Abstract
Background
The natural course of abdominal aortic aneurysms (AAA) is growth and rupture if left untreated. Numerous markers have been investigated; however, none are broadly acknowledged. Our aim was to identify potential prognostic markers for AAA growth and rupture.
Methods and Results
Potential circulating, biomechanical, and genetic markers were studied. A comprehensive search was conducted in PubMed, Embase, and Cochrane Library in February 2017, following Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines. Study selection, data extraction, and methodological quality assessment were conducted by 2 independent researchers. Plausibility of markers was based on the amount of publications regarding the marker (more than 3), pooled sample size (more than 100), bias risk and statistical significance of the studies. Eighty‐two studies were included, which examined circulating (n=40), biomechanical (n=27), and genetic markers (n=7) and combinations of markers (n=8). Factors with an increased expansion risk included: AAA diameter (9 studies; n=1938; low bias risk), chlamydophila pneumonia (4 studies; n=311; medium bias risk), S‐elastin peptides (3 studies; n=205; medium bias risk), fluorodeoxyglucose uptake (3 studies; n=104; medium bias risk), and intraluminal thrombus size (5 studies; n=758; medium bias risk). Factors with an increased rupture risk rupture included: peak wall stress (9 studies; n=579; medium bias risk) and AAA diameter (8 studies; n=354; medium bias risk). No meta‐analysis was conducted because of clinical and methodological heterogeneity.
Conclusions
We identified 5 potential markers with a prognostic value for AAA growth and 2 for rupture. While interpreting these data, one must realize that conclusions are based on small sample sizes and clinical and methodological heterogeneity. Prospective and methodological consonant studies are strongly urged to further study these potential markers.
Keywords: abdominal aortic aneurysm, biomechanical marker, circulating biomarker, genetic marker, growth, rupture
Subject Categories: Aneurysm, Prognosis
Clinical Perspective
What Is New?
In the management of abdominal aortic aneurysm (AAA) disease, the use of prognostic parameters is still limited to current AAA diameter and growth speed.
In this article, we have systematically reviewed the literature for prognostic markers of aneurysm growth and rupture. In addition to AAA diameter, also chlamydophila pneumonia, S‐elastin peptides, 18F‐fluorodeoxyglucose uptake, and intraluminal thrombus have potential to predict AAA expansion.
Peak wall stress measurement in AAA and S‐elastin peptides appear useful tools for predicting aneurysm rupture, along with AAA diameter.
What Are the Clinical Implications?
Because of heterogeneity in threshold values, the aforementioned markers are not yet ready for clinical use, although intraluminal thrombus and peak wall stress appear closest to clinical application.
The current article provides insight into multiple promising markers that can help predict aneurysm growth and rupture in patients with AAA.
Introduction
The natural course of an abdominal aortic aneurysm (AAA) is a steady increase of the diameter, and eventually, if left untreated, the aneurysm might rupture.1 In most cases of AAA, this pathophysiological process remains asymptomatic until rupture. Such an event can be prevented by surgical AAA repair. The decision to perform surgery is commonly based on 3 characteristics being the: (1) maximum AAA diameter exceeding 5.0 cm in women and 5.5 cm in men; (2) experience of symptoms; or (3) aneurysm growth rate exceeds 1 cm/year.2, 3 The first 2 characteristics are relatively easy to identify by imaging or by questioning the patient. However, AAA growth rate can only be considered retrospectively, because a prognostic value for expansion has not yet been acknowledged.
In the current AAA management, no marker for aneurysm progression or rupture has been implemented as common practice. This might be explained by little existing evidence and lack of experience with prognostic markers. Although numerous potential markers of aneurysm growth and rupture have been examined, a systematic review with a detailed and structured evaluation of markers for AAA expansion and rupture is lacking.
The aim of this systematic review was to identify promising markers of aneurysm expansion and rupture to aid clinicians in AAA management. We searched for retro‐ and prospective observational studies in which the prognostic value of circulating bloodmarkers, biomechanical properties, and genetic variations for AAA expansion or rupture are investigated.
Methods
The data, analytical methods, and study materials will be available from the corresponding author upon reasonable request for purposes of reproducing the results.
Search Strategy
A comprehensive search was conducted following Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) guidelines.4 Separate searches were performed in PubMed, Embase, and Cochrane Library on February 27, 2017 exploring: circulating, biomechanical, and genetic markers. The search strategies can be found in Data S1. Study titles and abstracts were screened, and full texts were examined when a study appeared to fulfill the inclusion criteria. In addition, reference lists were searched to identify potentially missing studies.
Study Selection and Data Extraction
Studies were independently selected by 2 reviewers, and differences in selected studies were discussed. In case of disagreement during the selection process, a third author would make the final decision.
Studies examining markers for a correlation with AAA expansion or rupture were included. No limits were placed on year of publication. Inclusion was limited to studies published in English and full publications. No attempt was performed to search for “gray literature.” Case reports, reviews, animal studies, and studies regarding inflammatory AAA were excluded.
Data extraction was performed independently by 2 reviewers and merged by consensus. Using data extraction forms, the following data were extracted: study population (sex, age), sample size, results reported either as Pearson or Spearman correlations, area under curve, odds ratio, fold increase/decrease, means or medians alongside a measure of variance (eg, range, interquartile range, and SDs), and statistical significance (P values).
Quality Appraisal of Individual Studies
Risk of bias was assessed using guidelines provided by Hayden et al for evaluating the quality of prognosis studies in systematic reviews.5 Accordingly, 6 potential bias items were addressed: (1) study participation; (2) study attrition; (3) prognostic factor measurement; (4) outcome measurement; (5) measurement and account of confounders; and (6) analysis methods. Every item has 3 to 7 questions; per item, an equal amount of points were attributed, resulting in a total percentile score of bias items excluded. We classified studies as low risk (75% or more bias items excluded), intermediate risk (50–75% bias items excluded), or high risk of bias (less than 50% of bias items excluded). Risk of bias is presented and studies are sorted accordingly.
Statistical Analysis
Reported outcomes of studies include correlation coefficients, statistical significance, sample size, and quality appraisal. The principal measure reported for each study was the correlation between the given biomarkers (ie, circulating, biomechanical, or genetic) and a presented outcome change with growth or rupture of AAA. Factors that pose an increased risk of growth or rupture were considered plausible if it was: (1) demonstrated to be a marker in 3 or more publications and these publications demonstrated consistent results; (2) a pooled sample size of more than 100 patients; (3) demostrated as a low risk of bias in at least one third of the studies; and (4) statistically significant in two thirds of the studies.
In consensus, the authors concluded that a meta‐analysis could not be performed because of clinical and methodological heterogeneity, which is consistent with current thought.6 Additionally, a meta‐analysis of correlation coefficients is only considered to be reliable if more than 30 studies are able to be pooled for the same outcome.7 In the present review, a maximum of 9 studies were able to be identified per marker.
Results
Search Results
The searches resulted in 760 studies (Figure), of which 605 were excluded based on title or abstract (no AAA [n=352]; no biomarker of growth or rupture [n=141]; case report, comment or oral presentation only [n=34]; not English [n=37]; not human [n=9]; or other [n=32]). Consequently, 155 articles were retrieved for full‐text evaluation, of which 73 were excluded (no biomarker of growth or rupture [n=54]; review [n=14], no AAA [n=4]; or inflammatory AAA [n=1]). A total of 82 articles were included: 40 studies concerned circulating biomarkers; 27 studies concerned biomechanical markers; 7 studies concerned genetic markers; and 8 studies described a circulating biomarker together with a biomechanical or a genetic marker.
Figure 1.
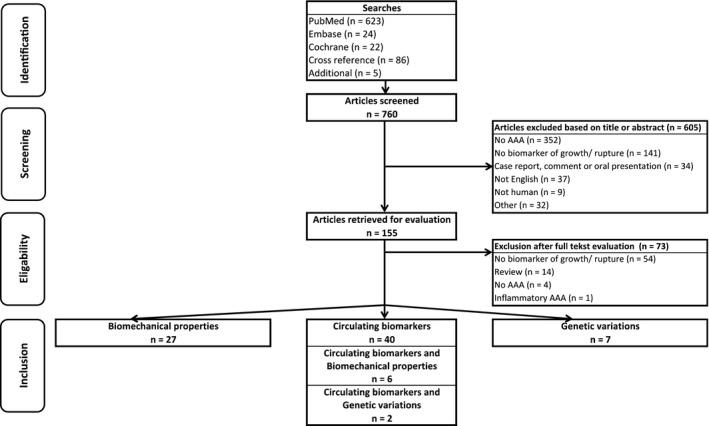
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) diagram showing the literature search. AAA indicates abdominal aortic aneurysm.
Circulating Biomarkers
In 48 studies, 63 circulating biomarkers were investigated (Table 1). Most investigated circulating markers are part of the immune response (18 markers); then the coagulation cascade (14 markers); connective tissue turnover (12 markers); and lipids (9 markers). Remaining categories concerned smoking, kidney function, hormones, and others. The following focuses on markers described in 3 or more publications.
Table 1.
