Abstract
Background
Diabetes mellitus (DM) is a major risk factor for cardiovascular events. We aimed to investigate the coronary plaque phenotype of diabetic patients who presented with acute coronary syndromes by optical coherence tomography.
Methods and Results
A total of 322 patients with acute coronary syndromes who underwent preintervention optical coherence tomography imaging of the culprit lesion were included. Culprit plaque characteristics were compared between patients with DM (n=95) and those without DM (n=227). In the subgroup of 250 patients in whom sufficient length of nonculprit region in the culprit vessel was imaged by optical coherence tomography, the characteristics of nonculprit plaques were also evaluated. Patients with DM had a higher prevalence of lipid‐rich plaque (58.9% versus 44.9%, P=0.030) and macrophage accumulation (60.0% versus 44.9%, P=0.019) in the culprit lesion compared with patients without DM. The prevalence of plaque rupture (33.7% versus 30.4%, P=0.896) and plaque erosion (21.1% versus 22.0%, P=0.458) was similar. In the nonculprit lesions, the DM group had greater maximal lipid arc (248.9°±83.9° versus 179.9°±58.3°, P=0.006), thinner fibrous cap thickness (103.3±56.2 μm versus 140.7±70.0 μm, P=0.013), and a higher prevalence of thin‐cap fibroatheroma (17.2% versus 6.3%, P=0.031), compared with the non‐DM group.
Conclusions
Compared with patients without DM, those with DM had more vulnerable features in both culprit and nonculprit lesions, thus indicating a higher level of panvascular instability.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT01110538.
Keywords: acute coronary syndrome, diabetes mellitus, optical coherence tomography, plaque
Subject Categories: Optical Coherence Tomography (OCT)
Clinical Perspective
What Is New?
The present study represents the largest series so far among the optical coherence tomography studies in patients with diabetes mellitus. Our results demonstrated that patients with diabetes mellitus had a higher prevalence of lipid‐rich plaque and macrophage accumulation at the culprit plaques and greater lipid arc, thinner fibrous cap, and more prevalent thin‐cap fibroatheroma at the nonculprit regions, indicating a higher level of panvascular plaque instability.
What Are the Clinical Implications?
Our results suggest that plaques in patients with diabetes mellitus and acute coronary syndromes have a higher level of pancoronary vulnerability.
Therefore, more aggressive risk including tight control of glucose, lipid, and blood pressure is particularly important in patients with diabetes mellitus who present with acute coronary syndromes.
Introduction
Diabetes mellitus (DM) is a major risk factor for coronary artery disease and cardiovascular events.1, 2 Coronary arteries of patients with DM are angiographically characterized as showing diffuse narrowing and multivessel disease.3, 4 Pathology studies have demonstrated that patients with DM have plaques with larger necrotic cores, increased presence of inflammation, and advanced coronary artery calcification.5 Recently, several small studies have reported in vivo plaque characteristics in patients with DM using optical coherence tomography (OCT), but with conflicting results.6, 7, 8 In the present study, we compared the plaque characteristics of patients with DM who presented with acute coronary syndromes (ACS) in both the culprit and the nonculprit lesions to those of patients without DM from a multicenter experience. We also compared the culprit plaque morphologies according to the underlying culprit lesion pathology (rupture versus erosion) and the clinical presentation between patients with and without DM.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Study Population
Between August 2010 and May 2014, 799 patients with ACS who had undergone preintervention OCT imaging of the culprit plaque were identified in the Massachusetts General Hospital OCT Registry (http://ClinicalTrials.gov: NCT01110538). Patients with in‐stent restenosis (n=150), imaging after predilatation (n=75), poor image quality (n=45), short pullback (n=12), and incomplete demographic, clinical, or imaging data (n=195) were excluded. Finally, 322 patients with ACS were included in the analysis. Diagnosis of ACS included ST‐segment–elevation myocardial infarction or non‐ST‐segment–elevation acute coronary syndrome (NSTE‐ACS) as previously described.9 The culprit lesion was identified based on angiographic findings, ECG changes, and/or left ventricular wall motion abnormalities. In patients with multiple stenoses, the culprit lesion was identified as the lesion having the most severe stenosis or with evidence of recent plaque disruption, such as a filling defect suggestive of thrombus on angiogram. Patients were assigned to the DM group if receiving insulin or oral hypoglycemic agents, had fasting plasma glucose level ≥126 mg/dL, 2‐hour plasma glucose level ≥200 mg/dL by oral glucose tolerance test, classic symptoms with casual plasma glucose level ≥200 mg/dL, or hemoglobin A1c (HbA1c) level ≥6.5%.10, 11 The DM group was further divided into 2 subgroups: a high‐HbA1c group (HbA1c≥8%) and a low‐HbA1c group (HbA1c<8%). Plaque characteristics were compared between DM patients and non‐DM patients. The Massachusetts General Hospital OCT Registry was approved by the Institutional Review Board at each participating site, and all patients provided written informed consent before enrollment.
