Abstract
Background
Infective endocarditis (IE) after transcatheter pulmonary valve implantation (TPVI) in dysfunctioning right ventricular outflow tract conduits has evoked growing concerns. We aimed to investigate the incidence and the natural history of IE after TPVI with the Melody valve through a systematic review of published data.
Methods and Results
PubMed, EMBASE, and Web of Science databases were systematically searched for articles published until March 2017, reporting on IE after TPVI with the Melody valve. Nine studies (including 851 patients and 2060 patient‐years of follow‐up) were included in the analysis of the incidence of IE. The cumulative incidence of IE ranged from 3.2% to 25.0%, whereas the annualized incidence rate ranged from 1.3% to 9.1% per patient‐year. The median (interquartile range) time from TPVI to the onset of IE was 18.0 (9.0–30.4) months (range, 1.0–72.0 months). The most common findings were positive blood culture (93%), fever (89%), and new, significant, and/or progressive right ventricular outflow tract obstruction (79%); vegetations were detectable on echocardiography in only 34% of cases. Of 69 patients with IE after TPVI, 6 (8.7%) died and 35 (52%) underwent surgical and/or transcatheter reintervention. Death or reintervention was more common in patients with new/significant right ventricular outflow tract obstruction (69% versus 33%; P=0.042) and in patients with non‐streptococcal IE (73% versus 30%; P=0.001).
Conclusions
The incidence of IE after implantation of a Melody valve is significant, at least over the first 3 years after TPVI, and varies considerably between the studies. Although surgical/percutaneous reintervention is a common consequence, some patients can be managed medically, especially those with streptococcal infection and no right ventricular outflow tract obstruction.
Keywords: congenital heart disease, endocarditis, Melody valve, percutaneous pulmonary valve implantation, percutaneous valve, pulmonary valve, transcatheter valve implantation
Subject Categories: Infectious Endocarditis, Congenital Heart Disease, Valvular Heart Disease
Clinical Perspective
What Is New?
The risk of infective endocarditis after implantation of the Melody valve is significant, extending at least over the first 3 years after the procedure.
The diagnosis is challenging, and the modified Duke criteria have a modest diagnostic yield in this setting.
Approximately 52% of patients require reintervention, either surgically or percutaneously.
The outcome is favorable when streptococci are the causative organism and the right ventricular outflow tract is not obstructed.
What Are the Clinical Implications?
More attention should be paid to prevent and early detect infective endocarditis in patients who receive Melody valve implantation.
Documentation of valvular involvement on echocardiography is challenging, and failure to visualize vegetations should not exclude the diagnosis of infective endocarditis when the clinical suspicion is high.
The causative organism and the pressure gradient across the valve can be used for risk stratification of the patients.
One decade after Bonhoeffer et al1 demonstrated the feasibility of transcatheter pulmonary valve implantation (TPVI) in a right ventricular outflow tract (RVOT) conduit, the US Food and Drug Administration approved the Melody valve (a modified version of the original device of Bonhoeffer et al,1 manufactured by Medtronic Inc, Minneapolis, MN)2 in 2010.
TPVI was subsequently shown to provide satisfactory hemodynamic and clinical outcomes and a low rate of primary valve dysfunction, especially with the marked reduction in the rates of stent fracture after the wide adoption of routine prestenting.3, 4 However, valve dysfunction secondary to infective endocarditis (IE) is increasingly recognized as a threat to valve function and patients’ outcomes3, 5 after TPVI. This risk of IE should be kept in mind when considering the expansion of the indications of TPVI to patients with native RVOT and small/large pulmonary conduits,6 and as a valve‐in‐valve treatment.7
The incidence rate of Melody valve IE varies considerably among the studies and is, reportedly, higher than the rate of pulmonary homograft IE.5, 8 Beyond the incidence rate, many other basic aspects of this disease entity remain obscure, including microbiologic features, presentation, and outcomes.
We aimed at investigating the incidence and natural history of IE after Melody valve implantation through a systematic review and pooled analysis of published data.
Methods
Because all results are derived from published data, the study materials will not be made available. The search method will be made available to other researchers for purposes of reproducing the results.
Literature Search
An electronic search in PubMed, EMBASE, and Web of Science databases for studies published until March 2017 was conducted to identify studies reporting on the incidence and/or the natural history of IE in patients treated with a Melody valve in the pulmonary/RVOT position. The details of the PubMed search items are shown in Data S1. A thorough review of the reference lists of retrieved studies as well as relevant review articles and editorials was conducted for the sake of completeness.
Eligibility Criteria of Studies and Data Extraction
The analysis consisted of 2 parts (the search strategy is shown as PRISMA (Preferred Reporting Items for Systematic reviews and Meta‐Analyses) flowchart9 in Figure S1):
The study‐level nonpooled analysis of the incidence rate of IE: For inclusion in this analysis, the studies were required to: (1) include ≥20 consecutive patients; (2) involve an average follow‐up of at least 12 months after TPVI; and (3) be published in peer‐reviewed literature (conference abstracts were excluded, conforming to Cochrane guidelines). As shown in Figure S1, 329 studies were initially identified, of which 248 were excluded after title/abstract review. Of the remaining 56 studies, 33 did not meet the inclusion criteria (nonconsecutive series, n=13; short follow‐up, n=11; mixed cohort with other devices/nonpulmonary position, n=2; and congress abstracts without published details, n=7). Inclusion criteria were fulfilled by 23 studies, of which 14 were excluded because of overlapping population. Nine studies (including 851 patients) were eventually included in this analysis.
The patient‐level pooled analysis: The individual‐patient characteristics were extracted from studies reporting detailed individual patients’ data (individual case reports/case series). Extracted data included baseline patient characteristics, TPVI periprocedural data, and details of IE, including time of onset, presentation, microbiologic studies, management, and outcome. A total of 22 studies (comprising 69 case reports) were included in this analysis. Special attention was exercised to avoid inclusion of duplicate cases (the same case reported in >1 publication).
Definitions
Overall, IE was defined as any documented blood‐borne infection treated with intravenous antimicrobial therapy for at least 2 weeks, presumed to be related to Melody valve in the absence of an alternative focus of infection. Endocarditis was reported according to the authors’ judgment or using the modified Duke criteria.10, 11 Definite TPV‐related IE was confirmed by the presence of vegetation(s) seen on the Melody valve leaflets, stent, or adjacent RVOT conduit wall, as documented by transthoracic/transesophageal/intracardiac echocardiography, positron emission tomography (PET), surgical explant examination, or autopsy. IE episodes occurring within 1 year after TPVI were defined as “early IE.”12
New/progressive RVOT obstruction was defined as a ≥15 mm Hg increase in RVOT pressure gradient and/or worsening of stenosis severity to moderate‐severe on the first echo‐Doppler study after IE onset compared with the latest study before IE. New/worsening pulmonary regurgitation was defined as pulmonary regurgitation that progressed from mild or less to moderate‐severe pulmonary regurgitation on the first echo‐Doppler study after IE onset compared with the latest study before IE. Death attributable to IE was defined as cardiac death during the same hospital admission for IE.
Statistical Analysis
The systematic review was performed according to the Meta‐Analysis of Observational Studies in Epidemiology (Research‐Checklist) guidelines.13 Continuous variables are expressed as mean±SD or as median and interquartile range, as appropriate. Categorical variables are presented as frequencies and percentages. Linear correlation between the rate of IE in the individual studies and the sample size/length of follow up/years of enrollment was assessed using the nonparametric Spearman's correlation coefficient. Binary logistic regression analysis was used to study the predictors of IE‐related death/reintervention, and the odds ratio and its 95% confidence interval (CI) were presented. Independent variables were included in the final multivariable model if associated with death/reintervention in univariable analysis (P<0.10).
Statistical analysis was performed with SPSS 23 (IBM, Armonk, NY). All probability values were 2 tailed, and P<0.05 was considered significant.
Results
Incidence of Melody Valve IE
A total of 9 studies14, 15, 16, 17, 18, 19, 20, 21, 22 were included in the analysis of the incidence of IE after TPVI using the Melody valve. The characteristics of the studies are summarized in Table 1. The publication year was 2008 to 2017, and the reported cases received TPVI from 2000 to 2015. A total of 851 patients received Melody valve in the pulmonary/RVOT position, mostly (89%) after prestenting. The median follow‐up was 31 (range, 20–59) months, and a total of 2060 patient‐years of follow‐up were included.
Table 1.
