Elevated levels of low‐density lipoprotein cholesterol (LDL‐C) are well established to be associated with the development of atherosclerotic cardiovascular disease (ASCVD), defined as acute coronary syndrome, a history of myocardial infarction, stable or unstable angina, coronary or other arterial revascularization, ischemic stroke, transient ischemic attack, or peripheral artery disease (all presumed to be of atherosclerotic origin).1 ASCVD is the leading cause of morbidity and mortality in individuals with diabetes mellitus.2 Individuals with diabetes mellitus and elevated levels of LDL‐C are at higher absolute risk of cardiovascular disease compared with those with high LDL‐C without diabetes mellitus.3 Statin therapy is recommended as the first‐line lipid‐lowering drug therapy for the management of dyslipidemia in individuals with diabetes mellitus (unless contraindicated) in current major US guidelines and recommendations (summarized in Table S1).1, 2, 4, 5 However, some patients with high cardiovascular risk either do not achieve adequate LDL‐C reductions on statins, or are intolerant to statins and therefore receive suboptimal statin doses or discontinue statin therapy, and thus remain at increased risk of cardiovascular events. For such patients, additional and/or alternative nonstatin lipid‐lowering treatment options should be considered.4, 6, 7, 8, 9, 10, 11, 12
Several nonstatin therapies are currently available, including the cholesterol absorption inhibitors (ezetimibe), bile acid sequestrants, nicotinic acid (niacin), and fibrates.4 Previous studies with statins and ezetimibe have shown that patients with diabetes mellitus benefit from tight lipid control at least in the same way (if not more) as patients with other risk factors, as well as those without diabetes mellitus.11, 13 The Cholesterol Treatment Trialists’ Collaboration meta‐analysis of 14 randomized statin trials found that the cardiovascular benefits of LDL‐C lowering with statin therapy were similar in those with (n=18 686) and without diabetes mellitus (n=71 370).13 In the IMPROVE‐IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) trial evaluating the addition of ezetimibe concomitant with statin therapy, which lowered LDL‐C levels below previous targets to a median level of 53 mg/dL in 18 144 patients with recent acute coronary syndromes (27% of whom had diabetes mellitus),11 individuals with diabetes mellitus had significantly greater relative and absolute benefit in improved cardiovascular outcomes than those without diabetes mellitus.14 Clinical outcomes studies for niacin (AIM‐HIGH [Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes] and HPS2‐THRIVE [Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events]) and fenofibrate (ACCORD [Action to Control Cardiovascular Risk in Diabetes] and FIELD [Fenofibrate Intervention and Event Lowering in Diabetes]) did not demonstrate significant cardiovascular benefits in individuals with diabetes mellitus, although there was a suggestion of benefit in subgroups with very high triglyceride levels in the fenofibrate studies.15, 16, 17, 18 Because of increased risk of adverse events (AEs) and lack of evidence of meaningful benefits as seen in cardiovascular outcomes trials,15, 16, 17 the US Food and Drug Administration (FDA) has recently rescinded its approval of the combined use of statins with niacin extended‐release tablets or fenofibric acid delayed‐release capsules.19 These nonstatin therapies only produce moderate LDL‐C‐lowering effects and have side effects that limit their use.11, 15, 16, 17
Recently, monoclonal antibodies that inhibit proprotein convertase subtilisin/kexin type 9 (PCSK9) have received considerable attention as promising nonstatin therapeutic options for the management of lipid disorders in patients with persistent cardiovascular risk, including in patients with diabetes mellitus. In this review, we discuss the results of studies investigating lipid‐lowering efficacy, safety, and cardiovascular outcomes in patients with diabetes mellitus and elevated LDL‐C, and recently released data and forthcoming clinical trials with a focus on 2 FDA‐approved monoclonal antibodies that inhibit PCSK9: alirocumab (Praluent®, Sanofi‐Aventis US LLC, Bridgewater, NJ, and Regeneron Pharmaceuticals, Inc, Tarrytown, NY)20 and evolocumab (Repatha®, Amgen Inc, Thousand Oaks, CA).21
Mechanism of Action of PCSK9 Inhibitors
The PCSK9 protein is an important regulator of circulating LDL‐C levels, through its inhibitory action on recycling of the LDL receptor (LDLR). LDLR on the liver cell surface binds to LDL and the LDLR–LDL complex is then internalized, after which the LDLR is normally recycled back to the cell surface up to 150 times.22 Secreted PCSK9 binds to the LDLR on the surface of the hepatocyte, leading to the internalization and degradation of the LDLR in the lysosomes, and reducing the number of LDLRs on the cell surface. Inhibition of secreted PCSK9 should therefore increase the number of available LDLRs on the cell surface and increase uptake of LDL‐C into the cell. PCSK9 inhibition thus offers a novel therapeutic mechanism for the lowering of LDL‐C levels.23
The relevance of PCSK9 to coronary heart disease was determined from human genetic studies that identified gain‐of‐function mutations in the PCSK9 gene associated with elevated serum LDL‐C levels and premature coronary heart disease.24, 25, 26 Conversely, loss‐of‐function PCSK9 mutations are associated with lower serum LDL‐C levels, lower lifelong exposure of vascular structures to LDL, and marked reduction of risk of coronary heart disease.27, 28, 29 Moreover, healthy subjects with severe loss of PCSK9 function have been shown to have serum LDL‐C concentrations as low as 14 mg/dL without apparent adverse health effects.28, 30
In addition to the well‐established role of PCSK9 in LDL metabolism, a recent study suggested that it could play a significant role in the metabolism of triglyceride‐rich lipoproteins also through interaction with the LDLR.31 This has important implications for individuals with type 2 diabetes mellitus (T2D), and for those with type 1 diabetes mellitus (T1D) with poor glycemic control, who typically have a pattern of lipid abnormalities related to insulin resistance that is characterized by reduced hepatic clearance of triglyceride‐rich lipoproteins, increased hepatic production of very‐low‐density lipoproteins, and enhanced intestinal production of chylomicrons.32 These lipid abnormalities, termed diabetic (or mixed) dyslipidemia (Figure), account for their elevated levels of non‐high‐density lipoprotein cholesterol, triglycerides, and small dense LDLs.32, 33 Remnants of triglyceride‐rich lipoproteins, which include chylomicrons and very‐low‐density lipoproteins, have enhanced atherogenic potential since they contain more cholesterol per particle than LDL,34 and have been shown to have a substantial and independent causal association with cardiovascular risk.35 Whereas the LDLR binds to LDLs via apolipoprotein‐B100 (apoB100),36 LDLR binds triglyceride‐rich lipoprotein remnants through interactions with apolipoprotein‐E (apoE), and clearance of these particles occurs along with other receptors such as LDLR‐related protein 1 and Syndecan‐1.37, 38 The recent study showed lower levels of fasting and postprandial triglycerides, apoB48 (an indicator of remnant lipoprotein metabolism), and total apoB (a surrogate of apoB100) in individuals carrying loss‐of‐function PCSK9 genetic variants, supporting a role of PCSK9 in the reduction of uptake of apoE‐containing remnant particles as well as LDL.31 Recent kinetic studies in healthy subjects showed that PCSK9 inhibitors decreased fractional production rate of LDL and intermediate‐density lipoprotein, and increased fractional clearance rates of very‐low‐density lipoprotein, intermediate‐density lipoprotein, and LDL particles, which may reflect a much higher expression of hepatic LDLRs than with statin treatment.39, 40 Similarly, lipoprotein (a) levels were also decreased with PCSK9 inhibitors, which was previously not seen with statins.40, 41 Thus, PCSK9 inhibitors could be especially potent in the treatment of dyslipidemia in those with diabetes mellitus.
Figure 1.
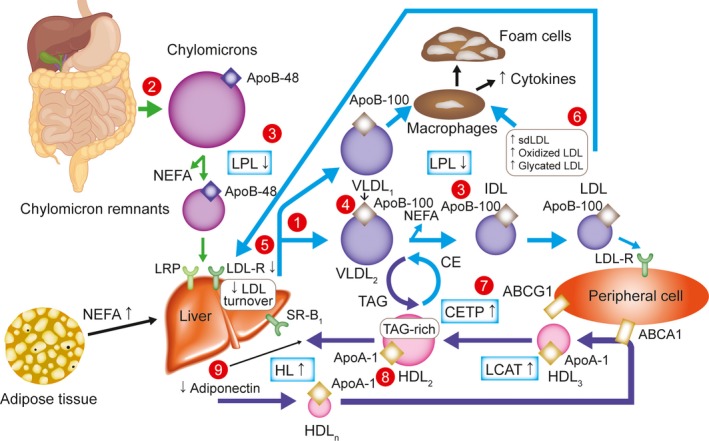
Overview of lipid abnormalities in T2DM.32 Triacylglycerols (hypertriglyceridemia, qualitative and kinetic abnormalities): (1) increased VLDL production (mostly VLDL1); (2) increased chylomicron production; (3) reduced catabolism of both chylomicrons and VLDLs (diminished LPL activity); (4) increased production of large VLDL (VLDL1), preferentially taken up by macrophages; LDL (qualitative and kinetic abnormalities); (5) reduced LDL turnover (decreased LDL B/E receptors); (6) increased number of glycated LDLs, small, dense LDLs (TAG‐rich) and oxidized LDLs, which are preferentially taken up by macrophages; HDL (low HDL‐C, qualitative and kinetic abnormalities); (7) increased CETP activity (increased transfer of triacylglycerols from TAG‐rich lipoproteins to LDLs and HDLs); (8) increased TAG content of HDLs, promoting HL activity and HDL catabolism; (9) low plasma adiponectin favoring the increase in HDL catabolism. ABCA1 indicates ATP‐binding cassette A1; ABCG1, ATP‐binding cassette G1; Apo, apolipoprotein; CE, cholesterol ester; CETP, CE transfer protein; HDL, high‐density lipoprotein; HDL‐C, HDL cholesterol; HDLn, nascent HDL; HL, hepatic lipase; LCAT, lecithin–cholesterol acyltransferase; LDL, low‐density lipoprotein; LDL‐R, LDL receptor; LPL, lipoprotein lipase; LRP, LDL receptor‐related protein; NEFA, nonesterified fatty acid; sdLDL, small, dense LDL; SR‐B1, scavenger receptor B1; T2DM, type 2 diabetes mellitus; TAG, triacylglycerol; VLDL, very low‐density lipoprotein.
