Abstract
Background
Ischemic cardiomyopathy (ICM) and dilated cardiomyopathy (DCM) differ in histopathology and prognosis. Although transendocardial delivery of mesenchymal stem cells is safe and provides cardiovascular benefits in both, a comparison of mesenchymal stem cell efficacy in ICM versus DCM has not been done.
Methods and Results
We conducted a subanalysis of 3 single‐center, randomized, and blinded clinical trials: (1) TAC‐HFT (Transendocardial Autologous Mesenchymal Stem Cells and Mononuclear Bone Marrow Cells in Ischemic Heart Failure Trial); (2) POSEIDON (A Phase I/II, Randomized Pilot Study of the Comparative Safety and Efficacy of Transendocardial Injection of Autologous Mesenchymal Stem Cells Versus Allogeneic Mesenchymal Stem Cells in Patients With Chronic Ischemic Left Ventricular Dysfunction Secondary to Myocardial Infarction); and (3) POSEIDON‐DCM (Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis in Dilated Cardiomyopathy). Baseline and 1‐year cardiac structure and function and quality‐of‐life data were compared in a post hoc pooled analysis including ICM (n=46) and DCM (n=33) patients who received autologous or allogeneic mesenchymal stem cells. Ejection fraction improved in DCM by 7% (within‐group, P=0.002) compared to ICM (1.5%; within‐group, P=0.14; between‐group, P=0.003). Similarly, stroke volume increased in DCM by 10.59 mL (P=0.046) versus ICM (−0.2 mL; P=0.73; between‐group, P=0.02). End‐diastolic volume improved only in ICM (10.6 mL; P=0.04) and end‐systolic volume improved only in DCM (17.8 mL; P=0.049). The sphericity index decreased only in ICM (−0.04; P=0.0002). End‐diastolic mass increased in ICM (23.1 g; P<0.0001) versus DCM (−4.1 g; P=0.34; between‐group, P=0.007). The 6‐minute walk test improved in DCM (31.1 m; P=0.009) and ICM (36.3 m; P=0.006) with no between‐group difference (P=0.79). The New York Heart Association class improved in DCM (P=0.005) and ICM (P=0.02; between‐group P=0.20). The Minnesota Living with Heart Failure Questionnaire improved in DCM (−19.5; P=0.002) and ICM (−6.4; P=0.03; δ between‐group difference P=0.042) patients.
Conclusions
Mesenchymal stem cell therapy is beneficial in DCM and ICM patients, despite variable effects on cardiac phenotypic outcomes. Whereas cardiac function improved preferentially in DCM patients, ICM patients experienced reverse remodeling. Mesenchymal stem cell therapy enhanced quality of life and functional capacity in both etiologies.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifiers: TAC‐HFT: NCT00768066, POSEIDON: NCT01087996, POSEIDON‐DCM: NCT01392625.
Keywords: functional capacity impairment, mesenchymal stem cell, remodeling heart failure, stem cell
Subject Categories: Heart Failure
Clinical Perspective
What Is New?
Transendocardial delivery of mesenchymal stem cells provides cardiovascular benefits in ischemic cardiomyopathy as well as nonischemic dilated cardiomyopathy via distinct phenotypic modes of action.
Patients with nonischemic dilated cardiomyopathy primarily recovered parameters of cardiac systolic function, including ejection fraction and stroke volume.
Patients with ischemic cardiomyopathy experienced an improvement in parameters of cardiac remodeling, including sphericity index, end‐diastolic volume, and end‐diastolic mass.
Quality‐of‐life and functional capacity parameters improved significantly in both patient cohorts.
What Are the Clinical Implications?
These findings illustrate that quality‐of‐life and functional capacity parameters improve with cell‐based therapy even if classic measures of cardiac function, such as ejection fraction, do not.
Introduction
Over the past 20 years, cell‐based therapy has emerged as a potentially safe therapeutic option that addresses an unmet need for heart disease.1, 2 However, despite numerous phase I and II clinical trials, there is ongoing controversy as to the ideal patient population for cell treatment. Considering that ischemic cardiomyopathy (ICM) is a leading cause of death worldwide and nonischemic dilated cardiomyopathy (DCM) is a progressive disorder with a high morbidity and mortality, often culminating in heart transplant, novel regenerative therapies are required to treat both underlying causes of left ventricular (LV) dysfunction and heart failure (HF).3 Bone marrow–derived human mesenchymal stem cells (MSCs) are particularly promising because they possess antifibrotic, proangiogenic, and immunomodulatory properties that stimulate repair of damaged tissues.4 MSCs are immunoprivileged secondary to their lack of major histocompatibility complex class II and costimulatory factors,5 which allows their use as an allogeneic graft. While the safety of both autologous and allogeneic MSCs is reasonably well established, studies have demonstrated that allogeneic MSCs derived from young, healthy donors are more efficacious.1, 5, 6 Notably, these cells are shown to be a safe method to improve cardiac structure and function in preclinical and clinical trials.1, 2, 7, 8, 9, 10, 11, 12 The question remains: In which pathologic setting are MSCs most effective? Whether ischemic or nonischemic cardiac pathologies are more amenable to MSC administration remains unknown, and a deeper understanding of the differences in response to cell‐based therapies can help focus research and therapeutic efforts.