Circulating Biomarkers That Have Been Investigated for an Association With AAA Expansion or Rupture
| Marker | Total Studies (n) | Significant Outcome | Total Patients (n) |
|---|---|---|---|
| Coagulation | |||
| Activated protein C—protein C inhibitor32 | 1 | 0 of 1 studies | 163 |
| Activated prothrombin time (APTT)27 | 1 | 1 of 1 studies | 44 |
| D‐dimer (see Table 4)26, 27, 28 | 3 | 3 of 3 studies | 438 |
| Factor XII40 | 1 | 1 of 1 studies | 48 |
| Fibrinogen (see Table 4)22, 23, 27 | 3 | 3 of 3 studies | 381 |
| Plasmingon activator inhibitor 1 (PAI‐1; see Table 4)13, 27, 28, 35 | 4 | 4 of 4 studies | 304 |
| Plasmin‐antiplasmin‐ complex36 | 1 | 1 of 1 studies | 70 |
| Platelets27 | 1 | 0 of 1 studies | 44 |
| Prothrombin time27 | 1 | 0 of 1 studies | 44 |
| Prothrombin fragment 1+227 | 1 | 1 of 1 studies | 44 |
| Serpine‐132 | 1 | 0 of 1 studies | 163 |
| Tissue plasminogen activator (tPA; see Table 4)13, 27, 28, 35 | 4 | 4 of 4 studies | 304 |
| tPA serpine‐132 | 1 | 0 of 1 studies | 163 |
| Urokinase‐like PA13 | 1 | 0 of 1 studies | 70 |
| Connective tissue | |||
| Aminoterminal propeptide of type III procollagen (see Table 4)9, 10, 12 | 3 | 1 of 3 studies | 190 |
| Carboxyterminal propeptide of type 1 procollagen41 | 1 | 0 of 1 studies | 86 |
| Elastase25 | 1 | 1 of 1 studies | 79 |
| Matrix metalloproteinase 1 (MMP‐1)34 | 1 | 1 of 1 studies | 68 |
| MMP‐232, 34 | 2 | 0 of 2 studies | 231 |
| MMP‐334 | 1 | 0 of 1 studies | 68 |
| MMP‐9 (see Table 4)10, 18, 32, 34 | 4 | 3 of 4 studies | 285 |
| S‐elastin peptides (see Table 4)8, 10, 36, 37, 38 | 5 | 5 of 5 studies | 365 |
| Transforming growth factor beta‐113 | 1 | 0 of 1 studies | 70 |
| Tissue inhibtor metalloproteinase‐1 (TIMP‐1; see Table 4)18, 32, 34 | 3 | 0 of 3 studies | 249 |
| α‐1 antitrypsine10, 18, 39, 40 (see Table 4) | 4 | 2 of 4 studies | 127 |
| α‐1 antitrypsine, Factor XII, D‐dimer, and IgG40 | 1 | 0 of 1 studies | 48 |
| Lipids | |||
| Albumin23 | 1 | 1 of 1 studies | 51 |
| Apolipoprotein A142 | 1 | 1 of 1 studies | 180 |
| Apolipoprotein B42 | 1 | 1 of 1 studies | 180 |
| Cholesterol42, 59 | 2 | 0 of 2 studies | 295 |
| Glycosylphosphatidylinositol phospholipase D43 | 1 | 1 of 1 studies | 133 |
| High‐density lipoprotein21, 59 | 2 | 0 of 2 studies | 295 |
| Low‐density lipoprotein59 | 1 | 0 of 1 studies | 117 |
| Lipoprotein A42 | 1 | 0 of 1 studies | 180 |
| Triglyceride42, 59 | 2 | 2 of 2 studies | 297 |
| Immune response system | |||
| Chlamydophila pneumoniae (see Table 4)11, 12, 13, 14, 15, 16 | 6 | 4 of 6 studies | 465 |
| CRP (see Table 4)17, 18, 19, 20, 21, 22, 23 | 7 | 4 of 7 studies | 1421 |
| Cytomegalovirus44 | 1 | 0 of 1 studies | 119 |
| Helicobacter pylori45 | 1 | 0 of 1 studies | 119 |
| Herpes simplex 116 | 1 | 0 of 1 studies | 119 |
| Interleukin‐1ß30 | 1 | 0 of 1 studies | 90 |
| Interleukin‐230 | 1 | 0 of 1 studies | 90 |
| Interleukin‐6 (see Table 4)21, 30, 31 | 3 | 0 of 3 studies | 734 |
| Interleukin‐830 | 1 | 1 of 1 studies | 90 |
| Interferon gamma95 | 1 | 1 of 1 studies | 50 |
| Leukocytes22 | 1 | 1 of 1 studies | 225 |
| Macrophage inhibiting factor13, 47 | 2 | 1 of 2 studies | 168 |
| Neutrophil gelastinase‐ associated lipocalin48 | 1 | 1 of 1 studies | 40 |
| Osteopontin84 | 1 | 1 of 1 studies | 198 |
| Osteoprotegerin49 | 1 | 1 of 1 studies | 146 |
| Peroxiredoxin50 | 1 | 1 of 1 studies | 80 |
| Tumor necrosis factor‐α21, 30 | 2 | 1 of 2 studies | 268 |
| Tumor necrosis factor–like weak inducer of apoptosis51 | 1 | 1 of 1 studies | 43 |
| Smoking | |||
| Cotinine (see Table 4)13, 24, 25 | 3 | 2 of 3 studies | 596 |
| Smoking25 | 1 | 1 of 1 studies | 79 |
| Kidney function | |||
| Creatinine21, 52 | 2 | 2 of 2 studies | 274 |
| Cystatine C52, 53 | 2 | 2 of 2 studies | 238 |
| Hormones | |||
| Endothelin‐1,254 | 1 | 0 of 1 studies | 65 |
| Endothelin‐121 | 1 | 0 of 1 studies | 178 |
| Insulin‐like growth factor 155 | 1 | 1 of 1 studies | 115 |
| Insulin‐like growth factor 255 | 1 | 0 of 1 studies | 115 |
| Others | |||
| Forced expiratory volume in 1 sec25 | 1 | 0 of 1 studies | 79 |
| Homocysteine (see Table 4)13, 21, 29 | 3 | 1 of 3 studies | 356 |
Markers are categorized by its (patho)physiological system. Per marker, the amount of included studies with significant outcomes are shown, as well as the total number of patients in studies pooled. AAA indicates abdominal aortic aneurysm.
Aminoterminal Propeptide of Type III Procollagen
A significant correlation with expansion was found in 1 study (r=0.24), in which 99 follow‐up patients were included.8 The quality appraisal attributed this study with medium bias risk. In 2 studies (1 medium and 1 high bias risk) no correlation was found in 91 follow‐up patients in total.9, 10 However, Satta et al did reach significance after 2 years of follow‐up.9
Chlamydophila Pneumoniae
In 4 studies, chlamydophila pneumoniae was investigated as a marker for expansion11, 12, 13, 14 and in 2 as a marker for rupture.15, 16 In none of the patients was an inflammatory AAA suspected. All studies on expansion had significant outcomes. Lindholt et al demonstrated in 2 separate studies (total patients n=194) that AAA expansion rate was faster in patients with a higher immunoglobulin A titer. Falkensammer et al found the same results for seropositive versus seronegative patients. In a third separate publication, Lindholt et al demonstrated a significant correlation (r=0.29) with expansion in 70 follow‐up patients. Nyberg et al found no difference in seropositivity between ruptured AAA patients and controls.15 A second study of Nyberg et al on the same cohort demonstrated that AAA patients had no increased risk of rupture as compared with controls when these patients were also seropositive for Helicobacter Pylori, Herpes Simplex, or Cytomegalovirus.16 Overall, the quality of studies was intermediate: 4 had medium risk, 1 had low risk, and 1 had high risk of bias.
Complement Reactive Protein
Complement reactive protein was examined as marker for expansion in 5 studies17, 18, 19, 20, 21 and in 2 as marker for rupture.22, 23 De Haro et al and Wiernicki et al were the only groups to demonstrate significant correlations with expansion. De Haro et al included 260 patients, had a low risk of bias, and measured a strong correlation (r=0.71; P<0.05). According to Norman and Flondell‐Sité et al, who included 723 patients in total and were both qualified as low risk of bias, complement reactive protein levels did not differ between follow‐up patients with high versus low expansion rate. Speelman et al also found no correlation, but included only 18 follow‐up patients and had a medium risk of bias. Domanovits et al measured higher complement reactive protein levels in patients presenting with a ruptured AAA than in patients preceding elective repair (low risk of bias and total n=225). Tambyraja et al, also with a low bias risk, measured 4 times higher complement reactive protein levels in symptomatic patients than in asymptomatic patients (total n=112).
Cotinine
Cotinine was examined in 3 studies as marker for AAA expansion. Wilmink et al,24 whose study was appraised with a medium bias risk, followed 447 AAA patients and found no difference in cotinine levels between follow‐up patients with an expanding AAA (growth >2 mm per year) versus a stable AAA. Lindholt et al demonstrated significant correlations (r=0.23 and r=0.24) in 2 separate studies13, 25 (low and medium bias risks), after including 149 follow‐up patients in total from the same screening program.
D‐Dimer
The association between D‐dimer and expansion was demonstrated by Golledge et al (r=0.39; n=299).26 In 2 studies, an increased D‐dimer level was found in patients suffering from AAA rupture (total n=139).27, 28 All studies had a low risk of bias.
Fibrinogen
Levels of fibrinogen were measured in ruptured AAA patients versus symptomatic and asymptomatic patients. All studies had a low risk of bias. In 2 studies, fibrinogen was lower in ruptured than in nonruptured patients (total n=269),22, 27 whereas Tambyraja et al measured higher levels in 12 symptomatic than in 39 asymptomatic AAA patients.23
Homocysteine
Homocysteine and AAA expansion were investigated in 3 studies, all with a low risk of bias. Halazun et al29 were the only group to describe a significant correlation (r=0.28; n=108). The other 2 studies observed no association between homocysteine and AAA expansion (total n=248).13, 21
Interleukin‐6
Interleukin‐6 and AAA expansion were examined in 3 studies, but none observed a significant association.21, 30, 31 Jones et al found no correlation in 466 follow‐up patients (low bias risk). Flondell‐Sité et al (low bias risk) observed no difference in interleukin‐6 between 178 high‐ versus low‐expansion‐rate AAA patients. Treska et al (high bias risk) included 90 patients and demonstrated no difference between patients who required surgery during follow‐up versus asymptomatic patients.
Matrix Metalloproteinase 9
In 3 studies, circulating matrix metalloproteinase 9 was tested as a marker for expansion. Flondell‐Sité et al, the largest study with the lowest risk of bias, found no correlation with AAA expansion in 163 follow‐up patients.32 In 2 smaller studies (medium bias risk), with 54 patients in total, significant correlations were described (r=0.32 and r=0.33).10, 33 Wilson et al (medium bias risk) demonstrated higher matrix metalloproteinase 9 levels in patients with a ruptured AAA than in patients preceding elective repair.34
Plasminogen Activator Inhibitor 1
Lindholt et al observed a significant, but weak, correlation between plasminogen activator inhibitor 1 (PAI‐1) and AAA expansion (r=0.02; n=70; low bias risk).13 In 3 studies (total n=234; 1 medium risk of bias, 2 low risk), ≈4‐fold higher levels of PAI‐1 were found in patients with a ruptured AAA than in nonruptured AAA patients.27, 28, 35
S‐Elastin Peptides
In 3 studies, S‐elastin peptides (SEP) was investigated as a marker for expansion8, 10, 36 and 2 as a marker for rupture.37, 38 Lindholt et al performed 3 different studies, including 205 follow‐up patients in total, all demonstrating significant correlations with expansion (r=0.51 [medium bias risk], r=0.33 [medium bias risk], and r=0.31 [low bias risk]). In 100 AAA patients with a rupture during follow‐up, SEP had a significantly predictive value (area under curve=0.68; medium bias risk).37 Petersen et al, appraised with a low risk of bias, found a significant difference between 15 patients with a ruptured AAA versus 45 patients preceding elective repair.38 Note that 1 research group, using patients from the same AAA screening cohort, performed 4 of 5 studies. The degree of patient overlap between studies, if any, is not clear.
