Abstract
Recent evidence has suggested that extracellular microRNAs have crucial roles in intercellular communications and are promising as minimally invasive biomarkers for various diseases including cancers. Oxidative stress also plays an essential role in homeostasis and disease development. This systematic review aims to clarify the current evidence on the interaction between oxidative stress and extracellular microRNAs. We identified 32 studies that provided information regarding the association between oxidative stress and extracellular microRNAs: 9 focused on the central nervous system, 11 focused on cardiovascular diseases, and 4 focused on liver injury. Endothelial cell-specific miR-126-3p was the most studied extracellular miRNA associated with oxidative stress. In addition, we highlight some reports that describe the mechanisms of how oxidative stress affects extracellular microRNA profiles in liver injury. In liver injury, the levels of miR-122-5p, miR-192-5p, miR-223-3p, and miR-1224-5p were reported to be elevated in the sera. The release of miR-122-5p, miR-192-5p, and miR-1224-5p from hepatocytes may be attributed to oxidative stress. miR-223-3p could be released from neutrophils and suppress oxidative stress in the liver. Elucidation of the mechanisms of the interaction between extracellular microRNAs and oxidative stress would improve our pathophysiological understanding as well as future medical practice.
Keywords: microRNA, extracellular vesicle, liquid biopsy, liver injury
Introduction
In multicellular organisms, cells can exchange information using single molecules such as hormones and cytokines. In addition, recent evidence has revealed that extracellular vesicles (EVs; also known as exosomes and microvesicles) can transport various molecules, such as nucleic acids, proteins, and lipids, between cells and play pathophysiological roles.(1,2) MicroRNAs (miRNAs) are the most investigated EV components thus far.(3–5) miRNAs are endogenous, short regulatory RNA molecules of 19–25 nucleotides in length. They modulate target gene expression at the post-translational level by guiding the RNA-induced silencing complex to miRNA target sites in the 3' untranslated region of mRNAs, leading to mRNA degradation or the inhibition of translation.(6) Currently, 2,588 mature human miRNAs are listed in the miRNA registry (miRBase release 21(7)). Among them, 300–500 miRNAs can be detected as stable extracellular miRNAs in circulation. In addition to those encapsulated within EVs, some extracellular miRNAs are bound to either RNA-binding proteins(8) or high-density lipoproteins.(9) As changes in extracellular miRNA profiles are associated with various disease conditions, they are attractive candidates for minimally invasive biomarkers.(10) Furthermore, studies on extracellular miRNA have revealed functional cell-to-cell miRNA transfer.(11,12)
Oxidative stress resulting from the increased production or inadequate removal of reactive oxygen species (ROS) plays a key role in the pathogenesis of aging, atherosclerosis, Alzheimer’s disease, cancer, etc. As both extracellular miRNAs and oxidative stress are essential in homeostasis and disease development, knowledge about the interaction between them could provide new physiological insights. Here, we systematically reviewed the literature to clarify the current progress in the field and highlight some notable studies on digestive diseases, which can enhance our understanding of the field and point out future directions for investigation.
Methods
We searched MEDLINE for studies published until 21 December 2017 using a search strategy (("oxidative stress"[MeSH Terms] OR ("oxidative"[All Fields] AND "stress"[All Fields]) OR "oxidative stress"[All Fields]) AND ("micrornas"[MeSH Terms] OR "micrornas"[All Fields] OR "microrna"[All Fields])) AND (circulating[All Fields] OR ("serum"[MeSH Terms] OR "serum"[All Fields]) OR ("plasma"[MeSH Terms] OR "plasma"[All Fields])) OR ("exosomes"[MeSH Terms] OR "exosomes"[All Fields] OR "exosome"[All Fields]) OR extracellular[All Fields] OR salivary[All Fields] OR ("urinary tract"[MeSH Terms] OR ("urinary"[All Fields] AND "tract"[All Fields]) OR "urinary tract"[All Fields] OR "urinary"[All Fields]) OR juice[All Fields] OR fluid[All Fields] OR ("ascites"[MeSH Terms] OR "ascites"[All Fields]) OR effusion[All Fields] OR ("feces"[MeSH Terms] OR "feces"[All Fields] OR "fecal"[All Fields])) NOT ("review"[Publication Type] OR "review literature as topic"[MeSH Terms] OR "review"[All Fields]).
