Abstract
Background
Obstructive sleep apnea is associated with high blood pressure. The magnitude of blood pressure effects from sleep apnea treatment is unclear. We aimed to determine the effect of mandibular advancement device therapy on ambulatory nighttime and daytime blood pressure in women and men with daytime sleepiness and snoring or mild to moderate sleep apnea (apnea‐hypopnea index, <30).
Methods and Results
In this 4‐month, double‐blind, randomized controlled trial comprising 96 untreated patients, 27 women and 58 men, aged 31 to 70 years, completed the study. The active group received individually made adjustable mandibular advancement devices, and the control group was given individually made sham devices, to be used during sleep. Polysomnographic sleep recordings and ambulatory 24‐hour blood pressure measurements were performed at baseline and at follow‐up. In women with mandibular advancement devices, the mean nighttime systolic blood pressure was 10.8 mm Hg (95% confidence interval, 4.0–17.7 mm Hg; P=0.004) lower than in the women in the sham group, adjusted for baseline blood pressure, age, body mass index, and the apnea‐hypopnea index. The mean nighttime adjusted diastolic blood pressure was 6.6 mm Hg (95% confidence interval, 2.7–10.4 mm Hg; P=0.002) lower in the mandibular advancement device group. In men, there were no significant differences in blood pressure at night or during the daytime between the intervention groups.
Conclusions
A mandibular advancement device for obstructive sleep apnea reduces nocturnal blood pressure in women.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT00477009.
Keywords: hypertension, mandibular advancement device, obstructive sleep apnea, women
Subject Categories: High Blood Pressure, Hypertension, Vascular Disease
Clinical Perspective
What Is New?
Mandibular advancement device therapy reduces nighttime blood pressure in women.
This effect was not found in men.
What Are the Clinical Implications?
Obstructive sleep apnea is usually regarded as a male disorder.
This trial highlights that women with symptoms of sleep apnea should be investigated and subsequently treated for sleep apnea.
The risk of hypertension is doubled in subjects with obstructive sleep apnea.1 Hypertension is also more prevalent in women with sleep apnea, whereas diabetes mellitus type 2 and ischemic heart disease are more prevalent in men with sleep apnea.2
We identified 6 randomized controlled trials using mandibular advancement devices for sleep apnea with 24‐hour blood pressure as the outcome.3, 4, 5, 6, 7, 8 Three trials reported no effect versus placebo,3, 5, 6 one study reported a reduction in daytime blood pressure,7 and another reported a reduction in nocturnal diastolic blood pressure.4 The sixth study reported no difference between a mandibular advancement device and continuous positive airway pressure, but there was no difference in blood pressures versus baseline with either treatment.8 None of these studies analyzed men and women separately, and they contained 15% to 21% women. A meta‐analysis of the effects of antihypertensive drugs reveals slightly more pronounced reductions in blood pressure in women than in men.9 A recent viewpoint highlights the fact that women's responses to therapeutic interventions often differ significantly from those of men.10 We were interested in studying the effect of mandibular advancement devices on blood pressure during the daytime and at night separately in men and women, because the effect of mandibular advancement devices varies between different trials and the treatment is only used at night. Furthermore, to date, no one has studied possible sex differences in effect.
Previously, in a randomized controlled trial, we reported that mandibular advancement devices are effective in reducing sleep apneas.11 Herein, we report a secondary prespecified outcome, blood pressure during the daytime and nighttime, separately in women and men.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Study Design
A randomized, sham‐controlled, double‐blind, parallel‐group study of the efficacy of a mandibular advancement device was designed according to the CONSORT (Consolidated Standards of Reporting Trials) Statement and conducted at the Department of Orthodontics and Department of Medicine, Umeå University, and Umeå University Hospital. The patients were randomized to receive an active device or a sham device. They were stratified on whether they had a diagnosis of sleep apnea or had an apnea‐hypopnea index of <5. The randomization was based on a computer‐generated table in blocks with 4 designs created by a statistician, delivered by a third party, and with the randomization key kept secret. The study was double blinded, and all patients and all personnel, including those scoring the blood pressure and polysomnography, were blinded to the treatment assignment. At baseline and follow‐up, after 4 months, all the patients underwent polysomnographic sleep recordings and 24‐hour ambulatory blood pressure monitoring (ABPM SpaceLabs Medical 90217 ambulatory blood pressure monitor).12 Blood pressure was measured every 20 minutes. Daytime was defined as 6 am to 10 pm, and nighttime was defined as 10 pm to 6 am. Blood pressures >240/150 or <70/40 mm Hg were regarded as unreliable and were excluded from the results. Body mass index was calculated from measures of height and weight before the study start.
Polysomnographic sleep recordings (Embla; Natus Neurology) were made at home and included continuous recordings of electroencephalograms (channels C3/M2 and C4/M1), electro‐oculograms, submental electromyography, nasal flow pressure sensor, piezoelectric belts (Resp‐EZ; EPM Systems), pulse oximetry (NoninXPOD + 8000J SensorAdult Flex System; NoninMedical), piezo respiratory effort sensor (Pro‐Tech; Philips), ECGs (V5), and a body position sensor. All the recordings were scored manually, according to the American Academy of Sleep Medicine Task Force.13, 14
At baseline and follow‐up, the patients responded to questionnaires about daytime sleepiness,15, 16 and they assessed adherence to the mandibular advancement device or the sham device.
Ethical Approval and Registration
The study protocol was approved by the Ethics Review Board at Umeå University and registered. All the patients gave their written consent to participate in the study.
Patients
Patients who were referred from the Department of Medicine to the Department of Orthodontics for treatment with mandibular advancement devices were asked to participate in the study. The criteria for inclusion were snoring; daytime sleepiness, defined as at least 1 positive answer on 4 different scales11; and an apnea‐hypopnea index <30. Patients with severe psychiatric illnesses, including dementia; an inability to protrude the mandible for ≥5 mm; active periodontal disease or caries; few teeth for anchoring the device; tonsil hypertrophy (grade 3 or 4 on the Friedman Scale); participation in other studies; or a bias with regard to the study (ie, physicians or nurses at the clinic) were excluded.
Interventions/Treatment
A custom‐made adjustable mandibular advancement device, the Herbst device, was used as active treatment. It consisted of 2 parts made of elastomer and connected by 2 lateral screws that enabled the continuous titration of the mandible forward. A mandibular advancement of 6 to 7 mm was intended for all patients. The sham device consisted of an acrylic plate in the palate and did not influence the position of the mandible.11
Outcome
The outcome was the blood pressure at follow‐up, separately in men and women, with adjustments for baseline blood pressure, age, body mass index, and the apnea‐hypopnea index.