Characteristics of 9 Studies14, 15, 16, 17, 18, 19, 20, 21, 22 Included for the Estimation of the Rate of IE After TPVI
| Study, Year of Publication | Country | Period of Patient Enrollment | No. of Patients | Follow‐Up, mo | Patient‐Years of Follow‐Up | Age, ya | Male Sex, % | TOF, % | TA, % | AS (S/P Ross Procedure), % | Homograft, % | Prestenting, % | IE cummulative Incidence, % | IE Incidence Rate/Patient‐Year, % |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biernacka et al,20 2015 | Poland | 2008–2012 | 26 | 20.4 | 44 | NA | NA | NA | NA | NA | NA | 100.0 | 15.4 | 9.1 |
| O'Donnell et al,18 2016 | New Zealand | 2009–2015 | 25 | 35 | 73 | 18 (11–51) | 60.0 | 64.0 | 24.0 | 12.0 | 92.0 | 96.0 | 16.0 | 5.5 |
| Cheung et al,21 2013 | Denmark | 2006–2012 | 42 | 27 | 95 | 25 (6–67) | NA | NA | NA | NA | NA | NA | 14.3 | 6.3 |
| Hascoet et al,22 2017 | France | 2008–2014 | 31 | 58.8 | 151.9 | 19 (15.8–28.9)b | 53.0 | 81.0 | 9.0 | 25.0 | 91.0 | 25.0 | 5.3 | |
| Butera et al,17 2013 | Italy | 2007–2010 | 61 | 30 | 153 | 24 (11–65) | 52.0 | 44.0 | 8.0 | 15.0 | 54.0 | 87.0 | 3.2 | 1.3 |
| Malekzadeh‐Milani et al,16 2014 | France | 2009–2013 | 93 | 23.8 | 184 | 20.1 (18.2–21.8) | 56.0 | 26.0 | 12.0 | 14.0 | 20.0 | NA | 8.6 | 4.4 |
| Van Dijck et al,19 2015 | Belgium | 2006–2013 | 107 | 24 | 214 | 14.3 (4.5–80.5) | 66.0 | 52.0 | 7.0 | 19.0 | 57.0 | NA | 7.5 | 3.7 |
| Lurz et al,15 2008 | UK‐France | 2000–2007 | 155 | 28.4 | 367 | 21.2 (7–71) | 58.0 | 61.0 | 11.0 | 8.0 | 81.0 | NA | 3.2 | 1.4 |
| McElhinney et al,14 2013 | United States, Canada, and Europe | 2007–2012 | 311 | 30 | 778 | 18.2 (5–59) | NA | 44.0 | 18.0 | 70.0 | 73.0 | 5.1 | 2.1 | |
| Total | … | 2000–2015 | 851 | 30.8 | 2059.9 | 20.0 | 57.5 | 48.5 | 12.4 | 13.6 | 57.0 | 89.4 | 10.92 | 4.33 |
AS indicates aortic stenosis; IE, infective endocarditis; NA, not applicable; RVOT, right ventricular outflow tract; S/P, status post; TA, truncus arteriosus; TOF, tetralogy of Fallot; TPVI, transcatheter pulmonary valve implantation.
Average (range).
Median (interquartile range).
The cumulative incidence of IE varied between the studies (range, 3.2%–25.0%), as did the annualized incidence rate (range, 1.3%–9.1% per patient‐year). In studies applying the modified Duke criteria (n=4 studies16, 19, 21, 22), the cumulative incidence of IE ranged from 7.5% to 25.0%, and the annualized incidence rate ranged from 3.7% to 6.3%. Both cumulative and annualized incidences of IE were higher in studies with lower numbers of patients (Figure 1A and 1B14, 15, 16, 17, 18, 19, 20, 21, 22). On the other hand, the incidence was not influenced by the follow‐up duration (Figure 2A and 2B14, 15, 16, 17, 18, 19, 20, 21, 22). Figure 3 shows a summary of the time period of patient enrollment in each study and the respective cumulative (Figure 3A14, 15, 16, 17, 18, 19, 20, 21, 22) and annualized incidence rate of IE (Figure 3B14, 15, 16, 17, 18, 19, 20, 21, 22), both trending to be higher in later than in earlier studies.
Figure 1.
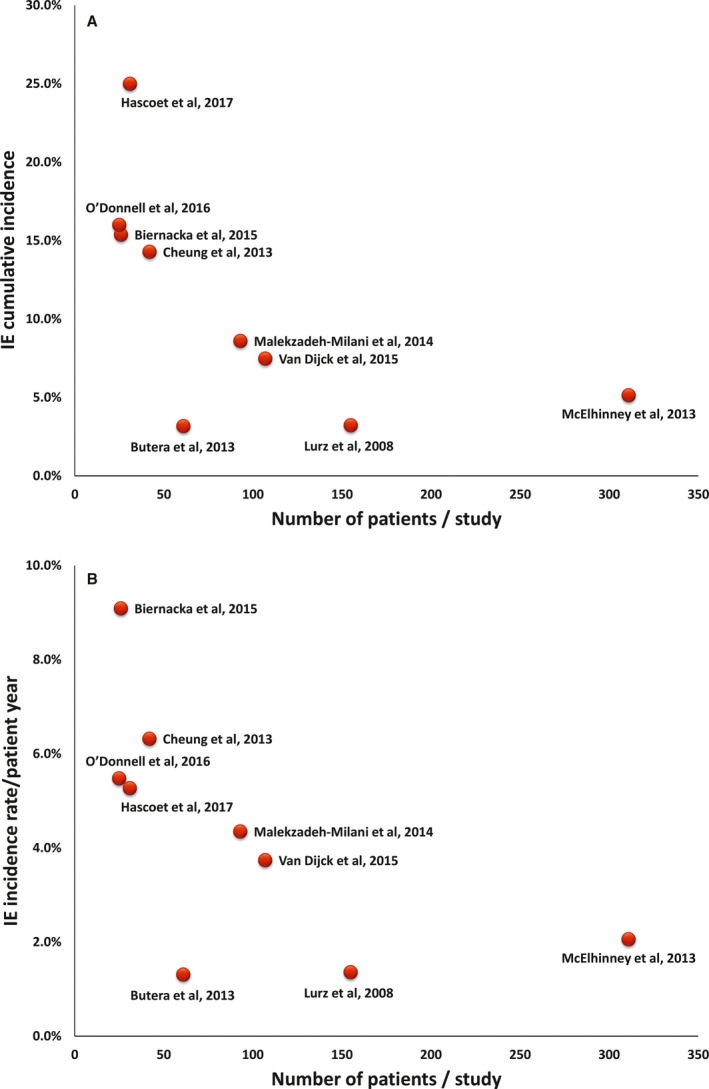
Scatter plots of the cumulative (A) and annualized (B) incidence rate of infective endocarditis (IE) plotted against the sample size (number of patients) of the individual studies14, 15, 16, 17, 18, 19, 20, 21, 22 (Spearman correlation coefficient=−0.80 and −0.73, P=0.010 and P=0.025, respectively).
Figure 2.
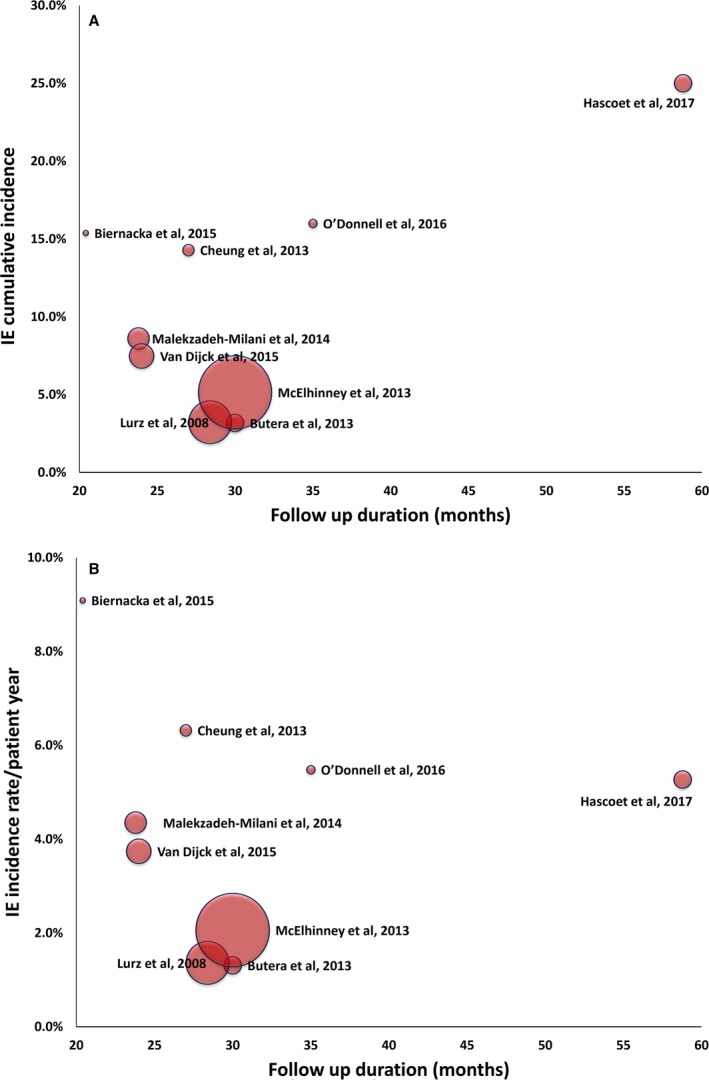
Scatter plots of the cumulative (A) and annualized (B) incidence rate of infective endocarditis (IE) plotted against the follow‐up duration (in months) of the individual studies14, 15, 16, 17, 18, 19, 20, 21, 22 (Spearman correlation coefficient=0.16 and −0.27, P>0.05 for both). Dot size is proportionate to the sample size (number of patients) of each study.