We included English-written original articles that described the association between circulating miRNAs and oxidative stress in their assessment. The exclusion criteria employed are as follows: papers that were not written in English, review articles, papers that only concerned intracellular miRNAs, papers that mentioned the association between circulating miRNAs and oxidative stress only based on in silico prediction, and papers that did not focus on human health or diseases.
Results
Result of literature search
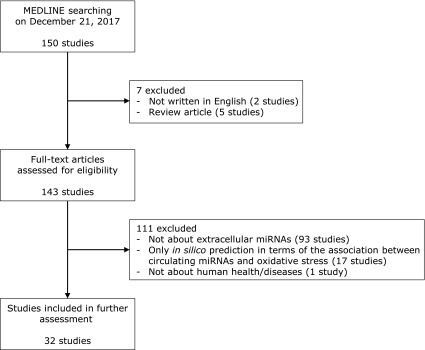
We obtained 150 published studies from the initial search, 32 of which were identified as focusing on the association between extracellular miRNAs and oxidative stress (Fig. 1). The organs/diseases in focus and reported miRNAs in these 32 studies are listed in Table 1.(13–44) These were published after 2013, and the number of studies increased every year. More than half of the identified studies were focused on cardiovascular diseases and/or the central nervous system.
Fig. 1.
Workflow of the systematic review.
Table 1.
Oxidative-stress associated extracellular miRNAs
| Focused organs/diseases | miRNAs | References |
|---|---|---|
| Central nervous system/Stroke | miR-4639-3p, miR-1-3p, miR-17-5p, miR-20a-5p, miR-20b-5p, miR-99a-5p, miR-106b-5p, miR-126-3p, miR-129-5p, miR-181c-5p, miR-300-3p, miR-369-3p, miR-410-3p, miR-451a, miR-499a-5p, miR-219a-5p | (Pusic, 2014; Pusic, 2015; Tao, 2015; Ma, 2016; Pusic, 2016; Chen, 2017a; Chen, 2017b; Li, 2017b; Phillips, 2017) |
| Cardiovascular diseases | miR-92a-3p, miR-126-3p, miR-129-5p, miR-155-5p, miR-210-3p, miR-21-5p, miR-29a-3p, miR-17-3p | (Wang, 2013; Curti, 2014; Chen, 2015; da Silva, 2015; Wang, 2015; Yamaguchi, 2015; DuPont, 2016; Wu, 2016; Xiao, 2016; Liu, 2017; Ramachandran, 2017) |
| Liver | miR-122-5p, miR-192-5p, miR-223-3p, miR-1224-5p | (Roy, 2016; Li, 2017a; Mosedale, 2017; Roy, 2017) |
| Kidney | miR-15a-5p, miR-92a-3p | (Kamalden, 2017; Shang, 2017) |
| Peripheral blood, bone marrow | miR-96-5p, miR-182-5p, miR-183-5p, miR-17-3p | (Curti, 2014; Davis, 2017) |