The etiologies of ICM and DCM are inherently different, primarily because of the presence of atherosclerotic lesions in the former, which leads to ischemia and cardiac necrosis, while infectious, autoimmune, and genetic factors contribute to DCM.13, 14, 15, 16 Nevertheless, both diseases are marked by some degree of fibrosis, remodeling, and a variable amount of viable, yet dysfunctional, myocardium.13, 17, 18 In addition, both are characterized by immune dysregulation with elevated circulating levels of inflammatory cytokines.19, 20 Persistent inflammation is a possible mechanism for the increased reactive oxygen species, nitroso‐redox imbalance, fibrosis, apoptosis, and impaired angiogenesis seen in LV remodeling.17, 21 Given that MSCs secrete numerous paracrine factors that attenuate these mechanisms and possess immunomodulatory properties, they are a promising therapy for both forms of cardiomyopathy.5
In the clinical trials included in this study, transendocardial stem cell injection (TESI) of MSCs was tested in patients with ICM and DCM. The TAC‐HFT (Transendocardial Autologous Mesenchymal Stem Cells and Mononuclear Bone Marrow Cells in Ischemic Heart Failure Trial) evaluated the impact of autologous MSCs versus bone marrow mononuclear cells versus placebo, whereas the POSEIDON (Phase I/II, Randomized Pilot Study of the Comparative Safety and Efficacy of Transendocardial Injection of Autologous Mesenchymal Stem Cells Versus Allogeneic Mesenchymal Stem Cells in Patients With Chronic Ischemic Left Ventricular Dysfunction Secondary to Myocardial Infarction) trial compared autologous versus allogeneic MSCs in ICM patients.7, 8 These single‐center trials demonstrated that TESI of MSCs significantly improved LV function and reduced scar size in patients with ICM.7, 8 Despite the difference in etiology, patients with DCM underwent TESI with autologous versus allogeneic MSCs in the POSEIDON‐DCM (Percutaneous Stem Cell Injection Delivery Effects on Neomyogenesis in Dilated Cardiomyopathy) trial and exhibited significant anti‐inflammatory effects and improved endothelial function, as well as enhanced parameters of cardiac function and quality of life.1 Given that differences exist in the etiology and pathophysiology of ICM and DCM, it is likely that MSCs work differently for each of these diseases.
Two recent large meta‐analyses highlighted the paucity of high quality clinical trials assessing the evidence of MSC therapy in ICM and DCM.22, 23 Similarly, to date, there has not been a comparison between MSC therapy in patients with ICM and DCM. Therefore, the goal of this subanalysis was to compare the effects of MSCs in patients with ICM and DCM from trials performed at a single institution (TAC‐HFT, POSEIDON, and POSEIDON‐DCM). The novelty of this study lies not only in the comparison of the treatment of 2 leading causes of cardiomyopathy and HF, but these 3 clinical trials conducted at the same institution utilized the same injection technique, along with similar inclusion criteria and outcome measurements.1, 7, 8 Accordingly, we performed a comparative analysis of quality of life, functional outcome, and cardiac phenotypic outcomes from these 3 trials to evaluate potential differences in responses to MSC intracardiac administration.
Given the results from prior studies, and the greater improvement in ejection fraction (EF) reported in POSEIDON‐DCM,1 we hypothesized that TESI of MSCs would be most efficacious in patients with DCM as compared to those with ICM. Results from this post hoc analysis compare the therapeutic effects of MSCs between both HF subtypes, to better inform the field of cardiac regenerative medicine on the clinical indications and limitations of MSC therapy in HF.
Methods
The data, analytic methods, and study materials will be made available, as per National Institutes of Health guidelines, to other researchers for purposes of reproducing the results or replicating the procedure. The 3 clinical trials have been previously published.1, 7, 8
Study Design
This is a post hoc analysis of 3 phase I/II clinical trials conducted at the University of Miami Hospital. The clinical trials included were TAC‐HFT (NCT00768066);8 POSEIDON (NCT01087996), which randomly assigned and tested 20, 100, and 200 million cells7; and POSEIDON‐DCM (NCT01392625), which tested 100 million autologous versus 100 million allogeneic MSCs.1 All 3 studies administered bone marrow–derived human MSCs by transendocardial injection utilizing the NOGA catheter system. The institutional review board of the University of Miami Miller School of Medicine approved all of the studies. All subjects provided a written informed consent.
Inclusion Criteria
Inclusion and exclusion criteria were similar in TAC‐HFT and POSEIDON for enrolling patients with ICM. Briefly, patients with chronic LV dysfunction secondary to myocardial infarction, as documented by confirmed coronary artery disease with corresponding areas of wall motion abnormalities, were included. EF was <50% confirmed within 6 months of study recruitment, without a recent ischemic event. The POSEIDON‐DCM study included patients with an EF of 40% or lower and either an LV end‐diastolic diameter >5.9 cm in male patients, an LV end‐diastolic diameter of >5.6 cm in female patients, or LV end‐diastolic volume index >125 mL/m2. History or evidence of ischemic heart disease was an exclusion criterion. Only patients who were injected with MSCs were included in this post hoc analysis.
Cardiac Functional Capacity and Quality of Life Analysis
Cardiac contrast enhanced computed tomography or delayed enhancement magnetic resonance imaging (depending on pacemaker) was obtained at baseline and 1 year after TESI. Cardiac imaging was used to assess EF, stroke volume (SV), end‐diastolic volume (EDV), end‐systolic volume (ESV), sphericity index (SI), and end‐diastolic mass (EDM). Quality‐of‐life parameters measured the 6‐minute walk test (6MWT), New York Heart Association (NYHA) class, and the Minnesota Living with Heart Failure Questionnaire (MLHFQ), all of which were obtained at baseline and 1 year. Data were collected using a central electronic data system.
Statistical Analysis
For continuous measures, normality of data was tested using the Shapiro‐Wilk test along with assessment of histograms, distribution of mean and median, Q‐Q plots, kurtosis, and skewness. For normally distributed outcome variables, we applied parametric tests and presented the data as mean and 95% confidence interval (CI); otherwise, nonparametric tests were used and data are presented as median and interquartile range. Differences within groups were analyzed by the paired t test for parametric values, and the Wilcoxon matched‐pairs signed rank test for nonparametric values. Differences between groups were analyzed by unpaired t test for parametric values and the Mann‐Whitney test for nonparametric values. For categorical variables, the Pearson chi‐squared test was used. P values <0.05, 2‐tailed, were considered statistically significant. Absolute values were reported with the exception of the between‐groups analysis of the SI, which measured percent change, given the small values for absolute indices. Analyses were done using GraphPad Prism7 (GraphPad Software, Inc, La Jolla, CA).
