Abstract
Background
Venous thromboembolism (VTE) is the third leading cause of vascular disease and accounts for $10 billion in annual US healthcare costs. The nationwide burden of 30‐day readmissions after such events has not been comprehensively assessed.
Methods and Results
We analyzed adults ≥18 years of age with hospitalizations associated with acute VTE between January 1, 2010, and December 31, 2014, in the Nationwide Readmissions Database. International Classification of Disease, Ninth Revision, Clinical Modification (ICD‐9‐ CM) codes were used to identify hospitalizations associated with acute pulmonary embolism or deep vein thrombosis. The primary outcome was the rate of unplanned 30‐day readmission. Hierarchical logistic regression was used to calculate hospital‐specific 30‐day risk‐standardized readmission rates, a marker of healthcare quality. Among 1 176 335 hospitalizations with acute VTE, in‐hospital death occurred in 6.2%. VTE was associated with malignancy in 19.7%, recent surgery in 19.3%, recent trauma in 4.6%, hypercoagulability in 3.3%, and pregnancy in 1.0%. Among survivors to discharge, the 30‐day readmission rate was 17.5%, with no significant difference in rates across study years (17.4%–17.7%; P=0.10 for trend). Major predictors of readmission were malignancy (relative risk, 1.49, 95% confidence interval 1.47‐1.50), Medicaid insurance (relative risk, 1.48, 95% confidence interval 1.46‐1.50), and nonelective index admission (relative risk, 1.31, 95% confidence interval 1.29‐1.33). Top causes of readmission included sepsis (9.6%) and procedural complications (8.1%). Median rehospitalization costs were $9781.7 (interquartile range, $5430.7–$18 784.1), and 8.1% died during readmission. The interquartile range in risk‐standardized readmission rates was 16.6% to 18.3%, suggesting modest interhospital heterogeneity in readmission risk.
Conclusions
Nearly 1 in 5 patients with acute VTE were readmitted within 30 days. Predictors and causes of readmission were primarily related to patient characteristics and complications from comorbid conditions, whereas healthcare quality had a moderate impact on readmission risk.
Keywords: deep vein thrombosis, pulmonary embolism, readmission, venous thromboembolism
Subject Categories: Embolism, Quality and Outcomes, Vascular Disease, Thrombosis
Clinical Perspective
What Is New?
Nearly 1 in 5 patients with acute venous thromboembolism in the United States are readmitted within 30 days.
These readmissions are associated with high healthcare costs and mortality.
Predictors and causes of readmission were primarily associated with patient characteristics and complications related to comorbid conditions, whereas a smaller proportion of readmissions were associated with recurrent venous thromboembolism or disease processes directly consequential to acute venous thromboembolism.
After standardizing for hospital case mix, there was only modest heterogeneity in readmission rates between institutions, suggesting that healthcare quality had only a moderate impact on readmission risk.
What Are the Clinical Implications?
Further research is needed to identify and validate effective interventions to reduce these frequent and costly readmissions that follow acute venous thromboembolism events.
Because variations between risk‐standardized readmission rates among studied institutions were only modest, programs designed to penalize institutional outliers, similar to the mandate of Centers for Medicare and Medicaid Services' Hospital Readmissions Reduction Program, may have less of an impact on improving outcomes for this disease process.
Introduction
Venous thromboembolism (VTE), consisting of pulmonary embolism (PE) and deep vein thrombosis (DVT), constitutes a major global health issue. With nearly 10 million annual cases worldwide and an individual lifetime risk of >8%, VTE represents the third leading cause of vascular disease.1, 2, 3 VTE is associated with significant morbidity and mortality, accounting for >200 000 deaths in the United States annually.4 The yearly economic burden of VTE is substantial, with estimated US healthcare costs totaling $10 billion.5
In September 2008, the US Surgeon General released a Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism,6 urging a coordinated multifaceted plan to reduce the number of VTE cases nationwide. At the same time, the Centers for Medicare and Medicaid Services discontinued reimbursing hospitals for the marginal cost of treating certain preventable hospital‐acquired conditions, including VTE after joint arthroplasty surgeries.7 More recently, the Centers for Medicare and Medicaid Services instituted the Hospital Readmissions Reduction Program,8, 9 penalizing hospitals for greater than expected 30‐day readmission rates after certain conditions and procedures. These programs have to date been successful10, 11 and have motivated interest in expanding the Hospital Readmissions Reduction Program to other disorders.
Despite the commonality of VTE and its significant costs to the healthcare system, the burden of nationwide readmissions has not yet been comprehensively assessed. With these data, practitioners can identify areas for intervention and opportunities for prevention. Therefore, we examined nationwide hospital readmission data from 2010 through 2014 to determine the rate, predictors, and causes of 30‐day readmission after acute VTE. In addition, we sought to examine whether variations in risk‐standardized readmission rates (RSRRs), a measure of healthcare quality, exist between US hospitals.
Methods
Data Source
The data used in this analysis are available to other researchers for purposes of reproducing the results or replicating the procedure. The data set is publicly available for purchase online.12 We examined data from the Nationwide Readmissions Database (NRD) between 2010 and 2014. The NRD is sponsored by the Agency for Healthcare Research and Quality as part of the Healthcare Cost and Utilization Project and collects discharge data from 22 geographically dispersed states. The NRD includes data from all payers and the uninsured and is composed of >100 clinical and nonclinical variables for each hospital stay. These data include International Classification of Disease, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis and procedure codes, Clinical Classification Software (CCS) diagnosis and procedure classifications, patient demographics, expected payment sources, and total charges and hospital costs based on cost/charge ratios. Weights are provided to calculate national estimates. Research using the NRD was deemed not to qualify as human subjects research by the Institutional Review Board at Beth Israel Deaconess Medical Center (Boston, MA) because of the deidentified nature of the data, and no informed consent was required.
Study Population
All adult hospitalizations (>18 years of age) between January 1, 2010, and December 31, 2014, associated with a diagnosis code for acute VTE were identified (N=1 431 552) (Figure S1). Acute VTE included PE and DVT. Diagnosis codes in any billing position were included to capture all admissions associated with acute VTE. These codes are shown in Table S1. Because the NRD does not track readmissions between years, data from December of each year did not contribute admissions (N=115 659), allowing 30 days of follow‐up for every patient. All analyses were performed using unweighted data. Of 1 315 893 hospitalizations associated with acute VTE, admissions were excluded if the hospitalization occurred in a state outside the patient's primary residence (N=66 873), because any readmission in a state different from the index hospital's state would not be captured by the NRD. Multiple VTE hospitalizations involving the same patient were allowed as long as they occurred >30 days after any previously included VTE hospitalization or readmission. However, a second VTE hospitalization within 30 days of another by the same patient was counted only as a readmission (excluded N=72 685).
