Abstract
The gastrointestinal tract is exposed to a variety of noxious factors, such as Helicobacter pylori, nonsteroidal anti-inflammatory drugs, gastric acid, ischemia-reperfusion, and mental stresses. Theses stressors generate free radicals within gastrointestinal tissues, causing organ injury and functional disturbance. Although the gastrointestinal tract can withstand such oxidative stresses to some extent by enhancing its antioxidant system via nuclear factor erythroid 2-related factor 2-Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1-mediated pathways, acute or chronic exposure to oxidative stress can cause several gastrointestinal tract disorders, such as inflammation, ulcers, cancers, and various functional disturbances. Recent studies have demonstrated that some natural compounds and drugs can upregulate the nuclear factor erythroid 2-related factor 2-mediated antioxidant system, ameliorating or preventing these disorders. Although these compounds may be useful as chemopreventive agents, sufficient evidence for their clinical efficacy has not yet been provided. In addition, it is important to note that excessive nuclear factor erythroid 2-related factor 2 stimulation can be harmful to human health, especially from the standpoint of tumor biology.
Keywords: nuclear factor erythroid 2-related factor 2, oxidative stress, gastrointestinal tract, Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1, antioxidant systems
Oxidative Stress Causes Cell and Tissue Injury
Oxidative stress involves the exposure of cells and/or tissues to reactive oxygen species (ROS) generated by various intrinsic and extrinsic factors. Exposure to acute or chronic oxidative stress causes cellular damage and impairs the normal physiological functions of various organs, causing a variety of diseases, such as acute organ failure, chronic degenerative diseases, and cancers. However, cells can withstand oxidative stress by activating systems that scavenge free radicals, protecting them from critical damage. The nuclear factor erythroid 2-related factor 2 (NRF2)-Kelch-like erythroid cell-derived protein with CNC homology-associated protein 1 (KEAP1) pathway, originally discovered by Itoh et al.,(1) is an important scavenger system that protects cells against oxidative stress.(2,3) In this review, I first provide some examples of gastrointestinal (GI) disorders caused by oxidative stress. Second, I outline the role of NRF2 in protecting GI organs from oxidative stress-induced diseases. Third, I discuss natural and synthetic chemical agents that enhance NRF2-mediated protection against oxidative GI disorders. Finally, I mention the negative aspects of NRF2 stimulation, especially from the viewpoint of tumor biology.
GI Disorders Caused by Oxidative Stress
Reflux esophagitis
The healthy gastric mucosa is protected from back-diffusion of luminal gastric acid by the impermeability of the apical membrane tight junctions of the gastric epithelial cells (GECs).(4) In contrast, the esophageal mucosa is relatively susceptible to gastric luminal acid.(5) Thus, reflux of gastric luminal acid into the esophageal lumen readily causes esophageal mucosal injury, manifested clinically as reflux esophagitis. It has been reported that H+ not only injures epithelial cells, but also causes free radical generation in their mitochondria,(6–8) exacerbating acid-induced injury and inflammation.(9) This suggests that free radicals generated by luminal H+ contribute to the pathophysiology of reflux esophagitis.
Helicobacter pylori-associated gastroduodenal disease
Although a number of epidemiological studies have shown a strong association between Helicobacter pylori (H. pylori) infection and various gastric diseases(10) the exact mechanisms by which H. pylori infection causes gastric mucosal injury were not well understood until GI investigators focused on its role in oxidative stress.(11,12) H. pylori colonization in GECs causes the accumulation of neutrophils and macrophages within the gastric mucosa, resulting in the generation of superoxide anion (O2−) and nitric oxide (NO).(13–15) These conditions are histopathologically recognized as H. pylori-induced gastritis. Continuous release of free radicals from mucosal neutrophils accumulated within the H. pylori-infected gastric mucosa gradually results in GEC apoptosis, leading to gastric atrophy and intestinal metaplasia. In some cases, long-term infection with H. pylori can cause various neoplasms, such as hyperplastic polyps, adenomas, and carcinomas. In addition to causing the accumulation of neutrophils in the gastric mucosa, H. pylori infection releases CagA protein into the cytoplasm of GECs, resulting in free radical generation in the mitochondria of CagA-infected cells.(16) Free radicals generated by H. pylori infection, together with gastric luminal acid, degrade the tight junction structures of the gastric and duodenal epithelia, resulting in enhanced back-diffusion of luminal acid into GECs. As a result, ulcers develop in the gastroduodenal mucosae.(17,18)
Nonsteroidal anti-inflammatory drug (NSAID)-induced GI ulcers
Recent global trends show increased ingestion of aspirin and/or NSAIDs with the aging of the human population.(10) Aspirin and/or NSAID intake frequently induces ulcers, erosions, and bleeding in the GI tract. It has been reported that aspirin and/or NSAIDs generate free radicals by several mechanisms.(19) NSAIDs enhance neutrophil adhesion to endothelial cells, inducing free radical generation in the endothelial cells.(20–22) In the upper GI tract, in addition to free radical generation, gastric acid is necessary for NSAID-induced ulcer formation,(23) since most NSAID-induced ulcers are mitigated or prevented by potent acid inhibitors, such as proton pump inhibitors (PPI)(24) and potassium-competitive acid blockers.(25) However, in the small intestine, acid inhibitors do not mitigate, but sometimes exacerbate aspirin-induced ulcer development.(26) Aspirin induces small intestinal ulceration by generating free radicals,(27–29) possibly in the mitochondria of small intestinal cells.(30) Dysbiosis induced by potent acid inhibitors may play a role in NSAID-induced injury to the small intestine.(26,31)
Inflammatory bowel disease (IBD)
The exact mechanisms of IBD have not yet been clarified. However, numerous studies have shown that excessive amounts of free radicals are generated in IBD patients by various factors, such as autoimmune abnormalities, changes in microbiota, and recent changes to Western style diets. In ulcerative colitis (UC), neutrophils accumulate within the colonic mucosa and generate microabscesses, which cause mucosal inflammation and ulcers.(32–35) Prolonged inflammation causes continuous exposure to free radicals, thereby increasing the risk of developing colon cancer.(36,37) In Crohn’s disease, intraluminal antigens derived from the diet and/or microbiota activate mucosal macrophages, which produce inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, and IL-8. These cytokines induce infiltration of polymorphonuclear leukocytes and mononuclear cells into the GI tissues, which causes overproduction of free radicals. This results in an imbalance between oxidative stress and the antioxidant systems, thereby causing transmural inflammation, ulcers, and fibrosis in the GI tract.(38–40)
Functional GI disorders
Recent studies have provided evidence that functional GI disorders, such as gastric motility disease(12) and irritable bowel syndrome (IBS),(41) are also associated with oxidative stress. It has been suggested that the disturbance of gastric motility is caused by damage to the intramural smooth muscle cells and by dysfunction of the neuromuscular junction, which is composed of enteric nerves and interstitial cells of Cajal (ICC). A number of previous studies in experimental animals has shown that dysfunction of these components is observed during sepsis, ischemia/reperfusion stress, and diabetes mellitus. Details in the pathogenesis of GI motility disorders have been provided in a previous review.(12) In addition, we have recently shown that daily intake of sulforaphane (SFN)-rich broccoli sprouts improves defecation in human patients with chronic constipation, which may also indicate that NRF2 stimulation by dietary intake of SFN strengthens antioxidative defense systems, thereby preserving ICC-dependent GI motility.(42) It has been reported that mild inflammation is associated with the pathogenesis of IBS, since serum levels of inflammatory cytokines are increased in patients with IBS compared with those in healthy subjects. In addition, serum cytokine levels correlate well with IBS symptom scores, indicating that oxidative stress may play some role in the pathogenesis of IBS.(41)
Mechanisms by which NRF2 Protects the GI Tract against Oxidative Stress
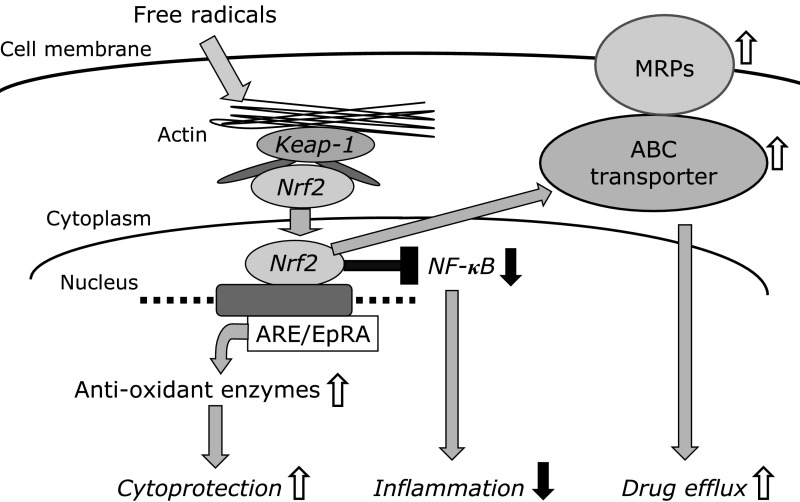
NRF2 protects cells from oxidative stress and subsequent inflammation through several mechanisms. In this chapter, three major and different mechanisms, illustrated in Fig. 1, are discussed.
