Abstract
Background
Hypertensive disorders complicating pregnancy are a major cause of maternal death. Our objective was to evaluate maternal clinical, hemodynamic, and placental prognostic indicators in a consolidated manner to identify women who develop hypertension in pregnancy.
Methods and Results
Twenty‐six normotensive pregnant women from a specialized Placenta Clinic at increased risk of developing de novo hypertension and 20 normotensive healthy pregnant controls were recruited at 22 to 26 weeks' gestation. Fourteen maternal clinical, hemodynamic, and placental characteristics were assessed in the second trimester and aggregated. Principal component analysis of this combined data set determined that 3 dimensions accounted for 56% of the cohort variability. The first dimension accounted for 31% of the cohort variability, with significant contributions from total peripheral resistance, endoglin, and cardiac output. The second dimension was predominantly influenced by body mass index and mean arterial pressure, while uric acid and myeloperoxidase mainly contributed to the third dimension. Unsupervised clustering identified 3 groups within this combined data set. Total peripheral resistance was the most significant distinguishing parameter between these groups (P<0.0001), followed by placental growth factor, endoglin, and cardiac output (P<0.0001). Using these 4 parameters, a receiver operating curve was constructed with an area under the curve of 0.975 (95% confidence interval 0.93–1) for the prediction of developing hypertension in pregnancy.
Conclusions
Consolidated assessment of prognostic indicators in the second trimester of pregnancy may be useful to characterize and distinguish pathways by which women may develop hypertension in pregnancy. This approach could contribute to the development of pathway‐specific preventative and antihypertensive treatment strategies.
Keywords: hemodynamics, hypertension, preeclampsia/pregnancy, pregnancy
Subject Categories: Hypertension, Pregnancy, Preeclampsia, Hemodynamics
Clinical Perspective
What Is New?
The development of hypertension in pregnancy places women at significantly higher risk of maternal and fetal adverse outcomes during pregnancy and increases their long‐term risk of cardiovascular disease and death.
This study identified unique groups of clinical, hemodynamic and placental variables that contribute to the development of hypertension in pregnancy amongst pre‐symptomatic pregnant women in the second trimester.
The phenotypes of pregnant women at low‐, moderate‐ and high‐risk of developing hypertension in pregnancy were characterized.
What Are the Clinical Implications?
Our findings emphasize that hypertension in pregnancy may evolve along several distinct pathways; this approach has the potential to develop more effective preventative and treatment strategies.
Consolidated assessment of clinical, hemodynamic and placental variables should be evaluated in a large‐scale prospective trial to assess the risk of pre‐symptomatic pregnant women for the development of a hypertensive disorder of pregnancy.
Introduction
Hypertension in pregnancy is the second leading global cause for maternal death following hemorrhage, accounting for 14% of all maternal deaths worldwide.1 Prevention and management of hypertension in pregnancy is a critical clinical initiative, as pregnant women presenting with hypertension can rapidly deteriorate into a life‐threatening hypertensive crisis that potentially requires hospitalization, intense monitoring, and iatrogenic preterm delivery to stabilize the maternal condition. In addition to the acute significant maternal and perinatal risk associated with hypertension during pregnancy, women with a history of hypertension in pregnancy are also at significantly higher risk of future cardiovascular disease.2
It is well accepted that the majority of pregnant women who subsequently develop hypertension in pregnancy exhibit abnormalities in maternal hemodynamics before the clinical development of hypertension, with or without aberrations in circulating angiogenic proteins that regulate vascular function early in pregnancy.3, 4, 5 The clinical syndromes of hypertension in pregnancy are distinctly heterogeneous. Hypertension may appear at different time points of pregnancy and may occur in isolation or have concurrent features of preeclampsia. The fetus may or may not be growth restricted, while the mother may or may not exhibit features of placental dysfunction, metabolic syndrome or have a family history of cardiovascular disease. Importantly, the natural history in terms of risks for mother and fetus are also highly variable, and the ability to predict these risks is an important priority. Consequently, the obstetric and cardiology communities have made joint calls to try and effectively utilize prognostic indicators as a strategy to identify, classify and guide treatment for hypertensive disorders of pregnancy.6, 7, 8 To effectively translate current knowledge and develop effective pathway‐specific therapeutic strategies, the predictive value of clinical characteristics, maternal hemodynamic and placental indicators must be evaluated.