Tissue Inhibitor Metalloproteinase 1
Speelman et al18 (n=18) and Flondell‐Sité et al32 (n=163) investigated tissue inhibitor metalloproteinase 1 as marker for expansion. Their studies had, respectively, low and medium bias risk. Wilson et al34 (medium bias risk) examined tissue inhibitor metalloproteinase 1 as marker for rupture in 68 patients. None found significant outcomes.
Tissue Plasminogen Activator
Lindholt et al demonstrated a significant correlation between circulating tissue plasminogen activator and AAA expansion (r=0.37; n=70; low bias risk).13 Remarkably, Adam et al and Hobbs et al measured lower levels of tissue plasminogen activator in patients with a ruptured AAA versus nonruptured (total n=139; low and medium risk of bias, respectively),27, 35 whereas Skagius et al observed 1.7‐fold higher levels in 50 ruptured AAA patients than in 45 electively treated AAA (low bias risk).28
α‐1 Antitrypsine
Significant correlations with expansion were found in 2 studies (1 low and 1 medium bias risk; r=0.55 and r=0.42) with 61 follow‐up patients in total,10, 39 whereas 2 studies (1 low and 1 medium bias risk) could not reproduce such significant correlations in 66 follow‐up patients.18, 40 Pulinx et al, however, did reach significance when initial AAA diameter was included in their multivariate model.40
Other included biomarkers that have not been mentioned above are markers in the field of connective tissue,41 lipids,42, 43 the immune system,44, 45, 46, 47, 48, 49, 50, 51 kidney function,52, 53 and hormones54, 55 (see Table 1).
Biomechanical Markers
A total of 33 studies investigated 28 biomechanical AAA properties as a marker for expansion or rupture (Table 2). Markers were categorized as anatomic properties (13 markers), radiographic properties (3 markers), or as vessel wall properties (9 markers). The fourth category contains 3 software‐calculated predictive indices. The following focuses on markers described in 3 or more publications.
Table 2.
Biomechanical Markers That Have Been Investigated for an Association With AAA Expansion or Rupture
| Marker | Total Studies (n) | Significant Outcome | Total Patients (n) |
|---|---|---|---|
| Anatomical properties | |||
| AAA diameter8, 17, 19, 21, 34, 37, 40, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66 | 18 | 15 of 18 studies | 2570 |
| AAA expansion76, 77 | 2 | 1 of 2 studies | 1125 |
| AAA surface area76 | 1 | 0 of 1 studies | 52 |
| AAA volume71 | 1 | 1 of 1 studies | 34 |
| Aortic diameter asymmetry78 | 1 | 1 of 1 studies | 200 |
| Aortic tortuosity78 | 1 | 1 of 1 studies | 200 |
| ILT area57, 76 | 2 | 2 of 2 studies | 469 |
| ILT circumference78 | 1 | 0 of 1 studies | 200 |
| ILT location79 | 1 | 1 of 1 studies | 34 |
| ILT thickness71, 78 | 2 | 1 of 2 studies | 234 |
| ILT volume33, 60, 71 | 3 | 3 of 3 studies | 139 |
| Lumbar 3 vertebral body diameter78 | 1 | 1 of 1 studies | 200 |
| Peak wall stress equivalent diameter75 | 1 | 0 of 1 studies | 243 |
| Predictive indices | |||
| PWRI60, 75 | 2 | 2 of 2 studies | 303 |
| PWRI equivalent diameter60, 75 | 2 | 1 of 2 studies | 303 |
| Rupture potential index61, 62 | 2 | 1 of 2 studies | 66 |
| Radiographical properties | |||
| LaPlace66, a | 1 | 0 of 1 studies | 48 |
| Medium filter texture parameter kurtosis67 | 1 | 1 of 1 studies | 40 |
| 18F‐FDG uptake67, 68, 69, 70, b | 4 | 4 of 4 studies | 119 |
| Vessel wall properties | |||
| Stiffness (ß)56, 65 | 2 | 0 of 2 studies | 108 |
| Minimal strenght61 | 1 | 0 of 1 studies | 53 |
| Mean wall stress18, 74 | 2 | 1 of 2 studies | 99 |
| Peak wall stress 60, 62, 63, 64, 66, 72, 73, 74, 75 | 9 | 7 of 9 studies | 579 |
| Pressure strain elastic modules (Ep)56, 65 | 2 | 0 of 2 studies | 108 |
| Von Mises strain61, c | 1 | 1 of 1 studies | 53 |
| Von Mises stress61, c | 1 | 1 of 1 studies | 53 |
| Wall displacement61 | 1 | 1 of 1 studies | 53 |
| Wall strength62 | 1 | 1 of 1 studies | 13 |
Markers are categorized by different properties, which can be measured after radiographic scanning. The total amount of studies and significant outcomes are presented as well as the total number of patients in studies pooled. AAA indicates abdominal aortic aneurysm; 18F‐FDG, Fluorodeoxyglucose; ILT, Intraluminal thrombus; PWRI, Peak wall rupture index.
LaPlace=law of LaPlace (pressure=surface tension/radius).
18F‐FDG uptake as measured by positron emission tomography.
Von Mises strain and stress are calculations of tensile stress according to Maximum Distortion Energy Theory of Failure.
AAA Diameter
In 9 studies, AAA diameter was described as a marker for expansion8, 17, 19, 21, 40, 56, 57, 58, 59 and in 9 as a marker for rupture.34, 37, 60, 61, 62, 63, 64, 65, 66 Overall, the data are reliable because 2570 patients in total were included and 8 studies were appraised with low bias risk, 7 with medium risk, and only 3 with high risk. In 7 studies, significant correlations with expansion were demonstrated in 958 patients in total (r=0.30–0.83),8, 21, 40, 56, 57, 58 and Norman et al measured faster growth in patients with a large (≥4 cm; n=112) versus small AAA (3–4 cm; n=433).19 In 6 studies, with a total of 552 patients, significant outcomes were demonstrated for AAA diameter as a marker for rupture. In 5 studies, larger diameters were measured in ruptured (and symptomatic) AAA when compared with asymptomatic patients,34, 60, 61, 64, 65 and 1 study demonstrated aneurysm diameter as a prognostic marker for rupture (area under curve=0.67).37 In 3 studies, of which 2 were with high bias risk, no difference was found in diameter between ruptured AAA patients versus patients preceding elective repair (total n=80).62, 63, 66
Fluorodeoxyglucose Uptake
Maximum fluorodeoxyglucose (18F‐FDG) uptake after positron emission tomography scanning was studied as a marker for expansion in 3 studies67, 68, 69 and in 1 study as a marker for rupture.70 All 3 studies demonstrated significant inverse correlations with aneurysm expansion (r=−0.50 [medium bias risk], r=−0.38 [low bias risk], and r=−0.32 [medium bias risk]; total n=104). Reeps et al, however, found higher uptake in symptomatic versus asymptomatic AAA patients (n=15; medium bias risk).
Intraluminal Thrombus Volume
In 3 studies, intraluminal thrombus (ILT) volume was focused on. In 2 studies as a marker for expansion33, 71 and in 1 as a marker for rupture,60 all studies had medium risk of bias. Speelman et al measured significantly higher expansion rates in patients with a large ILT volume (≥32% of the total aneurysm sac) versus a small ILT volume (total n=30). Kontopodis et al found a significant correlation (r=0.60) with expansion in 34 follow‐up patients. Erhart et al measured larger ILT volumes in ruptured AAA than in follow‐up patients (total n=75).
Peak Wall Stress
Aortic peak wall stress (PWS) was investigated as a marker for AAA rupture in 9 studies.60, 62, 63, 64, 66, 72, 73, 74, 75 In 7 studies, significantly higher PWS (ranging 1.29–1.66‐fold higher) was found in ruptured (and symptomatic) AAA patients than in asymptomatic AAA patients (2 low risk, 4 medium risk, and 1 high risk of bias; total n=536). According to Truijers et al, PWS was higher in 10 ruptured AAA than in 10 diameter‐matched asymptomatic patients. In 2 studies, no difference was found between ruptured and electively treated AAA. However, the latter 2 included only 43 patients in total and both had high risk of bias.
Other biomechanical markers that have not been mentioned above, but are included, concern anatomical properties (see Table 2).76, 77, 78, 79
Genetic Variations
In 9 studies, 20 genetic markers were elaborated on (Table 3). None of the following markers were described in more than 1 study. These genetic markers are therefore not evaluated as extensively as circulating and biomechanical markers in this review.
Table 3.
Genetic Variations That Have Been Investigated for an Association With AAA Expansion or Rupture
| Marker | Total Studies (n) | Significant Outcome | Total Patients (n) |
|---|---|---|---|
| APOE gene81 | 1 | 1 of 1 studies | 57 |
| IL‐6 gene31 | 1 | 0 of 1 studies | 466 |
| Cystatin C gene83 | 1 | 0 of 2 studies | 412 |
| CCR5 gene80 | 1 | 1 of 1 studies | 70 |
| OPN gene84 | 1 | 0 of 1 studies | 198 |
| Chromosome 9p2185 | 1 | 0 of 1 studies | 741 |
| Haptoglobin 2‐120 | 1 | 1 of 1 studies | 83 |
| LRP1 gene82 | 1 | 1 of 1 studies | 141 |
| MMP‐9 p‐2502 gene82 | 1 | 1 of 1 studies | 141 |
| MTHFR gene82 | 1 | 1 of 1 studies | 141 |
| miR‐125a‐5p86 | 1 | 1 of 1 studies | 169 |
| miR‐136‐5p86 | 1 | 0 of 1 studies | 169 |
| miR‐195‐5p86 | 1 | 1 of 1 studies | 169 |
| miR‐221‐3p86 | 1 | 1 of 1 studies | 169 |
| miR‐223‐3p86 | 1 | 1 of 1 studies | 169 |
| miR‐30a‐5p86 | 1 | 0 of 1 studies | 169 |
| miR‐32686 | 1 | 1 of 1 studies | 169 |
| miR‐335‐p86 | 1 | 1 of 1 studies | 169 |
| miR‐42186 | 1 | 1 of 1 studies | 169 |
| miR‐99a‐5p86 | 1 | 1 of 1 studies | 169 |
The total amount of studies and significant outcomes are presented as well as the total number of patients in studies pooled. AAA indicates abdominal aortic aneurysm.
