Abstract
There have been many reports on the roles of intestinal flora and intestinal environment in health promotion and disease prevention. Beneficial bacteria such as Bifidobacterium and lactic acid-producing bacteria have been shown to improve the intestinal environment, and yield a good effect on metabolism, immunity and nerve response. In this review, in addition to these beneficial bacteria, we introduced Akkermansia muciniphila as a next-generation beneficial microbe. Several reports indicate that Akkermansia muciniphila affects glucose metabolism, lipid metabolism, and intestinal immunity, and that certain food ingredients such as polyphenols may increase the abundance of Akkermansia muciniphila in the gut.
Keywords: Akkermansia muciniphila, diabetes, polyphenols, cancer immunotherapy
Introduction
In 2004, Muriel Derrien in her Ph.D. research at Wageningen University of the Netherlands isolated from a sample of healthy human feces a species of bacteria that can grow on a viscogenic substrate such as mucin and use it a single nutrient source, especially on the mucosal surface of the gastrointestinal tract.(1) From the name of microbial ecologist Antoon DL Akkermans and 6“preferring mucin”, this bacterium was named Akkermansia muciniphila (Akkermansia). It accounts for 1 to 4% of intestinal bacteria in adults and is a species of bacteria that inhabits the large intestine.(2) Akkermansia a is a gram-negative, obligate anaerobic, non-motile, nonspore-forming elliptical eubacterium, classified under the phylum Verrucomicrobia. In this review, we summarized recent studies that have indicated that Akkermansia is involved in obesity, glucose metabolism, and intestinal immunity, as well as reports on the associated role of food factors.(3,4)
Glucose Metabolism and Akkermansia
In humans with high body weight, body mass index (BMI), blood cholesterol level, and fasting blood glucose level, it is suggested that the abundance of Akkermansia in the gut is lower than that in the gut of healthy humans.(5) In addition, when overweight or obese people undergo calorie-restricted diet therapy, the effect of improving insulin resistance has been reported to be more pronounced in humans with a higher abundance of Akkermansia in the intestine. It is reported that Akkermansia increases when metformin, which is one of the therapeutic agents for diabetes, is administered to obese mice, and the action of metformin is partly mediated by the action of Akkermansia.(6) In recent years, it has been revealed that, in the diabetic state, the breakdown of the intestinal mucosal barrier mechanism modifies the pathological condition; it has been reported that Akkermansia promotes mucus secretion and makes the barrier mechanism more robust. Chelakkot et al.(7) demonstrated that Akkermansia-derived extracellular vesicles may act as functional moieties for controlling gut permeability and that the regulation of intestinal barrier integrity can improve metabolic functions in high-fat diet-fed mice. Blood lipopolysaccharide (LPS) concentration, an indicator of intestinal permeability, was also observed to be high in obese subjects (high-fat diet and diabetes mellitus mouse models), and the administration of Akkermansia was shown to decrease it.(8)In mice, many studies have been carried out towards showing how Akkermansia more directly influences glucose/lipid metabolism.
The precise molecular mechanisms underlying how Akkermansia physiologically influences the human body are gradually being elucidated. It is thought that Akkermansia produces short-chain fatty acids such as acetic acid from mucin and supplies energy to goblet cells that produce mucin. Metformin, an antidiabetic drug, is suggested to increase the number of goblet cells, thereby enhancing mucin production, thickening the intestinal mucus layer, and maintaining the intestinal barrier mechanism; this contributes to an anti-inflammatory effect and, consequently, its antidiabetic action.(6) Studies analyzing the bacterial cell proteins of Akkermansia have also been carried out. Amuc-1100, an outer membrane protein of Akkermansia, has been identified and found to activate intracellular signals mediated by the Toll-like receptor 2 (TLR2) of intestinal epithelial cells, contributing to the enhancement of the intestinal barrier.(9) It has also been demonstrated that Amuc-1100 of Akkermansia is involved in the immune response, specifically in the induction of the production of interleukin-10 (IL-10), which is an anti-inflammatory cytokine.(9) As previously mentioned, it has become clear that Akkermansia is either directly or indirectly involved in the metabolic and immune responses of humans, thus attracting attention as a next-generation beneficial bacterium.(3)
Polyphenol Functionality and Akkermansia
The health-promoting and disease-preventing effects of polyphenols have attracted attention. There are many polyphenols in nature, including catechins found in wine, tea, apples, grape leather, mussels and blueberries; isoflavones found in soybeans; and chlorogenic acid found in coffee. Polyphenols generally have low absorption rates, and there previously suggested to not work effectively in the body. However, it has recently been reported that polyphenols are metabolized by intestinal bacteria to change its absorption rate and bioavailability; conversely, polyphenols can change the composition of the intestinal bacterial bacteria. Intestinal bacteria that degrade polyphenols such as quercetin have been reported, and the relationship between polyphenols and the gut microbiota is becoming an important subject for the evaluation of food functionality.