Patient, Hospitalization, and Institutional Characteristics
Chronic conditions were determined on the basis of the 29 Elixhauser comorbidities provided by the Agency for Healthcare Research and Quality.13 Because tobacco use is not included in this list, we used ICD‐9‐CM diagnosis codes to define smoking status (Table S1). To ascertain comorbid conditions associated with acute VTE, we identified claims codes for surgical procedures, trauma, malignancy, hypercoagulability, and pregnancy charged during the same admission (Table S1). Acute VTE associated with recent surgery was defined by a surgical billing code charged during the same hospitalization as the VTE event. Surgical procedures were included if they met at least 2 of 3 criteria: (1) surgery that typically requires ≥1 night stay; (2) surgery that requires some period of immobility; and (3) surgery that is likely to require general anesthesia. Acute VTE associated with recent trauma was defined by a billing code for trauma charged during the same hospitalization as the VTE event. Characteristics of the index hospitalization included type of acute VTE, length of stay, discharge destination, and costs. Institutional characteristics included bed volume, ownership, and teaching status. In‐hospital procedures included systemic thrombolysis, pulmonary endarterectomy, mechanical ventilation, extracorporeal circulation, and inferior vena cava filter placement. In‐hospital adverse events included major bleeding, acute myocardial infarction or unstable angina, cardiogenic shock, cardiorespiratory arrest, and acute heart failure.
Study Outcomes
For the first objective, the outcome was unplanned all‐cause 30‐day readmission rate, defined as the occurrence of a hospitalization for any cause within 30 days of discharge after an index hospitalization. Readmissions that were coded as “nonelective” within the NRD were considered unplanned. To further explore this outcome, we analyzed predictors and causes of unplanned 30‐day readmission. Reasons for readmission were determined on the basis of CCS codes, a categorization scheme developed by the Agency for Healthcare Research and Quality based on ICD‐9‐CM coding14 (Data S1). We used CCS categories 103 (pulmonary heart disease) and 118 (phlebitis; thrombophlebitis and thromboembolism) to categorize causes of readmission attributable to recurrent VTE or disease processes directly related to acute VTE. The CCS categorization of “pulmonary heart disease” includes diagnosis codes for recurrent PE; chronic pulmonary heart disease, including pulmonary hypertension; and other diseases of the pulmonary circulation. The CCS categorization of “thrombophlebitis” includes diagnosis codes for thrombophlebitis, recurrent DVT, and chronic DVT.
For the second objective, the primary outcome was hospital‐specific 30‐day RSRRs. To make these estimates, a hierarchical logistic regression model accounting for differences in hospital case mix was developed, as described later.
Statistical Analysis
Categorical variables were reported as counts and percentages, and continuous variables were reported as means with SDs. Between‐group differences were assessed using Fisher's exact or χ2 tests for categorical variables and Student t or Wilcoxon rank sum tests for continuous variables. The Mantel‐Haenszel test for trend was used to assess for changes in readmission rates across study years.
To derive predictors of 30‐day readmission, hierarchical modified Poisson models were developed. We estimated risk ratios directly using modified Poisson regression models with robust standard errors instead of odds ratios because of the high readmission rates.15 These models were clustered by hospital per year, because the NRD does not track hospitals across years. Candidate variables for the models were determined on the basis of clinical experience and prior literature (Table S2). To establish parsimonious models, we used a backward selection process until 90% of the full model R2 was retained.16
To estimate RSRRs across hospitals, we used hospital data from 2014, because the NRD does not track hospitals across years. For this analysis, we adapted a previously established approach.17 First, we created a logistic regression model predicting 30‐day readmission to identify variables for risk adjustment. Candidate variables for the model included patient factors, as reported in Table S3. We excluded covariates, such as in‐hospital complications, certain patient demographics (eg, type of insurance), and discharge disposition, because these characteristics may be more related to hospital quality and resource availability. To inform variable selection, we used a modified stepwise logistic regression approach, which involved creating 500 bootstrap samples from the data set. For each sample, we ran a logistic stepwise regression, with both backward and forward selection. The P value to enter was set at 0.05, and the P value to exit was set at 0.01. We retained all risk‐adjustment variables above a 70% cutoff because these demonstrated a strong association with readmission and were clinically relevant. The results of this model are displayed in Table S4. Next, we used hierarchical generalized linear models to estimate RSRRs.17 This approach accounts for within‐hospital correlation of the observed readmissions and assumes that, after adjusting for patient risk and sampling variability, the remaining heterogeneity is attributable to hospital quality.18, 19
Analyses were stratified by type of acute VTE during the index hospitalization (PE versus DVT). For stratified analyses, patients who had both types of VTE during the index admission only contributed data to the PE group. In addition, we examined hospitalizations in which acute VTE was the primary billing diagnosis.
Because of the inability to track out‐of‐hospital deaths, we performed a sensitivity analysis examining predictors and causes of readmission among those discharged to home. This was to ensure that discharge to a care facility, which may be associated with greater risk of short‐term death, did not affect our primary findings.
A 2‐sided P<0.05 was considered significant. Statistical analyses were performed using SAS software v9.3 (SAS Institute, Cary, NC).
Results
Readmissions After Acute VTE
During the study period, there were 1 176 335 hospitalizations associated with acute VTE. In‐hospital death occurred in 6.2% (PE, 7.8%; DVT, 5.5%; both, 5.5%). Among 1 103 742 admissions in which patients survived to discharge, 193 632 were associated with nonelective readmissions at 30 days at a rate of 17.5% (PE, 15.8%; DVT, 19.4%; both, 14.3%). There were an additional 19 536 (1.8%) planned readmissions, which were excluded from the analysis. Weighted on a national level, there were 2 742 947 hospitalizations associated with acute VTE, with 2 577 387 involving patients who survived to discharge, and there were 441 562 readmissions at 30 days.