Statistical Analyses
The sample size was estimated at 60 patients plus 15 patients for potential loss, making a total of 75 patients in each group to detect changes of 25% in the occurrence of categorical data, with reports of daytime sleepiness according to the Epworth Sleepiness Scale and other ordinal and categorical data.11 The statistical tests had a power of 0.8, and P<0.05 was considered significant.
Descriptive continuous variables were presented as means and SDs, and categorical variables were presented as numbers and percentages. Independent and dependent t tests were used to calculate mean differences in blood pressure, with 95% confidence intervals (CIs), between the 2 groups and differences from baseline at the time of follow‐up. Fisher's exact test was used to analyze categorical data. Differences in blood pressure between the mandibular advancement device and the sham device were adjusted for baseline blood pressure, age, body mass index, and the apnea‐hypopnea index using linear regression analysis, separately in women and men. Multiple regression models were used to test if there was a significant interaction between sex and treatment. The regression models for nighttime systolic and diastolic blood pressures included sex, age, body mass index, apnea‐hypopnea index, treatment, and interaction between sex and treatment. The SPSS statistical software pack, version 24 for Mac, was used in the calculations.
Results
Ninety‐six patients were randomized, 48 in the mandibular advancement device group and 48 in the sham device group.11 Three patients in the active group and 2 patients in the sham group did not tolerate their devices, and they refused further participation (Figure 1). Of the 91 patients who completed the study, a further 6 subjects were excluded from analysis because of possible blood pressure effects from other sources: 1 because of shift work during the night of blood pressure registration, 1 because of aortic coarctation that might interfere with interpreting the results for blood pressure, and a further 4 because they changed their number of antihypertensive medications during the study. Baseline and follow‐up data on blood pressure were available in 27 women, 11 in the mandibular advancement device group and 16 in the sham group; and in 58 men, 31 in the mandibular advancement device group and 27 in the sham group (Figure 1). There was no difference in any baseline variable between women and men (Table 1).
Figure 1.
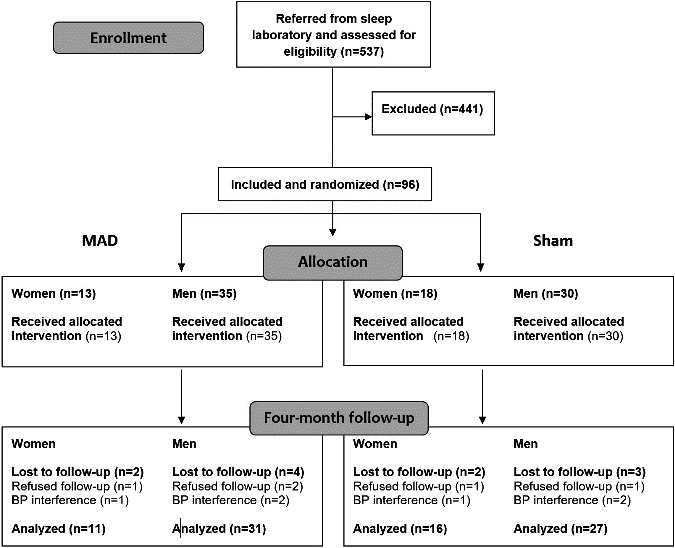
Flow chart showing the patients screened, randomized, and analyzed. Exclusions were made because of severe obstructive sleep apnea (OSA; n=27), body mass index ≥35 kg/m2 (n=20), <20 or ≥70 years of age (n=22), not sleepy (n=69), medical reasons (n=15), dental reasons (n=37), other ongoing OSA treatment or no treatment needed (n=109), professional driver (n=28), refused participation (n=71), and long distance to the clinic, old sleep recording, biased to the study, or included in other studies (n=43). BP indicates blood pressure; MAD, mandibular advancement device.
Table 1.
Baseline Characteristics for Women and Men Treated With MAD or a Sham Device
| Characteristics | All Patients | Women | Men | |||
|---|---|---|---|---|---|---|
| MAD (n=42) | Sham (n=43) | MAD (n=11) | Sham (n=16) | MAD (n=31) | Sham (n=27) | |
| Office systolic blood pressure, mm Hg | 126.5 (12.2)* | 133.8 (16.4) | 120.7 (12.0) | 131.1 (18.9) | 128.5 (11.8) | 135.4 (15.0) |
| Office diastolic blood pressure, mm Hg | 82.3 (9.3) | 85.3 (10.3) | 80.2 (9.9) | 83.5 (10.6) | 83.0 (9.1) | 86.4 (10.1) |
| Age, y | 49.6 (10.5)* | 54.5 (9.1) | 50.5 (8.6) | 54.0 (7.3) | 49.2 (11.3) | 54.9 (10.2) |
| Body mass index, kg/m2 | 27.4 (3.4) | 27.8 (3.6) | 27.4 (3.3) | 28.6 (3.5) | 27.4 (3.5) | 27.3 (3.7) |
| Apnea‐hypopnea index | 15.0 (9.5) | 14.8 (9.3) | 14.2 (10.7) | 10.4 (6.8) | 15.3 (9.2) | 17.4 (9.7) |
| ESS score | 11.1 (3.9) | 10.9 (3.6) | 10.6 (4.5) | 12.2 (3.4) | 11.3 (3.8) | 10.1 (3.5) |
| Hypertension at baseline, n/total | 21/42 | 30/43 | 5/11 | 12/16 | 16/31 | 18/27 |
| Hypertensive medication, n/total | 10/42 | 19/43 | 3/11 | 9/16 | 7/31 | 10/27 |
| Dippers, n/total | 31/42 | 31/43 | 8/11 | 10/16 | 23/31 | 21/27 |
Data are given as mean (SD). ESS indicates Epworth Sleepiness Scale; MAD, mandibular advancement device.
*P<0.05.
The mandibular advancement device group reported that they had used their devices for a mean of 87% (SD, 17%) of the nights, and the sham group had used their devices during 82% (SD, 22%) of the nights. There was no difference in adherence between the intervention groups in the whole sample or in any sex. Two women in the active group and 3 in the sham group and 2 men in the active group and 2 in the sham group did not use their devices during the night with blood pressure recordings. All men and women were included in the analysis regardless of whether they used the device or not during the recordings. Body mass index was unchanged during the study in both women and men in each intervention group.
