Abstract
Background
The usefulness of vascular function tests for management of patients with a history of coronary artery disease is not fully known.
Methods and Results
We measured flow‐mediated vasodilation (FMD) and brachial–ankle pulse wave velocity (baPWV) in 462 patients with coronary artery disease for assessment of the predictive value of FMD and baPWV for future cardiovascular events in a prospective multicenter observational study. The first primary outcome was coronary events, and the second primary outcome was a composite of coronary events, stroke, heart failure, and sudden death. During a median follow‐up period of 49.2 months, the first primary outcome occurred in 56 patients and the second primary outcome occurred in 66 patients. FMD above the cutoff value of 7.1%, derived from receiver‐operator curve analyses for the first and second primary outcomes, was significantly associated with lower risk of the first (hazard ratio, 0.27; 95% confidence interval, 0.06–0.74; P=0.008) and second (hazard ratio, 0.32; 95% confidence interval, 0.09–0.79; P=0.01) primary outcomes. baPWV above the cutoff value of 1731 cm/s was significantly associated with higher risk of the first (hazard ratio, 1.86; 95% confidence interval, 1.01–3.44; P=0.04) and second (hazard ratio, 2.19; 95% confidence interval, 1.23–3.90; P=0.008) primary outcomes. Among 4 groups stratified according to the combination of cutoff values of FMD and baPWV, stepwise increases in the calculated risk ratio for the first and second primary outcomes were observed.
Conclusions
In patients with coronary artery disease, both FMD and baPWV were significant predictors of cardiovascular events. The combination of FMD and baPWV provided further cardiovascular risk stratification.
Clinical Trial Registration
URL: http://www.umin.ac.jp. Unique identifier: UMIN000012950.
Keywords: arterial stiffness, coronary artery disease, endothelial function, flow‐induced dilation, pulse wave velocity
Subject Categories: Biomarkers, Endothelium/Vascular Type/Nitric Oxide, Coronary Artery Disease
Clinical Perspective
What Is New?
Flow‐mediated vasodilation above the cutoff value of 7.1% was significantly associated with lower risk of cardiovascular events in patients with a history of coronary artery disease (CAD).
Brachial‐ankle pulse wave velocity above the cutoff value of 1731 cm/s was significantly associated with higher risk of cardiovascular events in patients with CAD.
The combination of flow‐mediated vasodilation and brachial–ankle pulse wave velocity provided further risk stratification of patients with CAD.
What Are the Clinical Implications?
Measurements of both flow‐mediated vasodilation and brachial–ankle pulse wave velocity are recommended for cardiovascular risk assessment in patients with CAD.
Introduction
Patients with a history of coronary artery disease (CAD) are at high risk for subsequent cardiovascular events and need intensive risk‐reduction therapies to prevent recurrent cardiovascular events.1, 2, 3, 4 However, despite recent advances in the understanding and management of CAD, some optimally treated patients with CAD still have recurrent cardiovascular events.5, 6 Since the number of evidence‐based therapies that reduce cardiovascular morbidity and mortality in high‐risk patients receiving standard therapy has been increasing, identification of individuals at especially high risk of recurrent cardiovascular events is necessary to select candidates for individualized intensive risk‐reduction therapies in patients with established CAD for secondary prevention. However, risk stratification strategies in patients with CAD are not well established.
Noninvasive vascular function tests have been developed and performed for assessment of functional vascular damage and severity of atherosclerosis.7, 8, 9 Impairment of vascular function, such as endothelial dysfunction and increased arterial stiffness, is closely associated with the development and maintenance of atherosclerotic conditions, leading to target organ damage and cardiovascular complications.10 Therefore, vascular function tests could be used not only as markers of atherosclerosis but also as prognostic markers of cardiovascular events.11 Recent meta‐analyses have shown that flow‐mediated vasodilation (FMD), an index of endothelial function, and brachial–ankle pulse wave velocity (baPWV), an index of arterial stiffness, are significant predictors of cardiovascular events independent of conventional cardiovascular risk factors.12, 13, 14, 15, 16 However, there have only been a few studies in which the predictive values of FMD, baPWV, and a combination of FMD and baPWV in patients with established CAD were investigated. Therefore, unfortunately, the usefulness of FMD and baPWV for risk stratification of patients with CAD has not been fully investigated. FMD‐J (Flow‐Mediated Dilation Japan) Study A was a prospective multicenter observational study designed to assess the predictive value of FMD for future cardiovascular events in patients with CAD independent of conventional cardiovascular risk factors and to evaluate the usefulness of a multimarker strategy to assess the prognosis of patients with CAD.17 The purpose of this multicenter study was to determine whether FMD, baPWV, and a combination of FMD and baPWV could be used as independent markers to predict the risk of recurrent cardiovascular events in patients with established CAD.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for the purpose of reproducing the results or replicating the procedure.
Study Design
The rationale and design of FMD‐J Study A have been described previously.17 This study was a prospective multicenter observational cohort study conducted at 22 university hospitals and affiliated clinics in Japan to examine the usefulness of FMD assessment for the management of Japanese patients with CAD with a 3‐year follow‐up period.17 The ethical committees of the participating institutions approved the study protocol. The study was executed in accordance with the Good Clinical Practice guidelines. Informed consent for participation in the study was obtained from all subjects. The protocol was registered in the University Hospital Medical Information Network Clinical Trials Registry (UMIN000012950).
Study Patients
Patients aged 20 to 74 years who had a diagnosis of CAD and who had been under regular follow‐up at any of the participating institutions for at least 6 months were eligible for enrollment in FMD‐J Study A. CAD was defined as myocardial infarction, angina pectoris with organic stenosis of at least 1 coronary artery confirmed by diagnostic imaging (ie, coronary angiography, cardiac nuclear scintigraphy, or coronary computed tomography), or previous percutaneous coronary intervention. The exclusion criteria were as follows: a history of coronary bypass surgery; severe valvular heart disease; arrhythmia that requires treatment (ie, atrial fibrillation, atrial flutter, permanent pacemaker implantation or frequent ventricular premature beats); severe chronic heart failure (New York Heart Association level of >Level III); malignancy; undergoing treatment with steroids, nonsteroidal anti‐inflammatory drugs, or immunosuppressive drugs; a serum creatinine level >2.5 mg/dL; a history of stroke, aortic disease (except peripheral artery disease), or serious liver disease; and judgment of an attending physician that an individual is ineligible for inclusion in the study.
Study Procedures
FMD and PWV measurements and blood examinations were conducted at the start of the study. Cardiovascular events were monitored annually during the 3‐year follow‐up period. The participants were managed by their attending physicians, who were encouraged to treat cardiovascular risk factors, including hypertension, dyslipidemia, and diabetes mellitus, to achieve the best of available standard of care in accordance with guidelines.
Measurements of FMD and baPWV and Assessment of Cardiovascular Risk Factors
Subjects fasted the previous night and abstained from consuming alcohol, smoking, consuming caffeine, and taking antioxidant vitamins on the day of the examination. Each subject was kept in the supine position in a quiet, dark, and air‐conditioned room (constant temperature of 23–26°C) throughout the study. A 23‐gauge polyethylene catheter was inserted into the left deep antecubital vein to obtain blood samples. FMD and baPWV were measured at least 20 minutes after maintaining the supine position. The observers were blind to the form of examination.
Vascular response to reactive hyperemia in the brachial artery was used for assessment of endothelium‐dependent FMD. A high‐resolution linear artery transducer was coupled to computer‐assisted analysis software (UNEXEF18G, UNEX Co, Nagoya, Japan) that used an automated edge detection system for measurement of brachial artery diameter. A blood pressure cuff was placed around the forearm. The brachial artery was scanned longitudinally 5 to 10 cm above the elbow. When the clearest B‐mode image of the anterior and posterior intimal interfaces between the lumen and vessel wall was obtained, the transducer was held at the same point throughout the scan by a special probe holder (UNEX Co) to ensure consistency of the image. Depth and gain setting were set to optimize the images of the arterial lumen wall interface. When the tracking gate was placed on the intima, the artery diameter was automatically tracked, and the waveform of diameter changes over the cardiac cycle was displayed in real time using the FMD mode of the tracking system. This allowed the ultrasound images to be optimized at the start of the scan and the transducer position to be adjusted immediately for optimal tracking performance throughout the scan. Pulsed Doppler flow was assessed at baseline and during peak hyperemic flow, which was confirmed to occur within 15 s after cuff deflation. Blood flow velocity was calculated from the color Doppler data and was displayed as a waveform in real time. The baseline longitudinal image of the artery was acquired for 30 s, and then the blood pressure cuff was inflated to 50 mm Hg above systolic pressure for 5 minutes. The longitudinal image of the artery was recorded continuously until 5 minutes after cuff deflation. Pulsed Doppler velocity signals were obtained for 20 s at baseline and for 10 s immediately after cuff deflation. Changes in brachial artery diameter were immediately expressed as percentage change relative to the vessel diameter before cuff inflation. FMD was automatically calculated as the percentage change in peak vessel diameter from the baseline value. Percentage of FMD [(Peak diameter−Baseline diameter)/Baseline diameter] was used for analysis. Blood flow volume was calculated by multiplying the Doppler flow velocity (corrected for the angle) by heart rate and vessel cross‐sectional area (−r2). Reactive hyperemia was calculated as the maximum percentage increase in flow after cuff deflation compared with baseline flow. All of the sonographers specialized in FMD measurement at the participating institutions received training for a standard protocol of FMD measurement and training for scanning and analysis of the record at the core laboratory located in Tokyo Medical University. All recordings of brachial artery scans obtained during the measurement of FMD were sent from the participant institutions to the core laboratory in Tokyo Medical University by universal serial bus flash drives and were individually analyzed by a well‐experienced reader at the core laboratory without any information about the patients. The intraclass correlation coefficient between each participating institutions and the core laboratory has been previously described.18 The correlation coefficient between FMD analyzed at the core laboratory and participant institutions was 0.84 (P<0.001).
