Abstract
Background
Previously, we reported on associations between dietary patterns and incident acute coronary heart disease (CHD) in the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study. Here, we investigated the associations of dietary patterns and a dietary index with recurrent CHD events and all‐cause mortality in REGARDS participants with existing CHD.
Methods and Results
We included data from 3562 participants with existing CHD in REGARDS. We used Cox proportional hazards regression to examine the hazard of first recurrence of CHD events—definite or probable MI or acute CHD death—and all‐cause mortality associated with quartiles of empirically derived dietary patterns (convenience, plant‐based, sweets, Southern, and alcohol and salads) and the Mediterranean diet score. Over a median 7.1 years (interquartile range, 4.4, 8.9 years) follow‐up, there were 581 recurrent CHD events and 1098 deaths. In multivariable‐adjusted models, the Mediterranean diet score was inversely associated with the hazard of recurrent CHD events (hazard ratio for highest score versus lowest score, 0.78; 95% confidence interval, 0.62–0.98; PT rend=0.036). The Southern dietary pattern was adversely associated with the hazard of all‐cause mortality (hazard ratio for Q4 versus Q1, 1.57; 95% confidence interval, 1.28–1.91; P Trend<0.001). The Mediterranean diet score was inversely associated with the hazard of all‐cause mortality (hazard ratio for highest score versus lowest score, 0.80; 95% confidence interval, 0.67–0.95; PT rend=0.014).
Conclusions
The Southern dietary pattern was associated with a greater hazard of all‐cause mortality in REGARDS participants. Greater adherence to the Mediterranean diet was associated with both a lower hazard of recurrent CHD events and all‐cause mortality.
Keywords: cardiovascular disease prevention, diet, epidemiology, nutrition
Subject Categories: Diet and Nutrition, Epidemiology, Cardiovascular Disease
Clinical Perspective
What Is New?
Greater adherence to a “Southern” dietary pattern, characterized by added fats, fried food, eggs and egg dishes, organ meats, processed meats, and sugar‐sweetened beverages, was associated with a greater hazard of all‐cause mortality in community‐dwelling blacks and whites who were at least 45 years of age.
Greater adherence to the Southern dietary pattern was not associated with hazard of recurrent coronary heart disease events.
A higher Mediterranean diet score (indicating greater adherence to the Mediterranean diet) was associated with a lower hazard of both recurrent coronary heart disease events and all‐cause mortality.
Although these are observational data, this study was conducted in a large, population‐based, and diverse sample and included a comprehensive assessment of diet and a rigorous method for deriving dietary patterns.
What Are the Clinical Implications?
Based on these results, it would be reasonable to make recommendations to patients to reduce intakes of the main components of the Southern dietary pattern, and more closely adhere to the principles of the Mediterranean diet.
There are no known risks associated with these recommendations, and they may favorably influence the hazard of recurrent coronary heart disease and all‐cause mortality.
Introduction
Despite decades of research and improvements in treatment, coronary heart disease (CHD) remains an important cause of death in the United States, accounting for 1 of every 7 deaths in 2014—more than 364 000 deaths in total.1 Risk factors for CHD are well established and include dyslipidemia, diabetes mellitus, hypertension, overweight/obesity, cigarette smoking, and physical inactivity.2 Observational and intervention studies provide evidence that diet also influences risk of CHD, as well as the course of the disease, likely through its documented effects on several of these key risk factors.3
Studies of diet and CHD risk traditionally focus on dietary constituents such as individual foods and nutrients, resulting in important findings such as the adverse associations of red meat and saturated fat with CHD risk.4 However, interest in overall diet and CHD risk has increased in the past decade with the understanding—by both researchers and the public—that foods typically are eaten in combination, not in isolation.5 Therefore, a comprehensive dietary approach more closely reflects the way most humans actually eat.
Empirically deriving dietary patterns a posteriori has facilitated investigations into the role overall diet may play in the etiology of chronic diseases.6, 7 Factor analysis, a data‐driven exploratory method, assesses eating patterns in specified groups without preconceived judgments about which foods commonly are consumed together and has been used in previous studies to derive dietary patterns that subsequently were related to CHD risk.8, 9, 10, 11, 12, 13, 14, 15, 16 In a previous analysis, we derived dietary patterns with factor analysis within the REGARDS (Reasons for Geographic and Racial Differences in Stroke) study, a national, population‐based, longitudinal cohort.17 We then used Cox proportional hazards regression to examine hazard of incident acute CHD events—nonfatal myocardial infarction and acute CHD death—associated with quartiles of consumption of each pattern in participants free of CHD at baseline.18 After multivariable adjustment, the highest consumers of the Southern pattern (characterized by added fats, fried food, eggs, organ and processed meats, and sugar‐sweetened beverages) experienced a 56% higher hazard of incident acute CHD.
While examining risk factors for incident CHD is critical for primary prevention, individuals who suffer a myocardial infarction (MI) now have greater survival than in previous years.19 As a result, there is growing interest in examining risk factors for recurrent events among those with established disease. Therefore, we investigated the association of REGARDS dietary patterns, along with a dietary index—the Mediterranean diet score—with recurrent CHD events and all‐cause mortality in REGARDS participants with CHD at baseline.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
Details on the design and methods of REGARDS have been published.20 Briefly, REGARDS is a national, population‐based, longitudinal cohort of 30 239 community‐dwelling black and white women and men ≥45 years of age, identified via mail and telephone using commercially available lists of US residents, and enrolled from 2003 to 2007. The sampling scheme included 30% of participants from the stroke belt (North Carolina, South Carolina, Georgia, Tennessee, Alabama, Mississippi, Arkansas, and Louisiana), 20% from the stroke buckle (the coastal plain of North Carolina, South Carolina, and Georgia), and 50% from elsewhere in the continental United States. The baseline cohort was 42% black and 55% women.
Exclusion criteria included race other than white or black, active treatment for cancer, chronic medical conditions precluding long‐term participation, cognitive impairment, current or impending residence in a nursing home, or inability to communicate in English. An initial telephone interview was used to survey participants and establish eligibility. Following verbal consent, demographic information and medical history (including risk factor evaluation) was collected by computer‐assisted telephone interviewing. Race was self‐classified by participants and included the following options defined by the investigators: white and black/African American. An in‐home examination was conducted to perform various physical measurements, medication inventory, ECG, phlebotomy, and urine collection among those eligible. The study was approved by the institutional review boards at all participating institutions, and written informed consent was obtained from all participants.
For this analysis, we included only those REGARDS participants with a history of CHD at baseline (n=5314), defined as self‐reported history of MI or coronary revascularization procedure, or evidence of MI on the baseline ECG.