PCSK9 Inhibitors and Their Effects in Patients With Diabetes Mellitus and High LDL‐C Levels
Currently, the only FDA‐approved PCSK9 inhibitors are 2 fully human monoclonal antibodies that bind extracellular PCSK9: alirocumab20 and evolocumab,21 administered via subcutaneous injections every 2 weeks (Q2W) or once monthly. Several other approaches to inhibit PCSK9 are in the early stages of clinical development, including small interfering ribonucleic acids, antisense oligonucleotides, small molecule inhibitors, and vaccines; these nonmonoclonal antibody approaches, which utilize alternative strategies to inhibit intracellular or extracellular PCSK9, could potentially provide greater convenience than use of monoclonal antibodies through oral administration, and less frequent dosing.42
Both alirocumab and evolocumab received FDA approval in 2015 as adjunct therapy to diet and maximally tolerated statin therapy to treat adults with heterozygous familial hypercholesterolemia or clinical ASCVD who need greater LDL‐C reduction.20, 21 Evolocumab is also indicated as adjunct therapy to diet and other lipid‐lowering therapies (eg, statins, ezetimibe, LDL apheresis) in patients with homozygous familial hypercholesterolemia who need additional LDL‐C reduction; additionally, as of 2017, evolocumab is indicated to reduce the risk of myocardial infarction, stroke, and coronary revascularization in adults with established cardiovascular disease.21 Both antibodies are approved by the FDA to be administered subcutaneously Q2W or once monthly. The recommended starting dose for alirocumab is 75 mg Q2W, or 300 mg every 4 weeks for patients who prefer less frequent dosing; with either starting dose, the alirocumab dose can be increased to 150 mg Q2W if patients did not have sufficient LDL‐C lowering within 4 to 8 weeks of initiating treatment. The FDA‐approved doses for evolocumab are 140 mg Q2W or 420 mg once monthly.20, 21 Currently, individuals with diabetes mellitus who have established ASCVD and need to reduce LDL‐C levels can receive treatment with PCSK9 inhibitors.
Alirocumab and evolocumab, either alone or in combination with statins and/or other lipid‐lowering therapies, have been shown in their respective phase 3 clinical trial programs (ODYSSEY and PROFICIO [Program to Reduce LDL‐C and Cardiovascular Outcomes Following Inhibition of PCSK9 In Different Populations]) to significantly reduce LDL‐C levels by up to 60% from baseline (depending on dosing regimen; Table) in patients with hypercholesterolemia, including those with familial hypercholesterolemia, moderate to very high cardiovascular risk, and statin intolerance.43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62 The inclusion/exclusion criteria and other details of each phase 3 ODYSSEY and PROFICIO trial are shown in Table S2. LDL‐C reductions in the placebo‐controlled phase 3 trials were consistent with those found in the FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) cardiovascular outcomes trial (Table), studying evolocumab versus placebo in 27 564 patients with clinically evident ASCVD and on a moderate‐to‐high‐intensity statin regimen over a median follow‐up duration of 2.2 years.63 The GLAGOV (Global Assessment of Plaque Regression With a PCSK9 Antibody as Measured by Intravascular Ultrasound) study (which included 968 statin‐treated patients with angiographic coronary disease to evaluate the effect of evolocumab versus placebo on the progression of coronary atherosclerosis) also showed comparable reductions in LDL‐C levels over 76 weeks of evolocumab treatment (from 93 mg/dL at baseline to 37 mg/dL at week 76).64 Moreover, in a 4‐year assessment of an ongoing open‐label extension of the phase 2 OSLER‐1 (Open‐Label Study of Long‐Term Evaluation against LDL Cholesterol 1) trial (the longest clinical trial exposure to date with a PCSK9 inhibitor), monthly doses of evolocumab treatment produced sustained LDL‐C reductions over 4 years of follow‐up without increased incidence of AEs.65
Table 1.
Alirocumab ODYSSEY and Evolocumab PROFICIO Phase 3 Studies Show Similar Reductions in Calculated LDL‐C Levels From Baseline to Primary End Point in Individuals With vs Without DM, Pre‐DM, and Metabolic Syndrome (ITT Analysis)
| % Change From Baseline (LDL‐C) to Primary End Point | Difference vs Control, LS Mean % Change From Baseline (SE or 95% CI If Published), Unless Otherwise Specified | Interaction P Value | |||||
|---|---|---|---|---|---|---|---|
| ALI or EVO | Control (PBO or EZE) | ||||||
| n | LS Mean (SE), Unless Otherwise Specified | n | LS Mean (SE), Unless Otherwise Specified | ||||
| ALIROCUMAB 150 mg Q2W vs PBO (with statin), 24 wks | |||||||
| LONG TERM, ALI 150 vs PBO62 | |||||||
| Overall | All (n=2310) | 1530 | −61.0 (0.7) | 780 | 0.8 (1.0) | −61.9 (1.3) | – |
| DM subanalysis | DM (n=818) | 545 | −60.0 (1.3) | 273 | −1.0 (1.8) | −59.0 | 0.0957 |
| Non‐DM (n=1492) | 985 | −61.6 (0.9) | 507 | 1.8 (1.3) | −63.4 | ||
| Pooled analysis of HIGH FH, LONG TERM, ALI 150 vs PBO | |||||||
| Overall59, 60 | All (n=2416) | 1601 | −60.4 (0.7) | 815 | 0.5 (1.0) | −60.9 (−63.3 to −58.5) | – |
| DM subanalysis69 | DM (n=833) | 554 | −59.7 (1.2) | 279 | −1.4 (1.7) | −58.3 (2.1) | 0.13 |
| Non‐DM (n=1583) | 1047 | −60.7 (0.9) | 536 | 1.5 (1.2) | −62.3 (1.5) | ||
| Pre‐DM subanalysis72 | Pre‐DM (n=876) | – | −61.8 (1.2) | – | 2.1 (1.6) | −63.9 | 0.1431 |
| NG (n=656) | – | −59.5 (1.4) | – | −0.1 (1.9) | −59.4 | ||
| DM+ASCVD subanalysis68 | DM+ASCVD (n=512) | 340 | −61.5 (1.6) | 172 | −1.0 (2.2) | −60.5 (−65.9 to −55.2) | – |
| ALIROCUMAB 75/150 mg Q2W vs PBO (with statins), 24 wks | |||||||
| Pool of FH I & II, ALI 75/150 vs PBO46 | |||||||
| Overall | All (n=732) | – | −48.8 (1.2) | – | 7.1 (1.7) | −55.9 | – |
| DM subanalysis | DM (n=66) | – | −51.4 (4.5) | – | 2.4 (5.5) | −53.8 | 0.7912 |
| Non‐DM (n=666) | – | −48.5 (1.3) | – | 7.6 (1.8) | −56.1 | ||
| COMBO I, ALI 75/150 vs PBO47 | |||||||
| Overall | All (n=311), estimated mean (95% CI) | 205 | −48.2 (−52.0 to −44.4) | 106 | −2.3 (−7.6 to 3.1) | −45.9 (−52.5 to −39.3) | – |
| DM subanalysis | DM (n=135), estimated mean (95% CI) | 94 | −42.2 (−47.8 to −36.6) | 41 | −2.6 (−11.1 to 5.8) | −39.6 | 0.0841 |
| Non‐DM (n=176), estimated mean (95% CI) | 111 | −53.2 (−58.4 to −48.1) | 65 | −2.0 (−8.8 to 4.8) | −51.2 | ||
| Pooled analysis of FH I & II, COMBO I, ALI 75/150 vs PBO | |||||||
| Overall59, 60 | All (n=1043) | 693 | −48.6 (1.0) | 350 | 4.2 (1.5) | −52.7 (−56.3 to −49.2) | – |
| DM subanalysis69 | DM (n=201) | 131 | −43.4 (2.6) | 70 | 0.3 (3.4) | −43.7 (4.1) | 0.02b |
| Non‐DM (n=842) | 562 | −49.8 (1.2) | 280 | 5.1 (1.6) | −54.8 (2.0) | ||
| Pre‐DM subanalysis72 | Pre‐DM (n=396) | – | −52.4 (1.7) | – | 3.3 (2.4) | −55.7 | 0.8451 |
