Abstract
Background
We compared the clinical characteristics and outcomes of young patients with myocardial infarction with nonobstructive coronary arteries (MINOCA) versus obstructive disease (myocardial infarction due to coronary artery disease [MI‐CAD]) and among patients with MINOCA by sex and subtype.
Methods and Results
Between 2008 and 2012, VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients) prospectively enrolled acute myocardial infarction patients aged 18 to 55 years in 103 hospitals at a 2:1 ratio of women to men. Using an angiographically driven taxonomy, we defined patients as having MI‐CAD if there was revascularization or plaque ≥50% and as having MINOCA if there was <50% obstruction or a nonplaque mechanism. Patients who did not have an angiogram or who received thrombolytics before an angiogram were excluded. Outcomes included 1‐ and 12‐month mortality and functional (Seattle Angina Questionnaire [SAQ]) and psychosocial status. Of 2690 patients undergoing angiography, 2374 (88.4%) had MI‐CAD, 299 (11.1%) had MINOCA, and 17 (0.6%) remained unclassified. Women had 5 times higher odds of having MINOCA than men (14.9% versus 3.5%; odds ratio: 4.84; 95% confidence interval, 3.29–7.13). MINOCA patients were more likely to be without traditional cardiac risk factors (8.7% versus 1.3%; P<0.001) but more predisposed to hypercoaguable states than MI‐CAD patients (3.0% versus 1.3%; P=0.036). Women with MI‐CAD were more likely than those with MINOCA to be menopausal (55.2% versus 41.2%; P<0.001) or to have a history of gestational diabetes mellitus (16.8% versus 11.0%; P=0.028). The MINOCA mechanisms varied: a nonplaque mechanism was identified for 75 patients (25.1%), and their clinical profiles and management also varied. One‐ and 12‐month mortality with MINOCA and MI‐CAD was similar (1‐month: 1.1% and 1.7% [P=0.43]; 12‐month: 0.6% and 2.3% [P=0.68], respectively), as was adjusted 12‐month SAQ quality of life (76.5 versus 73.5, respectively; P=0.06).
Conclusions
Young patients with MINOCA were more likely women, had a heterogeneous mechanistic profile, and had clinical outcomes that were comparable to those of MI‐CAD patients.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00597922.
Keywords: acute myocardial infarction, myocardial infarction with nonobstructive coronary arteries, nonobstructive, prognosis, sex, women
Subject Categories: Cardiovascular Disease, Epidemiology, Women, Mortality/Survival, Quality and Outcomes
Clinical Perspective
What Is New?
In a multicenter study of young patients (aged 18–55 years) diagnosed with acute myocardial infarction, we found myocardial infarction with nonobstructive coronary arteries (MINOCA) to be prevalent in 11%, predominantly among women and nonwhite patients.
Patients with MINOCA were less likely to have traditional cardiac risk factors and more likely to have hypercoaguable states than those with myocardial infarction due to coronary artery disease.
When tested for the underlying mechanism, MINOCA patients had variable causes such as coronary artery vasospasm, spontaneous coronary artery dissection, or coronary artery embolization. The clinical profile, management, and prognosis of these patients also varied based on the cause, necessitating further workup.
What Are the Clinical Implications?
In young patients with acute myocardial infarction, the course of MINOCA was not benign; 1‐ and 12‐month mortality and functional and psychosocial outcomes were similar to those of patients with myocardial infarction due to coronary artery disease.
Patients with acute myocardial infarction who are ruled out for obstructive coronary artery disease should undergo additional testing to elucidate the underlying cause of ischemia and to initiate appropriate treatment.
Introduction
Patients with myocardial infarction with nonobstructive coronary arteries (MINOCA) constitute 6% to 14% of all those with acute myocardial infarction (AMI).1, 2, 3, 4, 5, 6, 7, 8 Recent evidence demonstrates that patients with MINOCA are distinct from patients with AMI with the classic culprit lesion—namely, >50% plaque‐mediated occlusion of the coronary artery (myocardial infarction due to coronary artery disease [MI‐CAD])—by having lower prevalence of the traditional cardiac risk factors and a lower but clinically significant annual mortality rate.1, 5, 6 Seen more commonly in women and young patients, existing literature on MINOCA is extrapolated from studies enrolling predominantly men and older patients, and this may limit opportunities to fully describe the different phenotypes of disease defined as MINOCA, their clinical profiles, and their associated outcomes.3, 6, 9, 10, 11
Current knowledge regarding outcomes of MINOCA patients has been limited primarily to mortality. Little is known about the clinical profile of specific phenotypes or the functional, psychosocial, and health status of these patients.6 Confusion also exists regarding the definition of MINOCA.12 In contemporary literature, MINOCA is an umbrella term for all causes of troponin elevation inclusive of coronary causes of ischemia (eg, plaque rupture, spasm, spontaneous coronary artery dissection [SCAD], or embolization) and noncoronary causes (eg, myocarditis or takotsubo).13 More recently, however, it has been proposed to primarily describe patients with coronary‐related ischemia.12 The dual interpretation is reflected in the heterogeneity of the inclusion criteria of earlier studies and has resulted in disparities in describing the clinical profile, course, and prognosis of these patients.5, 6, 14, 15
Young women with AMI represent an ideal population in which to clarify MINOCA‐related questions. In this population, nonobstructive disease is common, yet outcomes including mortality are worse compared with men.16 The VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients) study, the largest prospective multicenter study that enrolled young adults under age 55 years with a diagnosis of AMI and had an oversampling of women,17 provides an ideal opportunity to look more carefully at MINOCA. One in 8 young women in VIRGO had no evidence of plaque rupture or thrombosis, allowing pathophysiologically driven insight into the study of ischemic MINOCA.18 Accordingly, we studied this sample to better characterize the presentation, sociodemographic and psychosocial data, clinical characteristics, sex‐specific factors (eg, perimenopausal and peripartum status), and outcomes in MINOCA versus MI‐CAD patients and among MINOCA patients by phenotypic mechanism and sex.
Methods
Setting and Participants
We analyzed the US VIRGO registry, with a study population of 2985 patients (2009 women, 976 men). The VIRGO investigators intend to share study data and are investigating mechanisms and funding to make that possible. We are currently working on 2 pilot data‐sharing efforts. VIRGO was a prospective observational study of patients aged 18 to 55 years presenting with an AMI in 103 geographically diverse hospitals from August 2008 to January 2012 using a strict 2:1 enrollment ratio of women to men. AMI was defined as (1) an increase in cardiac biomarkers (troponin I or T or creatine kinase‐MB) with at least 1 value >99th percentile of the upper reference limit within 24 hours of admission and (2) supporting evidence of acute myocardial ischemia, including symptoms or ECG changes.18 Patients with elevated cardiac markers due to a complication of elective coronary revascularization, presumed myocarditis, or takotsubo were not eligible for VIRGO. Only patients who underwent cardiac catheterization were included in our analysis. Patients who received thrombolytics before undergoing angiography were excluded. Institutional review board approval was obtained at each participating center, and all patients provided written informed consent to participate.
Patient Characteristics and Outcomes
Information was obtained by medical chart abstraction, and standardized in‐person interviews were performed by trained personnel during the index admission for the following variables. Self‐identified race was categorized as black, white, or other, and ethnicity as hispanic or non‐hispanic. Chest pain symptoms were defined as pain, pressure, tightness, or discomfort in the chest. For women, reproductive and menstrual history was obtained. Information on cardiac risk factors and cardiac procedures as listed in Table 1, hypercoaguable syndromes (as charted by the treatment team), clinical severity of AMI (peak troponin level, ejection fraction <40%), in‐hospital therapies received (revascularization, automatic implantable cardioverter‐defibrillator insertion), discharge medications, length of stay, disposition and final adjudicated ECG diagnosis (ST‐segment–elevation myocardial infarction [STEMI] versus non‐STEMI) were also collected. Validated standardized instruments were used to assess psychosocial and health status. These included (1) depression using the 9‐item Patient Health Questionnaire (PHQ‐9), with higher scores indicating increasing severity (range 0–27)19, 20, 21, 22; (2) perceived stress using the 14‐item Perceived Stress Scale (PSS), with higher scores indicating higher stress levels (range 0–40)23, 24; and (3) health status outcomes (patients’ physical limitations, angina frequency, and quality of life related to angina) using the Seattle Angina Questionnaire (SAQ; scores ranging from 0 to 100), with higher scores indicating better health status.25, 26, 27, 28, 29 In addition, detailed review of medical charts was conducted to better characterize the AMI phenotypes.
Table 1.
