Abstract
Background
Rapid ventricular pacing (RVP) is used commonly during transcatheter aortic valve replacement (TAVR). Little is known about the safety and clinical consequences of this step. The aim of this study was to assess the impact of RVP on immediate and long‐term clinical outcomes in a large cohort of non‐selected TAVR patients.
Method and Results
The study included 412 consecutive patients undergoing TAVR with a mean age of 82±7 years, of which 47% were male. Patients were divided according to the number of RVPs during the TAVR procedure comparing patients undergoing no pacing (0), 1 to 2, and ≥3 pacing episodes (3+). Patients undergoing 3+ pacing episodes were significantly more likely to develop new atrial fibrillation (5.6% versus 7.3% versus 15%, respectively, for 0, 1–2, and 3+ groups, P=0.047), acute kidney injury (AKI) (18% versus 18% versus 28%, respectively, P<0.001), prolonged procedural hypotension (0%, 16%, and 25%, respectively; P<0.001), and suffered greater in‐hospital mortality (1.7%, 1.7%, and 6.5%, respectively, P=0.045), and 1‐year mortality (11.1%, 7.7%, and 18%, respectively, P=0.015). Multivariate Cox regression analysis indicated that acute kidney injury (OR 3.27 [1.763–6.09], P<0.001), euroSCORE II (OR 1.06 per unit [1.01–1.12], P=0.03), and 3+ pacing episodes (OR 2.35 [1.18–4.7], P=0.02) were the only independent predictors for 1‐year mortality.
Conclusions
In patients undergoing TAVR, multiple RVP episodes and prolonged RVP duration are associated with adverse outcomes including short‐ and long‐term mortality. Thus, operators should attempt to minimize the use of RVP, especially in patients who are at risk for post‐procedural acute kidney injury.
Keywords: outcome, pacing, transcutaneous aortic valve implantation
Subject Categories: Catheter-Based Coronary and Valvular Interventions
Clinical Perspective
What Is New?
In this study, the largest to evaluate the effect of rapid pacing episodes on outcomes in patients undergoing transcatheter aortic valve replacement, patients undergoing ≥3 pacing episodes had worse outcomes including greater incidence of acute kidney injury, in‐hospital and 1‐year mortality.
What Are the Clinical Implications?
Transcatheter aortic valve replacement operators should aim to minimize the use of rapid ventricular pacing, especially in patients at risk of acute kidney injury.
Introduction
With expanding indications for transcatheter aortic valve replacement (TAVR), larger and younger patient populations are being referred for TAVR. Various aspects of the procedure, which have not been assessed in the past, now require evaluation. Rapid ventricular pacing (RVP) is a frequent step during TAVR which is required for temporary reduction in cardiac output during the procedure.
Despite the frequent use of RVP during TAVR, there is a paucity of data on the safety and the clinical consequences of this step. Indeed, prior case reports and small‐scale studies have reported conflicting data on the hemodynamic effects and clinical impact of RVP during TAVR.1, 2, 3, 4 Thus the aim of the present study was to assess the clinical impact of RVP on immediate and long‐term outcomes in a large cohort of non‐selected TAVR patients.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. Consecutive patients with symptomatic, severe aortic stenosis (AS) who underwent transfemoral TAVR from 2008 to 2016 at Sheba Medical Center were included in the analysis. The study was approved by the Institutional Review Board and all participants gave informed consent to participate in the study. Eligibility for TAVR was established based on the consensus of a multidisciplinary heart team. Selection of the transcatheter heart valve, approach and anesthesia was chosen at the discretion of the physician. Patients undergoing non‐transfemoral approach were excluded.
The full disclosure of procedural 3‐lead ECG tracings and invasive blood pressure recordings during the entire TAVR procedure were reviewed retrospectively and each episode of pacing was recorded, including number of pacing episodes during the procedure, duration of each pacing episode, and duration of recovery of blood pressure. For the purpose of this study patients were divided according to the number of rapid pacing episodes during the TAVR procedure comparing patients undergoing no pacing, 1 to 2 pacing episodes, and ≥ 3 (3+) pacing episodes.
To assess the clinical impact of cumulative pacing duration, a sub‐group analysis of paced patients (1–2 and 3+ pacing groups) was performed according to the cumulative pacing time (in paced seconds), dividing patients into tertiles of pacing duration.
