Abstract
Background
The increased adverse cardiac events in women undergoing coronary artery bypass grafting are multifactorial and may include clinical, psychosocial, and biological factors. Potential contributing biological factors could include vascular hyperreactivity of the internal mammary artery (IMA) to endogenous vasoconstrictors in women, resulting in a predilection to myocardial ischemia. This study evaluated sex differences in serotonin and thromboxane A2 dependent vasoconstriction in human isolated IMA, with the mechanistic role of (1) the endothelium, (2) nitric oxide (NO), (3) prostaglandins, and (4) receptor activity investigated for any observed sex difference.
Methods and Results
Viable isolated human IMA segments were obtained from 116 patients (44 women [mean age, 66.8±12.2 years] and 72 men [mean age, 66.6±10.4 years]) undergoing coronary artery bypass grafting. Cumulative concentration‐response curves for serotonin and thromboxane A2 mimetic, U46619, were determined and revealed an increased sensitivity to serotonin but not U46619 in women. This sex difference to serotonin was further assessed by the following: (1) endothelial denudation, (2) endothelial NO synthase inhibition and NO quantification using electron paramagnetic resonance, (3) cyclooxygenase inhibition and prostaglandin metabolite quantification using mass spectrometry, and (4) quantification of receptor activity status. The female hyperreactivity to serotonin was (1) abolished by endothelial denudation; (2) unaffected by NO synthase inhibition, with no difference in electron paramagnetic resonance–assessed NO levels; (3) abolished by cyclooxygenase inhibition (quantification of prostaglandins in IMA revealed a trend towards reduced 6‐keto prostaglandin F1α in female IMA; P=0.08); and (4) unrelated to receptor activity.
Conclusions
These data indicate that female IMAs are hyperreactive to serotonin but not U46619, with the former attributable to an endothelium‐dependent cyclooxygenase pathway.
Keywords: hypersensitivity, internal mammary artery, serotonin, thromboxane A2, vascular biology, vascular endothelium
Subject Categories: Coronary Artery Disease, Peripheral Vascular Disease, Vascular Disease
Clinical Perspective
What Is New?
Female internal mammary arteries (used in coronary artery bypass grafting) are more sensitive to serotonin vasoconstriction than their male counterparts, with a compensatory prostanoid mechanism in men accounting for the difference.
What Are the Clinical Implications?
This may contribute to the poorer outcomes observed in women undergoing coronary artery bypass grafting.
Introduction
Compared with men, women have poorer outcomes after coronary artery bypass grafting (CABG), including increased in‐hospital mortality and hospital readmissions.1, 2, 3, 4
Although the mechanisms responsible for these sex differences are likely to be multifactorial, they may include clinical (eg, smaller vessel size),5, 6 psychosocial (eg, depression),7 and biological (eg, increase platelet activity and postmenopausal status)8 factors in women compared with men. Biological factors may involve endothelial injury with the consequential platelet activation and aggregation, which are more evident in postmenopausal women.8, 9
More important, activated platelets liberate serotonin and thromboxane A2, both of which are potent vasoactive compounds implicated in several clinical situations, such as angina, acute coronary syndrome, and vasospasm.10, 11, 12 Serotonin and thromboxane A2 function through their cell surface receptors (serotonin2A+2B and thromboxane A2 receptors), coupled to both the Gq/11 and G12/13 families of G‐protein–coupled receptors, respectively. In turn, these receptors activate sarcoplasmic reticulum calcium release, activate voltage‐gated L‐type calcium entry, and increase calcium sensitivity via Rho A–mediated activation of Rho kinases and the protein kinase C–dependent activation of C‐kinase–activated protein phosphatase‐1 inhibitor for serotonin but not thromboxane A2 signalling.13, 14, 15, 16 Therefore, vascular response to serotonin and thromboxane A2 involves a balance of direct contractile effects, mainly attributable to activation of their respective cell surface receptors on smooth muscle, balanced with endothelial activation and the release of endothelial‐dependent vasodilator substances, including NO and prostanoids.17, 18, 19, 20
The female sex hormone, estrogen, has been reported to regulate vascular physiological features and function by modulating ion fluxes on smooth muscle cells and regulating endothelial‐dependent vasodilator production and activity.21, 22 Loss of these beneficial vascular effects of estrogen in menopause is, therefore, accompanied by increased systemic vascular sensitivity and platelet aggregation.23, 24 More important, activated platelets may liberate both serotonin and thromboxane A2, both of which are potent vasoactive compounds.25, 26
The objective of this study is to determine sex differences in vascular sensitivity (half maximal effective concentration [EC50]) of human internal mammary artery (IMA) graft segments to serotonin and thromboxane A2. Where a difference in vascular sensitivity is demonstrated, potential mechanisms will be evaluated by the abolition of the sex difference by the following: (1) endothelial denudation, (2) NO pathway inhibition, and (3) prostaglandin pathway inhibition.
Methods
The data, analytic methods, and study materials are not available to other researchers for purposes of reproducing the results or replicating the procedure, because patient consent for data sharing was not obtained. However, if researchers would like additional analyses, please contact the corresponding author who is eager to assist.
Patient Recruitment
The Royal Adelaide Hospital Human Ethics Committee approved the study, with patients scheduled for elective CABG consented preoperatively for the use of their discarded IMA tissue. The patients’ clinical history and medications were recorded. Anesthesia was undertaken using standard cardiac protocols, including fentanyl premedication, thiopentone induction, isoflurane maintenance anesthesia, and muscle relaxation with rocuronium. Surgery was undertaken via a median sternotomy approach, with the left IMA harvested as a pedicle graft and the excess vessel length trimmed. The remnant IMA (generally discarded) was available for the experimental studies. Patients were grouped according to biological sex.
Study Drugs and Chemicals
Serotonin, U46619 (stable analogue of thromboxane A2), A23187 (calcium ionophore), indomethacin, N‐w‐nitro‐l‐arginine methyl ester (L‐NAME), FeSO4, diethyldithiocarbamate, and sodium nitroprusside were all obtained from Sigma Chemical Company (St Louis, MO). Other chemicals used and their source included the following: SDS, 1× Complete protease inhibitor cocktail (Roche, Mannheim, Germany); di‐isopropylfluorophosphate (Sigma‐Aldrich, Caste Hill, Australia); and Phos‐tag Acrylamide (Wako Laboratory Chemicals, Japan). Prostanoids (5 prostaglandin mix containing prostaglandins E2, D2, and F2α, 6‐keto prostaglandin F1α, and thromboxane B2, (TXB2)), deuterated prostanoids (prostaglandin F2α‐d4), and diethylamine nonoate were obtained from Cayman Chemical Co (Ann Arbor, MI). All organic solvents for the mass spectrometry analysis were of liquid chromatography–mass spectroscopy grade.
Serotonin was prepared by dissolving the compound in 0.1 mol/L HCl, further diluted in double‐distilled water to the required concentration. L‐NAME was prepared in double‐distilled water, with U46619, indomethacin, and A23187 prepared in 100% ethanol. The Krebs‐bicarbonate solution contained the following (mmol/L): NaCl 118, KH2PO4 1.18, KCl 3.89, NaHCO3 25, MgCl2 1.05, CaCl2 2.34, EDTA 0.01, and glucose 5.56. The high K+ solution replaced NaCl in normal Krebs with an equimolar amount of KCl (yielding a final KCl concentration of 121.89 mmol/L); both solutions were aerated with carbogen gas (95% O2 and 5% CO2 to maintain pH 7.4). All drugs were diluted to the required final concentration in physiological Krebs‐bicarbonate solution previously aerated with carbogen gas (95% O2 and 5% CO2). The concentration of each drug was expressed as final concentration in μmol/L.