Risk Factor Profile and Clinical Characteristics in Patients With MI‐CAD and MINOCA
| MI‐CAD n=2374 (88.8%) | MINOCA n=299 (11.2%) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age, y, median (IQR) | 48 (44–52) | 46 (40–51) | <0.001a |
| Women | 1541 (64.9) | 269 (90.0) | <0.001a |
| White | 1824 (76.8) | 203 (67.9) | 0.008b |
| Hispanic origin | 169 (7.1) | 31 (10.4) | 0.022c |
| Risk factors—conventional | |||
| Hypertension | 1595 (67.2) | 164 (54.9) | <0.001a |
| Diabetes mellitus | 750 (31.6) | 52 (17.4) | <0.001a |
| Dyslipidemia | 1653 (69.6) | 164 (54.9) | <0.001a |
| Smoking in past 30 d | 1430 (60.3) | 103 (34.5) | <0.001a |
| Obesity | 1285 (54.1) | 126 (42.1) | <0.001a |
| Family history of CAD | 1785 (75.2) | 184 (61.5) | <0.001a |
| Any of above risk factors | 2342 (98.7) | 273 (91.3) | <0.001a |
| Stroke/TIA | 112 (4.7) | 9 (3.0) | 0.19 |
| Prior AMI | 517 (21.8) | 37 (12.4) | <0.001a |
| CHF | 107 (4.5) | 13 (4.4) | 0.93 |
| Prior PAD | 63 (2.7) | 0 (0) | 0.004b |
| Risk factors—unconventional | |||
| Depression | 788 (34.6) | 83 (28.6) | 0.06 |
| Perceived stress, median (IQR) | 26.0 (19.0–32.0) | 26.0 (19.0–32.0) | 0.65 |
| History of cocaine use | 110 (4.6) | 18 (6.0) | 0.28 |
| History of illicit drug use (not cocaine) | 192 (8.1) | 31 (10.4) | 0.16 |
| AMI triggered by cocaine use | 29 (1.2) | 4 (1.3) | 0.78 |
| Hypercoagulability syndrome | 31 (1.3) | 9 (3.0) | 0.036 |
| Venous thromboembolism | 62 (2.6) | 11 (3.7) | 0.27 |
| Autoimmune disease | 70 (2.6) | 12 (4.0) | 0.29 |
| Known renal dysfunction | 261 (11.0) | 27 (9.1) | 0.32 |
| Thyroid disorders | 159 (6.7) | 29 (9.7) | 0.05 |
| For women only (n=1808) | |||
| Polycystic ovarian disease | 11 (0.7) | 2 (0.7) | 0.95 |
| Menopause | 850 (55.2) | 110 (40.9) | <0.001a |
| Age at menarche, median (IQR) | 12.0 (11.0–13.0) | 13.0 (12.0–14.0) | 0.003b |
| OCP use (n=1395) | 39 (3.3) | 7 (3.4) | 0.90 |
| Ever got pregnant | 1361 (88.4) | 232 (86.2) | 0.49 |
| For women who have been pregnant (n=1592) | |||
| Preeclampsia | 360 (26.7) | 67 (29.1) | 0.43 |
| Gestational diabetes mellitus | 224 (16.8) | 25 (10.9) | 0.028c |
| Stillbirth | 68 (05.0) | 12 (5.2) | 0.91 |
| Miscarriage | 391 (29.0) | 65 (28.1) | 0.82 |
| Diagnosis | |||
| Prehospital ECG | 639 (27.1) | 64 (21.5) | 0.041c |
| Discharge diagnosis | |||
| STEMI | 1236 (52.1) | 64 (21.4) | <0.001a |
| Non‐STEMI | 1138 (47.9) | 235 (78.6) | |
| Length of hospital stay, d, median (IQR) | 3.0 (2.0–5.0) | 3.0 (2.0–4.0) | 0.010c |
| Severity of disease | |||
| Peak troponin, median (IQR) | 7.6 (1.6–29.7) | 3.4 (1.1–11.1) | <0.001a |
| Ejection fraction <40% | 265 (11.5) | 29 (9.7) | 0.39 |
| Interventions | |||
| PCI | 1945 (81.9) | 34 (11.4) | <0.001a |
| CABG | 248 (10.5) | 5 (1.7) | <0.001a |
| AICD (of patients eligible n=47) | 14 (36.8) | 4 (44.4) | 0.72 |
| Discharge management (of eligible patients) | |||
| Aspirin | 2314 (98.6) | 266 (93.7) | <0.001a |
| Beta blockers | 2222 (98.3) | 213 (85.9) | <0.001a |
| ACEI or ARB | 1573 (73.3) | 134 (50.2) | <0.001a |
| Statin | 2255 (96.9) | 202 (73.4) | <0.001a |
| Cardiac rehabilitation | |||
| Referred on discharge | 1151 (48.5) | 97 (32.4) | <0.001a |
| Reported referral at 1‐mo after AMI follow‐up | 1410 (65.1) | 127 (46.5) | <0.001a |
Data are shown as n (%), except as noted. ACEI indicates angiotensin‐converting enzyme inhibitor; AICD, automatic implantable cardioverter‐defibrillator; AMI, acute myocardial infarction; ARB, angiotensin receptor II blocker; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHF, congestive heart failure; IQR, interquartile range; MI‐CAD, myocardial infarction due to coronary artery disease; MINOCA, myocardial infarction with nonobstructive coronary arteries; OCP, oral contraceptives; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; TIA, transient ischemic attack.
P<0.001.
P<0.01.
P<0.05.
One‐ and 12‐month post‐AMI outcomes including data on mortality and functional and psychosocial outcomes were collected through follow‐up interviews with the patients. Mortality data were collected through telephone follow‐up, review of medical records at the primary site, and online searches.
MINOCA Versus MI‐CAD Classification
We used the previously published VIRGO taxonomy that classified patients into 5 phenotypes.18 Briefly, class I included patients with plaque‐mediated obstructive culprit lesions who underwent revascularization; class II included patients with obstructive coronary artery disease (≥50%) but without evident plaque rupture/thrombosis; class III included patients with nonobstructive coronary artery disease (<50%); class IV included patients with a nonplaque mechanism identified for ischemia by the primary treatment team, including coronary artery vasospasm (relieved by intracoronary nitroglycerin), SCAD (regardless of degree of stenosis), and coronary artery embolization; and class V included patients with undetermined classification. Using this taxonomy, we defined patients for our analysis as follows: Patients were considered to have MI‐CAD if classified as class I or II. Patients in class III or IV were described as having MINOCA.
Statistical Analyses
Descriptive statistics were calculated using counts and percentages for categorical variables and median and interquartile range for continuous variables. To assess statistical significance, χ2 and Fisher exact tests were used for categorical variables, and Wilcoxon rank sum tests were used for continuous variables. Symptoms were categorized as chest pain, other (non–chest pain symptoms) or none. Other variables were defined as follows: current smoking (within the past 30 days), obesity (body mass index ≥30; kg/m2), and depression (PHQ‐9 score ≥10). Least squares means and SEs from linear covariance pattern models were used to describe scores on the PSS and the SAQ at baseline and at 1 and 12 months after discharge.30 Models were selected a priori and included fixed main effects for time, MINOCA status (MINOCA versus MI‐CAD), or sex (men versus women) and the interaction of MINOCA or sex with time. An unstructured covariance pattern was assumed to account for correlation of repeated measures. Adverse health status was defined with SAQ scores as follows: physical limitation <75, angina frequency <100, and treatment satisfaction <75. Overall quality of life from the SAQ was compared for MINOCA and MI‐CAD at 12 months using linear contrasts within the covariance pattern model framework. For modeling functional outcomes, we focused on quality of life because it was significantly different between the 2 groups and served as a summary measure to reflect the influence of disease on a patient's perception, symptoms, and function. The influence of differential baseline characteristics between comparison groups was evaluated by sequentially adding covariates to the model and examining their influence on the estimated difference. Eight models were evaluated, sequentially adding groups of demographic, socioeconomic, cardiac risk, other cardiac illness, noncardiac illness, and measures of symptom severity to the model, selected a priori based on their association with quality of life. Differences in quality of life at 12 months are presented with 95% confidence intervals (CIs). A 2‐sided P<0.05 was considered statistically significant. All analyses were performed in SAS version 9.4 (SAS Institute).
