Abstract
Background
People with end‐stage renal disease (ESRD) are at risk for advanced heart failure, but little is known about use and outcomes of durable mechanical circulatory support in this setting. We examined use and outcomes of implantable ventricular assist devices (VADs) in a national ESRD cohort.
Methods and Results
We performed a retrospective cohort study of Medicare beneficiaries with ESRD who underwent implantable VAD placement from 2006 to 2014. We examined in‐hospital and 1‐year mortality, all‐cause and cause‐specific hospitalizations, and heart/kidney transplantation outcomes. We investigated as predictors demographic factors, time‐period of VAD implantation, primary or post‐cardiotomy implantation, and duration of ESRD before VAD implantation. We identified 96 people with ESRD who underwent implantable VAD placement. At time of VAD implantation, 74 (77.1%) were receiving hemodialysis, 10 (10.4%) were receiving peritoneal dialysis and 12 (12.5%) had renal transplant. Time from incident ESRD to VAD implantation was median 4.0 (interquartile range 1.1, 8.2) years. Mortality during the implantation hospitalization was 40.6%. Within 1 year of implantation 61.5% of people had died. On multivariable analysis, males had half the mortality risk of females. Lower mortality risk was also seen with VAD implantation in a primary setting, and with more recent year of implantation, but these results did not reach statistical significance.
Conclusions
Medicare beneficiaries with ESRD are undergoing durable VAD implantation, often several years after incident ESRD, although in low numbers. Mortality is high among these patients, highlighting the need for investigations to improve treatment selection and management.
Keywords: end‐stage renal disease, kidney, ventricular assist device
Subject Categories: Heart Failure, Clinical Studies, Mortality/Survival, Cardiovascular Surgery
Clinical Perspective
What Is New?
People with end‐stage renal disease (ESRD) are at risk for advanced heart failure, but little is known about use and outcomes of durable mechanical circulatory support in this setting.
Using Medicare data, the current study demonstrates that ventricular assist devices are being implanted in people with pre‐existing ESRD, sometimes several years after incident ESRD.
These people have high in‐hospital and 1‐year mortality.
What Are the Clinical Implications?
Clinicians should be aware of the high mortality among Medicare beneficiaries with ESRD who undergo ventricular assist device implantation.
Investigations are needed to improve outcomes of people with ESRD who require mechanical circulatory support for advanced heart failure.
Introduction
Heart failure (HF) affects ≈6.5 million people ≥20 years of age in the United States, and contributes to 1 in 8 deaths.1 Chronic kidney disease (CKD) affects >30 million people in the United States, and over 700 000 people have end‐stage renal disease (ESRD).2, 3 Cardiovascular disease is a primary cause of morbidity and death among people with CKD, who are at high risk for coronary heart disease, diastolic and systolic left ventricular dysfunction, valvular disease, and arrhythmias.4 Ventricular assist devices (VADs) are used for advanced HF that cannot be managed with conservative therapies, and can be used as life‐sustaining therapy until cardiac transplantation, as permanent or destination therapy, or as temporary support while cardiac function improves.5 Among people undergoing left ventricular assist device (LVAD) implantation, preimplantation kidney dysfunction, especially preimplantation dialysis, is associated with poor outcomes, although the chronicity of kidney dysfunction in reports is generally unclear and may reflect largely acute and subacute, rather than chronic, dysfunction.6
An American Heart Association statement from 2012 on the appropriate use of mechanical circulatory support stated that patients on long‐term dialysis should not be considered for durable support.7 Decisions on patient selection vary by center, with some centers having even more stringent kidney function requirements for LVAD placement.8 Several recent publications address long‐term dialysis management in people with LVADs, although this is often in the context of ESRD developing after LVAD implantation.9 With advancing technologies, management challenges change. Continuous flow devices, now used almost exclusively, are less preload dependent than the earlier pulsatile flow devices,10 perhaps making volume shifts with ultrafiltration in hemodialysis less concerning. However, they are associated with more gastrointestinal bleeding and cerebrovascular events.11, 12 ESRD and VADs may have overlapping and negatively synergistic risk profiles, related to bleeding complications, infections, and neurologic dysfunction.
Organizations and authors are increasingly advocating for an integrated palliative care approach to the care of people with advanced heart failure, given their substantial symptom burden and high mortality. An American Heart Association scientific statement suggests routine palliative care physician consultation before institution of mechanical circulatory support, and The Joint Commission requires palliative care specialist involvement for VAD destination therapy.13, 14, 15 Organizations and authors are also increasingly recognizing the important role palliative care plays in ESRD, and especially congestive heart failure complicating ESRD.16, 17 People with ESRD, and those with congestive heart failure, may receive lower quality end‐of‐life care than those with other life‐limiting conditions.18
We aimed to describe implantable VAD use among Medicare beneficiaries with ESRD, and their hospitalizations and survival. We hypothesized that mortality would decrease in more recent years and would vary between people who were receiving dialysis and those with kidney transplants. We investigated the use of implantable VADs in Medicare beneficiaries with ESRD, including dialysis‐dependent people (CKD 5D) and kidney transplant recipients (CKD T), using data from the United States Renal Data System (USRDS).
Methods
The data files are publicly available from the USRDS. The analytic methods and study materials will not be maintained in a publicly available repository for other researchers to reproduce the results or replicate the procedure.