Figure 3.
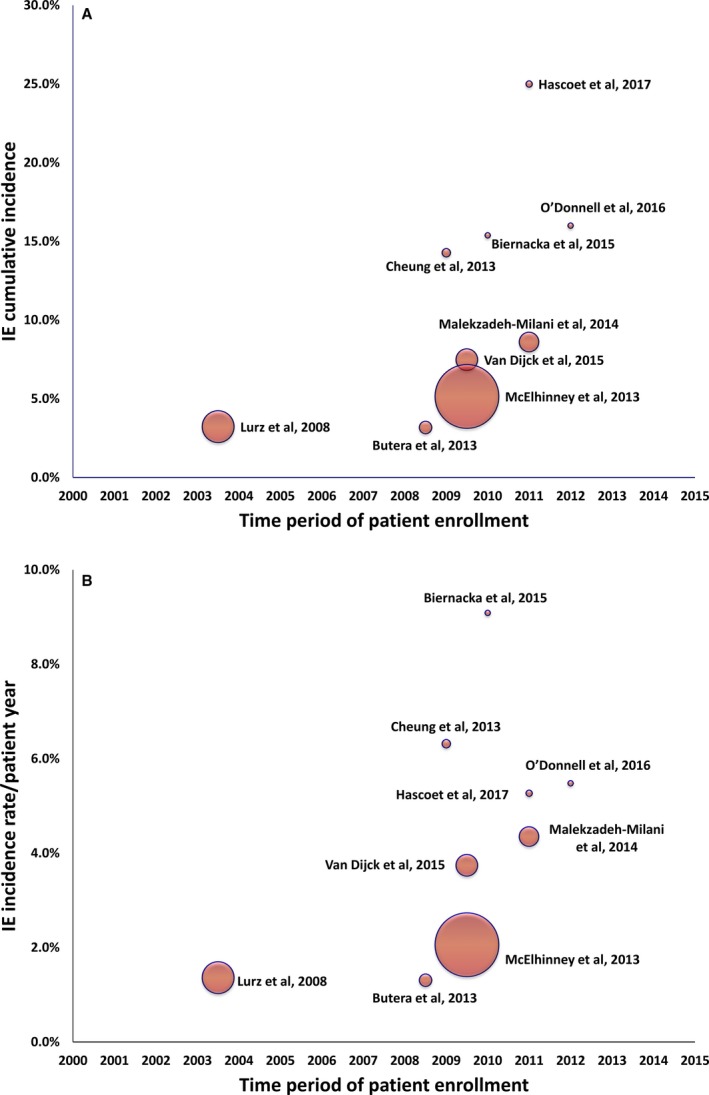
Scatter plots of the cumulative (A) and annualized (B) incidence rate of infective endocarditis (IE) plotted against the time period of patient enrollment of the individual studies14, 15, 16, 17, 18, 19, 20, 21, 22 (Spearman correlation coefficient=0.81 and 0.57, P=0.008 and P=0.11, respectively). Dots correspond to the middle of the enrollment time interval for each study, and dot size is proportionate to the sample size (number of patients) of each study. The characteristics of the 9 studies are summarized in Table 1.
Individual‐Patient Characteristics of Melody Valve Endocarditis Cases
In total, 69 detailed case reports of patients who developed Melody valve IE were identified.17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38 The characteristics of these patients are summarized in Table 2.
Table 2.
Characteristics of Patients With IE After TPVI (n=69)a
| Parameter | Value |
|---|---|
| Baseline characteristics | |
| Male sex | 84% |
| Age, y | 21.7±11.2 |
| Underlying initial pathologic feature | |
| TOF | 50% |
| AS (S/P Ross procedure) | 18% |
| TA | 15% |
| TGA | 10% |
| Other | 7% |
| RVOT conduit | |
| Homograft | 55% |
| Bioprosthetic valve/valved conduit | 38% |
| Bare stent/Melody valve | 7% |
| Manifestations of IE | |
| Onset after TPVI, mo | 23.6±19.7 |
| Fever | 89% |
| Vegetation | 34% |
| New/worsening greater than mild RVOT obstruction | 79% |
| PG increase in the setting of TPVI, mm Hg | 16.9±8.4 |
| New/worsening greater than mild PR | 6% |
| Blood culture | |
| Staphylococci | 42.0% |
| Streptococci | 30.4% |
| Corynebacterium groupb | 5.8% |
| HACEK group | 4.3% |
| Haemophilus groupc | 2.9% |
| Rothia dentocariosa | 1.4% |
| Aerococcus viridans | 1.4% |
| Escherichia coli | 1.4% |
| Enterococcus foecalis | 1.4% |
| Aspergillus fumigatus | 1.4% |
| Negative cultured | 7.2% |
Data are given as mean±SD unless otherwise indicated. AS indicates aortic stenosis; IE, infective endocarditis; HACEK, Haemophilus parainfluenzae, H. aphrophilus, H. paraphrophilus, H. influenzae, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae, and K. denitrificans; PG, pressure gradient; PR, pulmonary regurgitation; RVOT, right ventricular outflow tract; S/P, status post; TA, truncus arteriosus; TGA, transposition of the great arteries; TOF, tetralogy of Fallot; TPVI, transcatheter pulmonary valve implantation.
From 22 reports published between 2011 and 2017.17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38
Corynebacterium pseudodiphtheriticum in 3 cases and Corynebacterium striatum in 1 case.
Haemophilus influenzae in 1 case and Haemophilus parainfluenzae in another case.
In 3 cases, serologic findings and polymerase chain reaction of excised valves revealed Bartonella henselae.
Patients had a mean age of 22±11 (range, 4–56) years, and they were predominantly men (84%). Most had a pulmonary homograft (55%) or a bioprosthetic valve/valved conduit (38%) implanted in the setting of correction of tetralogy of Fallot (50%), aortic stenosis treated by a Ross procedure (18%), truncus arteriosus (15%), or transposition of the great arteries (10%).
Interval After TPVI
The median time from TPVI to onset of IE was 18.0 (interquartile range, 9.0–30.4; range, 1.0–72.0) months. As shown in Figure 4, 32% of cases occurred within 1 year after TPVI, 27% occurred in the second year, 18% occurred in the third year, and 23% occurred beyond 3 years after the procedure.
Figure 4.
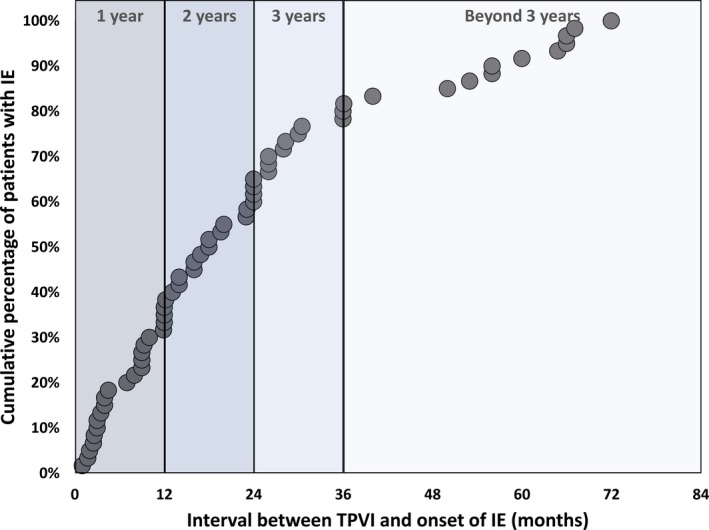
A cumulative curve of the individual patients with Melody valve endocarditis displaying (on the horizontal axis) the time interval between transcatheter pulmonary valve implantation (TPVI) and the onset of infective endocarditis (IE). The figure represents data from 59 (of 69) patients in whom the exact time interval from TPVI to IE was reported.