| NG (n=422) | – | −46.1 (1.6) | – | 8.9 (2.3) | −55.0 | ||
| DM+ASCVD subanalysis68 | DM+ASCVD (n=137) | 92 | −46.4 (3.0) | 45 | 6.3 (4.5) | −52.7 (−63.5 to −41.9) | – |
| DM‐INSULIN75 | |||||||
| DM+insulin | T2DM (n=429) | 287 | −48.2 (1.6) | 142 | 0.8 (2.2) | −49.0 (2.7) | – |
| T1DM (n=74) | 49 | −51.8 (3.7) | 25 | −3.9 (5.3) | −47.8 (6.5) | – | |
| ALIROCUMAB 75/150 mg Q2W vs EZE, 24 wks | |||||||
| COMBO II DM subanalysis, ALI 75/150 vs EZE (with statin) | |||||||
| Overall45 | All (n=707) | 467 | −50.6 (1.4) | 240 | −20.7 (1.9) | −29.8 (2.3) | – |
| DM subanalysis67 | DM (n=225a) | 148b | −49.1 | 77b | −18.4 | −30.7 | 0.8025 |
| Non‐DM (n=495a) | 331b | −51.2 | 164b | −21.8 | −29.5 | ||
| Pooled analysis of COMBO II, OPTIONS I & II, ALI 75/150 vs EZE (with statin) | |||||||
| Overall59, 60 | All (n=1105) | 669 | −48.9 (1.4) | 436 | −19.3 (1.7) | −29.6 (−33.8 to −25.3) | – |
| Pre‐DM subanalysis72 | Pre‐DM (n=432) | – | −51.7 (2.2) | – | −16.1 (2.6) | −35.6 | 0.0428 |
| NG (n=244) | – | −45.8 (2.8) | – | −21.8 (3.6) | −24.0 | ||
| DM+ASCVD subanalysis68 | DM+ASCVD (n=283) | 173 | −48.7 (2.6) | 110 | −20.6 (3.3) | −28.1 (−36.6 to −19.6) | – |
| ALTERNATIVE, ALI 75/150 vs EZE (without statin) | |||||||
| Overall48 | All (n=168) | 90 | −54.8 (1.4) | 78 | −20.1 (2.4) | −34.7 | |
| DM+ASCVD subanalysis68 | DM+ASCVD (n=34) | 23 | −54.9 (6.0) | 11 | 4.0 (8.8) | −58.9 (−80.9 to −36.8) | – |
| Pooled analysis of ALTERNATIVE & MONO, ALI 75/150 vs EZE (without statin) | |||||||
| Overall59, 60 | All (n=351) | 178 | −45.6 (1.8) | 173 | −14.8 (1.8) | −30.9 (−35.9 to −25.9) | – |
| Pre‐DM subanalysis72 | Pre‐DM (n=135) | – | −44.0 (2.9) | – | −16.0 (2.7) | −28.0 | 0.4073 |
| NG (n=147) | – | −46.3 (2.7) | – | −13.7 (2.6) | −32.6 | ||
| DM‐DYSLIPIDEMIAd, 77 | |||||||
| T2DM+mixed dyslipidemiad | All (n=409)d | 273d | −43.3d | 136d | −0.3d | −43.0d | – |
| EVOLOCUMAB 140 mg Q2W or 420 mg QM vs PBO | |||||||
| Pool of LAPLACE‐2 & RUTHERFORD‐2, EVO 140 or 420 vs PBO, 12 wks66 | |||||||
| T2DM subanalysis | T2DM (n=304) | 210 | – | 94 | – | −60 (−69 to −51)c | 0.27 |
| Non‐T2DM (n=1700) | 1127 | – | 573 | – | −66 (−70 to −62)c | ||
| DESCARTES, EVO 420 vs PBO, 52 wks | |||||||
| Overall61 | All (n=901) | 599 | – | 302 | – | −57.0 (2.1) | – |
| Subanalysis by glycemic status and MetS73 | T2DM (n=120) | 77 | – | 43 | – | −50.8 (6.0) | – |
| IFG (n=293) | 194 | – | 99 | – | −59.4 (3.4) | – | |
| MetS (n=289) | 182 | – | 107 | – | −55.0 (3.5) | – | |
| No dysglycemia or MetS (n=393) | 274 | – | 119 | – | −58.1 (3.5) | – | |
| FOURIER, EVO 140 or 420 vs PBO, 48 wks | |||||||
| Overall63 | All (n=27 563) | 13 784 | – | 13 779 | – | −59 (58 to 60) | – |
| DM subanalysis71 | DM (n=11 031) | 5515 | – | 5516 | – | −57 (56 to 58) | – |
| Non‐DM (n=16 533) | 8269 | – | 8264 | – | −60 (60 to 61) | ||
| EVOLOCUMAB 140 mg Q2W or 420 mg QM vs EZE | |||||||
| Pool of LAPLACE‐2 (atorvastatin cohorts only) & GAUSS‐2, EVO 140 or 420 vs EZE, 12 wks66 | |||||||
| T2DM subanalysis | T2DM (n=187) | 114 | – | 73 | – | −39 (−47 to −32)c | 0.79 |
| Non‐T2DM (n=780) | 530 | – | 250 | – | −40 (−45 to −36)c | ||
LS means and SEs taken from mixed‐effect model with repeated measures analysis. All values shown are as published in the respective referenced articles; if the values for difference vs control were not published, values were estimated based on the respective percent changes with alirocumab/evolocumab and controls. ALI indicates alirocumab; ASCVD, atherosclerotic cardiovascular disease; CI, confidence interval; DM, diabetes mellitus; DESCARTES, Durable Effect of PCSK9 Antibody Compared with Placebo Study; EVO, evolocumab; EZE, ezetimibe; FOURIER, Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk; GAUSS‐2, Goal Achievement after Utilizing an Anti‐PCSK9 Antibody in Statin Intolerant Subjects; HDL‐C, high‐density lipoprotein cholesterol; IFG, impaired fasting glucose; ITT, intention‐to‐treat; LAPLACE‐2, LDL‐C Assessment with PCSK9 Monoclonal Antibody Inhibition Combined With Statin Therapy; LDL‐C, low‐density lipoprotein cholesterol; LS, least squares; MetS, metabolic syndrome; NG, normoglycemia; PBO, placebo; Q2W, every 2 weeks; QM, once monthly; RUTHERFORD‐2, Reduction of LDL‐C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder‐2; SE, standard error; T1, type 1; T2, type 2.
Randomized population.
Because of a higher proportion of participants without DM receiving an alirocumab dose increase at wk 12 (36.5% vs 25.6%).
Random‐effects treatment difference (95% CI) between evolocumab and control (placebo or ezetimibe), generated by use of the DerSimonian and Laird random‐effect estimator.
The comparator in the DM‐DYSLIPIDEMIA trial was usual care, which included the option to continue on maximally tolerated statin therapy without adding another lipid‐lowering therapy at randomization, or with the addition of one of the following at randomization: ezetimibe, fenofibrate, omega‐3 fatty acids, or nicotinic acid. Mixed dyslipidemia was defined as non‐HDL‐C ≥100 mg/dL (2.59 mmol/L), and triglycerides ≥150 mg/dL (1.70 mmol/L) and <500 mg/dL (5.65 mmol/L) at the screening visit. The primary efficacy end point in this trial was non‐HDL‐C: at week 24, mean non‐HDL‐C changes were superior with alirocumab (−37.3%) vs usual care (−4.7%). The LDL‐C reduction (secondary end point) values shown for DM‐DYSLIPIDEMIA in this table are measured LDL‐C values, not calculated LDL‐C.76, 77
Lipid‐Lowering Efficacy of PCSK9 Inhibitors in Patients With Diabetes Mellitus
Subanalyses of the diabetes mellitus subpopulations in the alirocumab and evolocumab phase 3 trials (Table) showed significant reductions in LDL‐C that were generally similar between individuals with and without diabetes mellitus.46, 47, 62, 66, 67, 68, 69, 70 Findings were consistent in the prespecified diabetes mellitus subanalysis of FOURIER, which analyzed 11 031 patients with diabetes mellitus versus 16 533 patients without diabetes mellitus; compared with placebo, median LDL‐C levels were reduced by 57% in those with diabetes mellitus and by 60% in those without diabetes mellitus.71 Other subanalyses of ODYSSEY and PROFICIO phase 3 trials showed that LDL‐C reductions were also similar in those with and without prediabetes,72 impaired fasting glucose, and metabolic syndrome (Table).73 Similarly, a recent post‐hoc subanalysis of 9 ODYSSEY phase 3 trials (24–104 weeks’ treatment duration) showed significant LDL‐C reductions with alirocumab in patients with both diabetes mellitus and ASCVD (Table).68 Moreover, LDL‐C reductions in these subpopulations with alirocumab and evolocumab were comparable to those seen in the overall phase 3 patient populations (Table).
