Abstract
Background
Heart failure constitutes a high burden on patients and society, but although lifetime risk is high, it is difficult to predict without costly or invasive testing. We aimed to establish new risk factors of heart failure, which potentially could enable early diagnosis and preemptive treatment.
Methods and Results
We applied machine learning in the UK Biobank in an agnostic search of risk factors for heart failure in 500 451 individuals, excluding individuals with prior heart failure. Novel factors were then subjected to several in‐depth analyses, including multivariable Cox models of incident heart failure, and assessment of discrimination and calibration. Machine learning confirmed many known and putative risk factors for heart failure and identified several novel candidates. Mean reticulocyte volume appeared as one novel factor and leg bioimpedance another, the latter appearing as the most important new marker. Leg bioimpedance was lower in those who developed heart failure during an up to 9.8‐year follow‐up. When adjusting for known heart failure risk factors, leg bioimpedance was inversely related to heart failure (hazard ratio [95% confidence interval], 0.60 [0.48–0.73] and 0.75 [0.59–0.94], in age‐ and sex‐adjusted and fully adjusted models, respectively, comparing the upper versus lower quartile). A model including leg bioimpedance, age, sex, and self‐reported history of myocardial infarction showed good discrimination for future heart failure hospitalization (Concordance index [C‐index]=0.82) and good calibration.
Conclusions
Leg bioimpedance is inversely associated with heart failure incidence in the general population. A simple model of exclusively noninvasive measures, combining leg bioimpedance with history of myocardial infarction, age, and sex provides accurate predictive capacity.
Keywords: heart failure, machine learning, risk factors
Subject Categories: Risk Factors
Clinical Perspective
What Is New?
By leveraging the power of machine learning, we identified leg bioimpedance as a novel risk factor for incident heart failure in the general population.
A simple model combining leg bioimpedance, history of myocardial infarction, age, and sex provides accurate prediction of new‐onset heart failure.
What Are the Clinical Implications?
Given the simplicity of measurement, leg bioimpedance could potentially be used as a tool for screening in primary care facilities, providing independent validation of our findings.
Introduction
Heart failure is a common cause of mortality and is associated with substantial morbidity that greatly impacts quality of life of the affected individuals and their families. The lifetime risk of developing heart failure is high, and it is among the most common discharge diagnoses in the United States, with frequent costly readmissions for decompensation once disease has been established.1 While invasively measured biomarkers have improved prognostication, prediction of new‐onset heart failure remains challenging.2 Increased knowledge of risk factors for incident heart failure in the general population could enable earlier diagnosis, preemptive treatment, and risk factor control, to possibly mitigate disease development and improve outcomes. This is in line with recent heart failure guidelines, which outline several evidence‐based treatments that may delay or prevent the development of overt heart failure.3
We hypothesized that novel risk factors for incident heart failure in the UK Biobank, a population‐based longitudinal cohort study of >500 000 individuals, could be identified in an agnostic search by machine learning techniques, combined with confirmatory in‐depth analyses.
Methods
Study Design and Participants
The full UK Biobank data set is available to approved researchers.4, 5 Between 2006 and 2010, 502 639 individuals aged 49 to 69 years were enrolled in the UK Biobank, a longitudinal, population‐based cohort study that recruited volunteers across 21 centers in England, Wales, and Scotland. The study has been described in detail previously6 and online (http://www.ukbiobank.ac.uk). Briefly, participants were carefully investigated, using a series of questionnaires, physiological measures, imaging, blood and urine biomarkers, and genotype data. Importantly, the UK Biobank was merged with the national in‐hospital diagnosis and procedure registries, providing information about diagnoses before and after baseline of the study.6
For the main analyses, we excluded individuals with prevalent heart failure (either primary or secondary in‐hospital diagnosis of heart failure, International Classification of Diseases, Tenth Edition [ICD‐10], I50; or self‐reported history of heart failure, n=2188), leaving 500 451 individuals eligible. In secondary analyses of leg bioimpedance, we instead studied individuals with prevalent heart failure (n=2188). The UK Biobank study was approved by the North West Multi‐Centre Research Ethics Committee, and all participants provided written informed consent to participate in the study.
Leg Bioimpedance and Other Risk Factors
In our phenome‐wide scan of risk factors for incident heart failure, we considered all 3646 variables reflecting different aspects of lifestyle, health, and disease‐related factors in the UK Biobank (except a few administrative variables such as meta‐data regarding the genomics part of the UK Biobank, as well as genomics and raw imaging data), and all ICD‐10 diagnoses (excluding administrative, temporary, and unspecific codes [Chapters U, V, W, X, Y, and Z]) (Table S1).