Results
Clinical Characteristics
Figure S1 describes patient flow through the VIRGO study. Overall, 47% of eligible patients were not enrolled, and 47 (1%) were adjudicated as noncoronary AMI and did not complete enrollment. These patients were similar to enrolled patients, with a median age of 49 years (interquartile range: 44–52 years), 61% female, and 70% white. Reasons for not enrolling were as follows: 29% were discharged before being contacted by study coordinator, 50% refused consent, <1% were Spanish speaking and no translator was available, and 21% had other reasons.
Of the 2985 enrolled patients, 295 either did not have an angiogram or received thrombolytics before an angiogram, leaving 2690 participants for analysis. Of those, 2374 (88.4%) were classified as having MI‐CAD, 299 (11.1%) were classified as having MINOCA, and 17 (0.6%) remained unclassified. Using the VIRGO taxonomy, 75 (25.1%) of the MINOCA patients demonstrated a clear mechanism (class IV) with coronary artery vasospasm (n=11), SCAD (n=61), or coronary artery embolization (n=3), whereas the majority (n=224 from class III) had no cause attributed.
Table 1 depicts the overall characteristics and risk factor profiles of patients with MI‐CAD and MINOCA. MINOCA was associated with young age (median: 46 years) and 1.6 times greater likelihood of presenting as non‐STEMI than patients with MI‐CAD (78.6.1% versus 47.9%; P<0.001). Although prevalent overall, fewer MINOCA patients (91.3%) had ≥1 of the traditional cardiac risk factors than MI‐CAD patients (98.7%; P<0.001). Hypercoaguable states were uncommon but seen more frequently with MINOCA than MI‐CAD patients (3.0% versus 1.3%; P=0.036). Factors such as autoimmune conditions, psychosocial factors, or use of illicit drugs including cocaine did not differ by AMI type. Women with MI‐CAD were more likely to be menopausal at the time of AMI and to have a history of early menarche than MINOCA patients. They were also more likely to have history of gestational diabetes mellitus, but there were no differences in history of preeclampsia, stillbirth, or miscarriage. Chest pain was the most common presenting complaint for patients with MI‐CAD and MINOCA (87.3% versus 86.3%; P=0.63). This was true for both women and men with MINOCA (87.0% versus 80.0%) and with MI‐CAD (86.2% versus 89.3%).
Women had 5 times higher odds of presenting with MINOCA than men (14.9% versus 3.5%; unadjusted odds ratio: 4.84; 95% CI, 3.29–7.13). Nonwhite patients had 1.5 higher odds of having MINOCA than white patients (14.9% versus 10.0%; unadjusted odds ratio: 1.57; 95% CI, 1.21–2.04). With almost 90% of the MINOCA sample being women, further sex‐specific comparisons were limited (Table S1). Women and men appeared to be similar in age, cardiac risk profile, and severity of disease, but women received fewer cardioprotective medications.
Outcomes
At 1 month, a total of 10 participants were missing (9 with MI‐CAD [9/2374=0.003] and 1 with MINOCA [1/299=0.003]). At 12 months, a total of 58 were missing (51 with MI‐CAD [51/2374=0.02] and 7 with MINOCA [7/299=0.02]). Mortality was low for both MINOCA and MI‐CAD patients. Four patients with MI‐CAD and none with MINOCA died during the index hospitalization. The 1‐month mortality for patients with MINOCA was 1.1% compared with 0.6% in MI‐CAD patients (P=0.43), whereas 12‐month mortality was 0.6% and 2.3%, respectively (P=0.68). Functional and psychosocial outcomes showed a parallel trend in patients with MI‐CAD and MINOCA at baseline, 1 month, and 12 months (Figure 1). Adverse health status was similar between MINOCA and MI‐CAD at 12 months (7% versus 10%, respectively, for physical limitation [P=0.11]; 26% versus 31%, respectively, for angina frequency [P=0.17]; 28% versus 34%, respectively, for quality of life [P=0.07]), except for treatment satisfaction (15% versus 10%, respectively; P=0.03). Unadjusted quality of life for MI‐CAD patients at 12 months was lower compared with MINOCA patients (Table S2). When adjusted for sociodemographic, socioeconomic, clinical, and psychosocial factors, this was no longer true (76.5 versus 73.5 for MINOCA and MI‐CAD, respectively [P=0.06]; difference between means: 3.08; 95% CI, −0.11 to 6.27).
Figure 1.
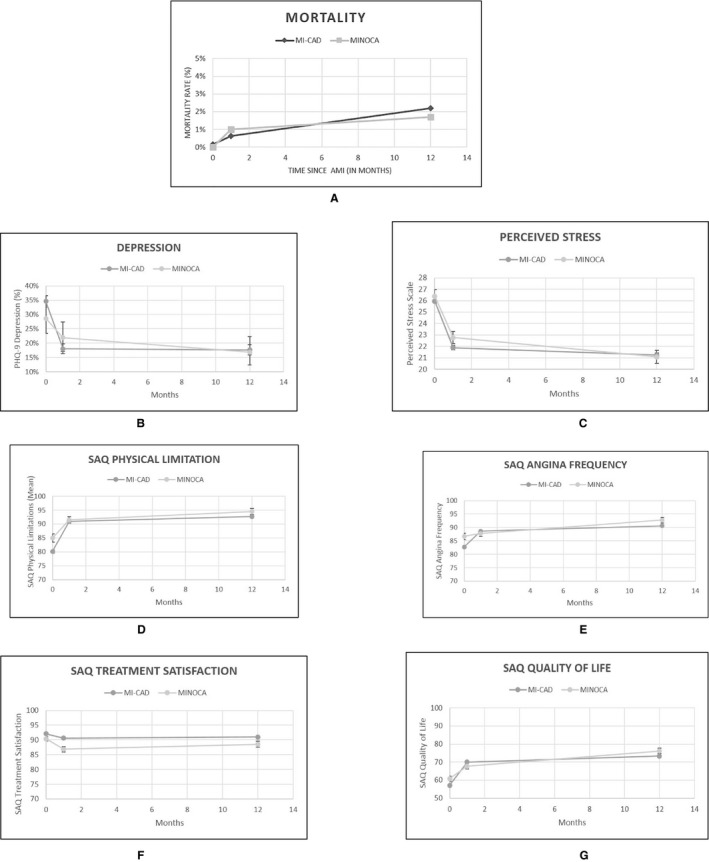
Comparison of young patients with myocardial infarction due to coronary artery disease (MI‐CAD) and myocardial infarction with nonobstructive coronary arteries (MINOCA) at baseline, 1 month, and 12 months for (A) mortality; psychosocial outcomes, including (B) depression and (C) perceived stress; and health status, including (D) physical limitations, (E) angina frequency, (F) treatment satisfaction, and (G) quality of life. PHQ‐9 indicates Patient Health Questionnaire; SAQ, Seattle Angina Questionnaire.
A total of 8 women with MINOCA died (1 with vasospasm, 2 with SCAD, and 5 with undefined MINOCA; Figure 2). No men with MINOCA died within the study period. Women reported lower functional status than men at baseline and at 1 month after AMI. Perceived stress was higher in women than men at baseline and at 12 months (mean score: 21.5 versus 17.3; P=0.03); however, these differences were no longer statistically significant at 1 month (mean score: 22.9 versus 20.3; P=0.09) or 12 months (mean score: 21.1 versus 18.7; P=0.20), after adjusting for baseline scores.
Figure 2.
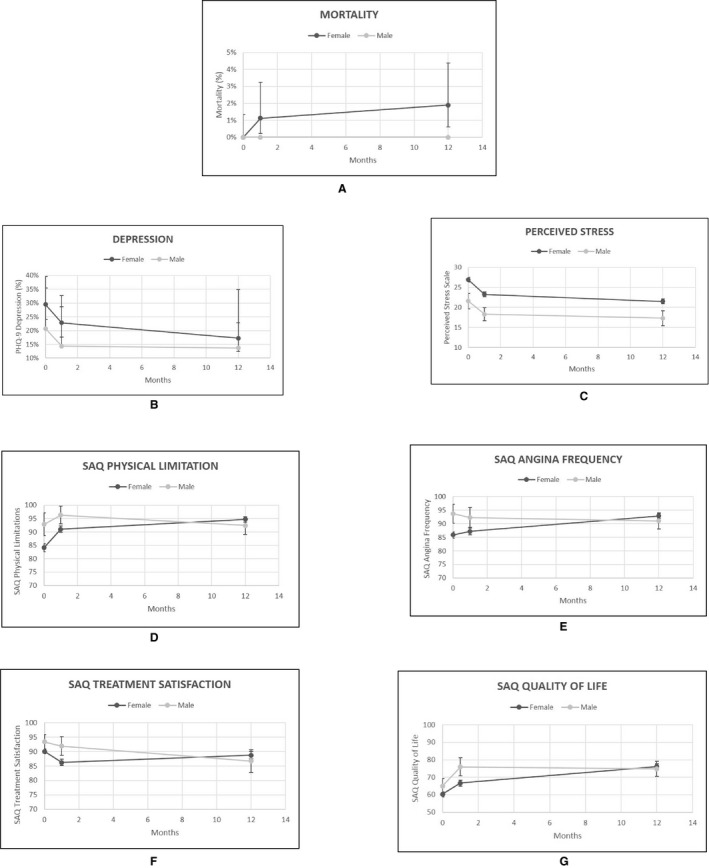
Comparison of young men and women with myocardial infarction with nonobstructive coronary arteries at baseline, 1 month, and 12 months for (A) mortality; psychosocial outcomes, including (B) depression and (C) perceived stress; and health status, including (D) physical limitations, (E) angina frequency, (F) treatment satisfaction, and (G) quality of life. PHQ‐9 indicates Patient Health Questionnaire; SAQ, Seattle Angina Questionnaire.