Precipitating Factors
An identifiable portal for IE was reported in 25 cases. An unprotected dental procedure/orthodontic/oral trauma was reported in 9 of the 25 cases (36%). Other portals included the following: an infected wound (n=3), cat scratches and bites/veterinary medical practice (n=2), paranasal sinusitis (2), cardiac catheterization (n=2), gastroenteritis (n=1), pneumonia (n=1), cystitis (n=1), dermatophytosis complex (n=1), nail biting and bad hygiene (n=1), hemodialysis (n=1), and tattooing (n=1).
Clinical Presentation
Fever was the most common presenting symptom (89%), whereas vegetations were visualized by echocardiography in only 34%. New, significant, and/or progressive RVOT obstruction occurred in 79% of cases, and the pressure gradient increase averaged 17±8 mm Hg. New significant pulmonary regurgitation was documented in 2 cases (Table 2). Other presentations of IE included right ventricular failure (n=5), severe sepsis (n=5), septic pulmonary embolism (n=5), gastrointestinal tract symptoms (n=4), glomerulonephritis (n=1), and macrophage activation syndrome (n=1).
Microbiologic Features
As shown in Table 2, most cases involved Gram‐positive cocci (42% staphylococci and 30% streptococci). This microbiologic profile was similar between early (47% staphylococci and 32% streptococci) and late (46% staphylococci and 27% streptococci; P>0.05) IE. Bacterial culture was negative in 5 cases, of which serologic features and/or polymerase chain reaction of excised valve revealed Bartonella henselae in 3.
Treatment and Outcome
Six patients died (8.7%) in the course of IE, and 11 patients required urgent intervention (percutaneous and/or surgical) to relieve critical RVOT obstruction or to remove the infected valve to control septicemia. Furthermore, 24 patients required intervention (mostly to relieve RVOT obstruction), either during the same hospital admission (n=10) or electively thereafter at a median of 4 months after IE onset (n=14). Overall, 39 reinterventions (31 surgical replacement, 3 percutaneous stenting, 3 balloon dilatation, and 2 Melody‐in‐Melody implantation) were performed, whereas antibiotic therapy (for 6.2±1.3 weeks) was sufficient for clinical stabilization without the need for any intervention in 23 patients (34%). Clinical outcome was not reported in 1 patient, and the mode of treatment was not specified in 5 patients.
In total, 59.7% of the patients either died (n=6) or required reintervention (n=35). In 1 of these patients, reintervention was followed by death. Death or reintervention was more common in patients with new, significant, and/or progressive RVOT obstruction (69% versus 33%; P=0.042) and was lower (P=0.015) in streptococcal IE (30%) than in staphylococcal (72%) and Gram‐negative bacterial (73%) IE. On multivariate regression analysis, nonstreptococcal IE was an independent predictor of death/reintervention (odds ratio, 4.28; 95% CI, 1.16–15.78; P=0.029).
Discussion
Clinical trials have shown the safety and efficacy of TPVI, with excellent short‐term outcomes2 and freedom from reintervention in most patients up to 5 years.3 However, the risk of IE of the Melody valve is increasingly recognized as a significant threat to the long‐term valve function.
Although IE is associated with high mortality and severe complications,12 its management is mostly based on expert opinion rather than on evidence.12 Shortage of evidence is basically because of the low incidence of the disease and the scarcity of randomized trials and meta‐analyses.12 Original studies are, definitely, more robust than inferences from reviews/meta‐analyses but only when original studies are adequately powered. Given the fact that IE is a relatively uncommon disease, frequently occurring long after the index procedure, and is challenging to diagnose in many instances, data from individual registries are often inadequate to derive evidence on the natural history and the management of IE. Because more and more reports from small single‐center registries are reporting an extraordinarily high IE risk with the Melody valve, we conducted this systematic review. Although desirable in the field of congenital heart diseases, in which studies are usually small scale, meta‐analysis of observational studies with differences in baseline characteristics and anatomic substrates, procedural characteristics, and follow‐up durations might introduce imprecision into the results, even when proper statistical methods are applied. Therefore, we opted to pool patient‐level data, whereas study‐level data were systematically reviewed without pooling.
Risk of IE After Melody Valve Implantation
The data provided in the present review highlight that only large, well‐designed, prospective studies will be able to assess the actual rate and the potential risk factors for IE after TPVI.
The phenomenon of reporting higher rates of IE in the later than the earlier studies (Figure 3) can be in part because of the increasing awareness of the risk of IE that led to a more careful surveillance and is in line with a global trend toward an increased incidence of IE.39 We found that early postimplantation Melody valve endocarditis is uncommon, that only one third (32%) of cases occur within the first year after TPVI, and that the risk persists thereafter, with 23% of cases developing beyond 3 years after the procedure. This comes in agreement with the findings of a large contemporary study of patients with adult congenital heart disease (n=14 224 patients),40 in whom the presence of valve‐containing prosthetics was independently associated with greater risk of IE that persists in the long‐term (hazard ratio of IE beyond 12 months after prosthesis implantation, 5.3; 95% CI, 3.5–7.9). The authors suggested that this reflects the fact that the risk is likely not attributable only to surgical factors associated with implantation but also to the mere long‐term presence of those prostheses.40
Patient‐ Versus Device‐Related Vulnerability to IE
We found that in many patients with Melody valve IE, an identifiable portal of bacteria was present and most frequently involved an unprotected dental procedure/orthodontic/oral trauma. This highlights the importance of pre‐TPVI counseling (including a meticulous dental review) and continuous education of patients and families to lower the exposure to portals of infection, as well as the necessity of routine antibiotic prophylaxis before any invasive maneuver. Notwithstanding, patients with Melody valve implantation combine 2 conditions predisposing to a higher rate of IE and a higher risk of adverse outcome from endocarditis: the use of a prosthetic cardiac valve in the setting of a repaired complex congenital heart disease and an unfavorable hemodynamic environment in the vicinity of that prosthesis (which could interfere with complete prosthesis endothelialization).41 The most recent US and European guidelines on the management of valvular heart diseases recommend antibiotic prophylaxis before dental procedures in patients with prosthetic cardiac valves, including transcatheter‐implanted prostheses.42, 43 Although some clinical guidelines, on the other hand, advise against routine antibiotic prophylaxis against IE even in patients with a high risk of acquiring IE and of having a worse outcome of IE, there have been some signals indicting this conservative antibiotic prophylaxis approach for a trend of IE rates to increase.39, 44 Our data give an example of a condition and a patient population with an exceptionally significant rate of IE, with many of the episodes being linked to dental procedures.
In addition to patient‐related risk factors (eg, older age,26 male sex,26 history of IE,17 discontinuation of antiplatelets,29 and exposure to unprotected medical procedure29) and procedure‐related risk factors (eg, longer procedure,26 higher number of stents,26 and higher residual RVOT gradient14), some inherent device characteristics have been indicated by histopathologic studies as risk factors predisposing to IE. A selective propensity of bovine jugular tissue (the precursor of the Melody valve leaflets) to histological lesions during procedural steps45 and to bacterial adherence46 was shown in in‐vitro studies, especially after procedural manipulation‐induced leaflet damage.46 These findings are in line with the observed higher propensity of valves with bovine jugular vein leaflets (Melody valve and Contegra graft) to IE than pulmonary homografts.18, 19, 47 The risk of IE after TPVI with the Sapien valve (Edwards Lifesciences, Irvine, CA), which has bovine pericardial tissue leaflets, albeit still present,48 seems to be lower than with the Melody valve.20, 22, 49 However, the number of implanted Sapien valves in the pulmonary position and the duration of follow‐up are far lower than those of the Melody valve, and this might lead to underestimating the incidence of IE in Sapien valves.