Pre‐specified clinical and laboratory data were collected for all patients at baseline before the procedure, immediately post‐procedure, during the index hospitalization, and during long term follow up. Collected data included medical history, ECG, echocardiography studies, laboratory tests, and clinical outcomes. In‐hospital outcomes were collected according to the Valve Academic Research Consortium (VARC)‐2 consensus document5 and included acute kidney injury (AKI), peri‐procedural myocardial infarction, stroke, bleeding, vascular complications, and death. In addition, data on new‐onset atrial fibrillation, myocardial biomarker levels post‐procedure (creatinine phosphokinase and troponin I) and sustained intra‐procedural hypotension (defined as a reduced systolic pressure under 80 mm Hg for over 1 minute or requiring administration of vasopressor drugs) was collected. All suspected events were adjudicated by a blinded interventional cardiologist.
Statistical analyses were performed with SPSS version 21. Categorical variables were reported as frequency and percentages, and continuous variables as means and standard deviations or medians and interquartile range (IQR). Categorical variables were compared using Chi‐square test and continuous variables using ANOVA (Scheffe's method for post hoc analysis) or Kruskal–Wallis test (Mann–Whitney for post hoc analysis). Univariate Cox regression was used to evaluate the association between low, intermediate and high risk categories and mortality. Multivariate Cox regression was used to evaluate the association while controlling for potential confounders. Age, sex, kidney injury, predilation, euroSCORE, and residual paravalvular leak were included in the multivariate cox regression block. Kaplan–Meier plot was used to describe the mortality between categories and log‐rank test to compare between them. A 2‐tailed P<0.05 were considered statistically significant.
Results
This study included 412 consecutive patients with mean age of 82±7 years, of which 47% were male, with a mean euroSCORE II of 4.9±5.2. Balloon expandable valves were used in 45% and self‐expandable valves in 55% of patients. RVP was used in the vast majority of the procedures (87%) among which 247 patients (60%) underwent 1 to 2 RVP episodes (mean pacing duration 24±13 seconds) and 111 patients (27%) underwent 3+ RVP episodes (mean pacing duration 48±45 seconds). Only 54 patients (13%) underwent TAVI with no RVP.
Baseline characteristics of patients undergoing no pacing, 1 to 2, and 3+ pacing episodes are shown in Table 1. Minor differences were found between groups. The 3+ pacing group had more males and a higher euroSCORE II, however no difference in Society of Thoracic Surgeons score was noted between groups. Prevalence of permanent pacemakers at baseline was higher among the no pacing group. Patients who were not paced during the procedure were universally implanted with a self‐expandable valve (Table 2). Balloon expandable valves were progressively more often used in the 1 to 2 and 3+ pacing groups. Predilation was performed significantly more often in the 3+ pacing group, whereas postdilation was performed in around a quarter of patients in the 1 to 2 and 3+ pacing groups. Importantly, no significant differences in incidence of final paravalvular leak (PVL) were found between groups. No significant differences between the groups were noted with respect to the majority of procedural complications including bleeding, vascular injury, and procedural death (Table 3). However, the rates of new atrial fibrillation (5.6% versus 7.3% versus 15%, respectively, for 0, 1–2, and 3+ groups, P=0.047) as well as the rates of any kidney injury (18% versus 18% versus 28%, respectively, P<0.001) were significantly higher among the 3+ pacing group compared with both the no‐pacing and 1‐ to 2‐pacing groups. Of the 37 patients who developed peri‐procedural atrial fibrillation, the vast majority had transient atrial fibrillation. Only 7 patients were in atrial fibrillation at hospital discharge and only 4 patients at 1 year follow‐up.
Table 1.
