Abstract
Background
Currently, acute ischemic stroke is still a leading cause of mortality and morbidity. Approximately 2 years ago, mechanical thrombectomy was proven beneficial as a revolutionary new therapy for stroke in the MR‐CLEAN trial (A Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands). However, the mechanisms by which the thrombectomy device, or stent‐retriever, interacts with the thrombus are largely unknown. A better understanding could lead to improved efficacy of mechanical thrombectomy devices.
Methods and Results
Seven stent‐retrievers with thrombi still entrapped were collected directly after thrombectomy. The stent‐retrievers were studied using micro computed tomography, followed by scanning electron microscopy and light microscopy. Two independent observers rated interaction type and thrombus surface structure (porous filamentous or dense) at the interaction sites.
A total of 79 interaction sites between thrombus and stent‐retriever were categorized. Thrombus‐stent‐retriever interaction was found to be adhesive (n=44; 56%) or mechanical (n=35; 44%). Adhesive interaction was most frequently observed at interaction sites with a dense surface, compared with interaction sites with a porous filamentous fibrin surface (38/58; 66% versus 6/21; 29%, P=0.011).
Conclusions
The interaction between thrombus and stent‐retriever was predominantly adhesive, not mechanical. Adhesive interaction was strongly associated with the presence of a dense thrombus surface without a porous filamentous fibrin network.
Keywords: ischemic stroke, mechanical thrombectomy, scanning electron microscopy, stent‐retriever, thrombus
Subject Categories: Cerebrovascular Disease/Stroke, Ischemic Stroke, Cerebrovascular Procedures, Stent
Clinical Perspective
What Is New?
This is the first report studying the interaction between thrombus and stent‐retriever after thrombectomy, in patients with acute ischemic stroke, using high‐resolution imaging.
Stent‐retrievers with thrombi still entrapped were studied using micro computed tomography, scanning electron microscopy, and light microscopy.
What Are the Clinical Implications?
We found that the interaction between thrombus and stent‐retriever was predominantly adhesive, not mechanical.
Adhesive interaction was strongly associated with a dense thrombus surface without a porous filamentous fibrin network.
A better understanding of the interaction between thrombus and stent‐retriever enables developments to optimize mechanical thrombectomy for patients with ischemic stroke.
Ischemic stroke is one of the most important causes of neurologic morbidity and mortality. Recently it was shown that intra‐arterial treatment (IAT) is highly effective in patients with acute ischemic stroke caused by a proximal intracranial arterial occlusion, and it has revolutionized stroke treatment.1, 2, 3, 4, 5, 6
Currently, the standard approach of IAT consists of intravenous thrombolysis followed by either mechanical thrombectomy with stent‐retrievers or by aspiration of thrombus with microcatheters.
While angiographic reperfusion has been reported in 70% to 90% of patients, good clinical outcome is observed only in ≈45%.7 A better understanding of the interaction between thrombus and stent‐retriever could lead to the design of strategies aimed to increase efficacy of mechanical thrombectomy.
Successful reperfusion with stent‐retrievers has been associated with the extent of thrombus integration in the stent‐retriever mesh.8 Pilot in vitro data indicate that thrombus interaction may be influenced by stent‐retriever design, specifically mesh size and the degree of expansion after 5 minutes.9 Thrombus characteristics also seem to influence IAT efficacy, because erythrocyte‐rich thrombi have been associated with successful reperfusion.10, 11, 12, 13 As such, efficacy of mechanical thrombectomy devices seems to be influenced by properties of both thrombus and stent‐retriever. Based on scanning electron microscopy (SEM) and light microscopy, it was suggested that different types of interaction occur between thrombus and stent‐retriever.14 However, not much is known about the mechanisms of interaction between thrombus and stent‐retriever. The appearance of these interactions seemed not merely mechanical but often displayed thrombus coalescing around the stent‐retriever struts with smooth covers of fibrin overlying erythrocyte‐rich areas.14
In this study we aimed to explore the characteristics of thrombus‐stent‐retriever interaction by stent‐retrievers with thrombi still entrapped, at a microscopic level using both light microscopy and SEM. We hypothesized that a correlation exists between specific thrombus surface characteristics and type of thrombus‐stent‐retriever interaction.
Materials and Methods
Sample Collection and Preparation
Seven stent‐retrievers with thrombus material still entrapped were prospectively collected from 7 patients treated with mechanical IAT for acute ischemic stroke in 2 different stroke centers in the MR‐CLEAN registry (A Multicenter Clinical Registry of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands). This registry was approved by the medical ethical committee of our hospital as a substudy of the MR‐CLEAN registry (IRB approval: MEC‐2014‐235). All patients were provided with a written explanation of the study. The patients or their representatives were given the opportunity to refuse participation. Clot presence after initial intravenous thrombolytic treatment was confirmed by digital subtraction angiography. Clot position was unchanged compared with initial CTA (Computed tomography angiography) in all patients, confirming failure of intravenous thrombolytic therapy to achieve recanalization. Thrombectomy was performed by passing the thrombus with the microcatheter, followed by deployment of a stent‐retriever. After a delay of 5 minutes to optimize integration of the thrombus into the stent‐retriever, the stent‐retriever was gently pulled back in the guiding catheter in the internal carotid artery. During stent retrieval, flow reversal in the internal carotid artery was established by balloon inflation and manual aspiration over this balloon‐guiding catheter. Directly after thrombectomy, the stent‐retrievers were cut from the wire, carefully rinsed in non‐heparinized saline, fixed in buffered formaldehyde‐glutaraldehyde for at least 48 hours in preparation for electron microscopy, rinsed and stored in 0.1 mol/L cacodylate buffer (pH 7.4, Sigma Aldrich, Zwijndrecht, The Netherlands). Samples were then photographed and stored until further processing (Figure 1A).
Figure 1.