In another study of explanted infected Melody valves,50 pathologic examination revealed the presence of granulocytes in the preexisting surgical conduit in all cases, denoting that the space between the Melody valve stent and the underlying conduit with little neovascularization might represent a blind spot that cannot be reached by antibiotics. This blind spot seems to harbor the microorganism leading to failure of antibiotics to eradicate the infection. This is further supported by the association between a higher number of stents in the RVOT and the risk of Melody valve IE reported in 1 study.25
In the same study,50 a thrombotic material was found at the basis of the leaflets or at the graft wall in most cases. Turbulent blood flow in the RVOT might lead to endothelial damage predisposing to nonbacterial thrombotic endocarditis that can turn infective after any transient bacteremia.5 These findings further support the association between discontinuation of antiplatelets and the development of endocarditis reported in 1 study.28 Data from transcatheter aortic valve replacement (TAVR) studies refer to a significant risk of clinical/subclinical leaflet thrombosis that can be prevented and effectively treated by oral anticoagulation.51, 52 It is, however, unknown whether the same phenomenon is relevant to TPVI. Notwithstanding, more rigorous use of multislice computed tomography (MSCT) in post‐TPVI surveillance (especially in cases with increasing RVOT pressure) might provide more insights into the actual incidence of hypoattenuated leaflet thickening after TPVI and its clinical significance.
Risk of IE After Transcatheter Versus Surgical Pulmonary Valve Replacement
In a large series (n=586 patients) reported by Mery et al,47 including a total of 792 valved pulmonary conduits, 23 conduits (2.9%) developed endocarditis at a median of 5 years (range, 19 days to 11 years) after surgery. Bovine jugular grafts were associated with a significantly greater risk of late endocarditis (hazard ratio, 9.05; 95% CI, 2.6–31.8 compared with homografts).47 None of the patients with endocarditis died of related causes, and 16 (70%) of infected conduits were surgically replaced. In another study by Van Dijck et al,19 Melody valves, homografts, and Contegra grafts were compared for the incidence of IE. The annualized rate of IE was >3‐fold higher after Melody valve implantation (3.0%; 95% CI, 1.3%–5.8% per patient‐year) than after homograft implantation (0.8%; 95% CI, 0.4%–1.3% per patient‐year), whereas it was 2.4% (95% CI, 1.0%–3.8%) per patient‐year in the Contegra group. In a recent meta‐analysis of 50 studies involving 7063 patients, bovine jugular vein valves, independent of implantation technique, were associated with a higher cumulative risk of IE compared with other types of right ventricle‐to‐pulmonary artery conduits (median cumulative incidence, 5.4% versus 1.2%; P<0.0001). There was no difference in the incidence of endocarditis between catheter‐based and surgically implanted bovine valves, suggesting that the substrate for infection is related to the tissue precursor of the valve.8 However, this meta‐analysis is limited by reporting the incidence of endocarditis, which is a time‐dependent event, in terms of a crude cumulative incidence.53
Risk of IE After Transcatheter Pulmonary Valve Replacement Versus TAVR
In a large study by Regueiro et al54 that included 20 006 patients with TAVR, 250 had definite IE (incidence, 1.1%; 95% CI, 1.1%–1.4% per patient‐year) at a median interval of 5.3 months (interquartile range, 1.5–13.4 months) after the procedure, and IE was early in 178 patients (71.2%). In‐hospital mortality and surgical conversion rates were 36% and 15%, respectively. In a pooled analysis of multiple studies, IE developed at 241±287 days after TAVR, 28% of patients required a surgical intervention, and 30‐day mortality was 22%.55 These studies, and others,56, 57, 58 highlight that IE seems to be remarkably less common and to occur earlier after TAVR than after transcatheter pulmonary valve replacement.
Microbiologic Features and Outcomes of Melody Valve IE
We found that post‐TPVI endocarditis involves staphylococci (42%) or streptococci (30%) in most cases. This comes in line with the overall microbiologic profile of IE in adults (staphylococci, 42%; and streptococci, 29%)59 and in pediatrics (staphylococci, 43%; and streptococci, 40%).60 Similar patterns were also reported in prosthetic valve endocarditis (PVE; staphylococci, 40%; and streptococci, 22%)59 and in pulmonary surgical conduit IE (staphylococci, 53%; and streptococci, 43%).47 Accordingly, it can be suggested that initial empirical antibiotic therapy in cases with suspected Melody valve IE should follow a similar protocol to that of PVE, which specifically targets the virulent Staphylococcus aureus.12 This microorganism was shown in the present study to be the single most common organism involved in Melody valve IE and to be associated with a worse outcome than streptococcal infection.
We found that Melody valve IE leads to reintervention in 52% of cases and death in 8.7% of cases, compared with a 65% to 70% rate of surgical reintervention35, 47 and a mortality rate of ≈13%16, 35 in the setting of pulmonary surgical conduit IE. This comparison emphasizes that although the rate of IE is generally lower in pulmonary surgical conduits than after TPVI, IE‐related morbidity and mortality risks might be higher.
We found that streptococcal infection and the lack of RVOT obstruction are associated with a significantly better outcome. A similar association between streptococcal involvement and the freedom from in‐hospital death was previously reported in adults with IE.59 It turns out that these 2 criteria (the type of the organism and the presence/absence of RVOT obstruction) can be used for risk stratification of patients presenting with Melody valve IE.
Diagnostic Criteria
In the initial phase of TPVI experience, reobstruction of the RVOT with subsequent reintervention was relatively common, and it was mostly attributable to stent fracture.3 In the more contemporary TPVI experience, with the adoption of routine prestenting, reintervention attributable to recurrent valve obstruction became uncommon.4, 24, 61, 62 In the present review, a strong association between IE and Melody valve obstructive dysfunction was obviated. The direction of causality of this relationship (IE leading to valve obstruction versus valve dysfunction predisposing to IE) is not easy to conclude on. Notwithstanding, in some reports,22, 30, 31, 36 in‐hospital serial Doppler studies have documented a progressive pressure gradient increase during the course of IE. RVOT obstruction was the reason for most reinterventions, and whether pressure increase can be, in part, attributable to leaflet thrombosis and can be resolved by anticoagulation treatment without reintervention is still an open question.
Although considered by Duke criteria as a sign of valve involvement in IE, new valve regurgitation was reported in only 2 of 69 cases. Histopathologic studies have confirmed the same finding,50 as have other reports,14, 19 concluding that insufficiency is a rare manifestation of Melody valve IE.
It is well acknowledged that echocardiography, especially transthoracic echocardiography (TTE), affords only modest sensitivity (50%) for the detection of vegetations in PVE.12 In the setting of IE after Melody valve implantation, echocardiographic detection of vegetations is limited to only one third of published case reports, likely because of the anterior position of the RVOT and artefacts from the prosthetic valve stent and the underlying, mostly degenerated calcified, conduit. Some relevant points of clinical importance are worth mentioning: First, failure to visualize vegetations using cardiac imaging does not exclude their presence, because they can still be visualized intraoperatively18, 22 or on autopsy27 in cases with initially negative imaging studies. Second, unlike classic left‐sided PVE, where transesophageal echocardiography (TEE) is superior to TTE in detecting vegetations, TEE does not always have an added value to TTE results in transcatheter and surgical prosthetic pulmonary valve endocarditis.21, 23, 33, 35, 38 Diagnostic accuracy of TEE can be improved using biplane imaging63 and by using dedicated acquisition views64 (eg, high esophageal interrogation of the distal valve stent). A systematic combination of TTE and TEE35 was suggested to improve diagnostic accuracy, compared with either modality used alone. In some studies,21, 23, 38 intracardiac echocardiography was successful in detecting vegetations missed by TTE/TEE. Third, other imaging tools (eg, MSCT29 and PET scan28) can detect vegetations missed by echocardiography.
The combination of MSCT with PET has been shown to have an added value over TTE/TEE in confirming PVE,65, 66 especially in patients with negative or doubtful echocardiographic results.67 On the basis of these data, the latest guidelines on the management of IE12 considered an abnormal activity around the site of the prosthetic valve detected on PET/MSCT as a “major criterion” of IE. There are few, but rather encouraging, reports on the success of PET/MSCT to provide a good alternative to echocardiography in IE after Melody valve implantation.28, 29
It turns out that the classic approach recommended by the practice guidelines12 to confirm valvular involvement in the setting of PVE (ie, detection of vegetation[s] and/or [para‐] prosthetic regurgitation by TTE/TEE) is inefficient in a large proportion of patients with Melody valve IE. This observation calls for a modified diagnostic approach in these cases, possibly including progressive RVOT obstruction and abnormal activity on MSCT/PET as important additional diagnostic criteria.
Finally, given the aforementioned diagnostic challenges, pathologic examination of tissue samples that are excised during surgery should be routinely applied. This histological examination of resected valvular tissue is considered by the guidelines12 as “the gold standard” for the diagnosis of IE.