Between years, readmission rates did not significantly differ, ranging from a low of 17.4% in 2013 to a high of 17.7% in 2011 (P=0.10 for trend). Readmissions accrued throughout the follow‐up period, with a readmission rate of 6.6% at 7 days, 8.7% at 10 days, and 14.0% at 20 days (Figure S2). Among cases with identifiable provoking factors associated with acute VTE present during admission, readmission rates were highest when associated with malignancy (23.7%), followed by recent surgery (19.9%), recent trauma (16.4%), hypercoagulability (16.4%), and pregnancy (10.7%). For those with acute DVT, readmission rates were similar among cases involving proximal and distal lower extremity vessels (16.7% versus 16.1%, respectively). During rehospitalization, an additional 8.1% of patients died.
Patient and Hospitalization Characteristics by Readmission Status
Of index hospitalizations associated with acute VTE, 54.6% were associated with DVT, 29.5% were associated with PE, and 15.9% were associated with both. Among cases with identifiable provoking factors associated with VTE present during admission, the plurality was related to malignancy (19.7%), followed by recent surgery (19.3%), recent trauma (4.6%), hypercoagulability (3.3%), and pregnancy (1.0%). Readmitted patients were older, were more often women, had more comorbid conditions, and were primarily insured by Medicare or Medicaid (Table 1). Readmissions occurred more frequently among index hospitalizations for nonelective indications, of longer durations, and in which patients were discharged to a subacute facility (Table 2). These hospitalizations more commonly involved mechanical ventilation and inferior vena cava filter placement, whereas use of systemic thrombolysis was associated with a lower frequency of readmission. Adverse events occurred with greater rates during hospitalizations associated with readmission, particularly major bleeds and acute heart failure.
Table 1.
Patient Characteristics of Index Admissions Associated With Acute VTE, Stratified by 30‐Day Readmission Status
| Variable | All Index Admissions (N=1 103 742) | Nonelective 30‐d Readmission | ||
|---|---|---|---|---|
| Yes (n=193 632) | No (n=910 110) | P Value | ||
| VTE | <0.001 | |||
| Pulmonary embolism | 325 850 (29.5) | 51 417 (26.6) | 274 433 (30.2) | <0.001 |
| Deep vein thrombosis | 602 126 (54.6) | 117 107 (60.5) | 485 019 (53.3) | <0.001 |
| Both | 175 766 (15.9) | 25 108 (13.0) | 150 658 (16.6) | <0.001 |
| Saddle pulmonary embolus | 10 901 (1.0) | 1095 (0.6) | 9806 (1.1) | <0.001 |
| Acute cor pulmonale | 1816 (0.2) | 238 (0.1) | 1578 (0.2) | <0.001 |
| Thrombophlebitis | 57 040 (5.2) | 9726 (5.0) | 47 314 (5.2) | 0.001 |
| Provoking factors associated with VTE present during admission | ||||
| Malignancy | 217 402 (19.7) | 51 565 (26.6) | 165 837 (18.2) | <0.001 |
| Recent surgery | 213 163 (19.3) | 42 454 (21.9) | 170 709 (18.8) | <0.001 |
| Recent trauma | 50 474 (4.6) | 8293 (4.3) | 42 181 (4.6) | <0.001 |
| Hypercoagulability | 36 821 (3.3) | 6046 (3.1) | 30 775 (3.4) | <0.001 |
| Pregnancy | 11 043 (1.0) | 1179 (0.6) | 9864 (1.1) | <0.001 |
| Age, mean±SD, y | 64.0±17.3 | 64.8±16.9 | 63.9±17.3 | <0.001 |
| Female sex | 580 632 (52.6) | 102 849 (53.1) | 477 783 (52.5) | <0.001 |
| Primary insurance | <0.001 | |||
| Medicare | 627 703 (57.0) | 119 763 (62.0) | 507 940 (55.9) | <0.001 |
| Medicaid | 122 082 (11.1) | 27 344 (14.1) | 94 738 (10.4) | <0.001 |
| Private | 267 365 (24.3) | 34 767 (18.0) | 232 598 (25.6) | <0.001 |
| Self‐pay | 40 725 (3.7) | 5464 (2.8) | 35 261 (3.9) | <0.001 |
| Other | 43 459 (3.9) | 5966 (3.1) | 37 493 (4.1) | <0.001 |
| Median household income by residential zip code | <0.001 | |||
| Lowest quartile | 313 413 (28.8) | 58 261 (30.5) | 255 152 (28.5) | <0.001 |
| Middle lowest quartile | 265 269 (24.4) | 46 036 (24.1) | 219 233 (24.5) | 0.001 |
| Middle highest quartile | 260 176 (23.9) | 44 513 (23.3) | 215 663 (24.1) | <0.001 |
| Highest quartile | 247 686 (22.8) | 41 980 (22.0) | 205 706 (23.0) | <0.001 |
| Residence by county location | <0.001 | |||
| Central counties in metropolitan areas with population ≥1 million | 364 183 (33.0) | 71 770 (37.1) | 292 413 (32.2) | <0.001 |
| Fringe counties in metropolitan areas with population ≥1 million | 274 168 (24.9) | 49 022 (25.4) | 225 146 (24.8) | <0.001 |
| Counties in metropolitan areas with population 250 000–999 999 | 222 597 (20.2) | 36 264 (18.8) | 186 333 (20.5) | <0.001 |
| Counties in metropolitan areas with population 50 000–249 999 | 94 783 (8.6) | 15 055 (7.8) | 79 728 (8.8) | <0.001 |
| Micropolitan counties | 86 689 (7.9) | 12 817 (6.6) | 73 872 (8.1) | <0.001 |
| Other | 59 858 (5.4) | 8338 (4.3) | 51 520 (5.7) | <0.001 |
| No. of chronic conditions, mean±SD | 5.6±3.2 | 6.6±3.2 | 5.5±3.1 | <0.001 |
| Varicose veins | 5950 (0.5) | 662 (0.3) | 5288 (0.6) | <0.001 |
| Obesity | 174 637 (15.8) | 28 723 (14.8) | 145 914 (16.0) | <0.001 |
| Current or prior tobacco use | 275 169 (24.9) | 48 893 (25.3) | 226 276 (24.9) | <0.001 |
| Hypertension | 640 841 (58.1) | 116 883 (60.4) | 523 958 (57.6) | <0.001 |
| Congestive heart failure | 138 558 (12.6) | 33 563 (17.3) | 104 995 (11.5) | <0.001 |
| Diabetes mellitus | 277 924 (25.2) | 58 271 (30.1) | 219 653 (24.1) | <0.001 |
| Chronic pulmonary disease | 238 531 (21.6) | 50 018 (25.8) | 188 513 (20.7) | <0.001 |
| Pulmonary circulation disorders | 216 282 (19.6) | 39 899 (20.6) | 176 383 (19.4) | <0.001 |
| Renal disease | 177 924 (16.1) | 43 225 (22.3) | 134 699 (14.8) | <0.001 |
| Liver disease | 32 656 (3.0) | 7810 (4.0) | 24 846 (2.7) | <0.001 |
| Coagulopathy | 99 598 (9.0) | 21 530 (11.1) | 78 068 (8.6) | <0.001 |
| Anemia | 303 960 (27.5) | 67 349 (34.8) | 236 611 (26.0) | <0.001 |
| Fluid and electrolyte disorders | 335 626 (30.4) | 74 315 (38.4) | 261 311 (28.7) | <0.001 |
| Neurologic disorders/paralysis | 143 567 (13.0) | 28 597 (14.8) | 114 970 (12.6) | <0.001 |
| Alcohol abuse | 40 838 (3.7) | 7992 (4.1) | 32 846 (3.6) | <0.001 |
| Illicit drug abuse | 35 702 (3.2) | 8337 (4.3) | 27 365 (3.0) | <0.001 |
Data are given as number (percentage) unless otherwise indicated. For most variables, 0% to 2.5% had missing values. VTE indicates venous thromboembolism.