Whole Sample
There were no significant effects on blood pressure from the mandibular advancement device compared with the sham device in the whole sample, neither in crude difference nor in the adjusted analysis, which included blood pressure at baseline, age, sex, body mass index, and the apnea‐hypopnea index (Table 2, Figure S1). There was a significant interaction between sex and treatment effect in nighttime systolic blood pressure (P=0.020) and nighttime diastolic blood pressure (P=0.025).
Table 2.
Effects of MAD on Blood Pressure, Heart Rate, and the Apnea‐Hypopnea Index in the Whole Sample
| Variable | MAD (n=42) | Sham (n=43) | Crude Difference: Adjusted Difference Mean (95% CI)* | |||
|---|---|---|---|---|---|---|
| Baseline | 4 mo | Baseline | 4 mo | Sham‐MAD | Adjusted Sham‐MAD* | |
| Nighttime | ||||||
| Systolic blood pressure, mm Hg | 110.0 (9.6) | 108.6 (8.9) | 111.2 (12.2) | 112.5 (11.6) | 4.0 (−0.5 to 8.4) | 3.1 (−0.4 to 6.6) |
| Diastolic blood pressure, mm Hg | 68.4 (7.1) | 68.1 (7.4) | 69.4 (8.5) | 71.1 (7.7) | 3.0 (−0.2 to 6.3) | 2.2 (−0.2 to 4.5) |
| Heart rate, bpm | 62.6 (7.4) | 62.0 (8.2) | 61.8 (9.5) | 61.5 (9.7) | −0.5 (−4.3 to 3.4) | 0.1 (−2.4 to 2.5) |
| Apnea‐hypopnea index | 15.0 (9.5) | 6.6 (5.0)† | 14.8 (9.3) | 16.8 (9.9) | 10.2 (6.8 to 13.6)† | 9.4 (6.5 to 12.3)† |
| Daytime | ||||||
| Systolic blood pressure, mm Hg | 126.9 (9.9) | 125.6 (8.8) | 129.2 (11.4) | 128.3 (11.4) | 2.7 (−1.7 to 7.1) | 1.8 (−1.6 to 5.2) |
| Diastolic blood pressure, mm Hg | 82.2 (8.6) | 81.3 (7.6) | 83.3 (8.3) | 83.6 (8.3) | 2.3 (−1.1 to 5.7) | 1.8 (−0.2 to 3.7) |
| Heart rate, bpm | 73.5 (8.3) | 73.4 (9.3) | 72.5 (9.2) | 73.3 (10.0) | −0.1 (−4.3 to 4.0) | 0.8 (−1.8 to 3.4) |
Data are given as mean (SD) unless otherwise indicated. BPM indicates beats per minute; CI, confidence interval; MAD, mandibular advancement device.
*Adjusted for baseline value, apnea‐hypopnea index, age, sex, and body mass index.
† P<0.001.
The apnea‐hypopnea index was 9.4 events an hour (95% CI, 6.5–12.3 events an hour; P<0.001) lower in the mandibular advancement device group compared with the sham device sample, adjusted for the baseline apnea‐hypopnea index, body mass index, and age.
Women
The mean adjusted nighttime systolic blood pressure was 10.8 mm Hg (95% CI, 4.0–17.7 mm Hg; P=0.004) lower in women using mandibular advancement devices versus sham devices, with adjustments for the baseline blood pressure values, the apnea‐hypopnea index, body mass index, and age. The mean adjusted nighttime diastolic blood pressure was 6.6 mm Hg (95% CI, 2.7–10.4 mm Hg; P=0.002) lower in the mandibular advancement device group (Table 3) (Figures 2, 3, 4 through 5).
Table 3.
Effects of MAD on Blood Pressure, Heart Rate, and the Apnea‐Hypopnea Index in Women
| Variable | MAD (n=11) | Sham (n=16) | Crude Difference: Adjusted Difference Mean (95% CI)* | |||
|---|---|---|---|---|---|---|
| Baseline | 4 mo | Baseline | 4 mo | Sham‐MAD | Adjusted Sham‐MAD* | |
| Nighttime | ||||||
| Systolic blood pressure, mm Hg | 107.4 (10.3) | 102.2 (7.9)† | 111.7 (15.5) | 114.1 (11.3) | 11.9 (4.3 to 19.6)† | 10.8 (4.0 to 17.7)† |
| Diastolic blood pressure, mm Hg | 67.0 (6.7) | 64.8 (5.5)‡ | 68.3 (10.1) | 72.0 (7.9)‡ | 7.2 (1.5 to 12.8)‡ | 6.6 (2.7 to 10.4)† |
| Heart rate, bpm | 61.1 (5.5) | 62.1 (9.2) | 59.3 (10.3) | 58.5 (8.6) | −3.6 (−10.8 to 3.6) | −4.1 (−10.3 to 2.0) |
| Apnea‐hypopnea index | 14.2 (10.7) | 6.7 (5.2)‡ | 10.4 (6.8) | 15.6 (11.4)‡ | 8.9 (1.3 to 16.5)‡ | 8.7 (1.9 to 15.4)‡ |
| Daytime | ||||||
| Systolic blood pressure, mm Hg | 123.6 (9.9) | 119.6 (8.4)‡ | 127.6 (12.7) | 126.3 (12.0) | 6.7 (−2.0 to 15.3) | 6.5 (−1.4 to 14.5) |
| Diastolic blood pressure, mm Hg | 81.0 (8.0) | 79.5 (7.0) | 82.3 (9.9) | 83.1 (10.5) | 3.7 (−3.8 to 11.1) | 2.7 (−1.6 to 7.0) |
| Heart rate, bpm | 73.2 (8.2) | 73.8 (6.7) | 70.4 (11.8) | 70.9 (12.7) | −2.9 (−11.6 to 5.7) | −0.1 (−5.3 to 5.0) |
Data are given as mean (SD) unless otherwise indicated. BPM indicates beats per minute; CI, confidence interval; MAD, mandibular advancement device.
*Adjusted for baseline value, apnea‐hypopnea index, age, and body mass index.
† P<0.01, ‡ P<0.05.
Figure 2.