Leg bioimpedance was measured using the Tanita BC418MA body composition analyzer. This device is reminiscent of a standard scale but also has handlebars; ie, there are 4 electrodes (1 for each foot, and 1 for each hand) between which small electric signals are passed. The resistance, ie, the impedance, for different parts of the body is calculated. A detailed description of how these measurements were made (including a photo of the device used) is available in the UK Biobank data showcase (http://biobank.ctsu.ox.ac.uk/crystal/). In our confirmatory analyses, we defined leg bioimpedance as the mean of left and right leg bioimpedance.
Blood pressure and body mass index were measured in a standardized fashion; as were medical history, smoking status, and alcohol consumption, through standardized questionnaires (full details available in the UK Biobank data showcase: http://biobank.ctsu.ox.ac.uk/crystal/). High alcohol intake was defined as “daily or almost daily” intake of alcohol. Chronic kidney failure (N18) and coronary heart disease (I20–I25), were defined as presence of these (primary or secondary) ICD‐10 diagnoses in hospitalizations before inclusion in the UK Biobank.
Outcome and Follow‐Up
The primary outcome was hospitalization for heart failure (ICD‐10: I50) as the primary cause during follow‐up, and for the Cox models, time to hospitalization for heart failure. This was based on the Hospital Episode Statistics register, that includes all hospitalizations in the United Kingdom, by linkage to the UK Biobank data set. Vital status is observed through the mandatory Death register. Thereby, loss‐of‐follow‐up is negligible. There were 1054 incident events of heart failure during follow‐up of up to 9.8 years, with a median follow‐up of 6.2 years (lower and upper quartile, 5.5 and 6.9).
Statistical Analysis
For discovery of new potential risk factors for heart failure, we used a gradient boosting machine model. This machine learning method allows the computer to learn how to classify individuals as having heart failure or not in an iterative fashion, considering all of the 3646 available variables, improving prediction by each iteration. In addition to providing a model for classification, it provides a measure of how important each variable is in this classification. We used hospitalization for heart failure (ie, our primary outcome) as the response variable. The data set was divided into training (60%), validation (20%), and test (20%) sets. The maximum tree depth was set to 3, the maximum number of trees to 50, and the learning rate to 0.01. Performance was assessed by the receiver operating characteristic area under the curve, and classification error was assessed by logloss. Results are presented as variable importance for the classification. Variable importance can be considered a measure of how important each feature is in improving the classification. An extended description of the gradient boosting machine method is found in Data S1.
Next, we assessed the relationships between the top 15 features associated with heart failure from the gradient boosting machine model and incident heart failure by using Cox proportional hazards regression in 2 sets of models: (1) adjusting for age and sex; and (2) additionally adjusting for body mass index, systolic and diastolic blood pressure, antihypertensive treatment, alcohol consumption, diabetes mellitus, smoking status, prevalent chronic renal failure, and prevalent coronary heart disease, all of which are established risk factors for heart failure.7, 8, 9, 10, 11 Continuous variables were entered as restricted cubic splines to account for any nonlinear associations. The association between features and heart failure are reported as hazard ratios and 95% confidence intervals for the upper versus the lower quartile for continuous variables, or for presence or absence for binary variables. The proportional hazards assumption was assessed by visual inspection of the Schoenfeld residuals, without any signs of violation of this assumption.
As leg bioimpedance was deemed of particular importance after our initial analyses, we proceeded with in‐depth analyses of this variable. First, we performed a sensitivity analysis, where all individuals with any primary or secondary inpatient diagnosis in Chapter 9 of ICD‐10 (“Diseases of the Circulatory System”) were excluded, as well as anyone with any of the following self‐reported conditions: hypertension, prior stroke or transient ischemic attack, high cholesterol, diabetes mellitus, angina pectoris, or myocardial infarction (as well as self‐reported heart failure) rendering a subsample of 309 079 individuals. This was to exclude any other prevalent cardiometabolic disorders that could influence bioimpedance. Further, we assessed leg bioimpedance in the full population in relation to incident or prevalent heart failure, and to any incident or prevalent cardiovascular disease, to further characterize the associations between leg bioimpedance and cardiovascular disease. Distributions are presented as density plots. The predicted rate of heart failure hospitalization up to 8 years after inclusion in the study was plotted in relation to leg bioimpedance in a spline plot. The x axis was truncated at the 0.1th percentile at the lower end, and the 99.9th percentile in the higher end. Normality was assessed with visual inspection of the distributions, and levels of leg bioimpedance were compared with t tests. Finally, we assessed the addition of leg bioimpedance to age and sex, and in a final model adding leg bioimpedance to age, sex, and self‐reported myocardial infarction. There were few missing observations of leg impedance (<2%), so we performed complete case analyses without any attempts of imputation.