Vascular Segment Preparation
IMA segments obtained during CABG were immediately placed in ice‐cold Krebs‐bicarbonate solution. Each artery segment was dissected free from perivascular fat and external loose tissues and cut into 3‐mm‐wide rings. Vascular reactivity was assessed using an organ bath preparation, with the isolated IMA rings mounted onto stainless steel hooks and the artery tension measured with a force transducer (AD Instruments, Australia). The organ bath preparation used a 15‐mL water‐jacketed organ bath containing carbogen‐bubbled Krebs solution. Isometric tension was recorded using Lab chart 6 software (AD Instruments), with the system being calibrated daily. Arteries were equilibrated at a resting tension of 19.6 mN for 60 minutes at 37°C before undergoing high K+ solution–dependent contraction 3 times. On reaching a plateau, the high K+ buffer was washed out 3 times and the segments were allowed to equilibrate for 30 minutes. This step was repeated 3 times, and arteries were subjected to cumulative doses of either serotonin (0.001–300 μmol/L) or thromboxane A2 receptor agonist (0.0001–300 μmol/L). After the maximum agonist‐dependent response, arteries were placed in normal Krebs solution for 60 minutes and subsequently contracted to a dose of the agonist (a concentration that produced 70% maximum constriction to that agonist). As previously described, the calcium ionophore (A23187) was used at a concentration of 2 μmol/L, to activate endothelial calcium entry and verify endothelium‐dependent vasodilatation.27 All arteries treated with papaverine hydrochloride during surgery or that produced a force <19.6 mN when submaximally contracted with high K+ solution were considered to be damaged during isolation and excluded from the study.
Mechanistic Studies
Vasoconstrictor agonists producing a sex difference in vascular responses underwent further investigation to elucidate the underlying vascular mechanism, including the role of the endothelium, NO pathway, prostaglandin pathway, and vascular receptors.
Endothelium
Endothelial denudation was performed by mechanical luminal shear abrasion, as previously described.28 Briefly, an effervescent solution, consisting of air and physiological Krebs buffer, was injected through the lumen of the artery, moving the tip of the catheter up and down for 90 seconds in a petri dish to ensure complete removal of the endothelial layer.
NO pathway
The role of NO on sex differences in vascular contractile responses was assessed by the following: (1) NO synthase (NOS) inhibition and (2) electron paramagnetic resonance (EPR)–spin trapped based quantification of stimulated NO release in endothelium‐intact IMA segments. The NOS inhibition studies were conducted using the above vascular segment preparation, with the NOS blocker L‐NAME (300 μmol/L) added for 60 minutes, after confirming isolated vessel viability and endothelial integrity. Thereafter, cumulative concentration‐response curves for the vasomotor agonist were generated.
EPR quantification of NO release was validated using sodium nitroprusside and the calcium ionophore A23187 (10 μmol/L; n=8). The spin trap included Fe (II) dithiocarbamate complexes containing diethyldithiocarbamate (Fe [diethyldithiocarbamate]2) prepared by dissolving 0.45 mg/mL FeSO4 or 0.72 mg/mL diethyldithiocarbamate in 0.9% NaCl bidistilled water (EPR grade) and aerated with nitrogen gas >20 minutes on ice. Three IMA segments (each 3 mm in length) were pooled, placed in Krebs/HEPES buffer before the addition of stimulus or inhibitors, and incubated with the spin trap (equal volume of FeSO4 and diethyldithiocarbamate) complex for 120 minutes at 37°C. Signals were measured using ESR‐Spectrometer e‐scan equipped with Temperature & Gas Controller and Shear Stress Controller (Noxygen Science Transfer & Diagnostics GmbH Bruker Bio Spin Corp). A triplet EPR spectrum of (diethyldithiocarbamate)2–Fe (II)–NO with a N=12.8 gauss and g=2.04 was observed, a characteristic signal for NO in the above spectrum.29 The (diethyldithiocarbamate)2–Fe (II)–NO signal in the IMA segment was markedly suppressed by endothelium denudation and preadministration of the NOS inhibitor, L‐NAME, in rat aorta, confirming that the NO detected from the artery was produced enzymatically by NOS. Calibration solutions of diethylamine nonoate were prepared at final concentrations of 2, 4, 8, and 16 μmol/L, and the signals were measured as NO concentrations/double integral.
Prostaglandin pathway
The role of the prostaglandin pathway on sex differences in vascular responses was assessed by the following: (1) cyclooxygenase inhibition and (2) quantification of stimulated prostaglandin release in endothelium‐intact IMA segments. Similar to the NO pathway studies, isolated IMA segments were incubated with the cyclooxygenase inhibitor (indomethacin, 10 μmol/L) for 60 minutes, after assessment of vessel viability and endothelial integrity; cumulative concentration‐response curves for the vasomotor agonist were generated.
Prostaglandin metabolite levels of 6‐keto prostaglandin F11α, TXB2, prostaglandin F2α, prostaglandin D2, and prostaglandin E2 were quantified in the IMA segment by liquid chromatography/electrospray ionization tandem mass spectrometry, following activation of calcium entry using the calcium ionophore A23187 (2 μmol/L; n=10–12), as previously described by Furugen et al.30 Prostaglandins were isolated from IMA segments 284.5±22.8 mg ground under liquid nitrogen and solubilized in 2 mL prostaglandin homogenizing buffer (containing 1× PBS, 1 mmol/L EDTA, and 10 μmol/L indomethacin, pH 7.4) in a precoated tube with 2 mg butylated hydroxytoluene. Samples were further sonicated for 30 seconds and followed by the addition of 200 ng/mL of internal standard prostaglandins F2α‐d4. After the addition of 1 mL acetone and a 2‐minute vortex, samples were allowed to stand on ice for 20 minutes and centrifuged at 3000g for 15 minutes at 4°C. The supernatant was transferred into a fresh tube, and the acetone layer was evaporated with nitrogen gas. The pH was adjusted to 3 before solid‐phase extraction. A hydrophilic lipophilic balance (HBL) column was used in solid‐phase extraction conditioned with 3 mL methanol and 2% formic acid. Samples were washed with 3 mL solution containing 5% methanol and 2% formic acid, followed by 3 mL 25% methanol and 3 mL hexane, before eluting with 2 mL liquid chromatography–mass spectroscopy grade methanol and dried under nitrogen gas. Samples were reconstituted in 100 μL 25% acetonitrile. Calibration curves for the prostaglandins were linear in the range from 0.2 to 50 ng/mL (r 2>0.99). Calibration curves were constructed by plotting peak area ratio (exogenous standard/internal standard) versus the nominal concentration and were fitted using least‐squares regression.
Chromatography was run using a Nexera X2 Ultra High Performance Liquid Chromatograph (Shimadzu). Column=Kinetex 1.7μ C18 100×2.1 mm with Security Guard ULTRA cartridge (Phenomenex). Gradient conditions of acetonitrile in water/0.1% formic acid were as follows: 18% acetonitrile (0.5 minutes), 18% to 45% acetonitrile (0.5–4 minutes), 45% acetonitrile (4–4.6 minutes), 45% to 63% acetonitrile (4.6–4.8 minutes), 63% acetonitrile (4.8–5.4 minutes), 63% to 100% acetonitrile (5.4–6.5 minutes), and back to 18% (6.6 minutes). The mobile phase flow rate was 0.3 mL/min, and the column temperature was 40°C. The mass spectrometry detection was performed on Shimadzu LCMS‐8050, electrospray ionization in negative ion mode.