Study Population
We identified Medicare beneficiaries with ESRD who received an implantable VAD between 2006 and 2014, using the USRDS database. The USRDS collects and distributes information about chronic kidney disease (CKD) and end‐stage renal disease (ESRD) in the United States, in collaboration with the Centers for Medicare & Medicaid Services.19 We used the Centers for Medicare & Medicaid Services ESRD Standard Analytical Files (SAFs) from 2006 to 2014, which include Part A and Part B claims for all people with traditional Medicare and ESRD, to identify VAD implantation among people with pre‐existing ESRD.19 We identified implantable VAD placement using Medicare Part A institutional claims with International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) procedure code 37.66 (insertion of implantable heart assist system). The claims also had to include diagnoses for HF or cardiogenic shock, in which case it was classified as primary therapy, or there had to be a claim within 30 days prior for a separate major cardiac surgery, in which case it was classified as post‐cardiotomy (Table S1).20, 21 In 2005, before our study period, ICD‐9‐CM procedure codes were refined to distinguish implantable (37.66) and non‐implantable (37.62 and 37.65) ventricular assist devices.20 We required the VAD index date to be >30 days after the ESRD index date, which was obtained from the ESRD Medicare Evidence Report (Centers for Medicare & Medicaid Services Form 2728). People who recovered kidney function before VAD implantation were excluded (n=24). Transplantation was identified from Medicare institutional claims with ICD‐9‐CM 37.51 (heart transplantation), or USRDS reported kidney transplantation event, which is based on Organ Procurement and Transplantation Network data. People were excluded from the cohort if they had heart transplantation within 30 days before, or on the same day of, the VAD implantation. We determined hemodialysis vascular access at time of VAD implantation using the Medical Evidence Report, Medicare procedural claims, and, for VAD implantation since July 2010, the Centers for Medicare & Medicaid Services Common Procedure Coding Standard (HCPCS) modifier codes from the Revenue Center Details Files.
Predictors
We divided VAD implantation years into ranges (2006–2009, 2010–2011, and 2012–2014), to reflect changing patterns of device technology and experience (eg, changes in device strategy from use as a bridge to heart transplantation in the earlier years, to use as destination therapy also in later years). From 2006 to 2009, pulsatile flow pumps were common, whereas continuous flow pumps have been used almost exclusively since 2010.22 Demographic factors, ESRD modality (hemodialysis, peritoneal dialysis, or kidney transplant), VAD implantation circumstance (primary or post‐cardiotomy), and cause of ESRD (diabetes mellitus, or other, based on the ESRD Medical Evidence Report) were also evaluated as predictors of mortality. We also evaluated the duration of ESRD before VAD implantation as a predictor of mortality (dichotomized into <1 year, or ≥1 year), because of possible clinical differences between those with early versus later VAD implantation after incident ESRD. For example, early VAD implantation may be more common in Type 2 cardiorenal syndrome, whereas late VAD implantation may reflect a Type 4 or 5 cardiorenal syndrome.23
Outcomes
We identified all‐cause mortality from the USRDS database, which combines death identification from several sources.19 In‐hospital mortality was identified by comparing hospitalization dates and dates of death. Duration of hospitalization was determined using Medicare claims. Hospitalizations with discharges followed by admission on the same day were counted as 1 hospitalization. Proportion of days hospitalized in the first year following the VAD implantation was calculated by the total number of days of hospitalization divided by the days before death, heart or heart‐kidney transplantation, or loss of Medicare Parts A and B coverage. Among patients discharged alive from the initial hospitalization and having continuous Medicare Parts A and B, we determined readmission causes by classifying the primary discharge diagnosis using the Healthcare Cost and Utilization Project Clinical Classification Software for most causes.24 Infection‐related readmissions were determined from the primary discharge diagnosis, using ICD‐9‐CM codes as outlined in Table S2.25
Covariates
We extracted demographic data from the USRDS Patients file. We identified comorbid conditions from inpatient and outpatient claims submitted to the Centers for Medicare and Medicaid Services within 2 years before VAD implantation (as early as 2004), among the subgroup of those with continuous Medicare Parts A and B coverage during that time, using ICD‐9‐CM diagnostic codes (Table S1). We verified that the ICD‐9‐CM codes used did not change substantively between 2004 and 2014. We obtained the cause of ESRD from the Medical Evidence Report.
Statistical Methods
Characteristics at the time of VAD implantation were described as mean (SD) or median (interquartile range) for continuous variables, and as number (%) for categorical variables. Descriptive statistics are provided for the overall cohorts, and for subgroups by ESRD modality and VAD implantation year. Subjects were right censored when they lost continuous Medicare Parts A and B coverage or survived 1 year after VAD implantation, whichever was earlier. Heart and/or kidney transplantation were considered as competing events. Cumulative mortality incidence in 1 year after VAD implantation was estimated considering competing events and compared between subgroups using Gray's test.26 Baseline factors hypothesized to be associated with mortality (age, dichotomized race, sex, cause of ESRD [diabetes mellitus or other], year‐range of VAD implantation, and implantation circumstance [primary or post‐cardiotomy]) were evaluated using univariable and multivariable cause‐specific hazards regression models and subdistribution hazard models, using transplantations as competing events, and are reported with hazard ratios (HR) and 95% confidence intervals. We limited the number of variables in the models to avoid overfitting. Readmission was calculated using cumulative incidence of first readmission over 1 year, with competing events of death and transplantation, among patients with continuous Medicare Parts A and B who survived the index hospitalization. We created a competing events graph for those on maintenance dialysis at VAD implantation, demonstrating the proportions alive on dialysis, transplanted, and deceased, over 1 year. We conducted the analyses using SAS 9.4 (SAS Institute, Cary, NC). Two‐sided significance at α=0.05 was used in all analyses.