Results
Patient Population
The TAC‐HFT study enrolled a total of 65 patients with ICM, of whom 19 were treated with autologous MSCs, 19 were treated with bone marrow mononuclear cells, and 21 received placebo. The 16 patients who were treated with autologous MSCs and completed baseline and follow‐up imaging parameters were included in this study. The POSEIDON study enrolled a total of 31 patients, of whom 30 were treated with autologous (n=15) or allogeneic (n=15) MSCs. One patient was excluded from the study secondary to an LV thrombus. Twenty‐seven patients who had repeat imaging performed were included in this study.
The POSEIDON‐DCM trial enrolled 37 patients (n=18 for autologous MSCs and n=19 for allogeneic MSCs), of whom 34 received TESI of MSCs (n=16 autologous and n=18 allogeneic). In this study, we included 24 patients who completed imaging, of whom 9 were treated with autologous MSCs and 15 were treated with allogeneic MSCs. Ten patients were excluded from the 34 who were treated: 2 were attributable to death (both unrelated to treatment), 2 became ineligible (automated implantable cardioverter‐defibrillator placement), 3 received a cardiac transplant, and 3 withdrew from the study.
Table 1 shows the baseline characteristics of the patients included in this study. The mean age of the pooled patients in this post hoc analysis was 61.4±10.0 for those with ICM (n=43) and 55.4±12.1 for those with DCM (n=24; P=0.04). As expected, there are more males in the ICM group. In addition, more patients with ICM had a history of arrhythmias and hyperlipidemia than did patients with DCM. The baseline 6MWT was better in the patients with DCM. With regards to autologous versus allogeneic MSC treatment, a greater number of patients with ICM received autologous MSCs in this study.
Table 1.
Baseline Characteristics
| DCM (n=24) | ICM (n=43) | P Value | |
|---|---|---|---|
| Age at cell delivery, y | 55.4±12.1 | 61.4±10.0 | 0.04 |
| Treatment cell type | 0.02 | ||
| Autologous | 9 (37.5%) | 29 (67.4%) | |
| Allogeneic | 15 (62.5%) | 14 (32.6%) | |
| Sex | 0.05 | ||
| Male | 16 (66.7%) | 38 (88.4%) | |
| Female | 8 (33.3%) | 5 (11.6%) | |
| AICD or BIV/CRT | 19 (79.2%) | 26 (60.5%) | 0.17 |
| Ethnicity: Hispanic or Latino | 9 (37.5%) | 12 (27.9%) | 0.43 |
| Race: White | 16 (66.7%) | 12 (27.9%) | 0.004 |
| History of hypertension | 8 (33.3%) | 23 (53.5%) | 0.13 |
| History of atrial or ventricular arrhythmia | 4 (16.7%) | 25 (58.1%) | 0.002 |
| History of hyperlipidemia | 5 (20.8%) | 35 (81.4%) | 0.0001 |
| History of smoking | 15 (62.5%) | 21 (48.8%) | 0.32 |
| History of diabetes mellitus | 1 (4.2%) | 9 (20.9%) | 0.08 |
| NYHA Class | 0.53 | ||
| Class I‐no limitation | 9 (28.1%) | 9 (19.6%) | |
| Class II‐slight limitation of physical activity | 16 (50.0%) | 26 (58.7%) | |
| Class III‐marked limitation of physical activity | 7 (21.9%) | 11 (23.9%) | |
| 6‐minute walk test, m | 439.8±92.6 | 389.6±86.1 | 0.03 |
| MLHFQ | 36.75±24.65 | 35.46±29 | 0.66 |
| LV size and function | |||
| Ejection fraction, % | 27.0±10.1 | 30.7±10.5 | 0.17 |
| Stroke volume, mL | 84.3±27.24 | 80.5±25.6 | |
| LV end‐diastolic volume, mL | 299.9 (257.0, 421.0) | 270 (206.0, 330.0) | 0.09 |
| LV end‐systolic volume, mL | 232.8 (170.3, 319.0) | 187 (128.0, 251.0) | 0.09 |
| Sphericity index | 0.53±0.1 | 0.48±.0.1 | 0.08 |
| End‐diastolic mass, g | 203.3 (170.8, 307.8) | 212.5 (178.6, 248.2) | 0.97 |
Values are n (%), mean±SD, or median (interquartile range). AICD indicates the automated cardioverter‐defibrillator; BIV/CRT, biventricular pacemaker/cardiac resynchronization therapy; DCM, dilated cardiomyopathy; ICM, ischemic cardiomyopathy; LV, left ventricular; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NYHA, New York Heart Association.
Cardiac Function
Patients with DCM had significant improvements in cardiac function (EF, SV) as compared to those with ICM. The baseline EF in patients with DCM was 27.0±10.0%, which improved by 7.0% (95% CI, 2.9, 11.1; within‐group, P=0.002) at 12‐month follow‐up. The baseline EF in patients with ICM was 30.5±10.5%, with no change at follow‐up (within‐group, P=0.14). There was also a between‐group difference favoring patients with DCM (P=0.003) (Figure 1A). At baseline, SV in the DCM group was 84.3±27.2 mL, which increased by 10.6 mL (95% CI, 0.2, 21.0; within‐group, P=0.046) in response to treatment. Patients with ICM had a baseline SV of 81.5±26.2 mL, and there was no change in SV at follow‐up (within‐group, P=0.73). SV improved more in patients with DCM than in patients with ICM (between‐group, P=0.02; Figure 1B).
Figure 1.