Fig. 1.
Mechanisms by which NRF2 affords cytoprotection against oxidative stress.
Upregulation of antioxidant and xenobiotic enzymes
Oxidative stress causes severe damage to GECs. However, it also dissociates the inactive form of NRF2 from KEAP1 in the cytoplasm, and induces its translocation into the nucleus. Once in the nucleus, NRF2 binds to antioxidant response elements and upregulates the expression of antioxidant enzymes, thereby strengthening the cell’s ability to neutralize several types of free radicals.(43) NRF2 also contributes to the preservation of the fine structures of tight junctions, and maintains epithelial polarity, which is essential for mucosal protection of the upper GI tract against gastric luminal acid.(44,45) NRF2 also upregulates xenobiotic-metabolizing enzymes such as glutathione-S-transferase and UDP-glucosidase, which are mainly expressed in the small intestine.(46,47)
Amelioration of inflammation by downregulation of nuclear factor κB (NFκB)
It has been shown that NRF2 not only enhances antioxidant enzyme activity, but also upregulates inhibitor of κB (IκB) and downregulates NFκB, thereby inhibiting proinflammatory signaling and mitigating inflammation.(48) Mitigation of the inflammatory response contributes to the protection of the GI mucosa against oxidative injury. An anti-inflammatory role of NRF2 has been reported in experimentally induced uremic rats, in which inhibition of NRF2 function exacerbates intestinal inflammation and disrupts epithelial barrier function.(49)
Stimulation of ATP-binding cassette (ABC) transporters and multidrug resistance-associated protein 2 (MRP2)
Recent studies have demonstrated NRF2-dependent induction of ABC transporters under oxidative stress,(50) indicating that NRF2 contributes to the efflux of various substances by ABC transporter upregulation.(51) With respect to bile acid transporters, NRF2 regulates MRP2 and the bile salt export pump in human hepatocytes, which excrete bile acids into bile.(52) These NRF2-mediated stimulations of normal hepatic or intestinal transport contribute to organ protection from oxidative injury. In contrast to normal healthy cells, however, NRF2-dependent induction of these transporters causes drug resistance in cancer cells, suggesting a negative role of NRF2 in clinical cancer chemotherapy, as discussed below.
Natural and Synthetic Chemical Compounds that Enhance NRF2-Dependent Protection of the GI Tract against Oxidative Stress
There are a number of chemical compounds that can upregulate the NRF2-dependent antioxidant system, protecting cells and tissues from oxidative injury. Some of these substances are natural compounds found in plants and animals, which can be ingested in the diet. Others are drugs previously developed for other functions. All of these compounds can stimulate NRF2 signaling in cells in vitro and/or in experimental animals in vivo. Although some of these compounds may be useful as chemopreventive agents, sufficient evidence of their clinical efficacy has not yet been provided. A summary of the previous basic and clinical reports regarding the efficacy of these compounds on oxidative stress-induced injuries is provided in Table 1.
Table 1.
Representative studies on the effects of various compounds on NRF2-mediated protection of gastrointestinal tract and liver against oxidative stresses
| Basic Study |
Clinical Study |
|||||
|---|---|---|---|---|---|---|
| In vitro | In vivo | Observational study | Intervention study | |||
| Isothiocyanates | Sulforaphane | 28, 29 | 28, 29, 56 | 42 (Constipation) 56 (H. pylori-gastritis) | ||
| Alyl-isothicyanate | 60 | 61 | ||||
| Polyphenols | Curcumin | 64, 65 | 66 (IBS), 67 (UC) | |||
| Catechin | 69 | 69, 70 | 71, 72, 73 | |||
| Quercetin | 76 | 77 | 78, 79 | |||
| Resveratrol | 81, 82 | 83 | 85 (UC) | |||
| Carotenoids | Lycopene | 87 | 88, 89 | |||
| Astaxanthin | 93, 95 | 93 | 98 (FD) | |||
| Drugs | Lansoprazole | 100, 101 | 102 (No effect), 103 | |||
| UDCA | 104 | 105 | 106 (Barret esophagus) | |||
| Sofalcon | 107 | 108 | 109 (Gastric uler) | |||
| Hormones | Ghrelin | 112, 113, 114, 115, 116 | 117 (Diabetic gastroparesis) | |||
| Melatonin | 119 | 120 | 121 (GERD), 122 (IBS) 123 (UC) | |||
Corresponding reference numbers in this paper are indicated in the Table. IBS, irriable bowel syndrome; UC, ulcerative colitis; GERD, gastroesophageal reflux disease; FD, functional dyspepsia.
Natural compounds in food
A variety of natural compounds in plants and animals, such as isothiocyanates, polyphenols, and carotenoids, possess anti-oxidant properties and can thereby mitigate oxidative stress. It has been suggested that daily intake of food containing these compounds ameliorates inflammation, and retards the progression of atherosclerosis, cancer development, diabetes mellitus, degenerative diseases, and aging in humans.(53–55) The mechanisms by which these compounds exhibit anti-oxidant properties have been studied extensively. For example, polyphenols and carotenoids exhibit anti-oxidant activity by functioning as ROS scavengers. Furthermore, recent studies have revealed that some types of isothiocyanates, polyphenols, and carotenoids enhance anti-oxidant activity via nrf2-keap1-mediated mechanisms in response to oxidative stress. Although some studies have shown the clinical efficacy of these compounds,(53–55) sufficient evidence on the clinical efficacy of most of the other compounds has not been well documented. In this chapter, the nrf2-mediated antioxidant effects of natural food components on the GI tract are mainly discussed.
Isothiocyanates
1) Sulforaphane (SFN)
SFN is an isothiocyanate compound generated from glucosinolates, which are rich in cruciferous vegetables such as broccoli, cabbage, and radishes, and especially broccoli sprouts.(46) SFN has been shown to prevent not only a variety of cancers, but also cardiovascular diseases, neurodegenerative diseases, diabetes, and aging.(53) We have previously shown that SFN stimulates the expression of NRF2-dependent antioxidant enzymes both in vitro and in vivo, and protects cells and tissues from H. pylori- and NSAID-induced oxidative injury.(28,29,56,57) In some of these studies, we also found that SFN inhibits H. pylori activity in the gastric mucosa,(56) and anaerobic enteric bacteria in the small intestinal mucosa.(28) Furthermore, our clinical trials have shown that dietary intake of sulforaphane glucosinolate (SGS), a precursor of SFN, stimulates antioxidant enzymes in the human GI tract, and ameliorates gastric inflammation in H. pylori-infected subjects.(56) We have also shown that dietary intake of SGS reduces H. pylori levels in the gastric lumen, thereby providing chemoprotection against gastric cancer.(56) Details regarding the basic mechanisms by which SFN protects against cancer can be found in Fuentes et al.(58) In addition, our recent study has shown that dietary intake of SGS improves defecation in human subjects, presumably by upregulating antioxidant enzyme activities.(42) Taken together, we believe that SFN is a promising compound in the protection of the GI tract from oxidative injury.
2) Brassica plant-derived isothiocyanates other than SFN
Several types of isothiocyanates, such as allyl isothiocyanate (AITC) and authentic 6-(methylsulfinyl)hexyl isothiocyanate (6-HITC) are found in cruciferous vegetables and are especially high in wasabi.(59,60) Both AITC and 6-HITC have been shown to activate NRF2.(60–62) For example, an in vitro study in rat liver epithelial cells demonstrated that 6-HITC potently stimulates antioxidant response element transcription, inducing phase 2 enzymes, and that these effects of 6-HITC were abrogated in NRF2-deficient cells.(60,61) A recent study in human volunteers showed that daily intake of food levels of AITC does not cause DNA strand breaks, estimated by measuring urinary levels of 8-hydroxy-2'-deoxyguanosine (8-OHdG).(62) However, no data have been reported so far, from either in vivo animal studies or human studies, which demonstrate the protective effects of these compounds on the GI tract against oxidative stress.