This study was approved by the Institutional Review Board at Baylor College of Medicine (protocol #H‐36408). Informed consent was waived.
Results
Patient Characteristics
We identified 96 people with ESRD who underwent VAD implantation (Tables 1 and 2). Seventy‐four people (77.1%) were on hemodialysis, 10 (10.4%) were on peritoneal dialysis, and 12 (12.5%) had a functioning kidney transplant at time of VAD implantation. Age at VAD implantation ranged from 25 to 80 years old, and mean (SD) age at implantation was 56.8 (12.5) years. Females made up 21.9% of the cohort, with similar sex distributions in subgroups based on ESRD modality. Most of the cohort was classified as white, and approximately one third of the cohort was black. Diabetes mellitus was the most common cause of ESRD. Time from incident ESRD to VAD implantation ranged from 0.1 to 27.6 years. Among those with kidney transplants, the interval was longer (median [interquartile range] 14.5 [8.4, 21.2] years), compared with those on hemodialysis or peritoneal dialysis (3.7 [0.9, 6.6] years) (Table 2). In each modality and era subgroup, >55% underwent VAD implantation for primary indications, except the 2006 to 2009 era, in which 44.7% had a primary indication (Table 2). Among those on maintenance hemodialysis at time of VAD implantation, 43.3% used a dialysis catheter, 41.9% used an arteriovenous dialysis access (fistula or graft), and 14.9% used unknown dialysis access.
Table 1.
Characteristics of the ESRD Cohort at Time of VAD Implantation (N=96)
| Age, y | 56.8±12.5 |
| Female | 21 (21.9%) |
| Race | |
| Black | 34 (35.4%) |
| White/Other | 62 (64.6%) |
| Hispanic ethnicity | 17 (17.7) |
| Cause of ESRD | |
| Diabetes mellitus | 41 (42.7%) |
| HTN/Large vessel disease | 22 (22.9%) |
| Glomerulonephritis | 15 (15.6%) |
| Other | 18 (18.8%) |
| Time from start of ESRD treatment to VAD, years | 4.0 (1.1, 8.2) |
| VAD implantation circumstance | |
| Primary therapy | 54 (56.3%) |
| Post‐cardiotomy | 42 (43.8%) |
| Continuous Medicare for 2 years before VAD implantation | 53 (55.2%) |
| Comorbiditiesa | |
| Atrial fibrillation/flutter | 25 (47.2%) |
| Lung disease | 26 (49.1%) |
| Coronary artery disease | 43 (81.1%) |
| Diabetes mellitus | 31 (58.5%) |
| Hypertension | 51 (96.2%) |
| Peripheral vascular disease | 24 (45.3%) |
| Valvular cardiac disease | 34 (64.2%) |
Values for categorical variables are given as number (percentage); continuous variables as mean±SD or median (interquartile range). ESRD indicates end‐stage renal disease; HTN, hypertension; VAD, ventricular assist device.
Among those with continuous Medicare Parts A and B for 2 years before VAD implantation.
Table 2.
Characteristics for Total Cohort, and Subgroups Based on ESRD Modality and Year of VAD Implantation
| Total (N=96) | ESRD Modality | Year of VAD Implantation | ||||
|---|---|---|---|---|---|---|
| HD/PD (n=84) | Kidney Transplant (n=12) | 2006–2009 (n=38) | 2010–2011 (n=25) | 2012–2014 (n=33) | ||
| Age, y | 60.0 (49.0, 65.0) | 60.0 (49.0, 64.0) | 60.0 (49.0, 70.0) | 56.5 (42.0, 63.0) | 61.0 (53.0, 68.0) | 61.0 (54.0, 65.0) |
| Time from ESRD to VAD implantation (y) | 4.0 (1.1, 8.2) | 3.7 (0.9, 6.6) | 14.5 (8.4, 21.2) | 5.0 (0.8, 8.1) | 2.6 (0.8, 8.3) | 4.2 (3.0, 7.8) |
| VAD placement indication | ||||||
| Primary indication | 54 (56.3%) | 47 (56.0%) | a | 17 (44.7%) | 15 (60.0%) | 22 (66.7%) |
| Post‐cardiotomy | 42 (43.8%) | 37 (44.0%) | a | 21 (55.3%) | 10 (40.0%) | 11 (33.3%) |
Categorical variables are number (percentage); continuous variables are median (interquartile range). ESRD indicates end‐stage renal disease; HD, hemodialysis; PD, peritoneal dialysis; VAD, ventricular assist device.
Cell counts suppressed for n<10.
Mortality and Transplantation
Mortality rates were high early after implantation, and subsequently declined (Figure 1). Thirty‐nine people (40.6%) died during the VAD implantation hospitalization, with similar in‐hospital mortality in the dialysis and kidney transplant subgroups. An additional 20 people died within 1 year of VAD implantation, for a total 1‐year mortality of 61.5%. This proportion did not vary significantly by whether the patient was receiving maintenance hemodialysis, peritoneal dialysis, or had a functional kidney transplant at time of implantation. Cumulative 1‐year mortality incidence was 48.4% in the primary implantation group, compared with 73.8% in the post‐cardiotomy group (P=0.003), due primarily to early mortality events (Figure S1). For the most recent VAD implantations (2012–2014), 1‐year cumulative mortality was 51.5%, compared with 63.4% in 2006 to 2009 (P=0.11). We also analyzed 1‐year cumulative mortality by the interval from incident ESRD to VAD implantation, dichotomized at 1 year, and found similar outcomes (interval <1 year: 61.7%, interval ≥1 year: 59.0%).