Dietary Assessment
Diet was assessed with the Block 98 food frequency questionnaire (FFQ), a validated semiquantitative FFQ that assessed usual dietary intake of 110 food items (NutritionQuest, Berkeley, CA).21, 22 For each line item on the FFQ, participants were asked how often, on average, they consumed the food (or group of foods) during the previous year, as well as the quantity of the food consumed. The FFQ included adjustment questions (eg, inquiring about the type of milk consumed—low‐fat, nonfat, etc). The FFQ was self‐administered after the in‐home visit and mailed to the REGARDS Operations Center, where they were checked for completeness, scanned, and forwarded to NutritionQuest for processing and analysis. Amounts of each food on the FFQ consumed by a participant were calculated by multiplying the frequency of consumption of that food by the usual amount consumed. Calculation of the total weight (g) of each line item on the FFQ was provided by NutritionQuest. A total of 56 food groups, on which dietary patterns were based, were derived using the 110 individual food variables on the FFQ using methods described elsewhere.17
Dietary Patterns
We used split sample replication to (1) derive the dietary patterns using exploratory factor analysis and (2) test the patterns using confirmatory factor analysis.23 We conducted 3 separate analyses: by sex (male/female), race (black/white), and region (southeastern US stroke belt/nonbelt), and coefficients of congruence were determined for each stratification pair. The final number of factors retained was chosen based on the eigenvalue (scree plot) and the solution providing the optimal congruence across sex, race, and region. As congruence between sex, race, and region was high, we calculated final factor loadings using factor analysis with varimax rotation of 5 factors on the full sample. We named patterns based on the factor loadings that contributed most highly to each pattern. Factor 1 loaded heavily on mixed dishes, pasta dishes, pizza, Mexican food, and Chinese food and was designated the “convenience” pattern. Factor 2 had high factor loadings for vegetables, fruits, fruit juice, cereal, beans, fish, poultry, and yogurt and was named the “plant‐based” pattern. Factor 3 loaded on added sugars, desserts, chocolate, candy, and sweetened breakfast foods and was named the “sweets” pattern. Factor 4 loaded heavily on added fats, fried food, eggs and egg dishes, organ meats, processed meats, and sugar‐sweetened beverages. This diet reflected a culinary pattern observed in the southeastern US and was named the “Southern” pattern. Factor 5 loaded highly on beer, wine, liquor, green leafy vegetables, tomatoes, and salad dressing. Accordingly, we named it the “alcohol and salads” pattern.
Mediterranean Diet Score
We included the Mediterranean diet score because it has been associated with reduced risk of chronic disease incidence and mortality in various populations.24 The Mediterranean diet score was derived according to previously published methods used in REGARDS.25 In brief, food group contributors to the Mediterranean diet score included those designated as “beneficial” (vegetables, fruits, legumes, cereals, fish), and those designated as “detrimental” (meat, dairy). One point was assigned for consumption that exceeded the median for the “beneficial” groups or was below the median for “detrimental” food groups. For fat intake (eighth food category) we used the ratio of daily consumption (in grams) of monounsaturated lipids to saturated lipids, and we calculated the median separately for each sex. Individuals with ratios at or above the sex‐specific median were assigned a value of 1, and those with ratios below the sex‐specific median were assigned a value of 0. Moderate alcohol (ninth food category) consumption was defined as >0 and ≤7 drinks per week for women and >0 and ≤14 drinks per week for men. More‐than‐moderate consumption was defined as >7 drinks per week for women and >14 drinks per week for men. Individuals were assigned a score of 1 for moderate consumption and a score of 0 for the other 2 categories (0 and more‐than‐moderate consumption). Summing scores for the 9 food groups resulted in a possible score of 0 to 9, with a higher score reflecting higher adherence to the Mediterranean diet.
Outcome Ascertainment
We defined recurrent CHD events as first occurrence of definite or probable MI or acute CHD death in participants with a history (defined above) of CHD at baseline. Recurrent cases of CHD were captured by participant report and adjudicated by clinicians with appropriate expertise. Participants were contacted by telephone every 6 months to assess vital status. If a suspected heart event was reported, medical records were pursued. MIs were adjudicated based on the presence of signs or symptoms suggestive of ischemia; diagnostic cardiac enzymes (rising and/or falling pattern in cardiac troponin or creatine phosphokinase‐MB isoenzyme concentrations over ≥6 hours with a peak concentration greater than twice the upper limit of normal); and ECG changes consistent with ischemia or MI, guided by the Minnesota Code and classified as evolving diagnostic, positive, nonspecific, or not consistent with ischemia.26 In the case of deaths, interviews with family members or other proxies, proximal hospitalizations, baseline medical history, death certificates, and the National Death Index were used to identify CHD as the underlying cause of death for analyses of recurrent CHD events or to identify any death for analyses of all‐cause mortality.
Statistical Analysis
Of the participants with a history of CHD at baseline (n=5314), we excluded 1671 participants who were missing FFQ data altogether, had >15% missing data on the FFQ, or had implausible reported energy intakes (<800 or >5000 kcal/day in men and <500 or >4500 kcal/day in women), precluding derivation of dietary patterns and/or Mediterranean diet score. In addition, we excluded 81 participants who were lost to follow‐up. This resulted in a final sample of 3562 (67.0% of the sample with a history of CHD at baseline) (see Figure).
Figure 1.
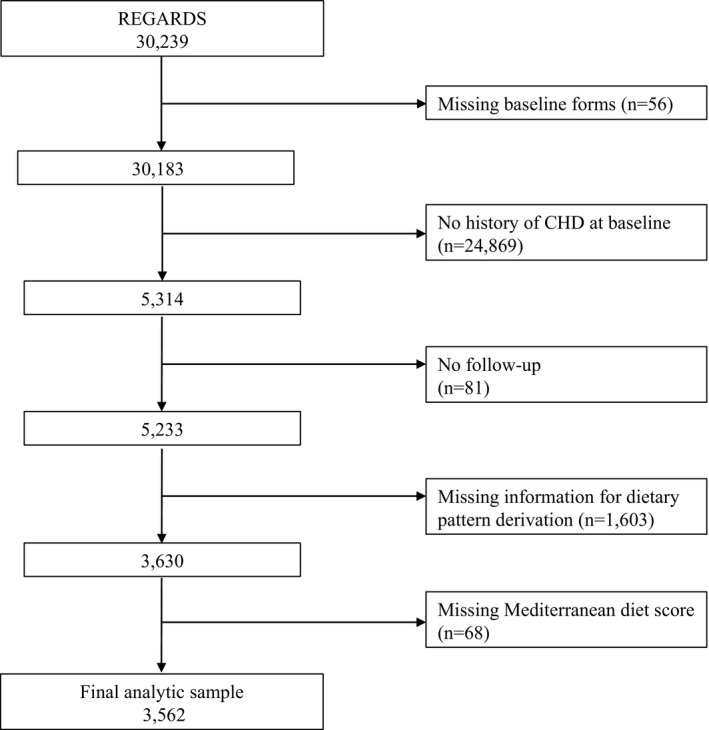
Participant exclusion cascade. CHD indicates coronary heart disease; REGARDS, Reasons for Geographic and Racial Differences in Stroke.