Baseline Characteristics
| No Pacing Episodes (n=54) | 1 to 2 Pacing Episodes (n=247) | 3+ Pacing Episodes (n=111) | P Value | |
|---|---|---|---|---|
| Age, y | 82±7 | 82±7 | 81±7 | 0.77 |
| Male | 18 (33) | 115 (47) | 61 (55) | 0.03 |
| Diabetes mellitus | 19 (35) | 95 (39) | 48 (44) | 0.48 |
| Hypertension | 43 (80) | 203 (82) | 97 (87) | 0.36 |
| Hyperlipidemia | 33 (61) | 173 (70) | 80 (73) | 0.27 |
| Chronic renal failure | 12 (23) | 61 (25) | 24 (22) | 0.82 |
| Dialysis | 2 (3.7) | 3 (1.2) | 2 (1.8) | 0.45 |
| Chronic lung disease | 9 (17) | 35 (14) | 23 (21) | 0.3 |
| Peripheral vascular disease | 4 (7.4) | 23 (9.3) | 17 (16) | 0.14 |
| Prior myocardial infarction | 13 (24) | 70 (29) | 23 (21) | 0.3 |
| Prior coronary bypass surgery | 9 (17) | 51 (21) | 25 (23) | 0.67 |
| Prior percutaneous coronary intervention | 16 (30) | 78 (32) | 29 (27) | 0.63 |
| Prior cerebrovascular accident | 9 (17) | 37 (15) | 17 (16) | 0.95 |
| euroSCORE II | 4.7±4.3 | 4.4±4.2 | 6.1±7 | 0.013 |
| STS score | 4.7±2.4 | 4.9±3 | 4.8±2.9 | 0.8 |
| Atrial fibrillation/flutter | 22 (41) | 69 (28) | 34 (31) | 0.19 |
| Permanent pacemaker | 11 (21) | 23 (9.3) | 5 (4.5) | 0.004 |
| Left ventricular ejection fraction >50% | 41 (80) | 183 (78) | 80 (73) | 0.52 |
| SPAP mm Hg | 49±19 | 47±14 | 47±13 | 0.53 |
All numbers expressed as mean±SD or n (%) unless otherwise stated. SPAP indicates estimated systolic pulmonary artery pressure; STS, Society of Thoracic Surgeons.
Table 2.
Procedural Characteristics
| No Pacing Episodes (n=54) | 1 to 2 Pacing Episodes (n=247) | 3+ Pacing Episodes (n=111) | P Value | |
|---|---|---|---|---|
| Self‐expandable valve | 54 (100) | 146 (59) | 26 (23) | <0.001 |
| Balloon expandable valve | 0 | 101 (41) | 85 (77) | <0.001 |
| Predilation | 0 | 141 (57) | 95 (86) | <0.001 |
| Postdilation | 0 | 57 (23) | 33 (30) | <0.001 |
| Contrast volume | 265±117 | 246±105 | 257±74 | 0.75 |
| Postprocedural paravalvular leak | 0.14 | |||
| None‐mild | 48 (89) | 212 (86) | 85 (77) | |
| Moderate | 6 (11) | 32 (13) | 24 (22) | |
| Severe | 0 | 3 (1) | 1 (1) |
All numbers expressed as percent unless otherwise stated.
Table 3.
In‐Hospital Outcomes and 1‐Year Mortality
| No Pacing Episodes (n=54) | 1 to 2 Pacing Episodes (n=247) | 3+ Pacing Episodes (n=111) | P Value | |
|---|---|---|---|---|
| AKI | 0.001 | |||
| Stage 1 | 5 (9.3) | 35 (14) | 18 (17) | |
| Stage 2 | 4 (7.4) | 7 (2.9) | 4 (3.7) | |
| Stage 3 | 1 (1.9) | 1 (0.4) | 9 (8.3) | |
| Vascular complications | 0.89 | |||
| Minor | 16 (30) | 58 (24) | 28 (26) | |
| Major | 1 (1.9) | 5 (2) | 3 (2.7) | |
| Bleeding | 0.6 | |||
| Minor | 9 (17) | 26 (11) | 13 (12) | |
| Major | 2 (3.7) | 11 (4.5) | 5 (4.5) | |
| Life threatening/disabling | 2 (3.7) | 4 (1.6) | 5 (4.5) | |
| Prolonged hypotension | 0 | 39 (16) | 28 (25) | <0.001 |
| New atrial fibrillation | 3 (5.6) | 18 (7.3) | 16 (15) | 0.047 |
| New onset left bundle branch block | 15 (28) | 81 (33) | 27 (25) | 0.3 |
| High‐degree atrioventricular block | 5 (9.3) | 41 (17) | 18 (17) | 0.38 |
| Permanent pacemaker implantation | 6 (12) | 46 (19) | 20 (19) | 0.46 |
| Postprocedure troponin >x15 ULN | 18 (33) | 128 (52) | 70 (63) | 0.002 |
| Postprocedural CPK >x5 ULN | 4 (7.4) | 18 (7.3) | 21 (19) | 0.003 |
| Stroke | 3 (5.6) | 6 (2.4) | 8 (7.3) | 0.09 |
| Peri‐procedural mortality | 0 | 0 | 1 (0.9) | 0.26 |
| In‐hospital mortality | 1 (2) | 4 (1.7) | 7 (6.5) | 0.045 |
| 1‐y death | 6 (11) | 19 (7.7) | 20 (18) | 0.015 |
All numbers expressed as n (%). AKI indicates acute kidney injury; CPK, creatine phosphokinase.