OCT Image Acquisition and Analysis
OCT examination was performed using either a frequency‐domain (C7‐XR OCT Intravascular Imaging System, St Jude Medical, St. Paul, MN) or time‐domain (M2/M3 Cardiology Imaging Systems, LightLab Imaging Inc, Westford, MA) OCT system, as previously reported.7, 12 All OCT images were submitted to the Cardiology Laboratory for Integrative Physiology and Imaging at Massachusetts General Hospital and analyzed by 2 independent investigators who were blinded to clinical, angiographic, and laboratory data using an offline review workstation (St Jude Medical). Any discordance was resolved by consensus with a third reviewer. Cross‐sectional OCT images were analyzed at 1‐mm intervals. Mean reference area was calculated from proximal and distal references, identified as sites with the largest lumen areas proximal and distal to the stenosis within the same segment.12, 13 Minimal lumen area was defined as the smallest lumen area within the length of the plaque.14 Percentage area stenosis was calculated as [(mean reference lumen area−minimal lumen area)/mean reference lumen area]×100.14 Lipid was defined as a signal‐poor region with a poorly defined or diffuse border, and the degree of lipid arc was measured in lipid plaques.12, 13 Fibrous cap thickness (FCT) overlying a lipid plaque was measured 3 times at its thinnest part, and the average value was calculated.7, 12 Lipid length was obtained by longitudinal view, and lipid index was calculated as the product of mean lipid arc and lipid length.7, 12 Lipid‐rich plaque was defined as a plaque with a maximal lipid arc >90°.7 Thin‐cap fibroatheroma (TCFA) was defined as a plaque with maximal lipid arc >90° and thinnest FCT ≤65 μm.7, 15 Macrophage accumulation was defined as the presence of highly backscattering focal regions within the fibrous cap.13 Microvessels were defined as the presence of signal‐poor structures with vesicular or tubular shapes.12, 13 Cholesterol crystals were identified as thin and linear regions of high signal intensity with high backscattering within a plaque.12, 13 Calcification was defined as signal‐poor or heterogeneous regions with sharply delineated borders.13 Spotty calcium was defined as the presence of lesions containing calcification arc <90° and extending in length for 1 to 4 mm.16, 17 Thrombus was defined as an irregular mass with minimum diameter >250 μm adherent to the vessel wall or floating within the lumen.12, 13 Plaque rupture was defined as fibrous cap discontinuity with cavity formation.13 Plaque erosion was identified by the presence of either an attached thrombus overlying an intact and visualized plaque, a luminal surface irregularity at the culprit lesion in the absence of thrombus, or attenuation of underlying plaque by thrombus without superficial lipid or calcification immediately proximal or distal to the site of thrombus.9 In patients with multiple stenoses, the culprit lesion was identified as the lesion having the most severe stenosis and/or with evidence of recent plaque disruption including thrombus. Plaque characteristics of the culprit lesions were compared between patients with DM and those without DM. Nonculprit plaques with area stenosis >50% on OCT were also identified.11 In the subgroup of patients in whom sufficient length (≥30 mm) of nonculprit region in the culprit vessel was imaged by OCT, the characteristics of nonculprit plaques were also evaluated.
Statistical Analysis
All analyses were performed using SPSS Statistics 23.0 software (IBM Corp, Armonk, NY). Categorical data were expressed as absolute frequencies and percentages, and compared using the chi‐squared test or Fisher exact test as appropriate. Continuous variables were expressed as mean±SD for normally distributed variables and as median (interquartile range) for nonnormally distributed variables and compared using the Student t test or Mann‐Whitney test as appropriate. Comparisons of plaque characteristics between different groups were carried out using generalized estimating equations to account for potential cluster effects of multiple nonculprit plaques in a single patient. A generalized linear model (ie, multivariable logistic model) for categorical variables and a general linear model (ie, multivariable regression) for continuous variables were used to adjust for differences in baseline patient characteristics between groups as appropriate. A P<0.05 was considered statistically significant.
Results
Patient Characteristics
A total of 322 patients with ACS who had undergone preintervention OCT imaging of the culprit lesion were investigated. Among them, 95 patients (29.5%) had DM. DM patients were older, more frequently observed in female, and had a higher prevalence of hypertension and a lower prevalence of current smoking than non‐DM patients. Patient characteristics are summarized in Table 1.
Table 1.
Patient Characteristics
| DM (n=95) | No DM (n=227) | P Value | |
|---|---|---|---|
| Age, y | 62.3±11.6 | 57.3±11.7 | 0.001a |
| Sex | 0.029a | ||
| Male | 65 (68.4) | 181 (79.7) | |
| Female | 30 (31.6) | 46 (20.3) | |
| BMI, kg/m2 | 26.1±4.6 | 26.0±7.3 | 0.874 |
| Clinical presentation | 0.231 | ||
| STEMI | 23 (24.2) | 70 (30.8) | |
| NSTE‐ACS | 72 (75.8) | 157 (69.2) | |
| Prior MI | 13 (13.7) | 29 (12.8) | 0.825 |
| Hypertension | 68 (71.6) | 125 (55.1) | 0.006a |
| Dyslipidemia | 72 (75.8) | 157 (69.2) | 0.231 |
| Current smoking | 22 (23.2) | 86 (37.9) | 0.011a |
| Creatinine, mg/dL | 1.02±0.63 | 0.92±0.20 | 0.151 |
| LDL cholesterol, mg/dL | 103.4±44.5 | 107.1±35.7 | 0.512 |
| HbA1c, % | 7.7±1.6 | 5.6±0.5 | <0.001a |
| hs‐CRP, mg/L | 2.0 (0.7‐4.0) | 1.0 (0.3‐4.0) | 0.402 |
| Medication | |||
| Aspirin | 41 (43.2) | 73 (32.2) | 0.060 |
| Clopidogrel | 24 (25.3) | 37 (16.3) | 0.061 |
| β‐Blockers | 21 (22.1) | 37 (16.3) | 0.216 |
| ACE‐I/ARBs | 30 (31.6) | 39 (17.2) | 0.004a |
| Statins | 36 (37.9) | 62 (27.3) | 0.060 |
| DM control | |||
| Insulin | 48 (50.5) | ||
| Oral hypoglycemic agents | 27 (28.4) | ||
| Diet only | 20 (21.1) | ||
Chi‐square test for categorical variables, and Student t test and Mann‐Whitney test for continuous variables were applied. Data are presented as number (%), mean±SD, or median (interquartile range). ACE‐I indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin II receptor blocker; BMI, body mass index; DM, diabetes mellitus; HbA1c, hemoglobin A1c; hs‐CRP, high‐sensitivity C‐reactive protein; LDL, low‐density lipoprotein; MI, myocardial infarction; NSTE‐ACS, non‐ST‐segment–elevation acute coronary syndrome; STEMI, ST‐segment–elevation myocardial infarction.