The objective of the current study was to explore the application of maternal clinical characteristics, hemodynamics, and circulating levels of angiogenic proteins through unsupervised classification to identify and characterize distinct phenotypes of pregnant women who are at increased risk of developing hypertensive disorders of pregnancy.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Selection of Pregnant Women
This is a secondary analysis of a prospective cohort study that recruited normotensive pregnant women at low‐ and high‐risk of developing de novo hypertension in pregnancy.9 Low‐risk pregnant women were recruited from the prenatal clinics at Mount Sinai Hospital, herein referred to as “healthy pregnant women”. Inclusion criteria of the healthy group included a singleton pregnancy at 22 to 26 weeks' gestation with no clinical risk factors, maternal health concerns or fetal abnormalities. Pregnant women at increased risk of hypertensive disorders of pregnancy were recruited from the Maternal‐Fetal Medicine Division, Placenta Clinic at Mount Sinai Hospital; this clinic specializes in placental complications, including preeclampsia and fetal growth restriction. This group is herein referred to as “screen‐positive women”. Inclusion criteria for this group included singleton pregnancy at 22 to 26 weeks' gestation accompanied by at least 2 of the following categories: (1) abnormal placental biochemistry; (2) abnormal placental shape or texture; (3) abnormal uterine artery Doppler; and (4) abnormal clinical risk factor score. Exclusion criteria included any additional maternal clinical risk factors or fetal health concerns, including pre‐existing maternal hypertension.9 Eligible subjects were invited to participate in the study and provided written informed consent. This study was reviewed and approved by the Human Subjects Review Committee of Mount Sinai Hospital (MSH REB 12‐0083‐A).
Hemodynamic Assessment
Blood pressure, heart rate and non‐invasive measures of maternal cardiac output were assessed after 15 minutes of rest in a quiet temperature‐ and humidity‐controlled environment. Transthoracic bioreactance was used to estimate stroke volume continuously for 15 minutes (Cheetah Medial, Vancouver, WA). Pulsatility index of the uterine arteries was determined using pulsed‐wave Doppler (Philips Medical Systems, Eindhoven, Netherlands).
Biochemical Analysis
Venous blood samples were collected from the antecubital vein.9 Serum and plasma samples were immediately sent for biochemical analysis through Mount Sinai laboratory services as part of routine clinical assessment, including complete blood count, creatinine, uric acid and liver function tests. Plasma samples were stored at −80°C and were later quantified using ELISAs for placental growth factor (PlGF), soluble fms‐like tyrosine kinase‐1 (sFlt‐1), soluble endoglin, myeloperoxidase and endothelin (R&D Systems, Burlington, Ontario, Canada), according to manufacturers' instructions.
Pregnancy Outcomes
Delivery information for all subjects was collected through electronic medical records. Information collected included gestational age at delivery, delivery mode, fetal demographics, placental pathology reports and incidence of adverse pregnancy outcomes. Hypertensive disorders of pregnancy were classified as gestational hypertension or preeclampsia. Gestational hypertension was defined as systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg on 2 occasions at least 4 hours apart after 20 weeks' gestation in women with previously normal blood pressure. Preeclampsia was defined as systolic blood pressure >140 mm Hg or diastolic blood pressure >90 mm Hg on 2 occasions at least 4 hours apart after 20 weeks' gestation in women with previously normal blood pressure and proteinuria, defined as >300 mg protein per 24‐hour urine collection, a protein/creatinine ratio >0.3 or a dipstick reading of 1+. In the absence of proteinuria, preeclampsia was also defined as hypertension accompanied by thrombocytopenia, renal insufficiency, impaired liver function, pulmonary edema or cerebral/visual symptoms.
Statistical Analysis
Statistical analysis and data visualization were performed by R‐Studio (1.1.383, RStudio, Inc 2016; Boston, MA, http://www.rstudio.com. Since the data sets are continuous numeric variables, FactoMineR (1.39) computed hierarchical clustering on principal components approach was applied to combine objective clustering technique with principal components analysis results. The random missing values were imputed by mice package (2.46.0). The optimized cluster number was selected by NbClust (3.0) according to the majority rule when 30 partitioning indices were calculated. Factoextra package (1.0.5) was used for visualizing the cluster results. Receiver operating curve plot was constructed according to the 4 most significant variables highlighted in principal components outcomes using ROCR (1.0–7) and pROC (1.10.0) packages. Kruskal–Wallis rank sum test was performed to compare statistical differences between multiple groups. Normally distributed data are presented as means±SD, whereas non‐normally distributed data are presented as median (interquartile range). P values of <0.05 were set as the threshold for significance.
Results
Demographic Characteristics and Pregnancy Outcomes of Cohort
Twenty healthy women and 26 screen‐positive women were recruited for study participation. Detailed demographic and delivery information has been previously reported (Table S1).9 While no women were clinically hypertensive in the second trimester, screen‐positive women demonstrated a low stroke volume, high peripheral resistance hemodynamic profile with lower circulating levels of the pro‐angiogenic protein PlGF, compared with healthy pregnant women (Table S1).9 Gestational age and birthweight were also significantly lower in screen‐positive women when compared with healthy pregnant women, while the subsequent incidence of hypertensive disorders of pregnancy was significantly higher (Table S1).9
This cohort therefore comprised of 46 pregnant women with 14 variables relating to maternal clinical characteristics (maternal age, body mass index, uric acid), hemodynamics (mean arterial pressure, heart rate, stroke volume, cardiac output, peripheral resistance, pulsatility index) and circulating levels of angiogenic proteins (PlGF, endoglin, sFlt‐1, myeloperoxidase, endothelin).