The discriminatory ability of the models is presented using c‐indices, and model fit was estimated by Akaike information criterion. We assessed calibration by visual inspection of calibration plots, and performed bootstrap validation using 100 bootstrap samples. All analyses were conducted using R versions 3.3.0 and 3.3.3, and the machine learning platform H2O version 3.10.4.1.
Results
Baseline characteristics of the study population are presented in Table 1. The 15 variables with the highest importance in the gradient boosting machine model are shown in Figure 1. Among these are some of the most well‐established risk factors for heart failure, such as manifestations of coronary artery disease and type 2 diabetes mellitus, as well as known correlates of heart failure, such as obesity, microalbuminuria, and anemia. There were also several novel variables associated with incident heart failure. Among the most important were left and right leg bioimpedance and mean reticulocyte volume. The receiver operating characteristic areas under the curve were similar in the training, validation, and test sets: 0.79, 0.78, and 0.81 (receiver operating characteristic curves shown in Figure S1); as were the logloss values: 0.0127, 0.0149, 0.0143, ie, acceptable discrimination and low error, which were very similar in the 3 sets, suggesting that the model was not overfitted.
Table 1.
Baseline Characteristics
| Characteristic | N | Without Incident Heart Failure (n=499 394) | With Incident Heart Failure (n=1054) |
|---|---|---|---|
| Leg bioimpedance, Ohms | 490 660 | 246.5 (223.5–270.5) | 221.5 (194.5–247.2) |
| Age, y | 500 488 | 58 (50–63) | 64 (60–67) |
| Male sex | 500 488 | 45% (226 815) | 68% (718) |
| Body mass index, kg/m2 | 497 382 | 26.7 (24.1–29.9) | 29.7 (26.2–34.6) |
| Systolic blood pressure, mm Hg | 499 157 | 136.5 (124.5–149.5) | 143.2 (130.5–157.0) |
| Diastolic blood pressure, mm Hg | 499 159 | 82.0 (75.3–89.0) | 82.0 (74.0–89.0) |
| Antihypertensive medication | 492 594 | 21% (104 123) | 59% (603) |
| Alcohol intake daily or almost daily | 498 969 | 21% (102 321) | 20% (205) |
| Diabetes mellitus | 497 858 | 5% (25 557) | 29% (305) |
| Current smoker | 497 576 | 11% (52 741) | 16% (163) |
| Prevalent chronic renal failure | 500 448 | 0.1% (619) | 2% (16) |
| Prevalent coronary heart disease | 500 448 | 4% (17 364) | 30% (314) |
For continuous variables, medians and interquartile ranges are reported; for categorical variables, percentages and frequencies. N is the number of nonmissing values.
Figure 1.
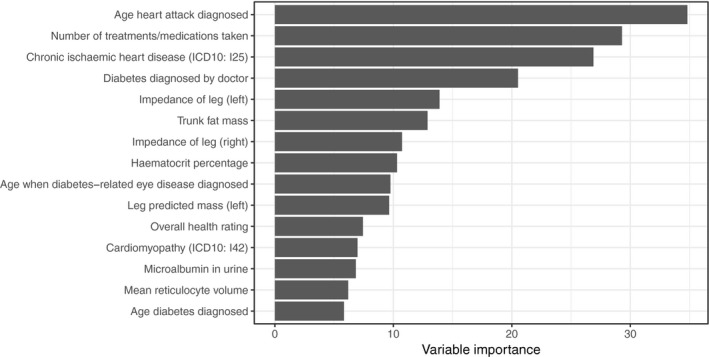
Variable importance for the top 15 variables in the gradient boosting machine model for incident hospitalization for heart failure.
For in‐depth analyses of the most important variables, we then performed 2 sets of Cox regression models (age‐ and sex‐adjusted; and adjusting for established heart failure risk factors) relating the top 15 features to incident heart failure during up to 9.8 years of follow‐up (Table 2). Among the independently associated variables were well‐established risk factors, such as ischemic heart disease and diabetes mellitus, but also other, novel markers, such as mean reticulocyte volume and leg bioimpedance.
Table 2.