P<0.05.
Optical Coherence Tomography Findings
Culprit plaque characteristics by OCT are described in Table 2. DM patients had a higher prevalence of lipid‐rich plaque (58.9% versus 44.9%, P=0.030) and macrophage accumulation (60.0% versus 44.9%, P=0.019) compared with non‐DM patients. The prevalence of plaque rupture (33.7% versus 30.4%, P=0.896) and plaque erosion (21.1% versus 22.0%, P=0.458) at the culprit site was similar between the groups.
Table 2.
Culprit Plaque Characteristics
| DM (n=95) | No DM (n=227) | P Valuea | |
|---|---|---|---|
| Location | 0.780 | ||
| RCA | 30 (31.6) | 66 (29.1) | |
| LAD | 53 (55.8) | 126 (55.5) | |
| LCX | 12 (12.6) | 35 (15.4) | |
| Maximal lipid arc, degrees | 253.6±84.4 | 246.9±91.7 | 0.995 |
| Mean lipid arc, degrees | 190.1±62.4 | 184.6±59.6 | 0.895 |
| Lipid length, mm | 8.0±4.8 | 8.1±6.4 | 0.864 |
| Lipid index | 1611.8±1135.7 | 1581.6±1337.9 | 0.915 |
| Thinnest FCT, μm | 95.8±61.1 | 97.9±64.0 | 0.825 |
| Minimal lumen area, mm2 | 1.75±1.47 | 1.85±1.45 | 0.979 |
| Reference lumen area, mm2 | 7.05±3.14 | 7.33±3.09 | 0.633 |
| Area stenosis, % | 75.0±15.4 | 73.9±16.8 | 0.913 |
| Lipid‐rich plaque | 56 (58.9) | 102 (44.9) | 0.030b |
| TCFA | 27 (28.4) | 55 (24.2) | 0.421 |
| Plaque rupture | 32 (33.7) | 69 (30.4) | 0.896 |
| Plaque erosion | 20 (21.1) | 50 (22.0) | 0.458 |
| Macrophage accumulation | 57 (60.0) | 102 (44.9) | 0.019b |
| Microvessels | 33 (34.7) | 78 (34.4) | 0.975 |
| Cholesterol crystals | 32 (33.7) | 64 (28.2) | 0.516 |
| Calcification | 55 (57.9) | 97 (42.7) | 0.308 |
| Spotty calcium | 20 (21.1) | 35 (15.4) | 0.248 |
| Thrombus | 52 (54.7) | 116 (51.1) | 0.301 |
Generalized linear model for categorical variables and general linear model for continuous variables were applied. Data are presented as number (%) or mean±SD.
Adjusted for differences in baseline characteristics. DM indicates diabetes mellitus; FCT, fibrous cap thickness; LAD, left anterior descending coronary artery; LCX, left circumflex coronary artery; RCA, right coronary artery; TCFA, thin‐cap fibroatheroma.
P<0.05.
Comparisons of the culprit plaque morphologies according to the culprit lesion pathology (rupture versus erosion) are shown in Table 3 and Figure. In patients with culprit plaque rupture, the prevalence of lipid‐rich plaque, macrophage accumulation, and TCFA was similar between patients with and without DM. The prevalence of microvessels was higher in patients with DM than in those without DM. In patients with culprit plaque erosion, compared with patients without DM, patients with DM had a significantly higher prevalence of lipid‐rich plaque, macrophage accumulation, and cholesterol crystals.
Table 3.
Comparisons of Culprit Plaque Morphologies Based on the Culprit Lesion Pathology
| Plaque Rupture (n=101) | Plaque Erosion (n=70) | |||||
|---|---|---|---|---|---|---|
| DM (n=32) | No DM (n=69) | P Value | DM (n=20) | No DM (n=50) | P Value | |
| Lipid‐rich plaque | 31 (96.9) | 62 (89.9) | 0.430 | 11 (55.0) | 11 (22.0) | 0.007a |
| TCFA | 22 (68.8) | 49 (71.0) | 0.817 | 3 (15.0) | 3 (6.0) | 0.343 |
| Macrophage accumulation | 23 (71.9) | 50 (72.5) | 0.951 | 12 (60.0) | 12 (24.0) | 0.004a |
| Microvessels | 17 (53.1) | 22 (31.9) | 0.041a | 4 (20.0) | 16 (32.0) | 0.390 |
| Cholesterol crystals | 10 (31.3) | 28 (40.6) | 0.368 | 10 (50.0) | 12 (24.0) | 0.034a |
| Calcification | 16 (50.0) | 28 (40.6) | 0.374 | 7 (35.0) | 11 (22.0) | 0.261 |
| Spotty calcium | 10 (31.3) | 13 (18.8) | 0.166 | 3 (15.0) | 4 (8.0) | 0.399 |
| Thrombus | 27 (84.4) | 58 (84.1) | 0.968 | 16 (80.0) | 46 (92.0) | 0.213 |
Chi‐squared test and Fisher exact test were applied. Data are presented as number (%) or mean±SD. DM indicates diabetes mellitus; TCFA, thin‐cap fibroatheroma.
P<0.05.
Figure 1.
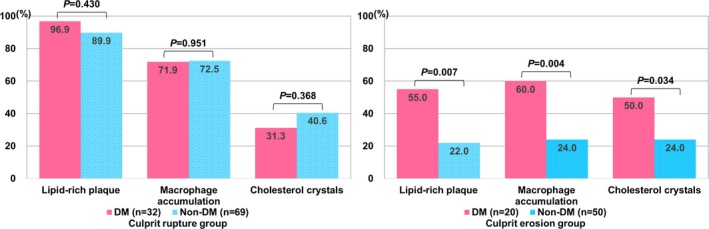
Comparisons of culprit plaque characteristics between patients with and without diabetes mellitus (DM) based on the culprit lesion pathology (rupture vs erosion). In patients with culprit plaque rupture (left column), the prevalence of lipid‐rich plaque, macrophage accumulation, and cholesterol crystals was similar between patients with DM and patients without DM. In patients with culprit plaque erosion (right column), those with DM had a higher prevalence of lipid‐rich plaque, macrophage accumulation, and cholesterol crystals. Chi‐squared test and Fisher exact test were applied.
