Abstract
To promote symptom relief from acid-related diseases, a medicine with a rapid-onset effect is ideal. The aim of this study was to investigate the early inhibitory effect on gastric acid secretion after a single oral administration of vonoprazan, which represents a new class of proton pump inhibitors, and to compare this effect with those of lansoprazole and famotidine. Ten Helicobacter pylori (HP)-negative male subjects participated in this randomized, three-way crossover study. A single oral administration of vonoprazan (20 mg), lansoprazole (30 mg) or famotidine (20 mg) was performed, and the intragastric pH was continuously monitored for 6 h. Each drug was administered at least seven days apart. The average intragastric pH during the 6-h period after the administration of famotidine was higher than that after the administration of lansoprazole (median: 4.45 vs 2.65; p = 0.0284). A similar result was observed for vonoprazan and lansoprazole (median: 4.30 vs 2.65; p = 0.0322). In conclusions, oral administration of vonoprazan and famotidine in HP-negative healthy male subjects caused the intragastric pH to rise more quickly than did lansoprazole. (Trial Registration: UMIN000020989)
Keywords: intragastric acidity, vonoprazan, lansoprazole, famotidine
Introduction
Recently, the number of Japanese patients with gastro-oesophageal reflux disease (GERD) has increased due to changes in eating habits and a decrease in Helicobacter pylori (HP) infections. In Japan, heartburn is a pervasive problem in Japan that interferes with daily life. Therefore, alleviating symptoms must be the primary objective in the treatment of symptomatic GERD patients.(1) Proton pump inhibitors (PPIs) are widely used worldwide as a treatment for acid-related diseases (such as GERD and peptic ulcer disease) and a component of eradication therapy for HP.(2–5) However, acid-suppressive therapy with PPIs has several limitations. Most GERD patients can be managed with standard PPI regimens; approximately 10–40% of patients have refractory symptoms.(6) For patients with acid-related diseases, rapid onset of a drug that leads to the alleviation of symptoms is important.
Potassium-competitive acid blockers (P-CABs) are a new class of gastric acid suppressants. Similarly to PPIs, P-CABs inhibit gastric hydrogen/potassium adenosine triphosphatase (H+/K+-ATPase); however, dissimilarly to PPIs, P-CABs inhibit K+ competitively and reversibly.(7) Vonoprazan is a new orally active P-CAB that was discovered and synthesized by Takeda Pharmaceutical Co., Ltd., Japan. Vonoprazan accumulates in the gastric tissue and remains for a long period; consequently, it has a long-lasting and potent anti-secretory effect on H+/K+-ATPase.(8)
To date, there have been few studies directly comparing the effects of vonoprazan and PPIs. Sakurai et al.(9) reported that the acid-inhibitory effect (pH 4 holding time ratio) of vonoprazan is significantly greater than that of PPIs. Our previous study demonstrated that H2-receptor antagonists increase the intragastric pH faster than PPIs during the early post-administration phase.(10–12) No study has directly compared the intragastric pH of vonoprazan (a new class of PPIs), lansoprazole (a conventional PPI) and famotidine (an H2-receptor antagonist; H2RA). Thus, we designed a three-way crossover study to compare the early effects of vonoprazan, lansoprazole and famotidine by using pH monitoring.
Materials and Methods
Subjects
We performed a randomized, three-way crossover study. It was conducted in 10 healthy male volunteers, aged between 20 and 37 years (mean age of 26.9 years) who did not take gastric acid secretion inhibitors such as H2Ras and/or PPIs. Prior to the study, we tested anti-HP immunoglobulin G (IgG) antibodies (SRL, Tokyo, Japan), and all subjects were negative.