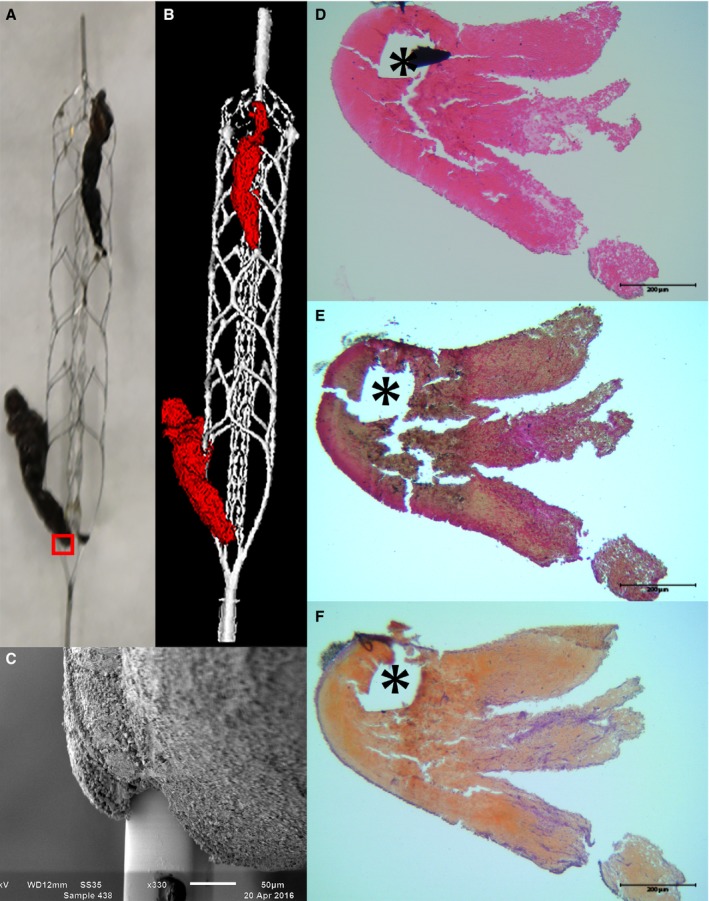
Macroscopy, micro‐CT, and microscopy of a stent retriever. After retrieval, thrombi were photographed (A) and imaged using micro‐CT (B). Each interaction site was visualized on a microscopic scale using SEM (C). Subsequently, thrombi were prepared for histology for assessment of thrombus composition: hematoxylin‐eosin (D), Resorcin‐Fuchsin (E), and Okajima (F). Erythrocytes are pink (D), beige (E), and orange (F). The small red box in 1A marks the location of the SEM (C) and LM images (D‐F). Stent strut voids are marked with an asterisk (*). LM indicates light microscopy; Micro‐CT, micro computed tomography; SEM, scanning electron microscopy.
Micro Computed Tomography
Micro computed tomography (micro‐CT) was performed on each stent‐retriever with 90 kV, 200 μA, and a field of view between 20 and 40 mm (Caliper Quantum FX, Perkin Elmer, Waltham, MA). Semiautomated analysis software was used to determine the total volume of the retrieved thrombi (AnalyzeDirect v11.0, Biomedical Imaging Resource; Mayo Clinic, Rochester, MN) (Figure 1B).
Scanning Electron Microscopy
Samples were dehydrated in a graded ethanol series, dried with hexamethyldisilazane (Sigma Aldrich, Zwijndrecht, The Netherlands), and sputter coated with gold (Agar Auto sputter coater; Agar Scientific, Stansted, UK). Samples were then studied at 3 kV (JSM 6100LV, JEOL, Japan) and all identifiable interactions between thrombus and stent‐retriever‐struts were photographed at both high‐ and low‐level magnification for later analysis. In a preliminary analysis in the first stent‐retriever (Figure S1), the following types of thrombus surface and thrombus‐stent‐retriever interaction were predominantly found:
Thrombus‐Stent‐Retriever Interaction
- Mechanical Interaction: Thrombus entwining around the struts, leaving spaces between strut and thrombus material (Figure 2A).
Figure 2.
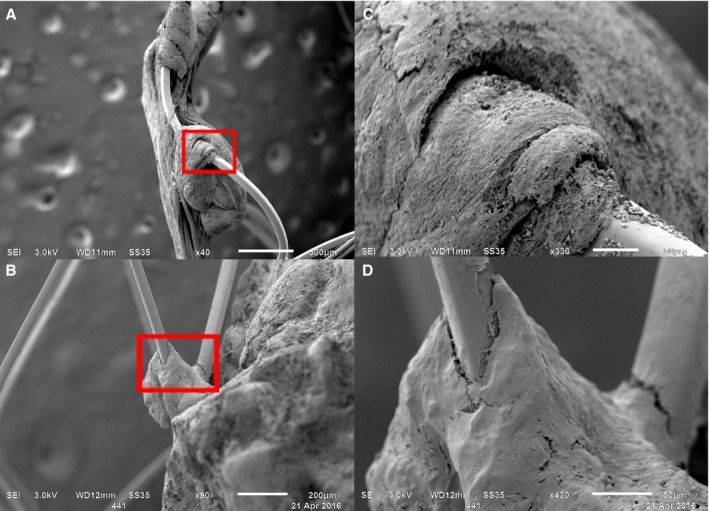 Stent–thrombus interaction. Per stent‐retriever, generally 2 interaction types were observed: mechanical (A) or adhesive (B). C and D, magnifications of the small red boxes in (A and B), respectively.
Stent–thrombus interaction. Per stent‐retriever, generally 2 interaction types were observed: mechanical (A) or adhesive (B). C and D, magnifications of the small red boxes in (A and B), respectively. Adhesive Interaction: Thrombus coalescing to a stent‐retriever strut, much like adhesion of a drop of water to a thread (Figure 2B).
Thrombus Surface
- Porous filamentous surface: Thrombus having a visible porous filamentous surface at a magnification factor of at least 200× using SEM, resembling fibrin networks described in previous publications15, 16 (Figure 3A).
Figure 3.