Comparisons of the culprit plaque characteristics according to the clinical presentation are shown in Table 4. In patients with ST‐segment–elevation myocardial infarction, patients with DM had a significantly smaller minimal lumen area and reference lumen area, a significantly higher prevalence of macrophage accumulation, calcification, and spotty calcium compared with patients without DM. In patients with NSTE‐ACS, patients with DM had a significantly higher prevalence of lipid‐rich plaque compared with those without DM.
Table 4.
Comparisons of Culprit Plaque Characteristics Based on the Clinical Presentation
| STEMI (n=93) | NSTE‐ACS (n=229) | |||||
|---|---|---|---|---|---|---|
| DM (n=23) | No DM (n=70) | P Value | DM (n=72) | No DM (n=157) | P Value | |
| Maximal lipid arc, degrees | 268.2±75.5 | 277.4±82.2 | 0.719 | 249.1±87.3 | 232.3±92.9 | 0.331 |
| Mean lipid arc, degrees | 195.8±58.0 | 204.1±57.7 | 0.650 | 188.3±64.2 | 175.3±58.6 | 0.262 |
| Lipid length, mm | 10.1±6.0 | 10.3±8.0 | 0.941 | 7.4±4.2 | 7.1±5.3 | 0.747 |
| Lipid index | 1928.6±1082.1 | 1975.0±1299.0 | 0.907 | 1513.3±1145.6 | 1393.0±1323.6 | 0.615 |
| Thinnest FCT, μm | 70.0±19.1 | 75.7±39.9 | 0.615 | 103.8±67.4 | 108.5±70.6 | 0.722 |
| Minimal lumen area, mm2 | 1.17±0.59 | 1.80±1.35 | 0.003a | 1.93±1.61 | 1.87±1.50 | 0.771 |
| Reference lumen area, mm2 | 5.84±2.21 | 7.31±3.03 | 0.035a | 7.43±3.31 | 7.33±3.12 | 0.828 |
| Area stenosis, % | 78.4±10.8 | 73.4±19.2 | 0.121 | 73.9±16.5 | 74.1±15.8 | 0.917 |
| Lipid‐rich plaque | 13 (58.5) | 34 (48.6) | 0.508 | 43 (59.7) | 68 (43.3) | 0.021a |
| TCFA | 8 (34.8) | 24 (34.3) | 0.965 | 19 (26.4) | 31 (19.7) | 0.259 |
| Plaque rupture | 9 (39.1) | 31 (44.3) | 0.665 | 23 (31.9) | 38 (24.2) | 0.219 |
| Macrophage accumulation | 20 (87.0) | 33 (47.1) | 0.001a | 37 (51.4) | 69 (43.9) | 0.294 |
| Microvessels | 7 (30.4) | 29 (41.4) | 0.348 | 26 (36.1) | 49 (31.2) | 0.463 |
| Cholesterol crystals | 8 (34.8) | 19 (27.1) | 0.484 | 24 (33.3) | 45 (28.7) | 0.474 |
| Calcification | 13 (56.5) | 19 (27.1) | 0.010a | 42 (58.3) | 78 (49.7) | 0.224 |
| Spotty calcium | 6 (26.1) | 6 (8.6) | 0.030a | 14 (19.4) | 29 (18.5) | 0.861 |
| Thrombus | 16 (69.6) | 46 (65.7) | 0.734 | 36 (50.0) | 70 (44.6) | 0.446 |
Chi‐squared test and Fisher exact test for categorical variables, and Student t test for continuous variables were applied. Data are presented as number (%) or mean±SD. DM indicates diabetes mellitus; FCT, fibrous cap thickness; NSTE‐ACS, non‐ST‐segment–elevation acute coronary syndrome; STEMI, ST‐segment–elevation myocardial infarction; TCFA, thin‐cap fibroatheroma.
P<0.05.
In the subgroup of 250 patients who had sufficient OCT imaging of the nonculprit regions of the culprit vessel, 108 nonculprit plaques were identified. Among 108 nonculprit plaques included in the subgroup analysis, 34 were also included in our previous study,7 and 74 new plaques were added. The total length of segment imaged by OCT was comparable between patients with and without DM (62.3±19.1 mm versus 64.8±21.5 mm, P=0.382). The number of nonculprit plaques was similar between the DM group and non‐DM group (0.4±0.6 versus 0.5±0.7 plaques per vessel, P=0.415). The DM group had greater maximal lipid arc (248.9°±83.9° versus 179.9°±58.3°, P=0.006), thinner FCT (103.3±56.2 μm versus 140.7±70.0 μm, P=0.013), and a higher prevalence of nonculprit TCFA (17.2% versus 6.3%, P=0.031) than the non‐DM group (Table S1).
Comparisons of the culprit and nonculprit plaque characteristics based on HbA1c level are shown in Tables S2 and S3. In the culprit lesions, DM groups (both high and low HbA1c) had a higher prevalence of lipid‐rich plaque and macrophage accumulation than the non‐DM group. In the nonculprit lesions, the high‐HbA1c group had the greatest lipid burden among the groups.
Discussion
This study demonstrated that (1) patients with, compared with those without, DM had a higher prevalence of lipid‐rich plaque and macrophage accumulation in the culprit lesion, and (2) the DM group had greater lipid arc, thinner FCT, and a higher prevalence of TCFA in the nonculprit plaques of the culprit vessel than did the non‐DM group.