Study protocol and pH measurement
In this three-way crossover study, all subjects received a single oral dosage of vonoprazan (20 mg) (Takecab®, Takeda Pharmaceutical Co., Ltd.), a single oral dosage of lansoprazole (30 mg) (Takepron®, Takeda Pharmaceutical Co., Ltd.) and a single oral dosage of famotidine (20 mg) (Gaster®, Astellas Pharmaceutical Co., Ltd., Japan) in a crossover manner with a random sequence. Each of these drugs was administered for more than seven days. The subjects were requested to fast on the night before treatment (at least 8 h) and for a period of 6 h after drug administration. All medicines were given in the morning.
Under local anaesthesia, the subjects were nasally inserted with the pH electrode. The electrode tip was placed in the stomach. The intragastric pH was measured at 10-s intervals by a portable pH meter equipped with an antimony pH electrode (Chemical Instrument Co. Ltd.). Prior to each study, the pH electrode was calibrated by using standard buffer solutions of pH 4.01 and 6.86. The obtained pH data were analysed using computer software (Chemical Instrument Co., Ltd.). We measured the average pH, the average pH per hour, and the pH holding time at 6 h after each drug administration.
Statistical analysis
We used Wilcoxon’s signed-rank test for the data. The significance level was set at a p value of <0.05. All statistical analyses used the StatView program (SAS Institute, Cary, NC).
Ethics
This study was enforced according to the Declaration of Helsinki. The Yokohama City University Medical School Ethics Committee approved the study protocol and received written informed consent from all participants. This study is registered to the University Hospital Medical Information Network (UMIN) clinical trials registry (UMIN000020989). All authors had access to the clinical data and approved the final version of the manuscript.
Results
Adverse events
All 10 volunteers completed the study protocol, and adverse events were not reported.
Average pH
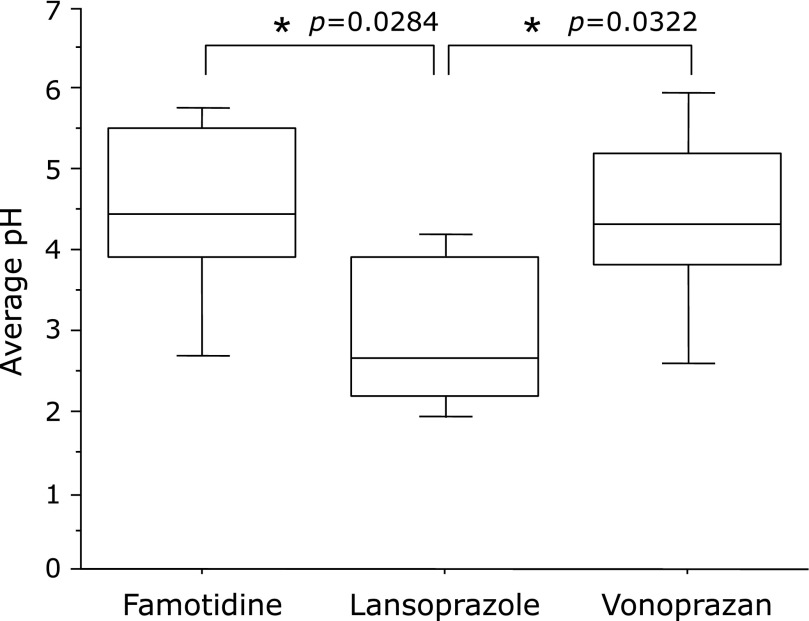
The average pH during the 6-h period after administration was significantly higher with famotidine than with lansoprazole (median: 4.45 vs 2.65; p = 0.0284). Similar results were found for vonoprazan and lansoprazole (median: 4.30 vs 2.65; p = 0.0322). No significant differences were found between famotidine and vonoprazan (median: 4.45 vs 4.30; p = 0.7581) (Fig. 1).
Fig. 1.
The average pH during the first 6 h was higher after the administration of famotidine and vonoprazan than after lansoprazole. *p<0.05 according to the Wilcoxon signed-ranks test.