| Sepsis | miR-1-3p, miR-25-3p | (Yao, 2015; Xu, 2017) |
| Diabetes | miR-126-3p | (Wu, 2016) |
| Lung | miR-320a, miR-221-3p | (Lee, 2016) |
| Hyperlipidemia | miR-33a-5p, miR-33b-5p, miR-200c-3p | (D'Agostino, 2017) |
| Skin/Vitiligo | miR-25-3p | (Shi, 2016) |
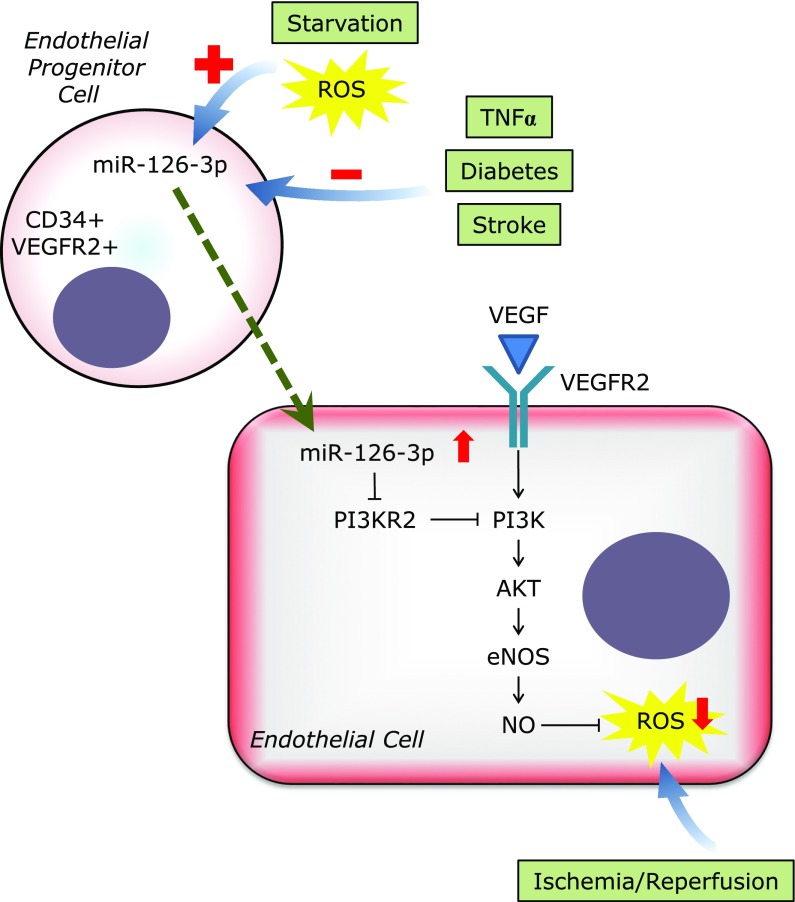
Among these 32 studies, the most frequently documented miRNA was miR-126-3p. As miR-126-3p is located within intron 7 of the EGF-like domain 7 (Egfl7), which is an endothelial-cell-derived secreted peptide, miR-126-3p is specifically expressed in endothelial cells.(45,46) In endothelial cells, miR-126-3p promotes angiogenesis by inhibiting endogenous VEGF repressors (SPRED1 and PIK3R2) (Fig. 2).(47) In addition, miR-126-3p in cardiomyocytes has the function of suppressing cardiac inflammation, macrophage infiltration, oxidative stress, and fibrosis according to the data from endothelial cell-specific conditional miR-126 knockout mice.(17) Circulating miR-126-3p is known to be suppressed in patients or animal models of cerebral ischemic stroke, diabetes, or peripheral arterial disease.(17,24,27) Furthermore, oxidative stress might play a role in releasing circulating miR-126-3p as oral treatment with the antioxidant N-acetylcysteine (NAC) was found to prevent the maximal exercise-induced increase of circulating miR-126-3p in patients with intermittent claudication.(24) Wang et al.(23) reported that miR-126-3p contained in endothelial progenitor cell-derived microvesicles (EPC-MVs) can suppress oxidative stress and promote angiogenesis of endothelial cells via the PI3K/eNOS/NO pathway. They also showed that miR-126-3p was increased in MVs released from EPCs cultured in a serum deprived medium (starvation stress), whereas it was decreased in MVs released from EPCs cultured in a serum-deprived medium containing tumor necrosis factor-α (TNFα) (apoptotic stress). This example clearly showed that oxidative stress can be a regulator of extracellular miRNAs, and can be regulated by extracellular miRNAs.
Fig. 2.
Function of endothelial progenitor cell-derived miR-126-3p in endothelial cells. ROS, reactive oxygen species.
