Abstract
Background
The relation between tea consumption and age‐related changes in high‐density lipoprotein cholesterol (HDL‐C) concentrations remains unclear, and longitudinal human data are limited. The aim of current study was to examine the relation between tea intake and longitudinal change in HDL‐C concentrations.
Methods and Results
Baseline (2006) tea consumption was assessed via a questionnaire, and plasma HDL‐C concentrations were measured in 2006, 2008, 2010, and 2012 among 80 182 individuals (49±12 years of age) who did not have cardiovascular diseases or cancer, or did not use cholesterol‐lowering agents both at baseline (2006) and during the follow‐up period (2006–2012). The associations between baseline tea consumption and rate of change in HDL‐C concentrations were examined using generalized estimating equation models. Tea consumption was inversely associated with a decreased rate of HDL‐C concentrations (P‐trend <0.0001) in the fully adjusted model. The adjusted mean difference in the HDL‐C decreased rate was 0.010 (95% confidence interval, 0.008, 0.012) mmol/L per year for tea consumers versus nonconsumers (never or less than once/month group). Interactions between tea consumption and age, sex, lifestyle scores, and metabolic syndrome (all P‐interaction <0.0001) were identified. The associations between greater tea consumption and slower decrease in HDL‐C concentrations were more pronounced in men, individuals aged 60 or older, individuals with a lower lifestyle score, and individuals with metabolic syndrome (all P‐trend <0.0001).
Conclusions
Tea consumption was associated with slower age‐related decreases in HDL‐C concentrations during 6 years of follow‐up.
Clinical Trial Registration
URL: http://www.chictr.org. Unique identifier: ChiCTR‐TNRC‐11001489.
Keywords: cardiovascular disease risk factors, catechins, high‐density lipoprotein cholesterol, lipids and lipoproteins, longitudinal cohort study, nutrition, polyphenols
Subject Categories: Epidemiology, Lifestyle, Diet and Nutrition, Risk Factors, Cardiovascular Disease
Clinical Perspective
What Is New?
To our knowledge, this is the first longitudinal cohort study to investigate the association between tea intake and time‐dependent change in high‐density lipoprotein cholesterol concentrations among over 80 000 participants with 6‐year follow‐up.
What Are the Clinical Implications?
Tea consumption was related to a slow decrease of high‐density lipoprotein cholesterol concentration during the follow‐up.
The potential impact of tea on high‐density lipoprotein cholesterol change during the 6‐year follow‐up period could be associated with 8% lower cardiovascular disease risk.
Further studies are warranted to replicate our finding.
Introduction
Cardiovascular disease (CVD) remains the leading cause of morbidity and mortality worldwide. In 2016, 17.6 million deaths were caused by CVD; the death from cardiovascular diseases increased by 14.5% from 2006 and 2016.1 Previous epidemiological studies suggest that greater tea consumption is related to lower cardiovascular disease (CVD) risk2, 3; 1 to 3 or more cups of green or black tea per day is associated with a 20% to 30% risk reduction in stroke and CVD.3, 4 Produced from Camellia sinensis and rich in polyphenols and catechins, green and black tea were reported to exert a cardioprotective effect via antioxidation of proteins, DNAs, and lipids; antiproliferation of vascular smooth muscle cell; anti‐inflammation in cardiovascular cells; and improvement in endothelial functions, blood pressure,5 and lipid profile.6
Clinical trials have reported that tea beverages lower total cholesterol (TC) and low‐density lipoprotein cholesterol concentrations.7, 8, 9 However, findings on the potential effects of tea on high‐density lipoprotein cholesterol (HDL‐C) are mixed. Some clinical trials reported a significant increase,10, 11, 12, 13 whereas other trials reported null effects14 on HDL‐C concentrations. Of note, no clinical trials examined the potential dose‐response effect of tea and were limited by the small sample sizes (10 to ≈200) and short intervention period (3 weeks to 3 months). The long‐term effect of tea on HDL‐C concentrations remains elucidated.
In this context, we hypothesized that tea consumption was associated with slower longitudinal decrease in HDL‐C concentrations. We tested this hypothesis in a large community‐based cohort including over 80 000 participants with 6‐year follow‐up. We also assessed the association between tea consumption and other lipid indices, including triglyceride (TG)/HDL‐C ratio and TC/HDL‐C ratio.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Population
The current study was based on the Kailuan Study (Chinese Clinical Trial Registry number: ChiCTR‐TNRC‐11001489), which is a prospective cohort study conducted in the Kailuan community in Tangshan city in China. Details of this study have been described previously.15, 16 Briefly, in 2006, 101 510 subjects (81 110 men and 20 400 women, aged 18–98 years) were recruited and underwent a standardized questionnaire survey and physical examination and laboratory assessments. Subsequent biennial evaluations of the above measurement continued through 2012. A total of 90 299 participants were followed at least twice and were included in the current study. We further excluded participants who had myocardial infarction, stroke, or cancer diagnosed both at baseline and during follow‐up (n=4, 932), reported to use lipid‐lowering agents (n=1904) or had missing information on tea consumption at baseline (n=3197) or missing information on HDL‐C reassessment during the follow‐up (n=235), leaving 80 182 participants included in the final analysis (Figure 1).
Figure 1.
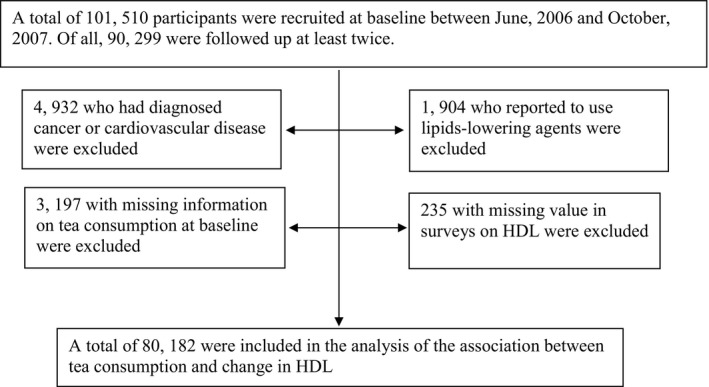
Flowchart of the study.