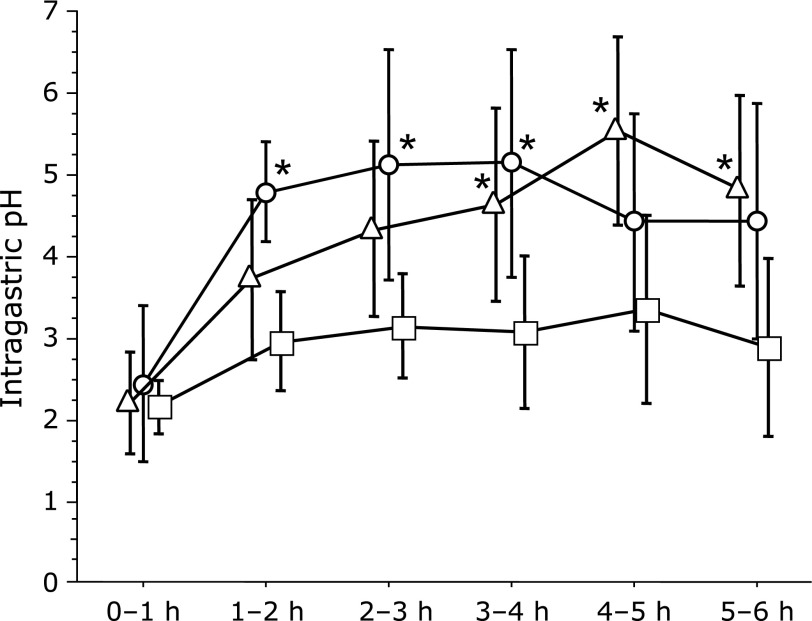
The average pH was significantly higher after the administration of famotidine than that after lansoprazole during the 1–2, 2–3 and 3–4 h study periods (median: 5.10 vs 3.10; p = 0.0069, 5.65 vs 3.25; p = 0.0217, 5.75 vs 2.85; p = 0.0208). No significant differences were observed in the 0–1, 4–5, and 5–6 h study periods. The average pH was significantly higher after the administration of vonoprazan than after lansoprazole during the 3–4, 4–5 and 5–6 h study periods (median: 5.20 vs 2.85; p = 0.0411, 6.00 vs 2.95; p = 0.0165, 5.00 vs 2.55; p = 0.0365). No significant differences were observed in the 0–1, 1–2 and 2–3 h study periods (Fig. 2).
Fig. 2.
Famotidine (20 mg) resulted in a higher average pH than did lansoprazole (30 mg) in the 1–2, 2–3 and 3–4 h study periods after administration. Vonoprazan (20 mg) resulted in a higher average pH than did lansoprazole (30 mg) in the 3–4, 4–5 and 5–6 h study periods after administration. Circles (famotidine), triangles (vonoprazan) and squares (lansoprazole), mean values; vertical lines, SD; horizontal line, ±SD. *p<0.05 according to the Wilcoxon signed-ranks test.