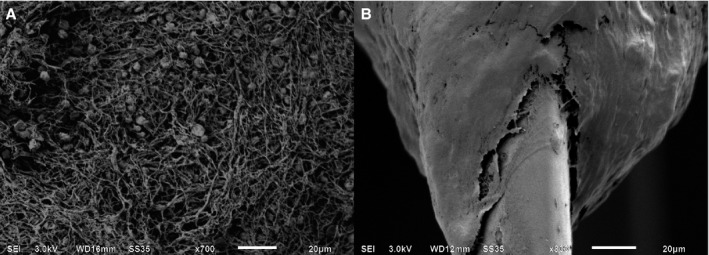 Thrombus surface by SEM. Two types of surfaces were recognized using SEM. A porous filamentous fibrin network was observed (A) vs a denser surface with the lack of such a network (B); magnification factors are 700 and 800, respectively. SEM indicates scanning electron microscopy.
Thrombus surface by SEM. Two types of surfaces were recognized using SEM. A porous filamentous fibrin network was observed (A) vs a denser surface with the lack of such a network (B); magnification factors are 700 and 800, respectively. SEM indicates scanning electron microscopy. Dense thrombus surface: Thrombus at which no porous filamentous fibrin network could be distinguished using SEM with a magnification factor of least 200× (Figure 3B).
Then, all stent‐retrievers with thrombus still entrapped were examined for sites of interaction between thrombus and stent‐retriever. These interaction sites were then evaluated for interaction type and surface properties based upon the aforementioned categories. Per thrombus, multiple interaction sites were found and rated independently. Each interaction site was allowed to have only 1 of the 2 predefined interaction and surface types.
Both interaction type and thrombus surface were independently classified by 2 researchers (A.A., H.B.) who specialized in thrombus histology. In case of disagreement, samples were reevaluated and consensus was reached.
Histologic Analysis
After SEM analysis, samples were placed in 100% ethanol and embedded in methyl methacrylate (Merck KgaA, Darmstadt, Germany) at −17° as published before.17 Following polymerization, thin 5‐μm sections were cut using a microtome (Microm 355S; Thermo Scientific, Walldorf, Germany) and mounted using butylglycol (Sigma Aldrich, Zwijndrecht, The Netherlands). Sections were dried overnight at 37°C, then deplasticized in xylene‐chloroform (1:1) and stained using hematoxylin‐eosin as an overview stain (Figure 1D), Resorcin‐Fuchsin as an elastin stain (Figure 1E), and Okajima (Figure 1F) as a hemoglobin stain.
Thrombus composition was assessed semiquantitatively by 2 researchers (H.B. and A.E.), estimating the amount of erythrocytes versus fibrin/platelets content at multiple locations in each thrombus.
Study Data
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. The patient consent forms do not allow for data and material sharing.
Statistical Analysis
Descriptive statistics are given as median with range for continuous variables, and as count with percentages for categorical variables. A multilevel regression analysis was performed since multiple observations originated from the same samples. A P<0.05 was considered statistically significant. Statistical analysis was performed using both Stata (StataCorp. 2017. Stata Statistical Software: Release 15. StataCorp LLC, College Station, TX) and SPSS (IBM Corp. Released 2013. IBM SPSS Statistics for Windows, Version 22.0. IBM Corp., Armonk, NY) software.
Results
Thrombus Surface Characteristics and Thrombus‐Stent‐Retriever Interaction
In total, 94 interaction sites (median number of interaction sites per stent‐retriever 12 [5–32]) were identified and photographed in the 7 stent‐retrievers using SEM. Upon analysis, 15 interaction sites were excluded, either because the specific interaction or surface type could not be established based on the photographs (n=13), or because they could not be classified under the prespecified distinctive categories (n=2), leaving 79 interaction sites (median per stent‐retriever 11 [4–27]) for further analysis.
The thrombus‐stent‐retriever interaction was of the adhesive type at 44 (56%) interaction sites (median per stent‐retriever 6 [1–12]) and of the mechanical type at 35 (44%) interaction sites (median per stent‐retriever 6 [0–15]).
Overall, a porous filamentous thrombus surface was observed at 21 (27%) interaction sites, with a median of 3 (0–7) interactions per stent retriever. A dense thrombus surface, lacking such a porous structure, was observed at 58 (73%) interaction sites, with a median of 5 (3–21) interactions per stent retriever.
Adhesive interaction was more frequently observed at interaction sites with a dense surface (38 of 58), as compared with adhesive interaction at sites with a porous filamentous surface (6 of 21) (P=0.011). Surface characteristics and interaction types for each individual stent‐retriever are shown in Table (see also Table S1 and Figures S1 through S7).
Table 1.
Thrombus Characteristics
| Thrombus | Volume (mm3) | Nr. Interaction Sites | Nr. Dense Surface at Interaction Sites (%) | Nr. Adhesive Interactions (%) |
|---|---|---|---|---|
| 1 | 40.9 | 14 | 11 (79) | 7 (50) |
| 2 | 75.3 | 27 | 21 (78) | 12 (44) |
| 3 | 31.4 | 4 | 4 (100) | 3 (75) |
| 4 | 48.4 | 11 | 4 (36) | 6 (55) |
| 5 | 18 | 11 | 10 (91) | 10 (91) |
| 6 | 11.9 | 5 | 5 (100) | 5 (100) |
| 7 | 10.3 | 7 | 3 (43) | 1 (14) |
| No. or avg±SD | 33.7±23 | 79 | 58 (73) | 44 (61) |
Thrombus volume, number of interaction sites, thrombus surface type, and interaction type per stent‐retriever.
Thrombus Composition
The retrieved thrombi consisted predominantly of erythrocytes (median 87%, range 47%–95%, Table S2), but also contained platelets, fibrin, and leukocytes. Erythrocytes showed either an intact discoid shape or loss of shape. Zahn lines, which indicate the formation of thrombi under arterial flow conditions, were observed in 2 thrombi. Areas with extracellular DNA on hematoxylin‐eosin staining with the same morphology as neutrophil extracellular traps suggested the presence of neutrophil extracellular traps and were observed in 5 thrombi. These areas were found in different patterns, either diffusely spread through the thrombus, in hotspots, or lining the thrombus. There was no predominant pattern observed. Cholesterol crystals were not observed. Nuclear fragmentation was present in all but 1 thrombus, indicating thrombi were older than 1 day according to the criteria by Rittersma et al.18 Hematoxylin‐eosin staining of thrombi revealed areas of different age with fibrin networks of variable density and both intact leukocytes and nonintact leukocytes.