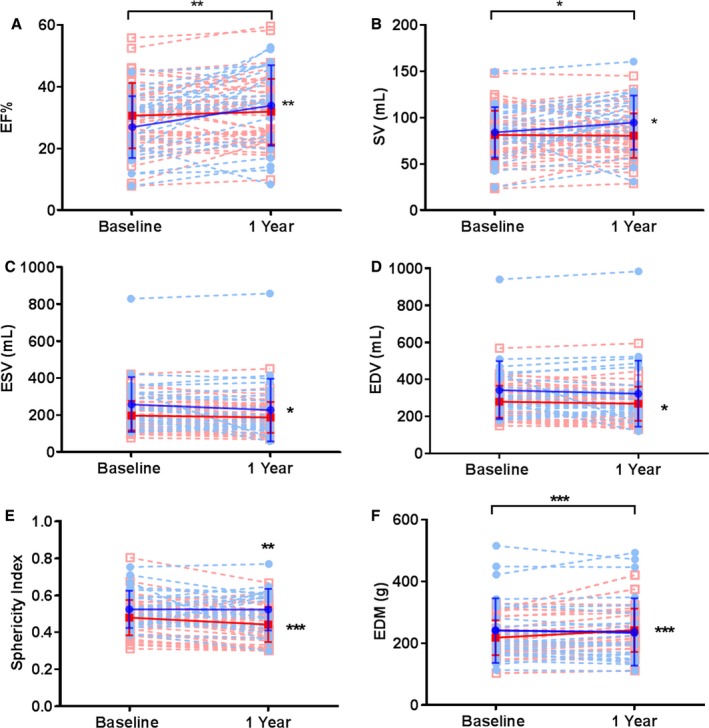
Changes in cardiac function in DCM (blue) and ICM (red) patients. A, EF increased from baseline in DCM (blue circles) by 7 EF units (2.9, 11.0; P=0.002), but not in ICM (red squares). DCM group showed a significant improvement over time in (B) stroke volume by 10.6 mL (95% CI, 0.2, 21.0; P=0.046) and (C) end‐systolic volume by −17.8 mL (interquartile range, −54.5, 17.0; P=0.049). However, the ICM group improved in (D) end‐diastolic volume by −8.32 mL (95% CI: −21.0, −0.3; P=0.05) from baseline, whereas DCM did not. E, Sphericity index improved in ICM by −0.04% (95% CI, −0.06, −0.02; P=0.0002). F, End‐diastolic mass increased in ICM by 23.1 g (95% 13.9, 32.2; P<0.0001) at follow‐up, with a significant difference between both groups (P=0.0003). DCM indicates dilated cardiomyopathy; ED, end diastolic; EDV, end‐diastolic volume; EF, ejection fraction; ESV, end‐systolic volume; ICM, ischemic cardiomyopathy; SV, stroke volume.
Cardiac Remodeling
Patients with ICM exhibited a negative remodeling effect, as indicated by significant improvements in EDV, SI, and EDM. Moreover, the improvements in SI and EDM were greater in patients with ICM compared to DCM. The ESV decreased in the DCM group by −17.8 mL (interquartile range, −54.5, 17.0; within‐group, P=0.049) and did not change in the ICM group (within group, P=0.1) (between‐group difference, P=0.39; Figure 1C). The EDV in patients with ICM was reduced after MSC treatment by −8.3 mL (95% CI, −21, −0.3; within‐group, P=0.05). In patients with DCM, there was no change in EDV at follow‐up (within‐group, P=0.58), and there was no difference in the change in EDV between patients with ICM and patients with DCM (P=0.85; Figure 1D). SI at baseline in ICM was 0.48±0.1 and decreased by 0.04 (95% CI, −0.06, −0.02; within‐group, P=0.0002) at follow‐up. Patients with DCM had a baseline SI of 0.53±0.1 with no change at follow‐up (within‐group, P=0.9). Furthermore, at 1‐year follow‐up there were greater improvements in SI in the ICM group versus DCM (P=0.004; Figure 1E). EDM at baseline was 212.5 (interquartile range, 178.6, 248.2) in ICM patients and increased by 23.1 g (95% CI, 13.9, 32.2; within‐group, P<0.0001). Patients with DCM had a baseline EDM of 241.6 g (interquartile range, 170.8, 307.8) without a change at follow‐up (within‐group, P=0.34). There was a significant δ difference between groups (P=0.0003), favoring patients with ICM (Figure 1F).
Functional Capacity and Quality of Life
Functional capacity measured by the 6MWT and quality‐of‐life parameters (NYHA, MLHFQ) significantly improved after TESI in both ICM and DCM groups. The 6MWT at baseline in DCM was 439.8±92.6 m and improved by 31.1 m (95% CI, 3.8, 58.4; within‐group, P=0.028). Patients with ICM had a baseline 6MWT of 389.6±86.1 m and improved by 36.3 m (95% CI, 10.9, 61.6; within‐group, P=0.0062), with no between‐group difference (P=0.79; Figure 2A). NYHA class improved in both DCM (46.9%; within‐group, P=0.005) and ICM (40.4%; within‐group, P=0.02) patients at follow‐up, with no between‐group difference (P=0.20; Figure 2B). The MLHFQ score at baseline in DCM was 36.75±24.65 and improved by −19.5 (95% CI, −30.8, −8.1 1; within‐group, P=0.002). The patients with ICM had a baseline MLHFQ of 35.46±29 and had a mean of difference from baseline to the end of the study of −6.4 (95% CI, −12.16, −0.6357; within‐group, P=0.03) and a median of −2.5 (95% CI, −12.16, 0.64; within‐group, P=0.024). Although both groups improved from baseline to 12‐month follow‐up, the MLHFQ improved to a greater extent in the DCM compared to the ICM group, with a mean difference between the groups of 11.05 (95% CI, 0.44, 21.67; P=0.042; Figure 2C).
Figure 2.