Holding time (%) of the various pH levels over the 6-h monitoring period
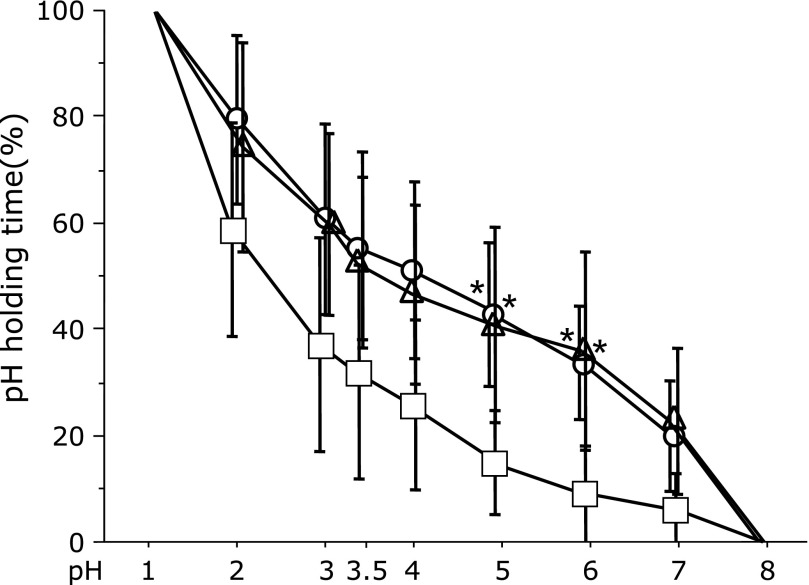
In the 6-h study period, the administration of famotidine caused a longer duration of pH >5 or 6 than did the administration of lansoprazole (median: 39.65% vs 10.6%; p = 0.0125, 35.3% vs 3.25%; p = 0.0125). Similar results were found for vonoprazan and lansoprazole (median: 45.6% vs 10.6%; p = 0.0367, 38.55% vs 3.25%; p = 0.0218) (Fig. 3).
Fig. 3.
During the 6-h study period, famotidine (20 mg) and vonoprazan (20 mg) yielded a longer duration of pH >5 and 6 than did lansoprazole (30 mg). Circles (famotidine), triangles (vonoprazan) and squares (lansoprazole), mean values; vertical lines, SD; horizontal line, ±SD. *p<0.05 according to the Wilcoxon signed-rank test.
Discussion
In this study, we examined the change in the intragastric pH in the early stage after a single oral administration of vonoprazan, lansoprazole or famotidine in HP-negative subjects. Vonoprazan and famotidine had a significantly faster onset of action and caused a stronger suppression of gastric acid secretion than did lansoprazole.
Lansoprazole is a benzimidazole class of anti-secretory agents and the second approved PPI in Japan. Lansoprazole inhibits gastric acid H+/K+-ATPase in the gastric parietal cells forming part of the proton pump that performs acid secretion. Lansoprazole is widely used for treating reflux oesophagitis, peptic ulcer diseases such as gastric and duodenal ulcers, and maintenance treatment of erosive oesophagitis. Most PPIs, including lansoprazole, are metabolized and inactivated by CYP2C19 and CYP3A4 of the liver enzyme cytochrome P450. There are genetic polymorphisms of extensive CYP2C19 metabolizers and poor metabolizers in CYP2C19, and significant differences in plasma drug concentration have been observed. These factors affect the degree of inhibition of acid secretion.(13–17)
By contrast, famotidine is metabolized by the kidney and not the liver.(18) Thus, the inhibition of acid secretion by famotidine is not affected by the CYP2C19 phenotype or genotype status.
Vonoprazan is a P-CAB that represents a new class of gastric-acid-suppressive agents. Vonoprazan was approved for use in HP eradication in addition to peptic ulcer and reflux esophagitis in Japan. Nishizawa et al.(19) reported that with clarithromycin-based triple therapy, Vonoprazan is a better choice of antisecretory agent compared to PPIs, especially in young to middle-aged patients.
Vonoprazan is metabolized and inactivated by CYP3A4 of the liver enzyme cytochrome P450. A phase I study of vonoprazan in healthy male volunteers has revealed that it rapidly and strongly inhibits gastric acid secretion within 24 h after a single dose in the range of 20–120 mg.(20) Furthermore, the pharmacokinetics of vonoprazan is not affected by the CYP2C19 genotype.(20,21)
The poor-metabolizer phenotype of CYP2C19 recognizes the difference in occurrence frequency among races. Poor metabolizers account for only 2% to 6% in Caucasians and 9% to 23% in Japanese individuals.(22–24) We also confirmed genetic polymorphisms in our study; three of the 10 subjects (33.3%) were poor metabolizers. Possibly, the plasma drug concentration of lansoprazole was high in these three subjects, but in this study, there was no significant difference between poor metabolizers and extensive metabolizers regarding intragastric acidity.