Procedural hypotension was progressively more common with greater RVP episodes (0%, 16%, and 25%, respectively, for no‐pacing, 1–2, and 3+ groups; P<0.001) (Table 3).
Post‐procedural elevated biomarkers (troponin levels >x15 ULN and CPK >x5 ULN) were progressively more common in the 1 to 2 and 3+ groups compared with the no‐pacing group.
There was only one procedural death recorded in the 3+ pacing group. In‐hospital mortality was greater in patients undergoing 3+ RVP as compared both to the no‐pacing and 1 to 2 pacing groups (1.7%, 1.7%, and 6.5%, respectively, for the no‐pacing, 1–2, and 3+ groups, P=0.045), as was the 1‐year mortality (11.1%, 7.7%, and 18%, respectively, P=0.015) (Table 3, Figure 1).
Figure 1.
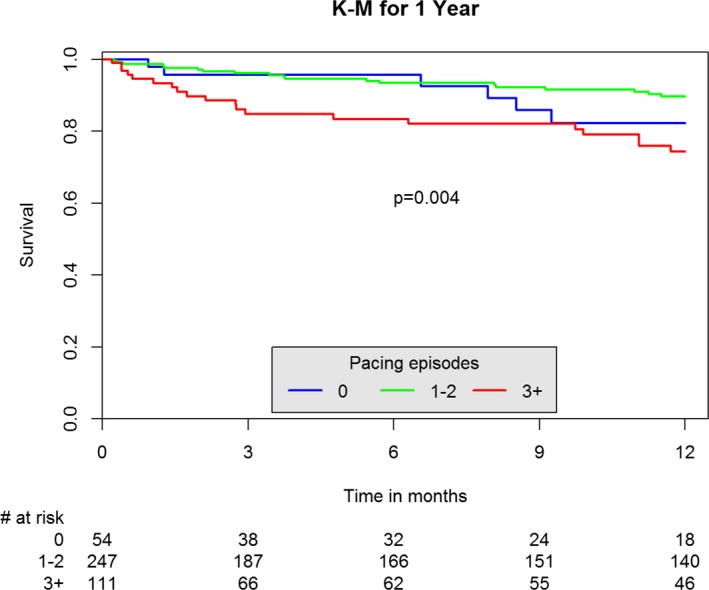
Kaplan–Meier (K‐M) of 1‐year mortality by number of pacing episodes.
A multivariate Cox regression indicated that AKI (OR 3.27 [1.763–6.09], P<0.001), euroSCORE II (OR 1.06 per unit [1.01–1.12], P=0.03), and 3+ pacing episodes (OR 2.35 [1.18–4.7], P=0.02) were the only independent predictors for 1‐year mortality.
Subgroup analysis including patients who underwent any RVP (groups 1–2 and 3+ pacing episodes) (n=352) showed a mean cumulative pacing duration of 30 seconds (IQR 17–41 seconds). Paced patients were divided into 3 tertiles based on the cumulative pacing duration (T1: 3–24 seconds; T2: 24–35 seconds; T3: >35 seconds). Baseline and procedural characteristics of patients in the 3 tertiles were comparable (Table S1). The rates of balloon expandable valve and balloon predilation, but not balloon postdilation increased with longer pacing duration (Table S2). Patients undergoing longer cumulative pacing duration (T3) were significantly more likely to suffer from AKI, new atrial fibrillation, sustained intra‐procedural hypotension, and stroke as compared with shorter pacing duration (Table 4). Also, post‐procedural elevated biomarkers were progressively more common with increasing pacing duration. No significant differences were found among groups with respect to vascular complications and bleeding (Table 4).
Table 4.