baPWV was measured using a volume‐plethysmographic apparatus (Form PWV/ABI, Omron Health Care Co, Kyoto, Japan). Four oscillometric cuffs were wrapped around both upper arms and lower legs. The cuffs were connected to an oscillometric pressure sensor for measurements of blood pressure and to a plethysmographic sensor for recordings of volume pulse form. Ankle–brachial pressure index values were automatically calculated by dividing the ankle systolic blood pressures of the right and left sides by the higher brachial systolic blood pressure of either arm, and the lower value of ankle–brachial pressure index was used for analysis. baPWV was calculated automatically according to the following formula: baPWV=(D1−D2)/T, where D1 is the distance between the suprasternal notch and the ankle obtained by using the equation D1=0.8129×height (in cm)+12.328, D2 is the distance between the suprasternal notch and the brachium obtained by using the equation D2=0.2195×height−2.0734, and T is the time interval between the wave front of the brachial waveform and that of the ankle waveform. The distance between sampling points of baPWV was calculated automatically by inputting the value of individual height. The baPWVs measured on the right side and left side were identical (r=0.95, P<0.001). Therefore, baPWV values on the left side were used for analysis.19
Hypertension was defined as treatment with oral antihypertensive agents or systolic blood pressure of ≥140 mm Hg and/or diastolic blood pressure of ≥90 mm Hg without medication. Diabetes mellitus was defined according to the American Diabetes Association recommendation.20 Dyslipidemia was defined according to the third report of the National Cholesterol Education Program.21 We defined smokers as those who had ever smoked.
Study Outcomes
The present study had 2 primary outcomes: The first primary outcome was coronary events, including fatal or nonfatal myocardial infarction, coronary artery restenosis, and de novo coronary artery stenosis as confirmed by diagnostic imaging (ie, coronary angiography, cardiac nuclear scintigraphy, or coronary computed tomography), and the second primary outcome was a composite of coronary events, stroke, heart failure, or sudden death. Definitions of the clinical outcomes have been provided previously.17 All cardiovascular events were reported to the Efficacy Endpoint Review Committee annually from each institution. The Committee, consisting of members blinded to any information with regard to vascular function, assessed the appropriateness of clinical judgment of cardiovascular events according to prespecified criteria. The Committee could request physicians to provide additional clinical information on cardiovascular events if needed. Any differences in opinion under assessment were resolved by discussion, and the Committee finally determined whether the cardiovascular events would be included as outcome events in the analysis.
Sample Size
The rationale for the planned sample size of the subjects has been described previously.17
Statistical Analysis
Results are presented as means±SD for continuous variables and as percentages for categorical variables. All reported probability values were 2‐sided, and a probability value of <0.05 was considered statistically significant. Categorical variables were compared by means of the χ2 test. Continuous variables were compared by using unpaired Student t test. Receiver‐operator characteristic curve analyses were performed to assess the sensitivity and specificity of measurements of FMD and baPWV for predicting cardiovascular events. Time‐to‐event end point analyses were performed by using the Kaplan–Meier method. We categorized subjects into 2 groups according to the cutoff values of FMD and baPWV. Cutoff values were determined according to the highest Youden index from the receiver‐operator characteristic curves for predicting the first and second primary outcomes. The log‐rank test was used to compare the groups. We evaluated the associations of cardiovascular events with FMD and baPWV after adjustment for age, sex, body mass index, left ventricular ejection fraction (LVEF), brain natriuretic peptide (BNP), and cardiovascular risk factors by using Cox proportional hazard regression analysis. In Model 1, hypertension, dyslipidemia, diabetes mellitus, and smoking were entered into the model as cardiovascular risk factors. In Model 2, to adjust potential confounding factors in which there were significant differences between the groups stratified according to the cutoff values of FMD and baPWV, systolic blood pressure and antihypertensive drug treatment instead of the presence of hypertension, statin use instead of dyslipidemia, and glucose level in addition to the presence of diabetes mellitus were entered into the model as cardiovascular risk factors. We examined the models with both markers (FMD and baPWV) and their interaction simultaneously but did not find any significant interaction between the biomarkers. We evaluated the proportional hazards assumption in each model using a test based on Schoenfeld residuals (ie, Stata's estat phtest) and found no violation. The data were processed using JMP version 11 (SAS Institute, Cary, NC) and Stata version 15 (Stata Corporation, College Station, TX).
Results
FMD and Clinical Outcomes
A total of 679 patients were registered from May 2010 to September 2012, and 662 patients (97.4%) completed the study. Of those patients, 462 were included in the analysis after excluding patients without any organic coronary artery stenosis (n=32), those with inadequate FMD recordings (n=35), those with ankle–brachial pressure index values <0.9 (n=29), and those without measurement of baPWV, in whom cardio‐ankle vascular index was measured using Vasera (Fukuda Denshi Co., Ltd., Tokyo, Japan) instead of baPWV for the assessment of arterial stiffness (n=104) (Figure 1). There were no significant differences in clinical parameters between patients who were included and those who were excluded except for high‐density lipoprotein cholesterol, glucose, BNP, and prevalence of prior myocardial infarction (Table S1). Therefore, 104 excluded patients without baPWV measurement were not systematically different. The baseline clinical characteristics are summarized in Table 1. Of the 462 subjects, 396 (85.7%) were men and 66 (14.3%) were women, 244 (52.8%) had a history of myocardial infarction, 404 (87.4%) had a history of percutaneous coronary intervention, 454 (98.3%) received antiplatelet drugs, 320 (69.3%) received angiotensin receptor blockers or angiotensin‐converting enzyme inhibitors, and 394 (85.3%) received statins. The mean value of systolic blood pressure was 129.0±16.2 mm Hg, that of low‐density lipoprotein cholesterol was 92.8±27.4 mg/dL, that of LVEF was 60.8±9.9%, that of BNP was 35.1±61.6 pg/mL, that of FMD was 4.8±2.6%, and that of baPWV was 1639±297 cm/s. During a median follow‐up period of 49.2 months (interquartile range, 43.2–56.1 months), 10 subjects had myocardial infarction, 15 had coronary artery restenosis, 31 had de novo coronary artery stenosis, 4 had stroke, 4 had heart failure, and 2 died suddenly (Table 2). Both of the cutoff values of FMD derived from receiver‐operator characteristic curves for predicting the first and second primary outcomes were 7.1%. Therefore, we divided subjects into 2 groups according to the cutoff value of FMD of 7.1%. Clinical characteristics of the subjects on the basis of FMD are summarized in Table 1. Kaplan–Meier analysis showed that patients with FMD above the cutoff value of 7.1% had significantly fewer first primary outcome events (coronary events) than those for patients with FMD below the cutoff value (log‐rank P=0.04; Figure 2A). Patients with FMD above the cutoff value also had significantly fewer second primary outcome events (a composite of coronary events, stroke, heart failure, or sudden death) than those for patients with FMD below the cutoff value (log‐rank P=0.04; Figure 2B). Multivariate Cox proportional hazard analyses revealed that FMD above the cutoff value of 7.1% was a significant predictor of lower risk of the first (hazard ratio, 0.27; 95% confidence interval [CI], 0.06–0.74; P=0.008) and second (hazard ratio, 0.32; 95% CI, 0.09–0.79; P=0.01) primary outcome events after adjustment of age, sex, body mass index, hypertension, dyslipidemia, diabetes mellitus, smoking, LVEF, and BNP (Model 1) (Table 3). FMD above the cutoff value of 7.1% was also a significant predictor of lower risk of the first (hazard ratio, 0.27; 95% CI, 0.07–0.76; P=0.01) and second (hazard ratio, 0.32; 95% CI, 0.10–0.79; P=0.01) primary outcome events after adjustment of age, sex, body mass index, systolic blood pressure, antihypertensive drug treatment, statin use, glucose, presence of diabetes mellitus, smoking, LVEF, and BNP (Model 2) (Table 3).