Figure 1.
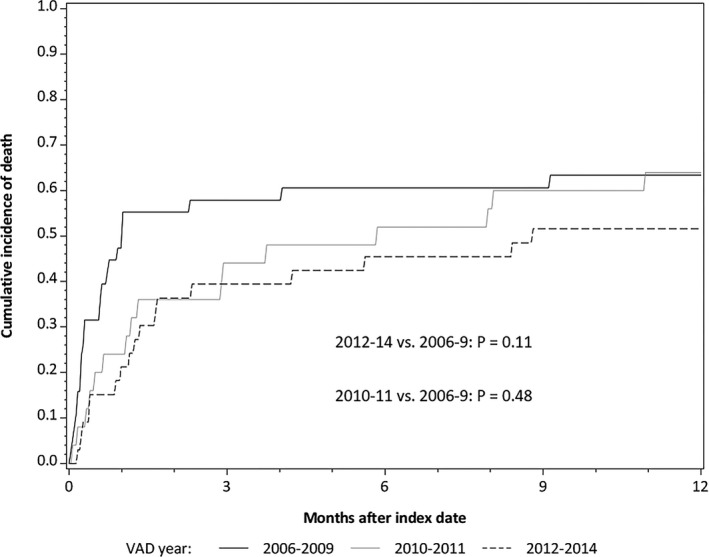
Cumulative incidence of mortality stratified by era of ventricular assist device implantation. (2012–2014 vs 2006–2009: P=0.11; 2010–2011 vs 2006–2009: P=0.48). VAD indicates ventricular assist device.
In the multivariable‐adjusted cause‐specific hazard model, males had less than half the risk of death (HR [95% confidence interval] 0.48 [0.26, 0.89]) compared with females (Table 3). VAD implantation in 2012 to 2014 was associated with a point estimate 45% lower risk compared with 2006 to 2009, but this was not statistically significant (HR [95% confidence interval]: 0.55 [0.29, 1.06]). Post‐cardiotomy VAD implantation was associated with nearly twice the mortality risk of primary VAD implantation in the univariable model, although this lost statistical significance in the multivariable model (HR [95% confidence interval] 1.77 [0.97, 3.23]). Subdistribution hazard models yielded similar findings (Table S3). Figure 2 shows the proportions of the initial cohort that remained alive and on dialysis, had received transplants, or had died before receiving transplants, over the first year following VAD implantation. Among those with CKD 5D at the time of VAD implantation, by 1 year following VAD implantation, 10.4% had received transplantation, and 30.2% remained on dialysis.
Table 3.
Cause Specific Hazards Models for Mortality
| Hazard Ratios (95% Confidence Intervals) | ||
|---|---|---|
| Univariable | Multivariable | |
| Age (per 1 y) | 1.01 (0.99, 1.03) | 1.01 (0.98, 1.03) |
| Race | ||
| Other | 1 (ref) | 1 (ref) |
| Black | 0.63 (0.36, 1.09) | 0.79 (0.44, 1.42) |
| Sex | ||
| Female | 1 (ref) | 1 (ref) |
| Male | 0.54 (0.28, 1.04) | 0.48 (0.26, 0.89) |
| Cause of ESRD | ||
| Other | 1 (ref) | 1 (ref) |
| Diabetes mellitus | 1.50 (0.87, 2.59) | 1.26 (0.73, 2.18) |
| VAD implantation year | ||
| 2006–2009 | 1 (ref) | 1 (ref) |
| 2010–2011 | 0.79 (0.41, 1.54) | 0.82 (0.43, 1.56) |
| 2012–2014 | 0.56 (0.29, 1.09) | 0.55 (0.29, 1.06) |
| VAD circumstance | ||
| Primary | 1 (ref) | 1 (ref) |
| Post‐cardiotomy | 1.96 (1.14, 3.37) | 1.77 (0.97, 3.23) |
ESRD indicates end‐stage renal disease; VAD, ventricular assist device.
Figure 2.
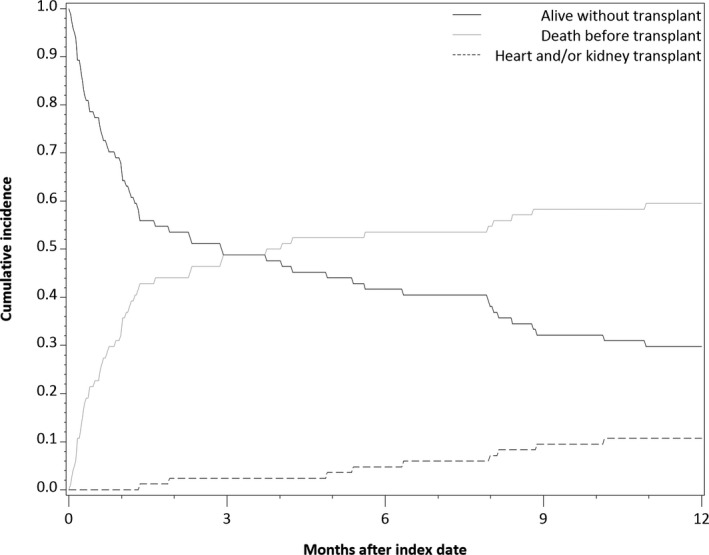
Cumulative incidence of competing outcomes of: remaining on dialysis (alive without transplant), transplantation, or death, among those with chronic kidney disease stage 5 on dialysis. The curves represent the proportions at each time that remain in the initial state (solid line: remaining on dialysis, alive without transplant), or have changed up to that time either to the state of having been transplanted (gray line), or having died (dashed line).