Cox Models of the Top 15 Associated Variables From the Machine Learning Approach
| Variable | Age‐ and Sex‐Adjusted HR (95% CI) | Fully Adjusteda HR (95% CI) |
|---|---|---|
| Prior myocardial infarctionb | 7.06 (6.06–8.23) | 4.29 (3.62–5.07) |
| Number of treatments/medications taken | 3.05 (2.44–3.82) | 1.96 (1.52–2.53) |
| Chronic ischemic heart disease (ICD‐10: I25) | 6.88 (5.95–7.95) | 4.04 (3.44–4.75) |
| History of diabetes mellitusb | 5.27 (4.60–6.03) | 2.45 (2.10–2.87) |
| Leg bioimpedance (left) (Ohms) | 0.59 (0.48–0.72) | 0.75 (0.60–0.94) |
| Trunk fat mass, kg | 1.73 (1.43–2.09) | 0.86 (0.64–1.17) |
| Leg bioimpedance (right) (Ohms) | 0.60 (0.49–0.73) | 0.78 (0.62–0.97) |
| Hematocrit, % | 0.56 (0.47–0.67) | 0.73 (0.61–0.87) |
| Diabetes mellitus–related eye diseaseb | 8.57 (6.01–12.21) | 2.01 (1.37–2.94) |
| Leg predicted mass (left) (kg) | 3.73 (2.74–5.07) | 1.45 (0.99–2.12) |
| Overall health rating | 3.14 (2.91–3.38) | 2.04 (1.86–2.22) |
| Cardiomyopathy (ICD‐10: I42) | 18.73 (11.59–30.27) | 9.61 (5.70–16.18) |
| Microalbumin in urine (log) (mg/L) | 2.12 (1.62–2.77) | 1.83 (1.38–2.42) |
| Mean reticulocyte volume (fL) | 1.28 (1.10–1.50) | 1.19 (1.01–1.39) |
CI indicates confidence interval; HR, hazard ratio (for continuous variables, HR represents upper vs lower quartile); ICD‐10, International Classification of Diseases, Tenth Edition.“Diabetes diagnosed by doctor” and “Age diabetes diagnosed,” was instead analyzed as history of diabetes mellitus.
Fully adjusted model includes age, sex, body mass index, systolic blood pressure, diastolic blood pressure, antihypertensive treatment, alcohol consumption, diabetes mellitus, smoking status, prevalent chronic renal failure, prevalent coronary heart disease.
Age when occurrence of myocardial infarction, diabetes mellitus, and diabetes mellitus–related eye disease were diagnosed were dichotomized to reflect the presence of these conditions or not.
As left and right leg bioimpedance emerged as novel, independent risk factors (Figure 1 and Table 2) and strong indicators of incident heart failure that can be assessed in a simple and noninvasive manner, we decided to focus the remaining analyses on leg bioimpedance, defined as the mean of left and right leg bioimpedance. The distribution of leg bioimpedance in relation to incident heart failure was investigated, and it showed fairly good separation between those who experienced future outcome compared with those who did not (Figure 2; P=1.1×10−72). Compared with individuals who were free from heart failure during follow‐up, individuals with incident heart failure had significantly lower leg bioimpedance (Figure 3).
Figure 2.
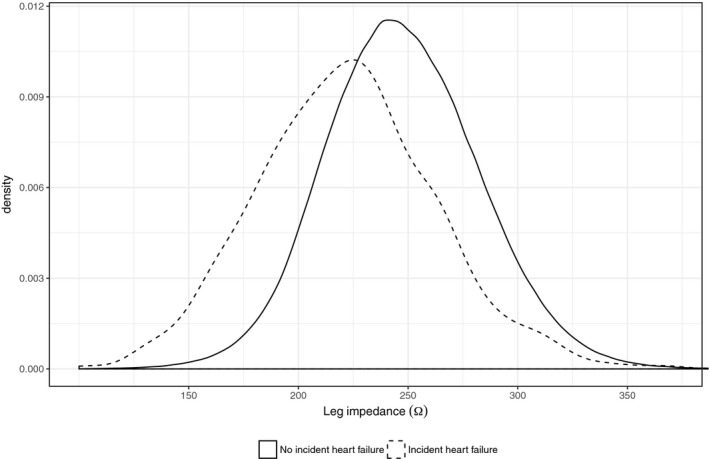
Distributions of leg bioimpedance in relation to incident heart failure.
Figure 3.
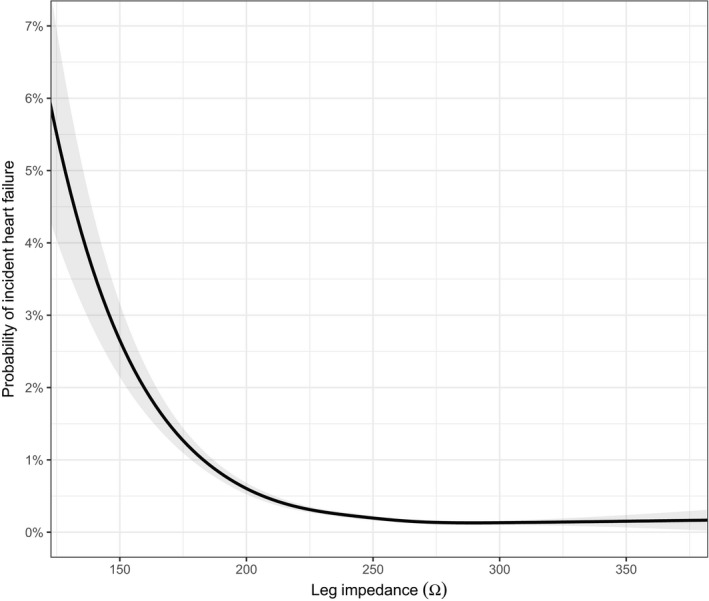
Spline plot of probability for heart failure hospitalization in relation to leg bioimpedance.