Table 2.
Hospitalization and Institutional Characteristics of Index Admissions Associated With Acute VTE, Stratified by 30‐Day Readmission Status
| Variable | All Index Admissions (N=1 103 742) | Nonelective 30‐d Readmission | ||
|---|---|---|---|---|
| Yes (n=193 632) | No (n=910 110) | P Value | ||
| Characteristics of index hospitalization | ||||
| Elective index admission | 117 726 (10.7) | 15 801 (8.2) | 101 925 (11.2) | <0.001 |
| Elective readmission | 19 536 (1.8) | ··· | ··· | <0.001 |
| Length of stay, mean±SD, d | 9.4±13.0 | 11.5±14.6 | 8.9±12.5 | <0.001 |
| Disposition at discharge | <0.001 | |||
| Home, no assistance | 568 369 (51.5) | 80 071 (41.4) | 488 298 (53.7) | <0.001 |
| Transfer to short‐term hospital | 14 514 (1.3) | 2960 (1.5) | 11 554 (1.3) | <0.001 |
| Transfer to subacute nursing or intermediate‐care facility | 256 964 (23.3) | 56 968 (29.4) | 199 996 (22.0) | <0.001 |
| Home with home health care | 253 187 (22.9) | 50 521 (26.1) | 202 666 (22.3) | <0.001 |
| Against medical advice | 8704 (0.8) | 2949 (1.5) | 5755 (0.6) | <0.001 |
| Cost of index hospitalization, median (IQR), $ | 11 208.4 (6127.2–23 529.9) | 14 739.0 (7917.0–30 518.5) | 10 573.3 (5851.9–22 043.2) | <0.001 |
| Cost of rehospitalization, median (IQR), $ | ··· | 9781.7 (5430.7–18 784.1) | ··· | ··· |
| Characteristics of index hospital | ||||
| Bed size of hospital | <0.001 | |||
| Small | 112 428 (10.2) | 17 033 (8.8) | 95 395 (10.5) | <0.001 |
| Medium | 267 782 (24.3) | 45 079 (23.3) | 222 703 (24.5) | <0.001 |
| Large | 723 532 (65.6) | 131 520 (67.9) | 592 012 (65.0) | <0.001 |
| Ownership of hospital | 0.013 | |||
| Government | 157 440 (14.3) | 28 116 (14.5) | 129 324 (14.2) | <0.001 |
| Private, nonprofit | 769 314 (69.7) | 134 513 (69.5) | 634 801 (69.7) | 0.014 |
| Private, for profit | 176 988 (16.0) | 31 003 (16.0) | 145 985 (16.0) | 0.751 |
| Teaching status | <0.001 | |||
| Metropolitan, nonteaching | 452 851 (41.0) | 76 030 (39.3) | 376 821 (41.4) | <0.001 |
| Metropolitan, teaching | 564 472 (51.1) | 106 161 (54.8) | 458 311 (50.4) | <0.001 |
| Nonmetropolitan | 86 419 (7.8) | 11 441 (5.9) | 74 978 (8.2) | <0.001 |
| Procedures during index hospitalization | ||||
| Systemic thrombolysis | 29 789 (2.7) | 4231 (2.2) | 25 558 (2.8) | <0.001 |
| Pulmonary endarterectomy | 131 (0.0) | 17 (0.0) | 114 (0.0) | 0.169 |
| Mechanical ventilation | 69 239 (6.3) | 15 395 (8.0) | 53 844 (5.9) | <0.001 |
| Extracorporeal circulation | 408 (0.0) | 82 (0.0) | 326 (0.0) | 0.174 |
| Inferior vena cava filter placement | 142 575 (12.9) | 29 453 (15.2) | 113 122 (12.4) | <0.001 |
| Adverse events during index hospitalization | ||||
| Major bleed | 239 633 (21.7) | 54 587 (28.2) | 185 046 (20.3) | <0.001 |
| Gastrointestinal hemorrhage | 47 628 (4.3) | 11 580 (6.0) | 36 048 (4.0) | <0.001 |
| Intracranial hemorrhage | 15 572 (1.4) | 2999 (1.5) | 12 573 (1.4) | <0.001 |
| Other bleeding event (including RBC transfusion) | 210 871 (19.1) | 48 438 (25.0) | 162 433 (17.8) | <0.001 |
| Acute myocardial infarction/unstable angina | 30 324 (2.7) | 6565 (3.4) | 23 759 (2.6) | <0.001 |
| Cardiogenic shock | 7686 (0.7) | 1652 (0.9) | 6034 (0.7) | <0.001 |
| Cardiorespiratory arrest | 9056 (0.8) | 1925 (1.0) | 7131 (0.8) | <0.001 |
| Acute heart failure | 174 146 (15.8) | 42 328 (21.9) | 131 818 (14.5) | <0.001 |
Data are given as number (percentage) unless otherwise indicated. For most variables, 0% to 2.5% had missing values. IQR indicates interquartile range; RBC, red blood cell; VTE, venous thromboembolism.
Overall, patient and hospitalization characteristics did not significantly differ from the overall group when stratified by PE and DVT (Tables S5 through S8).