Further information on the impact of alirocumab in patients with diabetes mellitus is available from the ODYSSEY DM‐INSULIN74, 75 and DM‐DYSLIPIDEMIA76, 77 trials, which were dedicated phase 3b and phase 4 studies, respectively, investigating alirocumab in individuals with diabetes mellitus. The placebo‐controlled DM‐INSULIN trial assessed the efficacy and safety of concomitant administration of 2 injectable biological agents (alirocumab and insulin) in insulin‐treated individuals with hypercholesterolemia and T1D or T2D at high cardiovascular risk, on background stable maximally tolerated statin therapy with or without other lipid‐lowering therapy (Table).74, 75 The DM‐DYSLIPIDEMIA trial assessed the efficacy and safety of alirocumab versus lipid‐lowering usual care (ezetimibe, fenofibrate, omega‐3 fatty acids, or nicotinic acid) in individuals with T2D and mixed dyslipidemia at high cardiovascular risk, on background stable maximally tolerated statin therapy without other lipid‐lowering therapies; this trial was the first trial of a PCSK9 inhibitor to evaluate non‐high‐density lipoprotein cholesterol as a primary efficacy end point (Table).76, 77 Two evolocumab phase 3 trials study individuals with T2D and hypercholesterolemia or mixed dyslipidemia (NCT02739984, NCT02662569).
Safety of PCSK9 Inhibitors in Patients With Diabetes Mellitus, and Impact On Risk of Diabetes Mellitus Development
In the overall phase 3 patient populations, pooled safety analyses of 14 alirocumab ODYSSEY trials (including 5234 patients with 8–104 weeks’ treatment duration),78 12 evolocumab PROFICIO parent trials (including 6026 patients with 6–52 weeks’ treatment duration), and the first year of 2 open‐label extension trials (which included 4465 of the 6026 patients who were included in this analysis in the parent trials),79 and the FOURIER trial63 showed that the incidence of overall treatment‐emergent AEs, serious treatment‐emergent AEs, discontinuations because of treatment‐emergent AEs, and deaths was similar with the PCSK9 inhibitors versus controls. Among alirocumab‐ or evolocumab‐treated patients, nasopharyngitis, injection‐site reactions, and upper respiratory tract infections were the most commonly occurring AEs.78, 79 Generally, a higher incidence of local injection‐site reactions (the majority of which were mild in nature) was seen with alirocumab/evolocumab versus controls.63, 78
Consistent with the overall patient populations mentioned above,63, 78 subanalyses by diabetes mellitus status demonstrated that overall alirocumab/evolocumab safety was comparable to that of control in those with and without diabetes mellitus.66, 67, 69, 71, 80 Overall safety was also comparable versus control between patients with diabetes mellitus and ASCVD,68 insulin‐treated patients with diabetes mellitus in the DM‐INSULIN study,75 patients with T2D and mixed dyslipidemia in the DM‐DYSLIPIDEMIA study,77 individuals with prediabetes and normoglycemia,72 and those with and without dysglycemia or metabolic syndrome.73 As shown in prior studies in the overall patient population,63, 78 higher rates of local injection‐site reactions (also generally mild) were typically seen with alirocumab/evolocumab compared with control for patients both with and without diabetes mellitus.69, 71, 80 However, in those with diabetes mellitus, several analyses showed lower rates of local injection‐site reactions with alirocumab/evolocumab versus control.66, 67, 75 Furthermore, alirocumab/evolocumab‐treated individuals with diabetes mellitus showed a lower incidence of local injection‐site reactions compared with alirocumab/evolocumab‐treated individuals without diabetes mellitus.66, 67, 69, 71, 80
Statin therapy and recent genetic epidemiology studies of PCSK9 loss‐of‐function genetic variants associated with LDL‐C reductions have suggested a small but statistically significant increased risk of the development of new‐onset diabetes mellitus.4, 81, 82, 83, 84 However, current clinical trial data for alirocumab and evolocumab do not suggest an association between PCSK9 inhibitors and loss of glycemic control. Analyses of ODYSSEY phase 3 trials with alirocumab with duration of 78 to 104 weeks of follow‐up showed no changes in fasting plasma glucose or hemoglobin A1c levels over time with alirocumab or control in patients with and without diabetes mellitus 67, 68, 75, 77, 80, 85 or in individuals with prediabetes or normoglycemia at baseline.72 Analyses of PROFICIO trials of 48 to 52 weeks of follow‐up and the diabetes mellitus subanalysis of the FOURIER trial of 168 weeks of follow‐up also did not show changes in fasting plasma glucose or hemoglobin A1c levels with evolocumab in patients with and without diabetes mellitus,71 high risk of diabetes mellitus,70 impaired fasting glucose, metabolic syndrome, or normoglycemia,73 although a small but statistically significant increase in fasting plasma glucose with evolocumab (but no change in hemoglobin A1c) at 78 weeks of treatment was found in the GLAGOV study.64 Furthermore, in contrast to the results seen in the statin and PCSK9 genetic variant studies mentioned above,4, 81, 82, 83, 84 no evidence of increased transition from normoglycemia to new‐onset diabetes mellitus following alirocumab or evolocumab treatment was found in pooled analyses.70, 73, 85 Findings from the FOURIER trial showed no significant differences in rates of adjudicated new‐onset diabetes mellitus cases between evolocumab and placebo over a median follow‐up of 2.2 years.63, 71 The lack of increased risk of developing new‐onset diabetes mellitus on a PCSK9 inhibitor was further confirmed in the longest‐running PCSK9 inhibitor trial to date (the 4‐year assessment of the ongoing open‐label extension of the phase 2 OSLER‐1 trial), which indicated an annualized incidence of new‐onset diabetes mellitus of 2.8% for the evolocumab group over up to 4 years of continued exposure (versus 4.0% for the control group).65 The lack of effect of PCSK9 inhibitors on new‐onset diabetes mellitus in contrast to the increased risk of new‐onset diabetes mellitus in those with PCSK9 loss‐of‐function genetic variants could be attributed to differences in biological effects of LDL‐C lowering associated with treatment with a PCSK9 inhibitor (ie, inhibiting circulating, extracellular PCSK9) versus the lifelong exposure to decreased LDL‐C levels because of PCSK9 loss‐of‐function genetic variants.81, 83 Indeed, PCSK9 monoclonal antibodies have been shown to affect the PCSK9 extracellular pathway without altering the PCSK9 intracellular pathway, which remains poorly characterized, especially in beta cells.86
Impact of PCSK9 Inhibitors on Atherosclerosis and Cardiovascular Outcomes in Patients With Diabetes Mellitus
The cardiovascular benefits of LDL‐C reductions with a PCSK9 inhibitor were first suggested by the post‐hoc analyses of the phase 3 LONG TERM and OSLER trials.58, 62 Recently, the GLAGOV study found that the addition of evolocumab to statin therapy in patients with angiographic coronary artery disease could lead to regression of atherosclerotic plaques after 76 weeks of treatment in those patients with LDL‐C reductions.64 In the subgroup analysis of GLAGOV by diabetes mellitus status, patients with diabetes mellitus had the same benefits as those without diabetes mellitus in the change in percent atheroma volume from baseline to week 78.64 Evidence of cardiovascular outcome benefits with a PCSK9 inhibitor was recently provided by the FOURIER trial, the first clinical outcomes trial to be reported for a PCSK9 inhibitor (evolocumab), which included 27 564 patients with clinically evident ASCVD and on a moderate‐to‐high‐intensity statin regimen over a median follow‐up duration of 2.2 years.63 FOURIER showed a statistically significant 15% reduction in occurrence of the primary composite end point of cardiovascular death, myocardial infarction, stroke, hospitalization for unstable angina, or coronary revascularization with evolocumab treatment relative to placebo (9.8% versus 11.3%; hazard ratio, 0.85; 95% confidence interval [CI], 0.79–0.92; P<0.001).63 The benefit was driven by a reduction of ischemic stroke, myocardial infarction, and revascularization. The magnitude of cardiovascular benefit of evolocumab in FOURIER (with a reduction in LDL‐C from 92 to 30 mg/dL) over 2.2 years63 was close to the range expected based on the Cholesterol Treatment Trialists’ meta‐analysis of statin trials, which reported a 22% relative risk reduction over 5 years per 1 mmol/L (39 mg/dL) LDL‐C reduction.87 Improved cardiovascular outcomes were observed down to LDL‐C levels as low as 8 mg/dL, with no significant associations between such very low LDL‐C levels and AEs.88 Together with the GLAGOV findings, these results show that patients with ASCVD benefit from LDL‐C lowering below current targets.63 As a result, the FDA indicated evolocumab to reduce the risk of myocardial infarction, stroke, and coronary revascularization in adults with established cardiovascular disease.21
Previous studies with other lipid‐lowering therapies have shown that patients with diabetes mellitus benefit from tight lipid control at least in the same way as patients with other risk factors,11, 13 or can often benefit significantly more than those without diabetes mellitus as shown in the recent IMPROVE‐IT analysis.14 The prespecified diabetes mellitus subanalysis of FOURIER (which, as previously mentioned, included 11 031 patients with diabetes mellitus versus 16 533 patients without diabetes mellitus, of whom 10 344 had prediabetes and 6189 had normoglycemia) found that evolocumab significantly reduced cardiovascular risk consistently in patients with and without diabetes mellitus at baseline71: the hazard ratios for the primary composite end point (defined as above) for patients with diabetes mellitus and without diabetes mellitus were 0.83 (95% CI , 0.75–0.93; P=0.0008) and 0.87 (95% CI , 0.79–0.96; P=0.0052), respectively (P‐interaction=0.60).71 However, attributed to their elevated cardiovascular risk at baseline, patients with diabetes mellitus tended to have a greater absolute risk reduction over time with evolocumab compared with those without diabetes mellitus (2.7% [95% CI , 0.7–4.8] versus 1.6% [95% CI, 0.1–3.2] reduction in the primary end point over 3 years).71
The long‐term cardiovascular outcomes trial for alirocumab (ODYSSEY OUTCOMES; NCT01663402), the primary results for which are now available (presentation by Dr Philippe Steg at the American College of Cardiology Annual Scientific Session 2018, Orlando, FL, March 10, 2018; unpublished data), enrolled 18 924 patients (≈29% of whom had diabetes mellitus) randomized 1 to 12 months after acute coronary syndrome, with a median follow‐up of 2.8 years. Data on diabetes mellitus and prediabetes parameters (reported by investigators and determined by serial hemoglobin A1c and fasting plasma glucose measurements) from OUTCOMES in this high and very high‐risk patient cohort are expected to be reported at a later date. Findings could provide further valuable information on the efficacy and safety of PCSK9 inhibitors in individuals with diabetes mellitus and high to very‐high cardiovascular risk, which will ultimately help to guide clinical decision‐making beyond statin therapy in this high‐risk patient population.