The Ethics Committee of the Kailuan General Hospital approved this study. All participants provided written informed consent.
Assessment of Tea Consumption
Information on tea consumption was collected via a questionnaire. Participants were asked to report the average frequency and type of tea they consumed in the past 5 years. For frequency of tea consumption, 5 choices were provided in the questionnaire, including never, less than once/month, 1 to 3 times/month, 1 to 3 times/week, and ≥4 times/week. Types of tea consumed included black tea, green tea, and other types of tea such as herbal tea, flowering tea, and non–Camellia sinensis tea.
Assessment of Lipid Profiles
Overnight fasting (8–12 hours) venous blood samples were collected using vacuum tubes containing EDTA during each study visit and were then separated and stored at −80°C for subsequent analyses. As described previously,15 plasma concentrations of TC and TG were measured using a colorimetric enzymatic method (Mind Bioengineering Co Ltd, Shanghai, China). The upper detectable limits were 20.7 and 11.3 mmol/L, respectively. HDL‐C and low‐density lipoprotein cholesterol were measured by the direct test method (Mind Bioengineering Co Ltd, Shanghai, China), and the upper detectable limits were 12.9 and 3.88 mmol/L, respectively. Less than 0.1% of measured values were above or below 5% of either limit. The interassay coefficients of variation for all measurements were <10%. All the plasma samples were analyzed using an auto‐analyzer (Hitachi 747; Hitachi, Tokyo, Japan) at the central laboratory at Kailuan General Hospital.
Assessment of Covariates
We collected information on age; sex; socioeconomic data including education level, monthly income level, and occupation; lifestyle behaviors including smoking, alcohol consumption, physical activity, and salt consumption; and medical history via a standardized questionnaire in 2006. Based on the physical and mental requirements of their tasks in their job and the working environment, we categorized the occupation type into “white collar,” who primarily do mental work, sitting in an office; “coal miners,” who do coal mining; and “other blue collar,” who do mostly physical work but not mine coal, either under or out of the ground. Physical activity was evaluated by self‐reported frequency of physical activity (20+ minutes per time) during leisure time, with response choices of never, sometimes, and 4+ times per week. According to participants’ responses, they were classified as inactive, moderately active, and active, as detailed previously.15, 16
Weight, height, waist circumference, and blood pressures were measured during the interview. Body mass index was calculated as weight (kg)/height (m2). Blood pressure was measured twice, on the left arm with participants in the seated position after at least 5 minutes’ rest, using a mercury sphygmomanometer. The average of the 2 readings was used for analysis. Hypertension was defined as having a systolic blood pressure ≥140 mm Hg or a diastolic blood pressure of ≥90 mm Hg, or self‐report history of hypertension. Prehypertension was defined as systolic blood pressure of 120 to 139 mm Hg or a diastolic blood pressure of 80 to 90 mm Hg.
Other biochemical parameters including blood glucose concentration, plasma high‐sensitivity C‐reactive protein, and alanine aminotransferases were measured using the aforementioned auto‐analyzer (Hitachi 747; Hitachi, Tokyo, Japan). Hyperglycemia was defined as fasting blood glucose of ≥7.0 mmol/L, or a self‐report history of diagnosis; impaired fasting blood glucose was defined as fasting blood glucose of 5.6 to 6.9 mmol/L. Fatty liver was diagnosed based on ultrasonographic liver features according to established criteria and classified as none, mild, and severe.17, 18
Statistical Analysis
Statistical analyses were performed using SAS software, version 9.4 (SAS institute, Cary, NC). Confidence intervals were estimated at the 95% level and P values <0.05 were regarded as significant for 2‐sided tests. Categorical data were presented as percentage, and continuous data were presented as mean ± standard error. Generalized estimating equation models were used to estimate the association between tea consumption and 6‐year longitudinal changes in HDL‐C and other lipid indices. Tea consumption was categorized into 4 groups: never or less than once/month, 1 to 3 times/month, 1 to 3 times/week, or ≥4 times/week. Types of tea consumed were grouped as “green tea,” “black tea,” and “other tea.” For each type of tea, participants who did not drink any tea or did not drink the indicated tea type were grouped as “never or less than once/month.” The group of never or less than once/month was treated as the reference group. We fit 3 multivariate models. In model 1, we adjusted for age and sex. In model 2, we further adjusted for the aforementioned covariates. In model 3, we adjusted for all the covariates in model 2 plus plasma concentrations of TG, TC, or low‐density lipoprotein cholesterol when appropriate. Trends in mean differences in HDL‐C change rate were tested for significance by treating the group number of tea consumption as an ordinal variable. For each model, we tested different working correlation structures including independent, exchangeable, autoregressive, and unstructured. We reported results based on the unstructured correlation structure because it requires minimum assumption. Robust standard errors were used for parameter estimates analysis. To identify the best‐fitting model, we also considered the spline function of age and interactions between covariates. However, these did not improve the model fit materially and similar results were observed (data not shown). We therefore did not include those models in our results.
To test the robustness of the main findings, we conducted several sensitivity analyses. First, we conducted fully adjusted models among participants after excluding smokers, alcohol consumers, or those who were overweight/obese (body mass index >24 kg/m2),19 hypertensive, hyperglycemic, or who had high C‐reactive protein (≥3 mg/L), or fatty liver because all these factors could affect HDL‐C concentrations. Second, we used propensity score adjustment to balance potential confounders.20 Propensity scores were derived from predicted probabilities of being in each tea consumption group estimated in logistic regression models that contained all the covariates listed in model 3. The generalized estimating equation models were stratified across fifths of the propensity score. We also adjusted the model using the inverse probability of treatment weight.