Predictors of 30‐Day Readmission
The strongest predictors of readmission included a diagnosis of malignancy (relative risk [RR], 1.49; 95% confidence interval [CI], 1.47–1.50), nonelective index admission (RR, 1.31; 95% CI, 1.29–1.33), and insurance status (Medicaid: RR, 1.48 [95% CI, 1.46–1.50]; Medicare: RR, 1.31 [95% CI, 1.29–1.33]) (Figure 1). Discharge to a subacute facility (RR, 1.25; 95% CI, 1.24–1.27) or home with additional care (RR, 1.19; 95% CI, 1.18–1.21) was also associated with increased readmission risk. Hospitalizations associated with acute DVT had a stronger relationship with need for readmission compared with those associated with acute PE (RR, 1.13; 95% CI, 1.11–1.14). Predictors of readmission did not significantly vary when stratified by PE and DVT (Figures S3 and S4), nor did they differ among those discharged to home (Figure S5).
Figure 1.
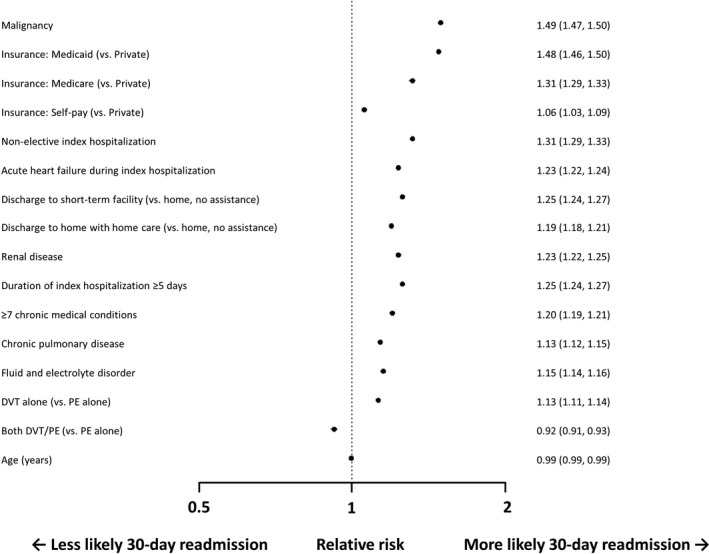
Displayed is a forest plot with predictors of nonelective 30‐day readmission after acute venous thromboembolism. The strongest predictors included malignancy, nonelective index hospitalization, insurance type (Medicaid and Medicare), acute heart failure exacerbation, and discharge to a short‐term facility. Risk estimates are relative risks with 95% confidence intervals. DVT indicates deep vein thrombosis; PE, pulmonary embolism.
Reasons for 30‐Day Readmission
Sepsis was the primary indication for readmission (9.6%), followed by procedural complications (8.1%) and issues related to malignancy (6.9%) (Figure 2). Recurrent VTE and disease processes directly related to acute VTE, which are categorized as “recurrent DVT/thrombophlebitis” and “pulmonary heart disease,” composed 9.4% of all causes for readmission. The most frequent reason for rehospitalization among those with acute PE was directly related to the disease process (pulmonary heart disease, 7.9%), whereas for acute DVT, causes were more often related to complications from comorbid conditions (sepsis, 10.8%; procedural complications, 9.7%). Reasons for readmission were overall similar among those discharged to home (Figure S6).
Figure 2.
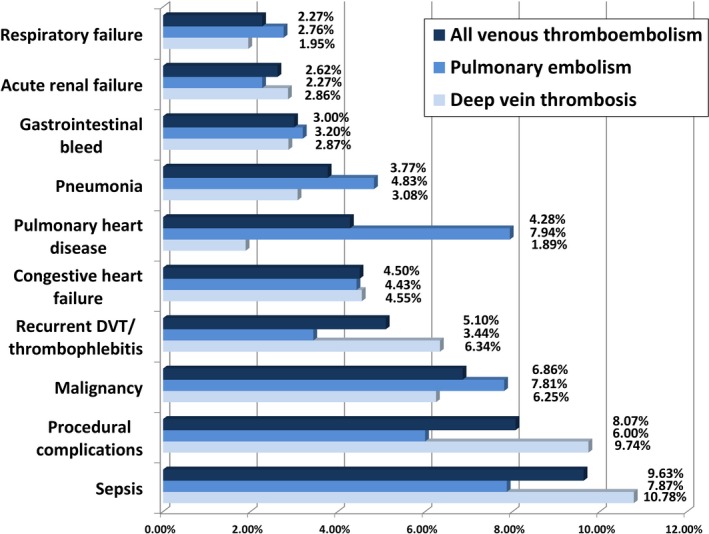
The figure displays the main reasons for 30‐day readmission. The most common cause of readmission was sepsis, followed by procedural complications and issues related to malignancy. DVT indicates deep vein thrombosis.
Healthcare Costs Associated With VTE Readmissions
The median cost of a readmission after a hospitalization for acute VTE was $9782 (interquartile range, $5431–$18 784), with a nationally weighted annual cost totaling $1.44 billion. Including the median cost of the index admission ($14 739; interquartile range, $7917–$30 519), the median total inpatient healthcare cost associated with an acute VTE hospitalization that required a readmission was $24 521 (interquartile range, $13 348–$49 303).
Heterogeneity in Readmission Risk Between Hospitals
After standardizing for hospital case mix, the median RSRR for all institutions in 2014 was 17.4%. RSRRs ranged from 11.0% to 27.4% across hospitals, with an interquartile range of 16.6% to 18.3% (Figure 3). The fifth percentile was 14.9%, and the 95th percentile was 21.3%.
Figure 3.
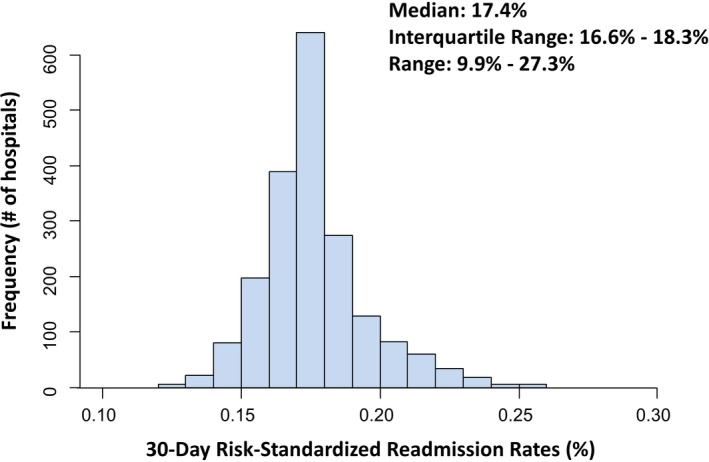
Frequency of 30‐day risk‐standardized readmission rates across institutions in 2014. Displayed on the X‐axis are the hospital‐specific 30‐day risk‐standardized readmission rates for each hospital included in the study. Displayed on the Y‐axis are the frequencies of hospitals with the specific risk‐standardized readmission rate.