Conclusion
Overall, clinical evidence shows that PCSK9 inhibitors are well tolerated and provide significant LDL‐C lowering in individuals with hyperlipidemia and diabetes mellitus on top of maximally tolerated statin therapy, without loss of glycemic control or increased risk of developing diabetes mellitus in those without pre‐existing diabetes mellitus, and can prevent or reduce further cardiovascular events.
Sources of Funding
Medical writing and editorial support in the preparation of this publication was funded by Sanofi US (Bridgewater, NJ) and Regeneron Pharmaceuticals, Inc. (Tarrytown, NY). The authors received no honoraria related to the development of this publication.
Disclosures
Dr Handelsman has received research grants from and is a consultant and compensated speaker for Aegerion, Amarin, Amgen, AstraZeneca, Bristol‐Myers Squibb, Boehringer Ingelheim, Gilead, Grifols, Intarcia, Janssen, Lexicon, Eli Lilly, Merck, Merck‐Pfizer, Novo Nordisk, Regeneron Pharmaceuticals, Inc., and Sanofi. Dr Lepor has received research grant support from Regeneron Pharmaceuticals, Inc., and participated in speaker's bureau for Amgen, Regeneron Pharmaceuticals, Inc., and Sanofi.
Supporting information
Table S1. Summary of Major US Guidelines/Consensus Statements for Individuals with DM
Table S2. Summary of Phase 3 Alirocumab ODYSSEY and Evolocumab PROFICIO Studies
Acknowledgments
Medical writing and editorial support in the preparation of this publication under the guidance of the authors was provided by Grace Shim, PhD, Prime (Knutsford, UK), according to Good Publication Practice guidelines. Employees of Sanofi and Regeneron Pharmaceuticals, Inc. were permitted to review the article and offer comments. However, the authors were responsible for all content and editorial decisions.
J Am Heart Assoc. 2018;7:e008953 DOI: 10.1161/JAHA.118.008953.
References
- 1. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. [DOI] [PubMed] [Google Scholar]
- 2. American Diabetes Association . Standards of medical care in diabetes—2017. Diabetes Care. 2017;40(suppl 1):S1–S135.27979885 [Google Scholar]
- 3. Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12‐yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. [DOI] [PubMed] [Google Scholar]
- 4. Bays HE, Jones PH, Orringer CE, Brown WV, Jacobson TA. National lipid association annual summary of clinical lipidology 2016. J Clin Lipidol. 2016;10:S1–S43. [DOI] [PubMed] [Google Scholar]
- 5. Garber AJ, Abrahamson MJ, Barzilay JI, Blonde L, Bloomgarden ZT, Bush MA, Dagogo‐Jack S, DeFronzo RA, Einhorn D, Fonseca VA, Garber JR, Garvey WT, Grunberger G, Handelsman Y, Hirsch IB, Jellinger PS, McGill JB, Mechanick JI, Rosenblit PD, Umpierrez GE. Consensus Statement By the American Association of Clinical Endocrinologists and American College of Endocrinology on the Comprehensive Type 2 Diabetes Management Algorithm—2017 Executive Summary. Endocr Pract. 2017;23:207–238. [DOI] [PubMed] [Google Scholar]
- 6. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Z, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL; Authors/Task Force Members, Additional Contributor . ESC/EAS guidelines for the management of dyslipidaemias. Eur Heart J. 2016;2016:2999–3058. [DOI] [PubMed] [Google Scholar]
- 7. Gidding SS, Champagne MA, de Ferranti SD, Defesche J, Ito MK, Knowles JW, McCrindle B, Raal F, Rader D, Santos RD, Lopes‐Virella M, Watts GF, Wierzbicki AS; American Heart Association Atherosclerosis, Hypertension, and Obesity in Young Committee of Council on Cardiovascular Disease in Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and Council on Lifestyle and Cardiometabolic Health . The agenda for familial hypercholesterolemia: a scientific statement from the American Heart Association. Circulation. 2015;132:2167–2192. [DOI] [PubMed] [Google Scholar]
- 8. Jacobson TA, Maki KC, Orringer CE, Jones PH, Kris‐Etherton P, Sikand G, La Forge R, Daniels SR, Wilson DP, Morris PB, Wild RA, Grundy SM, Daviglus M, Ferdinand KC, Vijayaraghavan K, Deedwania PC, Aberg JA, Liao KP, McKenney JM, Ross JL, Braun LT, Ito MK, Bays HE, Brown WV, Underberg JA; NLA Expert Panel . National lipid association recommendations for patient‐centered management of dyslipidemia: part 2. J Clin Lipidol. 2015;9:S1–S122. [DOI] [PubMed] [Google Scholar]
- 9. Lloyd‐Jones DM, Morris PB, Ballantyne CM, Birtcher KK, Daly DD Jr, DePalma SM, Minissian MB, Orringer CE, Smith SC Jr. 2016 ACC Expert Consensus Decision Pathway on the Role of Non‐Statin Therapies for LDL‐Cholesterol Lowering in the Management of Atherosclerotic Cardiovascular Disease Risk: A Report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2016;68:92–125. [DOI] [PubMed] [Google Scholar]
- 10. Orringer CE, Jacobson TA, Saseen JJ, Brown AS, Gotto AM, Ross JL, Underberg JA. Update on the use of PCSK9 inhibitors in adults: recommendations from an Expert Panel of the National Lipid Association. J Clin Lipidol. 2017;11:880–890. [DOI] [PubMed] [Google Scholar]
- 11. Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM; IMPROVE‐IT Investigators . Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 12. Jellinger PS, Handelsman Y, Rosenblit PD, Bloomgarden ZT, Fonseca VA, Garber AJ, Grunberger G, Guerin CK, Bell DSH, Mechanick JI, Pessah‐Pollack R, Wyne K, Smith D, Brinton EA, Fazio S, Davidson M. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocr Pract. 2017;23:1–87. [DOI] [PubMed] [Google Scholar]
- 13. Cholesterol Treatment Trialists Collaborators , Kearney PM, Blackwell L, Collins R, Keech A, Simes J, Peto R, Armitage J, Baigent C. Efficacy of cholesterol‐lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta‐analysis. Lancet. 2008;371:117–125. [DOI] [PubMed] [Google Scholar]
- 14. Giugliano RP, Cannon CP, Blazing MA, Nicolau JC, Corbalan R, Spinar J, Park JG, White JA, Bohula E, Braunwald E; IMPROVE‐IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) Investigators . Benefit of adding ezetimibe to statin therapy on cardiovascular outcomes and safety in patients with vs. without diabetes: results from IMPROVE‐IT. Circulation. 2018;137:1571–1582. [DOI] [PubMed] [Google Scholar]
- 15. AIM‐HIGH Investigators , Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes‐Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365:2255–2267. [DOI] [PubMed] [Google Scholar]
- 16. ACCORD Study Group , Ginsberg HN, Elam MB, Lovato LC, Crouse JR III, Leiter LA, Linz P, Friedewald WT, Buse JB, Gerstein HC, Probstfield J, Grimm RH, Ismail‐Beigi F, Bigger JT, Goff DC Jr, Cushman WC, Simons‐Morton DG, Byington RP. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362:1563–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. HPS‐THRIVE Collaborative Group , Landray MJ, Haynes R, Hopewell JC, Parish S, Aung T, Tomson J, Wallendszus K, Craig M, Jiang L, Collins R, Armitage J. Effects of extended‐release niacin with laropiprant in high‐risk patients. N Engl J Med. 2014;371:203–212. [DOI] [PubMed] [Google Scholar]
- 18. Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesaniemi YA, Sullivan D, Hunt D, Colman P, d'Emden M, Whiting M, Ehnholm C, Laakso M; Field Study Investigators . Effects of long‐term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. [DOI] [PubMed] [Google Scholar]
- 19. Food and Drug Administration . AbbVie Inc. et al.; Withdrawal of Approval of Indications Related to the Coadministration With Statins in Applications for Niacin Extended‐Release Tablets and Fenofibric Acid Delayed‐Release Capsules. Fed Reg. 2016;81:22612–22613. [Google Scholar]
- 20. sanofi‐aventis U.S. LLC . Highlights of PRALUENT prescribing information. Available at: http://products.sanofi.us/praluent/praluent.pdf. Accessed November 7, 2017.