Vascular receptors
Vascular receptors were quantified in relation to both total abundance and phosphorylation status. Mn2+ Phos‐tag phosphorylation‐dependent mobility shift analysis was used to quantify total and phosphorylated protein expression of serotonin2A and serotonin2B receptor subtypes.
Proteins were extracted from each 3‐mm IMA ring using 2× Laemmli sample buffer (containing 65.8 mmol/L Tris‐HCl, 26.3% [w/v] glycerol, 2.1% SDS, and 0.01% bromophenol blue), followed by SDS‐PAGE. They were Coomassie stained, and the entire lane of protein for each sample was quantified as an index of extractable vascular proteins; the concentration was adjusted to be equal for all arteries. After normalization of protein levels, samples were subject to a Phos‐tag SDS‐PAGE containing 7.5% polyacrylamide gel with 25 μmol/L Phos‐tag and 50 μmol/L MnCl2 at 150 V for 90 minutes, as described previously by Kinoshita et al, with some modification.31 Proteins were transferred, using the BioRad Transblot Turbo system, onto 0.22‐μm polyvinylidene difluoride at 25 V and 1.3 amps for 7 minutes. Nonspecific binding sites were blocked with 3% nonfat dry milk in 20 mmol/L Tris, 150 mmol/L NaCl, and 0.05% (v/v) Tween‐20 for 60 minutes, followed by incubation with 20 mmol/L Tris, 150 mmol/L NaCl, and 0.05% (v/v) Tween‐20 containing a 1:1000 dilution of either a mouse‐derived affinity‐purified anti‐serotonin2A or a rabbit‐derived serotonin2B antibody (Santa Cruz Biotechnology) for 60 minutes. Polyvinylidene difluoride membranes were washed 4 times in 20 mmol/L Tris, 150 mmol/L NaCl, and 0.05% (v/v) Tween‐20 and incubated for 60 minutes in 20 mmol/L Tris, 150 mmol/L NaCl, and 0.05% (v/v) Tween‐20 containing a 1:10 000 dilution of anti‐mouse (for the 2A receptor) or anti‐rabbit (for the 2B receptor) IgG coupled with Dylight 800 fluorochromes (Pierce Thermo Scientific, Rockford, IL).
Sample Size Calculation
On the basis of a previous isolated vessel study in which the effective concentration to elicit 50% of the maximal response (EC50, expressed as log EC50) was 6.2±0.529 μmol/L,32 to detect a 20% difference in EC50, a sample size of 4 patients is required per group to achieve 80% power with an α of 0.05. This was used in our pilot studies and applied to both the serotonin and thromboxane A2 mimetic (U46619).
Statistical Analysis
The concentration‐response curves of serotonin and the thromboxane mimetic (U46619) were expressed as the percentage of the final high K+ solution contraction. Sigmoid curves of best fit were constructed by performing nonlinear regression analyses using Graph Pad Prism, Version 6 (Graph Pad Software Inc, La Jolla, CA). Nonparametric frequency data, such as clinical characteristics, were compared between sexes using χ2 analysis. Parametric data, with confirmed normality distribution, were compared between sexes using unpaired t tests. An exception was the maximum contraction to high K+ solutions for both intact and endothelium‐denuded IMA, where a 2‐factor ANOVA was used. Data are presented as mean±SEM, with P<0.05 considered statistically significant.
Results
A total of 116 patients (44 women [mean age, 66.8±12.2 years] and 72 men [mean age, 66.6±10.4 years]) were recruited with viable IMA segments. The clinical characteristics of these patients, including their cardiovascular risk factors and regular maintenance medications before surgery, are recorded in the Table. There were no differences between men and women in these clinical characteristics, nor was there a difference in the maximum contraction to high K+ solutions for both intact IMA (men, 23.32±1.92 mN [n=52]; women, 25.31±2.54 mN [n=41]) and endothelium‐denuded IMA (men, 21.22±2.69 mN [n=27]; women, 22.69±1.93 mN [n=21]; P=0.87; Figure 1). Of importance, all women recruited to this study were postmenopausal.
Table 1.
Clinical Characteristics and Maintenance Medications of Study Patients
| Characteristics | Men (n=72) | Women (n=44) | P Value |
|---|---|---|---|
| Age, mean±SDa | 66.8±10.4 | 66.6±12.2 | 0.93 |
| Vascular risk factors | |||
| Current smoker | 19 (26) | 13 (29) | 0.83 |
| Ex‐smoker | 16 (22) | 12 (27) | 0.65 |
| Diabetic | 45 (62) | 25 (57) | 0.56 |
| Hypertension | 48 (67) | 34 (77) | 0.29 |
| Hypercholesterolemia | 47 (65) | 30 (68) | 0.84 |
| Maintenance medications | |||
| Antiplatelet | 62 (86) | 35 (80) | 0.44 |
| Statin | 68 (94) | 40 (91) | 0.48 |
| β Blocker | 58 (80) | 34 (77) | 0.81 |
| ACE inhibitor | 40 (56) | 22 (50) | 0.57 |
| Calcium channel blocker | 44 (61) | 28 (63) | 0.85 |
| Long‐acting nitrate | 45 (63) | 29 (66) | 0.84 |
| Diuretic | 26 (36) | 19 (43) | 0.55 |
| SSRI | 12 (17) | 6 (14) | 0.79 |
| Angiotensin receptor blocker | 21 (29) | 14 (32) | 0.84 |
ACE indicates angiotensin‐converting enzyme; SSRI, selective serotonin reuptake inhibitor.
Figure 1.
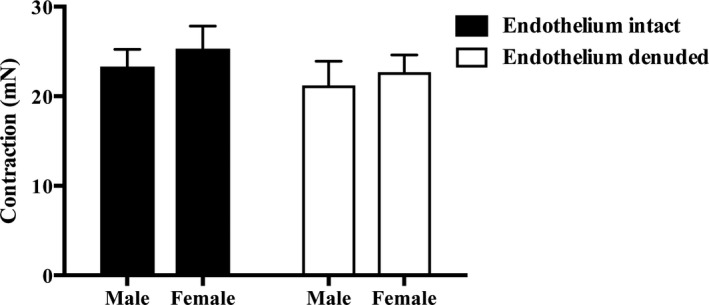
High K+‐mediated vasoconstriction reveals no sex difference in intact (41 women and 52 men) and denuded (21 women and 27 men) internal mammary artery rings.
Vascular Reactivity to Serotonin and U46619
In the endothelium‐intact human IMA segments, women exhibit increased sensitivity (log EC50: men, −6.29±0.07 [n=21]; and women, −6.83±0.09 [n=20]; P=0.001), with no sex difference in maximal contraction (Emax) (Emax: men, 88.27±6.14 [n=21]; and women, 97.80±6.18 [n=20]; P=0.281; Figure 2A) to serotonin.
Figure 2.