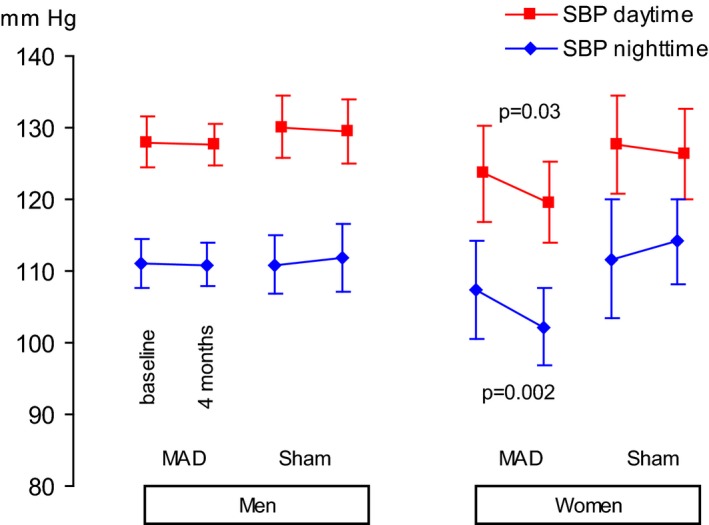
Systolic blood pressure (SBP) at baseline and at follow‐up after 4 months of using a mandibular advancement device (MAD) or a sham device. Values are expressed as the mean and 95% confidence interval.
Figure 3.
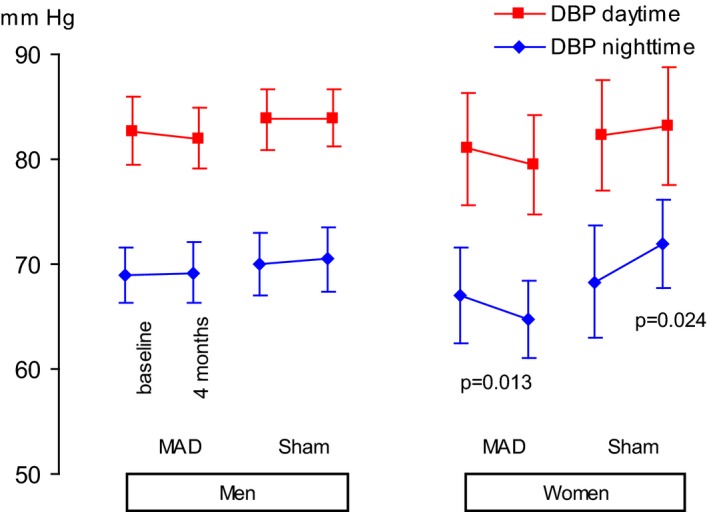
Diastolic blood pressure (DBP) at baseline and at follow‐up after 4 months of using a mandibular advancement device (MAD) or a sham device. Values are expressed as the mean and 95% confidence interval.
Figure 4.
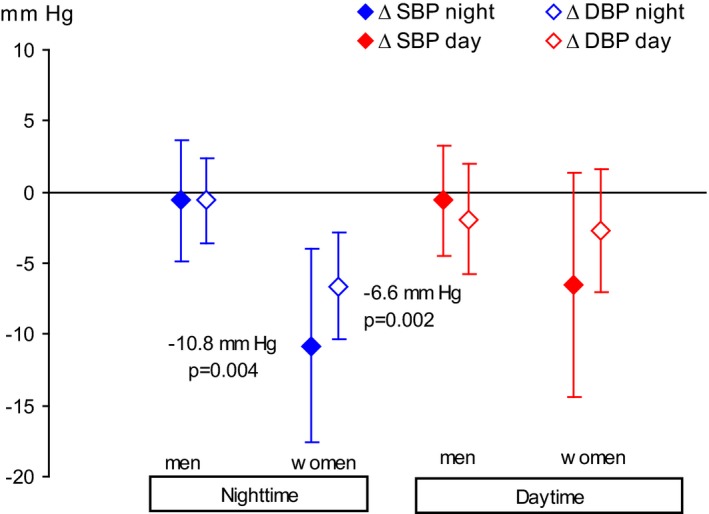
Adjusted differences in blood pressure at follow‐up using a mandibular advancement device vs a sham device. Adjusted for baseline blood pressure, age, body mass index, and apnea‐hypopnea index. Values are expressed as the mean and 95% confidence interval. DBP indicates diastolic blood pressure; SBP, systolic blood pressure.
Figure 5.
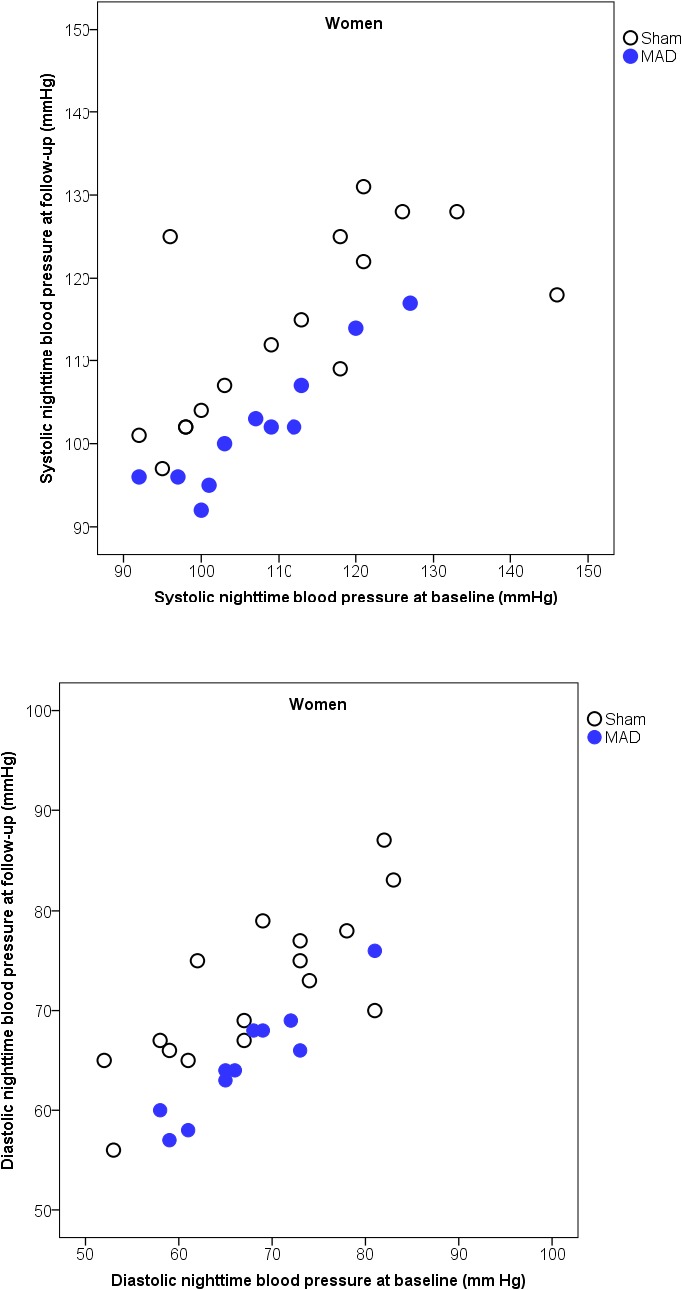
Scatterplots of nighttime systolic (above) and diastolic (below) blood pressure, with the baseline on the x axis and follow‐up on the y axis, with the 2 interventions in women. MAD indicates mandibular advancement device.