Principal Component Analysis
Principal component analysis is a statistical algorithm that transforms original variables into linear combinations of lower dimensionality variables, known as principal components. These principal components represent the largest possible variance in a data set that is then limited to the most relevant parameters, therefore increasing the interpretability of complex data.10 Principal component analysis suggested that the dimensionality of the current cohort could be reduced to 3 dominant principal components that accounted for 56% of variability among women for the characteristics assessed. The dominant principal component, dimension 1, accounted for 31% of the total variance (Table 1). Amongst the 14 variables assessed, the largest contributors to dimension 1 are peripheral resistance (20%), endoglin (15%), cardiac output (13%), and PlGF (10%) (Table 1; Figure 1). Dimension 2 was largely contributed to by maternal body mass index (35%) and mean arterial pressure (19%), while the main contributors to principal component 3 were uric acid (21%) and myeloperoxidase (21%) (Table 1; Figure 1).
Table 1.
Contribution of Significant Variables to the Top 3 Dimensions, Possibly Representing Unique Pathways to Hypertension in Pregnancy
| Variance | Dimension 1 31% | Dimension 2 13% | Dimension 3 11% | Hypertension Phenotype |
|---|---|---|---|---|
| Peripheral resistance | 20% | Placenta‐mediated | ||
| Endoglin | 15% | |||
| Cardiac output | 13% | |||
| BMI | 35% | Maternal clinical characteristics | ||
| Mean arterial pressure | 19% | |||
| Uric acid | 21% | Inflammation | ||
| Myeloperoxidase | 21% | |||
| Dimension variability | 48% | 54% | 42% |
BMI indicates body mass index.
Figure 1.
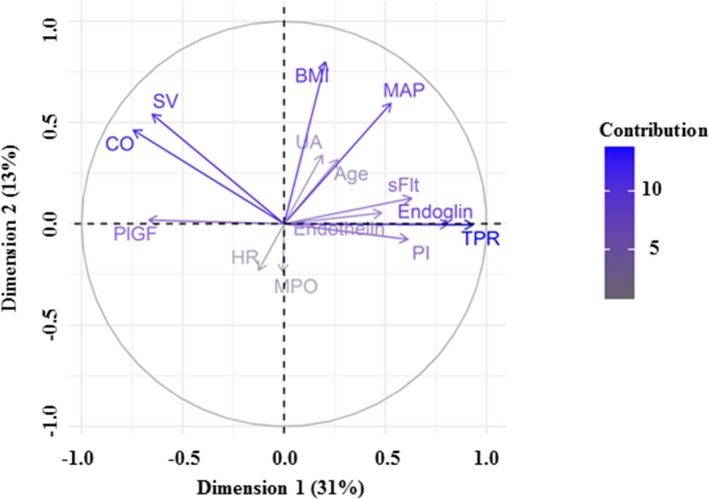
Visualization of dimension 1 and dimension 2, accounting for 44% of the cohort variability. Age indicates maternal age; BMI, body mass index; CO, cardiac output; HR, heart rate; MAP, mean arterial pressure; MPO, myeloperoxidase; PI, pulsatility index; PlGF, placental growth factor; SV, stroke volume; TPR, total peripheral resistance; UA, uric acid.
Cluster Analysis
Cluster analysis is an explorative technique used to separate a data set into natural groups, or “clusters,” that may have been previously unidentified. This is a statistical method to identify and characterize the subgroups that exist in a population. The Nbclust function was utilized for cluster validity to determine the optimal number of clusters.11 Of the 30 indices used by this function, the majority (12) of indices proposed that 3 clusters were the optimal partition pattern.
Cluster 1 was comprised of 26 pregnant women (18 healthy pregnant women and 8 screen‐positive women), Cluster 2 was comprised of 15 pregnant women (2 healthy pregnant women and 13 screen‐positive women), and Cluster 3 was comprised of 5 pregnant women (0 healthy pregnant women and 5 screen‐positive women). There were significant differences between the clusters in maternal hemodynamics (mean arterial pressure, stroke volume, cardiac output, peripheral resistance, pulsatility index) and circulating angiogenic proteins (PlGF, endoglin, sFlt‐1), while none of the maternal clinical characteristics were found to differ between the 3 subgroups (Table 2). Peripheral resistance, PlGF, cardiac output, and endoglin were the variables that differed most significantly between the 3 clusters (P<0.0001, Table 2).
Table 2.