Limitations
This review, although intended to be comprehensive, still bears the limitations inherent to a retrospective review of published data (eg, selection bias of patients with a relatively better outcome). In general, comparisons between patients with versus without IE were either missing or, when present, significantly heterogeneous and seriously limited by the small number in either/both groups, precluding appropriateness for pooled analysis.
Conclusions and Clinical Implications
The risk of IE after implantation of a Melody valve is significant, at least over the first 3 years after TPVI, with few cases occurring in the early postprocedural period. However, the reported incidence varies considerably between the studies. Diagnosis is challenging, especially in terms of the documentation of valvular involvement in the infective process. The classic modified Duke criteria, which heavily rely on echocardiographic signs, show a modest diagnostic yield in post‐TPVI endocarditis. Approximately 52% of patients require reintervention, either surgically or percutaneously, with the infection being controllable with antibiotics in some cases, especially when streptococci are involved and the RVOT is not obstructed.
Disclosures
None.
Supporting information
Data S1. PubMed search terms.
Figure S1. The PRISMA flow chart of the literature search.
Acknowledgments
We thank Brent C. Opmeer, PhD, and Jacqueline Limpens, PhD, for valued statistical and librarian support.
(J Am Heart Assoc. 2018;7:e008163 DOI: 10.1161/JAHA.117.008163.)
References
- 1. Bonhoeffer P, Boudjemline Y, Saliba Z, Merckx J, Aggoun Y, Bonnet D, Acar P, Le Bidois J, Sidi D, Kachaner J. Percutaneous replacement of pulmonary valve in a right‐ventricle to pulmonary‐artery prosthetic conduit with valve dysfunction. Lancet. 2000;356:1403–1405. [DOI] [PubMed] [Google Scholar]
- 2. Zahn EM, Hellenbrand WE, Lock JE, McElhinney DB. Implantation of the melody transcatheter pulmonary valve in patients with a dysfunctional right ventricular outflow tract conduit early results from the U.S. clinical trial. J Am Coll Cardiol. 2009;54:1722–1729. [DOI] [PubMed] [Google Scholar]
- 3. Cheatham JP, Hellenbrand WE, Zahn EM, Jones TK, Berman DP, Vincent JA, McElhinney DB. Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015;131:1960–1970. [DOI] [PubMed] [Google Scholar]
- 4. Chatterjee A, Bajaj NS, McMahon WS, Cribbs MG, White JS, Mukherjee A, Law MA. Transcatheter pulmonary valve implantation: a comprehensive systematic review and meta‐analyses of observational studies. J Am Heart Assoc. 2017;6:e006432 DOI: 10.1161/JAHA.117.006432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Uebing A, Rigby ML. The problem of infective endocarditis after transcatheter pulmonary valve implantation. Heart. 2015;101:749–751. [DOI] [PubMed] [Google Scholar]
- 6. Boshoff DE, Cools BL, Heying R, Troost E, Kefer J, Budts W, Gewillig M. Off‐label use of percutaneous pulmonary valved stents in the right ventricular outflow tract: time to rewrite the label? Catheter Cardiovasc Interv. 2013;81:987–995. [DOI] [PubMed] [Google Scholar]
- 7. Nordmeyer J, Coats L, Lurz P, Lee TY, Derrick G, Rees P, Cullen S, Taylor AM, Khambadkone S, Bonhoeffer P. Percutaneous pulmonary valve‐in‐valve implantation: a successful treatment concept for early device failure. Eur Heart J. 2008;29:810–815. [DOI] [PubMed] [Google Scholar]
- 8. Sharma A, Cote AT, Hosking MCK, Harris KC. A systematic review of infective endocarditis in patients with bovine jugular vein valves compared with other valve types. JACC Cardiovasc Interv. 2017;10:1449–1458. [DOI] [PubMed] [Google Scholar]
- 9. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. BMJ. 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings: Duke Endocarditis Service. Am J Med. 1994;96:200–209. [DOI] [PubMed] [Google Scholar]
- 11. Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG Jr, Ryan T, Bashore T, Corey GR. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis. 2000;30:633–638. [DOI] [PubMed] [Google Scholar]
- 12. Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del Zotti F, Dulgheru R, El Khoury G, Erba PA, Iung B, Miro JM, Mulder BJ, Plonska‐Gosciniak E, Price S, Roos‐Hesselink J, Snygg‐Martin U, Thuny F, Tornos Mas P, Vilacosta I, Zamorano JL, Erol C, Nihoyannopoulos P, Aboyans V, Agewall S, Athanassopoulos G, Aytekin S, Benzer W, Bueno H, Broekhuizen L, Carerj S, Cosyns B, De Backer J, De Bonis M, Dimopoulos K, Donal E, Drexel H, Flachskampf FA, Hall R, Halvorsen S, Hoen B, Kirchhof P, Lainscak M, Leite‐Moreira AF, Lip GY, Mestres CA, Piepoli MF, Punjabi PP, Rapezzi C, Rosenhek R, Siebens K, Tamargo J, Walker DM. 2015 ESC guidelines for the management of infective endocarditis: the Task Force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio‐Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur Heart J. 2015;36:3075–3128. [DOI] [PubMed] [Google Scholar]
- 13. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta‐analysis of observational studies in epidemiology: a proposal for reporting: Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. [DOI] [PubMed] [Google Scholar]
- 14. McElhinney DB, Benson LN, Eicken A, Kreutzer J, Padera RF, Zahn EM. Infective endocarditis after transcatheter pulmonary valve replacement using the Melody valve: combined results of 3 prospective North American and European studies. Circ Cardiovasc Interv. 2013;6:292–300. [DOI] [PubMed] [Google Scholar]
- 15. Lurz P, Coats L, Khambadkone S, Nordmeyer J, Boudjemline Y, Schievano S, Muthurangu V, Lee TY, Parenzan G, Derrick G, Cullen S, Walker F, Tsang V, Deanfield J, Taylor AM, Bonhoeffer P. Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008;117:1964–1972. [DOI] [PubMed] [Google Scholar]
- 16. Malekzadeh‐Milani S, Ladouceur M, Iserin L, Bonnet D, Boudjemline Y. Incidence and outcomes of right‐sided endocarditis in patients with congenital heart disease after surgical or transcatheter pulmonary valve implantation. J Thorac Cardiovasc Surg. 2014;148:2253–2259. [DOI] [PubMed] [Google Scholar]
- 17. Butera G, Milanesi O, Spadoni I, Piazza L, Donti A, Ricci C, Agnoletti G, Pangrazi A, Chessa M, Carminati M. Melody transcatheter pulmonary valve implantation: results from the registry of the Italian Society of Pediatric Cardiology. Catheter Cardiovasc Interv. 2013;81:310–316. [DOI] [PubMed] [Google Scholar]
- 18. O'Donnell C, Holloway R, Tilton E, Stirling J, Finucane K, Wilson N. Infective endocarditis following Melody valve implantation: comparison with a surgical cohort. Cardiol Young. 2016;27:1–8. [DOI] [PubMed] [Google Scholar]
- 19. Van Dijck I, Budts W, Cools B, Eyskens B, Boshoff DE, Heying R, Frerich S, Vanagt WY, Troost E, Gewillig M. Infective endocarditis of a transcatheter pulmonary valve in comparison with surgical implants. Heart. 2015;101:788–793. [DOI] [PubMed] [Google Scholar]
- 20. Biernacka EK, Ruzyllo W, Demkow M, Kowalski M, Spiewak M, Piotrowski W, Kusmierczyk M, Banas S, Rozanski J, Hoffman P. Transcatheter pulmonary valve implantation in patients with right ventricular outflow tract dysfunction: early and mid‐term results. J Invasive Cardiol. 2015;27:E82–E89. [PubMed] [Google Scholar]