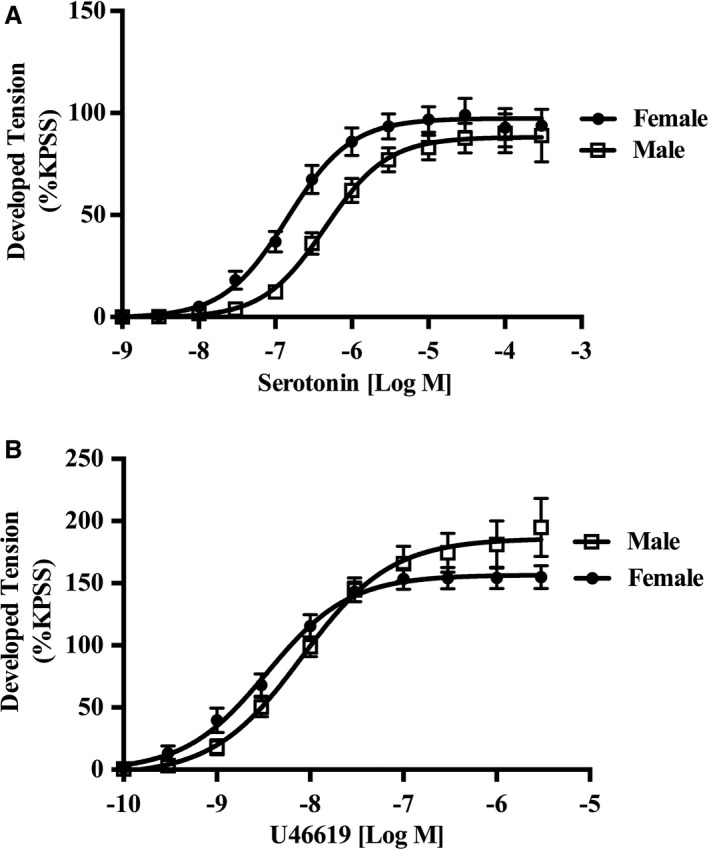
Female internal mammary arteries (IMAs) are hypersensitive to serotonin but not U46619. A, Cumulative dose‐response curves to serotonin (n=21 women, and n=22 men) in isolated rings of human IMAs. Women exhibit increased sensitivity to serotonin than aged matched men, with no sex difference in maximal contraction (see results for details). B, Cumulative dose‐response curves to U46619 (n=10 women, and n=9 men) in isolated rings of human IMAs. No sex differences were observed for both sensitivity and maximum response to U46619 (see results for details). KPSS indicates high K+ solution.
In contrast, there was no sex difference in IMA responses, both in vascular sensitivity (log EC50: men, −8.20±0.08 [n=9]; and women, −8.48±0.11 [n=10]; P=0.52) and maximum contraction (Emax: men, 179.6±17.67 [n=9]; and women, 155.2±8.80 [n=10]; P=0.218; Figure 2B) to thromboxane A2.
Accordingly, further experiments were undertaken to evaluate the mechanisms responsible for the sex differences in serotonin responses, but no further investigation of the thromboxane A2 mimetics response was performed.
Mechanisms for Sex Difference in Serotonin Vascular Reactivity
Role of endothelium in sex‐dependent vascular reactivity to serotonin
After endothelial denudation, the sex difference in vascular sensitivity observed with serotonin in endothelium‐intact vessels was abolished (log EC50: men, −7.04±0.13 [n=11]; and women, −7.02±0.11 [n=10]; P=0.92), with no change in maximal response (Emax: men, 125.3±9.03 [n=11]; and women, 124.9±19 [n=10]; P=0.98; Figure 3A). Hence, the sex difference in serotonin responses is endothelium dependent. As expected, the loss of NO production with denudation produced an increased sensitivity to serotonin in both men and women relative to the endothelium‐intact preparation (Figure 3B and 3C).
Figure 3.
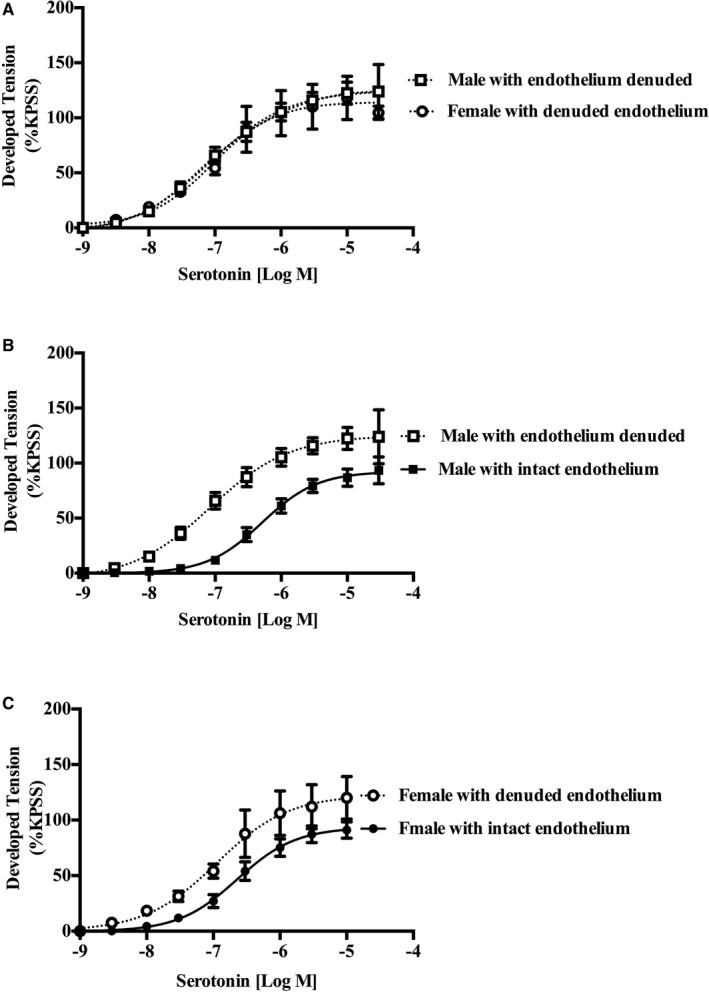
Female internal mammary artery (IMA) hypersensitivity to serotonin is endothelium dependent. Cumulative dose‐response curves to serotonin (n=10 women, and n=11 men) in isolated rings of endothelium denuded human IMA. Endothelium removal abolished female IMA hypersensitivity, with no change in maximum response to serotonin. Further analysis demonstrated that denudation (compared with intact vessel) increased sensitivity to serotonin (B and C). KPSS indicates high K+ solution.
Role of NO in endothelial sex‐dependent vascular reactivity to serotonin
Serotonin concentration‐response curves (0.001–300 μmol/L, n=7–10) were generated in the presence of L‐NAME in an endothelium‐intact human IMA. The NOS inhibition did not affect the sex difference in IMA sensitivity to serotonin (log EC50: men, −6.60±0.14 [n=10]; and women, −7.52±0.24 [n=7]; P=0.003) or maximal contractile response (Emax: men, 130.7±13.38 [n=10]; and women, 126.4±12.20 [n=7]; P=0.82; Figure 4A). Furthermore, direct measurement of NO release via EPR showed no sex difference in response to sodium nitroprusside (total moles of NO generated: men, 0.26±0.06 μmol/L; and women, 0.22±0.05 μmol/L; P=0.66 [n=8]; Figure 4B). Hence, these NOS inhibition and EPR measured NO release experiments provide supporting evidence that the endothelial NO pathway does not contribute to the endothelium‐dependent sex difference in serotonin responses.