Treatment with PPIs is known to be the most effective means of healing peptic ulcers. In addition, PPIs provide more effective and prompt ulcer healing than H2RAs, with respect to the alleviation of symptoms.(25) However, our study demonstrated that H2RA increased the intragastric pH more rapidly than a conventional PPI did. Prior studies have shown that the repeated oral or intravenous administration of PPIs was effective in suppressing acid secretion. In addition, the stable effects of PPIs are achieved after approximately 5 days.(26,27) Another study showed that a single initial dose of a PPI failed to achieve an intragastric pH >4 on the first day of treatment.(28) However, a PPI was more effective than an H2RA in suppressing acid secretion in healthy volunteers, patients with duodenal ulcers and patients with GERD after 5 to 7 days of treatment.(29,30) In an autoradiography study, H2RAs bound to all parietal cells uniformly, whereas PPIs bound only to young activated parietal cells. In the early period of drug treatment, the anti-secretory action of PPIs had a slower onset than that of H2RAs.(31) It appears that the suppression of gastric acid secretion by H2RAs is superior to that by PPIs. However, H2RAs have disadvantages of a relatively short duration of action, the development of tolerance, and an incomplete suppression of acid secretion in response to diet.
Some of GEED patients are reported to be refractory to PPI therapy. Kawai et al.(32) reported combination therapy with Rikkunshito and a PPI improves quality of life in patients with patients with PPI-refractory GERD. Studies for P-CAB, which strongly inhibits acid secretion than PPI, refractory GERD are also necessary in the future.
Patients receiving treatment at a therapeutic dose of conventional PPIs sometimes experience insufficient gastric acid inhibition at night. This phenomenon is generally called nocturnal acid breakthrough (NAB) and is defined as a stomach pH of <4 for over 1 h. Acid reflux at night can worsen GERD symptoms that are improving. Therefore, suppression of persistent acid reflux during the night is considered important for alleviating symptoms.(9) H2RAs are globally used to treat NAB, but it is known that the effects of H2RAs decrease with sequential administration.(33)
Most patients with mild GERD and infrequent symptoms use a PPI only when symptoms appear. On-demand therapy for patients with mild GERD after receiving initial therapy with PPIs improves the quality of daily life and is cost effective.(34,35) The rapid acid suppression effect is important in on-demand therapy aiming at improving heartburn. Although many patients experience heartburn after meals,(36–38) H2RAs are inappropriate for treatments to suppress acid secretion in response to diet because the condition of the stomach after meals slows the effectiveness of H2RAs.(39)
The main cause of GERD symptoms is believed to be acid reflux to the oesophagus. The long-term suppression of gastric acid control is necessary for GERD symptoms. However, the heartburn associated with mild GERD is a temporary symptom, mainly due to short-term gastric acid reflux. Therefore, rapid onset and long-term acid suppression play important roles in eliminating these symptoms.
Our results showed no significant differences between famotidine and vonoprazan. However, vonoprazan is characterized by not requiring activation by acid and is effective even when administered on an empty stomach. Therefore, vonoprazan is considered an agent useful for on-demand therapy and for patients with NAB because it can quickly suppress gastric acid secretion.
Conclusions
Oral administration of vonoprazan and famotidine in HP-negative healthy male subjects caused the intragastric pH to rise more quickly than did lansoprazole. This result shows that vonoprazan may be suitable for initial therapy, on-demand therapy and patients with NAB.
Acknowledgments
Special appreciation is extended to the medical staff of the Gastroenterology and Hepatology Division, Yokohama City University Hospital, Kanagawa, Japan.
Author Contributions
KO analysed and collected the clinical data and wrote the manuscript, with contributions from KF. AN was responsible for designing the study and collecting the clinical data. HI performed the statistical analyses. KO and HI analysed the clinical data and participated in the design and coordination of the study. All authors read and approved the final manuscript.