We explored potential interactions between tea consumption and age, sex, smoking status, and alcohol intake, in relation to changes in HDL‐C by adding multiplicative terms in the generalized estimating equation models. Because previous clinical studies suggested that the presence of metabolic symptoms could modify the relation between tea and lipid level,7, 9 we also examined the potential interactions between tea consumption and metabolic syndrome and lifestyle score. Metabolic syndrome was defined as the presence of 3 or more of the following: (1) elevated waist circumference of ≥90 cm in men or ≥80 cm in women; (2) triglyceride ≥1.7 mmol/L or drug treatment for elevated triglyceride; (3) reduced HDL‐C of <1.03 mmol/L in men or <1.3 mmol/L in women; (4) elevated blood pressure of ≥130 mm Hg systolic blood pressure or ≥85 mm Hg diastolic blood pressure, or using antihypertensive drug treatment; and (5) elevated fasting blood glucose of 5.6 mmol/L or drug treatment for elevated blood glucose.21 The lifestyle score was created based on body mass index (scored 0, 1, and 2 for ≥28, 24–27, and <24 kg/m2, respectively), physical activity (scored 0, 1, and 2 for inactive, moderately active, and active, respectively), smoking status (scored 0, 1, and 2 for 1+ times/day, occasional, and never/past, respectively), alcohol consumption (scored 0, 1, and 2 for never and 2+ servings/day, past, and 0.1–1.9 servings/day, respectively), and salt intake (scored 0, 1, and 2 for <6 g/day, 6 –12 g/day, and >12 g/day, respectively).22 This lifestyle score was significantly associated with risk of diabetes mellitus in previous studies.22
Results
Compared with the never or less than once/month group, other tea consumption groups had a higher education level and higher income level; were more likely to be white collar, smokers, or drinkers; and were more physically active (Table 1).
Table 1.
Baseline Characteristics in 80 182 Kailuan Study Participants by Tea Consumption in 2006a
| Tea Consumption | ||||
|---|---|---|---|---|
| Never or Less Than Once/Month (n=63 611) | 1 to 3 Times/Month (n=5141) | 1 to 3 Times/Week (n=4099) | ≥4 Times/Week (n=7331) | |
| Sex, % | ||||
| Men | 76.0 | 82.5 | 85.4 | 90.8 |
| Age, y | 49.4±0.05 | 43.8±0.17 | 46.6±0.19 | 51.8±0.14 |
| Education, % | ||||
| Illiterate | 8.9 | 7.6 | 8.2 | 13.0 |
| Middle | 75.5 | 48.0 | 51.5 | 54.6 |
| College | 16.6 | 44.3 | 40.2 | 32.3 |
| Occupation, % | ||||
| White collar | 5.8 | 15.2 | 15.7 | 14.7 |
| Coal miner | 21.1 | 31.2 | 28.0 | 27.4 |
| Other blue collar | 73.0 | 53.6 | 56.3 | 57.6 |
| Income level (RMB/month), % | ||||
| <600 | 26.6 | 37.9 | 37.0 | 38.6 |
| 600 to 1000 | 61.6 | 37.4 | 38.6 | 37.3 |
| 1000+ | 11.7 | 24.8 | 24.3 | 23.9 |
| Physical activity, % | ||||
| Inactive | 8.1 | 12.1 | 12.3 | 12.3 |
| Moderate active | 79.7 | 69.9 | 66.5 | 54.3 |
| Active | 12.1 | 17.9 | 21.0 | 33.1 |
| Smoking status, % | ||||
| Never | 67.1 | 37.9 | 36.6 | 29.5 |
| Past | 4.0 | 8.0 | 8.6 | 9.5 |
| Current | 28.8 | 54.0 | 54.7 | 60.8 |
| Alcohol drinking status, % | ||||
| Never | 66.8 | 27.4 | 26.7 | 27.0 |
| Past | 2.7 | 4.4 | 4.4 | 5.1 |
| Current | 30.4 | 68.0 | 68.8 | 67.7 |
| Fatty liver, % | ||||
| None | 89.6 | 89.9 | 89.0 | 85.9 |
| Mild | 8.3 | 8.0 | 8.8 | 11.1 |
| Severe | 1.7 | 1.6 | 1.7 | 2.6 |
| Blood glucose status, % | ||||
| Normoglycemia | 71.4 | 71.9 | 70.0 | 67.0 |
| Impaired fasting blood glucose | 20.2 | 20.8 | 22.0 | 22.0 |
| Hyperglycemia | 7.9 | 6.9 | 7.7 | 10.5 |
| Blood pressure status, % | ||||
| Normotensive | 21.0 | 29.8 | 27.2 | 19.4 |
| Prehypertension | 37.5 | 38.0 | 37.5 | 35.0 |
| Hypertension | 41.4 | 32.0 | 35.2 | 45.5 |
| C‐reactive protein,b mg/L | 0.71±1.01 | 0.83±1.02 | 0.79±1.02 | 0.79±1.02 |
| Body mass index, kg/m2 | 24.9±0.01 | 25.0±0.05 | 25.1±0.05 | 25.3±0.04 |
| Waist circumference, cm | 86.5±0.04 | 86.1±0.13 | 86.3±0.15 | 86.8±0.11 |
Means±SEs (all such values unless otherwise indicated) adjusted for age and sex.
C‐reactive protein values are geometric means±SEs.
Mean HDL‐C concentration decreased during 6 years of follow‐up. Frequent tea consumption was associated with a slower rate of decrease in HDL‐C concentrations in an age‐ and sex‐adjusted model (P‐trend <0.0001) (Table 2). With further adjustment for other potential confounders, results did not materially change. The adjusted mean difference in HDL‐C decrease rate was 0.010 (95% confidence interval, 0.008, 0.012) mmol/L per year, for tea consumers versus never or less than once/month. Consistently, significant improvement (lower rate of increase) in the TG/HDL‐C ratio and TC/HDL‐C ratio was observed in the most extreme tea consumption group (Table 2). Sensitivity analyses excluding participants who were smokers, alcohol users, hyperglycemic, hypertensive, or obese, or who had fatty liver or elevated high‐sensitivity C‐reactive protein, separately, generated similar results (Table S1). Using the propensity score did not change our results appreciably (Table S2). When different types of tea (ie, green tea, black tea, and other tea) were examined, similar significant associations were observed. The effect of green tea (adjusted mean difference=0.014 for green tea consumers versus never or less than once/month) was stronger than black (adjusted mean difference=0.009) and other tea (adjusted mean difference=0.006) (Table 3).
Table 2.