CCR5 gene was the only gene examined as a marker for rupture. Ghilardi et al demonstrated a higher percentage of CCR5 gene Δ32 deletion mutation in ruptured AAA patients (n=21) than in electively treated AAA patients (n=49; 48% versus 18%, respectively).80
The following markers were all investigated in AAA follow‐up patients and were associated with the aneurysm growth rate. Gerdes et al identified that APOE mutations are associated with higher growth rates in 57 patients.81 Wiernicki et al measured higher growth rates in 41 patients with a Haptoglobin 2‐1 phenotype than in 13 with a Haptoglobin 1‐1 phenotype.20 Duellman et al included 141 patients and demonstrated that mutations in the following genes are associated with a growth speed of 3.25 mm per year or more: LRP1 (odds ratio, 5.0), MMP9 p‐2502 (odds ratio, 2.2), and MTHFR (odds ratio, 3.0).82 No such differences were measured with the following genes: IL‐6 (n=466)31; Cystatin C (n=412)83; OPN (n=198)84; and 9p21 (n=741).85 Of 20 investigated genetic markers, 10 were investigated by Wanhainen et al86 in 169 follow‐up patients (all concerning microRNA as marker for expansion), of which 8 markers demonstrated significant differences between slow and fast growing AAA.
Discussion
Numerous markers have been investigated as a predictive factor for AAA expansion and rupture. All markers described in 3 or more studies were described in more detail and are summarized in Table 4. Thus, we focused on 14 markers, of which 5 were investigated as a marker for expansion, 1 as a marker for rupture, and 8 as a marker for both. Markers were qualified as high potential based on sample size, quality appraisal of the study, and significant outcomes. The highest potential as a prognostic marker for AAA expansion are in descending order: AAA diameter, chlamydophila pneumoniae; SEP; and 18F‐FDG uptake. Factors with high potential as marker for aneurysm rupture are in descending order: PWS, AAA diameter, and PAI‐1. The following 2 markers were described in only 2 studies, but had remarkable results and are therefore separately mentioned: ILT as a marker for expansion and S‐elastin peptides as a marker for rupture. Little research has been done on genetic markers for rupture and growth, given that this is a relatively new area of research. We therefore evaluated none of the genetic markers in detail.
Table 4.
All Markers for AAA Expansion or Rupture That Have Been Described in 3 or More Studies Have Been Evaluated in More Detail
| Marker Subject | Reference | Risk of Bias | Measurement | Study Group | Control Group | N (Total) | Correlation | Fold Change | P Value |
|---|---|---|---|---|---|---|---|---|---|
| Cicrculating markers | |||||||||
| Aminoterminal propeptide of type III procollagen (PIIINP) | |||||||||
| Expansion | Lindholt et al (2001)8 | Medium | Pearson | Follow‐up | … | 99 | 0.24 | … | Significant |
| Expansion | Lindholt et al (2000)10 | Medium | Spearman | Follow‐up | … | 36 | No correlation | … | 0.180 |
| Expansion | Satta et al (1997)9 | High | Pearson | Follow‐up | … | 55 | 0.15 | … | 0.260 |
| Chlamydophila pneumonia | |||||||||
| Expansion | Lindholt et al (2003)13 | Low | Spearman | Follow‐up | … | 70 | 0.29 | … | 0.006 |
| Expansion | Lindholt et al (1999)11 | Medium | Fold change | Follow‐up: IgA titre ≥20 | Follow‐up: IgA titer <20 | 139 | … | 1.48 | 0.003 |
| Expansion | Lindholt et al (2001)12 | Medium | Fold change | Follow‐up: IgA titer ≥64 | Follow‐up: IgA titer <64 | 55 | … | 1.69 | <0.050 |
| Expansion | Falkensammer et al (2007)14 | High | Fold change | Follow‐up: seropositive | Follow‐up: seronegative | 47 | … | 1.67 | 0.046 |
| Rupture | Nyberg et al (2007)15 | Medium | Fold change | Rupture | Controls | 77 | … | 1.01 | 0.397 |
| Rupture | Nyberg et al (2008)16 | Medium | Fold change | Rupture | Controls | 77 | … | NA | Ns |
| CRP | |||||||||
| Expansion | De Haro et al (2012)17 | Low | Spearman | Follow‐up | … | 260 | 0.71 | … | <0.050 |
| Expansion | Norman et al (2004)19 | Low | Fold change | Follow‐up: expansion ≥3 mm/year | Follow‐up: expansion <3 mm/year | 545 | … | NA | Ns |
| Expansion | Flondell‐Sité et al (2009)21 | Low | Fold change | Follow‐up: expansion ≥2.5 mm/year | Follow‐up: expansion <2.5 mm/year | 178 | … | 1.07 | 0.721 |
| Expansion | Wiernicki et al (2010)20 | Medium | Spearman | Follow‐up | 83 | … | 0.32 | 0.003 | |
| Expansion | Speelman et al (2010)18 | Medium | Partial correlation | Follow‐up | … | 18 | 0.06 | … | 0.720 |
| Rupture | Domanovits et al (2002)22 | Low | Fold change | Rupture | Elective | 225 | … | 4.80 | <0.050 |
| Rupture | Tambyraja et al (2007)23 | Low | Fold change | Symptomatic | Asymptomatic | 112 | … | 4.40 | <0.001 |
| Cotinine | |||||||||
| Expansion | Lindholt et al (2003)13 | Low | Spearman | Follow‐up | … | 70 | 0.23 | … | 0.038 |
| Expansion | Lindholt et al (2003)25 | Medium | Spearman | Follow‐up | … | 79 | 0.24 | … | 0.040 |
| Expansion | Wilmink et al (1999)24 | Medium | Fold change | Follow‐up: expansion ≥2 mm/year | Follow‐up: expansion <2 mm/year | 447 | … | 1.00 | Ns |
| D‐dimer | |||||||||
| Expansion | Golledge et al (2011)26 | Low | Spearman | Follow‐up | … | 299 | 0.39 | … | <0.001 |
| Rupture | Adam et al (2002)27 | Low | Fold change | Rupture | Symptomatic | 44 | … | 2.52 | 0.005 |
| Rupture | Skagius et al (2008)28 | Low | Fold change | Rupture | Elective | 95 | … | 4.53 | <0.001 |
| Fibrinogen | |||||||||
| Rupture | Adam et al (2002)27 | Low | Fold change | Rupture | Symptomatic | 44 | … | 0.53 | 0.033 |
| Rupture | Domanovits et al (2002)22 | Low | Fold change | Rupture | Asymptomatic | 225 | … | 0.94 | 0.049 |
| Rupture | Tambyraja et al (2007)23 | Low | Fold change | Symptomatic | Asymptomatic | 112 | … | 1.28 | <0.001 |
| Homocysteine | |||||||||
| Expansion | Lindholt et al (2003)13 | Low | Spearman | Follow‐up | … | 70 | 0.06 | … | 0.535 |
| Expansion | Halazun et al (2007)29 | Low | Spearman | Follow‐up | … | 108 | 0.28 | … | 0.003 |
| Expansion | Flondell‐Sité et al (2009)21 | Low | Fold change | Follow‐up: expansion ≥2.5 mm/year | Follow‐up: expansion <2.5 mm/year | 178 | … | 1.00 | 0.940 |
| IL‐6 | |||||||||
| Expansion | Jones et al (2001)31 | Low | Spearman | Follow‐up | … | 466 | No correlation | … | Ns |
| Expansion | Flondell‐Sité et al (2009)21 | Low | Fold change | Follow‐up: expansion ≥2.5 mm/year | Follow‐up: expansion <2.5 mm/year | 178 | … | 2.29 | 0.820 |
| Expansion | Treska et al (2000)30 | High | Fold change | Surgery during follow‐up | Asymptomatic | 90 | … | 2.19 | Ns |
| MMP‐9 | |||||||||
| Expansion | Flondell‐Sité et al (2010)32 | Low | Spearman | Follow‐up | … | 163 | No correlation | … | Ns |
| Expansion | Lindholt et al (2000)10 | Medium | Spearman | Follow‐up | … | 36 | 0.33 | … | 0.010 |
| Expansion | Speelman et al (2010)18 | Medium | Partial correlation | Follow‐up | … | 18 | 0.32 | … | <0.050 |
| Rupture | Wilson et al (2008)34 | Medium | Fold change | Rupture | Elective | 68 | … | 3.37 | 0.006 |
| Plasminogen activator inhibitor 1 | |||||||||
| Expansion | Lindholt et al (2003)13 | Low | Spearman | Follow‐up | … | 70 | 0.02 | … | 0.015 |
| Rupture | Adam et al (2002)27 | Low | Fold change | Rupture | Symptomatic | 44 | … | 4.92 | 0.023 |
| Rupture | Skagius et al (2008)28 | Low | Fold change | Rupture | Elective | 95 | … | 4.33 | 0.002 |
| Rupture | Hobbs et al (2007)35 | Medium | Fold change | Rupture | Elective | 95 | … | 3.73 | 0.001 |
| S‐elastin peptides | |||||||||
| Expansion | Lindholt et al (2001)36 | Low | Pearson | Follow‐up | … | 70 | 0.31 | … | 0.050 |
| Expansion | Lindholt et al (2001)8 | Medium | Pearson | Follow‐up | … | 99 | 0.33 | … | Significant |
| Expansion | Lindholt et al (2000)10 | Medium | Spearman | Follow‐up | … | 36 | 0.51 | … | 0.010 |
| Rupture | Petersen et al (2001)38 | Low | Fold change | Rupture | Elective | 60 | … | 0.80 | 0.001 |
| Rupture | Lindholt et al (2001)37 | Medium | AUC met 95% CI | Rupture | … | 100 | 0.68 | … | Significant |
| TIMP‐1 | |||||||||
| Expansion | Flondell‐Sité et al (2010)32 | Low | Spearman | Follow‐up | … | 163 | No correlation | … | Ns |
| Expansion | Speelman et al (2010)18 | Medium | Partial correlation | Follow‐up | … | 18 | 0.12 | … | 0.510 |
| Rupture | Wilson et al (2008)34 | Medium | Fold change | Rupture | Elective | 68 | … | 0.50 | 0.456 |
| Tissue plasminogen activator (tPA) | |||||||||
| Expansion | Lindholt et al (2003)13 | Low | Spearman | Follow‐up | … | 70 | 0.37 | … | 0.002 |
| Rupture | Adam et al (2002)27 | Low | Fold change | Rupture | Symptomatic | 44 | … | 0.16 | 0.023 |
| Rupture | Skagius et al (2008)28 | Low | Fold change | Rupture | Elective | 95 | … | 1.71 | <0.001 |
| Rupture | Hobbs et al (2007)35 | Medium | Fold change | Rupture | Elective | 95 | … | 0.22 | 0.036 |
| α‐1 antitrypsine | |||||||||
| Expansion | Vega de Céniga et al (2009)39 | Low | Spearman | Follow‐up | … | 25 | 0.55 | … | 0.004 |
| Expansion | Pulinx et al (2011)40 | Low | AUC met 95% CI | Follow‐up | … | 48 | No correlation | … | Ns |
| Expansion | Lindholt et al (2000)10 | Medium | Spearman | Follow‐up | … | 36 | 0.42 | … | 0.050 |
| Expansion | Speelman et al (2010)18 | Medium | Partial correlation | Follow‐up | … | 18 | 0.00 | … | 0.990 |