Culprit Plaque Characteristics in Diabetic Patients
Patients with DM have greater necrotic cores and increased levels of inflammation characterized as macrophages and T lymphocytes on pathology studies.18, 19 Moreover, poorly controlled DM is associated with a greater lipid burden.18 Intravascular ultrasound studies have shown that patients with DM and ACS have greater plaque burden and necrotic core volume at the culprit lesions compared with patients with ACS but not DM.20, 21 In contrast, previous OCT studies did not demonstrate the differences in the prevalence of lipid‐rich plaque or TCFA at the culprit lesion.6, 8, 22, 23 However, these studies reported only a small number (<80) of patients with a single‐ or 2‐center experience. The present study represents the largest series so far. OCT is the only intracoronary imaging modality that is capable of visualizing features of plaque vulnerability such as macrophage accumulation and TCFA that has been validated by histology studies.24, 25 Our results demonstrated that patients with DM had a higher prevalence of lipid‐rich plaque and macrophage accumulation at the culprit plaques, indicating a higher level of culprit plaque instability, consistent with previous pathology studies.18, 19
Some studies demonstrated that patients with DM had more frequent healed plaque rupture on pathology5 or ulceration detected by coronary angioscopy.26 However, in our study, the distribution of underlying pathology of ACS (rupture versus erosion) was not different between patients with and without DM. Previous autopsy studies demonstrated that plaque erosion was associated with younger age,27 female sex,27 and smoking status28 compared with plaque rupture. In our cohort, patients with DM and coronary artery disease were older, more frequently female, and had a lower prevalence of current smoking. These findings are consistent with previous clinical studies.3, 4 The adjustment for these differences in baseline patient characteristics should have corrected the confounder effects on our results. However, these confounders might explain the lack of difference in the prevalence of plaque rupture and plaque erosion between the 2 groups despite the greater plaque instability with higher prevalence of lipid‐rich plaque at the culprit lesions in patients with DM.
Because detailed analysis based on culprit etiology has not been reported so far, we divided the study patients into 2 groups based on the culprit lesion pathology: rupture and erosion. In our results, in the culprit rupture group, the prevalence of microvessels was higher in patients with DM than in those without. This finding is in line with pathology studies that reported that patients with DM had increased microvessel density, which indicates plaque progression evolving to plaque rupture.29, 30 In contrast, in the culprit rupture group, the prevalence of lipid‐rich plaque, macrophage accumulation, and cholesterol crystals was not different between patients with and without DM. Taken together, these findings suggest that plaque vulnerability at ruptured culprit plaques may be increased even in patients without DM. On the other hand, in the culprit erosion group, the prevalence of lipid‐rich plaque, macrophage accumulation, and cholesterol crystals was significantly higher in patients with DM compared with patients without DM. Although it is generally accepted that plaque erosion is caused by local endothelial damage rather than vascular inflammation, in a subset of patients with DM, inflammation may still play a significant role even in cases of plaque erosion. In patients with DM, insulin resistance not only impairs endothelial nitric oxide synthase activity, leading to endothelial dysfunction, but also causes reactive oxygen species accumulation triggering proinflammatory gene expression.31 It should be noted, however, that the overall features of vulnerability were still lower in the culprit erosion group compared with the culprit rupture group.
Hyperglycemia and DM promote vascular calcification in various mechanisms such as advanced glycation end products, oxidative stress, and endothelial cell dysfunction.5 The higher frequency of healed plaque rupture in patients with DM may also be associated with diffuse calcification.32 The patients with DM often develop diabetic nephropathy, and uremia‐related factors such as an increase in calcium‐phosphate products may also contribute to vascular calcification. Previous OCT studies have reported that older age and the presence of DM and chronic kidney disease were associated with the higher prevalence of calcified plaque.33 Patients with DM are also known to have greater calcification arcs and a higher prevalence of calcified nodules in NSTE‐ACS lesions.8 In contrast, the present study did not show a statistically significant difference in the prevalence of calcification at the culprit lesions between DM patients and non‐DM patients. Vascular calcification is associated with various factors such as age, sex, and hypertension.34, 35 In our cohort, patients with DM were older and had a higher prevalence of hypertension than those without DM. The adjustment for these confounders may have affected our results.
The comparison of the culprit plaque characteristics based on the clinical presentation showed that ST‐segment–elevation myocardial infarction patients with DM had a smaller minimal lumen area and reference lumen area and a higher prevalence of macrophage accumulation, calcification, and spotty calcium compared with those without DM, whereas NSTE‐ACS patients with DM had no significant difference except a higher frequency of lipid‐rich plaque compared with those without DM. This result suggests that patients with DM presenting with ST‐segment–elevation myocardial infarction, compared with those with NSTE‐ACS, had more severe luminal narrowing and greater vascular vulnerability.
Nonculprit Characteristics in Patients With DM
In the subgroup analysis of the nonculprit plaques of the culprit vessel, the DM group had greater lipid burden and thinner FCT and a higher prevalence of nonculprit TCFA than the non‐DM group. The high‐HbA1c group had the greatest lipid burden in the nonculprit region among the groups, indicating the importance of control of DM for plaque stabilization. However, this result should be interpreted with caution, as the number of patients in each group was small, and HbA1c level reflects an average of blood glucose levels only over several months. Our results are consistent with previous reports demonstrating that patients with DM have a greater lipid burden in nonculprit plaques7, 11, 36 and higher prevalence of nonculprit TCFA6 compared with patients without DM. Taken together, these findings are suggestive of pancoronary vulnerability in DM patients, which explains the worse prognosis.