Description of MINOCA Phenotypes
Overall, 4 patients with MINOCA presented in cardiac arrest and underwent automatic implantable cardioverter‐defibrillator placement. One had a history of familial thrombophilia, 1 had congenital long QTc syndrome, and 1 had coronary microvascular embolization. Despite fewer traditional risk factors, 37 MINOCA patients reported prior AMI (Table 1), listed as either non‐obstructive CAD (NOCAD) NOCAD or vasospasm on prior angiograms. Thirteen MINOCA patients had a history of heart failure, a third due to preserved ejection fraction. Those with low ejection fraction were older (median age: 50 years) and had a higher proportion of cardiac risk factors than patients with normal ejection fraction (Table S3).
Table 2 demonstrates interesting trends by MINOCA subtype. Patients with undefined MINOCA (class III) had lower peak troponin values than MI‐CAD patients but similar rates of reduced left ventricular function, automatic implantable cardioverter‐defibrillator placement, and length of hospital stay. Yet these patients were significantly less likely to receive secondary prevention medications and cardiac rehabilitation at discharge.
Table 2.
Clinical Characteristics and Outcomes in Patients With MI‐CAD Versus MINOCA (Undefined, Coronary Artery Vasospasm, Spontaneous Coronary Artery Dissection, or Coronary Artery Embolization)
| MI‐CAD (n=2374) | MINOCA Undefined (n=224) | MINOCA Spasm (n=11) | MINOCA Dissection (n=61) | MINOCA Embolization (n=3) | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, y, median (IQR) | 48.0 (44.0–52.0) | 47.0 (41.0–51.0) | 45.0 (37.0–50.0) | 44.0 (40.0–51.0) | 47.0 (31.0–54.0) |
| Women | 1541 (64.91) | 201 (89.73) | 10 (90.91) | 56 (91.80) | 2 (66.67) |
| Race | |||||
| Black | 412 (17.35) | 59 (26.34) | 3 (27.27) | 6 (9.84) | 1 (33.33) |
| White | 1824 (76.83) | 147 (65.63) | 7 (63.64) | 47 (77.05) | 2 (66.67) |
| American Indian | 32 (1.35) | 6 (2.68) | 1 (9.09) | 1 (1.64) | 0 (0.00) |
| Asian/Pacific Islander | 55 (2.32) | 4 (1.79) | 0 (0.00) | 3 (4.92) | 0 (0.00) |
| Other | 47 (1.98) | 8 (3.57) | 0 (0.00) | 4 (6.56) | 0 (0.00) |
| Hispanic ethnicity | 169 (7.12) | 23 (10.27) | 0 (0.00) | 8 (13.11) | 0 (0.00) |
| Risk factors—conventional | |||||
| Hypertension | 1595 (67.19) | 134 (59.82) | 7 (63.64) | 22 (36.07) | 1 (33.33) |
| Diabetes mellitus | 750 (31.59) | 46 (20.54) | 2 (18.18) | 4 (6.56) | 0 (0.00) |
| Dyslipidemia | 1653 (69.63) | 122 (54.46) | 6 (54.55) | 34 (55.74) | 2 (66.67) |
| Smoking | 1430 (60.26) | 85 (37.95) | 4 (36.36) | 13 (21.31) | 1 (33.33) |
| Obesity | 1285 (54.13) | 106 (47.32) | 1 (9.09) | 17 (27.87) | 2 (66.67) |
| Family history of CAD | 1785 (75.25) | 142 (63.39) | 8 (72.73) | 32 (52.46) | 2 (66.67) |
| Any of above risk factor | 2342 (98.65) | 210 (93.75) | 10 (90.91) | 50 (81.97) | 3 (100.00) |
| Stroke/TIA | 112 (4.72) | 8 (3.57) | 1 (9.09) | 0 (0.00) | 0 (0.00) |
| Prior AMI | 517 (21.78) | 26 (11.61) | 1 (9.09) | 10 (16.39) | 0 (0.00) |
| CHF | 107 (4.51) | 11 (4.91) | 1 (9.09) | 0 (0.00) | 1 (33.33) |
| Prior angina | 645 (27.20) | 44 (19.73) | 6 (54.55) | 13 (21.31) | 0 (0.00) |
| Known renal dysfunction | 261 (11.05) | 24 (10.76) | 2 (18.18) | 1 (1.64) | 0 (0.00) |
| Known PAD | 63 (2.66) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Risk factors—unconventional | |||||
| Prior cocaine use | 110 (4.65) | 17 (7.59) | 1 (9.09) | 0 (0.00) | 0 (0.00) |
| Prior other illicit drug use | 192 (8.13) | 28 (12.56) | 3 (27.27) | 0 (0.00) | 0 (0.00) |
| Hypercoagulability syndrome | 31 (1.31) | 7 (3.13) | 0 (0.00) | 1 (1.64) | 1 (33.33) |
| Venous thromboembolism | 62 (2.62) | 8 (3.57) | 1 (9.09) | 2 (3.28) | 0 (0.00) |
| Autoimmune disorder | 70 (2.95) | 9 (4.04) | 1 (9.09) | 2 (3.28) | 0 (0.00) |
| Thyroid disorders | 159 (6.71) | 23 (10.27) | 1 (9.09) | 5 (8.20) | 0 (0.00) |
| For women only (n=1808) | |||||
| Polycystic ovarian syndrome | 11 (0.72) | 1 (0.50) | 0 (0.00) | 1 (1.79) | 0 (0.00) |
| Menopause | 850 (55.19) | 88 (43.78) | 6 (60.00) | 16 (28.57) | 0 (0.00) |
| Age at menarche (IQR) | 12.0 (11.0–13.0) | 13.0 (11.0–14.0) | 15.0 (13.0–16.0) | 13.0 (12.0–14.0) | 9.5 (8.0–11.0) |
| OCP use (n=1395) | 39 (3.27) | 7 (4.61) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Ever got pregnant | 1361 (88.38) | 177 (88.06) | 8 (80.00) | 45 (80.36) | 2 (100.00) |
| For women who have been pregnant (n=1592) | |||||
| Preeclampsia | 360 (26.73) | 54 (30.86) | 3 (37.50) | 9 (20.00) | 1 (50.00) |
| Gestational diabetes mellitus | 224 (16.78) | 18 (10.29) | 2 (28.57) | 5 (11.36) | 0 (0.00) |
| Still birth | 68 (5.04) | 9 (5.11) | 0 (0.00) | 3 (6.67) | 0 (0.00) |
| Miscarriage | 391 (29.01) | 50 (28.41) | 4 (50.00) | 10 (22.22) | 1 (50.00) |
| Discharge diagnosis | |||||
| Non‐STEMI | 1138 (47.94) | 193 (86.16) | 10 (90.91) | 30 (49.18) | 2 (66.67) |
| STEMI | 1236 (52.06) | 31 (13.84) | 1 (9.09) | 31 (50.82) | 1 (33.33) |
| Length of stay, median (IQR) | 3.0 (2.0–5.0) | 3.0 (2.0–4.0) | 3.0 (2.0–5.0) | 4.0 (2.0–5.0) | 4.0 (3.0–5.0) |
| Severity of disease | |||||
| Peak troponin, median (IQR) | 7.6 (1.6–29.7) | 2.3 (0.8–6.7) | 1.5 (0.4–21.4) | 12.1 (5.2–35.3) | 33.1 (17.7–33.7) |