| Biomechanical markers | |||||||||
| AAA diameter | |||||||||
| Expansion | De Haro et al (2012)17 | Low | Spearman | Follow‐up | … | 435 | 0.31 | … | >0.050 |
| Expansion | Norman et al (2004)19 | Low | OR | Follow‐up ≥4 cm | Follow‐up <4 cm | 545 | … | 7.20 | 0.050 |
| Expansion | Tong et al (2015)58 | Low | Pearson | Elective and Rupture | … | 33 | 0.70 | … | 0.010 |
| Expansion | Flondell‐Sité et al (2010)21 | Low | Pearson | Follow‐up | … | 178 | 0.39 | … | 0.001 |
| Expansion | Pulinx et al (2011)40 | Low | AUC met 95% CI | Follow‐up | … | 48 | 0.83 | … | 0.001 |
| Expansion | Behr‐Rasmussen et al (2014)57 | Low | Pearson | Follow‐up | … | 416 | 0.30 | … | 0.001 |
| Expansion | Lindholt et al (2001)8 | Medium | Spearman | Follow‐up | … | 124 | 0.30 | … | 0.010 |
| Expansion | Lindholt et al (2001)8 | Medium | Pearson | Follow‐up | … | 99 | 0.48 | … | 0.000 |
| Expansion | Wilson et al (1999)56 | High | Spearman | Follow‐up | … | 60 | 0.60 | … | <0.050 |
| Rupture | Fillinger et al (2003)64 | Low | Fold change | Rupture and symptomatic | Elective | 61 | … | 1.03 | 0.000 |
| Rupture | Fillinger et al (2002)66 | Low | Fold change | Rupture | Elective | 40 | … | 1.13 | 0.100 |
| Rupture | Lindholt et al (2001)37 | Medium | ROC curve | Rupture | … | 100 | 0.67 | … | 0.011 |
| Rupture | Wilson et al (2003)65 | Medium | Fold change | Rupture | Follow‐up | 210 | … | 1.12 | 0.001 |
| Rupture | Maier et al (2010)61 | Medium | Fold change | Rupture and symptomatic | Elective | 53 | … | 1.33 | 0.006 |
| Rupture | Erhart et al (2015)60 | Medium | Fold change | Rupture | Follow‐up | 60 | … | 1.42 | <0.001 |
| Rupture | Wilson et al (2008)34 | Medium | Fold change | Rupture | Elective | 68 | … | 1.67 | <0.001 |
| Rupture | Venkatasubramaniam et al (2004)63 | High | Fold change | Rupture | Elective | 27 | … | 1.11 | 0.197 |
| Rupture | Vande Geest et al (2006)62 | High | Fold change | Rupture | Elective | 13 | … | 1.11 | 0.260 |
| Fluorodeoxyglucose (18F‐FDG) | |||||||||
| Expansion | Kotze et al (2014)67 | Low | Spearman | Follow‐up | … | 40 | −0.38 | … | 0.015 |
| Expansion | Morel et al (2015)69 | Medium | Spearman | Follow‐up | … | 39 | −0.32 | … | 0.049 |
| Expansion | Kotze et al (2011)68 | Medium | Spearman | Follow‐up | … | 25 | −0.50 | … | 0.011 |
| Rupture | Reeps et al (2008)70 | Medium | Fold change | Symptomatic | Elective | 15 | … | 2.14 | <0.001 |
| ILT volume | |||||||||
| Expansion | Speelman et al (2010)33 | Medium | Fold change | Follow‐up: ILT volume ≥32% | Follow‐up: ILT volume <32% | 30 | … | NA | <0.010 |
| Expansion | Kontopodis et al (2014)71 | Medium | Spearman | Follow‐up | … | 34 | 0.60 | … | 0.001 |
| Rupture | Erhart et al (2015)60 | Medium | Fold change | Rupture | Follow‐up | 75 | … | 2.00 | 0.015 |
| Peak wall stress (PWS) | |||||||||
| Rupture | Fillinger et al (2003)64 | Low | Fold change | Rupture and symptomatic | Elective | 61 | … | 1.38 | <0.001 |
| Rupture | Fillinger et al (2002)66 | Low | Fold change | Rupture | Elective | 40 | … | 1.29 | 0.030 |
| Rupture | Gasser et al (2014)75 | Medium | Fold change | Rupture | Follow‐up | 243 | … | 1.62 | <0.001 |
| Rupture | Erhart et al (2015)60 | Medium | Fold change | Rupture | Follow‐up | 75 | … | 1.57 | <0.001 |
| Rupture | Truijers et al (2007)72 | Medium | Fold change | Rupture | Follow‐up | 20 | … | 1.30 | 0.040 |
| Rupture | Heng et al (2008)73 | Medium | Fold change | Rupture | Elective | 70 | … | 1.66 | 0.008 |
| Rupture | Venkatasubramaniam et al (2004)63 | High | Fold change | Rupture | Elective | 27 | … | 1.65 | 0.004 |
| Rupture | Vande Geest et al (2006)62 | High | Fold change | Rupture | Elective | 13 | … | 1.08 | 0.620 |
| Rupture | Vande Geest et al (2008)74 | High | Fold change | Rupture | Elective | 30 | … | 1.09 | 0.550 |
Presented are: the subject of the marker (on which aspect the marker was investigated: AAA expansion or rupture); first author and date of publication of the reference; the risk of bias; statistical method of measurement; the moment of data retrieval (during conservative follow‐up of maximum aortic diameter, at time of presentation with symptomatic AAA or AAA rupture), and, if applicable, main clinical characteristic of the study and control groups (varying per study); the total sample size (cases and controls pooled); the correlation coefficient (negative correlation: −1 to 0; and positive correlation: 0 to 1) or the fold change (decrease: 0–1; and increase: above 1) of study group vs control group; and P values. Note that significant (P<0.05) outcomes are indicated by an asteriks. AAA indicates abdominal aortic aneurysm; AUC, area under the curve; CI, confidence interval; CRP, complement reactive protein; IL‐6, interleukin‐6; ILT, intraluminal thrombus; MMP‐9, matrix metalloproteinase 9; NA, not applicable; Ns, not significant; OR, odds ratio; ROC, receiver operating characteristic; TIMP‐1, tissue inhibitor of matrix metalloproteinase 1.
AAA diameter is broadly accepted as a predictive factor for both aneurysm growth and rupture and is thus implemented in important AAA follow‐up guidelines.2, 3 Our systematic review confirmed the strong prognostic value for expansion given that 8 of 9 studies had significant outcomes, with mainly low bias risks and low P values in a total of 1503 patients. However, correlation coefficients do have a relatively broad range, with values varying from r=0.30 to r=0.83. Overall, these studies demonstrate that large aneurysms grow faster than small AAA do.
Chlamydophila pneumoniae was already identified as a causative factor for inflammation and atherosclerosis of the aorta.87 The bacterial infection induces degenerative processes in the aortic wall, which might explain the strong correlation of antibodies against chlamydophila pneumoniae with AAA expansion. All 4 studies, with mainly medium bias risks, had significant outcomes and consistent results, of which 3 had very low P values. Therefore, it seems to be a reliable marker for AAA expansion in case of seropositivity.
SEP are derived from the enzymatic degradation of insoluble elastic polymers in the vessel wall by matrix metalloproteinase.88 In all studies, this marker was significantly correlated with AAA expansion and bias risks were medium. However, 1 group performed 4 of 5 studies using patients from the same AAA screening cohort. Therefore, other groups should first reproduce these data before SEP can be applied as a marker for expansion.
Metabolic activity in the aneurysm wall can be measured by positron emission tomography. Locations of high 18F‐FDG uptake in the aneurysm wall were demonstrated to accumulate MMP and other factors of aortic deterioration.89 It therefore seems contradictive that an inverse correlation was found between 18F‐FDG uptake and expansion in all 3 studies. The current explanation is that an inflammatory period precedes a phase of rapid growth and is then followed by a period of stasis with low metabolic activity.67, 68, 69, 70 However, this phenomenon is clearly not fully explained yet. Overall, 18F‐FDG uptake studies were appraised with medium bias risks and had consistent results with relatively low P values. Therefore, it seems a reliable marker for AAA expansion.
An ILT is the source of many pro‐proteolytic processes that stimulate aortic wall degradation.90 We designated this marker as promising because of a clear association of ILT volume with expansion, even though relatively small patient numbers were included in only 2 studies. However, Kontopodis, Nguyen, and Behr‐Rahsmussen et al also demonstrated the ILT to be correlated with AAA expansion in 694 follow‐up patients in total (ie, ILT thickness, signal intensity, and surface area, respectively).57, 71, 91 In total, 5 studies have elaborated on ILT size as a marker for expansion in 758 patients, with, on average, a medium bias risk. These data plead for the ILT size as a promising prognostic growth marker. However, there have been several studies demonstrating a correlation between ILT presence and AAA diameter.58, 71 The presented associations between ILT and AAA expansion might be the result of multicolinearity attributed to the strong correlation between AAA diameter and its growth speed. Therefore, before clinical implementation, more homogenous studies must be produced. In those studies, AAA diameter should be corrected for as a confounding factor before ILT can be considered a reliable growth marker.
A potential marker for rupture is PWS. To determine stress on the aneurysm wall, a technique called finite element analysis is used. This is a numerical method to approximate the forces that are applied on the aortic wall. Because aneurysms are not symmetrical dilations, pressure in the aneurysm sac is heterogeneously divided. Finite element analysis enables software programs to calculate the PWS on the aneurysm wall.63 In 7 of 9 studies, PWS retrospectively differentiated between ruptured and nonruptured AAA, but none investigated it as a prognostic value. In 2 studies, no significant differences were found, but both had high bias risks and a total patient number of only 43. Given that significant differences were found in 536 patients, we suggest that PWS has a high potential to contribute in AAA management.