In sensitivity analyses, we assessed leg bioimpedance in relation to incident heart failure in a subsample, excluding all individuals who had prior hospitalizations for any cardiovascular disease, as well as those with self‐reported presence of cardiovascular disease and/or cardiovascular risk factors (N=309 079 with 212 cases [0.07%] of incident heart failure). In this healthy subsample, associations of leg bioimpedance with heart failure were nonsignificant but displayed similar effect sizes as in the full study sample (hazard ratio, 0.74; 95% confidence interval, 0.47–1.15; comparing upper versus lower quartile in a multivariable‐adjusted model; Figure S2).
In additional sensitivity analyses in relation to prevalent cardiovascular disease, leg bioimpedance was consistently lower in those experiencing heart failure hospitalizations during follow‐up (Figures S3 and S4); and in those with known prior heart failure, lower leg bioimpedance was associated with having multiple rehospitalizations during follow‐up (Figure S5).
When adding leg bioimpedance to a model including only age and sex, the concordance index (C‐index) increased from 0.76 to 0.80, which should be compared with an increase from 0.76 to 0.79 when adding self‐reported myocardial infarction to the model (Table 3). Combining age, sex, leg bioimpedance, and self‐reported myocardial infarction (the variable with highest importance in the gradient boosting machine model; Figure 1) in a model led to a C‐index of 0.82. This model was well calibrated (calibration slope, 0.9985; Figure S6), and the optimism‐corrected C‐index was 0.82 (ie, essentially unchanged, obtained via bootstrap validation), indicating that overfitting was not an issue. When taking all known risk factors for heart failure into account, leg bioimpedance remained significantly inversely associated with incident heart failure, calibration was similar as for the smaller model (Figure S7), and the C‐index improved further to 0.85 (Table 3). The Akaike information criterion decreased (indicating better model fit) in a manner consistent with increases in the C‐index (Table 3).
Table 3.
Discrimination and Model Fit of Multivariable Models of Incident Heart Failure During Up to 8 Years of Follow‐Up
| Model | HR (95% CI) Upper vs Lower Quartile of Leg Bioimpedance | C‐Indexa | AICa |
|---|---|---|---|
| Age+sex | ··· | 0.76 | 26 241 |
| Age+sex+leg bioimpedance | 0.60 (0.48–0.73) | 0.80 | 23 531 |
| Age+sex+self‐reported MI | ··· | 0.79 | 25 820 |
| Age+sex+self‐reported MI+leg bioimpedance | 0.60 (0.49–0.74) | 0.82 | 23 204 |
| Fully adjustedb | 0.75 (0.59–0.94) | 0.85 | 21 615 |
AIC indicates Akaike Information Criterion; CI, confidence interval; HR, hazard ratio; MI, myocardial infarction.
Concordance index (C‐index) is a measure of discrimination, where higher is better (0.5 is random, 1 indicates perfect discrimination); Akaike Information Criterion (AIC) is a measure of model fit, where lower is better.
Fully adjusted model includes age, sex, self‐reported myocardial infarction, leg bioimpedance, body mass index, systolic blood pressure, diastolic blood pressure, antihypertensive treatment, alcohol consumption, diabetes mellitus, smoking status, prevalent chronic renal failure, and prevalent coronary heart disease.
Discussion
Using a contemporary, prospective cohort study of >500 000 individuals from the general population, we have performed an extensive investigation of factors associated with incident heart failure. Our key findings are several‐fold. First, using an agnostic machine learning approach in a phenome‐wide manner, we established the strongest features associated with incident heart failure, which included well‐established (eg, coronary artery disease, type 2 diabetes mellitus), previously suggested (eg, obesity, microalbuminuria, anemia), and novel (eg, leg bioimpedance, mean reticulocyte volume) potential risk factors of incident heart failure. Second, we report that leg bioimpedance was inversely associated with incident heart failure, even after taking known risk factors into account in multivariable‐adjusted Cox regression models. Third, a multivariable model including leg bioimpedance, age, sex, and self‐reported prior myocardial infarction provided accurate prediction of heart failure incidence, highlighting the potential of this simple, noninvasive, and cheap measure for clinical use. Given the simplicity of measurement, leg bioimpedance could potentially be used as a tool for screening in primary care facilities. In addition to independent prospective validation of leg bioimpedance in this setting, its role in established heart failure should be further investigated, especially assessing whether rehospitalizations could be predicted and ultimately prevented by preemptive treatment.