We categorized consumption of the 5 dietary patterns into quartiles, with quartile 1 representing the lowest consumption of each pattern and quartile 4 representing the highest consumption of the pattern. We categorized the Mediterranean diet score into 3 groups representing lowest to highest consumption of the Mediterranean diet. We calculated descriptive statistics (including proportions and measures of central tendency) for demographic, socioeconomic, lifestyle, anthropometric, medical history, and medication variables at the baseline assessment according to these quartiles/groups using the chi‐square test (for proportions) and ANOVA (for continuous variables). We used Cox proportional hazards regression to examine the hazard of recurrent CHD events and all‐cause mortality associated with consumption of each of the 5 dietary patterns and Mediterranean diet score, using the lowest quartile/group of consumption (quartile/group 1) as the referent quartile/group throughout. Quartiles proved not to be appropriate for analyses of the Mediterranean diet score. Specifically, attempting to categorize Mediterranean diet scores (originally on a scale of 0–9 to assess adherence) into 4 somewhat uniform groups was not possible because of the distribution of the scores—this would have resulted in at least 1 category with a very small number compared with the other groups. Therefore, 3 more equal groups were created based on the scores 0 to 3, 4 to 5, and 6 to 9. Years since study entry was the time metric, with participants censored at the date of a recurrent CHD event (for the recurrent CHD analysis); date of withdrawal from the study; date of death; or December 31, 2013, whichever came first. We examined Schoenfeld residuals and confirmed that proportional hazards assumptions were met. The base model (model 1) included the demographic variables age, sex, and race. The multivariable‐adjusted model (model 2) included factors in model 1 plus socioeconomic factors (education, household income), region, lifestyle factors (smoking, physical activity), total energy intake, anthropometric factors (body mass index [BMI], waist circumference), systolic blood pressure, medical history (hypertension, dyslipidemia, diabetes mellitus), and a physical health summary scale: the Physical Component Summary from the 12‐item Short‐Form Health Survey.
A Wald test was conducted to assess possible effect modification by sex and race. We conducted a further sensitivity analysis including antihypertensive, antidyslipidemic, and antidiabetic medications in model 2. To address concerns of collinearity between waist circumference and BMI, we ran all models using residualized variables. In a final sensitivity analysis, we used multiple imputation by chained equations to impute both the main exposure and covariates.
A total of 971 participants (27.3% of the 3562 participants) were missing information for at least 1 covariate of interest. Variables with the largest amount of missing data included income, antihypertensive medication and insulin use, and physical health summary scale. All other characteristics had ≤1% missing data. We performed analyses using SAS statistical software, version 9.4 (SAS Institute, Cary, NC) and Stata, version 14.2 (StataCorp, College Station, TX). A P value of <0.05 was considered statistically significant. One author (M.M.S.) had full access to all the data in the study and takes responsibility for its integrity and the data analysis.
Results
Over a median 7.1 years follow‐up (interquartile range, 4.4, 8.9 years), there were 581 (16.3%) recurrent CHD events and 1098 (30.8%) deaths. Compared with participants without recurrent CHD events, those with recurrent CHD events were older (70.0±9.2 versus 68.3±8.8 years), more likely to be male (70.1% versus 59.4%), more likely to not have graduated from high school (16.2% versus 12.9%), and more likely to be physically inactive (44.9% versus 34.6%) (Table 1). Participants with recurrent CHD events also had higher BMI (29.9±5.7 versus 29.0±5.9 kg/m2), waist circumference (102.2±15.3 versus 98.3±15.4 cm), and systolic blood pressure (132.3±17.9 versus 128.6±16.7 mm Hg); were more likely to have a history of hypertension (77.6% versus 69.3%), dyslipidemia (81.9% versus 77.2%), and diabetes mellitus (42.7% versus 27.3%); were more likely to report antihypertensive medication (72.2% versus 65.9%), regular aspirin (75.0% versus 69.7%), oral antidiabetic medication (30.5% versus 19.5%), and insulin (16.3% versus 8.7%) use; and had a lower physical health summary score (40.2±11.4 versus 43.5±11.4).
Table 1.
Characteristics of Study Participants Without and With Recurrent CHD Events in the REGARDS Cohort
| Characteristic | Total Mean±SD or n (%) | No Recurrent CHD Event Mean±SD or n (%) | Recurrent CHD Event Mean±SD or n (%) | P Difference |
|---|---|---|---|---|
| N=3562 | n=2981 | n=581 | ||
| Age, y | 68.6±8.9 | 68.3±8.8 | 70.0±9.2 | <0.001 |
| Sex, male | 2179 (61.2) | 1772 (59.4) | 407 (70.1) | <0.001 |
| Race, black | 962 (27.0) | 812 (27.2) | 150 (25.8) | 0.48 |
| Education, did not graduate from high school | 477 (13.4) | 383 (12.9) | 94 (16.2) | 0.031 |
| Household income <$20 000/y | 686 (21.7) | 557 (21.1) | 129 (24.4) | 0.092 |
| Resident of stroke belta | 2007 (56.3) | 1692 (56.8) | 315 (54.2) | 0.26 |
| Current smoker | 527 (14.9) | 434 (14.6) | 93 (16.0) | 0.38 |
| Physically inactiveb | 1276 (36.3) | 1020 (34.6) | 256 (44.9) | <0.001 |
| Total energy intake, kcal/day | 1685±688 | 1684±687 | 1694±693 | 0.74 |
| Body mass index, kg/m2 | 29.2±5.8 | 29.0±5.9 | 29.9±5.7 | 0.002 |
| Waist circumference, cm | 98.9±15.4 | 98.3±15.4 | 102.2±15.3 | <0.001 |
| Systolic blood pressure, mm Hg | 129.2±16.9 | 128.6±16.7 | 132.3±17.9 | <0.001 |
| Hypertensionc | 2506 (70.7) | 2058 (69.3) | 448 (77.6) | <0.001 |
| Dyslipidemiad | 2716 (78.0) | 2251 (77.2) | 465 (81.9) | 0.015 |
| Diabetes mellituse | 1030 (29.8) | 786 (27.3) | 244 (42.7) | <0.001 |
| Antihypertensive use | 2298 (66.9) | 1887 (65.9) | 411 (72.2) | 0.003 |
| Statin use | 2109 (59.2) | 1759 (59.0) | 350 (60.2) | 0.58 |
| Regular aspirin use | 2512 (70.5) | 2077 (69.7) | 435 (75.0) | 0.010 |
| Oral antidiabetic use | 759 (21.3) | 582 (19.5) | 177 (30.5) | <0.001 |
| Insulin use | 334 (9.9) | 245 (8.7) | 89 (16.3) | <0.001 |
| PCS‐12 | 43.0±11.5 | 43.5±11.4 | 40.2±11.4 | <0.001 |
CHD indicates coronary heart disease; PCS, Physical Component Summary; REGARDS, Reasons for Geographic and Racial Differences in Stroke study; SD, standard deviation.