Polyphenols
Polyphenols are secondary metabolites of plants, and are considered to enhance the defense system against human chronic diseases induced by prolonged oxidative stress. Recent studies have shown that dietary intake of polyphenols contributes to many types of chronic diseases induced by oxidative stress.(54) Hydroxyl groups linked with the benzene bond in many types of polyphenols reduce oxidative stress not only by oxidizing themselves, but also by chelating metals such as copper and iron, which oxidize the cells.(54) Details of these classic mechanisms and their association with various types of clinical diseases are described elsewhere.(54) This review focuses on the nrf2-dependent protection of the GI tract induced by some polyphenols during oxidative stress.
1) Curcumin
Curcumin is a polyphenol found at a high level in turmeric, which is used as a spice, food colorant, and traditional herbal medicine.(63) In vitro studies in rat renal epithelial cells(61) and mouse macrophages(64) have shown that both curcumin and its synthetic analog, dimethoxycurcumin, activate the expression of heme oxygenase-1 (HO-1) by stimulating the binding of NRF2 to its antioxidant response element.(64,65) Previous clinical trials on curcumin have shown its effectiveness in mitigating symptoms in patients with IBS.(66,67) A clinical trial in IBS patients has shown that daily intake of curcumin in combination with fennel essential oil for 30 days improved the symptom scores and quality of life for IBS patients.(66) Another clinical intervention study in UC patients has shown that induction with NCB-02 (curcumin) enema ameliorates symptoms in patients with mild-to-moderate distal UC.(67)
2) Catechin
Catechins are phytochemicals that are highly enriched in tea.(68) Basic studies have shown that catechin protects against ketoprofen-induced oxidative damage of the gastric mucosa by upregulating NRF2 in vitro and in vivo.(69) Epigallocatechin gallate upregulates NRF2 by disabling KEAP1, preventing diabetic nephropathy.(70) Several epidemiological studies have suggested that catechin intake may reduce the risk of human GI disorders. For example, higher phenolic acid concentrations in the plasma and urine of men consuming green or black tea have demonstrated potential chemopreventive properties for colon cancer.(71) A prospective cohort comparison of flavonoid treatment in patients with resected colorectal cancer to prevent recurrence has shown that sustained long-term treatment with a flavonoid mixture can reduce the recurrence rate of colon neoplasia in patients with resected colon cancer.(72) Observational studies conducted in Finland have shown that high flavonoid intake was associated with low risk of pancreatic cancer in male smokers.(73) Only one intervention trial has shown a protective effect of green tea polyphenols in liver cancer prevention among high-risk individuals.(74)
3) Quercetin
Quercetin is a flavonoid that is highly enriched in citrus fruits and onions.(75) An in vitro study in liver cancer-derived HepG2 cells has shown that quercetin modulates redox signaling by upregulation of NRF2 expression and downregulation of NFκB and cyclooxygenase-2 (COX-2), thereby mitigating oxidative injury induced by a cytotoxic agent, ochratoxin A.(76) A recent in vivo study in rats has demonstrated that pretreatment with quercetin mitigates indomethacin-induced GI injury via upregulation of NRF2 and downregulation of NFκB, supporting the protective role of quercetin against NSAID-induced oxidative injury in the GI tract.(77) Although some previous clinical trials have shown that daily intake of quercetin improves biomarkers of metabolic syndromes,(78,79) no clinical data have been reported regarding the protective effect of quercetin in the GI tract during oxidative stress in human subjects.
4) Resveratrol
Resveratrol (trans-3,4',5-trihydroxystilbene), a polyphenolic phytoalexin, is rich in grapes and other fruits and plants.(80,81) The protective effects of resveratrol against oxidative stress involve not only direct neutralization of reactive oxygen species, but also upregulation of NRF2-dependent antioxidant enzymes during oxidative stress.(81,82) A recent study showed that resveratrol enhances heat stress-induced upregulation of antioxidant enzymes via NRF2-dependent mechanisms, and protects quail hepatocytes from oxidative stress induced by high ambient temperatures.(83) Since resveratrol possesses anti-inflammatory and antioxidant activity, and it inhibits multiple immune responses in colonic mucosa, resveratrol may be useful as a treatment option for IBD.(84) A recent clinical trial demonstrated that 6 weeks of supplementation with 500 mg resveratrol decreases plasma levels of TNF-α and mitigates clinical colitis in patients with UC,(85) supporting the possibility of resveratrol as a treatment option for UC in the future.
Carotenoids
Carotenoids are organic pigments that are produced by plants and animals. Daily intake of carotenoids reduces the risk of chronic diseases induced by oxidative stress.(55) It has been well known that several types of carotenoids attenuate oxidative stress by scavenging free radicals.(55) Details of these mechanisms and their association with various types of clinical diseases have been described elsewhere.(55) This review addresses the roles of lycopene and astaxanthin in the nrf2-dependent protection of the GI tract against oxidative stress.
1) Lycopene
Lycopene is a bright red carotene enriched in tomatoes and other red fruits and vegetables, such as red carrots and watermelons.(86) An in vitro study using human bronchial epithelial cells has shown that enzymatic metabolites of lycopene induce NRF2-mediated expression of phase II antioxidant enzymes.(87) Recent in vivo studies have shown that lycopene not only ameliorates atrazine-induced oxidative damage in the adrenal cortex of male rats by activating the NRF2/HO-1 pathway,(88) but also attenuates oxidative stress-induced neuroinflammation and cognitive impairment via the NRF2/NFκB transcriptional pathway.(89) Although some prior clinical trials showed that administration of lycopene improves the profiles of oxidative biomarkers in humans,(90,91) no clinical trials have yet shown the clinical efficacy of lycopene in treating human GI disorders.
2) Astaxanthin
Astaxanthin is a carotenoid enriched in shrimp and salmon.(92) Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage, and early hepatocarcinogenesis in rats, via the actions of NRF2, p53, p38, and phase-II enzymes.(93) Astaxanthin and β-carotene can prevent H. pylori-induced gastric inflammation.(94) Astaxanthin and omega-3 fatty acids protect against oxidative stress via the NRF2-ARE pathway, both individually and in combination.(95) Although some clinical studies have shown that intake of astaxanthin improves biomarkers of systemic oxidative stress, the effects on GI diseases have not been extensively studied.(96,97) However, a recent clinical trial has demonstrated that higher dose of astaxanthin, 40 mg/day, reduced reflux symptoms in patients with gastroesophageal reflux disease (GERD), but had no curative effects on functional dyspepsia.(98) Further studies are required regarding the clinical efficacy of astaxanthin to treat oxidative stress-induced GI disorders.
Drugs & Hormones
Lansoprazole (LPZ)
LPZ is a PPI, originally developed in Japan.(99) LPZ not only inhibits gastric acid secretion,(99) but also shows anti-inflammatory effects.(100) An in vitro study using rat gastric mucosal cells demonstrated that LPZ, at a concentration of 1–100 µM, which approximates doses used to inhibit gastric acid secretion, upregulates HO-1 expression through an NRF2-KEAP1-meditated mechanism.(100) Another in vitro study showed that LPZ, at concentrations of 10–100 µM, inhibits mitochondrial superoxide production and cellular lipid peroxidation induced by indomethacin in GECs, supporting the possibility that LPZ enhances cellular defenses against oxidative stress.(101) However, an in vivo study in rat stomach demonstrated that LPZ failed to stimulate NRF2 expression, although it strongly inhibited indomethacin-induced gastric ulcers, suggesting that the acid inhibitory effect of LPZ is more important than the role of NRF2, at least in stomach.(102) In contrast, another in vivo study using rat small intestines have shown that LPZ, but not omeprazole, prevented indomethacin-induced small intestinal ulceration through induction of HO-1, suggesting that NRF2-mediated protection plays an important role against indomethacin-induced oxidative stress in the absence of luminal acid.(103) Although numerous clinical studies have shown that LPZ prevents NSAID/aspirin-induced injury in the upper GI tract, no clinical studies to date have clearly demonstrated that the protective effects of LPZ on the GI tract are mediated by NRF2-dependent mechanisms. Further studies are required to assess the role of LPZ.