- 21. Amgen Inc . Highlights of Repatha prescribing information. Available at: http://pi.amgen.com/~/media/amgen/repositorysites/pi-amgen-com/repatha/repatha_pi_hcp_english.ashx. Accessed December 6, 2017.
- 22. Brown MS, Anderson RG, Goldstein JL. Recycling receptors: the round‐trip itinerary of migrant membrane proteins. Cell. 1983;32:663–667. [DOI] [PubMed] [Google Scholar]
- 23. Seidah NG, Awan Z, Chretien M, Mbikay M. PCSK9: a key modulator of cardiovascular health. Circ Res. 2014;114:1022–1036. [DOI] [PubMed] [Google Scholar]
- 24. Abifadel M, Elbitar S, El Khoury P, Ghaleb Y, Chemaly M, Moussalli ML, Rabes JP, Varret M, Boileau C. Living the PCSK9 adventure: from the identification of a new gene in familial hypercholesterolemia towards a potential new class of anticholesterol drugs. Curr Atheroscler Rep. 2014;16:439. [DOI] [PubMed] [Google Scholar]
- 25. Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derre A, Villeger L, Farnier M, Beucler I, Bruckert E, Chambaz J, Chanu B, Lecerf JM, Luc G, Moulin P, Weissenbach J, Prat A, Krempf M, Junien C, Seidah NG, Boileau C. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. [DOI] [PubMed] [Google Scholar]
- 26. Kotowski IK, Pertsemlidis A, Luke A, Cooper RS, Vega GL, Cohen JC, Hobbs HH. A spectrum of PCSK9 alleles contributes to plasma levels of low‐density lipoprotein cholesterol. Am J Hum Genet. 2006;78:410–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cohen J, Pertsemlidis A, Kotowski IK, Graham R, Garcia CK, Hobbs HH. Low LDL cholesterol in individuals of African descent resulting from frequent nonsense mutations in PCSK9. Nat Genet. 2005;37:161–165. [DOI] [PubMed] [Google Scholar]
- 28. Zhao Z, Tuakli‐Wosornu Y, Lagace TA, Kinch L, Grishin NV, Horton JD, Cohen JC, Hobbs HH. Molecular characterization of loss‐of‐function mutations in PCSK9 and identification of a compound heterozygote. Am J Hum Genet. 2006;79:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Langsted A, Nordestgaard BG, Benn M, Tybjaerg‐Hansen A, Kamstrup PR. PCSK9 R46L loss‐of‐function mutation reduces lipoprotein(a), LDL cholesterol, and risk of aortic valve stenosis. J Clin Endocrinol Metab. 2016;101:3281–3287. [DOI] [PubMed] [Google Scholar]
- 30. Hooper AJ, Marais AD, Tanyanyiwa DM, Burnett JR. The C679X mutation in PCSK9 is present and lowers blood cholesterol in a Southern African population. Atherosclerosis. 2007;193:445–448. [DOI] [PubMed] [Google Scholar]
- 31. Ooi TC, Krysa JA, Chaker S, Abujrad H, Mayne J, Henry K, Cousins M, Raymond A, Favreau C, Taljaard M, Chretien M, Mbikay M, Proctor SD, Vine DF. The effect of PCSK9 loss‐of‐function variants on the postprandial lipid and apob‐lipoprotein response. J Clin Endocrinol Metab. 2017;102:3452–3460. [DOI] [PubMed] [Google Scholar]
- 32. Verges B. Pathophysiology of diabetic dyslipidaemia: where are we? Diabetologia. 2015;58:886–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mooradian AD. Dyslipidemia in type 2 diabetes mellitus. Nat Clin Pract Endocrinol Metab. 2009;5:150–159. [DOI] [PubMed] [Google Scholar]
- 34. Varbo A, Benn M, Nordestgaard BG. Remnant cholesterol as a cause of ischemic heart disease: evidence, definition, measurement, atherogenicity, high risk patients, and present and future treatment. Pharmacol Ther. 2014;141:358–367. [DOI] [PubMed] [Google Scholar]
- 35. Varbo A, Benn M, Tybjaerg‐Hansen A, Jorgensen AB, Frikke‐Schmidt R, Nordestgaard BG. Remnant cholesterol as a causal risk factor for ischemic heart disease. J Am Coll Cardiol. 2013;61:427–436. [DOI] [PubMed] [Google Scholar]
- 36. Brown MS, Goldstein JL. A receptor‐mediated pathway for cholesterol homeostasis. Science. 1986;232:34–47. [DOI] [PubMed] [Google Scholar]
- 37. Nakajima K, Nakano T, Tokita Y, Nagamine T, Inazu A, Kobayashi J, Mabuchi H, Stanhope KL, Havel PJ, Okazaki M, Ai M, Tanaka A. Postprandial lipoprotein metabolism: VLDL vs chylomicrons. Clin Chim Acta. 2011;412:1306–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen K, Wu Q, Hu K, Yang C, Wu X, Cheung P, Williams KJ. Suppression of Hepatic FLOT1 (Flotillin‐1) by type 2 diabetes mellitus impairs the disposal of remnant lipoproteins via Syndecan‐1. Arterioscler Thromb Vasc Biol. 2018;38:102–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Watts GF, Chan DC, Dent R, Somaratne R, Wasserman SM, Scott R, Burrows S, Barrett PHR. Factorial effects of evolocumab and atorvastatin on lipoprotein metabolism. Circulation. 2017;135:338–351. [DOI] [PubMed] [Google Scholar]
- 40. Reyes‐Soffer G, Pavlyha M, Ngai C, Thomas T, Holleran S, Ramakrishnan R, Karmally W, Nandakumar R, Fontanez N, Obunike J, Marcovina SM, Lichtenstein AH, Matthan NR, Matta J, Maroccia M, Becue F, Poitiers F, Swanson B, Cowan L, Sasiela WJ, Surks HK, Ginsberg HN. Effects of PCSK9 inhibition with alirocumab on lipoprotein metabolism in healthy humans. Circulation. 2017;135:352–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Watts GF, Chan DC, Somaratne R, Wasserman SM, Scott R, Marcovina SM, Barrett PHR. Controlled study of the effect of proprotein convertase subtilisin‐kexin type 9 inhibition with evolocumab on lipoprotein(a) particle kinetics. Eur Heart J. 2018. Available at: 10.1093/eurheartj/ehy122. Accessed May 25, 2018. [DOI] [PubMed] [Google Scholar]
- 42. Dixon DL, Trankle C, Buckley L, Parod E, Carbone S, Van Tassell BW, Abbate A. A review of PCSK9 inhibition and its effects beyond LDL receptors. J Clin Lipidol. 2016;10:1073–1080. [DOI] [PubMed] [Google Scholar]
- 43. Roth EM, Taskinen MR, Ginsberg HN, Kastelein JJ, Colhoun HM, Robinson JG, Merlet L, Pordy R, Baccara‐Dinet MT. Monotherapy with the PCSK9 inhibitor alirocumab versus ezetimibe in patients with hypercholesterolemia: results of a 24 week, double‐blind, randomized Phase 3 trial. Int J Cardiol. 2014;176:55–61. [DOI] [PubMed] [Google Scholar]
- 44. Bays H, Gaudet D, Weiss R, Ruiz JL, Watts GF, Gouni‐Berthold I, Robinson J, Zhao J, Hanotin C, Donahue S. Alirocumab as add‐on to atorvastatin versus other lipid treatment strategies: ODYSSEY OPTIONS I randomized trial. J Clin Endocrinol Metab. 2015;100:3140–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cannon CP, Cariou B, Blom D, McKenney JM, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM; ODYSSEY COMBO II Investigators . Efficacy and safety of alirocumab in high cardiovascular risk patients with inadequately controlled hypercholesterolaemia on maximally tolerated doses of statins: the ODYSSEY COMBO II randomized controlled trial. Eur Heart J. 2015;36:1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Kastelein JJ, Ginsberg HN, Langslet G, Hovingh GK, Ceska R, Dufour R, Blom D, Civeira F, Krempf M, Lorenzato C, Zhao J, Pordy R, Baccara‐Dinet MT, Gipe DA, Geiger MJ, Farnier M. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J. 2015;36:2996–3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kereiakes DJ, Robinson JG, Cannon CP, Lorenzato C, Pordy R, Chaudhari U, Colhoun HM. Efficacy and safety of the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab among high cardiovascular risk patients on maximally tolerated statin therapy: the ODYSSEY COMBO I study. Am Heart J. 2015;169:906–915. [DOI] [PubMed] [Google Scholar]
- 48. Moriarty PM, Thompson PD, Cannon CP, Guyton JR, Bergeron J, Zieve FJ, Bruckert E, Jacobson TA, Kopecky SL, Baccara‐Dinet MT, Du Y, Pordy R, Gipe DA; ODYSSEY ALTERNATIVE Investigators . Efficacy and safety of alirocumab vs ezetimibe in statin‐intolerant patients, with a statin rechallenge arm: the ODYSSEY ALTERNATIVE randomized trial. J Clin Lipidol. 2015;9:758–769. [DOI] [PubMed] [Google Scholar]