Previous Studies of Bioimpedance and Heart Failure
To our knowledge, this is the largest study of risk factors for heart failure hospitalization, and the only study of bioimpedance in relation to incident heart failure in the general population. There are a few previous studies that have assessed bioimpedance in relation to heart failure decompensation in patients with already established severe heart failure treated with cardiac resynchronization devices or implantable defibrillators, where impedance could be measured using the pacemaker leads. In that setting, decompensation is characterized by a decrease in intrathoracic impedance, indicative of volume overload, well before rehospitalization for congestion.12 There are also a few prior studies of noninvasive impedance measures in patients with established heart failure. In a phase II trial, treatment guided by lung impedance (assessed using external electrodes) reduced heart failure hospitalizations.13 In addition, bioimpedance measures have been suggested as an adjunctive test for patients presenting with dyspnea at the emergency department, to identify heart failure patients when other tests are equivocal.14, 15 Whether the information obtained from impedance measures in patients with established heart failure translates into improved outcomes is, however, uncertain.13, 16, 17
Machine Learning Studies of Heart Failure
In the present study, we employed machine learning techniques to identify novel factors associated with incident heart failure, and then studied these factors (especially the top new marker, leg bioimpedance) in more detail. Machine learning has been employed before in the heart failure setting, but with the primary goal to develop machine learning–based prediction models18, 19 in contrast to the present study, where we proceeded with standard Cox proportional hazards models after the top features had been identified. In a recent study, machine learning algorithms (random forests, gradient boosting machine, and support vector machine) were compared with standard regression models (logistic regression, and Poisson regression) to detect readmission of patients with established heart failure. Although performing better than the standard models, the machine learning models demonstrated only modest discriminatory ability, with a C‐index of 0.68 for the best‐performing model.18
Another study focused on prediction of new‐onset heart failure, as in the present study. Based on electronic health record data in the primary care setting, these models included information on demographics, vital signs, diagnoses, medications, laboratory values, Framingham heart failure signs and symptoms, hospitalizations, and imaging. Although taking a large number of features into account, the best‐performing model had a receiver operating characteristic area under the curve of about 0.80.19 In the present study, a simple model including only 4 features (of which leg bioimpedance was one) provided slightly higher discriminatory capacity for incident heart failure, which highlights the strong predictive value of the variables included.
Strengths and Limitations
Strengths of our study include the unprecedented sample size from the general population with consistent assessment of risk factors for heart failure, including leg bioimpedance in >500 000 individuals; the minimal loss‐to‐follow‐up; and our stringent, yet exploratory approach that allowed us to discover leg bioimpedance as a novel risk factor for heart failure in the general population. Our study also has some important limitations. Cardiac‐specific biomarkers, especially the natriuretic peptides, which are strongly associated with incident heart failure when screening the general population,20, 21 are not available in the UK Biobank. The factors included in our prediction models (Table 3) do not require any blood draw and could be easily assessed and rapidly in a primary care facility using simple and available instruments and self‐reported information. However, it would have been interesting both from a mechanistic and predictive standpoint to be able to study leg bioimpedance in relation to, for example, B‐type natriuretic peptide. Furthermore, incident heart failure was defined as a diagnosis of heart failure in national registries during follow‐up, rather than via review of journal records including echocardiography reports. As a consequence, the etiology of the heart failure, and whether the events represented heart failure with reduced or preserved ejection fraction could not be discerned, and milder heart failure never requiring hospitalization may not have been captured, which, in addition to a possible selection of healthier individuals who chose to participate in the UK Biobank, may explain that the event rate might be slightly lower than expected. Finally, even if the association between leg bioimpedance and incident heart failure was strong also when excluding individuals with any prior cardiovascular diagnoses or self‐reported cardiovascular history and risk factors (including self‐reported heart failure, which presumably also would capture milder cases of heart failure), reverse causation is always a possibility in any observational analysis. In this case, we cannot be certain whether the associations are attributable to subclinical heart failure, as impedance changes have been suggested to precede heart failure symptoms,22 or if this relates to specific biological attributes associated with increased risk of future heart failure. Along the same lines, even if we have adjusted our models for known risk factors for heart failure, there is certainly risk of residual confounding, so any conclusions regarding mechanistic and causal relations between leg bioimpedance and heart failure needs to be studied using other study designs. That said, our finding that leg bioimpedance is strongly associated with incident heart failure with good discrimination and calibration in the general population remains valid regardless of the above limitations.