Ursodeoxycholic acid (UDCA)
UDCA, a drug known to protect human liver function, stimulates NRF2-mediated hepatocellular transport, detoxification, and antioxidant stress systems in mice.(104) Studies in diabetic mice have shown that UDCA inhibits the expression of proinflammatory cytokines and foam cell formation via upregulation of ABC transporters, thereby blocking atherosclerosis (105). In patients with Barrett’s esophagus and in Barrett’s cell lines, UDCA increases expression of antioxidant enzymes and prevents DNA damage by bile acids.(106) All of these findings strongly suggest that NRF2-mediated stimulation of normal hepatic or intestinal transport by UDCA contributes to the protection of the liver and small intestine from oxidative injury. However, sufficient evidence for the protective role of UDCA in oxidative GI injury has not yet been provided. Thus, future clinical studies are required to address this possibility.
Sofalcone
Sofalcone, originally developed as a gastric mucosal protective agent, has been shown to increase vascular endothelial growth factor via the NRF2-HO-1 pathway in GECs.(107) It also has been shown that sofalcone increases mucus gel thickness and mucosal blood flow in the gastric mucosa.(108) Although previous clinical trials of sofalcone have shown that it promotes human gastric ulcer healing,(109) no studies have shown that this effect is mediated by an antioxidant system. Although the clinical efficacy of sofalcone against gastroduodenal ulcers is far less than that observed with potent acid inhibitors, such as PPIs or PCABs, the effects on small intestinal ulcers may be different. Therefore, it seems worthwhile to examine the effect of sofalcone on NSAID/aspirin-induced small intestinal injury in both animal models and human patients.
Ghrelin
Ghrelin, a gut-brain peptide hormone secreted from the gastric corpus as well as from brain tissues, was originally discovered as a gut hormone and plays an important role in appetite regulation.(110–112) Previous studies have demonstrated that ghrelin protects the GI mucosa from ethanol-induced injuries, effects mediated by crosstalk between endogenous prostaglandins and NO.(112,113) More recent in vivo studies in rats have shown that ghrelin upregulates HO-1 expression and protects gastric mucosa against indomethacin-induced injury(114) and ischemia/reperfusion injury,(115) suggesting the involvement of NRF2-mediated induction of the antioxidant system in the protection afforded by ghrelin. Another recent study from China showed that ghrelin protects lung tissues from oxidative injury induced by paraquat, and the authors demonstrated upregulation of NRF2 by ghrelin, supporting the possibility that ghrelin affords organ protection against oxidative stress by NRF2-mediated mechanism.(116) Furthermore, a recent clinical trial on relamorelin, a ghrelin receptor agonist, showed that it mitigates vomiting and accelerates gastric emptying in patients with diabetic gastroparesis, indicating that ghrelin may be useful as a therapeutic drug for GI disorders induced by various types of oxidative stress.(117) Further studies are required to assess this possibility.
Melatonin
Melatonin, known as N-acetyl-5-methoxytryptamine, is a hormone produced by the pineal gland, which regulates sleep and wakefulness.(118) Several studies have shown that melatonin enhances antioxidant properties, thereby protecting cells from oxidative stress by upregulating the NRF2-mediated antioxidant system.(119) A recent in vivo mouse study has shown that melatonin not only prevents dextran sodium sulfate (DSS)-induced colitis, but also prevents the formation of colitis-associated colonic carcinoma induced by a chemical carcinogen; both effects are mediated by NRF2-dependent mechanisms.(120) Several clinical trials have been conducted regarding the therapeutic effects of melatonin on oxidative stress-induced GI disorders. For example, treatment with melatonin, 3 mg/day, for 4 to 8 weeks caused significant improvement in GERD-related symptoms, although the effects were slightly smaller than those induced by omeprazole, 40 mg/day.(121) However, melatonin treatment in combination with omeprazole demonstrated enhanced efficacy compared with that observed with omeprazole alone, indicating that melatonin may be useful as a therapeutic drug for PPI-resistant GERD symptoms.(121) Another clinical study showed that treatment with melatonin, 8 mg/day, for 6 months significantly improved symptoms of IBS in postmenopausal women.(122) Furthermore, another clinical trial has shown that treatment with melatonin, 5 mg/day, in combination with mesalazine, 2 g/day, for 12 months, significantly reduced the relapse rate in patients with UC in remission.(123) All three studies have provided supportive data to indicate that melatonin may be useful as a therapeutic drug for GERD, IBS, and UC in the future. Further studies are required to confirm these findings, and to assess the safety of long-term use of melatonin in human subjects.
Negative Aspects of NRF2 Activation: NRF2 as a Double-Edged Sword
Numerous prior studies have revealed that NRF2 plays an important role in protecting the GI tract against various oxidative stresses, thereby contributing to chemoprevention against GI cancers. However, it is important to note the negative aspects of NRF2, with special emphasis on its effects on cancer cells.(124,125) In experimental mice, mutation of the gene encoding NRF2 or overexpression of NRF2 by KEAP1 knockout enhances cancer cell proliferation.(126,127) Stimulation of ABC transporters and multidrug resistance protein 2 (MDR2) by NRF2 facilitates the clearance of anticancer drugs, which in turn induces chemoresistance in cancer cells and promotes tumor growth.(128,129) Such cases have been reported clinically for several types of cancers. However, no report has shown that long-term intake of NRF2-stimulating compounds causes similar conditions. However, clinicians should be wary of this possibility, especially when administering these agents to cancer patients.
Conflicts of Interest
The author is an endowed chair, supported by Hitachi Co. Ltd.
The author received financial support from Murakami Farm, Co. Ltd, Japan.
References
- 1.Itoh K, Chiba T, Takashima S, et al. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 2.Suzuki T, Yamamoto M. Stress-sensing mechanisms and the physiological roles of the Keap1-Nrf2 system during cellular stress. J Biol Chem. 2017;292:16817–16824. doi: 10.1074/jbc.R117.800169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Itoh K, Tong KI, Yamamoto M. Molecular mechanism activating Nrf2-Keap1 pathway in regulation of adaptive response to electrophiles. Free Radic Biol Med. 2004;36:1208–1213. doi: 10.1016/j.freeradbiomed.2004.02.075. [DOI] [PubMed] [Google Scholar]
- 4.Silen W, Schiessel R, Kivilaakso E. The gastric mucosal barrier and ulceration. Brain Res Bull. 1980;5 Supple 1:3–6. doi: 10.1016/0361-9230(80)90296-8. [DOI] [PubMed] [Google Scholar]
- 5.Tobey NA, Powell DW, Schreiner VJ, Orlando RC. Serosal bicarbonate protects against acid injury to rabbit esophagus. Gastroenterology. 1989;96:1466–1477. doi: 10.1016/0016-5085(89)90514-3. [DOI] [PubMed] [Google Scholar]
- 6.Nagano Y, Matsui H, Tamura M, et al. NSAIDs and acidic environment induce gastric mucosal cellular mitochondrial dysfunction. Digestion. 2012;85:131–135. doi: 10.1159/000334685. [DOI] [PubMed] [Google Scholar]
- 7.Matsui H, Nagano Y, Shimokawa O, et al. Gastric acid induces mitochondrial superoxide production and lipid peroxidation in gastric epithelial cells. J Gastroenterol. 2011;46:1167–1176. doi: 10.1007/s00535-011-0434-6. [DOI] [PubMed] [Google Scholar]