Funding
The funding source had no involvement in the design, analysis, and writing of the paper or decision to publish this work.
Conflict of Interest
No potential conflicts of interest were disclosed.
References
- 1.Bytzer P. Goals of therapy and guidelines for treatment success in symptomatic gastroesophageal reflux disease patients. Am J Gastroenterol. 2003;98 (Suppl 3):S31–S39. doi: 10.1016/s0002-9270(03)00013-3. [DOI] [PubMed] [Google Scholar]
- 2.Graham DY. Critical effect of Helicobacter pylori infection on the effectiveness of omeprazole for prevention of gastric or duodenal ulcers among chronic NSAID users. Helicobacter. 2002;7:1–8. doi: 10.1046/j.1523-5378.2002.00048.x. [DOI] [PubMed] [Google Scholar]
- 3.Frazzoni M, De Micheli E, Grisendi A, Savarino V. Effective intra-oesophageal acid suppression in patients with gastro-oesophageal reflux disease: lansoprazole vs. pantoprazole. Aliment Pharmacol Ther. 2003;17:235–241. doi: 10.1046/j.1365-2036.2003.01405.x. [DOI] [PubMed] [Google Scholar]
- 4.Robinson M. Proton pump inhibitors: update on their role in acid-related gastrointestinal diseases. Int J Clin Pract. 2005;59:709–715. doi: 10.1111/j.1368-5031.2005.00517.x. [DOI] [PubMed] [Google Scholar]
- 5.Malfertheiner P, Mössner J, Fischbach W, et al. Helicobacter pylori eradication is beneficial in the treatment of functional dyspepsia. Aliment Pharmacol Ther. 2003;18:615–625. doi: 10.1046/j.1365-2036.2003.01695.x. [DOI] [PubMed] [Google Scholar]
- 6.Fass R, Shapiro M, Dekel R, Sewell J. Systematic review: proton-pump inhibitor failure in gastro-oesophageal reflux disease--where next? Aliment Pharmacol Ther. 2005;22:79–94. doi: 10.1111/j.1365-2036.2005.02531.x. [DOI] [PubMed] [Google Scholar]
- 7.Andersson K, Carlsson E. Potassium-competitive acid blockade: a new therapeutic strategy in acid-related diseases. Pharmacol Ther. 2005;108:294–307. doi: 10.1016/j.pharmthera.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 8.Hori Y, Imanishi A, Matsukawa J, et al. 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methylmethanamine monofumarate (TAK-438), a novel and potent potassium-competitive acid blocker for the treatment of acid-related diseases. J Pharmacol Exp Ther. 2010;335:231–238. doi: 10.1124/jpet.110.170274. [DOI] [PubMed] [Google Scholar]
- 9.Sakurai Y, Mori Y, Okamoto H, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects--a randomised open-label cross-over study. Aliment Pharmacol Ther. 2015;42:719–730. doi: 10.1111/apt.13325. [DOI] [PubMed] [Google Scholar]
- 10.Abe Y, Inamori M, Togawa J, et al. The comparative effects of single intravenous doses of omeprazole and famotidine on intragastric pH. J Gastroenterol. 2004;39:21–25. doi: 10.1007/s00535-003-1240-6. [DOI] [PubMed] [Google Scholar]
- 11.Inamori M, Togawa J, Iwasaki T, et al. Early effects of lafutidine or rabeprazole on intragastric acidity: which drug is more suitable for on-demand use? J Gastroenterol. 2005;40:453–458. doi: 10.1007/s00535-005-1569-0. [DOI] [PubMed] [Google Scholar]
- 12.Iida H, Inamori M, Akimoto K, et al. Early effects of intravenous administrations of lansoprazole and famotidine on intragastric pH. Hepatogastroenterology. 2009;56:551–554. [PubMed] [Google Scholar]