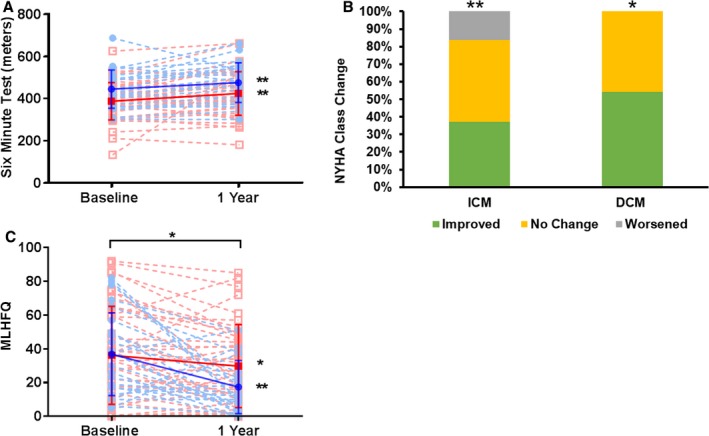
Functional capacity and quality of life in DCM (blue) and ICM (red) patients. A, 6MWT increased at follow‐up from baseline in both groups: DCM group by 31.1 m (95% CI, 3.8, 6.4; P=0.009) and ICM group by 36.3 m (95% CI, 10.9, 61.6; P=0.00062). B, New York Heart Association (NYHA) class improved in both, DCM (P=0.005) and ICM (P=0.02) groups, with no between group differences. C, Minnesota Living with Heart Failure Questionnaire (MLHFQ) total score improved from baseline to 12 months postinjection in both groups, with a difference between means of 11.05 (95% CI, 0.44, 21.67; P=0.042). DCM indicates dilated cardiomyopathy; ICM, ischemic cardiomyopathy.
Discussion
Although other studies have investigated the efficacy of cell‐based therapy in mixed patient populations of cardiomyopathy,24, 25, 26 this is the first study to directly compare clinical and phenotypic outcomes in response to MSCs delivered by TESI for the treatment of 2 different etiologies of cardiomyopathy, namely, DCM and ICM. Pooling the data from 3 single‐institution, phase I/II clinical trials demonstrates that TESI of MSCs provides clinical benefit in both ischemic and non‐ischemic LV dysfunction and HF. Interestingly, MSC administration improves cardiac structural and functional parameters in DCM and ICM in variable ways (Table 2). Patients with DCM primarily recovered parameters of cardiac systolic function––SV, EF, and ESV––whereas patients with ICM experienced a beneficial effect on cardiac remodeling, as evidenced by improved SI, EDV, and EDM. Importantly, quality of life and functional capacity (NYHA class, MLHFQ, and 6MWT) improved in both cohorts, illustrating that these parameters can improve even if classic measures of cardiac function (such as EF) do not.
Table 2.
DCM Versus ICM Study Summary
| Within‐DCM‐Group Changes | Within‐ICM‐Group Changes | Between‐Group Difference | |
|---|---|---|---|
| EF | ↑a | NS | P=0.005 |
| SV | ↑b | NS | P=0.02 |
| EDV | NS | ↓b | NS |
| ESV | ↓b | NS | NS |
| SI | NS | ↓c | P=0.004 |
| ED mass | NS | ↑a | P=0.0003 |
| 6MWT | ↑a | ↑a | NS |
| NYHA | ↓a | ↓b | NS |
| MLHFQ | ↓a | ↓b | 0.04 |
6MWT indicates 6‐minute walk test; DCM, dilated cardiomyopathy; ED, end diastolic; EDV, end‐diastolic volume; EF, ejection fraction; ESV, end‐systolic volume; ICM, ischemic cardiomyopathy; MLHFQ, Minnesota Living with Heart Failure Questionnaire; NS, not significant; NYHA, New York Heart Association; SI, sphericity index; SV, stroke volume.
P=0.001,
P=0.05,
P=0.0001.
It is important to note that these findings are in contrast to those in the IMPACT‐DCM (Use of Ixmyelocel‐T in Patients With Heart Failure Due to Dilated Cardiomyopathy) trial, in which patients with DCM were stratified by ischemic or nonischemic status and randomized to either ixmyelocel‐T, a multicellular therapy produced from autologous bone marrow mononuclear cells with selective expansion of MSCs and macrophages, administered intramyocardially, or standard of care.26 Notably, major adverse cardiovascular events during follow‐up were lower in the ischemic patients treated with ixmyelocel‐T compared to control patients, whereas this benefit was not found in the nonischemic patients. Moreover, ixmyelocel‐T treatment improved NYHA Class, 6MWT, and MLHFQ scores in the ischemic patients relative to control, but not in the nonischemic population. On the other hand, in a phase II dose‐escalation study investigating immunoselected allogeneic bone marrow–derived mesenchymal progenitor cells delivered by TESI in patients with ICM and DCM, no differences were observed in EF at 1 year of follow‐up, although the high‐dose group had a significant reduction in LV ESV and EDV, indicating improvement in reverse remodeling at 6 months and a nonsignificant decrease of both ESV and EDV at 12 months.24 More recently, the Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy showed that intravenously infused umbilical cord–derived MSCs produced significant improvements in EF, NYHA class, and MLHFQ at 3, 6, and 12 months of follow‐up in patients with HF attributable to either ischemic or nonischemic causes.25 The differences between these studies may be attributable, at least in part, to differences in the cell types studied and to the use of autologous versus allogeneic cells, underscoring the need to do further larger clinical studies.
Taken together, the results of this subanalysis do not support the hypothesis that patients with DCM respond better to MSCs than those with ICM. Although the findings here show a significant improvement in EF in DCM as compared to the ICM group, both groups experienced improvements in quality of life as measured by the MLHFQ, clinical symptoms (NYHA classification), and functional capacity as measured by 6MWT. An improvement in EF has historically been associated with a clinically meaningful improvement.27 Interestingly, this study provides evidence against that paradigm because both groups experienced improvements in quality of life, highlighting the importance of evaluating a variety of efficacy end points rather than a single functional parameter to determine the overall benefit from cell‐based therapies, an idea supported by several investigators in the field.27, 28, 29, 30 Similarly, several clinical trials evaluating cell‐based therapies for both forms of HF found that stem cells improved quality of life and functional parameters without sustained improvements in EF.31, 32, 33, 34 Although both patient populations experienced quality‐of‐life improvements, the MLHFQ score improved more in the DCM group. Larger studies are needed to further substantiate this finding. It is also important to consider that a major limitation of this analysis is that there may be further subsets of patients in both groups that may be identified in the future as being highly responsive to cell‐based therapy.