- 8.Jones MK, Zhu E, Sarino EV, et al. Loss of parietal cell superoxide dismutase leads to gastric oxidative stress and increased injury susceptibility in mice. Am J Physiol Gastrointest Liver Physiol. 2011;301:G537–G546. doi: 10.1152/ajpgi.00177.2011. [DOI] [PubMed] [Google Scholar]
- 9.Yoshida N. Inflammation and oxidative stress in gastroesophageal reflux disease. J Clin Biochem Nutr. 2007;40:13–23. doi: 10.3164/jcbn.40.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malfertheiner P, Chan FK, McColl KE. Peptic ulcer disease. Lancet. 2009;374:1449–1461. doi: 10.1016/S0140-6736(09)60938-7. [DOI] [PubMed] [Google Scholar]
- 11.Suzuki H, Hibi T. Oxidative stress in Helicobacter pylori-associated gastroduodenal diseases. J Clin Biochem Nutr. 2006;39:56–63. [Google Scholar]
- 12.Suzuki H, Nishizawa T, Tsugawa H, Mogami S, Hibi T. Roles of oxidative stress in stomach disorders. J Clin Biochem Nutr. 2012;50:35–39. doi: 10.3164/jcbn.11-115SR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gajewski A, Mnich E, Szymański K, et al. Helicobacter pylori antigens, acetylsalicylic acid, LDL and 7-ketocholesterol—their potential role in destabilizing the gastric epithelial cell barrier. An in vitro model of Kato III cells. Acta Biochim Pol. 2016;63:145–152. doi: 10.18388/abp.2015_1122. [DOI] [PubMed] [Google Scholar]
- 14.Shi LQ, Zheng RL. DNA damage and oxidative stress induced by Helicobacter pylori in gastric epithelial cells: protection by vitamin C and sodium selenite. Pharmazie. 2006;61:631–637. [PubMed] [Google Scholar]
- 15.Bhattacharjee M, Bhattacharjee S, Gupta A, Banerjee RK. Critical role of an endogenous gastric peroxidase in controlling oxidative damage in H. pylori-mediated and nonmediated gastric ulcer. Free Radic Biol Med. 2002;32:731–743. doi: 10.1016/s0891-5849(02)00757-8. [DOI] [PubMed] [Google Scholar]
- 16.Handa O, Naito Y, Yoshikawa T. Redox biology and gastric carcinogenesis: the role of Helicobacter pylori. Redox Rep. 2011;16:1–7. doi: 10.1179/174329211X12968219310756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang C, Zhang H, Yu L, Cao Y. Helicobacter pylori dwelling on the apical surface of gastrointestinal epithelium damages the mucosal barrier through direct contact. Helicobacter. 2014;19:330–342. doi: 10.1111/hel.12138. [DOI] [PubMed] [Google Scholar]
- 18.Amieva MR, Vogelmann R, Covacci A, Tompkins LS, Nelson WJ, Falkow S. Disruption of the epithelial apical-junctional complex by Helicobacter pylori CagA. Science. 2003;300:1430–1434. doi: 10.1126/science.1081919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laine L. The gastrointestinal effects of nonselective NSAIDs and COX-2-selective inhibitors. Semin Arthritis Rheum. 2002;32:25–32. doi: 10.1053/sarh.2002.37217. [DOI] [PubMed] [Google Scholar]
- 20.Wallace JL. Non-steroidal anti-inflammatory drug gastropathy and cytoprotection: pathogenesis and mechanisms re-examined. Scand J Gastroenterol Suppl. 1992;192:3–8. doi: 10.3109/00365529209095973. [DOI] [PubMed] [Google Scholar]
- 21.Wallace JL. Gastric ulceration: critical events at the neutrophil--endothelium interface. Can J Physiol Pharmacol. 1993;71:98–102. doi: 10.1139/y93-014. [DOI] [PubMed] [Google Scholar]
- 22.Fiorucci S, Santucci L, Gerli R, et al. NSAIDs upregulate beta 2-integrin expression on human neutrophils through a calcium-dependent pathway. Aliment Pharmacol Ther. 1997;11:619–630. doi: 10.1046/j.1365-2036.1997.00190.x. [DOI] [PubMed] [Google Scholar]
- 23.Laine L, Takeuchi K, Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology. 2008;135:41–60. doi: 10.1053/j.gastro.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 24.Kwiecień S, Magierowska K, Śliwowski Z, Wójcik D, Magierowski M, Brzozowski T. New insight into the mechanisms of gastroduodenal injury induced by nonsteroidal anti-inflammatory drugs: practical implications. Pol Arch Med Wewn. 2015;125:191–198. doi: 10.20452/pamw.2715. [DOI] [PubMed] [Google Scholar]
- 25.Garnock-Jones KP. Vonoprazan: first global approval. Drugs. 2015;75:439–443. doi: 10.1007/s40265-015-0368-z. [DOI] [PubMed] [Google Scholar]
- 26.Wallace JL, Syer S, Denou E, et al. Proton pump inhibitors exacerbate NSAID-induced small intestinal injury by inducing dysbiosis. Gastroenterology. 2011;141:1314–1322. doi: 10.1053/j.gastro.2011.06.075. [DOI] [PubMed] [Google Scholar]
- 27.Leung WK, Bjarnason I, Wong VW, Sung JJ, Chan FK. Small bowel enteropathy associated with chronic low-dose aspirin therapy. Lancet. 2007;369:614. doi: 10.1016/S0140-6736(07)60282-7. [DOI] [PubMed] [Google Scholar]
- 28.Yanaka A, Sato J, Ohmori S. Sulforaphane protects small intestinal mucosa from aspirin/NSAID-induced injury by enhancing host defense systems against oxidative stress and by inhibiting mucosal invasion of anaerobic enterobacteria. Curr Pharm Des. 2013;19:157–162. doi: 10.2174/13816128130120. [DOI] [PubMed] [Google Scholar]
- 29.Yanaka A. Role of sulforaphane in protection of gastrointestinal tract against H. pylori and NSAID-induced oxidative stress. Curr Pharm Des. 2017;23:4066–4075. doi: 10.2174/1381612823666170207103943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Handa O, Majima A, Onozawa Y, et al. The role of mitochondria-derived reactive oxygen species in the pathogenesis of non-steroidal anti-inflammatory drug-induced small intestinal injury. Free Radic Res. 2014;48:1095–1099. doi: 10.3109/10715762.2014.928411. [DOI] [PubMed] [Google Scholar]
- 31.Plé C, Breton J, Richoux R, et al. Combining selected immunomodulatory Propionibacterium freudenreichii and Lactobacillus delbrueckii strains: reverse engineering development of an anti-inflammatory cheese. Mol Nutr Food Res. 2016;60:935–948. doi: 10.1002/mnfr.201500580. [DOI] [PubMed] [Google Scholar]
- 32.Naito Y, Takagi T, Yoshikawa T. Neutrophil-dependent oxidative stress in ulcerative colitis. J Clin Biochem Nutr. 2007;41:18–26. doi: 10.3164/jcbn.2007003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novak EA, Mollen KP. Mitochondrial dysfunction in inflammatory bowel disease. Front Cell Dev Biol. 2015;3:62. doi: 10.3389/fcell.2015.00062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balmus IM, Ciobica A, Trifan A, Stanciu C. The implications of oxidative stress and antioxidant therapies in inflammatory bowel disease: clinical aspects and animal models. Saudi J Gastroenterol. 2016;22:3–17. doi: 10.4103/1319-3767.173753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tanida S, Mizoshita T, Mizushima T, et al. Involvement of oxidative stress and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) in inflammatory bowel disease. J Clin Biochem Nutr. 2011;48:112–116. doi: 10.3164/jcbn.10-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pereira C, Grácio D, Teixeira JP, Magro F. Oxidative stress and DNA damage: implications in inflammatory bowel disease. Inflamm Bowel Dis. 2015;21:2403–2417. doi: 10.1097/MIB.0000000000000506. [DOI] [PubMed] [Google Scholar]
- 37.Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353–362. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- 38.Zhu H, Li YR. Oxidative stress and redox signaling mechanisms of inflammatory bowel disease: updated experimental and clinical evidence. Exp Biol Med (Maywood) 2012;237:474–480. doi: 10.1258/ebm.2011.011358. [DOI] [PubMed] [Google Scholar]
- 39.Alzoghaibi MA. Concepts of oxidative stress and antioxidant defense in Crohn’s disease. World J Gastroenterol. 2013;19:6540–6547. doi: 10.3748/wjg.v19.i39.6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iborra M, Moret I, Rausell F, et al. Role of oxidative stress and antioxidant enzymes in Crohn’s disease. Biochem Soc Trans. 2011;39:1102–1106. doi: 10.1042/BST0391102. [DOI] [PubMed] [Google Scholar]