Clinical Implications
DM is associated with a higher incidence of myocardial infarction or cardiac death,2 and worse prognosis after ACS,37 percutaneous coronary intervention,38 and coronary artery bypass graft surgery.39 Panvascular inflammation in patients with DM presenting with ACS explains an increased risk for future nonculprit lesion‐related major adverse cardiovascular events in patients with DM.36 It may also explain the superiority of coronary artery bypass graft surgery to percutaneous coronary intervention in the clinical outcomes of patients with DM and multivessel disease.40, 41 Our results demonstrated a higher level of panvascular instability in patients with DM. These findings suggest that more aggressive risk management including tight control of glucose, lipid, and blood pressure is of utmost importance regardless of underlying culprit etiology in patients with DM and ACS.
Study Limitations
First, this is a retrospective observational study from a registry database, and therefore selection bias could not be excluded. Although the analysis was retrospectively performed, data were prospectively collected. Our registry was an all‐comers registry that enrolled patients who had undergone coronary angiography and OCT imaging. Second, this study used 2 different OCT systems (time‐domain and frequency‐domain OCT). However, the distribution of plaque morphology examined by each system did not differ significantly between the 2 groups. Third, due to its shallow penetration, plaque burden and arterial remodeling could not be evaluated by OCT. Fourth, data on the etiology and duration of DM, the types of oral hypoglycemic agents used, and serial HbA1c values were not collected in our registry. However, it is known that the duration of DM is often not accurate, and the delay between the onset and clinical diagnosis is estimated at 4 to 6 years.42 Fifth, the current OCT system cannot image an endothelial layer despite its high resolution. Therefore, diagnosis of OCT‐derived erosion is a diagnosis of exclusion in the absence of fibrous cap rupture. Finally, this study did not evaluate clinical outcomes.
Conclusions
In the present study, compared with patients without DM, patients with DM had a higher prevalence of lipid‐rich plaque and macrophage accumulation in the culprit plaques and had greater lipid arc, thinner FCT, and more frequent TCFA in the nonculprit plaques. These findings suggest that plaques in patients with DM who present with ACS have a higher level of pancoronary vulnerability.
Sources of Funding
Dr Sugiyama is supported in part by the grant from Strategic International Research Collaboration Program with Harvard Medical School to Cultivate Professionals with Knowledge and Humanity from Japan Society for the Promotion of Science, and support from the Sumitomo Life Welfare and Culture Foundation.
Disclosures
Dr Jang has received educational grants and consulting fees from Abbott Vascular and financial support from Medicure. The remaining authors have no disclosures to report.
Supporting information
Table S1. Characteristics of the Nonculprit Plaques of the Culprit Vessel
Table S2. Comparison of Culprit Plaque Characteristics Based on HbA1c Level
Table S3. Comparison of Nonculprit Plaque Characteristics of the Culprit Vessel Based on HbA1c Level
Acknowledgments
The authors thank all the contributors and all the institutions of the Massachusetts General Hospital OCT Registry. Dr Jang's research was supported by Michael and Kathryn Park, and by Gill and Allan Gray. Dr Jang had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
(J Am Heart Assoc. 2018;7:e009245 DOI: 10.1161/JAHA.118.009245.)
References
- 1. Kannel WB, McGee DL. Diabetes and cardiovascular disease: the Framingham study. JAMA. 1979;241:2035–2038. [DOI] [PubMed] [Google Scholar]
- 2. Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234. [DOI] [PubMed] [Google Scholar]
- 3. Natali A, Vichi S, Landi P, Severi S, L'Abbate A, Ferrannini E. Coronary atherosclerosis in type II diabetes: angiographic findings and clinical outcome. Diabetologia. 2000;43:632–641. [DOI] [PubMed] [Google Scholar]
- 4. Kip KE, Faxon DP, Detre KM, Yeh W, Kelsey SF, Currier JW. Coronary angioplasty in diabetic patients. The National Heart, Lung, and Blood Institute Percutaneous Transluminal Coronary Angioplasty Registry. Circulation. 1996;94:1818–1825. [DOI] [PubMed] [Google Scholar]
- 5. Yahagi K, Kolodgie FD, Lutter C, Mori H, Romero ME, Finn AV, Virmani R. Pathology of human coronary and carotid artery atherosclerosis and vascular calcification in diabetes mellitus. Arterioscler Thromb Vasc Biol. 2017;37:191–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fukunaga M, Fujii K, Nakata T, Shibuya M, Miki K, Kawasaki D, Masutani M, Kawabata‐Lee M, Ohyanagi M, Masuyama T. Multiple complex coronary atherosclerosis in diabetic patients with acute myocardial infarction: a three‐vessel optical coherence tomography study. EuroIntervention. 2012;8:955–961. [DOI] [PubMed] [Google Scholar]
- 7. Kato K, Yonetsu T, Kim SJ, Xing L, Lee H, McNulty I, Yeh RW, Sakhuja R, Zhang S, Uemura S, Yu B, Mizuno K, Jang IK. Comparison of nonculprit coronary plaque characteristics between patients with and without diabetes: a 3‐vessel optical coherence tomography study. JACC Cardiovasc Interv. 2012;5:1150–1158. [DOI] [PubMed] [Google Scholar]