Mean Differences (and 95% Confidence Intervals) in Change Rate in HDL‐C (mmol/L per year), TG/HDL‐C Ratio, and TC/HDL‐C Ratio During 2006–2012 by Tea Consumption in 2006a
| Tea Consumption | P for Trend | ||||
|---|---|---|---|---|---|
| Never or Less Than Once/Month (n=63 611) | 1 to 3 Times/Month (n=5141) | 1 to 3 Times/Week (n=4099) | ≥4 Times/Week (n=7331) | ||
| Change in HDL‐C | |||||
| Model 1 | 0 (ref) | 0.010 (0.007, 0.013) | 0.008 (0.005, 0.011) | 0.010 (0.007, 0.012) | <0.0001 |
| Model 2 | 0 (ref) | 0.010 (0.007, 0.013) | 0.008 (0.006, 0.011) | 0.010 (0.007, 0.012) | <0.0001 |
| Model 3 | 0 (ref) | 0.011 (0.008, 0.014) | 0.009 (0.006, 0.012) | 0.010 (0.008, 0.013) | <0.0001 |
| Change in TG/HDL‐C ratio | |||||
| Model 3 | 0 (ref) | 0.003 (−0.010, 0.016) | 0.011 (−0.013, 0.035) | −0.017 (−0.031, −0.003) | 0.16 |
| Change in TC/HDL‐C ratio | |||||
| Model 3 | 0 (ref) | 0.002 (−0.030, 0.033) | −0.007 (−0.020, 0.005) | −0.015 (−0.024, −0.005) | 0.007 |
HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TC, total cholesterol; and TG, triglyceride.
Model 1 adjusted age, sex (men or women); model 2 adjusted age, sex (men or women), education (illiterate, middle, or college), income (<600, 600–1000, or >1000 RMB/month), occupation (white collar, coal miner, or other blue collar), physical activity (inactive, moderate, or active), smoking status (never, past, or current), alcohol drinking status (never, past, or current), blood glucose status (normoglycemia, impaired fasting blood glucose, or hyperglycemia), blood pressure status (normotensive, prehypertension, or hypertension), body mass index (in kg/m2; <24, 24–27.9, 28–29.9, or ≥30), waist circumference (<85 or ≥85 cm for women, and <90 or ≥90 cm for men), C‐reactive protein (<1, 1–2.9, or ≥3 mg/L), fatty liver (none, mild, or severe); model 3 adjusted all the variables in model 2, plus TG and LDL‐C for change in HDL‐C, plus TG for change in TC/HDL‐C ratio, plus LDL‐C for change in TG/HDL ratio. Generalized estimating equation models were used to model change rates and to test differences in change rates compared with the never or less than once/month group.
Table 3.
Mean Differences (and 95% Confidence Intervals) in HDL‐C Change Rate From 2006 to 2012 by Tea Type and Consumption in 2006a
| Tea Consumption | P for Trend | ||||
|---|---|---|---|---|---|
| Never or Less Than Once/Month | 1 to 3 Times/Month | 1 to 3 Times/Week | ≥4 Times/Week | ||
| Black tea | |||||
| N | 79 450 | 282 | 194 | 256 | |
| Change in HDL‐C | 0 (ref) | 0.006 (−0.002, 0.015) | 0.008 (−0.004, 0.019) | 0.013 (0.003, 0.023) | 0.002 |
| Change in TG/HDL‐C ratio | 0 (ref) | −0.028 (−0.055, −0.001) | −0.009 (−0.043, 0.025) | −0.049 (−0.073, −0.025) | <0.0001 |
| Change in TC/HDL‐C ratio | 0 (ref) | −0.016 (−0.040, 0.009) | −0.004 (−0.044, 0.035) | −0.025 (−0.053, 0.003) | 0.07 |
| Green tea | |||||
| N | 75 885 | 1344 | 1143 | 1810 | |
| Change in HDL‐C | 0 (ref) | 0.014 (0.010, 0.019) | 0.014 (0.009, 0.019) | 0.013 (0.009, 0.017) | <0.0001 |
| Change in TG/HDL‐C ratio | 0 (ref) | −0.002 (−0.021, 0.016) | 0.008 (−0.027, 0.042) | −0.025 (−0.043, −0.007) | 0.07 |
| Change in TC/HDL‐C ratio | 0 (ref) | −0.017 (−0.030, −0.005) | −0.014 (−0.033, 0.005) | −0.025 (−0.036, −0.013) | <0.0001 |
| Other tea | |||||
| N | 69 669 | 3096 | 2475 | 4942 | |
| Change in HDL‐C | 0 (ref) | 0.008 (0.004, 0.012) | 0.003 (−0.0004, 0.006) | 0.007 (0.004, 0.011) | <0.0001 |
| Change in TG/HDL‐C ratio | 0 (ref) | 0.009 (−0.008, 0.025) | 0.023 (−0.012, 0.059) | −0.011 (−0.030, 0.007) | 0.81 |
| Change in TC/HDL‐C ratio | 0 (ref) | 0.015 (−0.036, 0.066) | −0.0003 (−0.018, 0.017) | −0.009 (−0.020, 0.001) | 0.38 |
HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; TC, total cholesterol; and TG, triglyceride.
All models adjusted age, sex (men or women), education (illiterate, middle, or college), income (<600, 600–1000, or >1000 RMB/month), occupation (white collar, coal miner, or other blue collar), physical activity (inactive, moderate, or active), smoking status (never, past, or current), alcohol drinking status (never, past, or current), blood glucose status (normoglycemia, impaired fasting blood glucose, or hyperglycemia), blood pressure status (normotensive, prehypertension, or hypertension), body mass index (in kg/m2; <24, 24–27.9, 28–29.9, or ≥30), waist circumference (<85 or ≥85 cm for women, and <90 or ≥90 cm for men), C‐reactive protein (<1, 1–2.9, or ≥3 mg/L), fatty liver (none, mild, or severe). Models were further adjusted for TG and LDL‐C for change in HDL‐C, for TG for change in TC/HDL‐C ratio, for LDL‐C for change in TG/HDL ratio. Generalized estimating equation models were used to model change rates and to test differences in change rates compared with the never or less than once/month group.