In‐Hospital Outcomes and 1‐Year Mortality by Cumulative Pacing Time
| T1 (3–24 seconds) n=119 | T2 (25–35 seconds) n=114 | T3 (>35 seconds) n=119 | P Value | |
|---|---|---|---|---|
| AKI | 0.017 | |||
| Stage 1 | 14 (12) | 15 (13) | 23 (20) | |
| Stage 2 | 6 (5.1) | 1 (0.9) | 4 (3.4) | |
| Stage 3 | 1 (0.9) | 1 (0.9) | 7 (6) | |
| Vascular complications | 0.12 | |||
| Minor | 21 (18) | 32 (28) | 32 (27) | |
| Major | 5 (4.2) | 0 | 2 (1.7) | |
| Bleeding | 0.55 | |||
| Minor | 11 (9) | 14 (12) | 11 (9.3) | |
| Major | 8 (6.7) | 2 (1.8) | 6 (5.1) | |
| Life threatening/disabling | 2 (1.7) | 2 (1.8) | 4 (3.4) | |
| Sustained hypotension | 9 (7.6) | 24 (21) | 33 (28) | <0.001 |
| New atrial fibrillation | 8 (6.7) | 8 (7) | 18 (16) | 0.037 |
| New onset left bundle branch block | 42 (35) | 40 (35) | 25 (22) | 0.03 |
| High‐degree atrioventricular block | 21 (18) | 17 (15) | 17 (15) | 0.79 |
| Permanent pacemaker implantation | 23 (19) | 20 (18) | 19 (17) | 0.85 |
| Postprocedure troponin >x15 ULN | 57 (48) | 63 (55) | 76 (64) | 0.046 |
| Postprocedural CPK >x5 ULN | 8 (6.7) | 8 (7) | 23 (19) | 0.002 |
| Stroke | 3 (2.5) | 2 (1.8) | 9 (7.6) | 0.044 |
| Peri‐procedural mortality | 1 (0.9) | 0 | 0 | 0.37 |
| In‐hospital mortality | 3 (2.6) | 1 (0.9) | 7 (6) | 0.077 |
| 1‐y death | 11 (9.2) | 8 (7) | 20 (17) | 0.043 |
All numbers expressed as n (%). AKI indicates acute kidney injury; CPK, creatine phosphokinase; ULN, upper limit of normal.
Among patients who underwent rapid pacing, those in the upper tertile of cumulative pacing time were more likely to die than those in the first and second tertiles (in‐hospital mortality: 2.6%, 0.9%, and 6%, P=0.077; 1‐year mortality: 9.2%, 6.8%, and 16.8%, P=0.043, respectively, for T1, T2, and T3). Kaplan–Meier analysis of 1‐year mortality according to tertiles of cumulative pacing time is shown in Figure 2.
Figure 2.
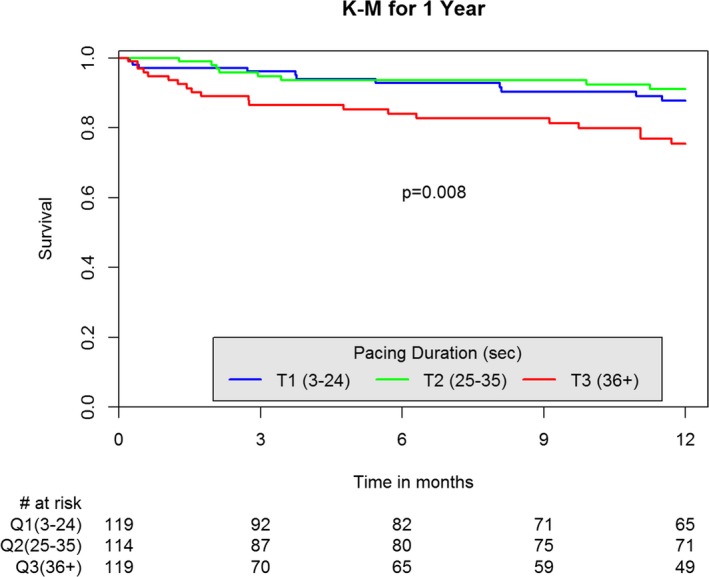
Kaplan–Meier (K‐M) of 1‐year mortality by cumulative pacing duration.
Discussion
The main findings of the present study indicate that the majority of TAVR patients undergo RVP during the procedure. Performance of 1 or 2 RVP episodes during the procedure seems safe and does not increase the risk for early or late adverse events. However, performance of ≥3 RVP episodes is associated with increased risk for peri‐procedural AKI, and atrial fibrillation, as well as higher in‐hospital and 1‐year mortality.