| Ejection fraction <40% | 265 (11.50) | 19 (8.52) | 1 (9.09) | 8 (13.11) | 1 (33.33) |
| Interventions | |||||
| PCI | 1945 (81.93) | 0 (0.00) | 0 (0.00) | 34 (55.74) | 0 (0.00) |
| CABG | 248 (10.52) | 0 (0.00) | 0 (0.00) | 5 (8.20) | 0 (0.00) |
| AICD | 14 (36.84) | 4 (44.44) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Discharge management (of eligible patients) | |||||
| Aspirin | 2314 (98.64) | 192 (91.43) | 10 (100.00) | 61 (100.00) | 3 (100.00) |
| Beta blocker | 2222 (98.32) | 156 (83.87) | 4 (66.67) | 50 (94.34) | 3 (100.00) |
| ACEI or ARB | 1573 (73.33) | 97 (48.26) | 1 (14.29) | 34 (60.71) | 2 (66.67) |
| Statins | 2255 (96.95) | 145 (70.39) | 5 (62.50) | 50 (84.75) | 2 (100.00) |
| Cardiac rehabilitation | |||||
| Referred on discharge | 1151 (48.48) | 62 (27.68) | 2 (18.18) | 31 (50.82) | 2 (66.67) |
| Referred 1 mo after AMI | 1410 (65.10) | 79 (39.11) | 2 (20.00) | 45 (77.59) | 1 (33.33) |
| Outcomes | |||||
| Mortality | |||||
| In‐hospital mortality | 4 (0.17) | 0 (0.00) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| 1‐mo mortality | 14 (0.59) | 2 (0.90) | 0 (0.00) | 1 (1.64) | 0 (0.00) |
| 12‐mo mortality | 53 (2.28) | 3 (1.37) | 1 (11.11) | 1 (1.64) | 0 (0.00) |
| Functional outcomes | |||||
| SAQ physical limitation score, mean (SD) | |||||
| Baseline | 80.12 (26.05) | 83.32 (24.52) | 94.19 (10.28) | 88.31 (20.65) | 100.00 (0.00) |
| 1‐mo after AMI | 91.20 (18.77) | 90.69 (18.75) | 85.28 (25.79) | 96.30 (11.48) | 100.00 (0.00) |
| 12‐mo after AMI | 93.00 (17.57) | 94.38 (16.42) | 84.92 (37.50) | 97.82 (8.89) | 94.44 (9.62) |
| SAQ angina frequency score, mean (SD) | |||||
| Baseline | 82.69 (20.85) | 86.28 (20.14) | 89.09 (13.00) | 87.87 (15.18) | 83.33 (15.28) |
| 1‐mo after AMI | 88.66 (18.09) | 86.90 (18.95) | 79.00 (32.81) | 91.90 (16.70) | 100.00 (0.00) |
| 12‐mo after AMI | 90.82 (17.45) | 92.81 (14.62) | 81.43 (30.78) | 96.07 (9.08) | 83.33 (15.28) |
| SAQ treatment satisfaction score, mean (SD) | |||||
| Baseline | 92.11 (12.47) | 89.83 (14.57) | 88.07 (12.64) | 92.42 (11.86) | 100.00 (0.00) |
| 1‐mo after AMI | 90.62 (14.09) | 86.73 (18.36) | 81.88 (19.86) | 88.04 (12.95) | 89.58 (13.01) |
| 12‐mo after AMI | 91.09 (15.13) | 88.23 (19.87) | 94.44 (4.87) | 89.58 (15.88) | 97.92 (3.61) |
| SAQ quality of life score, mean (SD) | |||||
| Baseline | 57.14 (25.15) | 60.54 (24.46) | 58.33 (15.81) | 61.61 (22.38) | 69.44 (26.79) |
| 1‐mo after AMI | 70.12 (25.12) | 67.00 (28.85) | 61.67 (31.48) | 71.84 (20.93) | 77.78 (9.62) |
| 12‐mo after AMI | 73.79 (23.34) | 75.65 (22.10) | 70.83 (19.54) | 80.71 (15.00) | 72.22 (9.62) |
| Psychosocial outcomes | |||||
| Depression—PHQ‐9 score >10 | |||||
| Baseline | 788 (34.58) | 64 (29.63) | 3 (27.27) | 15 (25.00) | 1 (33.33) |
| 1‐mo after AMI | 381 (18.00) | 48 (24.24) | 5 (50.00) | 6 (10.34) | 0 (0.00) |
| 12‐mo after AMI | 323 (17.67) | 34 (18.89) | 1 (16.67) | 6 (11.11) | 0 (0.00) |
| Perceived stress, median (IQR) | |||||
| Baseline | 26.0 (19.0–32.0) | 26.0 (20.0–32.5) | 30.5 (23.0–35.0) | 24.0 (16.0–31.0) | 15.0 (13.0–24.0) |
| 1‐mo after AMI | 21.0 (15.0–28.0) | 23.0 (17.0–29.0) | 29.0 (18.0–33.0) | 20.5 (15.0–25.0) | 15.0 (15.0–24.0) |
| 12‐mo after AMI | 20.0 (14.0–27.0) | 21.0 (16.0–27.0) | 20.5 (19.0–29.0) | 18.0 (13.0–22.0) | 23.0 (18.0–26.0) |
Data are shown as n (%), except as noted. ACEI indicates angiotensin‐converting enzyme inhibitor; AICD, automatic implantable cardioverter‐defibrillator; AMI, acute myocardial infarction; ARB, angiotensin receptor II blocker; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CHF, congestive heart failure; IQR, interquartile range; MI‐CAD, myocardial infarction due to coronary artery disease; MINOCA, myocardial infarction with nonobstructive coronary arteries; OCP, oral contraceptives; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; PHQ‐9, Patient Health Questionnaire; SAQ, Seattle Angina Questionnaire; STEMI, ST‐segment–elevation myocardial infarction; TIA, transient ischemic attack.
In total, 61 patients were diagnosed with SCAD. These patients were younger (median age: 44 years) and often without traditional cardiac risk factors compared with other MINOCA phenotypes (81.9% versus 93.7%); however, they presented often with STEMI (50.8%), had higher troponins, and had more referrals for cardiac rehabilitation than other MINOCA groups (77.6% versus 34.5%; P<0.001). We observed wide variation in revascularization practices for SCAD regardless of degree of stenosis.
Eleven MINOCA patients had vasospasm and 5 times higher odds of having prior angina than other MINOCA patients (odds ratio: 4.80; 95% CI, 1.41–16.28). Triggers identified by the primary treatment team included smoking, sumatriptan, pseudoephedrine, inotropes, hypertension, methamphetamine, and extreme stress. None tested positive for cocaine. None were treated with calcium channel blockers or automatic implantable cardioverter‐defibrillator placement.
Seventeen patients remained unclassified, with prior revascularization but no identifiable cause (Table S4). They were treated similarly to MI‐CAD patients with the exception of referral for cardiac rehabilitation.