Identified studies about liver injury
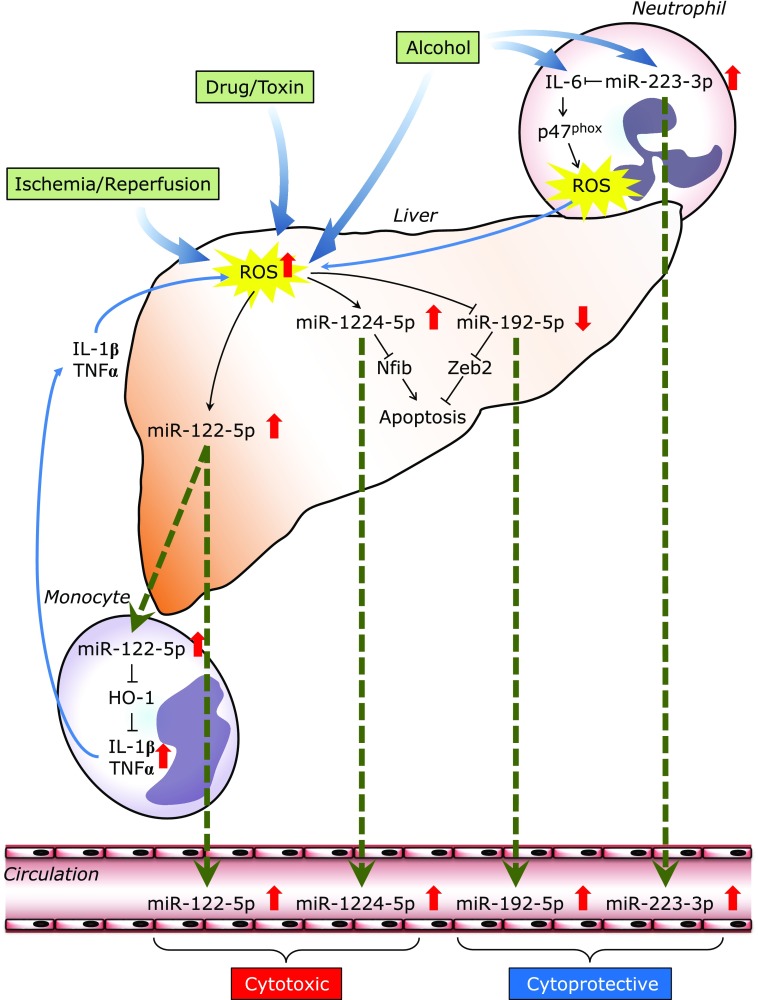
Regarding the digestive system, 4 studies were focused on liver injury as indicated in Table 1. Here, we introduced miRNAs that are associated with oxidative stress and released from cells during liver injury (Fig. 3). Nevertheless, the findings could be applied to all pathophysiological events in which ROS-mediated extracellular miRNAs are involved.
Fig. 3.
Regulatory mechanisms of circulating/extracellular miRNAs in liver injury. ROS, reactive oxygen species.
miR-122-5p is one of the most investigated miRNAs; it is abundantly expressed in hepatocytes but is absent or expressed at very low levels in other cell types.(48) Mice lacking the gene encoding miR-122-5p are viable but develop temporally controlled steatohepatitis, fibrosis, and hepatocellular carcinoma.(49) In healthy individuals, the number of exosomes containing miRNA-122-5p significantly increases in the serum after alcohol binge drinking.(50) Exosomes derived from ethanol-treated human hepatoma cells (Huh7.5 cells) are taken up by monocytes/macrophages and Kupffer cells and horizontally transfer miR-122-5p. In monocytes, exosome-transferred miR-122-5p inhibits the HO-1 pathway and increases the levels of pro-inflammatory cytokines such as IL-1β and TNFα. Mosedale et al.(44) reported that early increases in exosomal miR-122-5p tend to be associated with mitochondrial-induced apoptosis and oxidative stress during idiosyncratic drug-induced liver injury. Taken together, alcohol- or drug-induced cellular stress would promote the release of miR-122-5p-containing exosomes in hepatocytes in the absence of overt necrosis. Circulating miR-122-5p could be an early biomarker for damaged hepatocytes.
Although miR-1224-5p is not a liver-specific miRNA, Roy et al.(35) discovered that it is also up-regulated in the serum of patients with acute liver failure. Intracellular miR-1224-5p was found to be up-regulated in hepatocytes following in vivo and in vitro ischemia-reperfusion or H2O2 stimulation. They also demonstrated that miR-1224-5p could suppress the anti-apoptotic gene Nfib, leading to impaired proliferation and elevated apoptosis. More importantly, increased serum levels of miR-1224-5p were found to be associated with survival in acute liver failure (area under a receiver operating characteristic curve, 0.92).