The mean daytime systolic and diastolic blood pressures did not differ between the mandibular advancement device group and the sham group (Table 3).
The apnea‐hypopnea index was 8.7 units (95% CI, 1.9–15.4 units; P=0.014) lower in the mandibular advancement device group compared with the sham group, adjusted for the baseline apnea‐hypopnea index, body mass index, and age (Table 3).
Men
There were no significant differences in blood pressure at night or during the daytime in men using mandibular advancement devices versus sham devices (Table 4; Figures 2, 3 through 4 and 6). The mean difference between the intervention groups in nighttime systolic blood pressure was 0.6 mm Hg (95% CI, −3.8 to 4.9 mm Hg; P=0.78); and in nighttime diastolic blood pressure, it was 0.6 mm Hg (95% CI, −2.4 to 3.6 mm Hg; P=0.52).
Table 4.
Effects of MAD on Blood Pressure, Heart Rate, and the Apnea‐Hypopnea Index in Men
| Variable | MAD (n=31) | Sham (n=27) | Crude Difference: Adjusted Difference Mean (95% CI)* | |||
|---|---|---|---|---|---|---|
| Baseline | 4 mo | Baseline | 4 mo | Sham‐MAD | Adjusted Sham‐MAD* | |
| Nighttime | ||||||
| Systolic blood pressure, mm Hg | 111.0 (9.3) | 110.8 (8.2) | 111.0 (10.1) | 111.6 (11.9) | 0.8 (−4.7 to 6.3) | 0.6 (−3.8 to 4.9) |
| Diastolic blood pressure, mm Hg | 68.9 (7.2) | 69.2 (7.7) | 70.0 (7.6) | 70.5 (7.8) | 1.3 (−2.8 to 5.4) | 0.6 (−2.4 to 3.6) |
| Heart rate, bpm | 63.2 (8.0) | 62.0 (8.0) | 63.3 (8.8) | 63.3 (10.0) | 1.4 (−3.4 to 6.1) | 1.3 (−1.4 to 3.9) |
| Apnea‐hypopnea index | 15.3 (9.2) | 6.5 (5.1)† | 17.4 (9.7) | 17.5 (9.1) | 11.0 (7.0 to 15.0)† | 9.6 (6.4 to 12.9)† |
| Daytime | ||||||
| Systolic blood pressure, mm Hg | 128.0 (9.7) | 127.7 (8.1) | 130.1 (10.8) | 129.5 (11.1) | 1.8 (−3.3 to 6.8) | 0.6 (−3.4 to 4.5) |
| Diastolic blood pressure, mm Hg | 82.7 (8.9) | 82.0 (7.8) | 83.8 (7.3) | 83.9 (6.9) | 1.9 (−2.0 to 5.8) | 1.5 (−0.7 to 3.7) |
| Heart rate, bpm | 73.6 (8.5) | 73.3 (10.1) | 73.8 (7.2) | 74.7 (7.9) | 1.5 (−3.4 to 6.3) | 1.4 (−1.9 to 4.6) |
Data are given as mean (SD) unless otherwise indicated. BPM indicates beats per minute; CI, confidence interval; MAD, mandibular advancement device.
*Adjusted for baseline value, apnea‐hypopnea index, age, and body mass index.
† P<0.001.
Figure 6.
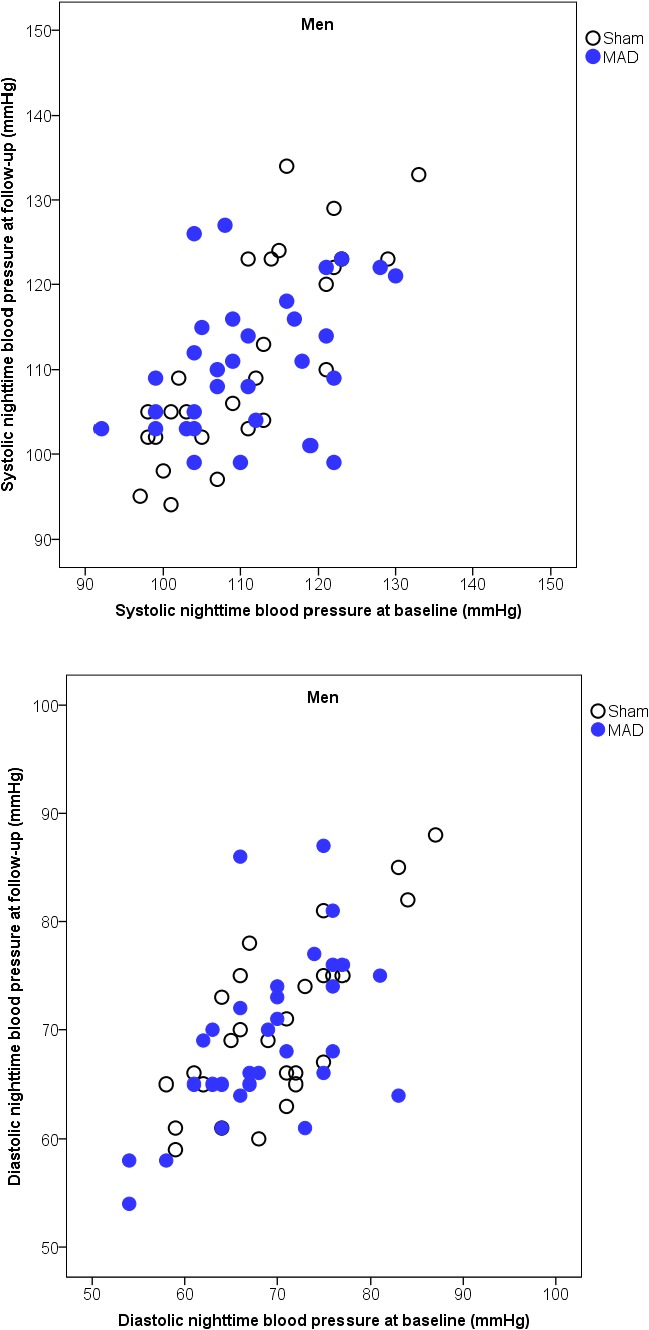
Scatterplots of nighttime systolic (above) and diastolic (below) blood pressure, with the baseline on the x axis and follow‐up on the y axis, with the 2 interventions in men. MAD indicates mandibular advancement device.