MSCs have been shown to exert their reparative effects by 4 main mechanisms: decrease in fibrosis, increase in neovascularization, and immunomodulation, as well as direct enhancement of endogenous stem cell activity.5, 35, 36 Few clinical trials have been conducted on patients with DCM,1, 37 further stressing the importance of understanding the differences in responses to cell therapy compared to ICM. Histopathologic analyses have revealed a greater prevalence of replacement fibrosis and a lesser degree of myocardial hypertrophy in patients with ICM compared to DCM.13 The mechanisms by which MSCs may have improved cardiac function in patients with DCM include the restoration of endothelial function, which enhances coronary circulation,6, 38 and the reduction of fibrosis and tumor necrosis factor–α, an inflammatory biomarker associated with worsening HF and contractility.39, 40 Those with ICM likely benefit mostly from the stem cells’ antifibrotic properties through reduction in scar size and subsequent reverse remodeling as indicated by a reduction in SI and ESV.7, 29 This may be explained in part by the enhancement of endogenous cardiomyocyte proliferation and a subsequent increase in ventricular mass.5
Both DCM and ICM share an important therapeutic target: the dysfunctional viable myocardium. Ischemic patients’ dysfunctional viable myocardium is limited by the area of scar, which contains very little viable tissue, whereas patients with DCM have a greater area of dysfunctional viable tissue free of scar.17 Previous analyses demonstrated that the increase in LV EF is related to a higher amount of dysfunctional viable tissue before treatment,41 correlating with our findings of improvement in LV systolic function among patients with DCM.
There are notable limitations in this study. The baseline disease severity of these patients varied. Those with cardiac defibrillators and those necessitating cardiac resynchronization usually suffered from more depressed cardiac function and advanced stages of chronic HF. In addition, this study combined cell dose groups (20 million, 100 million, and 200 million cells) and the donor source of the cell (autologous and allogeneic bone marrow–derived MSCs) in order to increase the power of the study with the increase in the number of treated patients. The numbers of patients assessed for the different end points differed from those reported in the original trial publications for various reasons (eg, lack of follow‐up in regards to quality of life, lack of repeat imaging, death, and/or ineligibility because of placement of an automated implantable cardioverter‐defibrillator). Future studies will allow for more rigorous assessment of those parameters and their therapeutic outcomes. Similarly, a lack of comparison against a placebo in both groups makes it difficult to draw firm conclusions about each disease process. However, despite these limitations, we believe that this analysis parallels other HF studies utilizing MSCs, while enhancing the understanding of how MSCs may modulate the structure and function of the heart regardless of disease process.
Conclusion
This subanalysis of 3 single‐center clinical trials suggests that MSCs benefit the treatment of patients with DCM and ICM via distinct phenotypic modes of action. Human MSCs exert antiremodeling effects in chronic ischemia, whereas functional parameters of HF are improved in DCM. Most importantly, quality‐of‐life symptoms and functional capacity are enhanced regardless of HF etiology. Given these outcomes, larger placebo‐controlled clinical trials evaluating the efficacy of MSC therapy in both HF types is warranted, and EF should not be used as the primary marker for stem cell efficacy.
Sources of Funding
This work was supported by National Institutes of Health grants UM1 HL113460, R01 HL110737, and 1R01HL134558‐01; the Starr Foundation; and the Soffer Family Foundation.
Disclosures
Hare reports having a patent for cardiac cell‐based therapy and holds equity in Vestion Inc and maintains a professional relationship with Vestion Inc as a consultant and member of the Board of Directors and Scientific Advisory Board. Vestion Inc did not play a role in the design, conduct, or funding of the study. Dr Hare is the chief scientific officer and a compensated consultant and advisory board member for Longeveron and holds equity in Longeveron. Dr Hare is also the coinventor of intellectual property licensed to Longeveron. Longeveron did not play a role in the design, conduct, or funding of the study. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2018;7:e008460 DOI: 10.1161/JAHA.117.008460.)
References
- 1. Hare JM, DiFede DL, Rieger AC, Florea V, Landin AM, El‐Khorazaty J, Khan A, Mushtaq M, Lowery MH, Byrnes JJ, Hendel RC, Cohen MG, Alfonso CE, Valasaki K, Pujol MV, Golpanian S, Ghersin E, Fishman JE, Pattany P, Gomes SA, Delgado C, Miki R, Abuzeid F, Vidro‐Casiano M, Premer C, Medina A, Porras V, Hatzistergos KE, Anderson E, Mendizabal A, Mitrani R, Heldman AW. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON‐DCM trial. J Am Coll Cardiol. 2017;69:526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Karantalis V, Hare JM. Use of mesenchymal stem cells for therapy of cardiac disease. Circ Res. 2015;116:1413–1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Global Burden of Disease 2016 Causes of Death Collaborators . Global, regional, and national age‐specific mortality for 264 causes of death, 1980‐2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hodgkinson CP, Bareja A, Gomez JA, Dzau VJ. Emerging concepts in paracrine mechanisms in regenerative cardiovascular medicine and biology. Circ Res. 2016;118:95–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Golpanian S, Wolf A, Hatzistergos KE, Hare JM. Rebuilding the damaged heart: mesenchymal stem cells, cell‐based therapy, and engineered heart tissue. Physiol Rev. 2016;96:1127–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Premer C, Blum A, Bellio MA, Schulman IH, Hurwitz BE, Parker M, Dermarkarian CR, DiFede DL, Balkan W, Khan A, Hare JM. Allogeneic mesenchymal stem cells restore endothelial function in heart failure by stimulating endothelial progenitor cells. EBioMedicine. 