Duration of Hospitalizations
Among those who survived the initial hospitalization, the time from VAD implantation until discharge was median (interquartile range) 27.5 (12.5, 48.5) days, with a maximum interval of 251 days. The proportion of the first year (mean [SD]) following VAD implantation spent in the hospital (censoring at death, heart or heart‐kidney transplant, or loss of Medicare Parts and/or B) was 61.8% (38.1%). This was similar across ESRD modalities but was 15.5% lower in more recent years (51.7% [36.2] in 2012–2014, compared with 67.2% [38.2] in 2006–2011). This corresponds to a reduction of 56.6 hospitalization days over a 1‐year risk period, comparing 2006 to 2011 with 2012 to 2014.
All‐Cause and Cause‐Specific Readmissions
Of the 43 people who survived the initial hospitalization and had continuous Medicare coverage, 38 (88.4%) had at least 1 readmission during the first year. The most common readmission causes during the first year were cardiovascular (62.8% of people had a cardiovascular readmission), infection (51.5%), and gastrointestinal or other non‐intracranial hemorrhage (30.3%).
Discussion
In this national cohort of people with ESRD, on either maintenance dialysis or with functioning kidney transplant, who received implantable VAD placement, we found that over half the patients died within 1 year. This cohort consisted of people with severe organ dysfunction, requiring dual organ support therapy, and as such, high mortality is expected. Nearly half the cohort underwent post‐cardiotomy implantation, which generally has higher mortality, and in our cohort had 77% higher mortality risk than primary implantation, although this did not reach statistical significance in multivariable analysis. We found that males had much lower mortality risk than females after VAD implantation, a pattern previously reported in non‐CKD LVAD cohorts.27 The hemodynamic instability and clinical status at time of implantation, as reflected by the Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) profile, is an important determinant of mortality,27 and is unknown in our cohort. It is likely, however, that the severe chronic kidney disease and associated comorbidities are major contributors to the high mortality.
Data from INTERMACS, a national database of people receiving VAD implantation, show much higher survival than our cohort, with 1‐year survival of 81% in continuous flow LVADs and 65% with pulsatile flow LVADs in 2006 to 2013.22 Bansal et al recently published an analysis of circulatory support use in people with ESRD from 2003 to 2013, based on Medicare claims.28 However, unlike our analysis, that analysis included both external circulatory support devices (ICD‐9‐CM 37.62 and 37.65) and implantable VADs.28, 29 Thus, temporary circulatory support devices were included, which have different indications and outcomes than durable VADs, including placement in dire circumstances such as acute cardiogenic shock or cardiopulmonary arrest.30 This may in part explain the higher reported in‐hospital mortality (51.6%) compared with our cohort (40.6%). Further, primary and post‐cardiotomy mechanical circulatory support were not distinguished in that report. Other publications on VADs in ESRD are limited to case reports and small case series.31, 32 Arteriovenous hemodialysis accesses, which divert a significant portion of the cardiac output from systemic perfusion and have been implicated in high‐output HF, have been a cause for concern, both in terms of whether maturation would occur for arteriovenous fistulas, and whether systemic perfusion would remain adequate.33, 34 Recent case reports have described successful arteriovenous fistulas maturation in hemodialysis patients with LVADs.35, 36 A large proportion of our patients had arteriovenous accesses at time of VAD implantation, but we were unable to investigate outcomes related to dialysis access because of limited numbers.
Kidney dysfunction at time of LVAD implantation is associated with worse outcomes in other cohorts, although in those cases the dysfunction may reflect the severity of cardiac decompensation, and may be primarily acute.6, 22, 37 Many other investigations have focused on acute kidney injury complicating the LVAD implantation procedure, and on post‐implantation reductions in estimated glomerular filtration rate, and both are associated with poor outcomes.38 Preimplantation proteinuria has recently been reported to be associated with mortality and need for renal replacement therapy among people receiving LVADs, which may reflect presence of parenchymal kidney disease and endothelial dysfunction.39
Our cohort included exclusively people with chronic kidney failure. To be included a nephrologist had to formally certify, at least 30 days before VAD implantation, that the patient had irreversible kidney failure requiring dialysis or kidney transplantation. For most of our cohort, the ESRD declaration antedated the VAD implantation by several years. The age and preexisting comorbidities reflect the expected case‐mix in a group with end‐stage heart and kidney disease. Diabetes mellitus is the most common cause of ESRD, and was the most common cause of ESRD among our cohort. Diabetes mellitus itself is associated with higher mortality and other adverse outcomes with VADs (including stroke, pump thrombosis, and device infection).21, 40 As VADs are more commonly used, they are being implanted in people with higher comorbidity burden. Because VAD placement in people with ESRD is not routine practice, the people in our cohort may have been carefully selected based on clinician expectation of recovery and life‐prolongation and placed in high‐volume centers comfortable with more complicated patients, factors which would make the observed high mortality more concerning.
The decreased mortality risk point estimate in more recent years mirrors improvements that have been reported in the non‐CKD VAD population.41 The decreasing mortality observed in the non‐CKD VAD population, likely because of improved technology and increasing experience with care of these patients, may be carrying over to people with ESRD. In April 2008, the HeartMate II (Abbott Laboratories, Abbott Park, IL) was approved for bridging to transplantation by the Food and Drug Administration, and in January 2010 received Food and Drug Administration approval as destination therapy.42, 43 This type of second generation, axial continuous flow device is smaller and more durable than prior pulsatile devices. These continuous flow devices have lower risk of intraoperative mortality, in addition to lower risk of infection.43 In 2012, the HeartWare HVAD (HeartWare Int, Framingham, MA) received Food and Drug Administration approval for bridge‐to‐transplant therapy, and in 2013 and 2014 significant numbers of centrifugal flow devices have been implanted. These devices may be more durable and have lower risk of pump thrombosis41 but may increase stroke risk.44
If clinicians consider VAD implantation in a person with ESRD, given the prolonged hospitalizations, frequent complications and readmissions, and substantial mortality observed in our study, integrated palliative care, with close cooperation with a palliative care physician, is essential.