There were significant interactions between tea consumption and age, sex, lifestyle scores, and presence of metabolic syndrome (all P‐interaction <0.0001), in relation to the changes in HDL‐C concentrations (Figure 2). Men, individuals aged ≥60, individuals with a lifestyle score of ≤5, or individuals with metabolic syndrome exhibited the strongest dose‐response association between higher tea consumption and slower decrease in HDL‐C concentrations.
Figure 2.
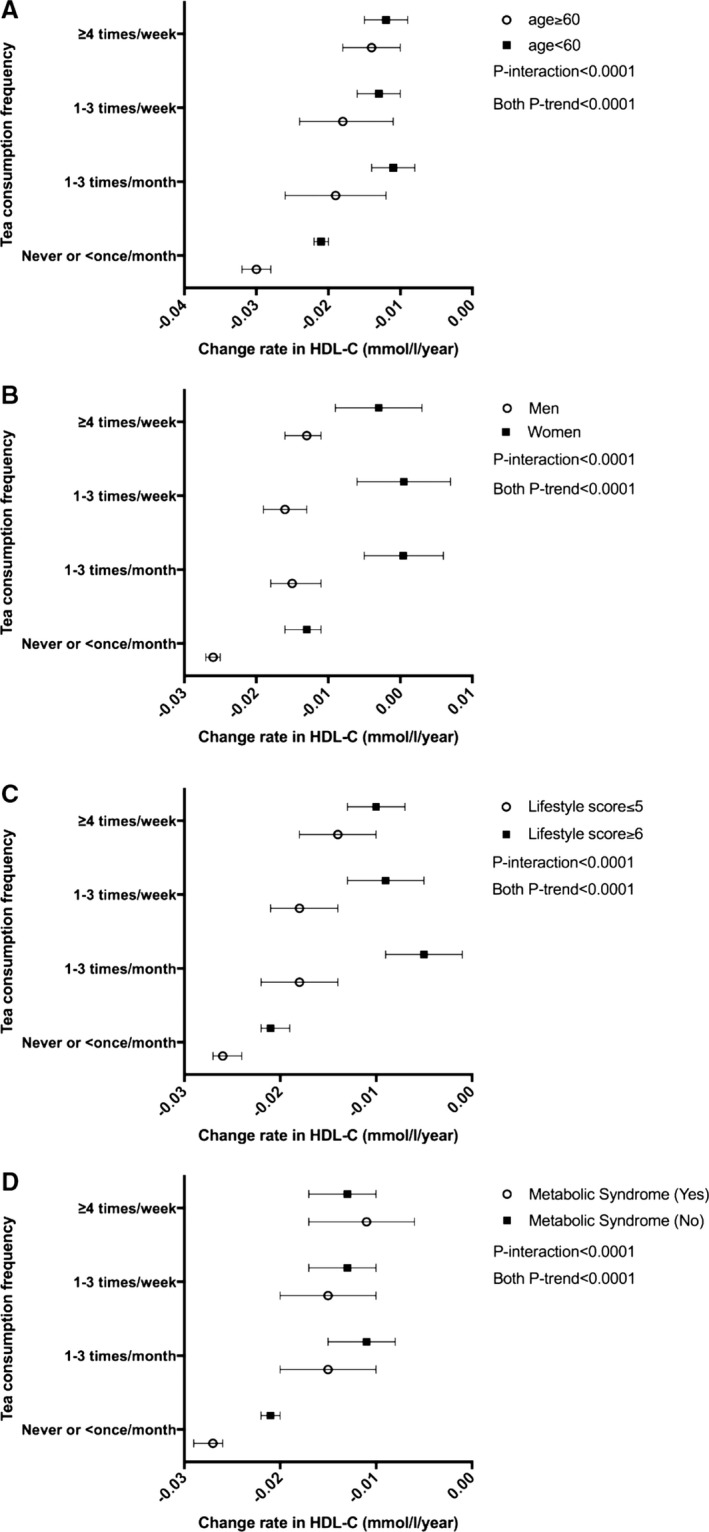
Time‐dependent decrease rate (95% confidence interval) in HDL‐C concentrations (mmol/L per year) by tea consumption frequency in subgroups by age (A), sex (B), lifestyle score (C), and metabolic syndrome (D). Tea consumption was categorized into 4 groups: never or less than once/month, 1 to 3 times/month, 1 to 3 times/week, or ≥4 times/week. The model was adjusted for age, sex (men or women), education (illiterate, middle, or college), income (<600, 600–1000, or >1000 RMB/month), occupation (white collar, coal miner, or other blue collar), physical activity (inactive, moderate, or active), smoking status (never, past, or current), alcohol drinking status (never, past, or current), blood glucose status (normoglycemia, impaired fasting blood glucose, or hyperglycemia), blood pressure status (normotensive, prehypertension, or hypertension), body mass index (in kg/m2; <24, 24–27.9, 28–29.9, or ≥30), waist circumference (<85 or ≥85 cm for women, and <90 or ≥90 cm for men), C‐reactive protein (<1, 1–2.9, or ≥3 mg/L), fatty liver (none, mild, or severe), low‐density lipoprotein cholesterol, and triglyceride. Generalized estimating equation models were used to model change rates and to test differences in change rates compared with the never or less than once/month group. Linear trends in HDL‐C change rate across all the groups were tested for significance by treating the group number of tea consumption as ordinal variable. All P‐interaction <0.01.
Discussion
In this study, we observed that tea consumption was associated with slower aged‐related decreases in HDL‐C concentration and slower increases in the TG/HDL‐C and TC/HDL‐C ratios. Previous clinical trials have reported that an increase in HDL‐C concentration of 0.1 mmol/L was associated with a risk reduction in CVD of 13.3%.23 In this study, we found that tea consumption was related to a slower decrease of ≈0.01 mmol/L per year in HDL‐C concentration, which accumulated to 0.06 mmol/L during the 6‐year follow‐up period. This would be predicted to reduce CVD risk by 8%. Compared to the mean difference in HDL‐C change rate between moderate alcohol drinkers and nondrinkers observed in the same population,15 the association between tea consumption and longitudinal change in HDL‐C is modest.