2015;2:467–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hare JM, Fishman JE, Gerstenblith G, DiFede Velazquez DL, Zambrano JP, Suncion VY, Tracy M, Ghersin E, Johnston PV, Brinker JA, Breton E, Davis‐Sproul J, Schulman IH, Byrnes J, Mendizabal AM, Lowery MH, Rouy D, Altman P, Wong Po Foo C, Ruiz P, Amador A, Da Silva J, McNiece IK, Heldman AW, George R, Lardo A. Comparison of allogeneic vs autologous bone marrow–derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heldman AW, DiFede DL, Fishman JE, Zambrano JP, Trachtenberg BH, Karantalis V, Mushtaq M, Williams AR, Suncion VY, McNiece IK, Ghersin E, Soto V, Lopera G, Miki R, Willens H, Hendel R, Mitrani R, Pattany P, Feigenbaum G, Oskouei B, Byrnes J, Lowery MH, Sierra J, Pujol MV, Delgado C, Gonzalez PJ, Rodriguez JE, Bagno LL, Rouy D, Altman P, Foo CW, da Silva J, Anderson E, Schwarz R, Mendizabal A, Hare JM. Transendocardial mesenchymal stem cells and mononuclear bone marrow cells for ischemic cardiomyopathy: the TAC‐HFT randomized trial. JAMA. 2014;311:62–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams AR, Suncion VY, McCall F, Guerra D, Mather J, Zambrano JP, Heldman AW, Hare JM. Durable scar size reduction due to allogeneic mesenchymal stem cell therapy regulates whole‐chamber remodeling. J Am Heart Assoc. 2013;2:e000140. doi: 10.1161/JAHA.113.000140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee HW, Lee HC, Park JH, Kim BW, Ahn J, Kim JH, Park JS, Oh JH, Choi JH, Cha KS, Hong TJ, Park TS, Kim SP, Song S, Kim JY, Park MH, Jung JS. Effects of intracoronary administration of autologous adipose tissue‐derived stem cells on acute myocardial infarction in a porcine model. Yonsei Med J. 2015;56:1522–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res. 2010;107:913–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bartunek J, Behfar A, Dolatabadi D, Vanderheyden M, Ostojic M, Dens J, El Nakadi B, Banovic M, Beleslin B, Vrolix M, Legrand V, Vrints C, Vanoverschelde JL, Crespo‐Diaz R, Homsy C, Tendera M, Waldman S, Wijns W, Terzic A. Cardiopoietic stem cell therapy in heart failure: the C‐CURE (Cardiopoietic stem Cell therapy in heart failURE) multicenter randomized trial with lineage‐specified biologics. J Am Coll Cardiol. 2013;61:2329–2338. [DOI] [PubMed] [Google Scholar]
- 13. Hare JM, Walford GD, Hruban RH, Hutchins GM, Deckers JW, Baughman KL. Ischemic cardiomyopathy: endomyocardial biopsy and ventriculographic evaluation of patients with congestive heart failure, dilated cardiomyopathy and coronary artery disease. J Am Coll Cardiol. 1992;20:1318–1325. [DOI] [PubMed] [Google Scholar]
- 14. Felker GM, Hu W, Hare JM, Hruban RH, Baughman KL, Kasper EK. The spectrum of dilated cardiomyopathy. The Johns Hopkins experience with 1,278 patients. Medicine (Baltimore). 1999;78:270–283. [DOI] [PubMed] [Google Scholar]
- 15. Felker GM, Thompson RE, Hare JM, Hruban RH, Clemetson DE, Howard DL, Baughman KL, Kasper EK. Underlying causes and long‐term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. [DOI] [PubMed] [Google Scholar]
- 16. Hare JM. The dilated, restrictive and infiltrative cardiomyopathies In: Bonow RO, Mann D, Zipes D, Libby P, eds. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 9th ed Philadephia, PA: Elsevier/Saunders; 2010:1561–1581. [Google Scholar]
- 17. Kelkar AA, Butler J, Schelbert EB, Greene SJ, Quyyumi AA, Bonow RO, Cohen I, Gheorghiade M, Lipinski MJ, Sun W, Luger D, Epstein SE. Mechanisms contributing to the progression of ischemic and nonischemic dilated cardiomyopathy: possible modulating effects of paracrine activities of stem cells. J Am Coll Cardiol. 2015;66:2038–2047. [DOI] [PubMed] [Google Scholar]
- 18. Nazarian S, Bluemke DA, Lardo AC, Zviman MM, Watkins SP, Dickfeld TL, Meininger GR, Roguin A, Calkins H, Tomaselli GF, Weiss RG, Berger RD, Lima JA, Halperin HR. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112:2821–2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aukrust P, Ueland T, Lien E, Bendtzen K, Muller F, Andreassen AK, Nordoy I, Aass H, Espevik T, Simonsen S, Froland SS, Gullestad L. Cytokine network in congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol. 1999;83:376–382. [DOI] [PubMed] [Google Scholar]
- 20. Kittleson MM, Minhas KM, Irizarry RA, Ye SQ, Edness G, Breton E, Conte JV, Tomaselli G, Garcia JG, Hare JM. Gene expression analysis of ischemic and nonischemic cardiomyopathy: shared and distinct genes in the development of heart failure. Physiol Genomics. 2005;21:299–307. [DOI] [PubMed] [Google Scholar]
- 21. Vrtovec B, Poglajen G, Sever M, Lezaic L, Socan A, Haddad F, Wu JC. CD34+ stem cell therapy in nonischemic dilated cardiomyopathy patients. Clin Pharmacol Ther. 2013;94:452–458. [DOI] [PubMed] [Google Scholar]
- 22. Fisher SA, Doree C, Mathur A, Taggart DP, Martin‐Rendon E. Stem cell therapy for chronic ischaemic heart disease and congestive heart failure. Cochrane Database Syst Rev. 2016;12:CD007888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lipinski MJ, Luger D, Epstein SE. Mesenchymal stem cell therapy for the treatment of heart failure caused by ischemic or non‐ischemic cardiomyopathy: immunosuppression and its implications. Handb Exp Pharmacol. 2017;243:329–353. [DOI] [PubMed] [Google Scholar]