The same researchers also reported that serum miR-192-5p levels are selectively elevated in patients with liver injury and closely correlated with serum miR-122-5p levels.(34) Supernatant levels of miR-192-5p were also found to be increased in a hypoxia/reoxygenation model of in vitro hepatocyte injury. However, in contrast to the up-regulation of miR-122-5p and miR-1224-5p in hepatocytes, miR-192-5p was reported to be down-regulated in injured livers in vivo and during H2O2 stimulation in vitro. Functional experiments confirmed the protective effect of miR-192-5p down-regulation in hepatocytes through the increase of a target gene (Zeb2), an important suppressor of apoptosis. Based on these results, the authors suggested that the decrease in intracellular miR-192-5p could be caused by the release of miR-192-5p from hepatocytes during acute liver injury. A limited number of reports also show reciprocal changes in intracellular and extracellular miRNAs, suggesting that some miRNAs might be actively and selectively released from cells in specific conditions.(51,52)
Li et al.(22) found that the serum miR-223-3p levels of alcoholics were elevated compared with those of healthy controls by miRNA microarray analysis, and miR-223-3p could also be a possible biomarker for alcoholic liver injury. However, miR-223-3p was not released from hepatocytes but present at high levels in neutrophils. In mice, the levels of miR-223-3p were found to be increased in both the serum and neutrophils upon ethanol intake. They also showed that miR-223-3p could directly inhibit IL-6 expression and subsequently inhibit p47phox expression in neutrophils. In miR-223-3p-deleted mice, the expression of IL-6 and the phagocytic oxidase p47phox was enhanced in the liver, leading to ROS generation, neutrophil infiltration, and hepatic injury upon ethanol administration. ROS production by neutrophils and ethanol-induced liver injury were suppressed by p47phox deletion. In summary, miR-223-3p in neutrophils could be an important regulator for blocking neutrophil infiltration in alcoholic liver disease.
Discussion
In this systematic review, we identified 23 studies indicating that oxidative stress could affect extracellular miRNA profiles and that some transported miRNAs could play cytotoxic or cytoprotective roles in recipient cells. Although a number of studies addressed the use of extracellular miRNAs as biomarkers for various diseases, the regulatory mechanisms of extracellular miRNAs remain unclear. Further studies on oxidative stress should be conducted to shed light on this issue.
In the case of digestive diseases, all studies on the association between oxidative stress and extracellular miRNAs were focused on liver injury. In acute liver injury and hepatitis, circulating miRNAs regulated by intrahepatic oxidative stress seem to be powerful assessment tools for determining the extent of liver damage. The most important requirement for the use of a biomarker of acute diseases, such as acute liver injury, acute pancreatitis, and acute myocardial infarction, is to facilitate rapid measurement. Since it takes 1 to 3 days to obtain results by conventional quantitative RT-PCR or microarray, novel methods are required to use circulating miRNAs for the evaluation of acute diseases.
In digestive carcinogenesis, exposure to oxidative stress plays crucial roles.(53) In addition, hundreds of previous reports have shown that circulating miRNA signatures are dramatically altered through the carcinogenic process.(10,54) Nevertheless, none of the studies demonstrate the importance of the association between oxidative stress and extracellular miRNAs in cancer development or progression. As the aberrant miRNA profiles in cancer tissues and their precursor lesions such as Barrett’s esophagus, gastric intestinal metaplasia, or the inflamed colonic mucosa in ulcerative colitis are well-known,(55–57) extracellular miRNAs released from malignant and pre-malignant lesions should be studied in detail with a focus on oxidative stress to promote cancer prevention and early detection.
In conclusion, although there are some well-conducted studies, knowledge of the association between oxidative stress and extracellular miRNAs is rapidly increasing but still limited. Further studies in this area would uncover unique cell-cell interactions and lead to changes in future clinical practice.
Acknowledgments
This study was supported by the “Development of Diagnostic Technology for Detection of miRNA in Body Fluids” grant from the Japan Agency for Medical Research and Development and New Energy and Industrial Technology Development Organization (to TO), and a Grant-in-Aid for Scientific Research C (17K09471, to JM).
Conflict of Interest
TO received research grant from Kyowa Medex, Kewpie Corporation, Takeda, Rohto Pharmaceutical Co., Ltd., Japan Atherosclerosis Research Foundation, Inter Stem, and BioMimetics Sympathies. JM has no conflict of interest.