Furthermore, assessment of RVP according to total pacing duration showed similar results, irrespective of number of pacing episodes. Longer pacing duration was associated with prolonged procedural hypotension, peri‐procedural AKI, atrial fibrillation, and stroke. Longer RVP duration was also associated with higher in‐hospital and 1‐year mortality.
The high rates of RVP shown in the present study (87% of the procedures) are attributable to the multiple indications for RVP during TAVR. Performance of RVP is required during implantation of balloon expandable transcatheter heart valves to transiently diminish cardiac output, thus allowing accurate deployment of the transcatheter heart valve without the risk of dislodgement because of cardiac output. RVP is also required during TAVR with other transcatheter heart valve systems, when balloon pre‐ or post‐dilatation of the aortic valve is required. Furthermore, several studies have suggested that a long period of RVP which allows for controlled deployment of balloon expandable valves may improve the positioning accuracy of the valve.6
Rapid ventricular pacing diminishes cardiac output, causing transient hypotension. As with any hypotensive period, RVP may have adverse clinical impact. However, even though RVP is routinely performed during the majority of TAVR procedures, current data on the clinical impact of RVP during TAVR are limited to small numbers of patients with surrogate end points.1, 2, 4, 7 Few studies have correlated RVP with a clinical end point. A sub‐analysis of the PARTNER trial found that in the subgroup of patients undergoing transapical TAVR, a greater number of pacing runs (not pacing duration) showed intermediate association with higher risk for stroke.3 Similarly, a report from the CoreValve trials indicated a higher risk of stroke in patients who underwent RVP for predilation of the aortic valve versus those who did not.8 The findings of the present study show a similar trend with increased rates of stroke with longer duration of RVP but further expand the scope of understanding the consequences of RVP.
Okitsu et al assessed correlation between RVP and elevation in myocardial biomarkers4 and found good correlation between duration of RVP and elevation of myocardial biomarkers, however, once corrected to TAVR approach (ie, femoral versus apical) no significant correlation was found between pacing duration and elevation in myocardial biomarkers. Indeed prior studies have shown that transapical approach is associated with higher levels of cardiac enzymes.9 Other studies suggested that RVP may have deleterious effect on the myocardium because of temporary right ventricular systolic and diastolic dysfunction,1 and impairment of the myocardial microcirculation after RVP.7 To the best of our knowledge, this study is the first to indicate that all these surrogate end points translate into poor short‐ and long‐term outcome among a non‐selected TAVR patient population.
Another interesting finding of the present study is the increased risk for AKI with prolonged RVP. This association may be attributed to the higher rates of procedural hypotension among patients who had prolonged RVP. Alternative mechanism for AKI may be related to myocardial damage associated with myocardial ischemia during RVP. Elevations in myocardial biomarkers have been shown to be correlated with higher rates of AKI and poor long‐term outcome.10 Accordingly, the present study shows that RVP duration is associated with increased levels of myocardial biomarkers and higher rates of AKI. Finally, AKI is a strong predictor for short‐ and long‐term mortality, thus partially explaining the association between RVP episodes and higher mortality that was shown in the present study.
Limitations
This study was a single center, non‐randomized, post hoc analysis and, as such, is subject to the limitations of retrospective analyses; results may possibly have been affected by unknown confounders. Its strengths lie in the relatively large number of consecutive patients recruited and the detailed analysis of pacing and hemodynamic data derived from the full disclosure of source data. Additional limitations include the lack of a comprehensive assessment of diastolic dysfunction by echocardiography or right heart catheterization, and lack of detailed cause of death.
Conclusions
The findings of the present study show that RVP is frequently performed during TAVR. Prolonged RVP duration and multiple RVP episodes are associated with adverse outcomes including short‐ and long‐term mortality. Thus, operators should attempt to minimize the use of RVP, especially in patients who are at risk for post‐procedural AKI.
Disclosures
Segev is a proctor for Medtronic Inc and Edwards Lifesciences. The remaining authors have no disclosures to report.
Supporting information
Table S1. Baseline Characteristics by Cumulative Pacing Time
Table S2. Procedural Characteristics by Cumulative Pacing Time
(J Am Heart Assoc. 2018;7:e009038 DOI: 10.1161/JAHA.118.009038.)