- 49. Farnier M, Jones P, Severance R, Averna M, Steinhagen‐Thiessen E, Colhoun HM, Du Y, Hanotin C, Donahue S. Efficacy and safety of adding alirocumab to rosuvastatin versus adding ezetimibe or doubling the rosuvastatin dose in high cardiovascular‐risk patients: the ODYSSEY OPTIONS II randomized trial. Atherosclerosis. 2016;244:138–146. [DOI] [PubMed] [Google Scholar]
- 50. Roth EM, Moriarty PM, Bergeron J, Langslet G, Manvelian G, Zhao J, Baccara‐Dinet MT, Rader DJ; ODYSSEY CHOICE I Investigators . A phase III randomized trial evaluating alirocumab 300 mg every 4 weeks as monotherapy or add‐on to statin: ODYSSEY CHOICE I. Atherosclerosis. 2016;254:254–262. [DOI] [PubMed] [Google Scholar]
- 51. Stroes E, Guyton JR, Lepor N, Civeira F, Gaudet D, Watts GF, Baccara‐Dinet MT, Lecorps G, Manvelian G, Farnier M; ODYSSEY CHOICE I I Investigators . Efficacy and safety of alirocumab 150 mg every 4 weeks in patients with hypercholesterolemia not on statin therapy: the ODYSSEY CHOICE II Study. J Am Heart Assoc. 2016;5:e003421 DOI: 10.1161/jaha.116.003421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Raal FJ, Hovingh GK, Blom D, Santos RD, Harada‐Shiba M, Bruckert E, Couture P, Soran H, Watts GF, Kurtz C, Honarpour N, Tang L, Kasichayanula S, Wasserman SM, Stein EA. Long‐term treatment with evolocumab added to conventional drug therapy, with or without apheresis, in patients with homozygous familial hypercholesterolaemia: an interim subset analysis of the open‐label TAUSSIG study. Lancet Diabetes Endocrinol. 2017;5:280–290. [DOI] [PubMed] [Google Scholar]
- 53. Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, Kim JB, Scott R, Wasserman SM, Bays H; Mendel Investigators . Anti‐PCSK9 monotherapy for hypercholesterolemia: the MENDEL‐2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531–2540. [DOI] [PubMed] [Google Scholar]
- 54. Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, Bruckert E, Cho L, Dent R, Knusel B, Xue A, Scott R, Wasserman SM, Rocco M; Investigators G . Anti‐PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS‐2 randomized, placebo‐controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541–2548. [DOI] [PubMed] [Google Scholar]
- 55. Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, Somaratne R, Legg JC, Nelson P, Scott R, Wasserman SM, Weiss R; LAPLACE‐2 Investigators . Effect of evolocumab or ezetimibe added to moderate‐ or high‐intensity statin therapy on LDL‐C lowering in patients with hypercholesterolemia: the LAPLACE‐2 randomized clinical trial. JAMA. 2014;311:1870–1882. [DOI] [PubMed] [Google Scholar]
- 56. Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, Ceska R, Roth E, Koren MJ, Ballantyne CM, Monsalvo ML, Tsirtsonis K, Kim JB, Scott R, Wasserman SM, Stein EA; Descartes Investigators . A 52‐week placebo‐controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–1819. [DOI] [PubMed] [Google Scholar]
- 57. Raal FJ, Honarpour N, Blom DJ, Hovingh GK, Xu F, Scott R, Wasserman SM, Stein EA; Tesla Investigators . Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2015;385:341–350. [DOI] [PubMed] [Google Scholar]
- 58. Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA; Open‐Label Study of Long‐Term Evaluation against L. D. L. Cholesterol Investigators . Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–1509. [DOI] [PubMed] [Google Scholar]
- 59. Bays HE, Leiter LA, Colhoun HM, Thompson D, Bessac L, Pordy R, Toth PP. Alirocumab treatment and achievement of non‐high‐density lipoprotein cholesterol and apolipoprotein b goals in patients with hypercholesterolemia: pooled results from 10 phase 3 ODYSSEY trials. J Am Heart Assoc. 2017;6:e005639 DOI: 10.1161/jaha.117.005639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Farnier M, Gaudet D, Valcheva V, Minini P, Miller K, Cariou B. Efficacy of alirocumab in high cardiovascular risk populations with or without heterozygous familial hypercholesterolemia: pooled analysis of eight ODYSSEY Phase 3 clinical program trials. Int J Cardiol. 2016;223:750–757. [DOI] [PubMed] [Google Scholar]
- 61. Blom DJ, Djedjos CS, Monsalvo ML, Bridges I, Wasserman SM, Scott R, Roth E. Effects of evolocumab on vitamin E and steroid hormone levels: results from the 52‐week, phase 3, double‐blind, randomized placebo‐controlled DESCARTES Study. Circ Res. 2015;117:731–741. [DOI] [PubMed] [Google Scholar]
- 62. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ; ODYSSEY LONG TERM Investigators . Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 63. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR; Fourier Steering Committee Investigators . Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 64. Nicholls SJ, Puri R, Anderson T, Ballantyne CM, Cho L, Kastelein JJ, Koenig W, Somaratne R, Kassahun H, Yang J, Wasserman SM, Scott R, Ungi I, Podolec J, Ophuis AO, Cornel JH, Borgman M, Brennan DM, Nissen SE. Effect of evolocumab on progression of coronary disease in statin‐treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373–2384. [DOI] [PubMed] [Google Scholar]
- 65. Koren MJ, Sabatine MS, Giugliano RP, Langslet G, Wiviott SD, Kassahun H, Ruzza A, Ma Y, Somaratne R, Raal FJ. Long‐term low‐density lipoprotein cholesterol‐lowering efficacy, persistence, and safety of evolocumab in treatment of hypercholesterolemia: results up to 4 years from the open‐label OSLER‐1 extension study. JAMA Cardiol. 2017;2:598–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Sattar N, Preiss D, Robinson JG, Djedjos CS, Elliott M, Somaratne R, Wasserman SM, Raal FJ. Lipid‐lowering efficacy of the PCSK9 inhibitor evolocumab (AMG 145) in patients with type 2 diabetes: a meta‐analysis of individual patient data. Lancet Diabetes Endocrinol. 2016;4:403–410. [DOI] [PubMed] [Google Scholar]
- 67. Leiter LA, Zamorano JL, Bujas‐Bobanovic M, Louie MJ, Lecorps G, Cannon CP, Handelsman Y. Lipid‐lowering efficacy and safety of alirocumab in patients with or without diabetes: a sub‐analysis of ODYSSEY COMBO II. Diabetes Obes Metab. 2017;19:989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ganda OP, Plutzky J, Sanganalmath SK, Bujas‐Bobanovic M, Koren A, Mandel J, Letierce A, Leiter LA. Efficacy and safety of alirocumab among individuals with diabetes mellitus and atherosclerotic cardiovascular disease in the ODYSSEY phase 3 trials. Diabetes Obes Metab. 2018. Available at: https://onlinelibrary.wiley.com/doi/abs/10.1111/dom.13384. Accessed May 25, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ginsberg HN, Farnier M, Robinson JG, Cannon CP, Sattar N, Baccara‐Dinet MT, Lorenzato C, Bujas‐Bobanovic M, Louie MJ, Colhoun HM. Efficacy and safety of alirocumab: pooled analyses of 1048 individuals with diabetes mellitus from five placebo‐controlled Phase 3 studies of at least 52 weeks duration. Circulation. 2015;132:A17070. [Google Scholar]
- 70. Sattar N, Toth PP, Blom DJ, Koren MJ, Soran H, Uhart M, Elliott M, Cyrille M, Somaratne R, Preiss D. Effect of the proprotein convertase subtilisin/kexin type 9 inhibitor evolocumab on glycemia, body weight, and new‐onset diabetes mellitus. Am J Cardiol. 2017;120:1521–1527. [DOI] [PubMed] [Google Scholar]
- 71. Sabatine MS, Leiter LA, Wiviott SD, Giugliano RP, Deedwania P, De Ferrari GM, Murphy SA, Kuder JF, Gouni‐Berthold I, Lewis BS, Handelsman Y, Pineda AL, Honarpour N, Keech AC, Sever PS, Pedersen TR. Cardiovascular safety and efficacy of the PCSK9 inhibitor evolocumab in patients with and without diabetes and the effect of evolocumab on glycaemia and risk of new‐onset diabetes: a prespecified analysis of the FOURIER randomised controlled trial. Lancet Diabetes Endocrinol. 2017;5:941–950. [DOI] [PubMed] [Google Scholar]