This analysis is the largest to our knowledge of people with ESRD who receive exclusively implantable VADs and is based on a national sample. The USRDS data set, with information on many people with ESRD, allowed for the analysis of a procedure that is not routine in this population. As such, it expands current knowledge. Nevertheless, there are important limitations. As it is based on administrative data, we are unable to determine details of devices and clinical circumstances. We are also unable to confirm that all patients underwent LVAD implantation; however, given the rarity of biventricular or right VADs, this is likely the case. We are also unable to confirm that these were the first VAD implantations, rather than device replacement, in all people. However, most had Medicare coverage before implantation, and among these we confirmed that there were no prior VAD implantations. Finally, because placement of VADs in people with ESRD remains uncommon, the cohort was small, and thus adjustment for potentially important comorbidities was limited. Despite this, this is the largest description to date of implantable VAD use in people with existing ESRD.
Conclusion
Implantable VAD placement in Medicare beneficiaries with ESRD is associated with high in‐hospital and 1‐year mortality, and readmissions. These risks may have improved in recent years, because of VAD technology improvements. Combined heart and kidney disease is a vexing problem, and people with ESRD and severe HF who undergo VAD implantation are among the most profoundly ill people. Our investigation highlights the need for further studies to determine optimal management of these coexisting conditions.
Disclosures
None.
Supporting information
Table S1. ICD‐9‐CM Codes Used for VAD Indication Classification, and for Comorbidities
Table S2. ICD‐9‐CM Codes to Identify Infection Related Hospitalizations
Table S3. Subdistribution Hazard Models for People With ESRD on Dialysis at Time of VAD Implantation, With Transplantation as a Competing Event
Figure S1. Cumulative incidence of mortality stratified by primary vs post‐cardiotomy VAD implantation.
Acknowledgments
The data reported here have been supplied by the United States Renal Data System (USRDS). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy or interpretation of the US government. The results presented in this paper have not been published previously in whole or part, except in abstract format.
(J Am Heart Assoc. 2018;7:e008664 DOI: 10.1161/JAHA.118.008664.)
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics C, Stroke Statistics S . Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Centers for Disease Control and Prevention . Chronic kidney disease surveillance system—United States website. Available at: http://www.cdc.gov/ckd. Accessed January 24, 2018.
- 3. United States Renal Data System . 2017 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2017. [Google Scholar]
- 4. Herzog CA, Asinger RW, Berger AK, Charytan DM, Diez J, Hart RG, Eckardt KU, Kasiske BL, McCullough PA, Passman RS, DeLoach SS, Pun PH, Ritz E. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011;80:572–586. [DOI] [PubMed] [Google Scholar]
- 5. Ward ST, Liang Q, Pagani FD, Zhang M, Kormos RL, Aaronson KD, Althouse AD, Nallamothu BK, Likosky DS. A roadmap for evaluating the use and value of durable ventricular assist device therapy. J Heart Lung Transplant. 2018;37:146–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kirklin JK, Naftel DC, Kormos RL, Pagani FD, Myers SL, Stevenson LW, Givertz MM, Young JB. Quantifying the effect of cardiorenal syndrome on mortality after left ventricular assist device implant. J Heart Lung Transplant. 2013;32:1205–1213. [DOI] [PubMed] [Google Scholar]
- 7. Peura JL, Colvin‐Adams M, Francis GS, Grady KL, Hoffman TM, Jessup M, John R, Kiernan MS, Mitchell JE, O'Connell JB, Pagani FD, Petty M, Ravichandran P, Rogers JG, Semigran MJ, Toole JM; American Heart Association Heart F, Transplantation Committee of the Council on Clinical C, Council on Cardiopulmonary CCP, Resuscitation, Council on Cardiovascular Disease in the Y, Council on Cardiovascular N, Council on Cardiovascular R, Intervention, Council on Cardiovascular S, Anesthesia . Recommendations for the use of mechanical circulatory support: device strategies and patient selection: a scientific statement from the American Heart Association. Circulation. 2012;126:2648–2667. [DOI] [PubMed] [Google Scholar]