Stroke belt consists of North Carolina, South Carolina, Georgia, Tennessee, Alabama, Mississippi, Arkansas, and Louisiana.
Physically active defined as ≥4 days of exercise (enough to work up a sweat) per week.
Hypertension defined as systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg or self‐reported current use of medication to control blood pressure.
Dyslipidemia defined as total cholesterol ≥6.22 mmol/L (240 mg/dL) and/or low‐density lipoprotein cholesterol ≥4.14 mmol/L (160 mg/dL) and/or high‐density lipoprotein cholesterol ≤1.04 mmol/L (40 mg/dL) or self‐reported current use of medication to control cholesterol.
Diabetes mellitus defined as fasting glucose ≥6.99 mmol/L (126 mg/dL) and/or nonfasting glucose ≥11.10 mmol/L (200 mg/dL) or self‐reported current use of medication to control blood sugar.
In multivariable‐adjusted models, the plant‐based dietary pattern demonstrated a trend for an increasing hazard of recurrent CHD events, although none of the hazard ratios (HRs) for quartile comparisons were statistically significant (HR for Q4 versus Q1, 1.28; 95% confidence interval [CI], 0.98–1.66; P Trend=0.048) (Table 2). The Southern pattern was adversely associated with the hazard of recurrent CHD events in the minimally adjusted model (HR for Q4 versus Q1, 1.35; 95% CI, 1.05–1.73; P Trend=0.011). However, the Southern pattern was not associated with the hazard of recurrent CHD events in the fully adjusted model (HR for Q4 versus Q1, 1.00; 95% CI, 0.76–1.31; P Trend=0.942). After multivariable adjustment, the alcohol and salads pattern was inversely associated with the hazard of recurrent CHD events (HR for Q4 versus Q1, 0.77; 95% CI, 0.59–1.00; P Trend=0.026). None of the other dietary patterns were significantly associated with hazard of recurrent CHD events. The multivariable‐adjusted Mediterranean diet score was inversely associated with the hazard of recurrent CHD events (HR for group 3 versus group 1, 0.78; 95% CI, 0.62–0.98; P Trend=0.036) (Table 3).
Table 2.
Hazard of Recurrent CHD Events by Quartile of Consumption of the Various Dietary Patterns
| Dietary Pattern | Model | Quartile 1 (Lowest Consumption), HR (95% CI) | Quartile 2, HR (95% CI) | Quartile 3, HR (95% CI) | Quartile 4 (Highest Consumption), HR (95% CI) | P Trend |
|---|---|---|---|---|---|---|
| Convenience | n=894 (158a) | n=890 (137) | n=888 (138) | n=890 (148) | ||
| 279.4b | 237.2 | 235.8 | 246.0 | |||
| 1c | 1 (referent) | 0.87 (0.69–1.09) | 0.85 (0.68–1.08) | 0.92 (0.73–1.16) | 0.472 | |
| 2d | 1 (referent) | 0.83 (0.66–1.05) | 0.86 (0.67–1.09) | 0.87 (0.67–1.13) | 0.296 | |
| Plant‐based | n=887 (135) | n=894 (146) | n=891 (155) | n=890 (145) | ||
| 238.9 | 251.0 | 261.5 | 245.6 | |||
| 1 | 1 (referent) | 0.99 (0.78–1.25) | 1.02 (0.81–1.29) | 0.97 (0.76–1.23) | 0.863 | |
| 2 | 1 (referent) | 1.08 (0.85–1.37) | 1.19 (0.93–1.51) | 1.28 (0.98–1.66) | 0.048 | |
| Sweets | n=892 (132) | n=888 (152) | n=890 (149) | n=892 (148) | ||
| 226.4 | 262.9 | 249.9 | 258.5 | |||
| 1 | 1 (referent) | 1.11 (0.88–1.41) | 1.05 (0.83–1.33) | 1.10 (0.87–1.39) | 0.590 | |
| 2 | 1 (referent) | 1.05 (0.83–1.33) | 1.00 (0.78–1.28) | 1.11 (0.83–1.48) | 0.640 | |
| Southern | n=895 (134) | n=888 (138) | n=891 (154) | n=888 (155) | ||
| 215.5 | 236.7 | 267.9 | 281.6 | |||
| 1 | 1 (referent) | 1.12 (0.88–1.42) | 1.26 (1.00–1.60) | 1.35 (1.05–1.73) | 0.011 | |
| 2 | 1 (referent) | 0.99 (0.78–1.27) | 1.02 (0.80–1.30) | 1.00 (0.76–1.31) | 0.942 | |
| Alcohol and salads | n=886 (149) | n=887 (158) | n=895 (148) | n=894 (126) | ||
| 263.6 | 281.0 | 252.1 | 204.7 | |||
| 1 | 1 (referent) | 1.07 (0.85–1.34) | 0.95 (0.75–1.20) | 0.75 (0.59–0.96) | 0.015 | |
| 2 | 1 (referent) | 1.11 (0.89–1.39) | 0.93 (0.73–1.17) | 0.77 (0.59–1.00) | 0.026 |
CHD indicates coronary heart disease; CI, confidence interval; HR, hazard ratio; PCS, Physical Component Summary.
Number of events.
Crude rate of recurrent coronary heart disease events per 10 000 person‐years.
Model 1 adjusts for age, sex, and race.
Model 2 adjusts for age, sex, race, education, household income, region, smoking, physical activity, total energy intake, body mass index, waist circumference, systolic blood pressure, history of hypertension, dyslipidemia, diabetes mellitus, and PCS‐12.
Table 3.
Hazard of Recurrent CHD Events by Mediterranean Diet Score Group
| Diet Score | Model | Group 1 (Score 0–3), HR (95% CI) | Group 2 (Score 4, 5), HR (95% CI) | Group 3 (Score 6–9), HR (95% CI) | P Trend |
|---|---|---|---|---|---|
| Mediterranean | n=1145 (208a) | n=1500 (248) | n=917 (125) | ||
| 291.2b | 252.4 | 197.4 | |||
| 1c | 1 (referent) | 0.81 (0.67–0.97) | 0.61 (0.49–0.76) | <0.001 | |
| 2d | 1 (referent) | 0.91 (0.76–1.10) | 0.78 (0.62–0.98) | 0.036 |
CHD indicates coronary heart disease; CI, confidence interval; HR, hazard ratio; PCS, Physical Component Summary.
Number of events.
Crude rate of recurrent coronary heart disease events per 10 000 person‐years.
Model 1 adjusts for age, sex, and race.
Model 2 adjusts for age, sex, race, education, household income, region, smoking, physical activity, total energy intake, body mass index, waist circumference, systolic blood pressure, history of hypertension, dyslipidemia, diabetes mellitus, and PCS‐12.