Thrombus surface most often resembled fibrin, even if the thrombus predominantly consisted of erythrocytes (Figure S4H). A statistically significant correlation between inner thrombus composition and thrombus surface by light microscopy was not found, most likely because of the small number of cross sections per thrombus. Also, because of the limited number of cross sections, exactly corresponding SEM photographs and light microscopy histology were available at only a few interaction sites. At these sites, a clear correlation was seen between thrombus surface structure on both imaging modalities, with corresponding porous filamentous and dense surfaces and pits that tended to contain erythrocytes (Figure 4).
Figure 4.
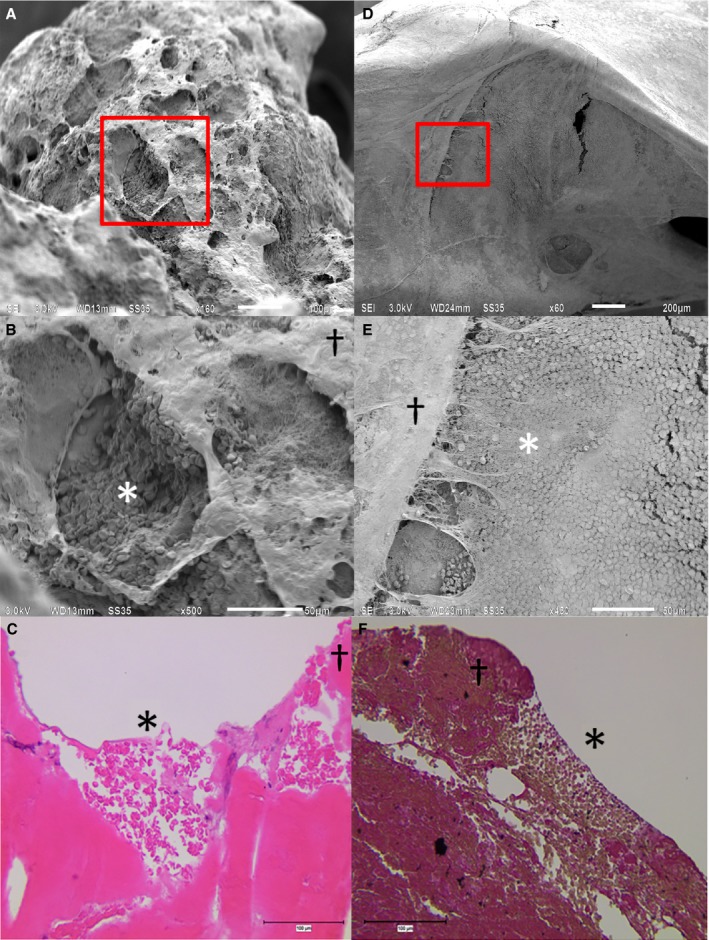
SEM and corresponding thrombus histology. Two examples of SEM and their corresponding LM shed light on the relation between the surface and interior of these thrombi. SEM is given as an overview (A, D). B and E, magnifications of the small red boxes in (A and B), respectively, and corresponding LM (C, F). SEM revealed both dense and porous filamentous networks on the surface of thrombi. Porous filamentous (*) and dense (†) surface areas on SEM corresponded with porous filamentous and dense surfaces on LM. Porous filamentous networks and pits (*) tended to contain erythrocytes. LM indicates light microscopy; SEM, scanning electron microscopy.
Discussion
We found that thrombus‐stent‐retriever interaction was predominantly adhesive, not merely mechanical. Previous studies hypothesized that the main capture mechanism is direct engagement of the clot between the crossings of stent‐retriever struts, resembling merely a mechanical interaction.8, 9, 19 This apparent contradiction is possibly because of the use of much higher magnifications in this study, at which these different types of interaction can be distinguished, and possibly also because of inherent differences among in vivo, in vitro, and our in situ study design.
Secondly, we found that adhesive interaction was associated with a dense thrombus surface. The density of fibrin networks is determined by many factors, among others the initiators of thrombus formation such as thrombin, tissue factor and calcium chloride, and their concentration.20 A dense fibrin network reduces the generation of plasmin activators and therefore reduces the degradation of the fibrin network.21 Whether sites of adhesive interaction with a dense thrombus surface consist of original thrombus or whether they are newly formed thrombi remains to be determined. If the observed surface of the retrieved thrombus reflects the original surface of the embolized thrombus, it might reflect resistance to thrombolysis.
It remains to be determined whether thrombus composition, and specifically fibrin content, affects thrombus behavior during mechanical thrombectomy. Recent in vitro data seem to suggest that fibrin‐rich thrombi containing relatively low amounts of erythrocytes are more resistant to thrombectomy, possibly because of their frictional properties.22, 23
Since our study was limited by sample size, it might represent only a small subgroup of the acute ischemic stroke population, and future studies will have to confirm our findings. Furthermore, a selection bias was inevitable because successful thrombolysis before IAT excluded thrombi sensitive to intravenous thrombolysis therapy from analysis. Hypothetically, thrombi fully resistant to mechanical IAT are also not included because they were not retrieved.
Although the interventional radiologist was aware of study inclusion at the time of the procedure, it is of course possible that thrombus surface characteristics have been altered by thrombolytic therapy before mechanical thrombectomy or by the thrombectomy procedure itself. However, thrombi were retrieved using a large‐bore guiding catheter in order to reduce mechanical strain on thrombus and stent‐retriever. Also, thrombi were immediately rinsed after retrieval to prevent new clot formation from aspirated blood outside of the patient. Of course, some uncertainty remains about whether a microscopically observed interaction accurately represents the original interaction, because of the in situ nature of this study.