Polyphenols derived from grapes act to increase the abundance of Akkermansia in the intestinal tract; as a result, they have been shown to enhance intestinal barrier function and incretin secretion from intestinal endocrine cells.(10,11) Polyphenols derived from cranberries have also been reported to increase the abundance of Akkermansia, as well as help suppress obesity, insulin resistance, and intestinal inflammation.(12) These indicate that there is an additional pathway that exerts its function by acting on the gastrointestinal tract without absorption of polyphenols, by influencing the intestinal bacterial flora and acting on intestinal mucosal cells. Masumoto et al.(13) demonstrated that apple-derived macromolecular procyanidins induce an increase in the abundance of intestinal Akkermansia and have anti-inflammatory and anti-metabolism effects in a mouse model of metabolic syndrome. Administration of macromolecular procyanidins suppressed changes in Inflammation in intestinal mucosa, weight gain, and abnormalities in liver lipid metabolism induced by a high-fat high-sucrose diet. In addition, 16S rRNA metagenomic analysis showed an improvement in the Firmicutes/Bacteroidetes ratio, as well as an increase in Akkermansia, leading to the anti-inflammatory action in the intestinal tract and the enhancement of the intestinal barrier function.
As far as the functionality of polyphenols has been deemed to contribute to the strong antioxidant effect observed in in vitro experiments, recent results have indicated that relatively poorly absorptive polyphenols directly affect intestinal bacteria and show the possibility that the antioxidant effect is mediated by so-called “good bacteria” such as Akkermansia. However, polyphenols have not been sufficiently analyzed in human subjects, and the evidence for its involvement in increasing the abundance of Akkermansia has not been sufficient.
Cancer Immunotherapy and Akkermansia
Therapeutic efficacy and its association with the intestinal microbiota were analyzed in 249 patients with lung, kidney, or bladder cancers treated with immune checkpoint inhibitors.(14) As a result, patients who used antibiotics before and after treatment had a poorer response to the immune checkpoint inhibitor PD-1 antibody and a shorter survival time compared to the patients who did not use antibiotics. In addition, in the intestine of a patient who responded positively to the immune checkpoint inhibitor, an increase in the abundance of Akkermansia was observed compared to a patient who did not respond positively to the immunotherapy. It was also confirmed that, using the stool of a patient that responded positively to the immunotherapy, fecal microbiome transplantation in a sterile mouse caused it to respond positively to the anti-PD-1 antibody. In future cancer immunotherapy, we should obtain detailed genetic information on the cancer tissue, identify the mutation of the mismatch repair gene, select an immune checkpoint inhibitor based on this information, and refer to metagenomic information on the gut microbiota of the patient.
Akkermansia in Japanese
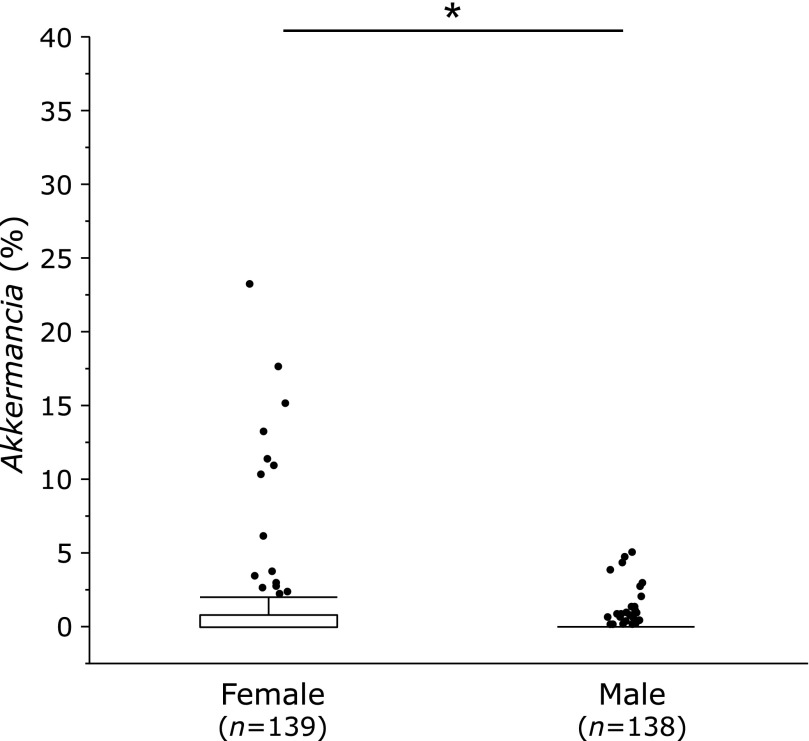
Recently, we conducted a 16S rRNA V3–V4 sequence analysis on the feces microbiota of about 300 Japanese people.(15) Interestingly, the relative abundance of Akkermansia in female was significantly higher than that in male (Fig. 1), however, the proportion of this bacteria being present at 5% or more was extremely low at 3.6% of females, which was similar to the previous report.(16) It is an important task for the future to study what kinds of meals and exercises can increase Akkermansia and to apply it to Japanese people.
Fig. 1.