In multivariable‐adjusted all‐cause mortality analyses, the Southern dietary pattern was adversely associated with the hazard of all‐cause mortality (HR for Q4 versus Q1, 1.57; 95% CI, 1.28–1.91; P Trend<0.001) (Table 4). The plant‐based pattern was inversely associated with the hazard of all‐cause mortality in the minimally adjusted model (HR for Q4 versus Q1, 0.71; 95% CI, 0.59–0.84; P Trend<0.001). While this association was attenuated in the fully adjusted model, evidence of an inverse association of the plant‐based pattern with the hazard of all‐cause mortality remained (HR for Q4 versus Q1, 0.84; 95% CI, 0.69–1.01; P Trend=0.150). None of the other empirically derived dietary patterns were associated with the hazard of all‐cause mortality. The multivariable‐adjusted Mediterranean diet score was inversely associated with the hazard of all‐cause mortality (HR for group 3 versus group 1, 0.80; 95% CI, 0.67–0.95; P Trend=0.014) (Table 5).
Table 4.
Hazard of All‐Cause Mortality by Quartile of Consumption of the Various Dietary Patterns
| Dietary Pattern | Model | Quartile 1 (Lowest Consumption), HR (95% CI) | Quartile 2, HR (95% CI) | Quartile 3, HR (95% CI) | Quartile 4 (Highest Consumption), HR (95% CI) | P Trend |
|---|---|---|---|---|---|---|
| Convenience | n=894 (306a) | n=890 (280) | n=888 (261) | n=890 (251) | ||
| 513.3b | 461.2 | 425.6 | 398.6 | |||
| 1c | 1 (referent) | 0.95 (0.81–1.12) | 0.92 (0.77–1.09) | 0.96 (0.80–1.14) | 0.527 | |
| 2d | 1 (referent) | 0.92 (0.78–1.09) | 0.97 (0.81–1.15) | 0.89 (0.74–1.08) | 0.342 | |
| Plant‐based | n=887 (269) | n=894 (282) | n=891 (294) | n=890 (253) | ||
| 454.8 | 459.5 | 476.0 | 405.9 | |||
| 1 | 1 (referent) | 0.85 (0.71–1.00) | 0.86 (0.73–1.01) | 0.71 (0.59–0.84) | <0.001 | |
| 2 | 1 (referent) | 0.90 (0.76–1.07) | 0.96 (0.81–1.15) | 0.84 (0.69–1.01) | 0.150 | |
| Sweets | n=892 (258) | n=888 (284) | n=890 (275) | n=892 (281) | ||
| 421.2 | 469.4 | 440.0 | 465.6 | |||
| 1 | 1 (referent) | 1.00 (0.85–1.19) | 0.94 (0.80–1.12) | 1.05 (0.88–1.24) | 0.781 | |
| 2 | 1 (referent) | 0.94 (0.79–1.12) | 0.87 (0.72–1.04) | 0.94 (0.76–1.16) | 0.358 | |
| Southern | n=895 (216) | n=888 (262) | n=891 (300) | n=888 (320) | ||
| 330.7 | 428.3 | 496.2 | 554.8 | |||
| 1 | 1 (referent) | 1.34 (1.12–1.61) | 1.61 (1.35–1.93) | 2.01 (1.67–2.41) | <0.001 | |
| 2 | 1 (referent) | 1.21 (1.01–1.46) | 1.37 (1.15–1.65) | 1.57 (1.28–1.91) | <0.001 | |
| Alcohol and salads | n=886 (292) | n=887 (291) | n=895 (279) | n=894 (236) | ||
| 489.7 | 487.0 | 452.9 | 370.9 | |||
| 1 | 1 (referent) | 1.03 (0.88–1.22) | 1.01 (0.85–1.19) | 0.82 (0.69–0.98) | 0.032 | |
| 2 | 1 (referent) | 1.09 (0.92–1.29) | 1.03 (0.86–1.22) | 0.86 (0.71–1.03) | 0.117 |
CI indicates confidence interval; HR, hazard ratio; PCS, Physical Component Summary.
Number of events.
Crude rate of all‐cause mortality per 10 000 person‐years.
Model 1 adjusts for age, sex, and race.
Model 2 adjusts for age, sex, race, education, household income, region, smoking, physical activity, total energy intake, body mass index, waist circumference, systolic blood pressure, history of hypertension, dyslipidemia, diabetes mellitus, and PCS‐12.
Table 5.
Hazard of All‐Cause Mortality by Mediterranean Diet Score Group
| Diet Score | Model | Group 1 (Score 0–3), HR (95% CI) | Group 2 (Score 4, 5), HR (95% CI) | Group 3 (Score 6–9), HR (95% CI) | P Trend |
|---|---|---|---|---|---|
| Mediterranean | n=1145 (379a) | n=1500 (481) | n=917 (238) | ||
| 499.9b | 468.7 | 359.6 | |||
| 1c | 1 (referent) | 0.84 (0.73–0.96) | 0.60 (0.51–0.71) | <0.001 | |
| 2d | 1 (referent) | 0.98 (0.85–1.13) | 0.80 (0.67–0.95) | 0.014 |
CI indicates confidence interval; HR, hazard ratio; PCS, Physical Component Summary.
Number of events.
Crude rate of all‐cause mortality per 10 000 person‐years.
Model 1 adjusts for age, sex, and race.
Model 2 adjusts for age, sex, race, education, household income, region, smoking, physical activity, total energy intake, body mass index, waist circumference, systolic blood pressure, history of hypertension, dyslipidemia, diabetes mellitus, and PCS‐12.
Sensitivity analyses solidified the results above. There was no statistically significant interaction by race or sex. Additionally, estimates were virtually unchanged when antihypertensive, antidyslipidemic, and antidiabetic medications were added to the final model; waist circumference and BMI were residualized; or the main exposure and covariates were imputed.
Discussion
In this follow‐up to our analysis of dietary patterns and incident acute CHD in the REGARDS cohort, a Southern dietary pattern (characterized by added fats, fried food, eggs, organ meats, processed meats, and sugar‐sweetened beverages) was not associated with the hazard of recurrent CHD events, in contrast to the adverse association of this dietary pattern with the hazard of incident acute CHD.18 The Southern pattern was adversely associated with the hazard of all‐cause mortality in REGARDS participants with existing CHD. Interestingly, greater adherence to the plant‐based dietary pattern showed a significant trend of a greater hazard of recurrent CHD events, although none of the HRs for quartile comparisons were statistically significant. Higher consumption of a Mediterranean diet was inversely associated with both hazard of recurrent CHD events and all‐cause mortality.