- 41.Choghakhori R, Abbasnezhad A, Hasanvand A, Amani R. Inflammatory cytokines and oxidative stress biomarkers in irritable bowel syndrome: association with digestive symptoms and quality of life. Cytokine. 2017;93:34–43. doi: 10.1016/j.cyto.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Yanaka A. Daily intake of broccoli sprouts normalizes bowel habits in human healthy subjects. J Clin Biochem Nutr. 2018;62:75–82. doi: 10.3164/jcbn.17-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pérez S, Taléns-Visconti R, Rius-Pérez S, Finamor I, Sastre J. Redox signaling in the gastrointestinal tract. Free Radic Biol Med. 2017;104:75–103. doi: 10.1016/j.freeradbiomed.2016.12.048. [DOI] [PubMed] [Google Scholar]
- 44.Chen H, Fang Y, Li W, Orlando RC, Shaheen N, Chen XL. NFkB and Nrf2 in esophageal epithelial barrier function. Tissue Barriers. 2013;1:e27463. doi: 10.4161/tisb.27463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen H, Hu Y, Fang Y, et al. Nrf2 deficiency impairs the barrier function of mouse oesophageal epithelium. Gut. 2014;63:711–719. doi: 10.1136/gutjnl-2012-303731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Talalay P, Fahey JW. Phytochemicals from cruciferous plants protect against cancer by modulating carcinogen metabolism. J Nutr. 2001;131 (11 Suppl):3027S–3033S. doi: 10.1093/jn/131.11.3027S. [DOI] [PubMed] [Google Scholar]
- 47.Buckley DB, Klaassen CD. Induction of mouse UDP-glucuronosyltransferase mRNA expression in liver and intestine by activators of aryl-hydrocarbon receptor, constitutive androstane receptor, pregnane X receptor, peroxisome proliferator-activated receptor alpha, and nuclear factor erythroid 2-related factor 2. Drug Metab Dispos. 2009;37:847–856. doi: 10.1124/dmd.108.024190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Puscheck EE, Awonuga AO, Yang Y, Jiang Z, Rappolee DA. Molecular biology of the stress response in the early embryo and its stem cells. Adv Exp Med Biol. 2015;843:77–128. doi: 10.1007/978-1-4939-2480-6_4. [DOI] [PubMed] [Google Scholar]
- 49.Lau WL, Liu SM, Pahlevan S, et al. Role of Nrf2 dysfunction in uremia-associated intestinal inflammation and epithelial barrier disruption. Dig Dis Sci. 2015;60:1215–1222. doi: 10.1007/s10620-014-3428-4. [DOI] [PubMed] [Google Scholar]
- 50.Vollrath V, Wielandt AM, Iruretagoyena M, Chianale J. Role of Nrf2 in the regulation of the Mrp2 (ABCC2) gene. Biochem J. 2006;395:599–609. doi: 10.1042/BJ20051518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adachi T, Nakagawa H, Chung I, et al. Nrf2-dependent and -independent induction of ABC transporters ABCC1, ABCC2, and ABCG2 in HepG2 cells under oxidative stress. J Exp Ther Oncol. 2007;6:335–348. [PubMed] [Google Scholar]
- 52.Weerachayaphorn J, Cai SY, Soroka CJ, Boyer JL. Nuclear factor erythroid 2-related factor 2 is a positive regulator of human bile salt export pump expression. Hepatology. 2009;50:1588–1596. doi: 10.1002/hep.23151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang L, Palliyaguru DL, Kensler TW. Frugal chemoprevention: targeting Nrf2 with foods rich in sulforaphane. Semin Oncol. 2016;43:146–153. doi: 10.1053/j.seminoncol.2015.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Costa C, Tsatsakis A, Mamoulakis C, et al. Current evidence on the effect of dietary polyphenols intake on chronic diseases. Food Chem Toxicol. 2017;110:286–299. doi: 10.1016/j.fct.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 55.Fiedor J, Burda K. Potential role of carotenoids as antioxidants in human health and disease. Nutrients. 2014;6:466–488. doi: 10.3390/nu6020466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yanaka A, Fahey JW, Fukumoto A, et al. Dietary sulforaphane-rich broccoli sprouts reduce colonization and attenuate gastritis in Helicobacter pylori-infected mice and humans. Cancer Prev Res (Phila). 2009;2:353–360. doi: 10.1158/1940-6207.CAPR-08-0192. [DOI] [PubMed] [Google Scholar]
- 57.Ushida Y, Suganuma H, Yanaka A. Low-dose of the sulforaphane precursor glucoraphanin as a dietary supplement induces chemoprotective enzymes in humans. Food and Nutrition Sciences. 2015;6:1603–1612. [Google Scholar]
- 58.Fuentes F, Paredes-Gonzalez X, Kong AN. Dietary glucosinolates sulforaphane, phenethyl isothiocyanate, indole-3-carbinol/3,3'-diindolylmethane: anti-oxidative stress/inflammation, Nrf2, epigenetics/epigenomics and in vivo cancer chemopreventive efficacy. Curr Pharmacol Rep. 2015;1:179–196. doi: 10.1007/s40495-015-0017-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang Y. Allyl isothiocyanate as a cancer chemopreventive phytochemical. Mol Nutr Food Res. 2010;54:127–135. doi: 10.1002/mnfr.200900323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Morimitsu Y, Nakagawa Y, Hayashi K, et al. A sulforaphane analogue that potently activates the Nrf2-dependent detoxification pathway. J Biol Chem. 2002;277:3456–3463. doi: 10.1074/jbc.M110244200. [DOI] [PubMed] [Google Scholar]
- 61.Munday R, Munday CM. Induction of phase II detoxification enzymes in rats by plant-derived isothiocyanates: comparison of allyl isothiocyanate with sulforaphane and related compounds. J Agric Food Chem. 2004;52:1867–1871. doi: 10.1021/jf030549s. [DOI] [PubMed] [Google Scholar]
- 62.Charron CS, Clevidence BA, Albaugh GA, et al. Assessment of DNA damage and repair in adults consuming allyl isothiocyanate or Brassica vegetables. J Nutr Biochem. 2013;24:894–902. doi: 10.1016/j.jnutbio.2012.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsuda T. Curcumin as a functional food-derived factor: degradation products, metabolites, bioactivity, and future perspectives. Food Funct. 2018;9:705–714. doi: 10.1039/c7fo01242j. [DOI] [PubMed] [Google Scholar]
- 64.Balogun E, Hoque M, Gong P, et al. Curcumin activates the haem oxygenase-1 gene via regulation of Nrf2 and the antioxidant-responsive element. Biochem J. 2003;371 (Pt 3):887–895. doi: 10.1042/BJ20021619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jeong SO, Oh GS, Ha HY, et al. Dimethoxycurcumin, a synthetic curcumin analogue, induces heme oxygenase-1 expression through Nrf2 activation in RAW264.7 macrophages. J Clin Biochem Nutr. 2009;44:79–84. doi: 10.3164/jcbn.08-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Portincasa P, Bonfrate L, Scribano ML, et al. Curcumin and fennel essential oil improve symptoms and quality of life in patients with irritable bowel syndrome. J Gastrointestin Liver Dis. 2016;25:151–157. doi: 10.15403/jgld.2014.1121.252.ccm. [DOI] [PubMed] [Google Scholar]
- 67.Singla V, Pratap Mouli V, Garg SK, et al. Induction with NCB-02 (curcumin) enema for mild-to-moderate distal ulcerative colitis - a randomized, placebo-controlled, pilot study. J Crohns Colitis. 2014;8:208–214. doi: 10.1016/j.crohns.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 68.Fan FY, Sang LX, Jiang M. Catechins and their therapeutic benefits to inflammatory bowel disease. Molecules. 2017;22. pii:E484. doi: 10.3390/molecules22030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cheng YT, Wu CH, Ho CY, Yen GC. Catechin protects against ketoprofen-induced oxidative damage of the gastric mucosa by up-regulating Nrf2 in vitro and in vivo. J Nutr Biochem. 2013;24:475–483. doi: 10.1016/j.jnutbio.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 70.Sun W, Liu X, Zhang H, et al. Epigallocatechin gallate upregulates NRF2 to prevent diabetic nephropathy via disabling KEAP1. Free Radic Biol Med. 2017;108:840–857. doi: 10.1016/j.freeradbiomed.2017.04.365. [DOI] [PubMed] [Google Scholar]