Figure 4.
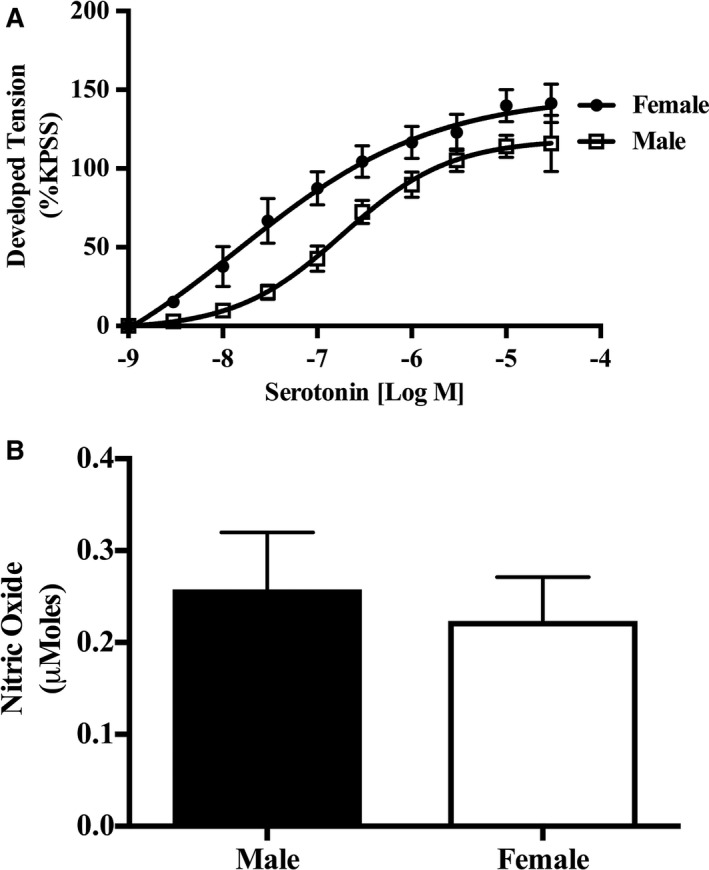
Female internal mammary artery (IMA) hypersensitivity to serotonin is NO synthase (NOS) independent. A, Cumulative dose‐response curves to serotonin in isolated rings of human IMA (n=7 women, and n=10 men) in the presence of the NOS inhibitor (N‐w‐nitro‐l‐arginine methyl ester). Compared with controls, NOS inhibition did not influence female IMA hypersensitivity or maximal contractile response to serotonin. B, NO quantification in isolated rings of human IMA in the presence of sodium nitroprusside reveals no sex difference in NO production in an ex vivo setup (n=8 women, and n=8 men). KPSS indicates high K+ solution.
Role of prostaglandins in endothelial sex‐dependent vascular reactivity to serotonin
The effect of indomethacin on serotonin concentration‐response curves (0.001–300 μmol/L) was assessed on endothelium‐intact human IMA segments. Indomethacin abolished the female IMA hypersensitivity (log EC50: men, −6.80±0.18 [n=9]; and women, −6.79±0.48 [n=6]; P=0.97), with increased female maximum contractile response (Emax: men, 133±9.0 [n=9]; and women, 144.9±10.37 [n=6]; P=0.01; Figure 5A), to serotonin. Furthermore, individual analysis within sex in the presence or absence of the cyclooxygenase inhibitor indomethacin revealed a significant shift in the male but not the female serotonin concentration response (Figure 5B).
Figure 5.
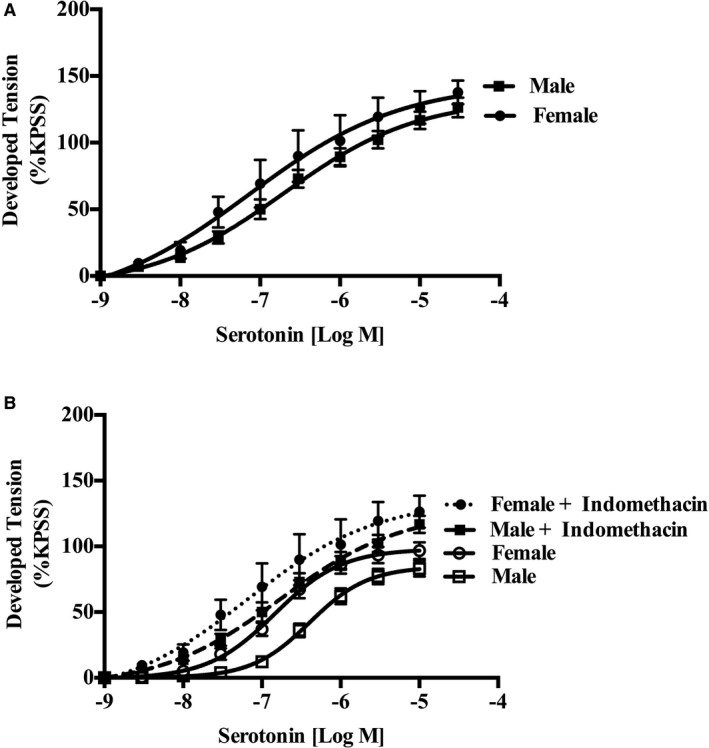
Female internal mammary artery (IMA) hypersensitivity to serotonin is cyclooxygenase (COX) dependent, with a significant shift in the male curve in the presence of indomethacin. A, Cumulative dose‐response curves to serotonin (n=6 women, and n=9 men) in isolated rings of human IMA in the presence of the nonselective COX inhibitor (indomethacin). COX inhibition abolished female IMA hypersensitivity, with increased female maximum response to serotonin. B, Evaluation of the male and female responses with and without indomethacin reveals a significant shift in the male, but not the female, serotonin concentration response (data derived from Figures 2A, 3, and 5A). KPSS indicates high K+ solution.
Direct prostaglandin metabolite measurement by mass spectrometry revealed no difference in the prostaglandin F2α metabolite level (men, 0.015±0.004 ng/mg [n=12]; and women, 0.011±0.004 ng/mg [n=10]; P=0.56; Figure 6A) but a trend towards reduced 6‐keto prostaglandin F1α in female compared with male IMAs (men, 0.035±0.001 ng/mg [n=12]; and women, 0.017±0.004 ng/mg [n=10]; P=0.08; Figure 6B). These data suggest that female IMAs produce less prostacyclin compared with male IMAs but comparable amounts of the prostaglandin F2α vasoconstrictor.
Figure 6.