Several observational studies have investigated the association between tea consumption and HDL‐C concentrations.24, 25, 26, 27, 28 The findings have been mixed. Some studies have reported no association between green or black tea and HDL‐C concentrations,26, 27, 28 whereas others have reported a significant positive association between tea consumption and HDL‐C concentration.24, 25 Of note, those observational studies were cross‐sectional study design, making it impossible to infer the temporal association between tea consumption and lipid profiles.
Meta‐analyses that have pooled clinical trials reported that there was no significant effect of green or black tea and HDL‐C concentrations.7, 8, 9 However, in the participants at highest cardiovascular disease risk, a nonsignificant trend was observed for a positive association between tea consumption and HDL‐C concentrations, whereas no such trend was observed in the healthy participants.7, 8, 9 Trials conducted in participants with chronic conditions including obesity, hypertension, or other metabolic symptoms and durations >12 weeks reported significant increases in HDL‐C concentrations resulting from tea consumption.11, 13, 29, 30 Consistent with those data, in the current study we examined the long‐term effects of tea consumption on HDL‐C concentrations and found a more pronounced association between higher tea consumption and slower decreases in HDL‐C concentration over a 6‐year period in individuals aged ≥60 years or at elevated risk of developing metabolic syndrome. This finding suggests that tea consumption could have important clinical implication in individuals at elevated risk for CVD. Because clinical trials targeting the use of pharmacotherapy to increase HDL‐C concentration failed to show improvement on CVD outcome, it would be of interest to determine whether the effect of tea or other lifestyle intervention on HDL‐C concentration would reduce CVD events.
We did not observe a clear dose‐response relationship between tea consumption and HDL‐C concentration changes in our primary analysis. This suggests an alternative interpretation: The observed association between tea consumption and HDL‐C concentration changes could be confounded by healthy lifestyle, and tea consumption is a marker for healthier lifestyles. We thus created a lifestyle score based on several factors22 and did subgroup analysis stratified by the score. We observed that tea consumption was associated with slower HDL‐C decrease in both lifestyle groups. Interestingly, we observed a significant dose‐response relationship between tea consumption and HDL‐C change rate among those who scored lower on the lifestyle pattern scale. These findings suggest that healthy lifestyle does not fully explain the observed tea‐HDL‐C relationship. The more pronounced dose‐response relationship between tea consumption and HDL‐C change in participants with a less healthy lifestyle could be due to their strong relationship with metabolic disorders or a modest association between tea consumption and change in HDL‐C concentrations. The presence of other healthy lifestyle factors, such as moderate alcohol consumption, physical activity, and nonsmoking, which have a stronger impact on HDL‐C, could contribute significantly for a better HDL‐C profile versus tea consumption.
TG/HDL‐C and TC/HDL‐C ratios increased more slowly in individuals with greater tea consumption relative to never or less than once/month tea drinkers. Similar results were observed in some12, 31 but not all previous studies.30, 32 Total/HDL cholesterol reflects the balance between the cholesterol carried by atherogenic and protective lipoproteins.33 TG/HDL‐C is a good atherogenic index of plasma and is inversely associated with low‐density lipoprotein cholesterol size. Lower ratio values are associated with more favorable for cardiovascular health.34 This observation is consistent with previous findings that tea consumption was associated with lower CVD risk.2, 3, 35
Consistent with findings from animal studies36 and clinical studies,8 we observed that green tea had a stronger effect than black tea on plasma HDL‐C concentrations. It is thought that polyphenols in tea (eg, phenols and catechins) could account for its benefits in lipid metabolism via antioxidation and inhibition of lipid absorption.6, 11, 37, 38 In vitro green tea has been reported to have ≈2.5 more antioxidant capacity than black tea.39 Caffeine, a component in both green and black tea, has been found to be associated with higher HDL‐C concentrations.40 Because Chinese generally have lower coffee consumption,41 tea is the major dietary contributor for caffeine. Relative to green or black tea, other teas appeared to have less effect on HDL‐C in our study. This could be attributable to lower amounts of tea‐related components (eg, catechins and caffeine) in these teas. Of note, in the current study, we did not collect data on the specific type of teas other than black and green.
Results from this study should be interpreted with caution because of several limitations. First, self‐reported tea consumption could introduce misclassification. However, nondifferential misclassification of the exposure would attenuate the observed association between tea intake and change in HDL‐C concentration. Preparation methods including the amount of the tea consumed each time, brewing time, and water temperature affect the polyphenol and caffeine content42 and thus affect HDL‐C concentrations. Furthermore, limited by the options provided in the questionnaire, we could not examine the effect of consuming tea more than once a day. However, because only 9% of participants consumed tea 4+ times/week, the percentage of the population who consumed more than once per day should be very low. We did not collect household information; thus, we could not evaluate if individuals’ tea consumption was clustered based on household. Although we controlled a wide range of potential confounders, some residual confounding might still exist. We did not collect coffee consumption information in the current study. However, coffee is not a popular beverage in the Chinese population41; daily coffee consumption was 1.1% based on the 2011 China Health and Nutrition Survey.41 Confounding caused by lack of information on coffee consumption should be modest in this context. Our study is also limited by lack of comprehensive dietary intake information such as fruits, vegetables, whole grains, nuts, or red and processed meat. However, in the primary analysis, we controlled some closely diet‐related factors, including body mass index, salt intake, and alcohol intake, which could reduce the confounding caused by diet to some extent. Finally, the participants were all from the Kailuan community. They are not nationally representative of the Chinese population. Compared with the general Chinese adult population, they are older, and the proportion of men is higher. These factors may limit the generalizability of our findings. However, the results represent a large cohort of individuals living in China who have a wide range of tea intake and a low intake of coffee.
In conclusion, we observed that tea consumption was associated with a slower age‐related decrease in blood HDL‐C concentrations during a 6‐year period. The data must be interpreted cautiously because the association is modest and might be confounded by other factors; however, over a long period of time, it could have a clinically significant impact on CVD risk. Future longitudinal studies from other populations with different socioeconomic backgrounds should be warranted to confirm the findings from the current study.