Conclusion
Two types of interaction between thrombus and stent‐retriever are observed after thrombectomy for acute ischemic stroke: adhesive and mechanical. The predominant type of interaction is adhesive, which correlates strongly with a denser thrombus surface. A better understanding of stent–thrombus interaction could lead to improved efficacy of mechanical thrombectomy devices.
Appendix
MR CLEAN Registry Group Members
Ivo G.H. Jansen, medical researcher, Department of Radiology, Academic Medical Centre, Amsterdam, Netherlands; Maxim J.H.L. Mulder, medical researcher, Department of Neurology, Erasmus MC University Medical Centre, Rotterdam, Netherlands; Department of Radiology, Erasmus MC University Medical Centre, Rotterdam, Netherlands; Robert‐Jan B. Goldhoorn, medical researcher, Department of Neurology, Maastricht University Medical Centre and Cardiovascular, Research Institute Maastricht (CARIM), Maastricht, Netherlands; Bart J. Emmer, interventional radiologist, Department of Radiology, Academic Medical Centre, Amsterdam, Netherlands; Department of Radiology, Erasmus MC University Medical Centre, Rotterdam, Netherlands; Adriaan C.G.M. van Es, interventional radiologist, Department of Radiology, Erasmus MC University Medical Centre, Rotterdam, Netherlands; Bob Roozenbeek, neurologist, Department of Neurology, Erasmus MC University Medical Centre, Rotterdam, Netherlands; Wouter J. Schonewille, neurologist, Department of Neurology, Sint Antonius Hospital, Nieuwegein, Netherlands; René van den Berg, interventional radiologist, Department of Radiology, Academic Medical Centre, Amsterdam, Netherlands; Jonathan M. Coutinho, neurologist, Department of Neurology, Academic Medical Centre, Amsterdam, Netherlands; Julie Staals, neurologist, Department of Neurology, Maastricht University Medical Centre and Cardiovascular, Research Institute Maastricht (CARIM), Maastricht, Netherlands; Alida A. Postma, interventional radiologist, Department of Radiology, Maastricht University Medical Centre and Cardiovascular, Research Institute Maastricht (CARIM), Maastricht, Netherlands; Geert J. Lycklama à Nijeholt, interventional radiologist, Department of Radiology, Haaglanden Medical Centre, The Hague, Netherlands; Jeannette Hofmeijer, neurologist, Department of Neurology, Rijnstate Hospital, Arnhem, Netherlands; Jasper M. Martens, interventional radiologist, Department of Radiology, Rijnstate Hospital, Arnhem, Netherlands; Heleen M. den Hertog, neurologist, Department of Neurology, Medisch Spectrum Twente, Enschede, Netherlands; Emiel J.C. Sturm, interventional radiologist, Department of Radiology, Medisch Spectrum Twente, Enschede, Netherlands; H. Bart van der Worp, neurologist, Department of Neurology, University Medical Centre Utrecht, Utrecht, Netherlands; Rob H. Lo, interventional radiologist, Department of Radiology, University Medical Centre Utrecht, Utrecht, Netherlands; Roel J.J. Heijboer, interventional radiologist, Department of Radiology, Atrium Medical Centre, Heerlen, Netherlands; Koos Keizer, neurologist, Department of Neurology, Catharina Hospital, Eindhoven, Netherlands; Maarten Uyttenboogaart, interventional neurologist, Department of Neurology, University Medical Centre Groningen, Groningen, Netherlands; Omid Eshghi, interventional radiologist, Department of Radiology, University Medical Centre Groningen, Groningen, Netherlands; Marieke J.H. Wermer, neurologist, Department of Neurology, Leiden University Medical Centre, Leiden, Netherlands; Marianne A.A. van Walderveen, interventional radiologist, Department of Radiology, Leiden University Medical Centre, Leiden, Netherlands; Ewoud J. van Dijk, neurologist, Department of Neurology, Radboud University Medical Centre, Nijmegen, Netherlands; Hieronymus D. Boogaarts, interventional radiologist, Department of Neurosurgery, Radboud University Medical Centre, Nijmegen, Netherlands; Sebastiaan F. de Bruijn, neurologist, Department of Neurology, HAGA Hospital, The Hague, Netherlands; Lukas C. van Dijk, interventional radiologist, Department of Radiology, HAGA Hospital, The Hague, Netherlands; Jan S.P. van den Berg, neurologist, Department of Neurology, Isala Klinieken, Zwolle, Netherlands; Boudewijn A.A.M. van Hasselt, interventional radiologist, Department of Radiology, Isala Klinieken, Zwolle, Netherlands; Paul L.M. de Kort, neurologist, Department of Neurology, Sint Elisabeth Hospital, Tilburg, Netherlands; Jo P.P. Peluso, interventional radiologist, Department of Radiology, Sint Elisabeth Hospital, Tilburg, Netherlands; Tobien H.C.M.L. Schreuder, neurologist, Department of Neurology, Atrium Medical Centre, Heerlen, Netherlands; Leo A.M. Aerden, neurologist, Department of Neurology, Reinier de Graaf Gasthuis, Delft, Netherlands; René J. Dallinga, interventional radiologist, Department of Radiology, Reinier de Graaf Gasthuis, Delft, Netherlands; Marieke E.S. Sprengers, interventional radiologist, Department of Radiology, Academic Medical Centre, Amsterdam, Netherlands; Lonneke S.F. Yo, interventional radiologist, Department of Radiology, Catharina Hospital, Eindhoven, Netherlands; Sjoerd F.M. Jenniskens, interventional radiologist, Department of Radiology, Radboud University Medical Centre, Nijmegen, Netherlands; Stefan D. Roosendaal, interventional radiologist, Department of Radiology, Academic Medical Centre, Amsterdam, Netherlands; Bas F.W. van der Kallen, interventional radiologist, Department of Radiology, Haaglanden Medical Centre, The Hague, Netherlands; Ido R. van den Wijngaard, interventional neurologist, Department of Radiology, Haaglanden Medical Centre, The Hague, Netherlands; Joost C.J. Bot, interventional