This work was presented as a moderated poster at the American College of Cardiology Scientific Sessions, March 17 to 19, 2017, in Washington, DC.
References
- 1. Axell RG, White PA, Giblett JP, Williams L, Rana BS, Klein A, O'Sullivan M, Davies WR, Densem CG, Hoole SP. Rapid pacing‐induced right ventricular dysfunction is evident after balloon‐expandable transfemoral aortic valve replacement. J Am Coll Cardiol. 2017;69:903–904. [DOI] [PubMed] [Google Scholar]
- 2. Iritakenishi T, Kamibayashi T, Torikai K, Maeda K, Kuratani T, Sawa Y, Fujino Y. Predictors of prolonged hemodynamic compromise after valve deployment during transcatheter aortic valve implantation. J Cardiothorac Vasc Anesth. 2015;29:868–874. [DOI] [PubMed] [Google Scholar]
- 3. Kapadia S, Agarwal S, Miller DC, Webb JG, Mack M, Ellis S, Herrmann HC, Pichard AD, Tuzcu EM, Svensson LG, Smith CR, Rajeswaran J, Ehrlinger J, Kodali S, Makkar R, Thourani VH, Blackstone EH, Leon MB. Insights into timing, risk factors, and outcomes of stroke and transient ischemic attack after transcatheter aortic valve replacement in the PARTNER trial (placement of aortic transcatheter valves). Circ Cardiovasc Interv. 2016;9:e002981. [DOI] [PubMed] [Google Scholar]
- 4. Okitsu K, Iritakenishi T, Imada T, Iwasaki M, Shibata SC, Fujino Y. A longer total duration of rapid ventricular pacing does not increase the risk of postprocedural myocardial injury in patients who undergo transcatheter aortic valve implantation. Heart Vessels. 2017;32:1117–1122. [DOI] [PubMed] [Google Scholar]
- 5. Kappetein AP, Head SJ, Généreux P, Piazza N, van Mieghem NM, Blackstone EH, Brott TG, Cohen DJ, Cutlip DE, van Es GA, Hahn RT, Kirtane AJ, Krucoff MW, Kodali S, Mack MJ, Mehran R, Rodés‐Cabau J, Vranckx P, Webb JG, Windecker S, Serruys PW, Leon MB. Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium‐2 consensus document. J Am Coll Cardiol. 2012;60:1438–1454. [DOI] [PubMed] [Google Scholar]
- 6. Mok M, Dumont E, Doyle D, Rodés‐Cabau J. Transcatheter aortic valve implantation using the slow balloon inflation technique: making balloon‐expandable valves partially repositionable. J Card Surg. 2012;27:546–548. [DOI] [PubMed] [Google Scholar]
- 7. Selle A, Figulla HR, Ferrari M, Rademacher W, Goebel B, Hamadanchi A, Franz M, Schlueter A, Lehmann T, Lauten A. Impact of rapid ventricular pacing during TAVI on microvascular tissue perfusion. Clin Res Cardiol. 2014;103:902–911. [DOI] [PubMed] [Google Scholar]
- 8. Kleiman NS, Maini BJ, Reardon MJ, Conte J, Katz S, Rajagopal V, Kauten J, Hartman A, McKay R, Hagberg R, Huang J, Popma J; for the CoreValve Investigators . Neurological events following transcatheter aortic valve replacement and their predictors. Circ Cardiovasc Interv. 2016;9:e003551. [DOI] [PubMed] [Google Scholar]
- 9. Barbash IM, Dvir D, Ben‐Dor I, Badr S, Okubagzi P, Torguson R, Corso PJ, Xue Z, Satler LF, Pichard AD, Waksman R. Prevalence and effect of myocardial injury after transcatheter aortic valve replacement. Am J Cardiol. 2013;111:1337–1343. [DOI] [PubMed] [Google Scholar]
- 10. Barbash IM, Ben‐Dor I, Dvir D, Maluenda G, Xue Z, Torguson R, Satler LF, Pichard AD, Waksman R. Incidence and predictors of acute kidney injury after transcatheter aortic valve replacement. Am Heart J. 2012;163:1031–1036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics by Cumulative Pacing Time
Table S2. Procedural Characteristics by Cumulative Pacing Time