Pregnancy Outcomes and Hemodynamic, Protein and Clinical Characteristics of the Clusters Derives Through Hierarchical Clustering on Principal Components
| Cluster 1 Low‐Risk (n=26) | Cluster 2 Moderate‐Risk (n=15) | Cluster 3 High‐Risk (n=5) | P‐Value | Assessment Type | |
|---|---|---|---|---|---|
| Incidence of hypertension | 1 (4) | 4 (27) | 5 (100) | <0.0001 | |
| Birthweight, kg | 3.2±0.5 | 1.9±0.8 | 1.5±0.3 | 0.0001 | |
| Peripheral resistance, dynes·s/cm5 | 883 (766–998) | 1156 (1095–1252) | 1603 (1528–1706) | <0.0001 | Hemodynamic |
| PlGF, pg/mL | 424±150 | 211±140 | 39±15 | <0.0001 | Protein |
| Cardiac output, L/min | 7.2±1.0 | 5.9±0.71 | 4.6±0.71 | <0.0001 | Hemodynamic |
| Endoglin, pg/mL | 5.9 (5–7) | 7.9 (7–10) | 32.3 (25–37) | <0.0001 | Protein |
| sFlt, pg/mL | 1042±435 | 2386±1701 | 4214±2009 | 0.0002 | Protein |
| Pulsatility index | 0.93 (0.82–1.46) | 1.8 (1.5–2.1) | 2.5 (2.0–2.8) | 0.0004 | Clinical |
| Stroke volume, mL | 93±15 | 79±15 | 63±7 | 0.0013 | Hemodynamic |
| Mean arterial pressure, mm Hg | 78±5 | 86±11 | 92±10 | 0.0021 | Clinical |
| Endothelin, pg/mL | 0.7 (0.6–1.0) | 1.1 (0.5–1.8) | 1.0 (0.7–3.1) | 0.1110 | Protein |
| BMI, kg/m2 | 26±4 | 28±5 | 28±5.5 | 0.3090 | Clinical |
| Maternal age, y | 33±3 | 34±4 | 33±6.0 | 0.4480 | Clinical |
| Heart rate, bpm | 79±9 | 76±9 | 75±10 | 0.5524 | Hemodynamic |
| Myeloperoxidase, pg/mL | 23±14 | 22±12 | 27±19 | 0.8345 | Protein |
| Uric acid, μmol/L | 201 (185–237) | 233 (186–285) | 260 (166–367) | 0.9250 | Clinical |
Data are presented as mean±SD or median (interquartile range), as appropriate. BMI indicates body mass index; PlGF, placental growth factor; sFlt‐1, soluble fms‐like tyrosine kinase‐1.
Cluster 1 (herein referred to as “low‐risk” of hypertension) was characterized by a high volume, low resistance hemodynamic profile with normal levels of circulating angiogenic proteins and maternal blood pressure, along with normal birthweight and 4% incidence of hypertensive disorders of pregnancy (Table 2). Cluster 2 (herein referred to as “moderate‐risk” of hypertension) was characterized by a lower volume, higher resistance hemodynamic profile compared with Cluster 1, with higher sFlt‐1 and blood pressure levels (Table 2). Women in Cluster 2 also had lower birthweight and a 27% incidence of hypertension during pregnancy. When compared with the women in Cluster 1, Cluster 3 (herein referred to as “high‐risk” of hypertension) exhibited a low volume, high resistance hemodynamic profile with an elevated anti‐angiogenic protein profile and higher blood pressure levels (Table 2). Pregnant women in Cluster 3 had a lower birthweight and 100% incidence of hypertension during pregnancy. A visualization exhibiting individual patients within the clusters on the respective principal components is provided (Figure 2).
Figure 2.
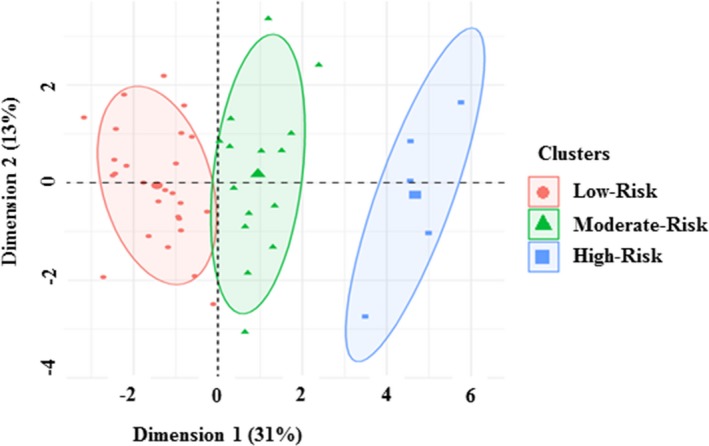
Visualization of individuals from the low‐, moderate‐, and high‐risk of hypertension clusters overlaid on a principal component space of dimension 1 vs dimension 2.
Prediction Value of Second Trimester Screening for the Development of Hypertension
The predictive value of second trimester maternal clinical, hemodynamic, and angiogenic protein characteristics were evaluated for the development of hypertension in pregnancy. After input of individual variables, generalized linear models were built to predict the potential pathological outcomes. Maternal peripheral resistance was the best single predictive factor for the development of hypertension in pregnancy (area under the receiver operating characteristic curve 0.95, specificity 0.89, sensitivity 0.90, threshold 0.19), followed by endoglin, mean arterial pressure and PlGF, sFlt‐1 (Figure 3A). The accuracy values of these variables were greater than 80% (Table S2).
Figure 3.