- 8. Daimee UA, Kutyifa V. Left ventricular assist devices in patients with renal dysfunction: where are we heading? Expert Rev Med Devices. 2017;14:413–415. [DOI] [PubMed] [Google Scholar]
- 9. Ross DW, Stevens GR, Wanchoo R, Majure DT, Jauhar S, Fernandez HA, Merzkani M, Jhaveri KD. Left ventricular assist devices and the kidney. Clin J Am Soc Nephrol. 2018;13:348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fukamachi K, Shiose A, Massiello A, Horvath DJ, Golding LA, Lee S, Starling RC. Preload sensitivity in cardiac assist devices. Ann Thorac Surg. 2013;95:373–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Joy PS, Kumar G, Guddati AK, Bhama JK, Cadaret LM. Risk factors and outcomes of gastrointestinal bleeding in left ventricular assist device recipients. Am J Cardiol. 2016;117:240–244. [DOI] [PubMed] [Google Scholar]
- 12. Moazami N, Dembitsky WP, Adamson R, Steffen RJ, Soltesz EG, Starling RC, Fukamachi K. Does pulsatility matter in the era of continuous‐flow blood pumps? J Heart Lung Transplant. 2015;34:999–1004. [DOI] [PubMed] [Google Scholar]
- 13. Braun LT, Grady KL, Kutner JS, Adler E, Berlinger N, Boss R, Butler J, Enguidanos S, Friebert S, Gardner TJ, Higgins P, Holloway R, Konig M, Meier D, Morrissey MB, Quest TE, Wiegand DL, Coombs‐Lee B, Fitchett G, Gupta C, Roach WH Jr; American Heart Association Advocacy Coordinating C . Palliative care and cardiovascular disease and stroke: a policy statement from the American Heart Association/American Stroke Association. Circulation. 2016;134:e198–e225. [DOI] [PubMed] [Google Scholar]
- 14. van Diepen S, Katz JN, Albert NM, Henry TD, Jacobs AK, Kapur NK, Kilic A, Menon V, Ohman EM, Sweitzer NK, Thiele H, Washam JB, Cohen MG; American Heart Association Council on Clinical C, Council on C, Stroke N, Council on Quality of C, Outcomes R, Mission L . Contemporary management of cardiogenic shock: a scientific statement from the American Heart Association. Circulation. 2017;136:e232–e268. [DOI] [PubMed] [Google Scholar]
- 15. Kavalieratos D, Gelfman LP, Tycon LE, Riegel B, Bekelman DB, Ikejiani DZ, Goldstein N, Kimmel SE, Bakitas MA, Arnold RM. Palliative care in heart failure: rationale, evidence, and future priorities. J Am Coll Cardiol. 2017;70:1919–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Davison SN, Levin A, Moss AH, Jha V, Brown EA, Brennan F, Murtagh FE, Naicker S, Germain MJ, O'Donoghue DJ, Morton RL, Obrador GT; Kidney Disease: Improving Global O . Executive summary of the KDIGO Controversies Conference on Supportive Care in Chronic Kidney Disease: developing a roadmap to improving quality care. Kidney Int. 2015;88:447–459. [DOI] [PubMed] [Google Scholar]
- 17. Grubbs V, Moss AH, Cohen LM, Fischer MJ, Germain MJ, Jassal SV, Perl J, Weiner DE, Mehrotra R; Dialysis Advisory Group of the American Society of N . A palliative approach to dialysis care: a patient‐centered transition to the end of life. Clin J Am Soc Nephrol. 2014;9:2203–2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wachterman MW, Pilver C, Smith D, Ersek M, Lipsitz SR, Keating NL. Quality of end‐of‐life care provided to patients with different serious illnesses. JAMA Intern Med. 2016;176:1095–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. U.S. Renal Data System . 2016 researcher's guide to the USRDS database. Bethesda, MD: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2016. [Google Scholar]
- 20. Hernandez AF, Shea AM, Milano CA, Rogers JG, Hammill BG, O'Connor CM, Schulman KA, Peterson ED, Curtis LH. Long‐term outcomes and costs of ventricular assist devices among Medicare beneficiaries. JAMA. 2008;300:2398–2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khazanie P, Hammill BG, Patel CB, Eapen ZJ, Peterson ED, Rogers JG, Milano CA, Curtis LH, Hernandez AF. Trends in the use and outcomes of ventricular assist devices among Medicare beneficiaries, 2006 through 2011. J Am Coll Cardiol. 2014;63:1395–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Miller MA, Baldwin JT, Young JB. Sixth INTERMACS annual report: a 10,000‐patient database. J Heart Lung Transplant. 2014;33:555–564. [DOI] [PubMed] [Google Scholar]
- 23. Ronco C. The cardiorenal syndrome: basis and common ground for a multidisciplinary patient‐oriented therapy. Cardiorenal Med. 2011;1:3–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Agency for Healthcare Research and Quality . Healthcare cost and utilization project clinical classifications software for ICD‐9‐CM. Available at: https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed December 11, 2017.