radiologist, Department of Radiology, VU Medical Centre, Amsterdam, Netherlands; Pieter J. van Doormaal, interventional radiologist, Department of Radiology, Erasmus MC University Medical Centre, Rotterdam, Netherlands; H. Zwenneke Flach, interventional radiologist, Department of Radiology, Isala Klinieken, Zwolle, Netherlands; Esmee Venema, medical researcher, Department of Neurology, Erasmus MC University Medical Centre, Rotterdam, Netherlands; Department of Public Health, Erasmus MC University Medical Centre, Rotterdam, Netherlands; Hester F. Lingsma, senior medical statistician, Department of Public Health, Erasmus MC University Medical Centre, Rotterdam, Netherlands; Ludo F.M. Beenen, radiologist, Department of Radiology, Academic Medical Centre, Amsterdam, Netherlands; Albert J. Yoo, interventional radiologist, Department of Radiology, Texas Stroke Institute, Plano, TX; Jelis Boiten, neurologist, Department of Neurology, Haaglanden Medical Centre, The Hague, Netherlands; Jan A. Vos, interventional radiologist, Department of Radiology, Sint Antonius Hospital, Nieuwegein, Netherlands; Yvo B.W.E.M. Roos, professor of acute neurology, Department of Neurology, Academic Medical Centre, Amsterdam, Netherlands; Robert J. van Oostenbrugge, professor of vascular neurology, Department of Neurology, Maastricht University Medical Centre and Cardiovascular, Research Institute Maastricht (CARIM), Maastricht, Netherlands; Aad van der Lugt, professor of neuroradiology and head/neck radiology, Department of Radiology, Erasmus MC University Medical Centre, Rotterdam, Netherlands; Wim H. van Zwam, interventional radiologist, Department of Radiology, Maastricht University Medical Centre and Cardiovascular, Research Institute Maastricht (CARIM), Maastricht, Netherlands; Diederik W.J. Dippel, professor of neurovascular diseases, Department of Neurology, Erasmus MC University Medical Centre, Rotterdam, Netherlands; Charles B.L.M. Majoie, professor of neuroradiology, Department of Radiology, Academic Medical Centre, Amsterdam, Netherlands; for the MR CLEAN Registry investigators.
Sources of Funding
This study and the MR‐CLEAN Registry were partly funded by unrestricted grants from TWIN, Erasmus MC, AMC, MUMC, and the Dutch Thrombosis Foundation.
Disclosures
Erasmus MC received funds from Stryker® for consultations by Diederik Dippel, Aad van der Lugt, Bart Emmer, and from Bracco Imaging® for consultations by Diederik Dippel. Amsterdam Medical Centre received funds from Stryker® for consultations by Charles Majoie, Yvo Roos, and Olvert Berkhemer. Maastricht University Medical Centre received funds from Stryker® for consultations by Wim van Zwam, Bart Emmer received funds from consultations for DEKRA b.v.
Supporting information
Table S1. Surface vs Interaction Using SEM. Adhesive interaction was more frequently observed at interaction sites with a dense surface (38 of 58), as compared with adhesive interaction at interaction sites with a porous filamentous surface (6 of 21) (P=0.011)
Table S2. Thrombus Characteristics. The presence of Zahn lines, cholesterol crystals, nuclear fragmentation is given per thrombus and determines thrombus age. In our study most thrombi were classified as lytic according to the criteria by Rittersma et al1
Figure S1. Stent 1. Thrombus entrapped in stent photographed (A) and visualized using micro‐CT (B). Both mechanical (C, D) and adhesive interactions (G) are observed using SEM. Details of the SEM images reveal both porous filamentous and dense surfaces (E and F). Histological staining using hematoxylin‐eosin (H), Okajima (I), and RF (J) show thrombus containing both fibrin and erythrocyte‐rich areas. The fibrin‐like surface is pink in (H), red in (I), and purple in (J).
Figure S2. Stent 2. Thrombus entrapped in stent photographed (A) and visualized using micro‐CT (B). Predominantly mechanical interaction is observed using SEM (C, D). Details of the SEM images reveal that the thrombus surface is mainly dense (E), but spots of porous filamentous surfaces also were observed (F). Histological staining using HE (G), Okajima (H), and RF (I) show thrombus containing mostly erythrocytes. The fibrin‐like surface is pink in (G), red in (H), and purple in (I).
Figure S3. Stent 3. Thrombus entrapped in stent photographed (A) and visualized using micro‐CT (B). Both adhesive (C) and mechanical interactions (D) are observed using SEM. Details of the SEM images reveal that the thrombus surface is mainly dense (E, F, G). Histological staining using hematoxylin‐eosin (H), Okajima (I), and RF (J) show thrombus containing mostly erythrocytes and little fibrin. The fibrin‐like surface is pink in (H), red in (I), and purple in (J).
Figure S4. Stent 4. Thrombus entrapped in stent photographed (A) and visualized using micro‐CT (B). Both mechanical (C) and adhesive interactions (D) are observed using SEM. Details of the SEM images reveal that the thrombus surface is mainly porous filamentous (E, F). Histological staining using hematoxylin‐eosin (G), Okajima (H), and RF (I) show thrombus containing mostly erythrocytes. The fibrin‐like surface is pink in (G), red in (H), and purple in (I).
Figure S5. Stent 5. Thrombus entrapped in stent photographed (A) and visualized using micro‐CT (B). Predominantly adhesive interaction is observed using SEM (C, E). Details of the SEM images reveal that the thrombus surface is mainly dense (D), but spots of porous filamentous surfaces also were observed (F). Histological staining using hematoxylin‐eosin (G), Okajima (H), and RF (I) show thrombus containing mostly erythrocytes. The fibrin‐like surface is pink in (G), red in (H), and purple in (I).