- 8. Niccoli G, Giubilato S, Di Vito L, Leo A, Cosentino N, Pitocco D, Marco V, Ghirlanda G, Prati F, Crea F. Severity of coronary atherosclerosis in patients with a first acute coronary event: a diabetes paradox. Eur Heart J. 2013;34:729–741. [DOI] [PubMed] [Google Scholar]
- 9. Jia H, Abtahian F, Aguirre AD, Lee S, Chia S, Lowe H, Kato K, Yonetsu T, Vergallo R, Hu S, Tian J, Lee H, Park SJ, Jang YS, Raffel OC, Mizuno K, Uemura S, Itoh T, Kakuta T, Choi SY, Dauerman HL, Prasad A, Toma C, McNulty I, Zhang S, Yu B, Fuster V, Narula J, Virmani R, Jang IK. In vivo diagnosis of plaque erosion and calcified nodule in patients with acute coronary syndrome by intravascular optical coherence tomography. J Am Coll Cardiol. 2013;62:1748–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Yonetsu T, Kato K, Uemura S, Kim BK, Jang Y, Kang SJ, Park SJ, Lee S, Kim SJ, Jia H, Vergallo R, Abtahian F, Tian J, Hu S, Yeh RW, Sakhuja R, McNulty I, Lee H, Zhang S, Yu B, Kakuta T, Jang IK. Features of coronary plaque in patients with metabolic syndrome and diabetes mellitus assessed by 3‐vessel optical coherence tomography. Circ Cardiovasc Imaging. 2013;6:665–673. [DOI] [PubMed] [Google Scholar]
- 12. Vergallo R, Uemura S, Soeda T, Minami Y, Cho JM, Ong DS, Aguirre AD, Gao L, Biasucci LM, Crea F, Yu B, Lee H, Kim CJ, Jang IK. Prevalence and predictors of multiple coronary plaque ruptures: In vivo 3‐vessel optical coherence tomography imaging study. Arterioscler Thromb Vasc Biol. 2016;36:2229–2238. [DOI] [PubMed] [Google Scholar]
- 13. Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho JM, Chowdhary S, Costa MA, de Silva R, Dijkstra J, Di Mario C, Dudek D, Falk E, Feldman MD, Fitzgerald P, Garcia‐Garcia HM, Gonzalo N, Granada JF, Guagliumi G, Holm NR, Honda Y, Ikeno F, Kawasaki M, Kochman J, Koltowski L, Kubo T, Kume T, Kyono H, Lam CC, Lamouche G, Lee DP, Leon MB, Maehara A, Manfrini O, Mintz GS, Mizuno K, Morel MA, Nadkarni S, Okura H, Otake H, Pietrasik A, Prati F, Raber L, Radu MD, Rieber J, Riga M, Rollins A, Rosenberg M, Sirbu V, Serruys PW, Shimada K, Shinke T, Shite J, Siegel E, Sonoda S, Suter M, Takarada S, Tanaka A, Terashima M, Thim T, Uemura S, Ughi GJ, van Beusekom HM, van der Steen AF, van Es GA, van Soest G, Virmani R, Waxman S, Weissman NJ, Weisz G. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–1072. [DOI] [PubMed] [Google Scholar]
- 14. Sugiyama T, Yamamoto E, Bryniarski K, Xing L, Lee H, Isobe M, Libby P, Jang IK. Nonculprit plaque characteristics in patients with acute coronary syndrome caused by plaque erosion vs plaque rupture: a 3‐vessel optical coherence tomography study. JAMA Cardiol. 2018;3:207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. [DOI] [PubMed] [Google Scholar]
- 16. Kataoka Y, Puri R, Hammadah M, Duggal B, Uno K, Kapadia SR, Tuzcu EM, Nissen SE, Nicholls SJ. Spotty calcification and plaque vulnerability in vivo: frequency‐domain optical coherence tomography analysis. Cardiovasc Diagn Ther. 2014;4:460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ong DS, Lee JS, Soeda T, Higuma T, Minami Y, Wang Z, Lee H, Yokoyama H, Yokota T, Okumura K, Jang IK. Coronary calcification and plaque vulnerability: an optical coherence tomographic study. Circ Cardiovasc Imaging. 2016;9:e003929. [DOI] [PubMed] [Google Scholar]
- 18. Burke AP, Kolodgie FD, Zieske A, Fowler DR, Weber DK, Varghese PJ, Farb A, Virmani R. Morphologic findings of coronary atherosclerotic plaques in diabetics: a postmortem study. Arterioscler Thromb Vasc Biol. 2004;24:1266–1271. [DOI] [PubMed] [Google Scholar]
- 19. Moreno PR, Murcia AM, Palacios IF, Leon MN, Bernardi VH, Fuster V, Fallon JT. Coronary composition and macrophage infiltration in atherectomy specimens from patients with diabetes mellitus. Circulation. 2000;102:2180–2184. [DOI] [PubMed] [Google Scholar]
- 20. Nicholls SJ, Tuzcu EM, Kalidindi S, Wolski K, Moon KW, Sipahi I, Schoenhagen P, Nissen SE. Effect of diabetes on progression of coronary atherosclerosis and arterial remodeling: a pooled analysis of 5 intravascular ultrasound trials. J Am Coll Cardiol. 2008;52:255–262. [DOI] [PubMed] [Google Scholar]
- 21. Hong YJ, Jeong MH, Choi YH, Ko JS, Lee MG, Kang WY, Lee SE, Kim SH, Park KH, Sim DS, Yoon NS, Yoon HJ, Kim KH, Park HW, Kim JH, Ahn Y, Cho JG, Park JC, Kang JC. Plaque characteristics in culprit lesions and inflammatory status in diabetic acute coronary syndrome patients. JACC Cardiovasc Imaging. 2009;2:339–349. [DOI] [PubMed] [Google Scholar]
- 22. Chia S, Raffel OC, Takano M, Tearney GJ, Bouma BE, Jang IK. Comparison of coronary plaque characteristics between diabetic and non‐diabetic subjects: an in vivo optical coherence tomography study. Diabetes Res Clin Pract. 2008;81:155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tian F, Chen Y, Chen L, Sun Z, Liu C, Guo J, Liu H. Assessment of coronary plaque characteristics by optical coherence tomography in patients with diabetes mellitus complicated with unstable angina pectoris. Atherosclerosis. 2010;213:482–485. [DOI] [PubMed] [Google Scholar]