Conclusions
Leg bioimpedance is inversely associated with incident heart failure in the general population. A model combining leg bioimpedance with self‐reported history of myocardial infarction, age, and sex shows high discrimination that may be useful in the clinical setting, provided independent prospective validation.
Sources of Funding
Dr Lindholm was supported by the Swedish Society of Medicine, the Swedish Cardiac Society, and the Royal Society of Arts and Sciences of Uppsala.
Disclosures
Lindholm reports institutional research grants from AstraZeneca and GlaxoSmithKline, and consultant fees from AstraZeneca to the institution, unrelated to the present project. Ingelsson is a scientific advisor for Precision Wellness, and Olink Proteomics for work unrelated to the present project. The remaining authors have no disclosures to report. All four co‐authors are listed as inventors on US Provisional Patent 62/522,601, “Systems and Methods for Predicting Heart Failure Using Leg Bioimpedance,” filed June 20, 2017.
Supporting information
Data S1. Supplemental Methods.
Table S1. Variable Importance in the Gradient Boosting Machine Model
Figure S1. Receiver operating characteristic curves for the gradient boosting machine model in the training, validation, and test sets.
Figure S2. Distribution of leg bioimpedance in relation to incident heart failure in the subset of individuals who did not have any prior hospitalization for cardiovascular disease (ie, any primary or secondary diagnosis in ICD‐10, Chapter 9, “Diseases of the Circulatory System”), nor any self‐reported history of hypertension, stroke, transient ischemic attack, high cholesterol, diabetes mellitus, angina pectoris, myocardial infarction, or heart failure (n=309 079).
Figure S3. Distributions of leg bioimpedance in full population (n=502 636) in relation to prevalent (primary or secondary I50 diagnosis or self‐reported heart failure) and incident (primary I50 diagnosis) heart failure.
Figure S4. Distributions of leg bioimpedance in full population (n=502 636) in relation to prevalent cardiovascular disease (any primary or secondary diagnosis in ICD‐10, Chapter 9, “Diseases of the Circulatory System”) and incident (primary I50 diagnosis) heart failure.
Figure S5. Distributions of leg bioimpedance in individuals with known prevalent heart failure at inclusion (Primary or secondary ICD‐10 I50 diagnosis or self‐reported history of heart failure; n=2188). A total of 41 individuals experienced 5 or more hospitalizations for heart failure during follow‐up and had lower leg bioimpedance than those with 0 to 4 hospitalizations.
Figure S6. Calibration of small Cox model (age+sex+history of myocardial infarction+leg bioimpedance).
Acknowledgments
This research was conducted using the UK Biobank Resource (http://www.ukbiobank.ac.uk) under Application Number 13 721. Computing for this project was performed on the Sherlock cluster. We thank the Stanford Research Computing Center for providing computational resources and support that have contributed to these research results.
(J Am Heart Assoc. 2018;7:e008970 DOI: 10.1161/JAHA.118.008970.)
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jiménez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics‐2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol. 2017;70:776–803. [DOI] [PubMed] [Google Scholar]
- 3. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola V‐P, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P; Authors/Task Force Members . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37:2129–2200. [DOI] [PubMed] [Google Scholar]
- 4. Allen NE, Sudlow C, Peakman T, Collins R; UK Biobank . UK Biobank data: come and get it. Sci Transl Med. 2014;6:224ed4. [DOI] [PubMed] [Google Scholar]
- 5. UK Biobank . Available at: http://www.ukbiobank.ac.uk. Accessed February 19, 2018.