- 21. Cheung G, Vejlstrup N, Ihlemann N, Arnous S, Franzen O, Bundgaard H, Sondergaard L. Infective endocarditis following percutaneous pulmonary valve replacement: diagnostic challenges and application of intra‐cardiac echocardiography. Int J Cardiol. 2013;169:425–429. [DOI] [PubMed] [Google Scholar]
- 22. Hascoet S, Mauri L, Claude C, Fournier E, Lourtet J, Riou JY, Brenot P, Petit J. Infective endocarditis risk after percutaneous pulmonary valve implantation with the melody and sapien valves. JACC Cardiovasc Interv. 2017;10:510–517. [DOI] [PubMed] [Google Scholar]
- 23. Georgievskaya Z, Nowalk AJ, Randhawa P, Picarsic J. Bartonella henselae endocarditis and glomerulonephritis with dominant C3 deposition in a 21‐year‐old male with a Melody transcatheter pulmonary valve: case report and review of the literature. Pediatr Dev Pathol. 2014;17:312–320. [DOI] [PubMed] [Google Scholar]
- 24. Fraisse A, Aldebert P, Malekzadeh‐Milani S, Thambo JB, Piechaud JF, Aucoururier P, Chatelier G, Bonnet D, Iserin L, Bonello B, Assaidi A, Kammache I, Boudjemline Y. Melody (R) transcatheter pulmonary valve implantation: results from a French registry. Arch Cardiovasc Dis. 2014;107:607–614. [DOI] [PubMed] [Google Scholar]
- 25. Buber J, Bergersen L, Lock JE, Gauvreau K, Esch JJ, Landzberg MJ, Valente AM, Sandora TJ, Marshall AC. Bloodstream infections occurring in patients with percutaneously implanted bioprosthetic pulmonary valve: a single‐center experience. Circ Cardiovasc Interv. 2013;6:301–310. [DOI] [PubMed] [Google Scholar]
- 26. Villafane J, Baker GH, Austin EH III, Miller S, Peng L, Beekman R III. Melody pulmonary valve bacterial endocarditis: experience in four pediatric patients and a review of the literature. Catheter Cardiovasc Interv. 2014;84:212–218. [DOI] [PubMed] [Google Scholar]
- 27. Patel M, Iserin L, Bonnet D, Boudjemline Y. Atypical malignant late infective endocarditis of Melody valve. J Thorac Cardiovasc Surg. 2012;143:e32–e35. [DOI] [PubMed] [Google Scholar]
- 28. Malekzadeh‐Milani S, Ladouceur M, Patel M, Boughenou FM, Iserin L, Bonnet D, Boudjemline Y. Incidence and predictors of Melody(R) valve endocarditis: a prospective study. Arch Cardiovasc Dis. 2015;108:97–106. [DOI] [PubMed] [Google Scholar]
- 29. Alsoufi B, Al‐joufan M, Al‐Omrani A, Bulbul Z. Obstruction of a percutaneous pulmonary valve by an Aspergillus mycotic thrombus mimicking massive pulmonary embolus. Ann Thorac Surg. 2012;94:e5–e6. [DOI] [PubMed] [Google Scholar]
- 30. Bhat DP, Forbes TJ, Aggarwal S. A case of life‐threatening Staphylococcus aureus endocarditis involving percutaneous transcatheter prosthetic pulmonary valve. Congenit Heart Dis. 2013;8:E161–E164. [DOI] [PubMed] [Google Scholar]
- 31. Johnson JN, Miller SG, Lodge AJ. Corynebacterium endocarditis of a percutaneously placed transcatheter pulmonary valve. Cardiol Young. 2014;24:932–934. [DOI] [PubMed] [Google Scholar]
- 32. Sosa T, Goldstein B, Cnota J, Bryant R, Frenck R, Washam M, Madsen N. Melody valve Bartonella henselae endocarditis in an afebrile teen: a case report. Pediatrics. 2016;137:e20151548. [DOI] [PubMed] [Google Scholar]
- 33. Yedidya I, Stein GY, Vaturi M, Blieden L, Bernstine H, Pitlik SD, Fuchs S. Positron emission tomography/computed tomography for the diagnosis of endocarditis in patients with pulmonic stented valve/pulmonic stent. Ann Thorac Surg. 2011;91:287–289. [DOI] [PubMed] [Google Scholar]
- 34. Chaudhry‐Waterman N, Bergersen L, Buber J. Bacterial endocarditis manifesting as outflow tract obstruction in two patients implanted with percutaneous prosthetic pulmonary valves. Can J Cardiol. 2015;31:1204.e1–3. [DOI] [PubMed] [Google Scholar]
- 35. Miranda WR, Connolly HM, Bonnichsen CR, DeSimone DC, Dearani JA, Maleszewski JJ, Greason KL, Wilson WR, Baddour LM. Prosthetic pulmonary valve and pulmonary conduit endocarditis: clinical, microbiological and echocardiographic features in adults. Eur Heart J Cardiovasc Imaging. 2016;17:936–943. [DOI] [PubMed] [Google Scholar]
- 36. Ramakrishnan KV, Olivieri L, Jonas RA. Acute endocarditis of a percutaneously placed pulmonary valve. Ann Pediatr Cardiol. 2015;8:225–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Atamanyuk I, Raja SG, Kostolny M. Bartonella henselae endocarditis of percutaneously implanted pulmonary valve. J Heart Valve Dis. 2012;21:682–685. [PubMed] [Google Scholar]
- 38. Bouajila S, Chalard A, Dauphin C. Usefulness of intracardiac echocardiography for the diagnosis of infective endocarditis following percutaneous pulmonary valve replacement. Cardiol Young. 2017;27:1406–1409. [DOI] [PubMed] [Google Scholar]
- 39. Pant S, Patel NJ, Deshmukh A, Golwala H, Patel N, Badheka A, Hirsch GA, Mehta JL. Trends in infective endocarditis incidence, microbiology, and valve replacement in the United States from 2000 to 2011. J Am Coll Cardiol. 2015;65:2070–2076. [DOI] [PubMed] [Google Scholar]
- 40. Kuijpers JM, Koolbergen DR, Groenink M, Peels KC, Reichert CL, Post MC, Bosker HA, Wajon EM, Zwinderman AH, Mulder BJ, Bouma BJ. Incidence, risk factors, and predictors of infective endocarditis in adult congenital heart disease: focus on the use of prosthetic material. Eur Heart J. 2017;38:2048–2056. [DOI] [PubMed] [Google Scholar]
- 41. Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, Bolger A, Cabell CH, Takahashi M, Baltimore RS, Newburger JW, Strom BL, Tani LY, Gerber M, Bonow RO, Pallasch T, Shulman ST, Rowley AH, Burns JC, Ferrieri P, Gardner T, Goff D, Durack DT. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116:1736–1754. [DOI] [PubMed] [Google Scholar]
- 42. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Fleisher LA, Jneid H, Mack MJ, McLeod CJ, O'Gara PT, Rigolin VH, Sundt TM III, Thompson A. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135:e1159–e1195. [DOI] [PubMed] [Google Scholar]
- 43. Baumgartner H, Falk V, Bax JJ, De Bonis M, Hamm C, Holm PJ, Iung B, Lancellotti P, Lansac E, Rodriguez Munoz D, Rosenhek R, Sjogren J, Tornos Mas P, Vahanian A, Walther T, Wendler O, Windecker S, Zamorano JL. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J. 2017;38:2739–2791. [DOI] [PubMed] [Google Scholar]
- 44. Dayer MJ, Jones S, Prendergast B, Baddour LM, Lockhart PB, Thornhill MH. Incidence of infective endocarditis in England, 2000–13: a secular trend, interrupted time‐series analysis. Lancet. 2015;385:1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jalal Z, Galmiche L, Beloin C, Boudjemline Y. Impact of percutaneous pulmonary valve implantation procedural steps on leaflets histology and mechanical behaviour: an in vitro study. Arch Cardiovasc Dis. 2016;109:465–475. [DOI] [PubMed] [Google Scholar]
- 46. Jalal Z, Galmiche L, Lebeaux D, Villemain O, Brugada G, Patel M, Ghigo JM, Beloin C, Boudjemline Y. Selective propensity of bovine jugular vein material to bacterial adhesions: an in‐vitro study. Int J Cardiol. 2015;198:201–205. [DOI] [PubMed] [Google Scholar]
- 47. Mery CM, Guzman‐Pruneda FA, De Leon LE, Zhang W, Terwelp MD, Bocchini CE, Adachi I, Heinle JS, McKenzie ED, Fraser CD Jr. Risk factors for development of endocarditis and reintervention in patients undergoing right ventricle to pulmonary artery valved conduit placement. J Thorac Cardiovasc Surg. 2016;151:432–439, 441.e1–2. [DOI] [PubMed] [Google Scholar]