Discussion
We demonstrated that young patients with ischemic MINOCA, representing 11% of our VIRGO population, were more likely to be women, nonwhite, and young; to present with non‐STEMI; and to have fewer traditional risk factors compared with MI‐CAD patients. Outcomes, including mortality and functional and psychosocial status, for patients with MINOCA and MI‐CAD were comparable. The strength and uniqueness of our study lie in the prospectively collected sex‐specific data, such as perinatal and menopausal history, and the detailed psychosocial and health status data that have not been reported previously, comparing MINOCA and MI‐CAD patients. Using a previously validated angiographically based VIRGO taxonomy, we were also able to describe the distinct features of MINOCA phenotypes once the underlying mechanism was defined.18
These findings advance our understanding of MINOCA, an area that is rapidly evolving as experts seek consensus on a precise definition.12 We classified MINOCA as describing patients with coronary‐related ischemia, either in the absence of coronary artery obstruction or from non–plaque‐mediated mechanisms.13, 31, 32, 33, 34, 35 The diverse pathophysiology likely explains the differences in clinical profile, severity, and prognosis that we observed in MINOCA subtypes. We found patients with SCAD, for example, to be younger, more often female, and with few cardiac risk factors but greater severity of disease compared with other MINOCA groups. In contrast, patients with vasospasm more often reported recurrent angina, illicit drug use, and pregnancy‐related complications. With 1 in 10 young patients with AMI diagnosed with MINOCA,3, 4, 5, 6, 7, 8 our findings highlight both the challenges and the importance of pursuing a systematic approach to identify the underlying cause (coronary‐ischemic, non–coronary‐ischemic or nonischemic, or noncardiac).13, 36
Our results also help us better understand the clinical profile of patients with MINOCA. In VIRGO, although these patients had fewer traditional risk factors compared with MI‐CAD patients, they had higher proportions relative to previously studied cohorts of MINOCA or the general population.5, 37, 38, 39 This could be due to systematic exclusion of patients with presumptive myocarditis and takotsubo, who are often healthier than patients with coronary ischemia such as those enrolled in VIRGO.37, 38, 39 We also noted some interesting nontraditional associations with MINOCA. Hypercoaguable conditions, although infrequent, were more common with MINOCA than MI‐CAD. Prothrombotic states associated with high fibrinogen, factor VII of homocysteine levels, decreased fibrinolytic activity, or deficiency of protein C or S can increase risk of coronary artery embolizations, one of the causes of MINOCA.40 There was also a sex‐specific association, with women having 4.8 times higher odds of having MINOCA than MI‐CAD in the younger age group (18–55 years). Earlier menarche was more common in MI‐CAD patients, who were also more likely to be obese. High body mass index among adolescents has been linked with early menarche and, later in life, with obesity, insulin resistance, metabolic syndrome, and dyslipidemia, possibly explaining this predisposition.41, 42 Other sex‐specific factors such as rates of polycystic ovarian syndrome or pregnancy‐related complications did not differ by type of AMI, but were almost 2 to 3 times higher than in the general population.43, 44 Endothelial dysfunction and procoagulant states have been implicated in increasing the long‐term risk of AMI in women with pregnancy‐related complications and warrant further investigation.45, 46, 47
We noted that the course of MINOCA patients was not benign. Similar proportions of patients with MINOCA and MI‐CAD presented in cardiac arrest, had reduced ejection fraction, or presented with heart failure. They also had similar lengths of hospital stay, possibly as a result of further testing of MINOCA patients to enhance management. Prior studies have shown lower mortality with MINOCA (3.2–4.5%) than with MI‐CAD.3, 6, 7, 9 We observed lower mortality in VIRGO patients.5, 48, 49, 50 This could be attributed to the young age of the cohort or the survivor bias for enrollment into VIRGO. Importantly, the 12‐month mortality for MINOCA was still 2 times higher than the 0.5% annual mortality rate observed for a 47‐year‐old woman in the United States.51 In addition, we noted that short‐ and long‐term functional outcomes of MINOCA and MI‐CAD patients were similar.52 Women with MINOCA showed a trend for worse functional outcomes than men.
Finally, we observed heterogeneity in MINOCA management, exposing a gap that needs attention.36 We noted variation in the revascularization strategy in SCAD patients regardless of the degree of stenosis and little use of proven therapies such calcium channel blockers in patients with angiographically demonstrable vasospasm.53 Recent data also suggest beneficial roles for statins and angiotensin‐converting enzyme inhibitors or angiotensin receptor II blockers in improving mortality and rates of recurrent myocardial infarction in patients with MINOCA, but they were underutilized in our study.49 The benefit of proven MI‐CAD treatments such as antiplatelet agents or reperfusion may not always apply to MINOCA patients, such as those with coronary artery embolization. Consequently, a standard protocol as used for MI‐CAD treatment may not apply uniformly to all MINOCA patients. These variations underscore the need for prospective pathophysiology‐driven studies that test primary and secondary treatments specific to MINOCA subtype.10, 54, 55, 56, 57
Our results should be interpreted in light of some limitations. First, the angiogram data were recorded by the clinician performing the angiogram and not by a centralized laboratory. Not all sites measured fractional flow reserve, which helps establish the clinical significance of 50% to 75% of lesions; therefore, MINOCA may be underestimated in our patients. However, the decision for treatment at each site was based on interpretation by the cardiologist and thus reflects real‐world clinical practice. Second, this study was voluntary as opposed to a registry, and thus not all patients with AMI were included at each site; consequently, there could be survivor bias in the VIRGO study. We also had low numbers of men with MINOCA in VIRGO, limiting sex‐specific comparisons for patients with MINOCA and nonplaque mechanisms. However, to our knowledge, this study remains the largest with prospectively collected data on young patients with AMI. Finally, VIRGO was originally designed to describe young patients with coronary causes of ischemia and, therefore, systematically excluded myocarditis and takotsubo based on the clinical impression of the treating provider. Correspondingly, we restricted the MINOCA patients in our report to describe coronary causes of ischemia only, consistent with a recent description of MINOCA.12 In addition, the determination of ischemia in the enrolled VIRGO population was based on close clinical scrutiny. This method, while rigorous and reflective of real‐world practice, still carried some limitations because not all patients had magnetic resonance imaging. It is possible that some patients with myocarditis were included in our cohort. We also recognize that the definition of coronary MINOCA is evolving, particularly concerning takotsubo, which some believe is ischemic but that contemporary belief labels as a “catecholamine cardiomyopathy,” as opposed to coronary ischemia. In VIRGO, only a few patients underwent formal provocative testing for coronary artery vasospasm or embolization, limiting our ability to fully characterize disease mechanisms in most patients. This might be due to the common belief that provocative testing in patients with AMI could be dangerous, and our data suggest that routine use of provocative testing in AMI patients is not yet standard of care in the United States. We recognize that the evidence in this space is evolving, and this practice might change, especially with new data from Europe suggesting both the safety and utility of provocative testing in AMI patients58; as such, the relative proportion of these various phenotypes should be interpreted with caution.
Conclusion
Presentation with MINOCA in this sample of young adults with AMI was more common in women, in younger patients, and in nonwhite adults; such patients were more likely to present non‐STEMI and to have fewer traditional cardiac risk factors than patients with MI‐CAD. Patients with MINOCA had similar outcomes, including mortality and psychosocial and functional status, to MI‐CAD patients. The clinical profile and management of MINOCA patients varied by sex and phenotype. Further work is needed to better characterize these patients based on the underlying mechanism.
Sources of Funding
VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients) was supported by grant R01 HL081153 from the National Heart, Lung, and Blood Institute. The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the article.
Disclosures
Dr Safdar is funded by a grant from Yale Center for Clinical Investigation. Dr Spatz is funded by a grant from the Women's Health Research at Yale. Dr Krumholz is a recipient of research agreements from Medtronic and from Johnson & Johnson (Janssen), through Yale, to develop methods of clinical trial data sharing; is the recipient of a grant from Medtronic and the Food and Drug Administration, through Yale, to develop methods for postmarket surveillance of medical devices; works under contract with the Centers for Medicare and Medicaid Services to develop and maintain performance measures that are publicly reported; chairs a cardiac scientific advisory board for UnitedHealth; is a participant/participant representative of the IBM Watson Health Life Sciences Board; is a member of the advisory board for Element Science and the physician advisory board for Aetna; and is the founder of Hugo, a personal health information platform. The remaining authors have no disclosures to report.
Supporting information
Table S1. Sex‐Specific Comparison of Sociodemographic and Clinical Profile of Patients With Myocardial Infarction With Nonobstructive Coronary Arteries
Table S2. Sequential Linear Regression Results for the Relationship Between Type of Myocardial Infarction (Myocardial Infarction With Nonobstructive Coronary Arteries or Myocardial Infarction Due to Coronary Artery Disease) and Quality of Life at 12 Months
Table S3. Risk Factor Profile and Clinical Characteristics in Patients With Myocardial Infarction With Nonobstructive Coronary Arteries and (A) History of Heart Failure and (B) Low Ejection Fraction (<40%)
Table S4. Clinical Characteristics and Outcomes in Patients With Myocardial Infarction Due to Coronary Artery Disease Versus Indeterminate Cause (Class V)
Figure S1. Flow of patients through the VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients) study.
(J Am Heart Assoc. 2018;7:e009174 DOI: 10.1161/JAHA.118.009174.)
These results were presented at the European Society of Cardiology‐Acute Cardiovascular Care Association (ESC‐ACCA) Conference, March 3 to 5, 2018, in Milan, Italy.