Figure 1.
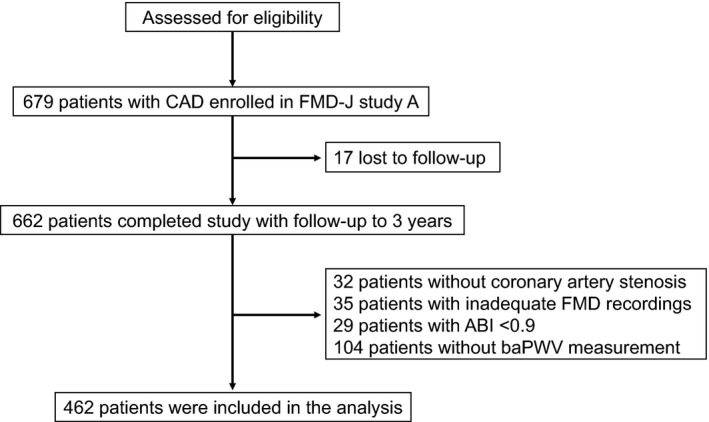
Flow chart of the study design from screening to completion of the trial. ABI indicates ankle–brachial pressure index; baPWV, brachial–ankle pulse wave velocity; CAD, coronary artery disease; FMD, flow‐mediated vasodilation; FMD‐J, Flow‐Mediated Dilation Japan.
Table 1.
Clinical Characteristics of the Subjects on the Basis of Cutoff Value of FMD
| Variables | All Subjects | High FMD (>7.1%) | Low FMD (≤7.1%) | P Value |
|---|---|---|---|---|
| (n=462) | (n=77) | (n=385) | ||
| Age, y | 63.8±8.7 | 61.1±9.9 | 64.3±8.3 | 0.003 |
| Men, n (%) | 396 (85.7) | 62 (80.5) | 334 (86.8) | 0.17 |
| Body mass index, kg/m2 | 24.8±3.6 | 24.7±4.3 | 24.8±3.5 | 0.85 |
| Systolic blood pressure, mm Hg | 129.0±16.2 | 128.2±16.2 | 129.1±16.2 | 0.66 |
| Diastolic blood pressure, mm Hg | 74.8±10.6 | 74.7±10.9 | 74.8±10.5 | 0.94 |
| Heart rate, bpm | 66.5±12.0 | 67.6±13.8 | 66.3±11.6 | 0.37 |
| Total cholesterol, mg/dL | 169.9±31.7 | 170.2±28.7 | 169.8±32.2 | 0.92 |
| Triglycerides, mg/dL | 138.0±94.0 | 145.4±78.1 | 136.6±96.9 | 0.45 |
| HDL cholesterol, mg/dL | 50.4±13.1 | 49.4±11.0 | 50.6±13.5 | 0.44 |
| LDL cholesterol, mg/dL | 92.8±27.4 | 92.3±26.0 | 92.9±27.7 | 0.84 |
| Glucose, mg/dL | 119.5±37.2 | 116.4±33.8 | 120.1±37.9 | 0.43 |
| HbA1c, % (n=347) | 6.4±1.0 | 6.2±0.8 | 6.5±1.0 | 0.02 |
| Creatinine, mg/dL | 0.85±0.24 | 0.83±0.17 | 0.86±0.25 | 0.28 |
| eGFR, mL/min per 1.73 m2 | 71.9±17.6 | 72.8±16.0 | 71.7±18.0 | 0.65 |
| Smoker, n (%) | 313 (70.2) | 47 (62.7) | 266 (71.7) | 0.13 |
| Complications, n (%) | ||||
| Hypertension | 433 (93.7) | 72 (93.5) | 361 (93.8) | 0.93 |
| Dyslipidemia | 436 (94.4) | 74 (96.1) | 362 (94.0) | 0.45 |
| Diabetes mellitus | 178 (38.5) | 23 (29.9) | 155 (40.3) | 0.08 |
| Prior myocardial infarction | 244 (52.8) | 39 (50.7) | 205 (53.3) | 0.68 |
| Prior coronary intervention | 404 (87.4) | 74 (96.1) | 330 (85.7) | 0.005 |
| Medication use, n (%) | ||||
| Antiplatelet drugs | 454 (98.3) | 76 (98.7) | 378 (98.2) | 0.74 |
| ARBs/ACEIs | 320 (69.3) | 49 (63.6) | 271 (70.4) | 0.25 |
| β‐Blockers | 219 (47.4) | 39 (50.7) | 180 (46.8) | 0.53 |
| Antidiabetic drugs | 145 (31.4) | 18 (23.4) | 127 (33.0) | 0.09 |
| Statins | 394 (85.3) | 68 (88.3) | 326 (84.7) | 0.40 |
| Left ventricular ejection fraction, % | 60.8±9.9 | 61.3±10.5 | 60.7±9.8 | 0.63 |
| BNP, pg/mL | 35.1±61.6 | 30.8±39.0 | 35.9±65.1 | 0.53 |
| FMD, % | 4.8±2.6 | 9.0±1.5 | 3.9±1.8 | <0.001 |
| baPWV, cm/s | 1639±297 | 1564±284 | 1654±297 | 0.02 |
All results are presented as mean±SD. ACEIs indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blocker; baPWV, brachial–ankle pulse wave velocity; BNP, brain natriuretic peptide; bpm, beats per minute; eGFR, estimated glomerular filtration rate; FMD, flow‐mediated vasodilation; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Table 2.
Clinical Outcomes of the Subjects on the Basis of Cutoff Value of FMD
| Variables, n (%) | All Subjects | High FMD (>7.1%) | Low FMD (≤7.1%) | P Value |
|---|---|---|---|---|
| (n=462) | (n=77) | (n=385) | ||
| Acute myocardial infarction | 10 (2.2) | 0 (0.0) | 10 (2.6) | 0.05 |
| Coronary artery restenosis | 15 (3.2) | 1 (1.3) | 14 (3.6) | 0.24 |
| de novo coronary artery stenosis | 31 (6.7) | 4 (5.2) | 27 (7.0) | 0.55 |
| Stroke | 4 (0.9) | 0 (0.0) | 4 (1.0) | 0.23 |
| Heart failure | 4 (0.9) | 0 (0.0) | 4 (1.0) | 0.23 |
| Sudden death | 2 (0.4) | 1 (1.3) | 1 (0.3) | 0.28 |
FMD indicates flow‐mediated vasodilation.
Figure 2.
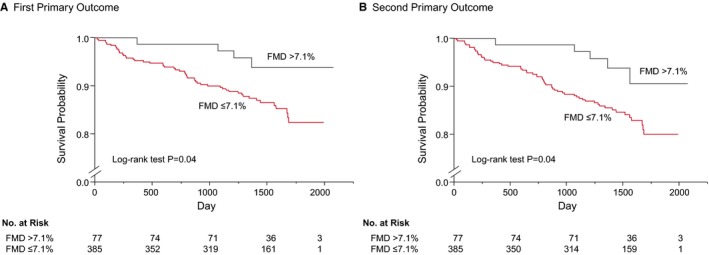
Kaplan–Meier curves of cumulative event‐free survival of the first primary outcome (coronary events) (A) and the second primary outcome (coronary events, stroke, heart failure, or sudden death) (B) in subgroups of subjects categorized according to the cutoff value of flow‐mediated vasodilation (FMD). The P value was calculated from the log‐rank test.
Table 3.