- 71.Henning SM, Wang P, Abgaryan N, et al. Phenolic acid concentrations in plasma and urine from men consuming green or black tea and potential chemopreventive properties for colon cancer. Mol Nutr Food Res. 2013;57:483–493. doi: 10.1002/mnfr.201200646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hoensch H, Groh B, Edler L, Kirch W. Prospective cohort comparison of flavonoid treatment in patients with resected colorectal cancer to prevent recurrence. World J Gastroenterol. 2008;14:2187–2193. doi: 10.3748/wjg.14.2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bobe G, Weinstein SJ, Albanes D, et al. Flavonoid intake and risk of pancreatic cancer in male smokers (Finland) Cancer Epidemiol Biomarkers Prev. 2008;17:553–562. doi: 10.1158/1055-9965.EPI-07-2523. [DOI] [PubMed] [Google Scholar]
- 74.Luo H, Tang L, Tang M, et al. Phase IIa chemoprevention trial of green tea polyphenols in high-risk individuals of liver cancer: modulation of urinary excretion of green tea polyphenols and 8-hydroxydeoxyguanosine. Carcinogenesis. 2006;27:262–268. doi: 10.1093/carcin/bgi147. [DOI] [PubMed] [Google Scholar]
- 75.Anand David AV, Arulmoli R, Parasuraman S. Overviews of biological importance of quercetin: a bioactive flavonoid. Pharmacogn Rev. 2016;10:84–89. doi: 10.4103/0973-7847.194044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramyaa P, Krishnaswamy R, Padma VV. Quercetin modulates OTA-induced oxidative stress and redox signalling in HepG2 cells - up regulation of Nrf2 expression and down regulation of NF-κB and COX-2. Biochim Biophys Acta. 2014;1840:681–692. doi: 10.1016/j.bbagen.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 77.Carrasco-Pozo C, Castillo RL, Beltrán C, Miranda A, Fuentes J, Gotteland M. Molecular mechanisms of gastrointestinal protection by quercetin against indomethacin-induced damage: role of NF-κB and Nrf2. J Nutr Biochem. 2016;27:289–298. doi: 10.1016/j.jnutbio.2015.09.016. [DOI] [PubMed] [Google Scholar]
- 78.McAnulty LS, Miller LE, Hosick PA, Utter AC, Quindry JC, McAnulty SR. Effect of resveratrol and quercetin supplementation on redox status and inflammation after exercise. Appl Physiol Nutr Metab. 2013;38:760–765. doi: 10.1139/apnm-2012-0455. [DOI] [PubMed] [Google Scholar]
- 79.Cialdella-Kam L, Nieman DC, Sha W, Meaney MP, Knab AM, Shanely RA. Dose-response to 3 months of quercetin-containing supplements on metabolite and quercetin conjugate profile in adults. Br J Nutr. 2013;109:1923–1933. doi: 10.1017/S0007114512003972. [DOI] [PubMed] [Google Scholar]
- 80.Khakimov B, Engelsen SB. Resveratrol in the foodomics era: 1:25,000. Ann N Y Acad Sci. 2017;1403:48–58. doi: 10.1111/nyas.13425. [DOI] [PubMed] [Google Scholar]
- 81.Thiel G, Rössler OG. Resveratrol regulates gene transcription via activation of stimulus-responsive transcription factors. Pharmacol Res. 2017;117:166–176. doi: 10.1016/j.phrs.2016.12.029. [DOI] [PubMed] [Google Scholar]
- 82.Truong VL, Jun M, Jeong WS. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors. 2018;44:36–49. doi: 10.1002/biof.1399. [DOI] [PubMed] [Google Scholar]
- 83.Sahin K, Orhan C, Akdemir F, Tuzcu M, Iben C, Sahin N. Resveratrol protects quail hepatocytes against heat stress: modulation of the Nrf2 transcription factor and heat shock proteins. J Anim Physiol Anim Nutr (Berl). 2012;96:66–74. doi: 10.1111/j.1439-0396.2010.01123.x. [DOI] [PubMed] [Google Scholar]
- 84.Shi Y, Zhou J, Jiang B, Miao M. Resveratrol and inflammatory bowel disease. Ann N Y Acad Sci. 2017;1403:38–47. doi: 10.1111/nyas.13426. [DOI] [PubMed] [Google Scholar]
- 85.Samsami-Kor M, Daryani NE, Asl PR, Hekmatdoost A. Anti-inflammatory effects of resveratrol in patients with ulcerative colitis: a randomized, double-blind, placebo-controlled pilot study. Arch Med Res. 2015;46:280–285. doi: 10.1016/j.arcmed.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 86.Martí R, Roselló S, Cebolla-Cornejo J. Tomato as a source of carotenoids and polyphenols targeted to cancer prevention. Cancers (Basel). 2016;8. pii:E58. doi: 10.3390/cancers8060058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lian F, Wang XD. Enzymatic metabolites of lycopene induce Nrf2-mediated expression of phase II detoxifying/antioxidant enzymes in human bronchial epithelial cells. Int J Cancer. 2008;123:1262–1268. doi: 10.1002/ijc.23696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Abass MA, Elkhateeb SA, Abd El-Baset SA, Kattaia AA, Mohamed EM, Atteia HH. Lycopene ameliorates atrazine-induced oxidative damage in adrenal cortex of male rats by activation of the Nrf2/HO-1 pathway. Environ Sci Pollut Res Int. 2016;23:15262–15274. doi: 10.1007/s11356-016-6637-x. [DOI] [PubMed] [Google Scholar]
- 89.Zhao B, Ren B, Guo R, et al. Supplementation of lycopene attenuates oxidative stress induced neuroinflammation and cognitive impairment via Nrf2/NF-κB transcriptional pathway. Food Chem Toxicol. 2017;109 (Pt 1):505–516. doi: 10.1016/j.fct.2017.09.050. [DOI] [PubMed] [Google Scholar]
- 90.Jacob K, Periago MJ, Böhm V, Berruezo GR. Influence of lycopene and vitamin C from tomato juice on biomarkers of oxidative stress and inflammation. Br J Nutr. 2008;99:137–146. doi: 10.1017/S0007114507791894. [DOI] [PubMed] [Google Scholar]
- 91.Harms-Ringdahl M, Jenssen D, Haghdoost S. Tomato juice intake suppressed serum concentration of 8-oxodG after extensive physical activity. Nutr J. 2012;11:29. doi: 10.1186/1475-2891-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ambati RR, Moi PS, Ravi S, Aswathanarayana RG. Astaxanthin: sources, extraction, stability, biological activities and its commercial applications—a review. Mar Drugs. 2014;12:128–152. doi: 10.3390/md12010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tripathi DN, Jena GB. Astaxanthin intervention ameliorates cyclophosphamide-induced oxidative stress, DNA damage and early hepatocarcinogenesis in rat: role of Nrf2, p53, p38 and phase-II enzymes. Mutat Res. 2010;696:69–80. doi: 10.1016/j.mrgentox.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 94.Kang H, Kim H. Astaxanthin and β-carotene in Helicobacter pylori-induced gastric inflammation: a mini-review on action mechanisms. J Cancer Prev. 2017;22:57–61. doi: 10.15430/JCP.2017.22.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Saw CL, Yang AY, Guo Y, Kong AN. Astaxanthin and omega-3 fatty acids individually and in combination protect against oxidative stress via the Nrf2-ARE pathway. Food Chem Toxicol. 2013;62:869–875. doi: 10.1016/j.fct.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 96.Baralic I, Djordjevic B, Dikic N, et al. Effect of astaxanthin supplementation on paraoxonase 1 activities and oxidative stress status in young soccer players. Phytother Res. 2013;27:1536–1542. doi: 10.1002/ptr.4898. [DOI] [PubMed] [Google Scholar]
- 97.Choi HD, Youn YK, Shin WG. Positive effects of astaxanthin on lipid profiles and oxidative stress in overweight subjects. Plant Foods Hum Nutr. 2011;66:363–369. doi: 10.1007/s11130-011-0258-9. [DOI] [PubMed] [Google Scholar]
- 98.Kupcinskas L, Lafolie P, Lignell A, et al. Efficacy of the natural antioxidant astaxanthin in the treatment of functional dyspepsia in patients with or without Helicobacter pylori infection: a prospective, randomized, double blind, and placebo-controlled study. Phytomedicine. 2008;15:391–399. doi: 10.1016/j.phymed.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 99.Barradell LB, Faulds D, McTavish D. Lansoprazole. A review of its pharmacodynamic and pharmacokinetic properties and its therapeutic efficacy in acid-related disorders. Drugs. 1992;44:225–250. doi: 10.2165/00003495-199244020-00007. [DOI] [PubMed] [Google Scholar]