The apnea‐hypopnea index was 9.6 units (95% CI, 6.4–12.9 units; P<0.001) lower in the men who had used the mandibular advancement devices compared with those who had used the sham devices, adjusted for the baseline apnea‐hypopnea index, body mass index, and age (Table 4).
Sleep Quality
Sleep quality did not differ between the mandibular advancement device group and the sham device group in the whole sample or in either sex (Tables 5 and 6 and Table S1).
Table 5.
Effects of MAD on Sleep Quality in Women
| Variable | MAD (n=11) | Sham (n=16) | Crude Difference: Adjusted Difference Mean (95% CI)* | |||
|---|---|---|---|---|---|---|
| Baseline | 4 mo | Baseline | 4 mo | Sham‐MAD | Adjusted Sham‐MAD* | |
| Sleep efficiency | 92.0 (9.3) | 93.3 (6.2) | 93.3 (4.3) | 94.6 (3.7) | 1.2 (−2.7 to 5.1) | 0.4 (−3.3 to 4.1) |
| Total sleep time | 424.1 (44.0) | 400.6 (53.7) | 471.0 (57.9) | 466.2 (47.2) | 65.6 (25.3 to 105.9)† | 45.6 (0.5 to 90.7)‡ |
| Stage 1 | 8.2 (2.9) | 8.2 (3.4) | 8.1 (4.1) | 8.4 (4.9) | 0.2 (−3.4 to 3.7) | 0.0 (−3.0 to 3.1) |
| Stage 2 | 55.8 (7.5) | 54.2 (8.0) | 54.2 (8.3) | 49.4 (9.6) | −4.8 (−12.1 to 2.5) | −4.8 (−12.8 to 3.2) |
| Stage 3–4 | 16.0 (5.3) | 14.5 (5.9) | 16.1 (8.1) | 18.0 (6.8) | 3.4 (−1.8 to 8.6) | 2.6 (−2.0 to 7.2) |
| REM | 21.6 (6.5) | 22.4 (5.6) | 22.0 (3.1) | 24.3 (6.6) | 1.9 (−3.1 to 6.9) | 2.9 (−2.2 to 8.0) |
Data are given as mean (SD) unless otherwise indicated. CI indicates confidence interval; MAD, mandibular advancement device; REM, rapid eye movement.
*Adjusted for baseline value, apnea‐hypopnea index, age, and body mass index.
† P<0.01, ‡ P<0.05.
Table 6.
Effects of MAD on Sleep Quality in Men
| Variable | MAD (n=31) | Sham (n=27) | Crude Difference: Adjusted Difference Mean (95% CI)* | |||
|---|---|---|---|---|---|---|
| Baseline | 4 mo | Baseline | 4 mo | Sham‐MAD | Adjusted Sham‐MAD* | |
| Sleep efficiency | 90.0 (8.1) | 90.6 (9.6) | 90.6 (6.4) | 89.9 (7.2) | −0.7 (−5.2 to 3.9) | 0.9 (−3.0 to 4.8) |
| Total sleep time | 405.4 (43.9) | 400.8 (60.1) | 415.6 (59.6) | 408.7 (55.9) | 7.9 (−22.7 to 38.6) | 8.0 (−23.2 to 39.2) |
| Stage 1 | 12.7 (7.0) | 11.4 (5.6) | 14.2 (7.3) | 16.9 (12.6) | 5.5 (0.5 to 10.5)† | 4.1 (−0.4 to 8.6) |
| Stage 2 | 52.7 (9.2) | 49.0 (6.5)† | 51.4 (11.6) | 49.0 (11.3) | 0.0 (−5.0 to 5.0) | 0.5 (−3.3 to 4.2) |
| Stage 3–4 | 15.2 (7.5) | 17.7 (7.4)† | 14.2 (7.9) | 14.8 (8.2) | −2.9 (−7.0 to 1.2) | −2.0 (−5.2 to 1.2) |
| REM | 19.3 (5.3) | 22.0 (6.0)‡ | 20.3 (6.7) | 19.4 (7.7) | −2.6 (−6.2 to 1.0) | −2.8 (−5.9 to 0.3) |
Data are given as mean (SD) unless otherwise indicated. CI indicates confidence interval; MAD, mandibular advancement device; REM, rapid eye movement.
*Adjusted for baseline value, apnea‐hypopnea index, age, and body mass index.
† P<0.05, ‡ P<0.01.
Discussion
Women who were treated with mandibular advancement devices at night experienced a reduction in their nighttime blood pressure compared with women who had used sham devices in this 4‐month, randomized, sham‐controlled trial; men did not experience a reduction. This sex difference is a new finding, and no previous study has investigated whether sex difference of this kind occurs in blood pressure when treating men and women for sleep apnea. The systolic nighttime adjusted blood pressure was a mean of 10.8 mm Hg (95% CI, 4.0–17.7 mm Hg; P=0.004) lower in the women using mandibular advancement devices versus the sham group, with adjustments for the baseline blood pressure values, the apnea‐hypopnea index, body mass index, and age. The mean adjusted nighttime diastolic blood pressure was 6.6 mm Hg (95% CI, 2.7–10.4 mm Hg; P=0.002) lower in the active group compared with the sham group. In men, there were no significant differences in blood pressure at night or during the daytime.
Individually fabricated mandibular advancement devices and continuous positive airway pressure reduce blood pressure when treating sleep apnea, according to meta‐analyses, but the effect varies between studies.17, 18, 19 The time and location of the blood pressure measurements varied in the studies that included an average of 20% women. Bratton et al17 report that mandibular advancement device therapy reduces the systolic blood pressure by 2.1 (95% CI, 0.8–3.4) mm Hg and the diastolic blood pressure by 1.9 (95% CI, 0.5–3.2) mm Hg, and a similar effect was found with continuous positive airway pressure. In a recent meta‐analysis, the use of positive airway pressure, compared with sham or no treatment, was not associated with reduced risks of cardiovascular outcomes or death for patients with sleep apnea.20 The samples included in that systematic review comprised only an average of 20% women. By investigating women and men separately during 24 hours of blood pressure measurements in the present study, we found a greater effect on blood pressure compared with the above‐mentioned studies of blood pressure effects and only during the night when the mandibular advancement devices were used.