Association of Primary End Points With FMD, baPWV, and FMD Combined With baPWV During Follow‐Up
| Variables | Hazard Ratio (95% Confidence Interval); P Value | |||||
|---|---|---|---|---|---|---|
| First Primary Outcome | Second Primary Outcome | |||||
| Unadjusted | Model 1a | Model 2b | Unadjusted | Model 1a | Model 2b | |
| FMD (%) | ||||||
| FMD ≤7.1% | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| FMD >7.1% | 0.36 (0.11–0.89); 0.02 | 0.27 (0.06–0.74); 0.008 | 0.27 (0.07–0.76); 0.01 | 0.39 (0.14–0.88); 0.02 | 0.32 (0.09–0.79); 0.01 | 0.32 (0.10–0.79); 0.01 |
| baPWV, cm/s | ||||||
| baPWV <1731 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| baPWV ≥1731 | 1.79 (1.05–3.03); 0.03 | 1.86 (1.01–3.44); 0.04 | 1.99 (1.04–3.82); 0.04 | 2.20 (1.35–3.59); 0.002 | 2.19 (1.23–3.90); 0.008 | 2.17 (1.18–4.00); 0.01 |
| FMD+baPWV | ||||||
| Group 1 (FMD >7.1%, baPWV <1731 cm/s) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| Group 2 (FMD >7.1%, baPWV ≥1731 cm/s) | 3.64 (0.44–30.35); 0.21 | 1.99 (0.09–21.47); 0.59 | 1.98 (0.09–21.65); 0.60 | 5.41 (0.90–41.09); 0.06 | 3.83 (0.45–32.81); 0.20 | 3.44 (0.40–29.84); 0.24 |
| Group 3 (FMD ≤7.1%, baPWV <1731 cm/s) | 3.59 (1.08–22.21); 0.03 | 3.79 (1.11–23.73); 0.03 | 3.61 (1.06–22.55); 0.04 | 3.83 (1.16–23.67); 0.02 | 4.05 (1.20–25.28); 0.02 | 3.87 (1.15–24.09); 0.03 |
| Group 4 (FMD ≤7.1%, baPWV ≥1731 cm/s) | 5.78 (1.17–35.99); 0.003 | 6.84 (1.87–44.31); 0.002 | 7.07 (1.89–46.35); 0.002 | 7.46 (2.25–46.15); <0.001 | 8.38 (2.34–53.77); <0.001 | 8.02 (2.21–51.79); <0.001 |
First primary end point is coronary events, including fatal or nonfatal myocardial infarction, coronary artery restenosis, or de novo coronary artery stenosis as confirmed by diagnostic imaging. Second primary end point is a composite of coronary events, death from cardiovascular causes, stroke, heart failure, and sudden death. baPWV indicates brachial–ankle pulse wave velocity; FMD, flow‐mediated vasodilation.
Model 1: adjusted for age, sex, body mass index, the presence of hypertension, dyslipidemia, and diabetes mellitus, smoking, left ventricular ejection fraction, and brain natriuretic peptide.
Model 2: adjusted for age, sex, body mass index, systolic blood pressure, antihypertensive drug treatment, statin use, glucose, the presence of diabetes mellitus, smoking, left ventricular ejection fraction, and brain natriuretic peptide.
baPWV and Clinical Outcomes
Both of the cutoff values of baPWV derived from receiver‐operator characteristic curves for predicting the first and second primary outcomes were 1731 cm/s. Therefore, we divided subjects into 2 groups according to the cutoff value of baPWV of 1731 cm/s. Clinical characteristics of the subjects and clinical events on the basis of baPWV are summarized in Tables 4 and 5. Kaplan–Meier analysis showed that patients with baPWV above the cutoff value of 1731 cm/s had significantly more first primary outcome events than those for patients with baPWV below the cutoff value (log‐rank P=0.03; Figure 3A). Patients with baPWV above the cutoff value also had significantly more second primary outcome events than those for patients with baPWV below the cutoff value (log‐rank P=0.001; Figure 3B). Multivariate Cox proportional hazard analyses revealed that baPWV above the cutoff value of 1731 cm/s was a significant predictor of the first (hazard ratio, 1.86; 95% CI, 1.01–3.44; P=0.04) and second (hazard ratio, 2.19; 95% CI, 1.23–3.90; P=0.008) primary outcome events in Model 1 (Table 3). baPWV above the cutoff value of 1731 cm/s was also a significant predictor of the first (hazard ratio, 1.99; 95% CI, 1.04–3.82; P=0.04) and second (hazard ratio, 2.17; 95% CI, 1.18–4.00; P=0.01) primary outcome events in Model 2 (Table 3).
Table 4.
Clinical Characteristics of the Subjects on the Basis of Cutoff Value of baPWV
| Variables | Low baPWV (<1731 cm/s) | High baPWV (≥1731 cm/s) | P Value |
|---|---|---|---|
| (n=312) | (n=150) | ||
| Age, y | 61.6±9.1 | 68.2±5.7 | <0.001 |
| Men, n (%) | 271 (86.9) | 125 (83.3) | 0.32 |
| Body mass index, kg/m2 | 25.2±3.9 | 23.9±2.7 | <0.001 |
| Systolic blood pressure, mm Hg | 125.9±15.4 | 135.4±16.0 | <0.001 |
| Diastolic blood pressure, mm Hg | 74.6±10.6 | 75.2±10.6 | 0.60 |
| Heart rate, bpm | 66.0±12.2 | 67.6±11.5 | 0.19 |
| Total cholesterol, mg/dL | 169.4±30.8 | 171.0±33.6 | 0.61 |
| Triglycerides, mg/dL | 138.8±99.5 | 136.5±81.7 | 0.81 |
| HDL cholesterol, mg/dL | 50.3±12.9 | 50.8±13.6 | 0.68 |
| LDL cholesterol, mg/dL | 92.4±26.0 | 93.7±30.1 | 0.65 |
| Glucose, mg/dL | 116.0±36.2 | 126.8±38.3 | 0.003 |
| HbA1c, % (n=347) | 6.3±0.9 | 6.7±1.0 | 0.004 |
| Creatinine, mg/dL | 0.84±0.23 | 0.88±0.26 | 0.06 |
| eGFR, mL/min per 1.73 m2 | 73.7±17.0 | 68.1±18.3 | 0.001 |
| Smoker, n (%) | 217 (71.9) | 96 (66.7) | 0.27 |
| Complications, n (%) | |||
| Hypertension | 290 (93.0) | 143 (95.3) | 0.31 |
| Dyslipidemia | 300 (96.2) | 136 (90.7) | 0.02 |
| Diabetes mellitus | 109 (34.9) | 69 (46.0) | 0.02 |
| Prior myocardial infarction | 170 (54.5) | 74 (49.3) | 0.30 |
| Prior coronary intervention | 278 (89.1) | 126 (84.0) | 0.13 |
| Medication use, n (%) | |||
| Antiplatelet drugs | 307 (98.4) | 147 (98.0) | 0.76 |
| ARBs/ACEIs | 213 (68.3) | 107 (71.3) | 0.50 |
| β‐Blockers | 157 (50.3) | 62 (41.3) | 0.07 |
| Antidiabetic drugs | 87 (27.9) | 58 (38.7) | 0.02 |
| Statins | 281 (90.1) | 113 (75.3) | <0.001 |
| Left ventricular ejection fraction, % | 60.6±10.2 | 61.4±9.3 | 0.44 |
| BNP, pg/mL | 28.6±33.7 | 49.4±97.0 | 0.001 |
| FMD, % | 5.0±2.6 | 4.3±2.4 | 0.01 |
| baPWV, cm/s | 1474±157 | 1981±215 | <0.001 |
All results are presented as mean±SD. ACEI indicates angiotensin‐converting enzyme inhibitor; ARBs, angiotensin receptor blockers; baPWV, brachial–ankle pulse wave velocity; BNP, brain natriuretic peptide; bpm, beats per minute; eGFR, estimated glomerular filtration rate; FMD, flow‐mediated vasodilation; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Table 5.
Clinical Outcomes of the Subjects on the Basis of Cutoff Value of baPWV
| Variables, n (%) | Low baPWV (<1731 cm/s) | High baPWV (≥1731 cm/s) | P Value |
|---|---|---|---|
| (n=312) | (n=150) | ||
| Acute myocardial infarction | 8 (2.6) | 2 (1.3) | 0.37 |
| Coronary artery restenosis | 7 (2.2) | 8 (5.3) | 0.09 |
| de novo coronary artery stenosis | 14 (4.5) | 17 (11.3) | 0.008 |
| Stroke | 1 (0.3) | 3 (2.0) | 0.08 |
| Heart failure | 0 (0.0) | 4 (2.7) | 0.003 |
| Sudden death | 1 (0.3) | 1 (0.7) | 0.61 |
baPWV indicates brachial–ankle pulse wave velocity.
Figure 3.
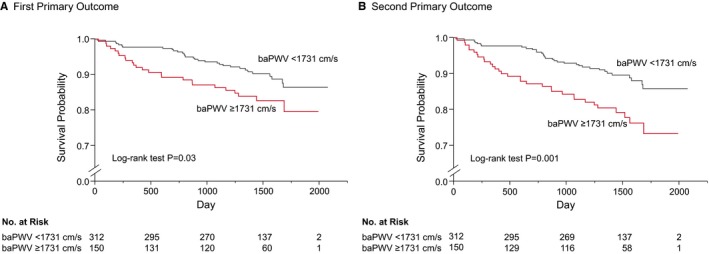
Kaplan–Meier curves of cumulative event‐free survival of the first primary outcome (coronary events) (A) and the second primary outcome (coronary events, stroke, heart failure, or sudden death) (B) in subgroups of subjects categorized according to the cutoff value of brachial–ankle pulse wave velocity (baPWV). The P value was calculated from the log‐rank test.