- 13.Williams MP, Sercombe J, Hamilton MI, Pounder RE. A placebo-controlled trial to assess the effects of 8 days of dosing with rabeprazole versus omeprazole on 24-h intragastric acidity and plasma gastrin concentrations in young healthy male subjects. Aliment Pharmacol Ther. 1998;12:1079–1089. doi: 10.1046/j.1365-2036.1998.00418.x. [DOI] [PubMed] [Google Scholar]
- 14.Shirai N, Furuta T, Moriyama Y, et al. Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment Pharmacol Ther. 2001;15:1929–1937. doi: 10.1046/j.1365-2036.2001.01108.x. [DOI] [PubMed] [Google Scholar]
- 15.Saitoh T, Fukushima Y, Otsuka H, et al. Effects of rabeprazole, lansoprazole and omeprazole on intragastric pH in CYP2C19 extensive metabolizers. Aliment Pharmacol Ther. 2002;16:1811–1817. doi: 10.1046/j.1365-2036.2002.01348.x. [DOI] [PubMed] [Google Scholar]
- 16.Andersson T. Pharmacokinetics, metabolism and interactions of acid pump inhibitors. Focus on omeprazole, lansoprazole and pantoprazole. Clin Pharmacokinet. 1996;31:9–28. doi: 10.2165/00003088-199631010-00002. [DOI] [PubMed] [Google Scholar]
- 17.Furuta T, Ohashi K, Kosuge K, et al. CYP2C19 genotype status and effect of omeprazole on intragastric pH in humans. Clin Pharmacol Ther. 1999;65:552–561. doi: 10.1016/S0009-9236(99)70075-5. [DOI] [PubMed] [Google Scholar]
- 18.Chremos AN. Pharmacodynamics of famotidine in humans. Am J Med. 1986;81:3–7. doi: 10.1016/0002-9343(86)90593-0. [DOI] [PubMed] [Google Scholar]
- 19.Nishizawa T, Suzuki H, Fujimoto A, et al. Effect of patient age and choice of antisecretory agent on success of eradication therapy for Helicobacter pylori infection. J Clin Biochem Nutr. 2017;60:208–210. doi: 10.3164/jcbn.16-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sakurai Y, Nishimura A, Kennedy G, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (vonoprazan) doses in healthy male Japanese/non-Japanese subjects. Clin Transl Gastroenterol. 2015;6:e94. doi: 10.1038/ctg.2015.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jenkins H, Sakurai Y, Nishimura A, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2015;41:636–648. doi: 10.1111/apt.13121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kubota T, Chiba K, Ishizaki T. Genotyping of S-mephenytoin 4'-hydroxylation in an extended Japanese population. Clin Pharmacol Ther. 1996;60:661–666. doi: 10.1016/S0009-9236(96)90214-3. [DOI] [PubMed] [Google Scholar]
- 23.Ishizaki T, Horai Y. Review article: cytochrome P450 and the metabolism of proton pump inhibitors--emphasis on rabeprazole. Aliment Pharmacol Ther. 1999;13 (Suppl 3):27–36. doi: 10.1046/j.1365-2036.1999.00022.x. [DOI] [PubMed] [Google Scholar]
- 24.Wedlund PJ, Aslanian WS, McAllister CB, Wilkinson GR, Branch RA. Mephenytoin hydroxylation deficiency in Caucasians: frequency of a new oxidative drug metabolism polymorphism. Clin Pharmacol Ther. 1984;36:773–780. doi: 10.1038/clpt.1984.256. [DOI] [PubMed] [Google Scholar]
- 25.Hunt RH, Malfertheiner P, Yeomans ND, Hawkey CJ, Howden CW. Critical issues in the pathophysiology and management of peptic ulcer disease. Eur J Gastroenterol Hepatol. 1995;7:685–699. [PubMed] [Google Scholar]