AAA diameter has long since also been acknowledged as a risk factor for aneurysm rupture and is used as an indicator for elective repair surgery.2, 3 Our results are in line with this common use, although 3 of 9 studies found no differences between ruptured AAA versus patients preceding elective repair. It must be noted that in those 3 studies, aneurysm diameters of the elective repair groups were all larger than current guidelines apply (6.8±1.5, 6.1±0.5, and 6.1±0.2 cm).
Another marker for rupture with promising results is PAI‐1, a known marker for coronary heart disease that plays an essential role in fibrinolysis.92 Its levels were ≈4 times higher in 102 patients with a ruptured AAA than in asymptomatic patients. However, given that the massive retroperitoneal hematoma and blood clotting could be the cause of PAI‐1 activation, its use a prospective marker for rupture must be reconsidered. Activation of this pathway should first be fully elucidated before it is investigated as a marker for AAA rupture in a prospective trial.
SEP have been investigated as a marker for rupture by 2 separate groups. Promising results were demonstrated given that both groups found highly significant associations. However, only 2 groups have reported on this marker yet in a total of 160 patients. Before it is implemented in a clinical setting, it should be studied more extensively.
Genetic variations and microRNA are relatively new markers for AAA expansion and rupture. Therefore, little is known about its potential as prognostic tools, when compared with circulating and biomechanical markers. Gene mutations in the FBN1 93 and COL3A1 94 genes, responsible for Marfan's disease and Ehlers‐Danlos vascular type disease, respectively, are perhaps the best‐known genetic disorders leading to aortic aneurysms. However, despite the broad amount of studies describing these 2 important genetic mutations, no studies about FBN1 or COL3A1 met our inclusion criteria. This might be explained by the fact that these disorders commonly cause thoracic and thoracoabdominal aortic aneurysms, and also that growth rate and rupture are often totally unpredictable in these cases.
One major limitation of this review is the inability to pool data attributed to high clinical and methodological heterogeneity. Also, we considered biomarkers in the evidence that demonstrated a statistically significant association with an outcome (AAA rupture or growth); however, we recognize that this may have severe limitations given that this choice is subject to type II errors, particularly in the case of studies with small sample sizes. Furthermore, the potential markers provided such heterogenic threshold values that direct clinical implementation is not possible based on the current data. More specifically, prospective and methodological consonant research is necessary for the promising markers that we have identified, in which threshold values for follow‐up and surgical intervention must be determined.
This review has identified several circulating and biomechanical markers with potential value for the prognosis of AAA expansion and rupture. As possible markers for expansion, we suggest the use of AAA diameter, chlamydophila pneumonia in case of seropositivity, SEP, inverse fluorodeoxyglucose uptake, and ILT size. Markers with the best prognostic value for rupture are PWS and AAA diameter. Prospective trials are now required to determine threshold values for the clinical implementation of these markers. In conclusion, there are several potential markers for AAA expansion and rupture, which could contribute to better decision making in the management of AAA.
Disclosures
None.
Supporting information
Data S1. MeSH Terms.
(J Am Heart Assoc. 2018;7:e007791 DOI: 10.1161/JAHA.117.007791.)
References
- 1. Kuivaniemi H, Ryer EJ, Elmore JR, Tromp G. Understanding the pathogenesis of abdominal aortic aneurysms. Expert Rev Cardiovasc Ther. 2015;13:975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chaikof EL, Brewster DC, Dalman RL, Makaroun MS, Illig KA, Sicard GA, Timaran CH, Upchurch GR, Veith FJ. SVS practice guidelines for the care of patients with an abdominal aortic aneurysm: executive summary. J Vasc Surg. 2009;50:880–896. [DOI] [PubMed] [Google Scholar]
- 3. Moll FL, Powell JT, Fraedrich G, Verzini F, Haulon S, Waltham M, Van Herwaarden JA, Holt PJE, Van Keulen JW, Rantner B, Schlosser FJV, Setacci F, Ricco JB. Management of abdominal aortic aneurysms clinical practice guidelines of the European society for vascular surgery. Eur J Vasc Endovasc Surg. 2011;41(suppl 1):S1–S58. [DOI] [PubMed] [Google Scholar]
- 4. Moher D, Liberati A, Tetzlaff J, Altman D. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. Int J Surg. 2010;8:336–341. [DOI] [PubMed] [Google Scholar]
- 5. Hayden JA, Cote P, Bombardier C. Annals of internal medicine academia and clinic evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med. 2006;144:427–438. [DOI] [PubMed] [Google Scholar]
- 6. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Field AP. Meta‐analysis of correlation coefficients: a Monte Carlo comparison of fixed‐ and random‐effects methods. Psychol Methods. 2001;6:161–180. [DOI] [PubMed] [Google Scholar]
- 8. Lindholt JS, Heickendorff L, Vammen S, Fasting H, Henneberg EW. Five‐year results of elastin and collagen markers as predictive tools in the management of small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2001;21:235–240. [DOI] [PubMed] [Google Scholar]
- 9. Satta J, Haukipuro K, Kairaluoma MI, Juvonen T. Aminoterminal propeptide of type III procollagen in the follow‐up of patients with abdominal aortic aneurysms. J Vasc Surg. 1997;25:909–915. [DOI] [PubMed] [Google Scholar]
- 10. Lindholt J, Vammen S, Fasting H, Henneberg E, Heickendorff L. The plasma level of matrix metalloproteinase 9 may predict the natural history of small abdominal aortic aneurysms. A preliminary study. Eur J Vasc Endovasc Surg. 2000;20:281–285. [DOI] [PubMed] [Google Scholar]
- 11. Lindholt JS, Juul S, Vammen S, Lind I, Fasting H, Henneberg EW. Immunoglobulin A antibodies against Chlamydia pneumoniae are associated with expansion of abdominal aortic aneurysm. Br J Surg. 1999;86:634–638. [DOI] [PubMed] [Google Scholar]
- 12. Lindholt JS, Ashton HA, Scott RAP. Indicators of infection with Chlamydia pneumoniae are associated with expansion of abdominal aortic aneurysms. J Vasc Surg. 2001;34:212–215. [DOI] [PubMed] [Google Scholar]
- 13. Lindholt JS, Jørgensen B, Shi GP, Henneberg EW. Relationships between activators and inhibitors of plasminogen, and the progression of small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2003;25:546–551. [DOI] [PubMed] [Google Scholar]
- 14. Falkensammer B, Duftner C, Seiler R, Pavlic M, Walder G, Wilflingseder D, Stoiber H, Klein‐Weigel P, Dierich M, Fraedrich G, Würzner R, Schirmer M. Lack of microbial DNA in tissue specimens of patients with abdominal aortic aneurysms and positive Chlamydiales serology. Eur J Clin Microbiol Infect Dis. 2007;26:141–145. [DOI] [PubMed] [Google Scholar]
- 15. Nyberg A, Skagius E, Nilsson I, Ljungh A, Henriksson AE. Lack of association between chlamydophila pneumoniae seropositivity and abdominal aortic aneurysm. Vasc Endovascular Surg. 2007;41:246–248. [DOI] [PubMed] [Google Scholar]
- 16. Nyberg A, Skagius E, Englund E, Nilsson I, Ljungh Å, Henriksson AE. Abdominal aortic aneurysm and the impact of infectious burden. Eur J Vasc Endovasc Surg. 2008;36:292–296. [DOI] [PubMed] [Google Scholar]
- 17. De Haro J, Acin F, Bleda S, Varela C, Medina FJ, Esparza L. Prediction of asymptomatic abdominal aortic aneurysm expansion by means of rate of variation of C‐reactive protein plasma levels. J Vasc Surg. 2012;56:45–52. [DOI] [PubMed] [Google Scholar]
- 18. Speelman L, Hellenthal FA, Pulinx B, Bosboom EMH, Breeuwer M, Van Sambeek MR, Van de Vosse FN, Jacobs MJ, Wodzig WKWH, Schurink GWH. The influence of wall stress on AAA growth and biomarkers. Eur J Vasc Endovasc Surg. 2010;39:410–416. [DOI] [PubMed] [Google Scholar]
- 19. Norman P, Spencer CA, Lawrence‐Brown MM, Jamrozik K. C‐reactive protein levels and the expansion of screen‐detected abdominal aortic aneurysms in men. Circulation. 2004;110:862–866. [DOI] [PubMed] [Google Scholar]
- 20. Wiernicki I, Safranow K, Baranowska‐Bosiacka I, Piatek J, Gutowski P. Haptoglobin 2‐1 phenotype predicts rapid growth of abdominal aortic aneurysms. J Vasc Surg. 2010;52:691–696. [DOI] [PubMed] [Google Scholar]
- 21. Flondell‐Sité D, Lindblad B, Gottsäter A. High levels of endothelin (ET)‐1 and aneurysm diameter independently predict growth of stable abdominal aortic aneurysms. Angiology. 2010;61:324–328. [DOI] [PubMed] [Google Scholar]
- 22. Domanovits H, Schillinger M, Müllner M, Hölzenbein T, Janata K, Bayegan K, Laggner AN. Acute phase reactants in patients with abdominal aortic aneurysm. Atherosclerosis. 2002;163:297–302. [DOI] [PubMed] [Google Scholar]
- 23. Tambyraja AL, Dawson R, Valenti D, Murie JA, Chalmers RT. Systemic inflammation and repair of abdominal aortic aneurysm. World J Surg. 2007;31:1210–1214. [DOI] [PubMed] [Google Scholar]