Combination of FMD and baPWV and Clinical Outcomes
We next divided the 462 subjects into 4 groups according to the cutoff values of FMD of 7.1% and baPWV of 1731 cm/s. Clinical characteristics of the subjects and clinical events according to the cutoff values of FMD and baPWV are summarized in Tables 6 and 7. There were significant differences between Kaplan–Meier curves of cumulative event‐free survival of the first primary outcome for the 4 subgroups of patients categorized according to the cutoff values of FMD and baPWV (log‐rank P=0.04; Figure 4A). Multivariate Cox proportional hazard analysis revealed that a combination of FMD below the cutoff value and baPWV below the cutoff value (hazard ratio, 3.79; 95% CI, 1.11–23.73; P=0.03) and a combination of FMD below the cutoff value and baPWV above the cutoff value (hazard ratio, 6.84; 95% CI, 1.87–44.31; P=0.002) were significant predictors of the first primary outcome events in Model 1 (Table 3). A combination of FMD below the cutoff value and baPWV below the cutoff value (hazard ratio, 3.61; 95% CI, 1.06–22.55; P=0.04) and a combination of FMD below the cutoff value and baPWV above the cutoff value (hazard ratio, 7.07; 95% CI, 1.89–46.35; P=0.002) were also significant predictors of the first primary outcome events in Model 2 (Table 3). There were also significant differences between Kaplan–Meier curves of cumulative event‐free survival of the second primary outcome (log‐rank P=0.003; Figure 4B). Multivariate Cox proportional hazard analysis revealed that a combination of FMD below the cutoff value and baPWV below the cutoff value (hazard ratio, 4.05; 95% CI, 1.20–25.28; P=0.02) and a combination of FMD below the cutoff value and baPWV above the cutoff value (hazard ratio, 8.38; 95% CI, 2.34–53.77; P<0.001) were significant predictors of the second primary outcome events in Model 1 (Table 3). A combination of FMD below the cutoff value and baPWV below the cutoff value (hazard ratio, 3.87; 95% CI, 1.15–24.09; P=0.03) and a combination of FMD below the cutoff value and baPWV above the cutoff value (hazard ratio, 8.02; 95% CI, 2.21–51.79; P<0.001) were also significant predictors of the second primary outcome events in Model 2 (Table 3).
Table 6.
Clinical Characteristics of the Subjects on the Basis of Cutoff Values of FMD and baPWV
| Variables | Group 1 | Group 2 | Group 3 | Group 4 | P Value |
|---|---|---|---|---|---|
| FMD >7.1% | FMD ≤7.1% | ||||
| baPWV <1731 cm/s | baPWV ≥1731 cm/s | baPWV <1731 cm/s | baPWV ≥1731 cm/s | ||
| (n=60) | (n=17) | (n=252) | (n=133) | ||
| Age, y | 59.2±10.0 | 67.8±6.1 | 62.2±8.7 | 68.3±5.7 | <0.001 |
| Men, n (%) | 48 (80.0) | 14 (82.4) | 223 (88.5) | 111 (83.5) | 0.28 |
| Body mass index, kg/m2 | 25.1±4.6 | 23.4±2.5 | 25.2±3.7 | 24.0±2.8 | 0.003 |
| Systolic blood pressure, mm Hg | 126.5±16.7 | 134.2±12.9 | 125.7±15.1 | 135.5±16.4 | <0.001 |
| Diastolic blood pressure, mm Hg | 74.6±10.1 | 75.0±13.9 | 74.6±10.7 | 75.2±10.2 | 0.96 |
| Heart rate, bpm | 67.8±14.2 | 66.9±12.5 | 65.5±11.7 | 67.6±11.5 | 0.32 |
| Total cholesterol, mg/dL | 169.6±28.1 | 172.6±31.7 | 169.3±31.4 | 170.8±34.0 | 0.96 |
| Triglycerides, mg/dL | 149.3±84.9 | 131.7±46.8 | 136.2±102.6 | 137.1±85.3 | 0.79 |
| HDL cholesterol, mg/dL | 49.2±11.4 | 50.2±9.7 | 50.5±13.2 | 50.9±14.0 | 0.87 |
| LDL cholesterol, mg/dL | 91.1±26.1 | 96.1±26.1 | 92.7±26.1 | 93.4±30.6 | 0.91 |
| Glucose, mg/dL | 111.8±29.6 | 133.0±42.8 | 117.0±37.6 | 126.0±37.8 | 0.02 |
| HbA1c, % (n=347) | 6.0±0.6 | 6.7±1.2 | 6.4±1.0 | 6.6±1.0 | 0.003 |
| Creatinine, mg/dL | 0.82±0.16 | 0.84±0.18 | 0.84±0.24 | 0.89±0.27 | 0.21 |
| eGFR, mL/min per 1.73 m2 | 73.5±15.4 | 70.2±18.2 | 73.8±17.4 | 67.9±18.4 | 0.01 |
| Smoker, n (%) | 36 (61.0) | 11 (68.8) | 181 (74.5) | 85 (66.4) | 0.15 |
| Complications, n (%) | |||||
| Hypertension | 56 (93.3) | 16 (94.1) | 234 (92.9) | 127 (95.5) | 0.78 |
| Dyslipidemia | 59 (98.3) | 15 (88.2) | 241 (95.6) | 121 (91.0) | 0.08 |
| Diabetes mellitus | 15 (25.0) | 8 (47.1) | 94 (37.3) | 61 (45.9) | 0.04 |
| Prior myocardial infarction | 32 (53.3) | 7 (41.2) | 138 (54.8) | 67 (50.4) | 0.65 |
| Prior coronary intervention | 58 (96.7) | 16 (94.1) | 220 (87.3) | 110 (82.7) | 0.02 |
| Medication use, n (%) | |||||
| Antiplatelet drugs | 59 (98.3) | 17 (100.0) | 248 (98.4) | 130 (97.7) | 0.84 |
| ARBs/ACEIs | 38 (63.3) | 11 (64.7) | 175 (69.4) | 96 (72.2) | 0.64 |
| β‐Blockers | 32 (53.3) | 7 (41.2) | 125 (49.6) | 55 (41.4) | 0.31 |
| Antidiabetic drugs | 11 (18.3) | 7 (41.2) | 76 (30.2) | 51 (38.4) | 0.03 |
| Statins | 54 (90.0) | 14 (82.4) | 227 (90.1) | 99 (74.4) | <0.001 |
| Left ventricular ejection fraction, % | 61.0±10.9 | 62.7±9.1 | 60.5±10.0 | 61.2±9.3 | 0.79 |
| BNP, pg/mL | 27.8±38.2 | 40.9±41.2 | 28.8±32.7 | 50.5±102.2 | 0.01 |
| FMD, % | 9.0±1.5 | 9.0±1.7 | 4.0±1.9 | 3.7±1.8 | <0.001 |
| baPWV, cm/s | 1447±149 | 1978±260 | 1481±159 | 1981±210 | <0.001 |
All results are presented as mean±SD. ACEIs indicates angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; baPWV, brachial–ankle pulse wave velocity; BNP, brain natriuretic peptide; bpm, beats per minute; eGFR, estimated glomerular filtration rate; FMD, flow‐mediated vasodilation; HbA1c, hemoglobin A1c; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein.
Table 7.
Clinical Outcome of the Subjects on the Basis of Cutoff Values of FMD and baPWV
| Variables | Group 1 | Group 2 | Group 3 | Group 4 | P Value |
|---|---|---|---|---|---|
| FMD >7.1% | FMD ≤7.1% | ||||
| baPWV <1731 cm/s | baPWV ≥1731 cm/s | baPWV <1731 cm/s | baPWV ≥1731 cm/s | ||
| (n=60) | (n=17) | (n=252) | (n=133) | ||
| Acute myocardial infarction | 0 (0.0) | 0 (0.0) | 8 (3.2) | 2 (1.5) | 0.19 |
| Coronary artery restenosis | 0 (0.0) | 1 (5.9) | 7 (2.8) | 7 (5.3) | 0.11 |
| de novo coronary artery stenosis | 2 (3.3) | 2 (11.8) | 12 (4.8) | 15 (11.3) | 0.06 |
| Stroke | 0 (0.0) | 0 (0.0) | 1 (0.4) | 3 (2.3) | 0.24 |
| Heart failure | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (3.0) | 0.02 |
| Sudden death | 0 (0.0) | 1 (5.9) | 1 (0.4) | 0 (0.0) | 0.16 |
baPWV indicates brachial–ankle pulse wave velocity; FMD, flow‐mediated vasodilation.