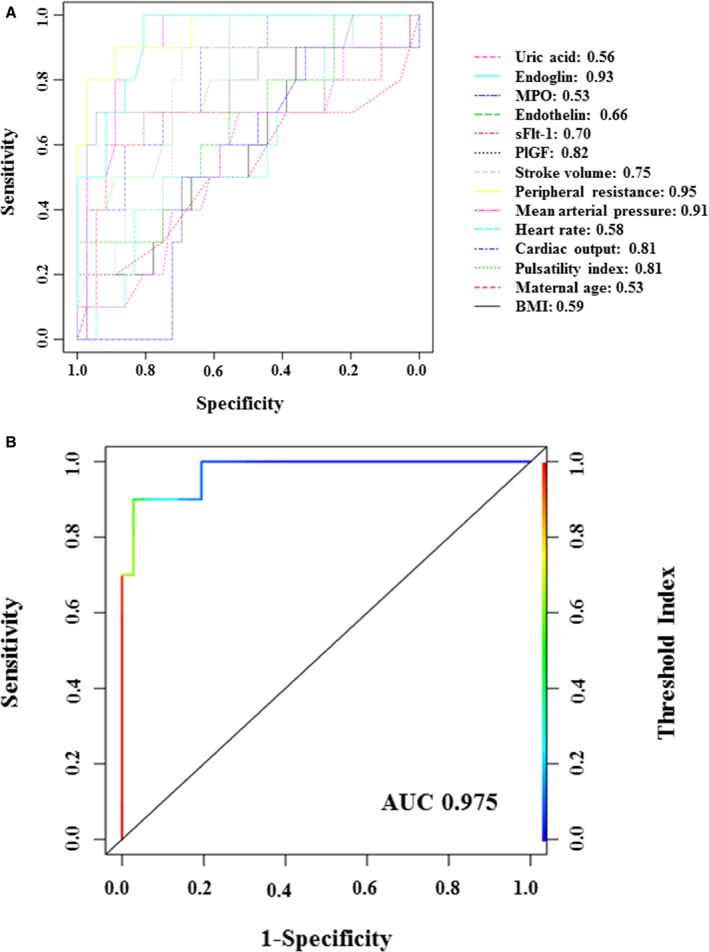
Receiver operating curves for the prediction of hypertension in pregnancy using individual maternal clinical, hemodynamic, and protein parameters (A) or the consolidated variables peripheral resistance, endoglin, cardiac output, and PlGF (B). BMI indicates body mass index; MPO, myeloperoxidase.
Because of the relatively small sample size of this cohort, the 4 most significant variables that differentiated women into 3 clusters were arbitrarily selected as the consolidated indicators for the prediction of hypertension, rather than the consolidated assessment of all 14 variables. These variables included a combination of hemodynamic and protein characteristics, including peripheral resistance, endoglin, cardiac output, and PlGF which were also supported by principal component analysis outcomes for their strong contribution to overall variability. The receiver operating curve performance of this combined model had an improved area under the curve value of 0.975 (specificity 0.97, sensitivity 0.90, threshold 0.39; Figure 3B).
Discussion
In the present study, we identified important hemodynamic, clinical, and circulating protein variables that differentiate pregnant women at low‐, moderate‐, and high‐risk of developing a hypertensive disorder of pregnancy. We then characterized the phenotypes of pregnant women in the second trimester who subsequently develop hypertension in pregnancy. Lastly, we determined the most significant clinical cardiovascular and circulating protein characteristics assessed in the second trimester demonstrating promising potential for predicting the development of hypertension in pregnancy.
The majority of variation in this cohort was accounted for by 3 principal components, thereby identifying the most relevant of the maternal clinical, hemodynamic and protein parameters. The first principal component was mainly contributed to by parameters that are typically aberrant in clinical cases of placenta‐mediated hypertension, including peripheral resistance, endoglin, cardiac output, and PlGF.12, 13 Conversely, the main contributors to the second and third principal components align with pathways to hypertension relating to maternal clinical characteristics and systemic inflammation, respectively.14, 15 These findings highlight the critical need for the development of pathway‐specific antihypertensive treatment strategies, with the understanding that hypertension in pregnancy does not involve only one common origin.7
Women at the highest risk of developing a hypertensive disorder of pregnancy exhibit a distinct cardiovascular, placental and clinical phenotype before the development of hypertension, consistent with previous reports.3, 9, 16, 17 To the best of our knowledge, this is the first analysis to evaluate clinical characteristics in a consolidated manner, rather than individually. Interestingly, this analysis identified a group of pregnant women who exhibited moderate abnormalities in hemodynamic and circulating proteins, with only 27% of these women subsequently developing hypertension in pregnancy. This observation illustrates the importance of the interaction between abnormalities in cardiovascular function and placental disease, which contrive together to mediate a clinically‐relevant hypertensive disorder of pregnancy.
Maternal characteristics in the second trimester were used to predict the subsequent development of hypertension in pregnancy. Principal component analysis identified the 4 most important variables that significantly differentiated the cohort of pregnant women into independent subgroups (peripheral resistance, PlGF, cardiac output, and endoglin) produced an area under the curve of 0.975 (95% confidence interval 0.93–1). This is consistent with a recent study investigating maternal biochemical and biophysical cardiovascular markers of pregnant women presenting with de novo hypertension, proteinuria, or clinical suspicion of preeclampsia; total peripheral resistance index, cardiac index and the sFlt‐1/PlGF ratio were identified as independent predictors of hypertension in pregnancy.18 An additional investigation used maternal hemodynamics to predict the development of preeclampsia in pregnant women with bilateral notching of the uterine artery Doppler; consistent with our findings, total peripheral resistance was identified as the best independent predictor of maternal and fetal complications.19 Relative wall thickness of the left ventricle and left ventricular mass were also identified as independent predictors of complications. Although the current study involved a small cohort, the observations provide support for further investigation into the role of second trimester maternal hemodynamic and placental screening for phenotypes of hypertensive disorders of pregnancy in a larger, heterogeneous population.