References
- 1.Kosaka N, Yoshioka Y, Fujita Y, Ochiya T. Versatile roles of extracellular vesicles in cancer. J Clin Invest. 2016;126:1163–1172. doi: 10.1172/JCI81130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Naito Y, Yoshioka Y, Yamamoto Y, Ochiya T. How cancer cells dictate their microenvironment: present roles of extracellular vesicles. Cell Mol Life Sci. 2017;74:697–713. doi: 10.1007/s00018-016-2346-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosaka N, Iguchi H, Yoshioka Y, Takeshita F, Matsuki Y, Ochiya T. Secretory mechanisms and intercellular transfer of microRNAs in living cells. J Biol Chem. 2010;285:17442–17452. doi: 10.1074/jbc.M110.107821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 5.Takahashi RU, Prieto-Vila M, Hironaka A, Ochiya T. The role of extracellular vesicle microRNAs in cancer biology. Clin Chem Lab Med. 2017;55:648–656. doi: 10.1515/cclm-2016-0708. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 7.Kozomara A, Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42 (Database Issue):D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arroyo JD, Chevillet JR, Kroh EM, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc Natl Acad Sci U S A. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vickers KC, Palmisano BT, Shoucri BM, Shamburek RD, Remaley AT. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat Cell Biol. 2011;13:423–433. doi: 10.1038/ncb2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuzaki J, Ochiya T. Circulating microRNAs and extracellular vesicles as potential cancer biomarkers: a systematic review. Int J Clin Oncol. 2017;22:413–420. doi: 10.1007/s10147-017-1104-3. [DOI] [PubMed] [Google Scholar]
- 11.Turchinovich A, Samatov TR, Tonevitsky AG, Burwinkel B. Circulating miRNAs: cell-cell communication function? Front Genet. 2013;4:119. doi: 10.3389/fgene.2013.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawamura Y, Yamamoto Y, Sato TA, Ochiya T. Extracellular vesicles as trans-genomic agents: emerging roles in disease and evolution. Cancer Sci. 2017;108:824–830. doi: 10.1111/cas.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li P, Shen M, Gao F, et al. An antagomir to microRNA-106b-5p ameliorates cerebral ischemia and reperfusion injury in rats via inhibiting apoptosis and oxidative stress. Mol Neurobiol. 2017;54:2901–2921. doi: 10.1007/s12035-016-9842-1. [DOI] [PubMed] [Google Scholar]
- 14.Pusic KM, Pusic AD, Kraig RP. Environmental enrichment stimulates immune cell secretion of exosomes that promote CNS myelination and may regulate inflammation. Cell Mol Neurobiol. 2016;36:313–325. doi: 10.1007/s10571-015-0269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ma Q, Zhao H, Tao Z, et al. MicroRNA-181c exacerbates brain injury in acute ischemic stroke. Aging Dis. 2016;7:705–714. doi: 10.14336/AD.2016.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Y, Gao C, Sun Q, et al. MicroRNA-4639 is a regulator of DJ-1 expression and a potential early diagnostic marker for parkinson’s disease. Front Aging Neurosci. 2017;9:232. doi: 10.3389/fnagi.2017.00232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen J, Cui C, Yang X, et al. MiR-126 affects brain-heart interaction after cerebral ischemic stroke. Transl Stroke Res. 2017;8:374–385. doi: 10.1007/s12975-017-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tao Z, Zhao H, Wang R, et al. Neuroprotective effect of microRNA-99a against focal cerebral ischemia-reperfusion injury in mice. J Neurol Sci. 2015;355:113–119. doi: 10.1016/j.jns.2015.05.036. [DOI] [PubMed] [Google Scholar]
- 19.Pusic AD, Kraig RP. Phasic treatment with interferon gamma stimulates release of exosomes that protect against spreading depression. J Interferon Cytokine Res. 2015;35:795–807. doi: 10.1089/jir.2015.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pusic AD, Pusic KM, Clayton BL, Kraig RP. IFNγ-stimulated dendritic cell exosomes as a potential therapeutic for remyelination. J Neuroimmunol. 2014;266:12–23. doi: 10.1016/j.jneuroim.