Author Contributions
Gao and Wu designed the research; Li, Wu, Xing, Zhao, and Wang conducted the research; Huang and Li analyzed the data; Ranjbar and Huang conducted literature review; Huang wrote the article; Bao helped with data analysis and revised article; Shearer and Lichtenstein provided critical study oversight and contributed critical revision and comments of the article for important intellectual content; Gao had primary responsibility for final content. All authors read and approved the final article.
Sources of Funding
This study was funded by NIH/NINDS 1R21NS08723501A1. None of the sponsors participated in the design of study or in the collection, analysis, or interpretation of the data. No commercial or industry funder provided financial support for the research.
Disclosures
None.
Supporting information
Table S1. Sensitivity Analyses for Mean Differences in HDL‐C Concentration, TC/HDL‐C Ratio and TG/HDL‐C Ratio Change During 6‐Year Follow‐Up by Tea Consumption in 2006*,†
Table S2. Longitudinal Change in HDL‐C (mmol/L per year) by Tea Consumption in 2006 Using Propensity Score
(J Am Heart Assoc. 2018;7:e008814 DOI: 10.1161/JAHA.118.008814.)
Contributor Information
Shouling Wu, Email: drwusl@163.com.
Xiang Gao, Email: xxg14@psu.edu.
References
- 1. GBD 2016 Causes of Death Collaborators G 2016 C of D . Global, regional, and national age‐sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arab L, Khan F, Lam H. Tea consumption and cardiovascular disease risk 1–3. Am J Clin Nutr. 2013;98:1651S–1659S. [DOI] [PubMed] [Google Scholar]
- 3. Pang J, Zhang Z, Zheng T, Bassig BA, Mao C, Liu X, Zhu Y, Shi K, Ge J, Yang Y, Huang D , Bai M , Peng Y. Green tea consumption and risk of cardiovascular and ischemic related diseases: a meta‐analysis. Int J Cardiol. 2016;202:967–974. [DOI] [PubMed] [Google Scholar]
- 4. Arab L, Liu W, Elashoff D. Green and black tea consumption and risk of stroke: a meta‐analysis. Stroke. 2009;40:1786–1792. [DOI] [PubMed] [Google Scholar]
- 5. Szulińska M, Stępień M, Kręgielska‐Narożna M, Suliburska J, Skrypnik D, Bąk‐Sosnowska M, Kujawska‐Łuczak M, Grzymisławska M, Bogdański P. Effects of green tea supplementation on inflammation markers, antioxidant status and blood pressure in NaCl‐induced hypertensive rat model. Food Nutr Res. 2017;61:1295525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stangl V, Dreger H, Stangl K, Lorenz M. Molecular targets of tea polyphenols in the cardiovascular system. Cardiovasc Res. 2007;73:348–358. [DOI] [PubMed] [Google Scholar]
- 7. Onakpoya I, Spencer E, Heneghan C, Thompson M. The effect of green tea on blood pressure and lipid profile: a systematic review and meta‐analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. 2014;24:823–836. [DOI] [PubMed] [Google Scholar]
- 8. Zheng X, Xu Y, Li S, Liu X, Hui R, Huang X. Green tea intake lowers fasting serum total and LDL cholesterol in adults: a meta‐analysis of 14 randomized controlled trials. Am J Clin Nutr. 2011;94:601–610. [DOI] [PubMed] [Google Scholar]
- 9. Zhao Y, Asimi S, Wu K, Zheng J, Li D. Black tea consumption and serum cholesterol concentration: systematic review and meta‐analysis of randomized controlled trials. Clin Nutr. 2015;34:612–619. [DOI] [PubMed] [Google Scholar]
- 10. Hsu C‐H, Tsai T‐H, Kao Y‐H, Hwang K‐C, Tseng T‐Y, Chou P. Effect of green tea extract on obese women: a randomized, double‐blind, placebo‐controlled clinical trial. Clin Nutr. 2008;27:363–370. [DOI] [PubMed] [Google Scholar]
- 11. Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek‐Musialik D, Jablecka A. Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutr Res. 2012;32:421–427. [DOI] [PubMed] [Google Scholar]
- 12. Bahorun T, Luximon‐Ramma A, Neergheen‐Bhujun VS, Gunness TK, Googoolye K, Auger C, Crozier A, Aruoma OI. The effect of black tea on risk factors of cardiovascular disease in a normal population. Prev Med. 2012;54:S98–S102. [DOI] [PubMed] [Google Scholar]
- 13. Suliburska J, Bogdanski P, Szulinska M, Stepien M, Pupek‐Musialik D, Jablecka A. Effects of green tea supplementation on elements, total antioxidants, lipids, and glucose values in the serum of obese patients. Biol Trace Elem Res. 2012;149:315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samavat H, Newman AR, Wang R, Yuan J‐M, Wu AH, Kurzer MS. Effects of green tea catechin extract on serum lipids in postmenopausal women: a randomized, placebo‐controlled clinical trial. Am J Clin Nutr. 2016;104:1671–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Huang S, Li J, Shearer GC, Lichtenstein AH, Zheng X, Wu Y, Jin C, Wu S, Gao X. Longitudinal study of alcohol consumption and HDL concentrations: a community‐based study. Am J Clin Nutr. 2017;105:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jin C, Chen S, Vaidya A, Wu Y, Wu Z, Hu FB, Kris‐Etherton P, Wu S, Gao X. Longitudinal change in fasting blood glucose and myocardial infarction risk in a population without diabetes. Diabetes Care. 2017;40:1565–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed). 1986;292:13–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Saadeh S, Younossi ZM, Remer EM, Gramlich T, Ong JP, Hurley M, Mullen KD, Cooper JN, Sheridan MJ. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123:745–750. [DOI] [PubMed] [Google Scholar]
- 19. Chen CM. Overview of obesity in Mainland China. Obes Rev. 2008;9:14–21. [DOI] [PubMed] [Google Scholar]