Hospitalizations With Acute VTE as Primary Billing Diagnosis
Of all hospitalizations, 49.7% (N=548 413) were associated with a primary diagnosis of acute VTE (Tables S9 and S10). The 30‐day unplanned readmission rate among these patients was 13.3%. Predictors of 30‐day nonelective readmission were similar to the overall study cohort (Figure S7), whereas the primary causes of readmission were more often related to VTE (recurrent DVT/thrombophlebitis, 8.8%; pulmonary heart disease, 8.1%) (Figure S8). Among patients readmitted, an additional 6.3% experienced an in‐hospital death.
Discussion
Among >1.1 million US hospitalizations associated with acute VTE, the short‐term readmission rate was substantial, with ≈1 in 5 rehospitalized within 30 days. These readmissions were costly, with ≈$10 000 in median additional healthcare expenditures per rehospitalization. Predictors and causes of readmission were primarily associated with patient characteristics and complications related to comorbid conditions. After standardizing for hospital case mix, there was only moderate heterogeneity in readmission rates between institutions.
Acute VTE is a major public health problem, with an incidence that is steadily increasing because of the aging population, increasing rates of comorbid conditions associated with VTE (eg, obesity and malignancy), and improved detection on imaging studies.1, 20 This disease process is also complex, involving the interaction between acquired and inherited predispositions for thrombosis. Many of these factors are related to external causes, such as hospitalization for surgery or trauma, pregnancy, prolonged immobilization, and active malignancy.1 This heterogeneity in presentation and risk factors makes caring for these patients challenging and has prompted the development of formalized, multifaceted, team‐based approaches.21
Beyond the direct costs accrued during the index hospitalization, our analysis demonstrates the substantial economic burden incurred by the US healthcare system because of short‐term readmissions related to acute VTE. Readmissions were frequent (17.5% by 30 days), costly (median, ≈$10 000/rehospitalization), and often fatal (8% in‐hospital death). Our findings complement a prior study, which found that the costs for VTE treatment are increasing faster than general inflation for medical care services.22 Notably, hospitalization costs were the primary cost driver in that analysis, with readmission‐related expenses representing a large component.
In this study, most readmissions were secondary to complications from an underlying illness or perioperative condition, whereas a smaller proportion was attributable to recurrent VTE or a directly related disease process (9.4%). This relationship was more pronounced among those with acute DVT, which tends to complicate acquired conditions more often than PE.23 Predictors of 30‐day readmission were primarily related to patient comorbidities (eg, malignancy and renal failure), socioeconomic status (eg, Medicaid insurance), and frailty (eg, Medicare insurance, longer index hospitalizations, and requiring discharge to subacute facilities). These results parallel a prior analysis of patients hospitalized with acute PE in the state of Pennsylvania.24
More important, when examining patients admitted with a primary VTE diagnosis, the readmission rate was lower, but remained substantial (13.3%). In addition, predictors of readmission were similar to the overall cohort. Although there was a greater prevalence of readmissions for illnesses directly related to VTE (16.9%), this remained a minority of all causes.
Between studied institutions, there was modest heterogeneity in standardized readmission rates. In comparison, greater variation has been observed for currently penalized conditions, such as heart failure.25 As such, programs designed to penalize institutional outliers, similar to the mandate of the Hospital Readmissions Reduction Program, may have less of an impact on improving outcomes for this disease process.
Taken together, the findings from this analysis should raise awareness to the large burden of readmissions that follow hospitalizations associated with acute VTE and the need to find effective strategies to reduce these events. Patient‐oriented interventions, including early postdischarge follow‐up, home health services, and confirmation of therapeutic anticoagulation, may be helpful for patients with high‐risk comorbidities and complicated hospitalizations. Similar out‐of‐hospital initiatives have been effective for other conditions.26 In addition, implementation of collaborative treatment teams for patients with acute VTE may be useful for coordinating in‐hospital care, determining safe therapeutic strategies, and organizing treatment plans after discharge.21
This analysis must be interpreted in the context of the study design. First, acute VTE cases were identified through use of ICD‐9‐CM codes, which may result in the misclassification of acute VTE cases. For instance, ICD‐9‐CM codes are lacking for acute DVT, whereas there are ICD‐9‐CM codes to document chronic DVT. However, prior studies of VTE have demonstrated that in up to 96% of cases, ICD‐9‐CM codes corresponded with documented disease on medical record review.23, 24 Second, we lacked data on the incidence of out‐of‐hospital death after discharge. As such, our results may have been influenced by survivorship bias. To support that this effect was minimal, we performed a sensitivity analysis examining only hospitalizations in which patients underwent routine discharge home, a cohort who may have a lower risk of short‐term death. These results did not vary significantly from our overall results (Figures S5 and S6). Third, we did not have data on the timing, type, and intensity of anticoagulation after the diagnosis of acute VTE, nor confirmatory testing among those with a diagnosis of primary hypercoagulability. Fourth, standardization of readmission rates for case mix using claims data may be subject to residual confounding. In addition, we chose to use risk standardization methods similar to those used by the Centers for Medicare and Medicaid Services that involve shrinkage of hospital estimates toward the mean. These methods may reduce the estimated variability in hospital readmission rates, particularly among lower‐volume institutions. Fifth, among those with primary admissions for conditions other than VTE, such as for surgery, we are unable to determine the extent to which VTE caused the readmission above the risk secondary to the primary condition. Last, this analysis included data from a sample of US states and may not be generalizable to other populations, including those abroad.
Conclusions
Short‐term readmission after acute VTE is common in the United States and associated with high healthcare costs and mortality. Readmissions are primarily related to patient characteristics and complications from comorbid conditions, whereas healthcare quality only modestly affects readmission risk. Further research is needed to identify and validate effective interventions to reduce these frequent and costly readmissions that follow acute VTE events.
Sources of Funding
This study was internally funded through the Smith Center for Outcomes Research in Cardiology at Beth Israel Deaconess Medical Center (Boston, MA).