- 24. Perin EC, Borow KM, Silva GV, DeMaria AN, Marroquin OC, Huang PP, Traverse JH, Krum H, Skerrett D, Zheng Y, Willerson JT, Itescu S, Henry TD. A phase II dose‐escalation study of allogeneic mesenchymal precursor cells in patients with ischemic or nonischemic heart failure. Circ Res. 2015;117:576–584. [DOI] [PubMed] [Google Scholar]
- 25. Bartolucci J, Verdugo FJ, Gonzalez PL, Larrea RE, Abarzua E, Goset C, Rojo P, Palma I, Lamich R, Pedreros PA, Valdivia G, Lopez VM, Nazzal C, Alcayaga‐Miranda F, Cuenca J, Brobeck MJ, Patel AN, Figueroa FE, Khoury M. Safety and efficacy of the intravenous infusion of umbilical cord mesenchymal stem cells in patients with heart failure: a phase 1/2 randomized controlled trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ Res. 2017;121:1192–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Henry TD, Traverse JH, Hammon BL, East CA, Bruckner B, Remmers AE, Recker D, Bull DA, Patel AN. Safety and efficacy of ixmyelocel‐T: an expanded, autologous multi‐cellular therapy, in dilated cardiomyopathy. Circ Res. 2014;115:730–737. [DOI] [PubMed] [Google Scholar]
- 27. Banovic M, Loncar Z, Behfar A, Vanderheyden M, Beleslin B, Zeiher A, Metra M, Terzic A, Bartunek J. Endpoints in stem cell trials in ischemic heart failure. Stem Cell Res Ther. 2015;6:159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tompkins B, Balkan W, Hare JM. Perspectives on the evolution of stem cell therapy for heart failure. EBioMedicine. 2015;2:1838–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Butler J, Hamo CE, Udelson JE, O'Connor C, Sabbah HN, Metra M, Shah SJ, Kitzman DW, Teerlink JR, Bernstein HS, Brooks G, Depre C, DeSouza MM, Dinh W, Donovan M, Frische‐Danielson R, Frost RJ, Garza D, Gohring UM, Hellawell J, Hsia J, Ishihara S, Kay‐Mugford P, Koglin J, Kozinn M, Larson CJ, Mayo M, Gan LM, Mugnier P, Mushonga S, Roessig L, Russo C, Salsali A, Satler C, Shi V, Ticho B, van der Laan M, Yancy C, Stockbridge N, Gheorghiade M. Reassessing phase II heart failure clinical trials: consensus recommendations. Circ Heart Fail. 2017;10:e003800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hare JM, Bolli R, Cooke JP, Gordon DJ, Henry TD, Perin EC, March KL, Murphy MP, Pepine CJ, Simari RD, Skarlatos SI, Traverse JH, Willerson JT, Szady AD, Taylor DA, Vojvodic RW, Yang PC, Moye LA. Phase II clinical research design in cardiology: learning the right lessons too well: observations and recommendations from the Cardiovascular Cell Therapy Research Network (CCTRN). Circulation. 2013;127:1630–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kalil RA, Ott D, Sant'Anna R, Dias E, Marques‐Pereira JP, Delgado‐Canedo A, Nardi NB, Sant'Anna JR, Prates PR, Nesralla I. Autologous transplantation of bone marrow mononuclear stem cells by mini‐thoracotomy in dilated cardiomyopathy: technique and early results. Sao Paulo Med J. 2008;126:75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang JA, Xie XJ, He H, Sun Y, Jiang J, Luo RH, Fan YQ, Dong L. [A prospective, randomized, controlled trial of autologous mesenchymal stem cells transplantation for dilated cardiomyopathy]. Zhonghua Xin Xue Guan Bing Za Zhi. 2006;34:107–110. [PubMed] [Google Scholar]
- 33. Chen S, Liu Z, Tian N, Zhang J, Yei F, Duan B, Zhu Z, Lin S, Kwan TW. Intracoronary transplantation of autologous bone marrow mesenchymal stem cells for ischemic cardiomyopathy due to isolated chronic occluded left anterior descending artery. J Invasive Cardiol. 2006;18:552–556. [PubMed] [Google Scholar]
- 34. Florea V, Rieger AC, DiFede DL, El‐Khorazaty J, Natsumeda M, Banerjee MN, Tompkins BA, Khan A, Schulman IH, Landin AM, Mushtaq M, Golpanian S, Lowery MH, Byrnes JJ, Hendel RC, Cohen MG, Valasaki K, Pujol MV, Ghersin E, Miki R, Delgado C, Abuzeid F, Vidro‐Casiano M, Saltzman RG, DaFonseca D, Caceres LV, Ramdas KN, Mendizabal A, Heldman AW, Mitrani RD, Hare JM. Dose comparison study of allogeneic mesenchymal stem cells in patients with ischemic cardiomyopathy (the TRIDENT Study). Circ Res. 2017;121:1279–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. White IA, Sanina C, Balkan W, Hare JM. Mesenchymal stem cells in cardiology. Methods Mol Biol. 2016;1416:55–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hatzistergos KE, Saur D, Seidler B, Balkan W, Breton M, Valasaki K, Takeuchi LM, Landin AM, Khan A, Hare JM. Stimulatory effects of mesenchymal stem cells on cKit+ cardiac stem cells are mediated by SDF1/CXCR4 and SCF/cKit signaling pathways. Circ Res. 2016;119:921–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Huynh K. Stem cells: allogenic mesenchymal stem cells for dilated cardiomyopathy. Nat Rev Cardiol. 2017;14:190–191. [DOI] [PubMed] [Google Scholar]
- 38. Marti CN, Gheorghiade M, Kalogeropoulos AP, Georgiopoulou VV, Quyyumi AA, Butler J. Endothelial dysfunction, arterial stiffness, and heart failure. J Am Coll Cardiol. 2012;60:1455–1469. [DOI] [PubMed] [Google Scholar]
- 39. Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990;323:236–241. [DOI] [PubMed] [Google Scholar]
- 40. Haudek SB, Taffet GE, Schneider MD, Mann DL. TNF provokes cardiomyocyte apoptosis and cardiac remodeling through activation of multiple cell death pathways. J Clin Invest. 2007;117:2692–2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bello D, Shah DJ, Farah GM, Di Luzio S, Parker M, Johnson MR, Cotts WG, Klocke FJ, Bonow RO, Judd RM, Gheorghiade M, Kim RJ. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing β‐blocker therapy. Circulation. 2003;108:1945–1953. [DOI] [PubMed] [Google Scholar]