Figure S6. Stent 6. Thrombus entrapped in stent photographed (A) and visualized using micro‐CT (B). Predominantly adhesive interaction is observed using SEM (C and D). Details of the SEM images reveal that the thrombus surface is mainly dense (F), but spots of porous filamentous surfaces also were observed (E). Histological staining using hematoxylin‐eosin (G), Okajima (H), and RF (I) shows thrombus containing both fibrin and erythrocyte‐rich areas. The fibrin‐like surface is pink in (G), red in (H), and purple in (I).
Figure S7. Stent 7. Thrombus entrapped in stent photographed (A) and visualized using micro‐CT (B). Predominantly mechanical interaction is observed using SEM (C and D). Details of the SEM images reveal both porous filamentous and dense surfaces (E and F). Histological staining using hematoxylin‐eosin (G), Okajima (H), and RF (I) show thrombus containing both fibrin and erythrocyte‐rich areas. The fibrin‐like surface is pink in (G), red in (H), and purple in (I).
(J Am Heart Assoc. 2018;7:e008563 DOI: 10.1161/JAHA.118.008563.)
Contributor Information
Heleen M. M. van Beusekom, Email: h.vanbeusekom@erasmusmc.nl.
on behalf of the MR CLEAN Registry Investigators:
Ivo G.H. Jansen, Maxim J.H.L. Mulder, Robert‐Jan B. Goldhoorn, Bart J. Emmer, Adriaan C.G.M. van Es, Bob Roozenbeek, Wouter J. Schonewille, René van den Berg, Jonathan M. Coutinho, Julie Staals, Alida A. Postma, Geert J. Lycklama à Nijeholt, Jeannette Hofmeijer, Jasper M. Martens, Heleen M. den Hertog, Emiel J.C. Sturm, H. Bart van der Worp, Rob H. Lo, Roel J.J. Heijboer, Koos Keizer, Maarten Uyttenboogaart, Omid Eshghi, Marieke J.H. Wermer, Marianne A.A. van Walderveen, Ewoud J. van Dijk, Hieronymus D. Boogaarts, Sebastiaan F. de Bruijn, Lukas C. van Dijk, Jan S.P. van den Berg, Boudewijn A.A.M. van Hasselt, Paul L.M. de Kort, Jo P.P. Peluso, Tobien H.C.M.L. Schreuder, Leo A.M. Aerden, René J. Dallinga, Marieke E.S. Sprengers, Lonneke S.F. Yo, Sjoerd F.M. Jenniskens, Stefan D. Roosendaal, Bas F.W. van der Kallen, Ido R. van den Wijngaard, Joost C.J. Bot, Pieter J. van Doormaal, H. Zwenneke Flach, Esmee Venema, Hester F. Lingsma, Ludo F.M. Beenen, Albert J. Yoo, Jelis Boiten, Jan A. Vos, Yvo B.W.E.M. Roos, Robert J. van Oostenbrugge, Aad van der Lugt, Wim H. van Zwam, Diederik W.J. Dippel, and Charles B.L.M. Majoie
References
- 1. Berkhemer OA, Fransen PS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJ, van Walderveen MA, Staals J, Hofmeijer J, van Oostayen JA, Lycklama à Nijeholt GJ, Boiten J, Brouwer PA, Emmer BJ, de Bruijn SF, van Dijk LC, Kappelle LJ, Lo RH, van Dijk EJ, de Vries J, de Kort PL, van Rooij WJ, van den Berg JS, van Hasselt BA, Aerden LA, Dallinga RJ, Visser MC, Bot JC, Vroomen PC, Eshghi O, Schreuder TH, Heijboer RJ, Keizer K, Tielbeek AV, den Hertog HM, Gerrits DG, van den Berg‐Vos RM, Karas GB, Steyerberg EW, Flach HZ, Marquering HA, Sprengers ME, Jenniskens SF, Beenen LF, van den Berg R, Koudstaal PJ, van Zwam WH, Roos YB, van der Lugt A, van Oostenbrugge RJ, Majoie CB, Dippel DW; MR CLEAN Investigators . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 2. Bracard S, Ducrocq X, Mas JL, Soudant M, Oppenheim C, Moulin T, Guillemin F; THRACE investigators . Mechanical thrombectomy after intravenous alteplase versus alteplase alone after stroke (THRACE): a randomised controlled trial. Lancet Neurol. 2016;15:1138–1147. [DOI] [PubMed] [Google Scholar]
- 3. Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, Wu TY, Brooks M, Simpson MA, Miteff F, Levi CR, Krause M, Harrington TJ, Faulder KC, Steinfort BS, Priglinger M, Ang T, Scroop R, Barber PA, McGuinness B, Wijeratne T, Phan TG, Chong W, Chandra RV, Bladin CF, Badve M, Rice H, de Villiers L, Ma H, Desmond PM, Donnan GA, Davis SM; EXTEND‐IA Investigators . Endovascular therapy for ischemic stroke with perfusion‐imaging selection. N Engl J Med. 2015;372:1009–1018. [DOI] [PubMed] [Google Scholar]
- 4. Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, Dowlatshahi D, Frei DF, Kamal NR, Montanera WJ, Poppe AY, Ryckborst KJ, Silver FL, Shuaib A, Tampieri D, Williams D, Bang OY, Baxter BW, Burns PA, Choe H, Heo JH, Holmstedt CA, Jankowitz B, Kelly M, Linares G, Mandzia JL, Shankar J, Sohn SI, Swartz RH, Barber PA, Coutts SB, Smith EE, Morrish WF, Weill A, Subramaniam S, Mitha AP, Wong JH, Lowerison MW, Sajobi TT, Hill MD; ESCAPE Trial Investigators . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. [DOI] [PubMed] [Google Scholar]
- 5. Saver JL, Goyal M, Bonafe A, Diener HC, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, Jansen O, Jovin TG, Mattle HP, Nogueira RG, Siddiqui AH, Yavagal DR, Baxter BW, Devlin TG, Lopes DK, Reddy VK, du Mesnil de Rochemont R, Singer OC, Jahan R; SWIFT PRIME Investigators . Stent‐retriever thrombectomy after intravenous t‐PA vs. t‐PA alone in stroke. N Engl J Med. 2015;372:2285–2295. [DOI] [PubMed] [Google Scholar]