Current standard‐of‐care screening for hypertensive disorders of pregnancy mostly relies on clinical risk factors, although some centers have integrated PlGF testing into their clinical platforms for preeclampsia screening.20, 21 In a nulliparous population, the addition of PlGF assessment in the first trimester of pregnancy improved the identification of pregnant women at risk of preeclampsia, when compared with clinical risk factors alone.21 Comprehensive cardiovascular assessment is not currently standard‐of‐care in high‐risk pregnancy clinics, which merely measure maternal blood pressure. Interestingly, the current study determined that the variables that differed most significantly between the subgroups of pregnant women at low‐, moderate‐, and high‐risk of subsequent hypertension development were peripheral resistance, PlGF, cardiac output, and endoglin. In addition, the majority of standard maternal characteristics, such as body mass index, maternal age, and uric acid, were not identified as important variables for this prediction, although pulsatility index of the uterine artery was established as a relevant ultrasound‐derived parameter. The consolidated assessment of maternal hemodynamics, clinical characteristics and circulating levels of angiogenic proteins may provide critical insight into the key pathways that mediate hypertensive disorders of pregnancy, integrating maternal placental development during pregnancy and the response of the maternal cardiovascular system to placental function. This cluster analysis approach was recently used to identify novel subgroups of patients with diabetes mellitus with unique characteristics and distinct risk of disease complication; this information could result in specific treatment pathways for patients with diabetes mellitus.22
The development of effective early pregnancy screening programs for hypertensive disorders of pregnancy is a critically important clinical initiative. As demonstrated in the current study, non‐invasive hemodynamic monitoring could be a valuable addition to current methods available for the screening and management of hypertensive disorders of pregnancy. This suggests that consolidated screening strategies may be superior to current standard‐of‐care screening tools that are confined to maternal clinical risk factors and placental function, although large screening trials are required to validate the clinical utility of the current findings. It is hypothesized that the inclusion of non‐invasive hemodynamic monitoring would translate into increased knowledge of maternal cardiovascular function of each individual patient, as well as the potential opportunity to initiate tailored, personalized antihypertensive therapy.7 While there are various antihypertensive therapies available for use during pregnancy, these therapies can have differing effects on maternal heart rate, cardiac output, and systemic vascular resistance.23 Blood pressure control rates in non‐pregnant hypertensive populations were determined to be superior when antihypertensive therapy was guided by patient hemodynamics versus specialist care alone.24, 25 In these trials, predefined algorithms recommended increased vasodilator therapy with reductions in β‐blockers for patients with low cardiac index and high vascular resistance; conversely, increased β‐blocker therapy with reductions in vasodilators were recommended for patients with high cardiac index and low systemic vascular resistance index. As recently reviewed, few trials have evaluated hemodynamic‐guided antihypertensive therapy in pregnant women.7 Similar to the non‐pregnant literature, antihypertensive therapy based on cardiac output and total peripheral resistance may be an effective therapeutic strategy to improve maternal blood pressure control.23, 26, 27, 28, 29 Hemodynamic‐guided antihypertensive therapy for pregnant women presenting with any type of hypertension significantly reduced the rates of severe maternal hypertension from 18% to 3.5%, when compared with standard care.30 Early detection and characterization of hypertensive disorders of pregnancy should also be investigated from a cost‐savings perspective, as maternal cardiovascular and circulating protein information could impact decision making and disease trajectory.31, 32 From a feasibility perspective, commercial non‐invasive hemodynamic monitoring systems are now available for the convenient and safe monitoring maternal hemodynamics in pregnant women. Such devices are of great clinical utility, allowing clinicians to assess hemodynamic information that would otherwise be unavailable and provide information that is directly relevant to clinical care of women with high‐risk pregnancies in a safe manner during gestation.33 Future research should focus on large, prospective studies that investigate the usefulness of maternal hemodynamic, placental, and clinical indicators for the screening and prediction of hypertensive disorders of pregnancy in a heterogeneous population.
It is important to acknowledge the limitations of the current study. The sample size of this study was small and recruited women exclusively from a specialty placenta clinic, targeting women at high‐risk of preeclampsia. In addition, this investigation excluded pregnant women with chronic hypertension, an important subset of hypertensive pregnant women. We also acknowledge that the high prevalence of white women in this study may represent a potential selection bias. Lastly, the pregnant women recruited for this study were at low‐and high‐risk of developing de novo hypertension in pregnancy on the basis of specific inclusion criteria. Future research should therefore focus on evaluating the clinical usefulness of consolidated screening strategies across the general pregnant population.