References
- 1. Beltrame JF. Assessing patients with myocardial infarction and nonobstructed coronary arteries (MINOCA). J Intern Med. 2013;273:182–185. [DOI] [PubMed] [Google Scholar]
- 2. DeWood MA, Spores J, Notske R, Mouser LT, Burroughs R, Golden MS, Lang HT. Prevalence of total coronary occlusion during the early hours of transmural myocardial infarction. N Engl J Med. 1980;303:897–902. [DOI] [PubMed] [Google Scholar]
- 3. Patel MR, Chen AY, Peterson ED, Newby LK, Pollack CV Jr, Brindis RG, Gibson CM, Kleiman NS, Saucedo JF, Bhatt DL, Gibler WB, Ohman EM, Harrington RA, Roe MT. Prevalence, predictors, and outcomes of patients with non–ST‐segment elevation myocardial infarction and insignificant coronary artery disease: results from the Can Rapid risk stratification of Unstable angina patients Suppress ADverse outcomes with Early implementation of the ACC/AHA Guidelines (CRUSADE) initiative. Am Heart J. 2006;152:641–647. [DOI] [PubMed] [Google Scholar]
- 4. Dokainish H, Pillai M, Murphy SA, DiBattiste PM, Schweiger MJ, Lotfi A, Morrow DA, Cannon CP, Braunwald E, Lakkis N; TACTICS‐TIMI‐18 Investigators . Prognostic implications of elevated troponin in patients with suspected acute coronary syndrome but no critical epicardial coronary disease: a TACTICS‐TIMI‐18 substudy. J Am Coll Cardiol. 2005;45:19–24. [DOI] [PubMed] [Google Scholar]
- 5. Pasupathy S, Air T, Dreyer RP, Tavella R, Beltrame JF. Systematic review of patients presenting with suspected myocardial infarction and nonobstructive coronary arteries. Circulation. 2015;131:861–870. [DOI] [PubMed] [Google Scholar]
- 6. Gehrie ER, Reynolds HR, Chen AY, Neelon BH, Roe MT, Gibler WB, Ohman EM, Newby LK, Peterson ED, Hochman JS. Characterization and outcomes of women and men with non‐ST‐segment elevation myocardial infarction and nonobstructive coronary artery disease: results from the Can Rapid Risk Stratification of Unstable Angina Patients Suppress Adverse Outcomes with Early Implementation of the ACC/AHA Guidelines (CRUSADE) quality improvement initiative. Am Heart J. 2009;158:688–694. [DOI] [PubMed] [Google Scholar]
- 7. Planer D, Mehran R, Ohman EM, White HD, Newman JD, Xu K, Stone GW. Prognosis of patients with non‐ST‐segment‐elevation myocardial infarction and nonobstructive coronary artery disease: propensity‐matched analysis from the Acute Catheterization and Urgent Intervention Triage Strategy trial. Circ Cardiovasc Interv. 2014;7:285–293. [DOI] [PubMed] [Google Scholar]
- 8. Diver DJ, Bier JD, Ferreira PE, Sharaf BL, McCabe C, Thompson B, Chaitman B, Williams DO, Braunwald E. Clinical and arteriographic characterization of patients with unstable angina without critical coronary arterial narrowing (from the TIMI‐IIIA Trial). Am J Cardiol. 1994;74:531–537. [DOI] [PubMed] [Google Scholar]
- 9. Roe MT, Harrington RA, Prosper DM, Pieper KS, Bhatt DL, Lincoff AM, Simoons ML, Akkerhuis M, Ohman EM, Kitt MM, Vahanian A, Ruzyllo W, Karsch K, Califf RM, Topol EJ. Clinical and therapeutic profile of patients presenting with acute coronary syndromes who do not have significant coronary artery disease. The platelet glycoprotein IIb/IIIa in unstable angina: receptor suppression using integrilin therapy (PURSUIT) trial investigators. Circulation. 2000;102:1101–1106. [DOI] [PubMed] [Google Scholar]
- 10. Maddox TM, Stanislawski MA, Grunwald GK, Bradley SM, Ho PM, Tsai TT, Patel MR, Sandhu A, Valle J, Magid DJ, Leon B, Bhatt DL, Fihn SD, Rumsfeld JS. Nonobstructive coronary artery disease and risk of myocardial infarction. JAMA. 2014;312:1754–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patel MR, Peterson ED, Dai D, Brennan JM, Redberg RF, Anderson HV, Brindis RG, Douglas PS. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010;362:886–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pasupathy S, Tavella R, Beltrame JF. Myocardial infarction with nonobstructive coronary arteries (MINOCA): the past, present, and future management. Circulation. 2017;135:1490–1493. [DOI] [PubMed] [Google Scholar]
- 13. Agewall S, Beltrame JF, Reynolds HR, Niessner A, Rosano G, Caforio AL, De Caterina R, Zimarino M, Roffi M, Kjeldsen K, Atar D, Kaski JC, Sechtem U, Tornvall P; Pharmacotherapy WGoC . ESC working group position paper on myocardial infarction with non‐obstructive coronary arteries. Eur Heart J. 2017;38:143–153. [DOI] [PubMed] [Google Scholar]
- 14. Lanza GA, Crea F. Acute coronary syndromes without obstructive coronary atherosclerosis: the tiles of a complex puzzle. Circ Cardiovasc Interv. 2014;7:278–281. [DOI] [PubMed] [Google Scholar]
- 15. Bugiardini R, Manfrini O, De Ferrari GM. Unanswered questions for management of acute coronary syndrome: risk stratification of patients with minimal disease or normal findings on coronary angiography. Arch Intern Med. 2006;166:1391–1395. [DOI] [PubMed] [Google Scholar]
- 16. Vaccarino V, Parsons L, Every NR, Barron HV, Krumholz HM. Sex‐based differences in early mortality after myocardial infarction. National Registry of Myocardial Infarction 2 participants. N Engl J Med. 1999;341:217–225. [DOI] [PubMed] [Google Scholar]
- 17. Lichtman JH, Lorenze NP, D'Onofrio G, Spertus JA, Lindau ST, Morgan TM, Herrin J, Bueno H, Mattera JA, Ridker PM, Krumholz HM. Variation in recovery: role of gender on outcomes of young AMI patients (VIRGO) study design. Circ Cardiovasc Qual Outcomes. 2010;3:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Spatz ES, Curry LA, Masoudi FA, Zhou S, Strait KM, Gross CP, Curtis JP, Lansky AJ, Barreto‐Filho JA, Lampropulos JF, Bueno H, Chaudhry SI, D'Onofrio G, Safdar B, Dreyer RP, Murugiah K, Spertus JA, Krumholz HM. The VIRGO classification system: a taxonomy for young women with acute myocardial infarction. Circulation. 2015;132:1710–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mallik S, Spertus JA, Reid KJ, Krumholz HM, Rumsfeld JS, Weintraub WS, Agarwal P, Santra M, Bidyasar S, Lichtman JH, Wenger NK, Vaccarino V. Depressive symptoms after acute myocardial infarction: evidence for highest rates in younger women. Arch Intern Med. 2006;166:876–883. [DOI] [PubMed] [Google Scholar]
- 20. Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health‐related quality of life: the Heart and Soul Study. JAMA. 2003;290:215–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Whooley MA, de Jonge P, Vittinghoff E, Otte C, Moos R, Carney RM, Ali S, Dowray S, Na B, Feldman MD, Schiller NB, Browner WS. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300:2379–2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kroenke K, Spitzer RL. The PHQ‐9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509–521. [Google Scholar]
- 23. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24:385–396. [PubMed] [Google Scholar]
- 24. Arnold SV, Smolderen KG, Buchanan DM, Li Y, Spertus JA. Perceived stress in myocardial infarction: long‐term mortality and health status outcomes. J Am Coll Cardiol. 2012;60:1756–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mozaffarian D, Bryson CL, Spertus JA, McDonell MB, Fihn SD. Anginal symptoms consistently predict total mortality among outpatients with coronary artery disease. Am Heart J. 2003;146:1015–1022. [DOI] [PubMed] [Google Scholar]
- 26. Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25:333–341. [DOI] [PubMed] [Google Scholar]
- 27. Spertus JA, Jones P, McDonell M, Fan V, Fihn SD. Health status predicts long‐term outcome in outpatients with coronary disease. Circulation. 2002;106:43–49. [DOI] [PubMed] [Google Scholar]
- 28. Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Fihn SD. Monitoring the quality of life in patients with coronary artery disease. Am J Cardiol. 1994;74:1240–1244. [DOI] [PubMed] [Google Scholar]
- 29. Arnold SV, Morrow DA, Lei Y, Cohen DJ, Mahoney EM, Braunwald E, Chan PS. Economic impact of angina after an acute coronary syndrome: insights from the MERLIN‐TIMI36 trial. Circ Cardiovasc Qual Outcomes. 2009;2:344–353. [DOI] [PubMed] [Google Scholar]
- 30. Brown H, Prescott R. Applied Mixed Models in Medicine. Chichester, England: John Wiley & Sons, LTD.; 1999. [Google Scholar]
- 31. Niccoli G, Scalone G, Crea F. Acute myocardial infarction with no obstructive coronary atherosclerosis: mechanisms and management. Eur Heart J. 2015;36:475–481. [DOI] [PubMed] [Google Scholar]
- 32. Lerman A, Holmes DR, Herrmann J, Gersh BJ. Microcirculatory dysfunction in ST‐elevation myocardial infarction: cause, consequence, or both? Eur Heart J. 2007;28:788–797. [DOI] [PubMed] [Google Scholar]
- 33. Hermens JA, van Es J, von Birgelen C, Op den Akker JW, Wagenaar LJ. Evidence of myocardial scarring and microvascular obstruction on cardiac magnetic resonance imaging in a series of patients presenting with myocardial infarction without obstructed coronary arteries. Int J Cardiovasc Imaging. 2014;30:1097–1103. [DOI] [PubMed] [Google Scholar]
- 34. Falk E. Unstable angina with fatal outcome: dynamic coronary thrombosis leading to infarction and/or sudden death. Autopsy evidence of recurrent mural thrombosis with peripheral embolization culminating in total vascular occlusion. Circulation. 1985;71:699–708. [DOI] [PubMed] [Google Scholar]
- 35. Niccoli G, Scalone G, Lerman A, Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J. 2016;37:1024–1033. [DOI] [PubMed] [Google Scholar]
- 36. Sheikh AR, Sidharta S, Worthley MI, Yeend R, Di Fiore DP, Beltrame JF. The importance of evaluating patients with MINOCA (myocardial infarction with non‐obstructive coronary arteries). Int J Cardiol. 2015;199:386–388. [DOI] [PubMed] [Google Scholar]
- 37. Yoon SS, Carroll MD, Fryar CD. Hypertension prevalence and control among adults: United States, 2011–2014. NCHS Data Brief. 2015;220:1–8. [PubMed] [Google Scholar]
- 38. CDC . National Diabetes Statistics Report: estimates of diabetes and its burden in the United States. 2017. Available at: https://www.cdc.gov/diabetes/pdfs/data/statistics/national-diabetes-statistics-report.pdf. Accessed January 1, 2018.