- 20. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) SM , Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Spertus JA, Costa F. Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 22. Hu FB, Manson JE, Stampfer MJ, Colditz G, Liu S, Solomon CG, Willett WC. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–797. [DOI] [PubMed] [Google Scholar]
- 23. Jacobs DR, Mebane IL, Bangdiwala SI, Criqui MH, Tyroler HA. High density lipoprotein cholesterol as a predictor of cardiovascular disease mortality in men and women: the follow‐up study of the Lipid Research Clinics Prevalence Study. Am J Epidemiol. 1990;131:32–47. [DOI] [PubMed] [Google Scholar]
- 24. Aro A, Pietinen P, Uusitalo U, Tuomilehto J. Coffee and tea consumption, dietary fat intake and serum cholesterol concentration of Finnish men and women. J Intern Med. 1989;226:127–132. [DOI] [PubMed] [Google Scholar]
- 25. Imai K, Nakachi K. Cross sectional study of effects of drinking green tea on cardiovascular and liver diseases. BMJ. 1995;310:693–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tokunaga S, White IR, Frost C, Tanaka K, Kono S, Tokudome S, Akamatsu T, Moriyama T, Zakouji H. Green tea consumption and serum lipids and lipoproteins in a population of healthy workers in Japan. Ann Epidemiol. 2002;12:157–165. [DOI] [PubMed] [Google Scholar]
- 27. Uemura H, Katsuura‐Kamano S, Yamaguchi M, Nakamoto M, Hiyoshi M, Arisawa K. Consumption of coffee, not green tea, is inversely associated with arterial stiffness in Japanese men. Eur J Clin Nutr. 2013;67:1109–1114. [DOI] [PubMed] [Google Scholar]
- 28. Grosso G, Stepaniak U, Micek A, Topor‐Mądry R, Pikhart H, Szafraniec K, Pająk A. Association of daily coffee and tea consumption and metabolic syndrome: results from the Polish arm of the HAPIEE study. Eur J Nutr. 2015;54:1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stendell‐Hollis NR, Thomson CA, Thompson PA, Bea JW, Cussler EC, Hakim IA. Green tea improves metabolic biomarkers, not weight or body composition: a pilot study in overweight breast cancer survivors. J Hum Nutr Diet. 2010;23:590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang T‐Y, Chou JI, Ueng K‐C, Chou M‐Y, Yang J‐J, Lin‐Shiau S‐Y, Hu M‐E, Lin J‐K. Weight reduction effect of Puerh tea in male patients with metabolic syndrome. Phytother Res. 2014;28:1096–1101. [DOI] [PubMed] [Google Scholar]
- 31. Miyazaki R, Kotani K, Ayabe M, Tsuzaki K, Shimada J, Sakane N, Takase H, Ichikawa H, Yonei Y, Ishii K. Minor effects of green tea catechin supplementation on cardiovascular risk markers in active older people: a randomized controlled trial. Geriatr Gerontol Int. 2013;13:622–629. [DOI] [PubMed] [Google Scholar]
- 32. Troup R, Hayes JH, Raatz SK, Thyagarajan B, Khaliq W, Jacobs DR, Key NS, Morawski BM, Kaiser D, Bank AJ, Gross M. Effect of black tea intake on blood cholesterol concentrations in individuals with mild hypercholesterolemia: a diet‐controlled randomized trial. J Acad Nutr Diet. 2015;115:264–271.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lemieux I, Lamarche B, Couillard C, Pascot A, Cantin B, Bergeron J, Dagenais GR, Després J‐P. Total cholesterol/HDL cholesterol ratio vs LDL cholesterol/HDL cholesterol ratio as indices of ischemic heart disease risk in men. Arch Intern Med. 2001;161:2685–2692. [DOI] [PubMed] [Google Scholar]
- 34. Millan J, Pinto X, Munoz A, Zuniga M, Rubies‐Prat J, Pallardo LF, Masana L, Mangas A, Hernandez‐Mijares A, Gonzalez‐Santos P, Ascaso JF, Pedro‐Botet J. Lipoprotein ratios: physiological significance and clinical usefulness in cardiovascular prevention. Vasc Health Risk Manag. 2009;5:757–765. [PMC free article] [PubMed] [Google Scholar]
- 35. Wang Z‐M, Zhou B, Wang Y‐S, Gong Q‐Y, Wang Q‐M, Yan J‐J, Gao W, Wang L‐S. Black and green tea consumption and the risk of coronary artery disease: a meta‐analysis. Am J Clin Nutr. 2011;93:506–515. [DOI] [PubMed] [Google Scholar]
- 36. Vinson JA, Dabbagh YA. Effect of green and black tea supplementation on lipids, lipid oxidation and fibrinogen in the hamster: mechanisms for the epidemiological benefits of tea drinking. FEBS Lett. 1998;433:44–46. [DOI] [PubMed] [Google Scholar]
- 37. Bursill CA, Abbey M, Roach PD. A green tea extract lowers plasma cholesterol by inhibiting cholesterol synthesis and upregulating the LDL receptor in the cholesterol‐fed rabbit. Atherosclerosis. 2007;193:86–93. [DOI] [PubMed] [Google Scholar]
- 38. Koo S, Noh S. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid‐lowering effect. J Nutr Biochem. 2007;18:179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Langley‐Evans SC. Antioxidant potential of green and black tea determined using the ferric reducing power (FRAP) assay. Int J Food Sci Nutr. 2000;51:181–188. [DOI] [PubMed] [Google Scholar]
- 40. Kempf K, Herder C, Erlund I, Kolb H, Martin S, Carstensen M, Koenig W, Sundvall J, Bidel S, Kuha S, Jaakko T. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am J Clin Nutr. 2010;91:950–957. [DOI] [PubMed] [Google Scholar]
- 41. Lee Y‐H, Wang Z, Chiang T, Liu C‐T. Beverage intake, smoking behavior, and alcohol consumption in contemporary china—a cross‐sectional analysis from the 2011 China Health and Nutrition Survey. Int J Environ Res Public Health. 2017;14:493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rains TM, Agarwal S, Maki KC. Antiobesity effects of green tea catechins: a mechanistic review. J Nutr Biochem. 2011;22:1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sensitivity Analyses for Mean Differences in HDL‐C Concentration, TC/HDL‐C Ratio and TG/HDL‐C Ratio Change During 6‐Year Follow‐Up by Tea Consumption in 2006*,†
Table S2. Longitudinal Change in HDL‐C (mmol/L per year) by Tea Consumption in 2006 Using Propensity Score