- 24. Wilmink TBM, Quick CRG, Day NE. The association between cigarette smoking and abdominal aortic aneurysms. J Vasc Surg. 1999;30:1099–1105. [DOI] [PubMed] [Google Scholar]
- 25. Lindholt JS, Jørgensen B, Klitgaard NA, Henneberg EW. Systemic levels of cotinine and elastase, but not pulmonary function, are associated with the progression of small abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2003;26:418–422. [DOI] [PubMed] [Google Scholar]
- 26. Golledge J, Muller R, Clancy P, McCann M, Norman PE. Evaluation of the diagnostic and prognostic value of plasma D‐dimer for abdominal aortic aneurysm. Eur Heart J. 2011;32:354–364. [DOI] [PubMed] [Google Scholar]
- 27. Adam DJ, Haggart PC, Ludlam CA, Bradbury AW. Hemostatic markers before operation in patients with acutely symptomatic nonruptured and ruptured infrarenal abdominal aortic aneurysm. J Vasc Surg. 2002;35:661–665. [DOI] [PubMed] [Google Scholar]
- 28. Skagius E, Siegbahn A, Bergqvist D, Henriksson AE. Fibrinolysis in patients with an abdominal aortic aneurysm with special emphasis on rupture and shock. J Thromb Haemost. 2008;6:147–150. [DOI] [PubMed] [Google Scholar]
- 29. Halazun KJ, Bofkin KA, Asthana S, Evans C, Henderson M, Spark JI. Hyperhomocysteinaemia is associated with the rate of abdominal aortic aneurysm expansion. Eur J Vasc Endovasc Surg. 2007;33:391–394. [DOI] [PubMed] [Google Scholar]
- 30. Třeška V, Topolčan O, Pecen L. Cytokines as plasma markers of abdominal aortic aneurysm. Clin Chem Lab Med. 2000;38:1161–1164. [DOI] [PubMed] [Google Scholar]
- 31. Jones KG, Brull DJ, Brown LC, Sian M, Greenhalgh RM, Humphries SE, Powell JT. Interleukin‐6 (IL‐6) and the prognosis of abdominal aortic aneurysms. Circulation. 2001;103:2260–2265. [DOI] [PubMed] [Google Scholar]
- 32. Flondell‐Sité D, Lindblad B, Kölbel T, Gottsäter A. Markers of proteolysis, fibrinolysis, and coagulation in relation to size and growth rate of abdominal aortic aneurysms. Vasc Endovascular Surg. 2010;44:262–268. [DOI] [PubMed] [Google Scholar]
- 33. Speelman L, Schurink GWH, Bosboom EMH, Buth J, Breeuwer M, Van de Vosse FN, Jacobs MH. The mechanical role of thrombus on the growth rate of an abdominal aortic aneurysm. J Vasc Surg. 2010;51:19–26. [DOI] [PubMed] [Google Scholar]
- 34. Wilson WRW, Anderton M, Choke EC, Dawson J, Loftus IM, Thompson MM. Elevated plasma MMP1 and MMP9 are associated with abdominal aortic aneurysm rupture. Eur J Vasc Endovasc Surg. 2008;35:580–584. [DOI] [PubMed] [Google Scholar]
- 35. Hobbs SD, Haggart P, Fegan C, Bradbury AW, Adam DJ. The role of tissue factor in patients undergoing open repair of ruptured and nonruptured abdominal aortic aneurysms. J Vasc Surg. 2007;46:682–686. [DOI] [PubMed] [Google Scholar]
- 36. Lindholt JS, Jørgensen B, Fasting H, Henneberg EW. Plasma levels of plasmin‐antiplasmin‐complexes are predictive for small abdominal aortic aneurysms expanding to operation‐recommendable sizes. J Vasc Surg. 2001;34:611–615. [DOI] [PubMed] [Google Scholar]
- 37. Lindholt JS, Ashton HA, Heickendorff L, Scott RAP. Serum elastin peptides in the preoperative evaluation of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2001;22:546–550. [DOI] [PubMed] [Google Scholar]
- 38. Petersen E, Gineitis A, Wågberg F, Ängquist KA. Serum levels of elastin‐derived peptides in patients with ruptured and asymptomatic abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2001;22:48–52. [DOI] [PubMed] [Google Scholar]
- 39. Vega de Céniga M, Esteban M, Quintana JM, Barba A, Estallo L, De la Fuente N, Viviens B, Martin‐Ventura JL. Search for serum biomarkers associated with abdominal aortic aneurysm growth—a pilot study. Eur J Vasc Endovasc Surg. 2009;37:297–299. [DOI] [PubMed] [Google Scholar]
- 40. Pulinx B, Hellenthal FAMVI, Hamulyák K, Van Dieijen‐Visser MP, Schurink GWH, Wodzig WKWH. Differential protein expression in serum of abdominal aortic aneurysm patients: a proteomic approach. Eur J Vasc Endovasc Surg. 2011;42:563–570. [DOI] [PubMed] [Google Scholar]
- 41. Treska V, Topolcan O. Plasma and tissue levels of collagen types I and III markers in patients with abdominal aortic aneurysms. Int Angiol. 2000;19:64–68. [PubMed] [Google Scholar]
- 42. Watt HC, Law MR, Wald NJ, Craig WY, Ledue TB, Haddow JE. Serum triglyceride: a possible risk factor for ruptured abdominal aortic aneurysm. Int J Epidemiol. 1998;27:949–952. [DOI] [PubMed] [Google Scholar]
- 43. Lindqvist M, Wallinder J, Bergström J, Henriksson AE. Plasma glycosylphosphatidylinositol phospholipase D (GPI‐PLD) and abdominal aortic aneurysm. Int J Clin Exp Med. 2012;5:306–309. [PMC free article] [PubMed] [Google Scholar]
- 44. Nyberg A, Skagius E, Nilsson I, Ljungh A, Henriksson AE. Abdominal aortic aneurysm and cytomegalovirus infection. J Med Virol. 2008;80:667–669. [DOI] [PubMed] [Google Scholar]
- 45. Nyberg A, Skagius E, Nilsson I, Ljungh A, Henriksson AE. Abdominal aortic aneurysm and infection with CagA positive strains of Helicobacter pylori. Scand J Infect Dis. 2008;40:204–207. [DOI] [PubMed] [Google Scholar]
- 46. Martelli‐Junior H, Cotrim P, Graner E, Sauk JJ, Coletta RD. Effect of transforming growth factor‐ß1, interleukin‐6, and interferon‐γ on the expression of type I collagen, heat shock protein 47, matrix metalloproteinase (MMP)‐1 and MMP‐2 by fibroblasts from normal gingiva and hereditary gingival fibromatosis. J Periodontol. 2003;74:296–306. [DOI] [PubMed] [Google Scholar]
- 47. Pan JH, Lindholt JS, Sukhova GK, Baugh JA, Henneberg EW, Bucala R, Donnelly SC, Libby P, Metz C, Shi GP. Macrophage migration inhibitory factor is associated with aneurysmal expansion. J Vasc Surg. 2003;37:628–635. [DOI] [PubMed] [Google Scholar]
- 48. Ramos‐Mozo P, Madrigal‐Matute J, Vega de Ceniga M, Blanco‐Colio LM, Meilhac O, Feldman L, Michel JB, Clancy P, Golledge J, Norman PE, Egido J, Martin‐Ventura JL. Increased plasma levels of NGAL, a marker of neutrophil activation, in patients with abdominal aortic aneurysm. Atherosclerosis. 2012;220:552–556. [DOI] [PubMed] [Google Scholar]
- 49. Moran CS, McCann M, Karan M, Norman P, Ketheesan N, Golledge J. Association of osteoprotegerin with human abdominal aortic aneurysm progression. Circulation. 2005;111:3119–3125. [DOI] [PubMed] [Google Scholar]
- 50. Martinez‐Pinna R, Ramos‐Mozo P, Madrigal‐Matute J, Blanco‐Colio LM, Lopez JA, Calvo E, Camafeita E, Lindholt JS, Meilhac O, Delbosc S, Michel JB, De Ceniga MV, Egido J, Martin‐Ventura JL. Identification of peroxiredoxin‐1 as a novel biomarker of abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2011;31:935–943. [DOI] [PubMed] [Google Scholar]
- 51. Martín‐Ventura JL, Lindholt JS, Moreno JA, Vega de Céniga M, Meilhac O, Michel JB, Egido J, Blanco‐Colio LM. Soluble TWEAK plasma levels predict expansion of human abdominal aortic aneurysms. Atherosclerosis. 2011;214:486–489. [DOI] [PubMed] [Google Scholar]
- 52. Vega de Ceniga M, Esteban M, Barba A, Estallo L, Blanco‐Colio LM, Martin‐Ventura JL. Assessment of biomarkers and predictive model for short‐term prospective abdominal aortic aneurysm growth‐A pilot study. Ann Vasc Surg. 2014;28:1642–1648. [DOI] [PubMed] [Google Scholar]
- 53. Lindholt JS, Erlandsen EJ, Henneberg EW. Cystatin C deficiency is associated with the progression of small abdominal aortic aneurysms. Br J Surg. 2001;88:1472–1475. [DOI] [PubMed] [Google Scholar]
- 54. Třeška V, Wenham PW, Valenta J, Topolčan O, Pecen L. Plasma endothelin levels in patients with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 1999;17:424–428. [DOI] [PubMed] [Google Scholar]
- 55. Lindholt JS, Martin‐Ventura JL, Urbonavicius S, Ramos‐Mozo P, Flyvbjerg A, Egido J, Henneberg EW, Frystyk J. Insulin‐like growth factor I—a novel biomarker of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2011;42:560–562. [DOI] [PubMed] [Google Scholar]
- 56. Wilson K, Whyman M, Hoskins P, Lee AJ, Bradbury AW, Fowkes FGR, Ruckley CV. The relationship between abdominal aortic aneurysm wall compliance, maximum diameter and growth rate. Cardiovasc Surg. 1999;7:208–213. [DOI] [PubMed] [Google Scholar]
- 57. Behr‐Rasmussen C, Grøndal N, Bramsen MB, Thomsen MD, Lindholt JS. Mural thrombus and the progression of abdominal aortic aneurysms: a large population‐based prospective cohort study. Eur J Vasc Endovasc Surg. 2014;48:301–307. [DOI] [PubMed] [Google Scholar]
- 58. Tong J, Cohnert T, Holzapfel GA. Diameter‐related variations of geometrical, mechanical, and mass fraction data in the anterior portion of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2015;49:262–270. [DOI] [PubMed] [Google Scholar]
- 59. Lindholt JS, Heegaard NHH, Vammen S, Fasting H, Henneberg EW, Heickendorff L. Smoking, but not lipids, lipoprotein (a) and antibodies against oxidised LDL, is correlated to the expansion of abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2001;21:51–56. [DOI] [PubMed] [Google Scholar]
- 60. Erhart P, Hyhlik‐Dürr A, Geisbüsch P, Kotelis D, Müller‐Eschner M, Gasser TC, Von Tengg‐Kobligk H, Böckler D. Finite element analysis in asymptomatic, symptomatic, and ruptured abdominal aortic aneurysms: In search of new rupture risk predictors. Eur J Vasc Endovasc Surg. 2015;49:239–245. [DOI] [PubMed] [Google Scholar]