Figure 4.
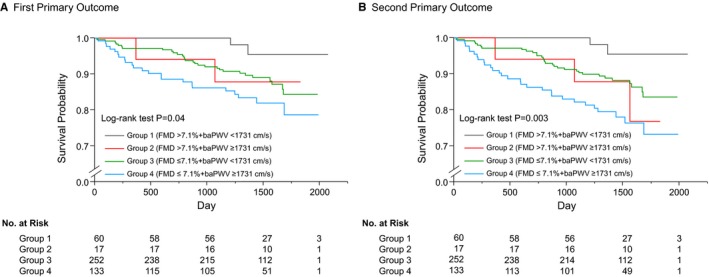
Kaplan–Meier curves of cumulative event‐free survival of the first primary outcome (coronary events) (A) and the second primary outcome (coronary events, stroke, heart failure, or sudden death) (B) in subgroups of subjects categorized as being above or below the cutoff values of flow‐mediated vasodilation (FMD) and brachial–ankle pulse wave velocity (baPWV). The P value was calculated from the log‐rank test.
Discussion
In the present study, we demonstrated that FMD and baPWV were significant predictors of cardiovascular events independent of conventional cardiovascular risk factors in patients with established CAD. In addition, we demonstrated that the combination of FMD and baPWV provided further cardiovascular risk stratification of patients with CAD. To our knowledge, this is the first prospective multicenter study showing the usefulness of FMD measurement alone, baPWV measurement alone, and the combination of FMD and baPWV measurements for predicting cardiovascular events in patients with CAD. Participants enrolled in the present study were well‐managed stable CAD patients who had high rates of adherence to guideline‐based therapies and who had been under regular follow‐up for at least 6 months. Therefore, our findings suggest that measurements of FMD and baPWV might be useful for risk assessment in well‐managed stable CAD patients receiving guideline‐based standard therapies.
For asymptomatic patients without a history of atherosclerotic cardiovascular disease (ASCVD), risk stratification tools have been developed and validated to provide the foundation for targeted preventive efforts based on the individual's predicted risk with the concept of targeting the intensity of drug treatment interventions to the severity of the patient's cardiovascular risk.22, 23, 24, 25 On the other hand, patients with ASCVD have been referred to as high‐risk patients for whom prompt initiation of guideline‐recommended therapies should be considered to reduce the risk. Therefore, risk stratification strategies have not been well established. However, in the context of the growing number of evidence‐based therapies that reduce cardiac morbidity and mortality in patients with high risk for cardiovascular events who are receiving standard therapy, risk stratification of high‐risk patients, such as those with CAD, may be helpful to select candidates for individualized intensive therapies who could gain the greatest benefit from the emerging therapies.
Measurement of FMD of the brachial artery has been used as a method for assessment of endothelial function in humans.26, 27 In addition, several lines of evidence have demonstrated that FMD could be used not only as an index of endothelial function but also as a prognostic marker of cardiovascular events.11, 28 Recent meta‐analyses have shown that FMD is a significant predictor of cardiovascular events independent of conventional cardiovascular risk factors.12, 13, 14 However, there have been very few studies in which the predictive value of FMD in patients with established CAD was investigated.29, 30 In addition, the predictive value of FMD in patients with high risk for cardiovascular events is controversial. Witte et al31 reported that FMD was significantly related to the principal cardiovascular risk factors and estimated 10‐year risk of CAD in low‐risk populations but not in medium‐ and high‐risk populations categorized according to the Framingham risk score, suggesting that measurement of FMD is not useful for risk assessment in individuals at high risk for cardiovascular events, such as those with a history of CAD. However, Gokce et al32 showed that patients who had peripheral artery disease undergoing vascular surgery and who were in the upper tertile of FMD (>8.1%) had significantly fewer cardiovascular events than those in patients in the lowest and middle tertiles of FMD with no difference in event‐free survival rates between the lowest and middle tertiles, suggesting that FMD could be used for identifying individuals at low risk for cardiovascular events among patients generally considered to be at high cardiovascular risk. In the present study, CAD patients with FMD >7.1% had significantly fewer cardiovascular events than those in CAD patients with FMD ≤7.1%. In addition, FMD >7.1% was a significant predictor of lower risk of cardiovascular events independent of conventional cardiovascular risk factors, LVEF, and BNP (hazard ratio, 0.27; 95% CI, 0.06–0.74; P=0.008 for the first primary outcomes in Model 1; hazard ratio, 0.32; 95% CI, 0.09–0.79; P=0.01 for the second primary outcomes in Model 1; hazard ratio, 0.27; 95% CI, 0.07–0.76; P=0.01 for the first primary outcomes in Model 2; hazard ratio, 0.32; 95% CI, 0.10–0.79; P=0.01 for the second primary outcomes in Model 2). According to a previous study conducted in 4533 subjects from the FMD‐J study cohort, reference values of FMD were 6.5% in men and 7.4% in women.18 Taken together, these findings suggest that measurement of FMD in patients with CAD might be clinically useful for identifying individuals with normally maintained endothelial function who are at low risk for recurrent cardiovascular events.
We have no information on the reproducibility of FMD measurements within each participant institution. However, the correlation coefficients between FMD analyzed at the core laboratory and institutions with a small number of enrolled study subjects were not satisfactory (R<0.60), raising the possibility that the reproducibility of FMD measurements performed at a less experienced institution was low, which could provide misleading information for risk stratification of patients with CAD.18 Although the concept of the endothelial function test seems simple, FMD measurement is technically challenging. Therefore, it is recommended that FMD measurement be performed by a skilled and trained operator with a comprehensive understanding of the principle of FMD.
Recently, baPWV has been used for assessment of arterial stiffness in humans. It has been shown that baPWV correlates closely with directly measured aortic PWV and carotid–femoral PWV used as the criterion standard for noninvasive assessment of central arterial stiffness.33, 34 Compared with the measurement of carotid–femoral PWV requiring a skilled technique and exposure of the inguinal region during measurement, baPWV measurement is a simple method using a separate oscillometric cuff for each of the 4 extremities. Several lines of evidence have demonstrated that baPWV could be used not only as an index of atrial stiffness but also as a prognostic marker of cardiovascular events.35 However, there have been few studies in which the predictive value of baPWV in patients with established CAD was investigated.30, 36 In the present study, CAD patients with baPWV ≥1731 cm/s had significantly more cardiovascular events than those in patients with baPWV <1731 cm/s. In addition, baPWV ≥1731 cm/s was a significant predictor of increased risk of cardiovascular events independent of conventional cardiovascular risk factors, LVEF, and BNP (hazard ratio, 1.86; 95% CI, 1.01–3.44; P=0.004 for the first primary outcomes in Model 1; hazard ratio, 2.19; 95% CI, 1.23–3.90; P=0.008 for the second primary outcomes in Model 1; hazard ratio, 1.99; 95% CI, 1.04–3.82; P=0.04 for the first primary outcomes in Model 2; hazard ratio, 2.17; 95% CI, 1.18–4.00; P=0.01 for the second primary outcomes in Model 2), being consistent with the results of a previous study showing that baPWV ≥1730 cm/s was independently associated with increased risk of cardiovascular events in patients with type 2 diabetes mellitus with CAD.36 These findings suggest that measurement of baPWV in patients with CAD might be clinically useful for identifying individuals who have increased arterial stiffness and who are at high risk for recurrent cardiovascular events. A literature‐based meta‐analysis, in which more than half of the study participants were very high‐risk patients, such as those with end‐stage renal disease or ASCVD, and a recent individual participant data meta‐analysis investigating the association of baPWV with the risk of development of ASCVD in subjects without a history of ASCVD, have demonstrated that elevated baPWV is associated with increased risk of cardiovascular events independent of conventional risk factors.15, 16 Taken together, the findings of our study support the broad applicability of baPWV for risk stratification in general clinical settings regardless of cardiovascular risk.