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Phillips TJ, Scott H, Menassa DA, et al. Treating the placenta to prevent adverse effects of gestational hypoxia on fetal brain development. Sci Rep. 2017;7:9079. doi: 10.1038/s41598-017-06300-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li M, He Y, Zhou Z, et al. MicroRNA-223 ameliorates alcoholic liver injury by inhibiting the IL-6-p47phox-oxidative stress pathway in neutrophils. Gut. 2017;66:705–715. doi: 10.1136/gutjnl-2016-311861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Chen S, Ma X, et al. Effects of endothelial progenitor cell-derived microvesicles on hypoxia/reoxygenation-induced endothelial dysfunction and apoptosis. Oxid Med Cell Longev. 2013;2013:572729. doi: 10.1155/2013/572729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.da Silva ND Jr, Roseguini BT, Chehuen M, et al. Effects of oral N-acetylcysteine on walking capacity, leg reactive hyperemia, and inflammatory and angiogenic mediators in patients with intermittent claudication. Am J Physiol Heart Circ Physiol. 2015;309:H897–H905. doi: 10.1152/ajpheart.00158.2015. [DOI] [PubMed] [Google Scholar]
- 25.Chen Z, Wen L, Martin M, et al. Oxidative stress activates endothelial innate immunity via sterol regulatory element binding protein 2 (SREBP2) transactivation of microRNA-92a. Circulation. 2015;131:805–814. doi: 10.1161/CIRCULATIONAHA.114.013675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramachandran S, Lowenthal A, Ritner C, Lowenthal S, Bernstein HS. Plasma microvesicle analysis identifies microRNA 129-5p as a biomarker of heart failure in univentricular heart disease. PLoS One. 2017;12:e0183624. doi: 10.1371/journal.pone.0183624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu K, Yang Y, Zhong Y, et al. The effects of microvesicles on endothelial progenitor cells are compromised in type 2 diabetic patients via downregulation of the miR-126/VEGFR2 pathway. Am J Physiol Endocrinol Metab. 2016;310:E828–E837. doi: 10.1152/ajpendo.00056.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DuPont JJ, McCurley A, Davel AP, et al. Vascular mineralocorticoid receptor regulates microRNA-155 to promote vasoconstriction and rising blood pressure with aging. JCI Insight. 2016;1:e88942. doi: 10.1172/jci.insight.88942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu H, Wang J, Chen Y, et al. NPC-EXs alleviate endothelial oxidative stress and dysfunction through the miR-210 downstream Nox2 and VEGFR2 pathways. Oxid Med Cell Longev. 2017;2017:9397631. doi: 10.1155/2017/9397631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiao J, Pan Y, Li XH, et al. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 2016;7:e2277. doi: 10.1038/cddis.2016.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Y, Zhang L, Li Y, et al. Exosomes/microvesicles from induced pluripotent stem cells deliver cardioprotective miRNAs and prevent cardiomyocyte apoptosis in the ischemic myocardium. Int J Cardiol. 2015;192:61–69. doi: 10.1016/j.ijcard.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yamaguchi T, Izumi Y, Nakamura Y, et al. Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. Int J Cardiol. 2015;178:239–246. doi: 10.1016/j.ijcard.2014.10.144. [DOI] [PubMed] [Google Scholar]
- 33.Curti V, Capelli E, Boschi F, et al. Modulation of human miR-17-3p expression by methyl 3-O-methyl gallate as explanation of its in vivo protective activities. Mol Nutr Food Res. 2014;58:1776–1784. doi: 10.1002/mnfr.201400007. [DOI] [PubMed] [Google Scholar]
- 34.Roy S, Benz F, Alder J, et al. Down-regulation of miR-192-5p protects from oxidative stress-induced acute liver injury. Clin Sci (Lond). 2016;130:1197–1207. doi: 10.1042/CS20160216. [DOI] [PubMed] [Google Scholar]
- 35.Roy S, Bantel H, Wandrer F, et al. miR-1224 inhibits cell proliferation in acute liver failure by targeting the antiapoptotic gene Nfib. J Hepatol. 2017;67:966–978. doi: 10.1016/j.jhep.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 36.Kamalden TA, Macgregor-Das AM, Kannan SM, et al. Exosomal microRNA-15a transfer from the pancreas augments diabetic complications by inducing oxidative stress. Antioxid Redox Signal. 2017;27:913–930. doi: 10.1089/ars.2016.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shang F, Wang SC, Hsu CY, et al. MicroRNA-92a mediates endothelial dysfunction in CKD. J Am Soc Nephrol. 2017;28:3251–3261. doi: 10.1681/ASN.2016111215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yao L, Liu Z, Zhu J, Li B, Chai C, Tian Y. Clinical evaluation of circulating microRNA-25 level change in sepsis and its potential relationship with oxidative stress. Int J Clin Exp Pathol. 2015;8:7675–7684. [PMC free article] [PubMed] [Google Scholar]