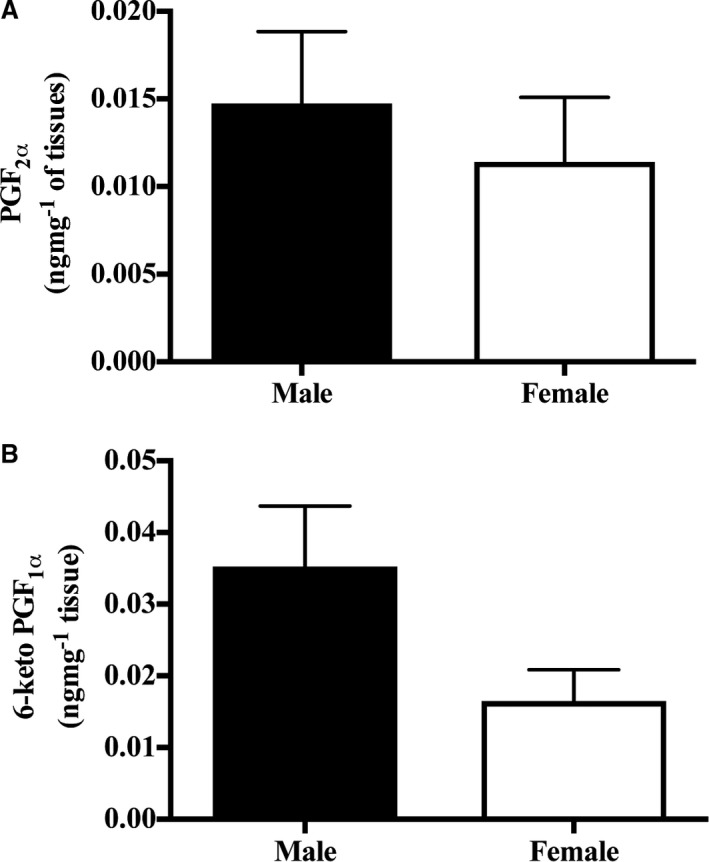
Male and female internal mammary artery expresses comparable amounts of prostaglandin F2α (PGF 2α) and 6‐keto prostaglandin F1α (PGF 1α). Quantification of the PGF 2α vasoconstrictor metabolite (A) and the 6‐keto PGF 1α vasodilatory metabolite (n=10 women, and n=12 men; B).
Quantification of total serotonin2A+2B abundance and phosphorylation status
Phos‐tag coupled with Western blot analysis revealed equal phosphorylation status and abundance of both serotonin2A receptor (phosphorylation: men, 39.90±12.35 [n=6]; and women, 33.26±6.39 [n=5] [P=0.66]; and abundance: men, 2.3×104±7.4×103 [n=6]; and women, 2.2×104±4.5×103 [n=5] [P=0.99]; Figure 7A, 7C, and 7E) and serotonin2B receptor (phosphorylation: men, 22.93±3.3 [n=6]; and women, 29.32±3.96 [n=5] [P=0.24]; and abundance: men, 2.2×106±4.1×105 [n=6]; and women, 2.1×106±5.5×105 [n=5] [P=0.87]; Figure 7B, 7D, and 7F) isoforms.
Figure 7.
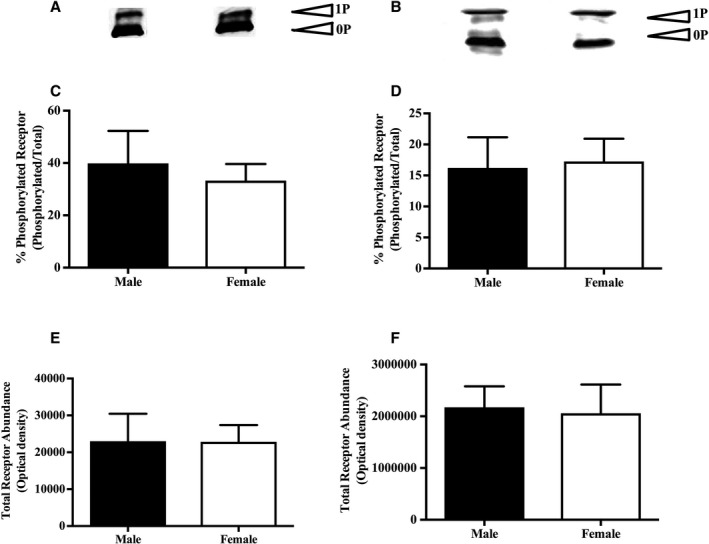
Female internal mammary artery (IMA) hypersensitivity is independent of serotonin2A and serotonin2B receptor activity. A and B, Representative Western blot of serotonin2A and serotonin2B receptors, respectively, in the phosphorylated and unphosphorylated state (n=5 women, and n=6 men). C and D, Cumulative data of the rate of phosphorylated to total serotonin2A and serotonin2B receptors, respectively (n=5 women, and n=6 men). E and F, Total serotonin2A and serotonin2B receptor abundance, respectively, in male and female IMAs (n=5 women, and n=6 men). 0P indicates unphosphorylated receptor; 1P, phosphorylated receptor.
Discussion
We report several novel findings in this study. First, female IMAs are hypersensitive to serotonin but not U46619, suggesting that sex‐dependent female IMA hypersensitivity is agonist specific. Second, the mechanism responsible for this sex difference in vascular reactivity to serotonin involves endothelium‐dependent factors that are independent of vascular NOS but dependent on the vascular cyclooxygenase pathway, and unrelated to vascular serotonin receptor abundance or phosphorylation. This endothelial cyclooxygenase‐mediated female IMA hypersensitivity to serotonin could be an important biological mechanism contributing to postoperative IMA graft spasm in women, thereby resulting in major adverse cardiac events.
Species‐Specific Sex Differences in Vascular Reactivity
Few studies have examined sex differences in responses to serotonin and/or thromboxane in an isolated vessel preparation. Nuno et al33 and Lamping and Faraci34 previously revealed male hypersensitivity to serotonin but not thromboxane A2 in mice. However, no sex difference to serotonin responses was observed in studies using porcine vessels.35, 36 Furthermore, Barber and Miller reported sex differences to endothelin‐1 contraction but not prostaglandin F2α in porcine coronary artery.37 This suggests species‐specific sex differences in vascular responses to serotonin, necessitating the study of human vessels.
In isolated human IMA segments, a previous study revealed increased female maximum contraction (Emax) but not hypersensitivity (EC50) to serotonin.38 The lack of female IMA sensitivity may be attributable to the experimental preparation because the present study excluded samples in which papaverine (a potent vasodilator) was administered by the surgeons. This could potentially blunt the vasoconstrictor responses, whereas this factor was not addressed in the previous study.
Absence of Sex Difference in Thromboxane Vascular Reactivity
The lack of a sex difference in IMA responses to the thromboxane analogue may be attributed to homeostatic regulatory pathways involving thromboxane and endothelial prostaglandin I2 production. Endothelial cell culture studies have demonstrated that high concentrations of thromboxane agonists may inhibit endothelial prostaglandin I2 production,39 thereby mimicking the use of indomethacin and masking any endothelial cyclooxygenase‐mediated female IMA hypersensitivity in the presence of the thromboxane analogue.
Mechanism of Sex Difference in Serotonin Vascular Reactivity
To the best of our knowledge, this is the first study to further evaluate the vascular mechanism responsible for the observed sex difference to serotonin in human IMA. The conclusion from our mechanistic studies is that this difference is dependent on the endothelial cyclooxygenase pathway, given its abolition by endothelial denudation and indomethacin. Moreover, within sex, analysis revealed a significant shift in the male but not the female serotonin concentration‐response curve with indomethacin (Figure 5B), suggesting that the abolition of the serotonin sex difference is attributable to the men becoming more sensitive to serotonin (ie, becoming more like women) in the presence of indomethacin. Hence, indomethacin may block male endothelium‐dependent vasodilatory prostanoids, which account for the sex difference in serotonin responses.
The mechanism of sex difference in porcine coronary artery relaxations to bradykinin and the α2‐adrenergic agonist (UK‐14304), studied by Barber and Miller, is mediated by cyclooxygenase products other than thromboxane A2 or prostacyclin.37 However, the lack of this sex difference not mediated by prostaglandin I2 would be a result of difference in organism; also, porcine did not age in the same way as human.