- 100.Takagi T, Naito Y, Okada H, et al. Lansoprazole, a proton pump inhibitor, mediates anti-inflammatory effect in gastric mucosal cells through the induction of heme oxygenase-1 via activation of NF-E2-related factor 2 and oxidation of kelch-like ECH-associating protein 1. J Pharmacol Exp Ther. 2009;331:255–264. doi: 10.1124/jpet.109.152702. [DOI] [PubMed] [Google Scholar]
- 101.Rai K, Matsui H, Kaneko T, et al. Lansoprazole inhibits mitochondrial superoxide production and cellular lipid peroxidation induced by indomethacin in RGM1 cells. J Clin Biochem Nutr. 2011;49:25–30. doi: 10.3164/jcbn.10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Luo C, Chen H, Wang Y, et al. Protective effect of coptisine free base on indomethacin-induced gastric ulcers in rats: characterization of potential molecular mechanisms. Life Sci. 2018;193:47–56. doi: 10.1016/j.lfs.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 103.Yoda Y, Amagase K, Kato S, et al. Prevention by lansoprazole, a proton pump inhibitor, of indomethacin-induced small intestinal ulceration in rats through induction of heme oxygenase-1. J Physiol Pharmacol. 2010;61:287–294. [PubMed] [Google Scholar]
- 104.Okada K, Shoda J, Taguchi K, et al. Ursodeoxycholic acid stimulates Nrf2-mediated hepatocellular transport, detoxification, and antioxidative stress systems in mice. Am J Physiol Gastrointest Liver Physiol. 2008;295:G735–G747. doi: 10.1152/ajpgi.90321.2008. [DOI] [PubMed] [Google Scholar]
- 105.Chung J, An SH, Kang SW, Kwon K. Ursodeoxycholic acid (UDCA) exerts anti-atherogenic effects by inhibiting RAGE signaling in diabetic atherosclerosis. PLoS One. 2016;11:e0147839. doi: 10.1371/journal.pone.0147839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Peng S, Huo X, Rezaei D, et al. In Barrett’s esophagus patients and Barrett’s cell lines, ursodeoxycholic acid increases antioxidant expression and prevents DNA damage by bile acids. Am J Physiol Gastrointest Liver Physiol. 2014;307:G129–G139. doi: 10.1152/ajpgi.00085.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shibuya A, Onda K, Kawahara H, et al. Sofalcone, a gastric mucosa protective agent, increases vascular endothelial growth factor via the Nrf2-heme-oxygenase-1 dependent pathway in gastric epithelial cells. Biochem Biophys Res Commun. 2010;398:581–584. doi: 10.1016/j.bbrc.2010.06.124. [DOI] [PubMed] [Google Scholar]
- 108.Konturek SJ, Mrzozowski T, Drozdowicz D, Pawlik W, Sendur R. Gastroprotective and ulcer healing effects of solon, a synthetic flavonoid derivative of sophoradin. Hepatogastroenterology. 1987;34:164–170. [PubMed] [Google Scholar]
- 109.Higuchi K, Watanabe T, Tanigawa T, Tominaga K, Fujiwara Y, Arakawa T. Sofalcone, a gastroprotective drug, promotes gastric ulcer healing following eradication therapy for Helicobacter pylori: a randomized controlled comparative trial with cimetidine, an H2-receptor antagonist. J Gastroenterol Hepatol. 2010;25 Suppl 1:S155–S160. doi: 10.1111/j.1440-1746.2010.06232.x. [DOI] [PubMed] [Google Scholar]
- 110.Kojima M, Hosoda H, Matsuo H, Kangawa K. Ghrelin: discovery of the natural endogenous ligand for the growth hormone secretagogue receptor. Trends Endocrinol Metab. 2001;12:118–122. doi: 10.1016/s1043-2760(00)00362-3. [DOI] [PubMed] [Google Scholar]
- 111.Suzuki H, Matsuzaki J, Hibi T. Ghrelin and oxidative stress in gastrointestinal tract. J Clin Biochem Nutr. 2011;48:122–125. doi: 10.3164/jcbn.10-16GFR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sibilia V, Rindi G, Pagani F, et al. Ghrelin protects against ethanol-induced gastric ulcers in rats: studies on the mechanisms of action. Endocrinology. 2003;144:353–359. doi: 10.1210/en.2002-220756. [DOI] [PubMed] [Google Scholar]
- 113.Sibilia V, Pagani F, Rindi G, et al. Central ghrelin gastroprotection involves nitric oxide/prostaglandin cross-talk. Br J Pharmacol. 2008;154:688–697. doi: 10.1038/bjp.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Allam MM, El-Gohary OA. Gastroprotective effect of ghrelin against indomethacin-induced gastric injury in rats: possible role of heme oxygenase-1 pathway. Gen Physiol Biophys. 2017;36:321–330. doi: 10.4149/gpb_2016056. [DOI] [PubMed] [Google Scholar]
- 115.Liu ZB, Fei SJ, Zhu SP, et al. Protection of ghrelin postconditioning on hypoxia/reoxygenation in gastric epithelial cells. World J Gastroenterol. 2012;18:5377–5388. doi: 10.3748/wjg.v18.i38.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu Y, Guo R, Wang F, Zhao G, Lu Z, Qui Q. Protective effect of ghrelin against paraquat-induced acute lung injury in mice. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za Zhi. 2014;32:190–194. (in Chinese) [PubMed] [Google Scholar]
- 117.Lembo A, Camilleri M, McCallum R, et al. Relamorelin reduces vomiting frequency and severity and accelerates gastric emptying in adults with diabetic gastroparesis. Gastroenterology. 2016;151:87–96. e6. doi: 10.1053/j.gastro.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 118.Hardeland R, Pandi-Perumal SR, Cardinali DP. Melatonin. Int J Biochem Cell Biol. 2005;38:313–316. doi: 10.1016/j.biocel.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 119.Luchetti F, Canonico B, Betti M, et al. Melatonin signaling and cell protection function. FASEB J. 2010;24:3603–3624. doi: 10.1096/fj.10-154450. [DOI] [PubMed] [Google Scholar]
- 120.Trivedi PP, Jena GB, Tikoo KB, Kumar V. Melatonin modulated autophagy and Nrf2 signaling pathways in mice with colitis-associated colon carcinogenesis. Mol Carcinog. 2016;55:255–267. doi: 10.1002/mc.22274. [DOI] [PubMed] [Google Scholar]
- 121.Kandil TS, Mousa AA, El-Gendy AA, Abbas AM. The potential therapeutic effect of melatonin in gastro-esophageal reflux disease. BMC Gastroenterol. 2010;10:7. doi: 10.1186/1471-230X-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chojnacki C, Walecka-Kapica E, Lokieć K, et al. Influence of melatonin on symptoms of irritable bowel syndrome in postmenopausal women. Endokrynol Pol. 2013;64:114–120. [PubMed] [Google Scholar]
- 123.Chojnacki C, Wisniewska-Jarosinska M, Walecka-Kapica E, Klupinska G, Jaworek J, Chojnacki J. Evaluation of melatonin effectiveness in the adjuvant treatment of ulcerative colitis. J Physiol Pharmacol. 2011;62:327–334. [PubMed] [Google Scholar]
- 124.Jeddi F, Soozangar N, Sadeghi MR, Somi MH, Samadi N. Contradictory roles of Nrf2/Keap1 signaling pathway in cancer prevention/promotion and chemoresistance. DNA Repair (Amst). 2017;54:13–21. doi: 10.1016/j.dnarep.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 125.Bao J, Li J, Li D, Li Z. Correlation between expression of NF-E2-related factor 2 and progression of gastric cancer. Int J Clin Exp Med. 2015;8:13235–13242. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 126.Shibata T, Kokubu A, Saito S, et al. NRF2 mutation confers malignant potential and resistance to chemoradiation therapy in advanced esophageal squamous cancer. Neoplasia. 2011;13:864–873. doi: 10.1593/neo.11750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sadeghi MR, Jeddi F, Soozangar N, Somi MH, Samadi N. The role of Nrf2-Keap1 axis in colorectal cancer, progression, and chemoresistance. Tumour Biol. 2017;39:1010428317705510. doi: 10.1177/1010428317705510. [DOI] [PubMed] [Google Scholar]
- 128.Zhao XQ, Zhang YF, Xia YF, Zhou ZM, Cao YQ. Promoter demethylation of nuclear factor-erythroid 2-related factor 2 gene in drug-resistant colon cancer cells. Oncol Lett. 2015;10:1287–1292. doi: 10.3892/ol.2015.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bai X, Chen Y, Hou X, Huang M, Jin J. Emerging role of NRF2 in chemoresistance by regulating drug-metabolizing enzymes and efflux transporters. Drug Metab Rev. 2016;48:541–567. doi: 10.1080/03602532.2016.1197239. [DOI] [PubMed] [Google Scholar]