The relative abundance of Akkermansia miciniphila in female and male subjects. Microbiota in fecal samples were analyzes by 16S rRNA V3–V4 gene sequencing. *p<0.05.
Conclusion
In addition to traditional beneficial bacteria such as Bifidobacterium and lactic acid-producing bacteria, we introduced the next-generation “beneficial bacteria” called Akkermansia muciniphila. It is considered as a target of the functionality of polyphenols as well as cancer immunotherapy, and it is important to verify its effect in human clinical trials in the future.
Author Contributions
YN, TT and KU were involved in editing the manuscript. All authors discussed the results and commented on the manuscript.
Acknowledgments
This work was supported by a grant-in-aid for Scientific Research (KAKENHI) (C) to TT (No. 16K09322) and (B) to YN (No. 16H05289) from the Japan Society for the Promotion of Science, and by Grant of Industry-Academia-Government Collaboration of “Field for Knowledge Integration and Innovation” (FKII) to YN (No. 16824414) from the Ministry of Agriculture, Forestry and Fisheries of Japan.
Conflict of Interest
YN received scholarship fund from EA Pharma. Co. Ltd. and collaboration research fund from Fujifilm Medical Co. Ltd., and has been paid lecture fees by Janssen Pharma KK, Mylan EPD Co., Takeda Pharma. Co. Ltd., Mochida Pharm. Co. Ltd., EA Pharma. Co. Ltd., Otsuka Pharma. Co. Ltd., and Astellas Pharma. Co. Ltd. TT received lecture fees by Mochida Pharm. Co. Ltd. and Mitsubishi Tanabe Pharama Co. The other authors have no conflicts of interest to declare.
References
- 1.Derrien M, Vaughan EE, Plugge CM, de Vos WM. Akkermansia muciniphila gen. nov., sp. nov., a human intestinal mucin-degrading bacterium. Int J Syst Evol Microbiol. 2004;54 (Pt 5):1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 2.Belzer C, de Vos WM. Microbes inside--from diversity to function: the case of Akkermansia. ISME J. 2012;6:1449–1458. doi: 10.1038/ismej.2012.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cani PD, de Vos WM. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front Microbiol. 2017;8:1765. doi: 10.3389/fmicb.2017.01765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou K. Strategies to promote abundance of Akkermansia muciniphila, an emerging probiotics in the gut, evidence from dietary intervention studies. J Funct Foods. 2017;33:194–201. doi: 10.1016/j.jff.2017.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tilg H, Moschen AR. Microbiota and diabetes: an evolving relationship. Gut. 2014;63:1513–1521. doi: 10.1136/gutjnl-2014-306928. [DOI] [PubMed] [Google Scholar]
- 6.Shin NR, Lee JC, Lee HY, et al. An increase in the Akkermansia spp. population induced by metformin treatment improves glucose homeostasis in diet-induced obese mice. Gut. 2014;63:727–735. doi: 10.1136/gutjnl-2012-303839. [DOI] [PubMed] [Google Scholar]
- 7.Chelakkot C, Choi Y, Kim DK, et al. Akkermansia muciniphila-derived extracellular vesicles influence gut permeability through the regulation of tight junctions. Exp Mol Med. 2018;50:e450. doi: 10.1038/emm.2017.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Everard A, Belzer C, Geurts L, et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plovier H, Everard A, Druart C, et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat Med. 2017;23:107–113. doi: 10.1038/nm.4236. [DOI] [PubMed] [Google Scholar]
- 10.Roopchand DE, Carmody RN, Kuhn P, et al. Dietary polyphenols promote growth of the gut bacterium Akkermansia muciniphila and attenuate high-fat diet-induced metabolic syndrome. Diabetes. 2015;64:2847–2858. doi: 10.2337/db14-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reunanen J, Kainulainen V, Huuskonen L, et al. Akkermansia muciniphila adheres to enterocytes and strengthens the integrity of the epithelial cell layer. Appl Environ Microbiol. 2015;81:3655–3662. doi: 10.1128/AEM.04050-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anhê FF, Marette A. A microbial protein that alleviates metabolic syndrome. Nat Med. 2017;23:11–12. doi: 10.1038/nm.4261. [DOI] [PubMed] [Google Scholar]
- 13.Masumoto S, Terao A, Yamamoto Y, Mukai T, Miura T, Shoji T. Non-absorbable apple procyanidins prevent obesity associated with gut microbial and metabolomic changes. Sci Rep. 2016;6:31208. doi: 10.1038/srep31208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1-based immunotherapy against epithelial tumors. Science. 2018;359:91–97. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 15.Takagi T, Naito Y, Inoue R, et al. Age/Gender- and stool consistency-associated differences in gut microbiota in healthy Japanese subjects. J Gastroenterol. 2018; in press. [DOI] [PubMed] [Google Scholar]
- 16.Escobar JS, Klotz B, Valdes BE, Agudelo GM. The gut microbiota of Colombians differs from that of Americans, Europeans and Asians. BMC Microbiol. 2014;14:311. doi: 10.1186/s12866-014-0311-6. [DOI] [PMC free article] [PubMed] [Google Scholar]