Considering the findings of our research, it could be hypothesized that male IMA segments should produce more prostacyclin than their female counterparts; however, measurement of IMA prostacyclin metabolite production showed no statistically significant sex difference, although there was a trend. The lack of statistical significance may be interpreted as the assay having limited sensitivity in detecting the sex difference in prostacyclin production and/or a type 2 error. Thus, it remains possible that increased endothelial prostacyclin production in men accounts for the observed sex difference in serotonin IMA responses.
This impaired endothelial prostacyclin production in women (relative to men) is consistent with umbilical vein cord studies, which demonstrate reduced prostacyclin production in women.40 It is also consistent with the high prevalence of primary pulmonary hypertension in women, a condition treated with prostacyclin infusion. In addition, it may contribute to the sex difference in CABG outcomes. Thus, platelet activation during surgery may result in the serotonin release, stimulating both platelet aggregation and endothelium‐dependent vasoconstriction, which would be exaggerated in women because of their impaired endothelial prostaglandin I2 production. This could result in more prevalent and/or more extensive ischemia than their male counterpart, thereby contributing to the observed sex differences in CABG outcomes.
Study Limitations
There are several limitations to the previously described studies, which should be considered in interpreting the clinical relevance of the findings. First, only postmenopausal women were recruited, possibly reflecting the protective role of endogenous female sex hormones against coronary artery disease. Whether there is a sex difference in IMA reactivity between premenopausal and postmenopausal women or men is unknown; however, Steinleitner et al demonstrated a decreased arterial production of prostacyclin in postmenopausal women compared with their premenopausal counterparts.41
Second, the ex vivo preparation allowed independent evaluation of vascular responses to individual agonists, demonstrating a sex difference in vasomotor reactivity to serotonin. However, the clinical in vivo setting is more complex, with multiple intraluminal agonists influencing vascular tone as well as autacoids, neural inputs, and counterregulatory homeostatic mechanisms. Thus, whether an isolated biological sex difference in serotonin vascular reactivity could result in significant clinical events is open to speculation. However, it may potentially contribute to sex differences in clinical outcomes in combination with other biological, psychosocial, and clinical factors.
Conclusion
This study identified biological sex differences in vascular reactivity of the IMA to serotonin but not U46619, with the former attributable to an endothelium‐dependent cyclooxygenase pathway involving possible impaired prostaglandin I2 productions in women. This sex difference may predispose women to developing IMA graft spasm when exposed to platelet‐derived serotonin and may potentially contribute to the increased post‐CABG adverse cardiac events experienced by women.
Sources of Funding
This research was supported by the Discipline of Medicine, University of Adelaide, and Cardiology Research Unit, Basil Hetzel Institute for Translational Health Research, The Queen Elizabeth Hospital.
Disclosures
None.
Acknowledgments
We thank the surgical team of D'Arcy Sutherland Cardiothoracic Surgical Unit, Royal Adelaide Hospital, for their support during sample collection. Furthermore, we acknowledge the expertise of Dr John Licari in the electron paramagnetic resonance assay development.
(J Am Heart Assoc. 2018;7:e007126 DOI: 10.1161/JAHA.117.007126.)
References
- 1. Vaccarino V, Abramson JL, Veledar E, Weintraub WS. Sex differences in hospital mortality after coronary artery bypass surgery: evidence for a higher mortality in younger women. Circulation. 2002;105:1176–1181. [DOI] [PubMed] [Google Scholar]
- 2. Saxena A, Dinh D, Smith JA, Shardey G, Reid CM, Newcomb AE. Sex differences in outcomes following isolated coronary artery bypass graft surgery in Australian patients: analysis of the Australasian Society of Cardiac and Thoracic Surgeons cardiac surgery database. Eur J Cardiothorac Surg. 2012;41:755–762. [DOI] [PubMed] [Google Scholar]
- 3. O'Connor GT, Morton JR, Diehl MJ, Olmstead EM, Coffin LH, Levy DG, Maloney CT, Plume SK, Nugent W, Malenka DJ;; The Northern New England Cardiovascular Disease Study Group . Differences between men and women in hospital mortality associated with coronary artery bypass graft surgery. Circulation. 1993;88:2104–2110. [DOI] [PubMed] [Google Scholar]
- 4. Jaglal SB, Tu JV, Naylor CD; Provincial Adult Cardiac Care Network of Ontario . Higher in‐hospital mortality in female patients following coronary artery bypass surgery: a population‐based study. Clin Invest Med. 1995;18:99–107. [PubMed] [Google Scholar]
- 5. Yusuf S, Hawken S, Ounpuu S, Dans T, Avezum A, Lanas F, McQueen M, Budaj A, Pais P, Varigos J, Lisheng L. Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case‐control study. Lancet. 2004;364:937–952. [DOI] [PubMed] [Google Scholar]
- 6. Fiebach NH, Viscoli CM, Horwitz RI. Differences between women and men in survival after myocardial infarction: biology or methodology? JAMA. 1990;263:1092–1096. [PubMed] [Google Scholar]
- 7. Frasure‐Smith N, Lesperance F, Juneau M, Talajic M, Bourassa MG. Gender, depression, and one‐year prognosis after myocardial infarction. Psychosom Med. 1999;61:26–37. [DOI] [PubMed] [Google Scholar]
- 8. Roshan TM, Normah J, Rehman A, Naing L. Effect of menopause on platelet activation markers determined by flow cytometry. Am J Hematol. 2005;80:257–261. [DOI] [PubMed] [Google Scholar]
- 9. Bobbert P, Stellbaum C, Steffens D, Schutte C, Bobbert T, Schultheiss HP, Rauch U. Postmenopausal women have an increased maximal platelet reactivity compared to men despite dual antiplatelet therapy. Blood Coagul Fibrinolysis. 2012;23:723–728. [DOI] [PubMed] [Google Scholar]
- 10. Robich MP, Araujo EG, Feng J, Osipov RM, Clements RT, Bianchi C, Sellke FW. Altered coronary microvascular serotonin receptor expression after coronary artery bypass grafting with cardiopulmonary bypass. J Thorac Cardiovasc Surg. 2010;139:1033–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mohammad‐Zadeh LF, Moses L, Gwaltney‐Brant SM. Serotonin: a review. J Vet Pharmacol Ther. 2008;31:187–199. [DOI] [PubMed] [Google Scholar]
- 12. Metais C, Bianchi C, Li J, Li J, Simons M, Sellke FW. Serotonin‐induced human coronary microvascular contraction during acute myocardial ischemia is blocked by COX‐2 inhibition. Basic Res Cardiol. 2001;96:59–67. [DOI] [PubMed] [Google Scholar]