Although previous studies have investigated the association of dietary patterns with incident CHD events, CHD risk factors, post‐MI prognosis and cardiovascular mortality, and coronary procedures such as angioplasty or coronary artery bypass graft surgery, ours is among the first to investigate a posteriori–derived dietary patterns and hazard of recurrent CHD events using adjudication, a more rigorous assessment than self‐report. This is in contrast to the Mediterranean diet, where clinical trial data from the Lyon Diet Heart Study have shown that patients with a previous MI who were randomized to consume a Mediterranean diet had a lower rate of recurrent MI compared with patients randomized to a prudent‐type diet.27 However, because of the relatively small sample size and short duration of follow‐up in the Lyon Study, as well as the selective nature of recruitment in this and other clinical trials, observational data from cohorts of community‐dwelling people, especially those such as REGARDS, which include a significant proportion of black participants, remain relevant. A previous analysis investigating various lifestyle modifications and recurrent CHD events in REGARDS showed an inverse association between the Mediterranean diet score and risk of recurrent CHD events, although this finding was of borderline statistical significant (HR [95% CI] for Q4 versus Q1, 0.77 [0.55–1.06]; P Trend=0.084).28 However, that analysis included outcome data through December 31, 2009, while the current analysis includes outcomes occurring over 4 additional years (through December 31, 2013).
Previous studies of dietary patterns and all‐cause mortality in various populations have produced mixed results. Western‐type diets (high‐fat, meat‐rich, low‐fiber), which have similarities to our Southern dietary pattern, have shown adverse associations with all‐cause mortality in several cohorts, including the US Nurse's Health study,29 older British men,30 and Chinese men and women (but only in ever smokers).31 However, a Western dietary pattern showed no association with all‐cause mortality in a Spanish cohort,32 in English civil service employees in the Whitehall II study,33 or in a cohort of Danish men and women.34 There was a surprising inverse association of a Western dietary pattern and all‐cause mortality in a cohort of Japanese men and women.35 In a recent systematic review and meta‐analysis of 13 prospective cohort studies, a Western dietary pattern was not significantly associated with risk of all‐cause mortality.36
In contrast to the lack of an association with the plant‐based pattern and all‐cause mortality in our analysis, a prudent diet (which generally is characterized by high intakes of fruits and vegetables, and low loadings of meats and sweets) was associated with a significantly lower risk of all‐cause mortality in the Nurse's Health Study cohort,29 a Chinese population,31 Danish men and women,34 and a cohort of Japanese men and women.35 However, there was no association of healthy dietary patterns with all‐cause mortality in a study of British men,30 or in the Whitehall II study.33 In the previously noted systematic review and meta‐analysis, a prudent dietary pattern was inversely associated with risk of all‐cause mortality.36
In agreement with our results, a Mediterranean dietary pattern was inversely associated with all‐cause mortality in the US Multiethnic Cohort,37 the US National Institutes of Health–AARP Diet and Health Study,38 Spanish cohorts,32, 39 an Italian cohort,40 the UK‐based EPIC (European Prospective Investigation of Cancer)–Norfolk study,41 a Danish cohort,42 and a cohort of elderly European men and women.43 However, there was no association of a Mediterranean‐type diet with all‐cause mortality in the UK‐based Whitehall II study.33 A previous analysis investigating various lifestyle modifications and recurrent CHD events in REGARDS showed an inverse association between the Mediterranean diet score and risk of all‐cause mortality, although this finding was of borderline statistical significance (HR [95% CI] for Q4 versus Q1, 0.84 [0.66–1.07]; P Trend=0.061).28 This analysis included outcome data through December 31, 2009, while the current analysis includes outcomes occurring over 4 additional years (through December 31, 2013). In another recent analysis from REGARDS, the Mediterranean diet pattern was inversely associated with all‐cause mortality44; however, this analysis was not restricted to participants with CHD at baseline, as in the current analysis.
There are several mechanisms potentially contributing to an association between greater consumption of the Southern dietary pattern and increased hazard of CHD. Examples include the high sodium content of processed meats that can contribute to hypertension, a risk factor for CHD; nitrite preservatives in processed meats, which have been shown experimentally to promote atherosclerosis and vascular dysfunction45; and the high sugar‐sweetened beverage consumption characteristic of the Southern pattern, which not only negatively impacts BMI but also increases glycemic load, resulting in insulin resistance, β‐cell dysfunction, and inflammation, all setting the stage for atherosclerosis.46 However, while the Southern pattern was adversely associated with the hazard of incident CHD events in our previous analysis,18 it was not associated with the hazard of recurrent CHD events in the current analysis. There are several possible explanations for this. First, those participants who experienced a recurrent CHD event may be different in important ways from those who did not, including having different susceptibilities to recognized CHD risk factors, including diet. Second, it is conceivable that risk factors and/or strength of associations vary between incident and recurrent events, such that while an adverse dietary pattern plays an important role in the initiation of CHD, once CHD is established, its importance in the risk factor profile is diminished relative to other concomitant factors. Under either scenario, different covariates would be declared statistically significant between the 2 analyses. Finally, those at greatest risk may have changed their diet to a healthier pattern after their initial CHD event with the intent of reducing the risk of recurrent disease. If this were to occur in a sizable segment of the study population, the risk of recurrent CHD in those adhering to a Southern pattern at baseline might have been underestimated. It is important to note that dietary assessment was conducted only at baseline, so we were unable to assess whether participants changed their dietary patterns between their incident and recurrent CHD events. Changing dietary habits from what are perceived as less healthy to healthier in response to a major life event, such as the diagnosis of an incident CHD event, potentially could have resulted in misclassification of the exposure (dietary intake) in reference to recurrent events, which could have attenuated the results. Unfortunately, we were unable to address this directly in the current study because dietary assessment was not repeated in REGARDS participants.
Possible reasons for disagreement in the results among previous studies noted above include differences in critical study elements such as sample size and population characteristics, dietary assessment instrument and methodology utilized, dietary pattern derivation and selection, and rigorousness of end point determination. The strengths of this study included the large, population‐based sample with sociodemographic and regional diversity, including a sizable proportion of black participants, which distinguishes it from most previous studies; a comprehensive assessment of diet with a recognized instrument; derivation of dietary patterns using a rigorous method—factor analysis—based on the actual dietary data collected in the population of interest rather than dietary patterns created a priori based on the authors’ opinions on what defines various dietary patterns; and expert adjudication of study end points. Limitations include the recognized potential for measurement error with any dietary assessment instrument that relies on recall of dietary intake, as is the case with FFQs, which could result in misclassification of dietary intake. However, if random, this would tend to bias results toward the null, potentially reducing the magnitude of the associations between dietary patterns/score and recurrent CHD events and all‐cause mortality observed in this analysis. Additionally, those who did not provide a completed FFQ showed no differences in sex or race compared with those who completed the FFQ but were more likely to be black, less educated, and have a lower income. Noncompleters also were slightly more likely to be current smokers and inactive, and had a slightly lower BMI, compared with those who completed the FFQ. In order to reduce potential bias and increase the power of our sample tests, we multiply imputed those who did not complete the FFQ in a sensitivity analysis. Finally, the results observed may not be generalizable to groups other than whites and blacks in the United States.
In summary, the Mediterranean diet score was inversely associated with the hazard of recurrent CHD events and all‐cause mortality, while the Southern dietary pattern was associated with the hazard of all‐cause mortality but not recurrent CHD events in this diverse sample of white and black adults. Because diet is a modifiable factor, identifying dietary patterns that may contribute to mortality or recurrent CHD events may pave the way for the development of specific nutritional health messages aimed at changing dietary choices made by individuals who have survived CHD.
