamoto S, Hanaoka N, et al. Endoscopic submucosal dissection for superficial Barrett's esophageal cancer in the Japanese state and perspective. Ann Transl Med 2014;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stein HJ, Feith M, Bruecher BL, et al. Early esophageal cancer: pattern of lymphatic spread and prognostic factors for long-term survival after surgical resection. Ann Surg 2005;242:566-73; discussion 73-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leers JM, DeMeester SR, Oezcelik A, et al. The prevalence of lymph node metastases in patients with T1 esophageal adenocarcinoma a retrospective review of esophagectomy specimens. Ann Surg 2011;253:271-8. 10.1097/SLA.0b013e3181fbad42 [DOI] [PubMed] [Google Scholar]
- 24.Barbour AP, Jones M, Brown I, et al. Risk stratification for early esophageal adenocarcinoma: analysis of lymphatic spread and prognostic factors. Ann Surg Oncol 2010;17:2494-502. 10.1245/s10434-010-1025-0 [DOI] [PubMed] [Google Scholar]