- 25. Dalrymple LS, Mu Y, Romano PS, Nguyen DV, Chertow GM, Delgado C, Grimes B, Kaysen GA, Johansen KL. Outcomes of infection‐related hospitalization in Medicare beneficiaries receiving in‐center hemodialysis. Am J Kidney Dis. 2015;65:754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gray RJ. A class of K‐sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 27. Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB, Naftel DC. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. J Heart Lung Transplant. 2017;36:1080–1086. [DOI] [PubMed] [Google Scholar]
- 28. Bansal N, Hailpern SM, Katz R, Hall YN, Tamura MK, Kreuter W, O'Hare AM. Outcomes associated with left ventricular assist devices among recipients with and without end‐stage renal disease. JAMA Intern Med. 2018;178:204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Thomas SS, Zern EK, D'Alessandro DA. The renal challenge with left ventricular assist device therapy‐when enough is enough. JAMA Intern Med. 2018;178:210–211. [DOI] [PubMed] [Google Scholar]
- 30. Rihal CS, Naidu SS, Givertz MM, Szeto WY, Burke JA, Kapur NK, Kern M, Garratt KN, Goldstein JA, Dimas V, Tu T; Society for Cardiovascular A, Interventions, Heart Failure Society of A, Society of Thoracic S, American Heart A, American College of C . 2015 SCAI/ACC/HFSA/STS clinical expert consensus statement on the use of percutaneous mechanical circulatory support devices in cardiovascular care: endorsed by the American Heart Assocation, the Cardiological Society of India, and Sociedad Latino Americana de Cardiologia Intervencion; Affirmation of Value by the Canadian Association of Interventional Cardiology‐Association Canadienne de Cardiologie d'intervention. J Am Coll Cardiol. 2015;65:e7–e26. [DOI] [PubMed] [Google Scholar]
- 31. Quader MA, Kumar D, Shah KB, Fatani YI, Katlaps G, Kasirajan V. Safety analysis of intermittent hemodialysis in patients with continuous flow left ventricular assist devices. Hemodial Int. 2014;18:205–209. [DOI] [PubMed] [Google Scholar]
- 32. Guglielmi AA, Guglielmi KE, Bhat G, Siemeck R, Tatooles AJ. Peritoneal dialysis after left ventricular assist device placement. ASAIO J. 2014;60:127–128. [DOI] [PubMed] [Google Scholar]
- 33. Patel AM, Adeseun GA, Ahmed I, Mitter N, Rame JE, Rudnick MR. Renal failure in patients with left ventricular assist devices. Clin J Am Soc Nephrol. 2013;8:484–496. [DOI] [PubMed] [Google Scholar]
- 34. Reddy YN, Melenovsky V, Redfield MM, Nishimura RA, Borlaug BA. High‐output heart failure: a 15‐year experience. J Am Coll Cardiol. 2016;68:473–482. [DOI] [PubMed] [Google Scholar]
- 35. Chin AI, Tong K, McVicar JP. Successful hemodialysis arteriovenous fistula creation in a patient with continuous‐flow left ventricular assist device support. Am J Kidney Dis. 2017;69:314–316. [DOI] [PubMed] [Google Scholar]
- 36. Schaefers JF, Ertmer C. Native arteriovenous fistula placement in three patients after implantation of a left ventricular assist device with non‐pulsatile blood flow. Hemodial Int. 2017;21:E54–E57. [DOI] [PubMed] [Google Scholar]
- 37. Cowger J, Sundareswaran K, Rogers JG, Park SJ, Pagani FD, Bhat G, Jaski B, Farrar DJ, Slaughter MS. Predicting survival in patients receiving continuous flow left ventricular assist devices: the HeartMate II risk score. J Am Coll Cardiol. 2013;61:313–321. [DOI] [PubMed] [Google Scholar]
- 38. Brisco MA, Kimmel SE, Coca SG, Putt ME, Jessup M, Tang WW, Parikh CR, Testani JM. Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ Heart Fail. 2014;7:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Muslem R, Caliskan K, Akin S, Sharma K, Gilotra NA, Brugts JJ, Houston B, Whitman G, Tedford RJ, Hesselink DA, Bogers A, Manintveld OC, Russell SD. Pre‐operative proteinuria in left ventricular assist devices and clinical outcome. J Heart Lung Transplant. 2018;37:124–130. [DOI] [PubMed] [Google Scholar]
- 40. Asleh R, Briasoulis A, Schettle SD, Tchantchaleishvili V, Pereira NL, Edwards BS, Clavell AL, Maltais S, Joyce DL, Joyce LD, Daly RC, Kushwaha SS, Stulak JM. Impact of diabetes mellitus on outcomes in patients supported with left ventricular assist devices: a single institutional 9‐year experience. Circ Heart Fail. 2017;10:e004213. [DOI] [PubMed] [Google Scholar]
- 41. Kirklin JK, Naftel DC, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB. Seventh INTERMACS annual report: 15,000 patients and counting. J Heart Lung Transplant. 2015;34:1495–1504. [DOI] [PubMed] [Google Scholar]
- 42. Starling RC, Naka Y, Boyle AJ, Gonzalez‐Stawinski G, John R, Jorde U, Russell SD, Conte JV, Aaronson KD, McGee EC Jr, Cotts WG, DeNofrio D, Pham DT, Farrar DJ, Pagani FD. Results of the post‐U.S. Food and Drug Administration‐approval study with a continuous flow left ventricular assist device as a bridge to heart transplantation: a prospective study using the INTERMACS (interagency registry for mechanically assisted circulatory support). J Am Coll Cardiol. 2011;57:1890–1898. [DOI] [PubMed] [Google Scholar]
- 43. Jorde UP, Kushwaha SS, Tatooles AJ, Naka Y, Bhat G, Long JW, Horstmanshof DA, Kormos RL, Teuteberg JJ, Slaughter MS, Birks EJ, Farrar DJ, Park SJ; HeartMate IICI . Results of the destination therapy post‐food and drug administration approval study with a continuous flow left ventricular assist device: a prospective study using the INTERMACS registry (interagency registry for mechanically assisted circulatory support). J Am Coll Cardiol. 2014;63:1751–1757. [DOI] [PubMed] [Google Scholar]
- 44. Rogers JG, Pagani FD, Tatooles AJ, Bhat G, Slaughter MS, Birks EJ, Boyce SW, Najjar SS, Jeevanandam V, Anderson AS, Gregoric ID, Mallidi H, Leadley K, Aaronson KD, Frazier OH, Milano CA. Intrapericardial left ventricular assist device for advanced heart failure. N Engl J Med. 2017;376:451–460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. ICD‐9‐CM Codes Used for VAD Indication Classification, and for Comorbidities
Table S2. ICD‐9‐CM Codes to Identify Infection Related Hospitalizations
Table S3. Subdistribution Hazard Models for People With ESRD on Dialysis at Time of VAD Implantation, With Transplantation as a Competing Event
Figure S1. Cumulative incidence of mortality stratified by primary vs post‐cardiotomy VAD implantation.