- 24. Tearney GJ, Yabushita H, Houser SL, Aretz HT, Jang IK, Schlendorf KH, Kauffman CR, Shishkov M, Halpern EF, Bouma BE. Quantification of macrophage content in atherosclerotic plaques by optical coherence tomography. Circulation. 2003;107:113–119. [DOI] [PubMed] [Google Scholar]
- 25. Kume T, Akasaka T, Kawamoto T, Okura H, Watanabe N, Toyota E, Neishi Y, Sukmawan R, Sadahira Y, Yoshida K. Measurement of the thickness of the fibrous cap by optical coherence tomography. Am Heart J. 2006;152:755.e1–4. [DOI] [PubMed] [Google Scholar]
- 26. Silva JA, Escobar A, Collins TJ, Ramee SR, White CJ. Unstable angina. A comparison of angioscopic findings between diabetic and nondiabetic patients. Circulation. 1995;92:1731–1736. [DOI] [PubMed] [Google Scholar]
- 27. Farb A, Burke AP, Tang AL, Liang TY, Mannan P, Smialek J, Virmani R. Coronary plaque erosion without rupture into a lipid core. A frequent cause of coronary thrombosis in sudden coronary death. Circulation. 1996;93:1354–1363. [DOI] [PubMed] [Google Scholar]
- 28. Burke AP, Farb A, Pestaner J, Malcom GT, Zieske A, Kutys R, Smialek J, Virmani R. Traditional risk factors and the incidence of sudden coronary death with and without coronary thrombosis in blacks. Circulation. 2002;105:419–424. [DOI] [PubMed] [Google Scholar]
- 29. Purushothaman KR, Purushothaman M, Muntner P, Lento PA, O'Connor WN, Sharma SK, Fuster V, Moreno PR. Inflammation, neovascularization and intra‐plaque hemorrhage are associated with increased reparative collagen content: implication for plaque progression in diabetic atherosclerosis. Vasc Med. 2011;16:103–108. [DOI] [PubMed] [Google Scholar]
- 30. Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, Badimon JJ, O'Connor WN. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta. Circulation. 2004;110:2032. [DOI] [PubMed] [Google Scholar]
- 31. Paneni F, Beckman JA, Creager MA, Cosentino F. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: part I. Eur Heart J. 2013;34:2436–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burke AP, Weber DK, Kolodgie FD, Farb A, Taylor AJ, Virmani R. Pathophysiology of calcium deposition in coronary arteries. Herz. 2001;26:239–244. [DOI] [PubMed] [Google Scholar]
- 33. Sugiyama T, Kimura S, Ohtani H, Yamakami Y, Kojima K, Sagawa Y, Hishikari K, Hikita H, Ashikaga T, Takahashi A, Isobe M. Impact of chronic kidney disease stages on atherosclerotic plaque components on optical coherence tomography in patients with coronary artery disease. Cardiovasc Interv Ther. 2017;32:216–224. [DOI] [PubMed] [Google Scholar]
- 34. McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi‐Ethnic Study of Atherosclerosis (MESA). Circulation. 2006;113:30–37. [DOI] [PubMed] [Google Scholar]
- 35. Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. 2004;24:331–336. [DOI] [PubMed] [Google Scholar]
- 36. Marso SP, Mercado N, Maehara A, Weisz G, Mintz GS, McPherson J, Schiele F, Dudek D, Fahy M, Xu K, Lansky A, Templin B, Zhang Z, de Bruyne B, Serruys PW, Stone GW. Plaque composition and clinical outcomes in acute coronary syndrome patients with metabolic syndrome or diabetes. JACC Cardiovasc Imaging. 2012;5:S42–S52. [DOI] [PubMed] [Google Scholar]
- 37. Malmberg K, Yusuf S, Gerstein HC, Brown J, Zhao F, Hunt D, Piegas L, Calvin J, Keltai M, Budaj A. Impact of diabetes on long‐term prognosis in patients with unstable angina and non‐Q‐wave myocardial infarction: results of the OASIS (Organization to Assess Strategies for Ischemic Syndromes) Registry. Circulation. 2000;102:1014–1019. [DOI] [PubMed] [Google Scholar]
- 38. Stone GW, Kedhi E, Kereiakes DJ, Parise H, Fahy M, Serruys PW, Smits PC. Differential clinical responses to everolimus‐eluting and paclitaxel‐eluting coronary stents in patients with and without diabetes mellitus. Circulation. 2011;124:893–900. [DOI] [PubMed] [Google Scholar]
- 39. Carson JL, Scholz PM, Chen AY, Peterson ED, Gold J, Schneider SH. Diabetes mellitus increases short‐term mortality and morbidity in patients undergoing coronary artery bypass graft surgery. J Am Coll Cardiol. 2002;40:418–423. [DOI] [PubMed] [Google Scholar]
- 40. Bangalore S, Guo Y, Samadashvili Z, Blecker S, Xu J, Hannan EL. Everolimus eluting stents versus coronary artery bypass graft surgery for patients with diabetes mellitus and multivessel disease. Circ Cardiovasc Interv. 2015;8:e002626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hakeem A, Garg N, Bhatti S, Rajpurohit N, Ahmed Z, Uretsky BF. Effectiveness of percutaneous coronary intervention with drug‐eluting stents compared with bypass surgery in diabetics with multivessel coronary disease: comprehensive systematic review and meta‐analysis of randomized clinical data. J Am Heart Assoc. 2013;2:e000354 DOI: 10.1161/JAHA.113.000354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Porta M, Curletto G, Cipullo D, Rigault de la Longrais R, Trento M, Passera P, Taulaigo AV, Di Miceli S, Cenci A, Dalmasso P, Cavallo F. Estimating the delay between onset and diagnosis of type 2 diabetes from the time course of retinopathy prevalence. Diabetes Care. 2014;37:1668–1674. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Characteristics of the Nonculprit Plaques of the Culprit Vessel
Table S2. Comparison of Culprit Plaque Characteristics Based on HbA1c Level
Table S3. Comparison of Nonculprit Plaque Characteristics of the Culprit Vessel Based on HbA1c Level