- 39.Xu L, Zhang W, Sun R, et al. IGF-1 may predict the severity and outcome of patients with sepsis and be associated with microRNA-1 level changes. Exp Ther Med. 2017;14:797–804. doi: 10.3892/etm.2017.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D'Agostino M, Martino F, Sileno S, et al. Circulating miR-200c is up-regulated in paediatric patients with familial hypercholesterolaemia and correlates with miR-33a/b levels: implication of a ZEB1-dependent mechanism. Clin Sci (Lond). 2017;131:2397–2408. doi: 10.1042/CS20171121. [DOI] [PubMed] [Google Scholar]
- 41.Shi Q, Zhang W, Guo S, et al. Oxidative stress-induced overexpression of miR-25: the mechanism underlying the degeneration of melanocytes in vitiligo. Cell Death Differ. 2016;23:496–508. doi: 10.1038/cdd.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee H, Zhang D, Zhu Z, Dela Cruz CS, Jin Y. Epithelial cell-derived microvesicles activate macrophages and promote inflammation via microvesicle-containing microRNAs. Sci Rep. 2016;6:35250. doi: 10.1038/srep35250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Davis C, Dukes A, Drewry M, et al. MicroRNA-183-5p Increases with age in bone-derived extracellular vesicles, suppresses bone marrow stromal (stem) cell proliferation, and induces stem cell senescence. Tissue Eng Part A. 2017;23:1231–1240. doi: 10.1089/ten.tea.2016.0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mosedale M, Eaddy JS, Trask OJ Jr, et al. miR-122 release in exosomes precedes overt tolvaptan-induced necrosis in a primary human hepatocyte micropatterned coculture model. Toxicol Sci. 2018;161:149–158. doi: 10.1093/toxsci/kfx206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Parker LH, Schmidt M, Jin SW, et al. The endothelial-cell-derived secreted factor Egfl7 regulates vascular tube formation. Nature. 2004;428:754–758. doi: 10.1038/nature02416. [DOI] [PubMed] [Google Scholar]
- 46.Wang S, Aurora AB, Johnson BA, et al. The endothelial-specific microRNA miR-126 governs vascular integrity and angiogenesis. Dev Cell. 2008;15:261–271. doi: 10.1016/j.devcel.2008.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fish JE, Santoro MM, Morton SU, et al. miR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. doi: 10.1016/j.devcel.2008.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschi T. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 49.Tsai WC, Hsu SD, Hsu CS, et al. MicroRNA-122 plays a critical role in liver homeostasis and hepatocarcinogenesis. J Clin Invest. 2012;122:2884–2897. doi: 10.1172/JCI63455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Momen-Heravi F, Bala S, Kodys K, Szabo G. Exosomes derived from alcohol-treated hepatocytes horizontally transfer liver specific miRNA-122 and sensitize monocytes to LPS. Sci Rep. 2015;5:9991. doi: 10.1038/srep09991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic Acids Res. 2010;38:7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boon RA, Vickers KC. Intercellular transport of microRNAs. Arterioscler Thromb Vasc Biol. 2013;33:186–192. doi: 10.1161/ATVBAHA.112.300139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cardin R, Piciocchi M, Bortolami M, et al. Oxidative damage in the progression of chronic liver disease to hepatocellular carcinoma: an intricate pathway. World J Gastroenterol. 2014;20:3078–3086. doi: 10.3748/wjg.v20.i12.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baffy G. MicroRNAs in nonalcoholic fatty liver disease. J Clin Med. 2015;4:1977–1988. doi: 10.3390/jcm4121953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Catănă CS, Berindan Neagoe I, Cozma V, et al. Contribution of the IL-17/IL-23 axis to the pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2015;21:5823–5830. doi: 10.3748/wjg.v21.i19.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matsuzaki J, Suzuki H, Tsugawa H, et al. Bile acids increase levels of microRNAs 221 and 222, leading to degradation of CDX2 during esophageal carcinogenesis. Gastroenterology. 2013;145:1300–1311. doi: 10.1053/j.gastro.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 57.Matsuzaki J, Suzuki H. MicroRNAs in Barrett’s esophagus: future prospects. Front Genet. 2014;5:69. doi: 10.3389/fgene.2014.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]