- 39. Jamal A, Phillips E, Gentzke AS, Homa DM, Babb SD, King BA, Neff LJ. Current cigarette smoking among adults—United States 2016. MMWR Morb Mortal Wkly Rep. 2018;67:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kardasz I, De Caterina R. Myocardial infarction with normal coronary arteries: a conundrum with multiple aetiologies and variable prognosis: an update. J Intern Med. 2007;261:330–348. [DOI] [PubMed] [Google Scholar]
- 41. Stockl D, Doring A, Peters A, Thorand B, Heier M, Huth C, Stockl H, Rathmann W, Kowall B, Meisinger C. Age at menarche is associated with prediabetes and diabetes in women (aged 32–81 years) from the general population: the KORA F4 study. Diabetologia. 2012;55:681–688. [DOI] [PubMed] [Google Scholar]
- 42. Stockl D, Meisinger C, Peters A, Thorand B, Huth C, Heier M, Rathmann W, Kowall B, Stockl H, Doring A. Age at menarche and its association with the metabolic syndrome and its components: results from the KORA F4 study. PLoS One. 2011;6:e26076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sirmans SM, Parish RC, Blake S, Wang X. Epidemiology and comorbidities of polycystic ovary syndrome in an indigent population. J Investig Med. 2014;62:868–874. [DOI] [PubMed] [Google Scholar]
- 44. Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990–2008. Natl Vital Stat Rep. 2012;60:1–21. [PubMed] [Google Scholar]
- 45. Germain AM, Romanik MC, Guerra I, Solari S, Reyes MS, Johnson RJ, Price K, Karumanchi SA, Valdes G. Endothelial dysfunction: a link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension. 2007;49:90–95. [DOI] [PubMed] [Google Scholar]
- 46. Kharazmi E, Dossus L, Rohrmann S, Kaaks R. Pregnancy loss and risk of cardiovascular disease: a prospective population‐based cohort study (EPIC‐Heidelberg). Heart. 2011;97:49–54. [DOI] [PubMed] [Google Scholar]
- 47. Ananth CV, Keyes KM, Wapner RJ. Pre‐eclampsia rates in the United States, 1980–2010: age‐period‐cohort analysis. BMJ. 2013;347:f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bugiardini R, Bairey Merz CN. Angina with “normal” coronary arteries: a changing philosophy. JAMA. 2005;293:477–484. [DOI] [PubMed] [Google Scholar]
- 49. Lindahl B, Baron T, Erlinge D, Hadziosmanovic N, Nordenskjold A, Gard A, Jernberg T. Medical therapy for secondary prevention and long‐term outcome in patients with myocardial infarction with nonobstructive coronary artery disease. Circulation. 2017;135:1481–1489. [DOI] [PubMed] [Google Scholar]
- 50. Feldman L, Steg PG, Amsallem M, Puymirat E, Sorbets E, Elbaz M, Ritz B, Hueber A, Cattan S, Piot C, Ferrieres J, Simon T, Danchin N; FAST‐MI investigators . Editor's Choice‐Medically managed patients with non‐ST‐elevation acute myocardial infarction have heterogeneous outcomes, based on performance of angiography and extent of coronary artery disease. Eur Heart J Acute Cardiovasc Care. 2017;6:262–271. [DOI] [PubMed] [Google Scholar]
- 51. Kochanek KD, Murphy SL, Xu J, Tejada‐Vera B. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65:1–122. [PubMed] [Google Scholar]
- 52. Mommersteeg PM, Arts L, Zijlstra W, Widdershoven JW, Aarnoudse W, Denollet J. Impaired health status, psychological distress, and personality in women and men with nonobstructive coronary artery disease: sex and gender differences: the TWIST (Tweesteden Mild Stenosis) Study. Circ Cardiovasc Qual Outcomes. 2017;10:e003387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mitchell LB. Use of the implantable cardioverter‐defibrillator in patients with coronary artery spasm as the apparent cause of spontaneous life‐threatening ventricular tachycardia or ventricular fibrillation: crossing the spasm sudden death chasm. J Am Coll Cardiol. 2012;60:914–916. [DOI] [PubMed] [Google Scholar]
- 54. Lindahl B, Baron T, Erlinge D, Hadziosmanovic N, Nordenskjold AM, Gard A, Jernberg T. Medical therapy for secondary prevention and long‐term outcome in patients with myocardial infarction with non‐obstructive coronary artery (MINOCA) disease. Circulation. 2017;135:1481–1489. [DOI] [PubMed] [Google Scholar]
- 55. Puymirat E, Riant E, Aissoui N, Soria A, Ducrocq G, Coste P, Cottin Y, Aupetit JF, Bonnefoy E, Blanchard D, Cattan S, Steg G, Schiele F, Ferrieres J, Juilliere Y, Simon T, Danchin N. beta blockers and mortality after myocardial infarction in patients without heart failure: multicentre prospective cohort study. BMJ. 2016;354:i4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Maddox TM, Ho PM, Roe M, Dai D, Tsai TT, Rumsfeld JS. Utilization of secondary prevention therapies in patients with nonobstructive coronary artery disease identified during cardiac catheterization: insights from the National Cardiovascular Data Registry Cath‐PCI Registry. Circ Cardiovasc Qual Outcomes. 2010;3:632–641. [DOI] [PubMed] [Google Scholar]
- 57. Dwyer JP, Redfern J, Freedman SB. Low utilisation of cardiovascular risk reducing therapy in patients with acute coronary syndromes and non‐obstructive coronary artery disease. Int J Cardiol. 2008;129:394–398. [DOI] [PubMed] [Google Scholar]
- 58. Montone RA, Niccoli G, Fracassi F, Russo M, Gurgoglione F, Camma G, Lanza GA, Crea F. Patients with acute myocardial infarction and non‐obstructive coronary arteries: safety and prognostic relevance of invasive coronary provocative tests. Eur Heart J. 2018;39:91–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sex‐Specific Comparison of Sociodemographic and Clinical Profile of Patients With Myocardial Infarction With Nonobstructive Coronary Arteries
Table S2. Sequential Linear Regression Results for the Relationship Between Type of Myocardial Infarction (Myocardial Infarction With Nonobstructive Coronary Arteries or Myocardial Infarction Due to Coronary Artery Disease) and Quality of Life at 12 Months
Table S3. Risk Factor Profile and Clinical Characteristics in Patients With Myocardial Infarction With Nonobstructive Coronary Arteries and (A) History of Heart Failure and (B) Low Ejection Fraction (<40%)
Table S4. Clinical Characteristics and Outcomes in Patients With Myocardial Infarction Due to Coronary Artery Disease Versus Indeterminate Cause (Class V)
Figure S1. Flow of patients through the VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients) study.