- 13. Webb RC. Smooth muscle contraction and relaxation. Adv Physiol Educ. 2003;27:201–206. [DOI] [PubMed] [Google Scholar]
- 14. Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5‐hydroxytryptamine (serotonin). Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 15. Hirata T, Ushikubi F, Kakizuka A, Okuma M, Narumiya S. Two thromboxane A2 receptor isoforms in human platelets: opposite coupling to adenylyl cyclase with different sensitivity to Arg60 to Leu mutation. J Clin Invest. 1996;97:949–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Somlyo AP, Somlyo AV. Signal transduction and regulation in smooth muscle. Nature. 1994;372:231–236. [DOI] [PubMed] [Google Scholar]
- 17. Van Nueten JM, Janssens WJ, Vanhoutte PM. Serotonin and vascular reactivity. Pharmacol Res Commun. 1985;17:585–608. [DOI] [PubMed] [Google Scholar]
- 18. Gelosa P, Ballerio R, Banfi C, Nobili E, Gianella A, Pignieri A, Brioschi M, Guerrini U, Castiglioni L, Blanc‐Guillemaud V, Lerond L, Tremoli E, Sironi L. Terutroban, a thromboxane/prostaglandin endoperoxide receptor antagonist, increases survival in stroke‐prone rats by preventing systemic inflammation and endothelial dysfunction: comparison with aspirin and rosuvastatin. J Pharmacol Exp Ther. 2010;334:199–205. [DOI] [PubMed] [Google Scholar]
- 19. Shirahase H, Fujiwara M, Usui H, Kurahashi K. A possible role of thromboxane A2 in endothelium in maintaining resting tone and producing contractile response to acetylcholine and arachidonic acid in canine cerebral arteries. Blood Vessels. 1987;24:117–119. [DOI] [PubMed] [Google Scholar]
- 20. Gude NM, Boura AL, King RG, Brennecke SP, Jamal OS, Smith R, Walters WA. Evidence for inhibition by endothelium‐derived relaxing factor of thromboxane A2 receptor‐mediated vasoconstriction in the fetal vessels of the human perfused placenta. Placenta. 1992;13:597–605. [DOI] [PubMed] [Google Scholar]
- 21. Tostes RC, Nigro D, Fortes ZB, Carvalho MH. Effects of estrogen on the vascular system. Braz J Med Biol Res. 2003;36:1143–1158. [DOI] [PubMed] [Google Scholar]
- 22. Guo X, Razandi M, Pedram A, Kassab G, Levin ER. Estrogen induces vascular wall dilation: mediation through kinase signaling to nitric oxide and estrogen receptors alpha and beta. J Biol Chem. 2005;280:19704–19710. [DOI] [PubMed] [Google Scholar]
- 23. Sherwood A, Park SB, Hughes JW, Blumenthal JA, Hinderliter A, Trivedi R, McFetridge‐Durdle J. Cardiovascular hemodynamics during stress in premenopausal versus postmenopausal women. Menopause. 2010;17:403–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jensen‐Urstad K, Johansson J. Gender difference in age‐related changes in vascular function. J Intern Med. 2001;250:29–36. [DOI] [PubMed] [Google Scholar]
- 25. Willerson JT, Golino P, Eidt J, Campbell WB, Buja LM. Specific platelet mediators and unstable coronary artery lesions: experimental evidence and potential clinical implications. Circulation. 1989;80:198–205. [DOI] [PubMed] [Google Scholar]
- 26. Hirsh PD, Hillis LD, Campbell WB, Firth BG, Willerson JT. Release of prostaglandins and thromboxane into the coronary circulation in patients with ischemic heart disease. N Engl J Med. 1981;304:685–691. [DOI] [PubMed] [Google Scholar]
- 27. Huraux C, Makita T, Kurz S, Yamaguchi K, Szlam F, Tarpey MM, Wilcox JN, Harrison DG, Levy JH. Superoxide production, risk factors, and endothelium‐dependent relaxations in human internal mammary arteries. Circulation. 1999;99:53–59. [DOI] [PubMed] [Google Scholar]
- 28. Lamin V, Worthington M, Edwards J, Viana F, Stuklis R, Wilson D, Beltrame J. Endothelial denudation of isolated human internal mammary artery segments. Cardiovasc Pharm Open Access. 2016;5:180. [Google Scholar]
- 29. Jackson SK, Thomas MP, Smith S, Madhani M, Rogers SC, James PE. In vivo EPR spectroscopy: biomedical and potential diagnostic applications. Faraday Discuss. 2004;126:103–117; discussion 69–83. [DOI] [PubMed] [Google Scholar]
- 30. Furugen A, Yamaguchi H, Tanaka N, Ito H, Miyamori K, Fujikawa A, Takahashi N, Ogura J, Kobayashi M, Yamada T, Mano N, Iseki K. Quantification of intracellular and extracellular prostanoids stimulated by A23187 by liquid chromatography/electrospray ionization tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879:3378–3385. [DOI] [PubMed] [Google Scholar]
- 31. Kinoshita E, Kinoshita‐Kikuta E, Ujihara H, Koike T. Mobility shift detection of phosphorylation on large proteins using a Phos‐tag SDS‐PAGE gel strengthened with agarose. Proteomics. 2009;9:4098–4101. [DOI] [PubMed] [Google Scholar]
- 32. Jarajapu YP, Coats P, McGrath JC, MacDonald A, Hillier C. Increased alpha(1)‐ and alpha(2)‐adrenoceptor‐mediated contractile responses of human skeletal muscle resistance arteries in chronic limb ischaemia. Cardiovasc Res. 2001;49:218–225. [DOI] [PubMed] [Google Scholar]
- 33. Nuno DW, Korovkina VP, England SK, Lamping KG. RhoA activation contributes to sex differences in vascular contractions. Arterioscler Thromb Vasc Biol. 2007;27:1934–1940. [DOI] [PubMed] [Google Scholar]
- 34. Lamping KG, Faraci FM. Role of sex differences and effects of endothelial NO synthase deficiency in responses of carotid arteries to serotonin. Arterioscler Thromb Vasc Biol. 2001;21:523–528. [DOI] [PubMed] [Google Scholar]
- 35. Miller VM, Lewis DA, Barber DA. Gender differences and endothelium‐ and platelet‐derived factors in the coronary circulation. Clin Exp Pharmacol Physiol. 1999;26:132–136. [DOI] [PubMed] [Google Scholar]
- 36. Cox DA, Cohen ML. Influence of gender on vasomotor effects of oxidized low‐density lipoprotein in porcine coronary arteries. Am J Physiol. 1997;272(pt 2):H2577–H2583. [DOI] [PubMed] [Google Scholar]
- 37. Barber DA, Miller VM. Gender differences in endothelium‐dependent relaxations do not involve NO in porcine coronary arteries. Am J Physiol. 1997;273(pt 2):H2325–H2332. [DOI] [PubMed] [Google Scholar]
- 38. Dignan RJ, Yeh T Jr, Dyke CM, Lutz HA III, Wechsler AS. The influence of age and sex on human internal mammary artery size and reactivity. Ann Thorac Surg. 1992;53:792–797. [DOI] [PubMed] [Google Scholar]
- 39. Nicholson NS, Smith SL, Fuller GC. Effect of the stable endoperoxide analog U‐46619 on prostacyclin production and cyclic AMP levels in bovine endothelial cells. Thromb Res. 1984;35:183–192. [DOI] [PubMed] [Google Scholar]
- 40. Batres RO, Dupont J. Gender differences in prostacyclin and prostaglandin E2 synthesis by human endothelial cells. Prostaglandins Leukot Med. 1986;22:159–171. [DOI] [PubMed] [Google Scholar]
- 41. Steinleitner A, Stanczyk FZ, Levin JH, d'Ablaing G III, Vijod MA, Shahbazian VL, Lobo RA. Decreased in vitro production of 6‐keto‐prostaglandin F1 alpha by uterine arteries from postmenopausal women. Am J Obstet Gynecol. 1989;161:1677–1681. [DOI] [PubMed] [Google Scholar]