We also investigated the predictive value of the combination of FMD and baPWV for cardiovascular events. Although a previous single‐center study showed the predictive value of the combination of FMD and baPWV in patients with chronic CAD, the optimal cutoff values of FMD and baPWV for predicting cardiovascular events were not assessed.30 In the present study, there were significant differences between the Kaplan–Meier curves for the first and second primary outcome events among the 4 groups categorized according to the cutoff values of FMD and baPWV. In addition, multivariate regression analysis showed a stepwise increase in the calculated risk ratio for the first primary outcome events (hazard ratio, 3.79; 95% CI, 1.11–23.73; P=0.03 for Group 3 in Model 1; hazard ratio, 6.84; 95% CI, 1.87–44.31; P=0.002 for Group 4 in Model 1; hazard ratio, 3.61; 95% CI, 1.06–22.55; P=0.04 for Group 3 in Model 2; hazard ratio, 7.07; 95% CI, 1.89–46.35; P=0.002 for Group 4 in Model 2) and the second primary outcome events (hazard ratio, 4.05; 95% CI, 1.20–25.28; P=0.02 for Group 3 in Model 1; hazard ratio, 8.38; 95% CI, 2.34–53.77; P<0.001 for Group 4 in Model 1; hazard ratio, 3.87; 95% CI, 1.15–24.09; P=0.03 for Group 3 in Model 2; hazard ratio, 8.02; 95% CI, 2.21–51.79; P<0.001 for Group 4 in Model 2). These findings suggest that the combination of FMD and baPWV provides further risk stratification of patients with CAD for recurrent cardiovascular events than does FMD alone or baPWV alone. Therefore, recommended procedures for risk assessment in patients with CAD could be as follows. First, FMD is measured to identify individuals with normally maintained endothelial function (FMD >7.1%) who are considered to be at low risk for recurrent cardiovascular events. Second, in patients with impaired endothelial function (FMD ≤7.1%), measurement of baPWV is recommended to identify individuals with increased arterial stiffness (baPWV ≥1731 cm/s) who are at especially high risk for recurrent cardiovascular events.
There are some limitations in this study. First, there is a wide range of variations in the protocols for measurement of FMD because of differences in testing modality, position of cuff placement, cuff inflation pressure for artery occlusion, cuff inflation time, and timing of measurement of peak artery diameter, resulting in a difference in diagnostic criteria among clinical studies. Therefore, the cutoff value of FMD obtained in this study is applicable only to Japanese CAD patients in whom FMD was measured in accordance with the same protocol as that used in this study. Second, there were significant differences in hemoglobin A1c (HbA1c) levels between the groups stratified according to the cutoff values of FMD and baPWV, which may be a potential confounding factor associated with changes in FMD and baPWV. Although HbA1c levels should be entered into the multivariate model, information on HbA1c levels was available in only 347 of the 462 participants, leading to a low level of statistical power with a decreased number of subjects in the multivariate analysis. Therefore, we did not enter HbA1c levels into the multivariate analyses and could not, therefore, exclude the possibility of a confounding effect of HbA1c levels on vascular function in multivariate analyses. Third, a previous study showed that CAD patients with improved FMD after 6 months of optimized therapy (responders) had significantly fewer cardiovascular events than those for patients with persistently impaired FMD despite the optimized therapy (nonresponders) during 36 months of follow‐up.37 In the present study, angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers and statins, which are known to improve vascular function, were all highly utilized. In addition, CAD patients who had been under regular follow‐up for at least 6 months were enrolled in this study. Although we have no information on FMD and baPWV before the initiation of evidence‐based therapies, we cannot deny the possibility that the difference in the response of vascular function to evidence‐based therapies predisposed to cardiovascular events with elevated risk in the nonresponders.
In conclusion, in CAD patients with high adherence to guideline‐based therapies, measurements of FMD and baPWV improved cardiovascular risk assessment. Both FMD and baPWV were significant predictors of recurrent cardiovascular events independent of conventional risk factors. In addition, the combination of FMD and baPWV provided further risk stratification of patients with CAD. FMD of 7.1% and baPWV of 1731 cm/s may be considered as reference values for recurrent cardiovascular events in patients with CAD. Measurements of both FMD and baPWV are recommended for risk assessment in patients with CAD. Further studies are needed for the justification for and validation of the cutoff values of FMD and baPWV to differentiate high‐ and low‐risk groups for clinical implementation and to determine whether the cutoff values of FMD and baPWV are universally valid, whether individualized intensive therapies for patients with CAD who are judged to be at especially high risk for recurrent cardiovascular events by measurements of FMD and baPWV improve cardiovascular outcomes, and whether those vascular function tests eventually contribute to lower management costs.
Sources of Funding
This work was supported by a grant‐in‐Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (18590815 and 21590898 to Higashi) and a Grant in Aid of Japanese Arteriosclerosis Prevention Fund (to Higashi).
Disclosures
None.
Supporting information
Table S1. Clinical Characteristics of the Included Subjects and Excluded Subjects Without baPWV Measurement
Acknowledgments
We thank Megumi Wakisaka, Miki Kumiji, Ki‐ichiro Kawano, and Satoko Michiyama for their excellent secretarial assistance.
(J Am Heart Assoc. 2018;7:e008588 DOI: 10.1161/JAHA.118.008588.)
References
- 1. Matsuzaki M, Kita T, Mabuchi H, Matsuzawa Y, Nakaya N, Oikawa S, Saito Y, Sasaki J, Shimamoto K, Itakura H; J‐LIT Study Group. Japan Lipid Intervention Trial . Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low‐dose simvastatin therapy in Japanese patients with hypercholesterolemia. Circ J. 2002;66:1087–1095. [DOI] [PubMed] [Google Scholar]
- 2. Mabuchi H, Kita T, Matsuzaki M, Matsuzawa Y, Nakaya N, Oikawa S, Saito Y, Sasaki J, Shimamoto K, Itakura H; J‐LIT Study Group. Japan Lipid Intervention Trial . Large scale cohort study of the relationship between serum cholesterol concentration and coronary events with low‐dose simvastatin therapy in Japanese patients with hypercholesterolemia and coronary heart disease: secondary prevention cohort study of the Japan Lipid Intervention Trial (J‐LIT). Circ J. 2002;66:1096–1100. [DOI] [PubMed] [Google Scholar]
- 3. Cholesterol Treatment Trialists C , Fulcher J, O'Connell R, Voysey M, Emberson J, Blackwell L, Mihaylova B, Simes J, Collins R, Kirby A, Colhoun H, Braunwald E, La Rosa J, Pedersen TR, Tonkin A, Davis B, Sleight P, Franzosi MG, Baigent C, Keech A. Efficacy and safety of LDL‐lowering therapy among men and women: meta‐analysis of individual data from 174,000 participants in 27 randomised trials. Lancet. 2015;385:1397–1405. [DOI] [PubMed] [Google Scholar]
- 4. Smith SC Jr, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd‐Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA; World Heart Federation and the Preventive Cardiovascular Nurses Association . AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. [DOI] [PubMed] [Google Scholar]
- 5. Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R; Cholesterol Treatment Trialists' (CTT) Collaborators . Efficacy and safety of cholesterol‐lowering treatment: prospective meta‐analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 6. LaRosa JC, Grundy SM, Waters DD, Shear C, Barter P, Fruchart JC, Gotto AM, Greten H, Kastelein JJ, Shepherd J, Wenger NK; Treating to New Targets I . Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005;352:1425–1435. [DOI] [PubMed] [Google Scholar]
- 7. Tomiyama H, Yamashina A. Non‐invasive vascular function tests: their pathophysiological background and clinical application. Circ J. 2010;74:24–33. [DOI] [PubMed] [Google Scholar]
- 8. Vlachopoulos C, Xaplanteris P, Aboyans V, Brodmann M, Cifkova R, Cosentino F, De Carlo M, Gallino A, Landmesser U, Laurent S, Lekakis J, Mikhailidis DP, Naka KK, Protogerou AD, Rizzoni D, Schmidt‐Trucksass A, Van Bortel L, Weber T, Yamashina A, Zimlichman R, Boutouyrie P, Cockcroft J, O'Rourke M, Park JB, Schillaci G, Sillesen H, Townsend RR. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation: endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis. 2015;241:507–532. [DOI] [PubMed] [Google Scholar]