- 72. Leiter LA, Muller‐Wieland D, Baccara‐Dinet MT, Letierce A, Samuel R, Cariou B. Efficacy and safety of alirocumab in people with prediabetes vs those with normoglycaemia at baseline: a pooled analysis of 10 phase III ODYSSEY clinical trials. Diabet Med. 2018;35:121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Blom DJ, Koren MJ, Roth E, Monsalvo ML, Djedjos CS, Nelson P, Elliott M, Wasserman SM, Ballantyne CM, Holman RR. Evaluation of the efficacy, safety and glycaemic effects of evolocumab (AMG 145) in hypercholesterolaemic patients stratified by glycaemic status and metabolic syndrome. Diabetes Obes Metab. 2017;19:98–107. [DOI] [PubMed] [Google Scholar]
- 74. Cariou B, Leiter LA, Muller‐Wieland D, Bigot G, Colhoun HM, Del Prato S, Henry RR, Tinahones FJ, Letierce A, Aurand L, Maroni J, Ray KK, Bujas‐Bobanovic M. Efficacy and safety of alirocumab in insulin‐treated patients with type 1 or type 2 diabetes and high cardiovascular risk: rationale and design of the ODYSSEY DM‐INSULIN trial. Diabetes Metab. 2017;43:453–459. [DOI] [PubMed] [Google Scholar]
- 75. Leiter LA, Cariou B, Muller‐Wieland D, Colhoun HM, Del Prato S, Tinahones FJ, Ray KK, Bujas‐Bobanovic M, Domenger C, Mandel J, Samuel R, Henry RR. Efficacy and safety of alirocumab in insulin‐treated individuals with type 1 or type 2 diabetes and high cardiovascular risk: the ODYSSEY DM‐INSULIN randomized trial. Diabetes Obes Metab. 2017;19:1781–1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Muller‐Wieland D, Leiter LA, Cariou B, Letierce A, Colhoun HM, Del Prato S, Henry RR, Tinahones FJ, Aurand L, Maroni J, Ray KK, Bujas‐Bobanovic M. Design and rationale of the ODYSSEY DM‐DYSLIPIDEMIA trial: lipid‐lowering efficacy and safety of alirocumab in individuals with type 2 diabetes and mixed dyslipidaemia at high cardiovascular risk. Cardiovasc Diabetol. 2017;16:70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ray KK, Leiter LA, Müller‐Wieland D, Cariou B, Colhoun HM, Henry RR, Tinahones FJ, Bujas‐Bobanovic M, Domenger C, Letierce A, Samuel R, Del Prato S. Alirocumab vs usual lipid‐lowering care as add‐on to statin therapy in individuals with type 2 diabetes and mixed dyslipidaemia: the ODYSSEY DM‐DYSLIPIDEMIA randomized trial. Diabetes Obes Metab. 2018;20:1479–1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jones PH, Bays HE, Chaudhari U, Pordy R, Lorenzato C, Miller K, Robinson JG. Safety of alirocumab (a PCSK9 monoclonal antibody) from 14 randomized trials. Am J Cardiol. 2016;118:1805–1811. [DOI] [PubMed] [Google Scholar]
- 79. Toth PP, Descamps O, Genest J, Sattar N, Preiss D, Dent R, Djedjos C, Wu Y, Geller M, Uhart M, Somaratne R, Wasserman SM; Proficio Investigators . Pooled safety analysis of evolocumab in over 6000 patients from double‐blind and open‐label extension studies. Circulation. 2017;135:1819–1831. [DOI] [PubMed] [Google Scholar]
- 80. Leiter LA, Tinahones FJ, Karalis DG, Bujas‐Bobanovic M, Letierce A, Mandel J, Samuel R, Jones PH. Alirocumab safety in individuals with and without diabetes mellitus: pooled data from 14 ODYSSEY trials. J Am Coll Cardiol. 2017;69:1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ference BA, Robinson JG, Brook RD, Catapano AL, Chapman MJ, Neff DR, Voros S, Giugliano RP, Davey Smith G, Fazio S, Sabatine MS. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375:2144–2153. [DOI] [PubMed] [Google Scholar]
- 82. Lotta LA, Sharp SJ, Burgess S, Perry JRB, Stewart ID, Willems SM, Luan J, Ardanaz E, Arriola L, Balkau B, Boeing H, Deloukas P, Forouhi NG, Franks PW, Grioni S, Kaaks R, Key TJ, Navarro C, Nilsson PM, Overvad K, Palli D, Panico S, Quiros JR, Riboli E, Rolandsson O, Sacerdote C, Salamanca EC, Slimani N, Spijkerman AM, Tjonneland A, Tumino R, van der A DL, van der Schouw YT, McCarthy MI, Barroso I, O'Rahilly S, Savage DB, Sattar N, Langenberg C, Scott RA, Wareham NJ. Association between low‐density lipoprotein cholesterol‐lowering genetic variants and risk of type 2 diabetes: a meta‐analysis. JAMA. 2016;316:1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Schmidt AF, Swerdlow DI, Holmes MV, Patel RS, Fairhurst‐Hunter Z, Lyall DM, Hartwig FP, Horta BL, Hypponen E, Power C, Moldovan M, van Iperen E, Hovingh GK, Demuth I, Norman K, Steinhagen‐Thiessen E, Demuth J, Bertram L, Liu T, Coassin S, Willeit J, Kiechl S, Willeit K, Mason D, Wright J, Morris R, Wanamethee G, Whincup P, Ben‐Shlomo Y, McLachlan S, Price JF, Kivimaki M, Welch C, Sanchez‐Galvez A, Marques‐Vidal P, Nicolaides A, Panayiotou AG, Onland‐Moret NC, van der Schouw YT, Matullo G, Fiorito G, Guarrera S, Sacerdote C, Wareham NJ, Langenberg C, Scott R, Luan J, Bobak M, Malyutina S, Pajak A, Kubinova R, Tamosiunas A, Pikhart H, Husemoen LL, Grarup N, Pedersen O, Hansen T, Linneberg A, Simonsen KS, Cooper J, Humphries SE, Brilliant M, Kitchner T, Hakonarson H, Carrell DS, McCarty CA, Kirchner HL, Larson EB, Crosslin DR, de Andrade M, Roden DM, Denny JC, Carty C, Hancock S, Attia J, Holliday E, O'Donnell M, Yusuf S, Chong M, Pare G, van der Harst P, Said MA, Eppinga RN, Verweij N, Snieder H; LifeLines Cohort study group , Christen T, Mook‐Kanamori DO, Gustafsson S, Lind L, Ingelsson E, Pazoki R, Franco O, Hofman A, Uitterlinden A, Dehghan A, Teumer A, Baumeister S, Dorr M, Lerch MM, Volker U, Volzke H, Ward J, Pell JP, Smith DJ, Meade T, Maitland‐van der Zee AH, Baranova EV, Young R, Ford I, Campbell A, Padmanabhan S, Bots ML, Grobbee DE, Froguel P, Thuillier D, Balkau B, Bonnefond A, Cariou B, Smart M, Bao Y, Kumari M, Mahajan A, Ridker PM, Chasman DI, Reiner AP, Lange LA, Ritchie MD, Asselbergs FW, Casas JP, Keating BJ, Preiss D, Hingorani AD; Ucleb consortium , Sattar N. PCSK9 genetic variants and risk of type 2 diabetes: a Mendelian randomisation study. Lancet Diabetes Endocrinol. 2017;5:97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Maki KC, Ridker PM, Brown WV, Grundy SM, Sattar N; The Diabetes Subpanel of the National Lipid Association Expert Panel . An assessment by the Statin Diabetes Safety Task Force: 2014 update. J Clin Lipidol. 2014;8:S17–S29. [DOI] [PubMed] [Google Scholar]
- 85. Colhoun HM, Ginsberg HN, Robinson JG, Leiter LA, Muller‐Wieland D, Henry RR, Cariou B, Baccara‐Dinet MT, Pordy R, Merlet L, Eckel RH. No effect of PCSK9 inhibitor alirocumab on the incidence of diabetes in a pooled analysis from 10 ODYSSEY Phase 3 studies. Eur Heart J. 2016;37:2981–2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cariou B, Si‐Tayeb K, Le May C. Role of PCSK9 beyond liver involvement. Curr Opin Lipidol. 2015;26:155–161. [DOI] [PubMed] [Google Scholar]
- 87. Cholesterol Treatment Trialists Collaboration , Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta‐analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Giugliano RP, Pedersen TR, Park JG, De Ferrari GM, Gaciong ZA, Ceska R, Toth K, Gouni‐Berthold I, Lopez‐Miranda J, Schiele F, Mach F, Ott BR, Kanevsky E, Pineda AL, Somaratne R, Wasserman SM, Keech AC, Sever PS, Sabatine MS; FOURIER Investigators . Clinical efficacy and safety of achieving very low LDL‐cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet. 2017;390:1962–1971. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Summary of Major US Guidelines/Consensus Statements for Individuals with DM
Table S2. Summary of Phase 3 Alirocumab ODYSSEY and Evolocumab PROFICIO Studies