- 61. Maier A, Gee MW, Reeps C, Pongratz J, Eckstein HH, Wall WA. A comparison of diameter, wall stress, and rupture potential index for abdominal aortic aneurysm rupture risk prediction. Ann Biomed Eng. 2010;38:3124–3134. [DOI] [PubMed] [Google Scholar]
- 62. Vande Geest JP, Di Martino ES, Bohra A, Makaroun MS, Vorp DA. A biomechanics‐based rupture potential index for abdominal aortic aneurysm risk assessment: demonstrative application. Ann N Y Acad Sci. 2006;1085:11–21. [DOI] [PubMed] [Google Scholar]
- 63. Venkatasubramaniam AK, Fagan MJ, Mehta T, Mylankal KJ, Ray B, Kuhan G, Chetter IC, McCollum PT. A comparative study of aortic wall stress using finite element analysis for ruptured and non‐ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2004;28:168–176. [DOI] [PubMed] [Google Scholar]
- 64. Fillinger MF, Marra SP, Raghavan ML, Kennedy FE. Prediction of rupture risk in abdominal aortic aneurysm during observation: wall stress versus diameter. J Vasc Surg. 2003;37:724–732. [DOI] [PubMed] [Google Scholar]
- 65. Wilson KA, Lee AJ, Hoskins PR, Fowkes FGR, Ruckley CV, Bradbury AW. The relationship between aortic wall distensibility and rupture of infrarenal abdominal aortic aneurysm. J Vasc Surg. 2003;37:112–117. [DOI] [PubMed] [Google Scholar]
- 66. Fillinger MF, Raghavan ML, Marra SP, Cronenwett JL, Kennedy FE. In vivo analysis of mechanical wall stress and abdominal aortic aneurysm rupture risk. J Vasc Surg. 2002;36:589–597. [DOI] [PubMed] [Google Scholar]
- 67. Kotze CW, Rudd JHF, Ganeshan B, Menezes LJ, Brookes J, Agu O, Yusuf SW, Groves AM. CT signal heterogeneity of abdominal aortic aneurysm as a possible predictive biomarker for expansion. Atherosclerosis. 2014;233:510–517. [DOI] [PubMed] [Google Scholar]
- 68. Kotze CW, Groves AM, Menezes LJ, Harvey R, Endozo R, Kayani IA, Ell PJ, Yusuf SW. What is the relationship between 18F‐FDG aortic aneurysm uptake on PET/CT and future growth rate? Eur J Nucl Med Mol Imaging. 2011;38:1493–1499. [DOI] [PubMed] [Google Scholar]
- 69. Morel O, Mandry D, Micard E, Kauffmann C, Lamiral Z, Verger A, Chevalier‐Mathias E, Mathias J, Karcher G, Meneroux B, Rossignol P, Marie PY. Evidence of cyclic changes in the metabolism of abdominal aortic aneurysms during growth phases: 18F‐FDG PET sequential observational study. J Nucl Med. 2015;56:1030–1035. [DOI] [PubMed] [Google Scholar]
- 70. Reeps C, Essler M, Pelisek J, Seidl S, Eckstein HH, Krause BJ. Increased 18F‐fluorodeoxyglucose uptake in abdominal aortic aneurysms in positron emission/computed tomography is associated with inflammation, aortic wall instability, and acute symptoms. J Vasc Surg. 2008;48:417–423. [DOI] [PubMed] [Google Scholar]
- 71. Kontopodis N, Metaxa E, Papaharilaou Y, Georgakarakos E, Tsetis D, Ioannou CV. Value of volume measurements in evaluating abdominal aortic aneurysms growth rate and need for surgical treatment. Eur J Radiol. 2014;83:1051–1056. [DOI] [PubMed] [Google Scholar]
- 72. Truijers M, Pol JA, SchultzeKool LJ, van Sterkenburg SM, Fillinger MF, Blankensteijn JD. Wall stress analysis in small asymptomatic, symptomatic and ruptured abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2007;33:401–407. [DOI] [PubMed] [Google Scholar]
- 73. Heng MS, Fagan MJ, Collier JW, Desai G, McCollum PT, Chetter IC. Peak wall stress measurement in elective and acute abdominal aortic aneurysms. J Vasc Surg. 2008;47:17–22. [DOI] [PubMed] [Google Scholar]
- 74. Vande Geest JP, Schmidt DE, Sacks MS, David A. The effects of anisotropy on the stress analyses of patient‐ specific abdominal aortic aneurysms. Ann Biomed Eng. 2008;36:921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Gasser TC, Nchimi A, Swedenborg J, Roy J, Sakalihasan N, Böckler D, Hyhlik‐Dürr A. A novel strategy to translate the biomechanical rupture risk of abdominal aortic aneurysms to their equivalent diameter risk: method and retrospective validation. Eur J Vasc Endovasc Surg. 2014;47:288–295. [DOI] [PubMed] [Google Scholar]
- 76. Stenbaek J, Kalin B, Swedenborg J. Growth of thrombus may be a better predictor of rupture than diameter in patients with abdominal aortic aneurysms. Eur J Vasc Endovasc Surg. 2000;20:466–469. [DOI] [PubMed] [Google Scholar]
- 77. Thompson AR, Cooper JA, Ashton HA, Hafez H. Growth rates of small abdominal aortic aneurysms correlate with clinical events. Br J Surg. 2010;97:37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fillinger MF, Racusin J, Baker RK, Cronenwett Jack L, Teutelink A, Schermerhorn ML, Zwolak RM, Powell RJ, Walsh DB, Rzucidlo EM. Anatomic characteristics of ruptured abdominal aortic aneurysm on conventional CT scans: implications for rupture risk. J Vasc Surg. 2004;39:1243–1252. [DOI] [PubMed] [Google Scholar]
- 79. Metaxa E, Kontopodis N, Tzirakis K, Ioannou CV, Papaharilaou Y. Effect of intraluminal thrombus asymmetrical deposition on abdominal aortic aneurysm growth rate. J Endovasc Ther. 2015;22:406–412. [DOI] [PubMed] [Google Scholar]
- 80. Ghilardi G, Biondi ML, Battaglioli L, Zambon A, Guagnellini E, Scorza R. Genetic risk factor characterizes abdominal aortic aneurysm from arterial occlusive disease in human beings: CCR5 Delta 32 deletion. J Vasc Surg. 2004;40:995–1000. [DOI] [PubMed] [Google Scholar]
- 81. Gerdes LU, Lindholt JS, Vammen S, Henneberg EW, Fasting H. Apolipoprotein E genotype is associated with differential expansion rates of small abdominal aortic aneurysms. Br J Surg. 2000;87:760–765. [DOI] [PubMed] [Google Scholar]
- 82. Duellman T, Warren CL, Matsumura J, Yang J. Analysis of multiple genetic polymorphisms in aggressive‐growing and slow‐growing abdominal aortic aneurysms. J Vasc Surg. 2014;60:613–621.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Eriksson P, Jones KG, Brown LC, Greenhalgh RM, Hamsten A, Powell JT. Genetic approach to the role of cysteine proteases in the expansion of abdominal aortic aneurysms. Br J Surg. 2004;91:86–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Golledge J, Muller J, Shephard N, Clancy P, Smallwood L, Moran C, Dear AE, Palmer LJ, Norman PE. Association between osteopontin and human abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 2007;27:655–660. [DOI] [PubMed] [Google Scholar]
- 85. Thompson AR, Golledge J, Cooper JA, Hafez H, Norman PE, Humphries SE. Sequence variant on 9p21 is associated with the presence of abdominal aortic aneurysm disease but does not have an impact on aneurysmal expansion. Eur J Hum Genet. 2009;17:391–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Wanhainen A, Mani K, Vorkapic E, De Basso R, Björck M, Lanne T, Wagsater D. Screening of circulating microRNA biomarkers for prevalence of abdominal aortic aneurysm and aneurysm growth. Atherosclerosis. 2017;256:82–88. [DOI] [PubMed] [Google Scholar]
- 87. Lindholt JS, Fasting H, Henneberg EW, Østergaard L. A review of Chlamydia pneumoniae and atherosclerosis. Eur J Vasc Endovasc Surg. 1999;17:283–289. [DOI] [PubMed] [Google Scholar]
- 88. Heinz A, Taddese S, Sippl W, Neubert RHH, Schmelzer CEH. Insights into the degradation of human elastin by matrilysin‐1. Biochimie. 2011;93:187–194. [DOI] [PubMed] [Google Scholar]
- 89. Courtois A, Nusgens BV, Hustinx R, Namur G, Gomez P, Somja J, Defraigne J‐O, Delvenne P, Michel JB, Colige AC, Sakalihasan N. 18F‐FDG uptake assessed by PET/CT in abdominal aortic aneurysms is associated with cellular and molecular alterations prefacing wall deterioration and rupture. J Nucl Med. 2013;54:1740–1747. [DOI] [PubMed] [Google Scholar]
- 90. Swedenborg J, Eriksson P. The intraluminal thrombus as a source of proteolytic activity. Ann N Y Acad Sci. 2006;1085:133–138. [DOI] [PubMed] [Google Scholar]
- 91. Nguyen VL, Leiner T, Hellenthal FAMVI, Backes WH, Wishaupt MCJ, Van der Geest RJ, Heeneman S, Kooi ME, Schurink GWH. Abdominal aortic aneurysms with high thrombus signal intensity on magnetic resonance imaging are associated with high growth rate. J Vasc Surg. 2014;60:1713. [DOI] [PubMed] [Google Scholar]
- 92. Song C, Burgess S, Eicher JD; CHARGE Consortium Hemostatic Factor Working Group, ICBP Consortium, CHARGE Consortium Subclinical Working Group , O'Donnell CJ, Johnson AD. Causal effect of plasminogen activator inhibitor type 1 on coronary heart disease. J Am Heart Assoc. 2017;6:e004918 DOI: 10.1161/JAHA.116.004918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Dietz HC. Marfan syndrome In: Pagon RA, Adam MP, Ardinger HH, Stephanie E Wallace. Marfan Syndrome. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 2001:1993–2017. [Google Scholar]
- 94. Beridze N, Frishman H. Vascular Ehlers‐Danlos syndrome: pathophysiology, diagnosis, and prevention and treatment of its complications. Cardiol Rev. 2012;20:4–7. [DOI] [PubMed] [Google Scholar]
- 95. Juvonen J, Surcel H‐M, Satta J, Teppo A‐M, Bloigu A, Syrjälä H, Airaksinen J, Leinonen M, Saikku P, Juvonen T. Elevated circulating levels of inflammatory cytokines in patients with abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol. 1997;17:2843–2847. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. MeSH Terms.