- 6. Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, Downey P, Elliott P, Green J, Landray M, Liu B, Matthews P, Ong G, Pell J, Silman A, Young A, Sprosen T, Peakman T, Collins R. UK Biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aune D, Sen A, Norat T, Janszky I, Romundstad P, Tonstad S, Vatten LJ. Body mass index, abdominal fatness, and heart failure incidence and mortality: a systematic review and dose‐response meta‐analysis of prospective studies. Circulation. 2016;133:639–649. [DOI] [PubMed] [Google Scholar]
- 8. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 9. Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. [DOI] [PubMed] [Google Scholar]
- 10. He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow‐up study. Arch Intern Med. 2001;161:996–1002. [DOI] [PubMed] [Google Scholar]
- 11. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW; American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention . Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108:2154–2169. [DOI] [PubMed] [Google Scholar]
- 12. Gudmundsson K, Lyngå P, Rosenqvist M, Braunschweig F. Monitoring of daily body weight and intrathoracic impedance in heart failure patients with a high risk of volume overload decompensation. Clin Cardiol. 2016;39:446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shochat MK, Shotan A, Blondheim DS, Kazatsker M, Dahan I, Asif A, Rozenman Y, Kleiner I, Weinstein JM, Frimerman A, Vasilenko L, Meisel SR. Non‐invasive lung IMPEDANCE‐guided preemptive treatment in chronic heart failure patients: a randomized controlled trial (IMPEDANCE‐HF Trial). J Card Fail. 2016;22:713–722. [DOI] [PubMed] [Google Scholar]
- 14. Génot N, Mewton N, Bresson D, Zouaghi O, Francois L, Delwarde B, Kirkorian G, Bonnefoy‐Cudraz E. Bioelectrical impedance analysis for heart failure diagnosis in the ED. Am J Emerg Med. 2015;33:1025–1029. [DOI] [PubMed] [Google Scholar]
- 15. Di Somma S, Lalle I, Magrini L, Russo V, Navarin S, Castello L, Avanzi GC, Di Stasio E, Maisel A. Additive diagnostic and prognostic value of bioelectrical impedance vector analysis (BIVA) to brain natriuretic peptide “grey‐zone” in patients with acute heart failure in the emergency department. Eur Heart J Acute Cardiovasc Care. 2014;3:167–175. [DOI] [PubMed] [Google Scholar]
- 16. van Veldhuisen DJ, Braunschweig F, Conraads V, Ford I, Cowie MR, Jondeau G, Kautzner J, Aguilera RM, Lunati M, Yu C‐M, Gerritse B, Borggrefe M; DOT‐HF Investigators . Intrathoracic impedance monitoring, audible patient alerts, and outcome in patients with heart failure. Circulation. 2011;124:1719–1726. [DOI] [PubMed] [Google Scholar]
- 17. Lyons KJ, Bischoff MK, Fonarow GC, Horwich TB. Noninvasive bioelectrical impedance for predicting clinical outcomes in outpatients with heart failure. Crit Pathw Cardiol. 2017;16:32–36. [DOI] [PubMed] [Google Scholar]
- 18. Mortazavi BJ, Downing NS, Bucholz EM, Dharmarajan K, Manhapra A, Li S‐X, Negahban SN, Krumholz HM. Analysis of machine learning techniques for heart failure readmissions. Circ Cardiovasc Qual Outcomes. 2016;9:629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ng K, Steinhubl SR, deFilippi C, Dey S, Stewart WF. Early detection of heart failure using electronic health records: practical implications for time before diagnosis, data diversity, data quantity, and data density. Circ Cardiovasc Qual Outcomes. 2016;9:649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Taylor CJ, Roalfe AK, Iles R, Hobbs FDR. The potential role of NT‐proBNP in screening for and predicting prognosis in heart failure: a survival analysis. BMJ Open. 2014;4:e004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. [DOI] [PubMed] [Google Scholar]
- 22. Adamson PB. Pathophysiology of the transition from chronic compensated and acute decompensated heart failure: new insights from continuous monitoring devices. Curr Heart Fail Rep. 2009;6:287–292. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods.
Table S1. Variable Importance in the Gradient Boosting Machine Model
Figure S1. Receiver operating characteristic curves for the gradient boosting machine model in the training, validation, and test sets.
Figure S2. Distribution of leg bioimpedance in relation to incident heart failure in the subset of individuals who did not have any prior hospitalization for cardiovascular disease (ie, any primary or secondary diagnosis in ICD‐10, Chapter 9, “Diseases of the Circulatory System”), nor any self‐reported history of hypertension, stroke, transient ischemic attack, high cholesterol, diabetes mellitus, angina pectoris, myocardial infarction, or heart failure (n=309 079).
Figure S3. Distributions of leg bioimpedance in full population (n=502 636) in relation to prevalent (primary or secondary I50 diagnosis or self‐reported heart failure) and incident (primary I50 diagnosis) heart failure.
Figure S4. Distributions of leg bioimpedance in full population (n=502 636) in relation to prevalent cardiovascular disease (any primary or secondary diagnosis in ICD‐10, Chapter 9, “Diseases of the Circulatory System”) and incident (primary I50 diagnosis) heart failure.
Figure S5. Distributions of leg bioimpedance in individuals with known prevalent heart failure at inclusion (Primary or secondary ICD‐10 I50 diagnosis or self‐reported history of heart failure; n=2188). A total of 41 individuals experienced 5 or more hospitalizations for heart failure during follow‐up and had lower leg bioimpedance than those with 0 to 4 hospitalizations.
Figure S6. Calibration of small Cox model (age+sex+history of myocardial infarction+leg bioimpedance).