In our study, the group of women who had been randomized to active treatment experienced a significant reduction in both their nighttime blood pressure and apnea‐hypopnea index. In contrast, the women who had been randomized to the sham intervention experienced an increase in both their nighttime diastolic blood pressure and their apnea‐hypopnea index during the 4 months. Among men receiving active treatment, there was no change in blood pressure, despite the fact that the apnea‐hypopnea index decreased significantly. In men using the sham device, there was no change in either blood pressure or the apnea‐hypopnea index. In neither the women nor the men did the mandibular advancement device have any effect on sleep quality. These findings indicate that there are some differences between women and men on their response to obstructive sleep apnea treatment in terms of blood pressure.
Different mechanisms have been proposed to mediate the increase in blood pressure observed in patients with obstructive sleep apnea. The most established mechanism is a blood pressure elevation through increased sympathetic activity.21, 22, 23 High sympathetic activity also stimulates the renin‐angiotensin system, and patients with obstructive sleep apnea and hypertension have increased aldosterone secretion.24 The treatment of obstructive sleep apnea with continuous positive airway pressure decreases the sympathetic activation.25
The mechanisms for a possible difference between women and men in the effect of mandibular advancement device therapy on high blood pressure remain to be proved. High aldosterone secretion increases the retention of sodium and water in the kidneys and is related to the severity of obstructive sleep apnea and blood pressure level.24, 26, 27 Hypertension in women is more sensitive to sodium intake than in men.27 However, it is not known whether aldosterone secretion decreases after the treatment of obstructive sleep apnea in women. Obstructive sleep apnea is related to the activation of the hypothalamic‐pituitary‐adrenal axis and increased cortisol levels, although these results vary between studies.28 Continuous positive airway pressure lowers the cortisol levels, but it is not known whether there are sex differences in this effect.28
The improvement in blood pressure as a result of using the mandibular advancement devices among the present women with sleep apnea and a worsening during the sham intervention occurred within only a few months. In the Wisconsin cohort study, hypertension had increased at the 4‐year follow‐up of untreated subjects with obstructive sleep apnea compared with healthy controls.1 The present trial indicates that blood pressure fluctuations in relation to nightly breathing stops take place during short time spans and stresses the indications for the effective treatment of obstructive sleep apnea. This may be particularly important for women, who often remain undiagnosed and untreated for obstructive sleep apnea.29
One limitation of the present study is that we only stratified for disease severity, but not for sex and hypertension. Adherence to treatment is also critical to ensure success. In the present study, the overall adherence to therapy was >80% in both groups. We used self‐assessments of adherence, which is a limitation in the present study. Compliance monitors were not widely available when the study was designed.30 A later study, comparing the results of objectively measured adherence with subjectively reported use, found only a 30‐minute longer sleeping time in the subjective reports.31
Women in the population often experience sleep apnea, related to hypertension.32 Sleep apnea is, however, often regarded as a male disorder, and sleep apnea is underdiagnosed in women in relation to men.33 This trial supports the hypothesis that women with symptoms of sleep apnea should be investigated and subsequently treated for sleep apnea. The trial also supports the belief that women's responses to therapeutic interventions differ significantly from those of men, and it further highlights the need for mechanistic studies and trials of sleep apnea separately in women and men.
Conclusion
A mandibular advancement device for obstructive sleep apnea reduces the nocturnal blood pressure in women.
Sources of Funding
The study was supported by grants from the Swedish Research Council, the Swedish Heart and Lung Foundation, and the County Council of Västerbotten.
Disclosures
None.
Supporting information
Table S1. Effects of the Mandibular Advancement Device on Sleep Quality in the Whole Sample
Figure S1. Systolic (SBP) and diastolic (DBP) blood pressure at baseline and at follow‐up after four months of using a mandibular advancement device or a sham device in the whole study sample.
(J Am Heart Assoc. 2018;7:e008642 DOI: 10.1161/JAHA.118.008642.)
References
- 1. Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep‐disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. [DOI] [PubMed] [Google Scholar]
- 2. Mokhlesi B, Ham SA, Gozal D. The effect of sex and age on the comorbidity burden of OSA: an observational analysis from a large nationwide US health claims database. Eur Respir J. 2016;47:1162–1169. [DOI] [PubMed] [Google Scholar]
- 3. Andren A, Hedberg P, Walker‐Engstrom ML, Wahlen P, Tegelberg A. Effects of treatment with oral appliance on 24‐h blood pressure in patients with obstructive sleep apnea and hypertension: a randomized clinical trial. Sleep Breath. 2013;17:705–712. [DOI] [PubMed] [Google Scholar]
- 4. Barnes M, McEvoy RD, Banks S, Tarquinio N, Murray CG, Vowles N, Pierce RJ. Efficacy of positive airway pressure and oral appliance in mild to moderate obstructive sleep apnea. Am J Respir Crit Care Med. 2004;170:656–664. [DOI] [PubMed] [Google Scholar]
- 5. Dal‐Fabbro C, Garbuio S, D'Almeida V, Cintra FD, Tufik S, Bittencourt L. Mandibular advancement device and CPAP upon cardiovascular parameters in OSA. Sleep Breath. 2014;18:749–759. [DOI] [PubMed] [Google Scholar]
- 6. Gagnadoux F, Pepin JL, Vielle B, Bironneau V, Chouet‐Girard F, Launois S, Meslier N, Meurice JC, Nguyen XL, Paris A, Priou P, Tamisier R, Trzepizur W, Goupil F, Fleury B. Impact of mandibular advancement therapy on endothelial function in severe obstructive sleep apnea. Am J Respir Crit Care Med. 2017;195:1244–1252. [DOI] [PubMed] [Google Scholar]