Conclusions
Consolidated assessment of prognostic indicators in the second trimester of pregnancy that include hemodynamic, placental, and clinical characteristics may be useful to precisely characterize and distinguish hypertensive women, leading to pathway‐specific screening programs with tailored preventative strategies. This strategy may be superior to current standard‐of‐care tools that solely assess maternal clinical risk factors and placental function; the focus of future research should be to validate these findings in a large‐scale screening trial.
Sources of Funding
J.C. Kingdom is supported by the Alva Foundation and Canadian Institutes of Health Research.
Disclosures
None.
Supporting information
Table S1. Demographic, Hemodynamic, Circulating Protein and Pregnancy Outcome Characteristics of Healthy Pregnant and Screen‐Positive
Table S2. Screening Test Characteristics of the Individual and Consolidated Variables (Peripheral Resistance, Endoglin, Cardiac Output and PlGF) for the Prediction of Hypertension in Pregnancy
(J Am Heart Assoc. 2018;7:e009595 DOI: 10.1161/JAHA.118.009595.)
References
- 1. Say L, Chou D, Gemmill A, Tuncalp O, Moller AB, Daniels J, Gulmezoglu AM, Temmerman M, Alkema L. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014;2:e323–e333. [DOI] [PubMed] [Google Scholar]
- 2. Tooher J, Thornton C, Makris A, Ogle R, Korda A, Hennessy A. All hypertensive disorders of pregnancy increase the risk of future cardiovascular disease. Hypertension. 2017;70:798–803. [DOI] [PubMed] [Google Scholar]
- 3. Valensise H, Vasapollo B, Gagliardi G, Novelli GP. Early and late preeclampsia: two different maternal hemodynamic states in the latent phase of the disease. Hypertension. 2008;52:873–880. [DOI] [PubMed] [Google Scholar]
- 4. Levine RJ, Maynard SE, Qian C, Lim KH, England LJ, Yu KF, Schisterman EF, Thadhani R, Sachs BP, Epstein FH, Sibai BM, Sukhatme VP, Karumanchi SA. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–683. [DOI] [PubMed] [Google Scholar]
- 5. Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, Stillman IE, Roberts D, D'Amore PA, Epstein FH, Sellke FW, Romero R, Sukhatme VP, Letarte M, Karumanchi SA. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–649. [DOI] [PubMed] [Google Scholar]
- 6. Ferrazzi E, Stampalija T, Monasta L, Di Martino D, Vonck S, Gyselaers W. Maternal hemodynamics: a method to classify hypertensive disorders of pregnancy. Am J Obstet Gynecol. 2018;218:124.e1–124.e11. [DOI] [PubMed] [Google Scholar]
- 7. McLaughlin K, Scholten RR, Kingdom JC, Floras JS, Parker JD. Should maternal hemodynamics guide antihypertensive therapy in preeclampsia? Hypertension. 2018;71:550–556. [DOI] [PubMed] [Google Scholar]
- 8. Ukah UV, Hutcheon JA, Payne B, Haslam MD, Vatish M, Ansermino JM, Brown H, Magee LA, von Dadelszen P. Placental growth factor as a prognostic tool in women with hypertensive disorders of pregnancy: a systematic review. Hypertension. 2017;70:1228–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McLaughlin K, Baczyk D, Potts A, Hladunewich M, Parker JD, Kingdom JC. Low molecular weight heparin improves endothelial function in pregnant women at high risk of preeclampsia. Hypertension. 2017;69:180–188. [DOI] [PubMed] [Google Scholar]
- 10. Ringner M. What is principal component analysis? Nat Biotechnol. 2008;26:303–304. [DOI] [PubMed] [Google Scholar]
- 11. Charrad M, Ghazzali N, Boiteau V, Niknafs A. Package ‘NbClust’. 2015.