Sources of Funding
This work was supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke and R01 HL080477 and K24 HL111154 from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Neurological Disorders and Stroke, National Heart, Lung, and Blood Institute, or the National Institutes of Health. Representatives of the funding agencies have been involved in the review of the manuscript but not directly involved in the collection, management, analysis, or interpretation of the data. General Mills Bell Institute of Health and Nutrition generously supported the scanning and analysis of the dietary questionnaires used in this study.
Disclosures
None.
Acknowledgments
The authors thank the other investigators, staff, and participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
(J Am Heart Assoc. 2018;7:e008078 DOI: 10.1161/JAHA.117.008078.)
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) . Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 3. Kromhout D, Menotti A, Kesteloot H, Sans S. Prevention of coronary heart disease by diet and lifestyle: evidence from prospective cross‐cultural, cohort, and intervention studies. Circulation. 2002;105:893–898. [DOI] [PubMed] [Google Scholar]
- 4. Mente A, de Koning L, Shannon HS, Anand SS. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch Intern Med. 2009;169:659–669. [DOI] [PubMed] [Google Scholar]
- 5. Hu FB, Rimm E, Smith‐Warner SA, Feskanich D, Stampfer MJ, Ascherio A, Sampson L, Willett WC. Reproducibility and validity of dietary patterns assessed with a food‐frequency questionnaire. Am J Clin Nutr. 1999;69:243–249. [DOI] [PubMed] [Google Scholar]
- 6. Newby PK, Tucker KL. Empirically derived eating patterns using factor or cluster analysis: a review. Nutr Rev. 2004;62:177–203. [DOI] [PubMed] [Google Scholar]
- 7. Van Horn L. Eating pattern analysis: the whole is more than the sum of its parts. J Am Diet Assoc. 2011;111:203. [DOI] [PubMed] [Google Scholar]
- 8. Hu FB, Rimm EB, Stampfer MJ, Acherio A, Spiegelman D, Willett WC. Prospective study of major dietary patterns and risk of coronary heart disease in men. Am J Clin Nutr. 2000;72:912–921. [DOI] [PubMed] [Google Scholar]
- 9. Fung TT, Willett WC, Stampfer MJ, Manson JE, Hu FB. Dietary patterns and the risk of coronary heart disease in women. Arch Intern Med. 2001;161:1857–1862. [DOI] [PubMed] [Google Scholar]
- 10. Martinez‐Ortiz JA, Fung TT, Baylin A, Hu FB, Campos H. Dietary patterns and risk of nonfatal acute myocardial infarction in Costa Rican adults. Eur J Clin Nutr. 2006;60:770–777. [DOI] [PubMed] [Google Scholar]
- 11. Iqbal R, Anand S, Ounpuu S, Islam S, Zhang X, Rangarajan S, Chifamba J, Al‐Hinai A, Keltai M, Yusuf S; INTERHEART Study Investigators . Dietary patterns and the risk of acute myocardial infarction in 52 countries: results of the INTERHEART study. Circulation. 2008;118:1929–1937. [DOI] [PubMed] [Google Scholar]
- 12. Guallar‐Castillón P, Rodríguez‐Artalejo F, Tormo MJ, Sanchez MJ, Rodriguez L, Quiros JR, Navarro C, Molina E, Martinez C, Marin P, Lopez‐Garcia E, Larrañaga N, Huerta JM, Dorronsoro M, Chirlaque MD, Buckland G, Barricarte A, Banegas JR, Arriola L, Ardanaz E, González CA, Moreno‐Iribas C. Major dietary patterns and risk of coronary heart disease in middle‐aged persons from a Mediterranean country: the EPIC‐Spain cohort study. Nutr Metab Cardiovasc Dis. 2012;22:192–199. [DOI] [PubMed] [Google Scholar]
- 13. Osler M, Helms Andreasen A, Heitmann B, Hoidrup S, Gerdes U, Morch Jorgensen L, Schroll M. Food intake patterns and risk of coronary heart disease: a prospective cohort study examining the use of traditional scoring techniques. Eur J Clin Nutr. 2002;56:568–574. [DOI] [PubMed] [Google Scholar]
- 14. Guo J, Li W, Wang Y, Chen T, Teo K, Liu LS, Yusuf S; INTERHEART China Study Investigators . Influence of dietary patterns on the risk of acute myocardial infarction in China population: the INTERHEART China study. Chin Med J. 2013;126:464–470. [PubMed] [Google Scholar]
- 15. Maruyama K, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, Inaba Y, Tamakoshi A; JACC Study Group . Dietary patterns and risk of cardiovascular deaths among middle‐aged Japanese: JACC Study. Nutr Metab Cardiovasc Dis. 2013;23:519–527. [DOI] [PubMed] [Google Scholar]
- 16. Stricker MD, Onland‐Moret NC, Boer JM, van der Schouw YT, Verschuren WM, May AM, Peeters PH, Beulens JW. Dietary patterns derived from principal component‐ and k‐means cluster analysis: long‐term association with coronary heart disease. Nutr Metab Cardiovasc Dis. 2013;23:250–256. [DOI] [PubMed] [Google Scholar]
- 17. Judd SE, Letter AJ, Shikany JM, Roth DL, Newby PK. Dietary patterns derived using exploratory and confirmatory factor analysis are stable and generalizable across race, region, and gender subgroups in the REGARDS study. Front Nutr. 2015;1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shikany JM, Safford MM, Newby PK, Durant RW, Brown TM, Judd SE. Southern dietary pattern is associated with hazard of acute coronary heart disease in the Reasons for Geographic and Racial Differences in Stroke (REGARDS) study. Circulation. 2015;132:804–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Krumholz HM, Normand SL, Wang Y. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999–2011. Circulation. 2014;130:966–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The Reasons for Geographic and Racial Differences in Stroke study: objectives and design. Neuroepidemiology. 2005;25:135–143. [DOI] [PubMed] [Google Scholar]
- 21. Block G, Woods M, Potosky A, Clifford C. Validation of a self‐administered diet history questionnaire using multiple diet records. J Clin Epidemiol. 1990;43:1327–1335. [DOI] [PubMed] [Google Scholar]
- 22. Mares‐Perlman JA, Klein BE, Klein R, Ritter LL, Fisher MR, Freudenheim JL. A diet history questionnaire ranks nutrient intakes in middle‐aged and older men and women similarly to multiple food records. J Nutr. 1993;123:489–501. [DOI] [PubMed] [Google Scholar]
- 23. Levine TR. Confirmatory factor analysis and scale validation in communication research. Commun Res Rep. 2005;22:335–338. [Google Scholar]
- 24. Dinu M, Pagliai G, Casini A, Sofi F. Mediterranean diet and multiple health outcomes: an umbrella review of meta‐analyses of observational studies and randomised trials. Eur J Clin Nutr. 2018;72:30–43. [DOI] [PubMed] [Google Scholar]