Disclosures
Jaff is a compensated advisor for Philips/Volcano and Venarum (modest); an equity investor for Embolitech, Vascular Therapies, PQ Bypass, MC10, Jana Care, and Sano V (modest); and a compensated board member of VIVA Physicians, a 501c3 not‐for‐profit education and research organization (modest). Rosenfield is a compensated advisor for Abbott Vascular, Cardinal Health, Surmodics, Inari Medical, Volcano/Phillips, and Proteon (modest); has received stock options for serving on advisory boards from Contego, Access Vascular, and MD Insider (modest); holds stock in Embolitech, Janacare, Primacea, and PQ Bypass (modest); has received grant support to his institution from Abbott Vascular, Atrium/Maquet, and Lutonix/Bard (modest); has served as a consultant to Capture Vascular, Shockwave, and the University of Maryland (modest); has received research grant support from Inari Medical and the National Institutes of Health (modest); has received equity or stock options from Capture Vascular, VORTEX, Micell, Shockwave, Cruzar Systems, Endospan, Eximo, Valcare, and Contego (modest); has received an honorarium from Cook (modest); and is a compensated board member of VIVA Physicians, a 501c3 not‐for‐profit education and research organization (modest). The remaining authors have no disclosures to report.
Supporting information
Data S1. Supplemental Methods.
Table S1. Diagnosis and Procedure Billing Codes
Table S2. Candidate Variables for 30‐Day Readmission Prediction Models
Table S3. Candidate Variables for 30‐Day Risk Standardization Readmissions Model
Table S4. 30‐Day Readmission Logistic Regression Model
Table S5. Patient Characteristics of Index Admissions Associated With Acute Pulmonary Embolism, Stratified by 30‐Day Readmission Status
Table S6. Hospitalization and Institutional Characteristics of Index Admissions Associated With Acute Pulmonary Embolism, Stratified By 30‐Day Readmission Status
Table S7. Patient Characteristics of Index Hospitalizations Associated With Acute Deep Vein Thrombosis, Stratified By 30‐Day Readmission Status
Table S8. Hospitalization and Institutional Characteristics of Index Admissions Associated With Acute Deep Vein Thrombosis, Stratified By 30‐day Readmission Status
Table S9. Patient Characteristics of Index Hospitalizations Associated With a Primary Diagnosis Code for Venous Thromboembolism, Stratified By 30‐Day Readmission Status
Table S10. Hospitalization and Institutional Characteristics of Index Admissions Associated With a Primary Diagnosis Code for Venous Thromboembolism, Stratified By 30‐Day Readmission Status
Figure S1. Flow diagram of selection of hospitalizations.
Figure S2. Cumulative incidences of readmission through 30 days after discharge following hospitalizations associated with acute venous thromboembolism.
Figure S3. Predictors of 30‐day readmission following hospitalizations associated with acute pulmonary embolism.
Figure S4. Predictors of 30‐day readmission following hospitalizations associated with acute deep vein thrombosis.
Figure S5. Predictors of 30‐day readmission following hospitalizations associated with acute venous thromboembolism for patients discharged to home.
Figure S6. Top causes of non‐elective 30‐day readmission following hospitalizations associated with acute venous thromboembolism for patients discharged to home.
Figure S7. Predictors of 30‐day readmission following hospitalizations associated with a primary diagnosis code for venous thromboembolism.
Figure S8. Top causes of non‐elective 30‐day readmission following hospitalizations associated with a primary diagnosis code for venous thromboembolism.
Acknowledgments
We thank Linda Valsdottir, MS, for editing assistance. The data reported in this study were provided by the Agency for Healthcare Quality and Research. Secemsky and Yeh have direct access to the data and take responsibility for their integrity and for the data analysis.
(J Am Heart Assoc. 2018;7:e009047 DOI: 10.1161/JAHA.118.009047.)
This article was awarded the Jay D. Coffman Early Career Investigator Award at the American Heart Association Scientific Sessions, November 11 to 15, 2017, in Anaheim, CA.
References
- 1. Raskob GE, Angchaisuksiri P, Blanco AN, Buller H, Gallus A, Hunt BJ, Hylek EM, Kakkar A, Konstantinides SV, McCumber M. Thrombosis: a major contributor to global disease burden. Arterioscler Thromb Vasc Biol. 2014;34:2363–2371. [DOI] [PubMed] [Google Scholar]
- 2. Bell EJ, Lutsey PL, Basu S, Cushman M, Heckbert SR, Lloyd‐Jones DM, Folsom AR. Lifetime risk of venous thromboembolism in two cohort studies. Am J Med. 2016;129:339.e19–339.e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Silverstein MD, Heit JA, Mohr DN, Petterson TM, O'fallon WM, Melton LJ. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25‐year population‐based study. Arch Intern Med. 1998;158:585–593. [DOI] [PubMed] [Google Scholar]
- 4. Weller WE, Gallagher BK, Cen L, Hannan EL. Readmissions for venous thromboembolism: expanding the definition of patient safety indicators. Jt Comm J Qual Saf. 2004;30:497–504. [DOI] [PubMed] [Google Scholar]
- 5. Grosse SD, Nelson RE, Nyarko KA, Richardson LC, Raskob GE. The economic burden of incident venous thromboembolism in the United States: a review of estimated attributable healthcare costs. Thromb Res. 2016;137:3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. The Surgeon General's Call to Action to Prevent Deep Vein Thrombosis and Pulmonary Embolism [Internet]. http://www.surgeongeneral.gov/topics/deepvein/calltoaction/call-to-action-on-dvt-2008.pdf. Accessed June 8, 2017.
- 7. Medicare and Medicaid Move Aggressively to Encourage Greater Patient Safety in Hospitals and Reduce Never Events [Internet]. https://www.cms.gov/Newsroom/MediaReleaseDatabase/Press-releases/2008-Press-releases-items/2008-07-313.html. Accessed June 8, 2017.
- 8. Centers for Medicare and Medicaid Services . Readmissions Reduction Program [Internet]. https://www.cms.gov/medicare/medicare-fee-for-service-payment/acuteinpatientpps/readmissions-reduction-program.html. Accessed March 31, 2017.