- 12. Goulopoulou S. Maternal vascular physiology in preeclampsia. Hypertension. 2017;70:1066–1073. [DOI] [PubMed] [Google Scholar]
- 13. Karumanchi SA. Angiogenic factors in preeclampsia: from diagnosis to therapy. Hypertension. 2016;67:1072–1079. [DOI] [PubMed] [Google Scholar]
- 14. Hall JE, do Carmo JM, da Silva AA, Wang Z, Hall ME. Obesity‐induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guzik TJ, Touyz RM. Oxidative stress, inflammation, and vascular aging in hypertension. Hypertension. 2017;70:660–667. [DOI] [PubMed] [Google Scholar]
- 16. Macdonald‐Wallis C, Lawlor DA, Fraser A, May M, Nelson SM, Tilling K. Blood pressure change in normotensive, gestational hypertensive, preeclamptic, and essential hypertensive pregnancies. Hypertension. 2012;59:1241–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Melchiorre K, Sutherland G, Sharma R, Nanni M, Thilaganathan B. Mid‐gestational maternal cardiovascular profile in preterm and term pre‐eclampsia: a prospective study. BJOG. 2013;120:496–504. [DOI] [PubMed] [Google Scholar]
- 18. Verlohren S, Perschel FH, Thilaganathan B, Droge LA, Henrich W, Busjahn A, Khalil A. Angiogenic markers and cardiovascular indices in the prediction of hypertensive disorders of pregnancy. Hypertension. 2017;69:1192–1197. [DOI] [PubMed] [Google Scholar]
- 19. Vasapollo B, Novelli GP, Valensise H. Total vascular resistance and left ventricular morphology as screening tools for complications in pregnancy. Hypertension. 2008;51:1020–1026. [DOI] [PubMed] [Google Scholar]
- 20. Walker MG, Bujold E, Kingdom JC. Is Canada ready to adopt maternal placental growth factor testing to improve clinical outcomes for women with suspected preeclampsia? J Obstet Gynaecol Canada. 2017;39:580–583. [DOI] [PubMed] [Google Scholar]
- 21. Myers JE, Kenny LC, McCowan LM, Chan EH, Dekker GA, Poston L, Simpson NA, North RA. Angiogenic factors combined with clinical risk factors to predict preterm pre‐eclampsia in nulliparous women: a predictive test accuracy study. BJOG. 2013;120:1215–1223. [DOI] [PubMed] [Google Scholar]
- 22. Ahlqvist E, Storm P, Karajamaki A, Martinell M, Dorkhan M, Carlsson A, Vikman P, Prasad RB, Aly DM, Almgren P, Wessman Y, Shaat N, Spegel P, Mulder H, Lindholm E, Melander O, Hansson O, Malmqvist U, Lernmark A, Lahti K, Forsen T, Tuomi T, Rosengren AH, Groop L. Novel subgroups of adult‐onset diabetes and their association with outcomes: a data‐driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361–369. [DOI] [PubMed] [Google Scholar]
- 23. Scardo JA, Vermillion ST, Newman RB, Chauhan SP, Hogg BB. A randomized, double‐blind, hemodynamic evaluation of nifedipine and labetalol in preeclamptic hypertensive emergencies. Am J Obstet Gynecol. 1999;181:862–866. [DOI] [PubMed] [Google Scholar]
- 24. Taler SJ, Textor SC, Augustine JE. Resistant hypertension: comparing hemodynamic management to specialist care. Hypertension. 2002;39:982–988. [DOI] [PubMed] [Google Scholar]
- 25. Smith RD, Levy P, Ferrario CM. Value of noninvasive hemodynamics to achieve blood pressure control in hypertensive subjects. Hypertension. 2006;47:771–777. [DOI] [PubMed] [Google Scholar]
- 26. Easterling TR, Benedetti TJ, Schmucker BC, Carlson KL. Antihypertensive therapy in pregnancy directed by noninvasive hemodynamic monitoring. Am J Perinatol. 1989;6:86–89. [DOI] [PubMed] [Google Scholar]
- 27. Vasapollo B, Novelli GP, Gagliardi G, Tiralongo GM, Pisani I, Manfellotto D, Giannini L, Valensise H. Medical treatment of early‐onset mild gestational hypertension reduces total peripheral vascular resistance and influences maternal and fetal complications. Ultrasound Obstet Gynecol. 2012;40:325–331. [DOI] [PubMed] [Google Scholar]
- 28. Cornette J, Buijs EA, Duvekot JJ, Herzog E, Roos‐Hesselink JW, Rizopoulos D, Meima M, Steegers EA. Hemodynamic effects of intravenous nicardipine in severely pre‐eclamptic women with a hypertensive crisis. Ultrasound Obstet Gynecol. 2016;47:89–95. [DOI] [PubMed] [Google Scholar]
- 29. Easterling TR, Brateng D, Schmucker B, Brown Z, Millard SP. Prevention of preeclampsia: a randomized trial of atenolol in hyperdynamic patients before onset of hypertension. Obstet Gynecol. 1999;93:725–733. [DOI] [PubMed] [Google Scholar]
- 30. Stott D, Papastefanou I, Paraschiv D, Clark K, Kametas NA. Serial hemodynamic monitoring to guide treatment of maternal hypertension leads to reduction in severe hypertension. Ultrasound Obstet Gynecol. 2017;49:95–103. [DOI] [PubMed] [Google Scholar]
- 31. Duckworth S, Chappell LC, Seed PT, Mackillop L, Shennan AH, Hunter R. Placental growth factor (PlGF) in women with suspected pre‐eclampsia prior to 35 weeks' gestation: a budget impact analysis. PLoS One. 2016;11:e0164276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Vatish M, Strunz‐McKendry T, Hund M, Allegranza D, Wolf C, Smare C. sFlt‐1/PlGF ratio test for pre‐eclampsia: an economic assessment for the UK. Ultrasound Obstet Gynecol. 2016;48:765–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McLaughlin K, Wright SP, Kingdom JCP, Parker JD. Clinical validation of non‐invasive cardiac output monitoring in healthy pregnant women. J Obstet Gynaecol Canada. 2017;39:1008–1014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Demographic, Hemodynamic, Circulating Protein and Pregnancy Outcome Characteristics of Healthy Pregnant and Screen‐Positive
Table S2. Screening Test Characteristics of the Individual and Consolidated Variables (Peripheral Resistance, Endoglin, Cardiac Output and PlGF) for the Prediction of Hypertension in Pregnancy