- 26.Howden CW, Forrest JA, Reid JL. Effects of single and repeated doses of omeprazole on gastric acid and pepsin secretion in man. Gut. 1984;25:707–710. doi: 10.1136/gut.25.7.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jansen JB, Lundborg P, Baak LC, et al. Effect of single and repeated intravenous doses of omeprazole on pentagastrin stimulated gastric acid secretion and pharmacokinetics in man. Gut. 1988;29:75–80. doi: 10.1136/gut.29.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cederberg C, Thomson AB, Mahachai V, et al. Effect of intravenous and oral omeprazole on 24-hour intragastric acidity in duodenal ulcer patients. Gastroenterology. 1992;103:913–918. doi: 10.1016/0016-5085(92)90025-t. [DOI] [PubMed] [Google Scholar]
- 29.Ohara S, Hongo M, Asaki S, et al. The effects of famotidine and omeprazole on 24-hour intragastric pH of normal subjects. Nihon Shokakibyo Gakkai Zasshi. 1988;85:1353–1359. [PubMed] [Google Scholar]
- 30.Ducrotté P, Guillemot F, Elouaer-Blanc L, et al. Comparison of omeprazole and famotidine on esophageal pH in patients with moderate to severe esophagitis: a cross-over study. Am J Gastroenterol. 1994;89:717–721. [PubMed] [Google Scholar]
- 31.Nakamura M, Oda M, Akiba Y, et al. Autoradiographic demonstration of lansoprazole uptake sites in rat antrum and colon. J Clin Gastroenterol. 1995;20 (Suppl 2):S8–S13. doi: 10.1097/00004836-199506002-00004. [DOI] [PubMed] [Google Scholar]
- 32.Kawai T, Hirayama Y, Oguchi A, et al. Effect of rikkunshito on quality of life in patients with gastroesophageal reflux disease refractory to proton pump inhibitor therapy. J Clin Biochem Nutr. 2017;60:143–145. doi: 10.3164/jcbn.16-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Komazawa Y, Adachi K, Mihara T, et al. Tolerance to famotidine and ranitidine treatment after 14 days of administration in healthy subjects without Helicobacter pylori infection. J Gastroenterol Hepatol. 2003;18:678–682. doi: 10.1046/j.1440-1746.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 34.Bardhan KD. Intermittent and on-demand use of proton pump inhibitors in the management of symptomatic gastroesophageal reflux disease. Am J Gastroenterol. 2003;98 (3 Suppl):S40–S48. doi: 10.1016/s0002-9270(03)00014-5. [DOI] [PubMed] [Google Scholar]
- 35.Ofman JJ. The economic and quality-of-life impact of symptomatic gastroesophageal reflux disease. Am J Gastroenterol. 2003;98 (3 Suppl):S8–S14. doi: 10.1016/s0002-9270(03)00010-8. [DOI] [PubMed] [Google Scholar]
- 36.Allen ML, Mellow MH, Robinson MG, Orr WC. The effect of raw onions on acid reflux and reflux symptoms. Am J Gastroenterol. 1990;85:377–380. [PubMed] [Google Scholar]
- 37.Vantrappen G, Janssens J. Recent studies of the pathophysiology and diagnosis of esophageal symptoms. Scand J Gastroenterol Suppl. 1990;175:34–41. doi: 10.3109/00365529009093125. [DOI] [PubMed] [Google Scholar]
- 38.Lanza FL, Smith V, Page-Castell JA, Castell DO. Effectiveness of foaming antacid in relieving induced heartburn. South Med J. 1986;79:327–330. doi: 10.1097/00007611-198603000-00017. [DOI] [PubMed] [Google Scholar]
- 39.Colin-Jones DG. The role and limitations of H2-receptor antagonists in the treatment of gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 1995;9 (Suppl 1):9–14. doi: 10.1111/j.1365-2036.1995.tb00778.x. [DOI] [PubMed] [Google Scholar]