- 9. Berenson RA, Paulus RA, Kalman NS. Medicare's readmissions‐reduction program: a positive alternative. N Engl J Med. 2012;366:1364–1366. [DOI] [PubMed] [Google Scholar]
- 10. Gidwani R, Bhattacharya J. CMS reimbursement reform and the incidence of hospital‐acquired pulmonary embolism or deep vein thrombosis. J Gen Intern Med. 2015;30:588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wasfy JH, Zigler CM, Choirat C, Wang Y, Dominici F, Yeh RW. Readmission rates after passage of the hospital readmissions reduction program: a pre‐post analysis. Ann Intern Med. 2017;166:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. HCUP Database Online Ordering [Internet]. https://www.distributor.hcup-us.ahrq.gov. Accessed April 5, 2018.
- 13. HCUP Elixhauser Comorbidity Software, Version 3.7 [Internet]. https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed March 31, 2017.
- 14. HCUP Clinical Classifications Software (CCS) for ICD‐9‐CM [Internet]. https://www.hcup-us.ahrq.gov/toolssoftware/ccs/ccs.jsp. Accessed March 31, 2017.
- 15. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 16. Harrell F. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis. Springer‐Verlag New York: Springer; 2001. [Google Scholar]
- 17. 2017 Condition‐Specific Measures Updates and Specifications Report: Hospital‐Level 30‐Day Risk‐Standardized Readmission Measures [Internet]. https://www.qualitynet.org/dcs/BlobServer?blobkey=id&blobnocache=true&blobwhere=1228890669335&blobheader=multipart%2Foctet‐stream&blobheadername1=Content‐Disposition&blobheadervalue1=attachment%3Bfilename%3D2017_Cond‐Spec_Rdmsn_MUS_Rpt.pdf&blobcol=urldata&blobtable=MungoBlobs. Accessed April 1, 2017.
- 18. Krumholz HM, Lin Z, Drye EE, Desai MM, Han LF, Rapp MT, Mattera JA, Normand SL. An administrative claims measure suitable for profiling hospital performance based on 30‐day all‐cause readmission rates among patients with acute myocardial infarction. Circ Cardiovasc Qual Outcomes. 2011;4:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Secemsky EA, Schermerhorn M, Carroll BJ, Kennedy KF, Shen C, Valsdottir LR, Landon B, Yeh RW. Readmissions after revascularization procedures for peripheral arterial disease: a Nationwide Cohort Study. Ann Intern Med. 2018;168:93–99. [DOI] [PubMed] [Google Scholar]
- 20. Huang W, Goldberg RJ, Anderson FA, Kiefe CI, Spencer FA. Secular trends in occurrence of acute venous thromboembolism: the Worcester VTE study (1985–2009). Am J Med. 2014;127:829–839e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kabrhel C, Rosovsky R, Channick R, Jaff MR, Weinberg I, Sundt T, Dudzinski DM, Rodriguez‐Lopez J, Parry BA, Harshbarger S, Chang Y, Rosenfield K. A multidisciplinary pulmonary embolism response team: initial 30‐month experience with a novel approach to delivery of care to patients with submassive and massive pulmonary embolism. Chest. 2016;150:384–393. [DOI] [PubMed] [Google Scholar]
- 22. Fernandez MM, Hogue S, Preblick R, Kwong WJ. Review of the cost of venous thromboembolism. Clinicoecon Outcomes Res. 2015;7:451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murin S, Romano PS, White RH. Comparison of outcomes after hospitalization for deep venous thrombosis or pulmonary embolism. Thromb Haemost. 2002;88:407–414. [PubMed] [Google Scholar]
- 24. Aujesky D, Mor MK, Geng M, Stone RA, Fine MJ, Ibrahim SA. Predictors of early hospital readmission after acute pulmonary embolism. Arch Intern Med. 2009;169:287–293. [DOI] [PubMed] [Google Scholar]
- 25. Krumholz HM, Merrill AR, Schone EM, Schreiner GC, Chen J, Bradley EH, Wang Y, Wang Y, Lin Z, Straube BM, Rapp MT, Normand SL, Drye EE. Patterns of hospital performance in acute myocardial infarction and heart failure 30‐day mortality and readmission. Circ Cardiovasc Qual Outcomes. 2009;2:407–413. [DOI] [PubMed] [Google Scholar]
- 26. Bradley EH, Curry L, Horwitz LI, Sipsma H, Wang Y, Walsh MN, Goldmann D, White N, Piña IL, Krumholz HM. Hospital strategies associated with 30‐day readmission rates for patients with heart failure. Circ Cardiovasc Qual Outcomes. 2013;6:444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental Methods.
Table S1. Diagnosis and Procedure Billing Codes
Table S2. Candidate Variables for 30‐Day Readmission Prediction Models
Table S3. Candidate Variables for 30‐Day Risk Standardization Readmissions Model
Table S4. 30‐Day Readmission Logistic Regression Model
Table S5. Patient Characteristics of Index Admissions Associated With Acute Pulmonary Embolism, Stratified by 30‐Day Readmission Status
Table S6. Hospitalization and Institutional Characteristics of Index Admissions Associated With Acute Pulmonary Embolism, Stratified By 30‐Day Readmission Status
Table S7. Patient Characteristics of Index Hospitalizations Associated With Acute Deep Vein Thrombosis, Stratified By 30‐Day Readmission Status
Table S8. Hospitalization and Institutional Characteristics of Index Admissions Associated With Acute Deep Vein Thrombosis, Stratified By 30‐day Readmission Status
Table S9. Patient Characteristics of Index Hospitalizations Associated With a Primary Diagnosis Code for Venous Thromboembolism, Stratified By 30‐Day Readmission Status
Table S10. Hospitalization and Institutional Characteristics of Index Admissions Associated With a Primary Diagnosis Code for Venous Thromboembolism, Stratified By 30‐Day Readmission Status
Figure S1. Flow diagram of selection of hospitalizations.
Figure S2. Cumulative incidences of readmission through 30 days after discharge following hospitalizations associated with acute venous thromboembolism.
Figure S3. Predictors of 30‐day readmission following hospitalizations associated with acute pulmonary embolism.
Figure S4. Predictors of 30‐day readmission following hospitalizations associated with acute deep vein thrombosis.
Figure S5. Predictors of 30‐day readmission following hospitalizations associated with acute venous thromboembolism for patients discharged to home.
Figure S6. Top causes of non‐elective 30‐day readmission following hospitalizations associated with acute venous thromboembolism for patients discharged to home.
Figure S7. Predictors of 30‐day readmission following hospitalizations associated with a primary diagnosis code for venous thromboembolism.
Figure S8. Top causes of non‐elective 30‐day readmission following hospitalizations associated with a primary diagnosis code for venous thromboembolism.