- 9. Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, Kajikawa M, Matsumoto T, Hidaka T, Kihara Y, Chayama K, Noma K, Nakashima A, Goto C, Higashi Y. Nitroglycerine‐induced vasodilation for assessment of vascular function: a comparison with flow‐mediated vasodilation. Arterioscler Thromb Vasc Biol. 2013;33:1401–1408. [DOI] [PubMed] [Google Scholar]
- 10. Higashi Y, Noma K, Yoshizumi M, Kihara Y. Endothelial function and oxidative stress in cardiovascular diseases. Circ J. 2009;73:411–418. [DOI] [PubMed] [Google Scholar]
- 11. Kajikawa M, Maruhashi T, Hida E, Iwamoto Y, Matsumoto T, Iwamoto A, Oda N, Kishimoto S, Matsui S, Hidaka T, Kihara Y, Chayama K, Goto C, Aibara Y, Nakashima A, Noma K, Higashi Y. Combination of flow‐mediated vasodilation and nitroglycerine‐induced vasodilation is more effective for prediction of cardiovascular events. Hypertension. 2016;67:1045–1052. [DOI] [PubMed] [Google Scholar]
- 12. Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow‐mediated vasodilatation of brachial artery: a meta‐analysis. Int J Cardiovasc Imaging. 2010;26:631–640. [DOI] [PubMed] [Google Scholar]
- 13. Ras RT, Streppel MT, Draijer R, Zock PL. Flow‐mediated dilation and cardiovascular risk prediction: a systematic review with meta‐analysis. Int J Cardiol. 2013;168:344–351. [DOI] [PubMed] [Google Scholar]
- 14. Matsuzawa Y, Kwon TG, Lennon RJ, Lerman LO, Lerman A. Prognostic value of flow‐mediated vasodilation in brachial artery and fingertip artery for cardiovascular events: a systematic review and meta‐analysis. J Am Heart Assoc. 2015;4:e002270 DOI: 10.1161/JAHA.115.002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vlachopoulos C, Aznaouridis K, Terentes‐Printzios D, Ioakeimidis N, Stefanadis C. Prediction of cardiovascular events and all‐cause mortality with brachial‐ankle elasticity index: a systematic review and meta‐analysis. Hypertension. 2012;60:556–562. [DOI] [PubMed] [Google Scholar]
- 16. Ohkuma T, Ninomiya T, Tomiyama H, Kario K, Hoshide S, Kita Y, Inoguchi T, Maeda Y, Kohara K, Tabara Y, Nakamura M, Ohkubo T, Watada H, Munakata M, Ohishi M, Ito N, Nakamura M, Shoji T, Vlachopoulos C, Yamashina A; Collaborative Group for J‐BAVEL (Japan Brachial‐Ankle Pulse Wave Velocity Individual Participant Data Meta‐Analysis of Prospective Studies) . Brachial‐ankle pulse wave velocity and the risk prediction of cardiovascular disease: an individual participant data meta‐analysis. Hypertension. 2017;69:1045–1052. [DOI] [PubMed] [Google Scholar]
- 17. Tomiyama H, Kohro T, Higashi Y, Takase B, Suzuki T, Ishizu T, Ueda S, Yamazaki T, Furumoto T, Kario K, Inoue T, Koba S, Watanabe K, Takemoto Y, Hano T, Sata M, Ishibashi Y, Node K, Maemura K, Ohya Y, Furukawa T, Ito H, Yamashina A. A multicenter study design to assess the clinical usefulness of semi‐automatic measurement of flow‐mediated vasodilatation of the brachial artery. Int Heart J. 2012;53:170–175. [DOI] [PubMed] [Google Scholar]
- 18. Tomiyama H, Kohro T, Higashi Y, Takase B, Suzuki T, Ishizu T, Ueda S, Yamazaki T, Furumoto T, Kario K, Inoue T, Koba S, Watanabe K, Takemoto Y, Hano T, Sata M, Ishibashi Y, Node K, Maemura K, Ohya Y, Furukawa T, Ito H, Ikeda H, Yamashina A. Reliability of measurement of endothelial function across multiple institutions and establishment of reference values in Japanese. Atherosclerosis. 2015;242:433–442. [DOI] [PubMed] [Google Scholar]
- 19. Kawai T, Ohishi M, Onishi M, Ito N, Takeya Y, Maekawa Y, Rakugi H. Cut‐off value of brachial‐ankle pulse wave velocity to predict cardiovascular disease in hypertensive patients: a cohort study. J Atheroscler Thromb. 2013;20:391–400. [DOI] [PubMed] [Google Scholar]
- 20. American Diabetes Association: clinical practice recommendations 1999. Diabetes Care. 1999;22(suppl 1):S1–S114. [PubMed] [Google Scholar]
- 21. Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III). JAMA. 2001;285:2486–2497. [DOI] [PubMed] [Google Scholar]
- 22. Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. [DOI] [PubMed] [Google Scholar]
- 23. Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren M, Albus C, Benlian P, Boysen G, Cifkova R, Deaton C, Ebrahim S, Fisher M, Germano G, Hobbs R, Hoes A, Karadeniz S, Mezzani A, Prescott E, Ryden L, Scherer M, Syvanne M, Scholte op Reimer WJ, Vrints C, Wood D, Zamorano JL, Zannad F; European Association for Cardiovascular Prevention & Rehabilitation (EACPR); ESC Committee for Practice Guidelines (CPG) . European guidelines on cardiovascular disease prevention in clinical practice (version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur Heart J. 2012;33:1635–1701. [DOI] [PubMed] [Google Scholar]
- 24. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 25. Califf RM, Armstrong PW, Carver JR, D'Agostino RB, Strauss WE. 27th Bethesda Conference: matching the intensity of risk factor management with the hazard for coronary disease events. Task Force 5. Stratification of patients into high, medium and low risk subgroups for purposes of risk factor management. J Am Coll Cardiol. 1996;27:1007–1019. [DOI] [PubMed] [Google Scholar]
- 26. Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, Lloyd JK, Deanfield JE. Non‐invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340:1111–1115. [DOI] [PubMed] [Google Scholar]
- 27. Maruhashi T, Soga J, Fujimura N, Idei N, Mikami S, Iwamoto Y, Kajikawa M, Matsumoto T, Hidaka T, Kihara Y, Chayama K, Noma K, Nakashima A, Goto C, Tomiyama H, Takase B, Yamashina A, Higashi Y. Relationship between flow‐mediated vasodilation and cardiovascular risk factors in a large community‐based study. Heart. 2013;99:1837–1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Patti G, Pasceri V, Melfi R, Goffredo C, Chello M, D'Ambrosio A, Montesanti R, Di Sciascio G. Impaired flow‐mediated dilation and risk of restenosis in patients undergoing coronary stent implantation. Circulation. 2005;111:70–75. [DOI] [PubMed] [Google Scholar]
- 30. Sugamata W, Nakamura T, Uematsu M, Kitta Y, Fujioka D, Saito Y, Kawabata K, Obata JE, Watanabe Y, Watanabe K, Kugiyama K. Combined assessment of flow‐mediated dilation of the brachial artery and brachial‐ankle pulse wave velocity improves the prediction of future coronary events in patients with chronic coronary artery disease. J Cardiol. 2014;64:179–184. [DOI] [PubMed] [Google Scholar]
- 31. Witte DR, Westerink J, de Koning EJ, van der Graaf Y, Grobbee DE, Bots ML. Is the association between flow‐mediated dilation and cardiovascular risk limited to low‐risk populations? J Am Coll Cardiol. 2005;45:1987–1993. [DOI] [PubMed] [Google Scholar]
- 32. Gokce N, Keaney JF Jr, Hunter LM, Watkins MT, Nedeljkovic ZS, Menzoian JO, Vita JA. Predictive value of noninvasively determined endothelial dysfunction for long‐term cardiovascular events in patients with peripheral vascular disease. J Am Coll Cardiol. 2003;41:1769–1775. [DOI] [PubMed] [Google Scholar]
- 33. Sugawara J, Hayashi K, Yokoi T, Cortez‐Cooper MY, DeVan AE, Anton MA, Tanaka H. Brachial‐ankle pulse wave velocity: an index of central arterial stiffness? J Hum Hypertens. 2005;19:401–406. [DOI] [PubMed] [Google Scholar]
- 34. Tanaka H, Munakata M, Kawano Y, Ohishi M, Shoji T, Sugawara J, Tomiyama H, Yamashina A, Yasuda H, Sawayama T, Ozawa T. Comparison between carotid‐femoral and brachial‐ankle pulse wave velocity as measures of arterial stiffness. J Hypertens. 2009;27:2022–2027. [DOI] [PubMed] [Google Scholar]
- 35. Tomiyama H, Matsumoto C, Shiina K, Yamashina A. Brachial‐ankle PWV: current status and future directions as a useful marker in the management of cardiovascular disease and/or cardiovascular risk factors. J Atheroscler Thromb. 2016;23:128–146. [DOI] [PubMed] [Google Scholar]
- 36. Nakamura M, Yamashita T, Yajima J, Oikawa Y, Sagara K, Koike A, Kirigaya H, Nagashima K, Sawada H, Aizawa T; Shinken Database Study Group . Brachial‐ankle pulse wave velocity as a risk stratification index for the short‐term prognosis of type 2 diabetic patients with coronary artery disease. Hypertens Res. 2010;33:1018–1024. [DOI] [PubMed] [Google Scholar]
- 37. Kitta Y, Obata JE, Nakamura T, Hirano M, Kodama Y, Fujioka D, Saito Y, Kawabata K, Sano K, Kobayashi T, Yano T, Nakamura K, Kugiyama K. Persistent impairment of endothelial vasomotor function has a negative impact on outcome in patients with coronary artery disease. J Am Coll Cardiol. 2009;53:323–330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Clinical Characteristics of the Included Subjects and Excluded Subjects Without baPWV Measurement