- 48. Vollroth M, Daehnert I, Kostelka M, Wagner R. First case of blood‐culture proven Staphylococcus aureus endocarditis of a Sapien(R) XT valve after percutaneous pulmonary valve implantation. Eur J Cardiothorac Surg. 2015;48:e124–e125. [DOI] [PubMed] [Google Scholar]
- 49. Wilson WM, Benson LN, Osten MD, Shah A, Horlick EM. Transcatheter pulmonary valve replacement with the Edwards Sapien system: the Toronto experience. JACC Cardiovasc Interv. 2015;8:1819–1827. [DOI] [PubMed] [Google Scholar]
- 50. Schneider H, Vogt M, Boekenkamp R, Hoerer J, Eicken A, Foth R, Kriebel T, Paul T, Sigler M. Melody transcatheter valve: histopathology and clinical implications of nine explanted devices. Int J Cardiol. 2015;189:124–131. [DOI] [PubMed] [Google Scholar]
- 51. Makkar RR, Fontana G, Jilaihawi H, Chakravarty T, Kofoed KF, de Backer O, Asch FM, Ruiz CE, Olsen NT, Trento A, Friedman J, Berman D, Cheng W, Kashif M, Jelnin V, Kliger CA, Guo H, Pichard AD, Weissman NJ, Kapadia S, Manasse E, Bhatt DL, Leon MB, Sondergaard L. Possible subclinical leaflet thrombosis in bioprosthetic aortic valves. N Engl J Med. 2015;373:2015–2024. [DOI] [PubMed] [Google Scholar]
- 52. Hansson NC, Grove EL, Andersen HR, Leipsic J, Mathiassen ON, Jensen JM, Jensen KT, Blanke P, Leetmaa T, Tang M, Krusell LR, Klaaborg KE, Christiansen EH, Terp K, Terkelsen CJ, Poulsen SH, Webb J, Botker HE, Norgaard BL. Transcatheter aortic valve thrombosis: incidence, predisposing factors, and clinical implications. J Am Coll Cardiol. 2016;68:2059–2069. [DOI] [PubMed] [Google Scholar]
- 53. Jones TK. The cow deserves a fair trial. JACC Cardiovasc Interv. 2017;10:1459–1461. [DOI] [PubMed] [Google Scholar]
- 54. Regueiro A, Linke A, Latib A, Ihlemann N, Urena M, Walther T, Husser O, Herrmann HC, Nombela‐Franco L, Cheema AN, Le Breton H, Stortecky S, Kapadia S, Bartorelli AL, Sinning JM, Amat‐Santos I, Munoz‐Garcia A, Lerakis S, Gutierrez‐Ibanes E, Abdel‐Wahab M, Tchetche D, Testa L, Eltchaninoff H, Livi U, Castillo JC, Jilaihawi H, Webb JG, Barbanti M, Kodali S, de Brito FS Jr, Ribeiro HB, Miceli A, Fiorina C, Dato GM, Rosato F, Serra V, Masson JB, Wijeysundera HC, Mangione JA, Ferreira MC, Lima VC, Carvalho LA, Abizaid A, Marino MA, Esteves V, Andrea JC, Giannini F, Messika‐Zeitoun D, Himbert D, Kim WK, Pellegrini C, Auffret V, Nietlispach F, Pilgrim T, Durand E, Lisko J, Makkar RR, Lemos PA, Leon MB, Puri R, San Roman A, Vahanian A, Sondergaard L, Mangner N, Rodes‐Cabau J. Association between transcatheter aortic valve replacement and subsequent infective endocarditis and in‐hospital death. JAMA. 2016;316:1083–1092. [DOI] [PubMed] [Google Scholar]
- 55. Loverix L, Juvonen T, Biancari F. Prosthetic endocarditis after transcatheter aortic valve implantation: pooled individual patient outcome. Int J Cardiol. 2015;178:67–68. [DOI] [PubMed] [Google Scholar]
- 56. Puls M, Eiffert H, Hunlich M, Schondube F, Hasenfuss G, Seipelt R, Schillinger W. Prosthetic valve endocarditis after transcatheter aortic valve implantation: the incidence in a single‐centre cohort and reflections on clinical, echocardiographic and prognostic features. EuroIntervention. 2013;8:1407–1418. [DOI] [PubMed] [Google Scholar]
- 57. Makkar RR, Fontana GP, Jilaihawi H, Kapadia S, Pichard AD, Douglas PS, Thourani VH, Babaliaros VC, Webb JG, Herrmann HC, Bavaria JE, Kodali S, Brown DL, Bowers B, Dewey TM, Svensson LG, Tuzcu M, Moses JW, Williams MR, Siegel RJ, Akin JJ, Anderson WN, Pocock S, Smith CR, Leon MB. Transcatheter aortic‐valve replacement for inoperable severe aortic stenosis. N Engl J Med. 2012;366:1696–1704. [DOI] [PubMed] [Google Scholar]
- 58. Genereux P, Head SJ, Van Mieghem NM, Kodali S, Kirtane AJ, Xu K, Smith C, Serruys PW, Kappetein AP, Leon MB. Clinical outcomes after transcatheter aortic valve replacement using valve academic research consortium definitions: a weighted meta‐analysis of 3,519 patients from 16 studies. J Am Coll Cardiol. 2012;59:2317–2326. [DOI] [PubMed] [Google Scholar]
- 59. Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG Jr, Bayer AS, Karchmer AW, Olaison L, Pappas PA, Moreillon P, Chambers ST, Chu VH, Falco V, Holland DJ, Jones P, Klein JL, Raymond NJ, Read KM, Tripodi MF, Utili R, Wang A, Woods CW, Cabell CH. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis‐Prospective Cohort Study. Arch Intern Med. 2009;169:463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gupta S, Sakhuja A, McGrath E, Asmar B. Trends, microbiology, and outcomes of infective endocarditis in children during 2000–2010 in the United States. Congenit Heart Dis. 2016;12:196–201. [DOI] [PubMed] [Google Scholar]
- 61. Borik S, Crean A, Horlick E, Osten M, Lee KJ, Chaturvedi R, Friedberg MK, McCrindle BW, Manlhiot C, Benson L. Percutaneous pulmonary valve implantation: 5 years of follow‐up: does age influence outcomes? Circ Cardiovasc Interv. 2015;8:e001745. [DOI] [PubMed] [Google Scholar]
- 62. Eicken A, Ewert P, Hager A, Peters B, Fratz S, Kuehne T, Busch R, Hess J, Berger F. Percutaneous pulmonary valve implantation: two‐centre experience with more than 100 patients. Eur Heart J. 2011;32:1260–1265. [DOI] [PubMed] [Google Scholar]
- 63. Winslow T, Foster E, Adams JR, Schiller NB. Pulmonary valve endocarditis: improved diagnosis with biplane transesophageal echocardiography. J Am Soc Echocardiogr. 1992;5:206–210. [DOI] [PubMed] [Google Scholar]
- 64. San Roman JA, Vilacosta I, Lopez J, Revilla A, Arnold R, Sevilla T, Rollan MJ. Role of transthoracic and transesophageal echocardiography in right‐sided endocarditis: one echocardiographic modality does not fit all. J Am Soc Echocardiogr. 2012;25:807–814. [DOI] [PubMed] [Google Scholar]
- 65. Saby L, Laas O, Habib G, Cammilleri S, Mancini J, Tessonnier L, Casalta JP, Gouriet F, Riberi A, Avierinos JF, Collart F, Mundler O, Raoult D, Thuny F. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F‐fluorodeoxyglucose uptake as a novel major criterion. J Am Coll Cardiol. 2013;61:2374–2382. [DOI] [PubMed] [Google Scholar]
- 66. Bartoletti M, Tumietto F, Fasulo G, Giannella M, Cristini F, Bonfiglioli R, Raumer L, Nanni C, Sanfilippo S, Di Eusanio M, Scotton PG, Graziosi M, Rapezzi C, Fanti S, Viale P. Combined computed tomography and fluorodeoxyglucose positron emission tomography in the diagnosis of prosthetic valve endocarditis: a case series. BMC Res Notes. 2014;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Pizzi MN, Roque A, Fernandez‐Hidalgo N, Cuellar‐Calabria H, Ferreira‐Gonzalez I, Gonzalez‐Alujas MT, Oristrell G, Gracia‐Sanchez L, Gonzalez JJ, Rodriguez‐Palomares J, Galinanes M, Maisterra‐Santos O, Garcia‐Dorado D, Castell‐Conesa J, Almirante B, Aguade‐Bruix S, Tornos P. Improving the diagnosis of infective endocarditis in prosthetic valves and intracardiac devices with 18F‐fluordeoxyglucose positron emission tomography/computed tomography angiography: initial results at an infective endocarditis referral center. Circulation. 2015;132:1113–1126. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. PubMed search terms.
Figure S1. The PRISMA flow chart of the literature search.