- 25. Tsivgoulis G, Judd S, Letter AJ, Alexandrov AV, Howard G, Nahab F, Unverzagt FW, Moy C, Howard VJ, Kissela B, Wadley VG. Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology. 2013;80:1684–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Safford MM, Brown TM, Muntner PM, Durant RW, Glasser S, Halanych JH, Shikany JM, Prineas RJ, Samdarshi T, Bittner VA, Lewis CE, Gamboa C, Cushman M, Howard V, Howard G; REGARDS Investigators . Association of race and sex with risk of incident acute coronary heart disease events. JAMA. 2012;308:1768–1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Lorgeril M, Salen P, Martin JL, Monjaud I, Delaye J, Mamelle N. Mediterranean diet, traditional risk factors, and the rate of cardiovascular complications after myocardial infarction: final report of the Lyon Diet Heart Study. Circulation. 1999;99:779–785. [DOI] [PubMed] [Google Scholar]
- 28. Booth JN III, Levitan EB, Brown TM, Farkouh ME, Safford MM, Muntner P. Effect of sustaining lifestyle modifications (nonsmoking, weight reduction, physical activity, and Mediterranean diet) after healing of myocardial infarction, percutaneous intervention, or coronary bypass (from the REasons for Geographic and Racial Differences in Stroke Study). Am J Cardiol. 2014;113:1933–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heidemann C, Schulze MB, Franco OH, van Dam RM, Mantzoros CS, Hu FB. Dietary patterns and risk of mortality from cardiovascular disease, cancer, and all causes in a prospective cohort of women. Circulation. 2008;118:230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Atkins JL, Whincup PH, Morris RW, Lennon LT, Papacosta O, Wannamethee SG. Dietary patterns and the risk of CVD and all‐cause mortality in older British men. Br J Nutr. 2016;116:1246–1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Odegaard AO, Koh WP, Yuan JM, Gross MD, Pereira MA. Dietary patterns and mortality in a Chinese population. Am J Clin Nutr. 2014;100:877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zazpe I, Sánchez‐Tainta A, Toledo E, Sánchez‐Villegas A, Martínez‐González MÁ. Dietary patterns and total mortality in a Mediterranean cohort: the SUN project. J Acad Nutr Diet. 2014;114:37–47. [DOI] [PubMed] [Google Scholar]
- 33. Brunner EJ, Mosdøl A, Witte DR, Martikainen P, Stafford M, Shipley MJ, Marmot MG. Dietary patterns and 15‐y risks of major coronary events, diabetes, and mortality. Am J Clin Nutr. 2008;87:1414–1421. [DOI] [PubMed] [Google Scholar]
- 34. Osler M, Heitmann BL, Gerdes LU, Jørgensen LM, Schroll M. Dietary patterns and mortality in Danish men and women: a prospective observational study. Br J Nutr. 2001;85:219–225. [DOI] [PubMed] [Google Scholar]
- 35. Nanri A, Mizoue T, Shimazu T, Ishihara J, Takachi R, Noda M, Iso H, Sasazuki S, Sawada N, Tsugane S; Japan Public Health Center‐Based Prospective Study Group . Dietary patterns and all‐cause, cancer, and cardiovascular disease mortality in Japanese men and women: the Japan Public Health Center‐Based Prospective Study. PLoS One. 2017;12:e0174848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li F, Hou LN, Chen W, Chen PL, Lei CY, Wei Q, Tan WL, Zheng SB. Associations of dietary patterns with the risk of all‐cause, CVD and stroke mortality: a meta‐analysis of prospective cohort studies. Br J Nutr. 2015;113:16–24. [DOI] [PubMed] [Google Scholar]
- 37. Harmon BE, Boushey CJ, Shvetsov YB, Ettienne R, Reedy J, Wilkens LR, Le Marchand L, Henderson BE, Kolonel LN. Associations of key diet‐quality indexes with mortality in the Multiethnic Cohort: the Dietary Patterns Methods Project. Am J Clin Nutr. 2015;101:587–597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mitrou PN, Kipnis V, Thiebaut AC, Reedy J, Subar AF, Wirfalt E, Flood A, Mouw T, Hollenbeck AR, Leitzmann MF, Schatzkin A. Mediterranean dietary pattern and prediction of all‐cause mortality in a US population: results from the NIH‐AARP Diet and Health Study. Arch Intern Med. 2007;167:2461–2468. [DOI] [PubMed] [Google Scholar]
- 39. Buckland G, Agudo A, Travier N, Huerta JM, Cirera L, Tormo MJ, Navarro C, Chirlaque MD, Moreno‐Iribas C, Ardanaz E, Barricarte A, Etxeberria J, Marin P, Quiros JR, Redondo ML, Larranaga N, Amiano P, Dorronsoro M, Arriola L, Basterretxea M, Sanchez MJ, Molina E, Gonzalez CA. Adherence to the Mediterranean diet reduces mortality in the Spanish cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC‐Spain). Br J Nutr. 2011;106:1581–1591. [DOI] [PubMed] [Google Scholar]
- 40. Prinelli F, Yannakoulia M, Anastasiou CA, Adorni F, Di Santo SG, Musicco M, Scarmeas N, Correa Leite ML. Mediterranean diet and other lifestyle factors in relation to 20‐year all‐ cause mortality: a cohort study in an Italian population. Br J Nutr. 2015;113:1003–1011. [DOI] [PubMed] [Google Scholar]
- 41. Tong TY, Wareham NJ, Khaw KT, Imamura F, Forouhi NG. Prospective association of the Mediterranean diet with cardiovascular disease incidence and mortality and its population impact in a non‐Mediterranean population: the EPIC‐Norfolk study. BMC Med. 2016;14:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tognon G, Lissner L, Saebye D, Walker KZ, Heitmann BL. The Mediterranean diet in relation to mortality and CVD: a Danish cohort study. Br J Nutr. 2014;111:151–159. [DOI] [PubMed] [Google Scholar]
- 43. Knoops KT, de Groot LC, Kromhout D, Perrin AE, Moreiras‐Varela O, Menotti A, van Staveren WA. Mediterranean diet, lifestyle factors, and 10‐year mortality in elderly European men and women: the HALE project. JAMA. 2004;292:1433–1439. [DOI] [PubMed] [Google Scholar]
- 44. Whalen KA, Judd S, McCullough ML, Flanders WD, Hartman TJ, Bostick RM. Paleolithic and Mediterranean diet pattern scores are inversely associated with all‐cause and cause‐specific mortality in adults. J Nutr. 2017;147:612–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta‐analysis. Circulation. 2010;121:2271–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang C, Huang J, Tian Y, Yang X, Gu D. Sugar sweetened beverages consumption and risk of coronary heart disease: a meta‐analysis of prospective studies. Atherosclerosis. 2014;234:11–16. [DOI] [PubMed] [Google Scholar]