- 7. Gotsopoulos H, Kelly JJ, Cistulli PA. Oral appliance therapy reduces blood pressure in obstructive sleep apnea: a randomized, controlled trial. Sleep. 2004;27:934–941. [DOI] [PubMed] [Google Scholar]
- 8. Phillips CL, Grunstein RR, Darendeliler MA, Mihailidou AS, Srinivasan VK, Yee BJ, Marks GB, Cistulli PA. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care Med. 2013;187:879–887. [DOI] [PubMed] [Google Scholar]
- 9. Paz MA, de‐La‐Sierra A, Saez M, Barcelo MA, Rodriguez JJ, Castro S, Lagaron C, Garrido JM, Vera P, Coll‐de‐Tuero G. Treatment efficacy of anti‐hypertensive drugs in monotherapy or combination: ATOM systematic review and meta‐analysis of randomized clinical trials according to PRISMA statement. Medicine (Baltimore). 2016;95:e4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Legato MJ, Johnson PA, Manson JE. Consideration of sex differences in medicine to improve health care and patient outcomes. JAMA. 2016;316:1865–1866. [DOI] [PubMed] [Google Scholar]
- 11. Marklund M, Carlberg B, Forsgren L, Olsson T, Stenlund H, Franklin KA. Oral appliance therapy in patients with daytime sleepiness and snoring or mild to moderate sleep apnea: a randomized clinical trial. JAMA Intern Med. 2015;175:1278–1285. [DOI] [PubMed] [Google Scholar]
- 12. Baumgart P, Kamp J. Accuracy of the spacelabs medical 90217 ambulatory blood pressure monitor. Blood Press Monit. 1998;3:303–307. [PubMed] [Google Scholar]
- 13. American Academy of Sleep Medicine Task Force . Sleep‐related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research: the report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–689. [PubMed] [Google Scholar]
- 14. Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System for Sleep Stages of Human Subjects. Los Angeles, CA: Barin Information Service/Brain Research Institute; 1968. [Google Scholar]
- 15. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–545. [DOI] [PubMed] [Google Scholar]
- 16. Partinen M, Gislason T. Basic Nordic Sleep Questionnaire (BNSQ): a quantitated measure of subjective sleep complaints. J Sleep Res. 1995;4:150–155. [DOI] [PubMed] [Google Scholar]
- 17. Bratton DJ, Gaisl T, Wons AM, Kohler M. CPAP vs mandibular advancement devices and blood pressure in patients with obstructive sleep apnea: a systematic review and meta‐analysis. JAMA. 2015;314:2280–2293. [DOI] [PubMed] [Google Scholar]
- 18. Liu T, Li W, Zhou H, Wang Z. Verifying the relative efficacy between continuous positive airway pressure therapy and its alternatives for obstructive sleep apnea: a network meta‐analysis. Front Neurol. 2017;8:289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ramar K, Dort LC, Katz SG, Lettieri CJ, Harrod CG, Thomas SM, Chervin RD. Clinical practice guideline for the treatment of obstructive sleep apnea and snoring with oral appliance therapy: an update for 2015. J Clin Sleep Med. 2015;11:773–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yu J, Zhou Z, McEvoy RD, Anderson CS, Rodgers A, Perkovic V, Neal B. Association of positive airway pressure with cardiovascular events and death in adults with sleep apnea: a systematic review and meta‐analysis. JAMA. 2017;318:156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J, Wallin BG. Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest. 1993;103:1763–1768. [DOI] [PubMed] [Google Scholar]
- 22. Hedner J, Ejnell H, Sellgren J, Hedner T, Wallin G. Is high and fluctuating muscle nerve sympathetic activity in the sleep apnoea syndrome of pathogenetic importance for the development of hypertension? J Hypertens Suppl. 1988;6:S529–S531. [DOI] [PubMed] [Google Scholar]
- 23. Somers VK, Dyken ME, Clary MP, Abboud FM. Sympathetic neural mechanisms in obstructive sleep apnea. J Clin Invest. 1995;96:1897–1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gonzaga CC, Gaddam KK, Ahmed MI, Pimenta E, Thomas SJ, Harding SM, Oparil S, Cofield SS, Calhoun DA. Severity of obstructive sleep apnea is related to aldosterone status in subjects with resistant hypertension. J Clin Sleep Med. 2010;6:363–368. [PMC free article] [PubMed] [Google Scholar]
- 25. Narkiewicz K, Kato M, Phillips BG, Pesek CA, Davison DE, Somers VK. Nocturnal continuous positive airway pressure decreases daytime sympathetic traffic in obstructive sleep apnea. Circulation. 1999;100:2332–2335. [DOI] [PubMed] [Google Scholar]
- 26. Dudenbostel T, Calhoun DA. Resistant hypertension, obstructive sleep apnoea and aldosterone. J Hum Hypertens. 2012;26:281–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ke X, Guo W, Peng H, Hu C, Zhang H, Peng C, Wang X. Association of aldosterone excess and apnea‐hypopnea index in patients with resistant hypertension. Sci Rep. 2017;7:45241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kritikou I, Basta M, Vgontzas AN, Pejovic S, Fernandez‐Mendoza J, Liao D, Bixler EO, Gaines J, Chrousos GP . Sleep apnoea and the hypothalamic‐pituitary‐adrenal axis in men and women: effects of continuous positive airway pressure. Eur Respir J. 2016;47:531–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lindberg E, Benediktsdottir B, Franklin KA, Holm M, Johannessen A, Jogi R, Gislason T, Real FG, Schlunssen V, Janson C. Women with symptoms of sleep‐disordered breathing are less likely to be diagnosed and treated for sleep apnea than men. Sleep Med. 2017;35:17–22. [DOI] [PubMed] [Google Scholar]
- 30. Marklund M, Verbraecken J, Randerath W. Non‐CPAP therapies in obstructive sleep apnoea: mandibular advancement device therapy. Eur Respir J. 2012;39:1241–1247. [DOI] [PubMed] [Google Scholar]
- 31. Dieltjens M, Braem MJ, Vroegop AV, Wouters K, Verbraecken JA, De Backer WA, Van de Heyning PH, Vanderveken OM. Objectively measured vs self‐reported compliance during oral appliance therapy for sleep‐disordered breathing. Chest. 2013;144:1495–1502. [DOI] [PubMed] [Google Scholar]
- 32. Franklin KA, Sahlin C, Stenlund H, Lindberg E. Sleep apnoea is a common occurrence in females. Eur Respir J. 2013;41:610–615. [DOI] [PubMed] [Google Scholar]
- 33. Young T, Peppard P, Palta M, Hla KM, Finn L, Morgan B, Skatrud J. Population‐based study of sleep‐disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157:1746–1752. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Effects of the Mandibular Advancement Device on Sleep Quality in the Whole Sample
Figure S1. Systolic (SBP) and diastolic (DBP) blood pressure at baseline and at follow‐up after four months of using a mandibular advancement device or a sham device in the whole study sample.