- 6. Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribó M, Millán M, Urra X, Cardona P, López‐Cancio E, Tomasello A, Castaño C, Blasco J, Aja L, Dorado L, Quesada H, Rubiera M, Hernandez‐Pérez M, Goyal M, Demchuk AM, von Kummer R, Gallofré M, Dávalos A; REVASCAT Trial Investigators . Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med. 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 7. Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, Davalos A, Majoie CB, van der Lugt A, de Miquel MA, Donnan GA, Roos YB, Bonafe A, Jahan R, Diener HC, van den Berg LA, Levy EI, Berkhemer OA, Pereira VM, Rempel J, Millan M, Davis SM, Roy D, Thornton J, Roman LS, Ribo M, Beumer D, Stouch B, Brown S, Campbell BC, van Oostenbrugge RJ, Saver JL, Hill MD, Jovin TG; HERMES collaborators . Endovascular thrombectomy after large‐vessel ischaemic stroke: a meta‐analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. [DOI] [PubMed] [Google Scholar]
- 8. Okawa M, Tateshima S, Liebeskind D, Rao N, Jahan R, Gonzalez N, Szeder V, Ali L, Kim D, Saver J, Duckwiler G. Early loss of immediate reperfusion while stent retriever in place predicts successful final reperfusion in acute ischemic stroke patients. Stroke. 2015;46:3266–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wenger KJ, Berkefeld J, Wagner M. Flat panel detector computed tomography for the interaction between contrast‐enhanced thrombi and stent retrievers in stroke therapy: a pilot study. Clin Neuroradiol. 2014;24:251–254. [DOI] [PubMed] [Google Scholar]
- 10. Hashimoto T, Hayakawa M, Funatsu N, Yamagami H, Satow T, Takahashi JC, Nagatsuka K, Ishibashi‐Ueda H, Kira JI, Toyoda K. Histopathologic analysis of retrieved thrombi associated with successful reperfusion after acute stroke thrombectomy. Stroke. 2016;47:3035–3037. [DOI] [PubMed] [Google Scholar]
- 11. Froehler MT, Tateshima S, Duckwiler G, Jahan R, Gonzalez N, Vinuela F, Liebeskind D, Saver JL, Villablanca JP; UCLA Stroke Investigators . The hyperdense vessel sign on CT predicts successful recanalization with the Merci device in acute ischemic stroke. J Neurointerv Surg. 2013;5:289–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moftakhar P, English JD, Cooke DL, Kim WT, Stout C, Smith WS, Dowd CF, Higashida RT, Halbach VV, Hetts SW. Density of thrombus on admission CT predicts revascularization efficacy in large vessel occlusion acute ischemic stroke. Stroke. 2013;44:243–245. [DOI] [PubMed] [Google Scholar]
- 13. Mokin M, Morr S, Natarajan SK, Lin N, Snyder KV, Hopkins LN, Siddiqui AH, Levy EI. Thrombus density predicts successful recanalization with solitaire stent retriever thrombectomy in acute ischemic stroke. J Neurointerv Surg. 2015;7:104–107. [DOI] [PubMed] [Google Scholar]
- 14. van Es AC, Autar AS, Emmer BJ, Lycklama À, Nijeholt GJ, van der Kallen BF, van Beusekom HM. Imaging stent‐thrombus interaction in mechanical thrombectomy. Neurology. 2017;88:216–217. [DOI] [PubMed] [Google Scholar]
- 15. Gersh KC, Nagaswami C, Weisel JW. Fibrin network structure and clot mechanical properties are altered by incorporation of erythrocytes. Thromb Haemost. 2009;102:1169–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaijzel EL, Koolwijk P, van Erck MG, van Hinsbergh VW, de Maat MP. Molecular weight fibrinogen variants determine angiogenesis rate in a fibrin matrix in vitro and in vivo. J Thromb Haemost. 2006;4:1975–1981. [DOI] [PubMed] [Google Scholar]
- 17. van der Giessen WJ, Sorop O, Serruys PW, Peters‐Krabbendam I, van Beusekom HM. Lowering the dose of sirolimus, released from a nonpolymeric hydroxyapatite coated coronary stent, reduces signs of delayed healing. JACC Cardiovasc Interv. 2009;2:284–290. [DOI] [PubMed] [Google Scholar]
- 18. Rittersma SZ, van der Wal AC, Koch KT, Piek JJ, Henriques JP, Mulder KJ, Ploegmakers JP, Meesterman M, de Winter RJ. Plaque instability frequently occurs days or weeks before occlusive coronary thrombosis: a pathological thrombectomy study in primary percutaneous coronary intervention. Circulation. 2005;111:1160–1165. [DOI] [PubMed] [Google Scholar]
- 19. Tsumoto T, Tsurusaki Y, Tokunaga S. Interaction between the stent strut and thrombus characterized by contrast‐enhanced high‐resolution cone beam CT during deployment of the solitaire stent retriever. J Neurointerv Surg. 2017;9:843–848. [DOI] [PubMed] [Google Scholar]
- 20. Wolberg AS. Thrombin generation and fibrin clot structure. Blood Rev. 2007;21:131–142. [DOI] [PubMed] [Google Scholar]
- 21. Mutch NJ, Engel R, Uitte de Willige S, Philippou H, Ariëns RA. Polyphosphate modifies the fibrin network and down‐regulates fibrinolysis by attenuating binding of tPA and plasminogen to fibrin. Blood. 2010;115:3980–3988. [DOI] [PubMed] [Google Scholar]
- 22. Gunning GM, McArdle K, Mirza M, Duffy S, Gilvarry M, Brouwer PA. Clot friction variation with fibrin content; implications for resistance to thrombectomy. J Neurointerv Surg. 2017;10:34–38. [DOI] [PubMed] [Google Scholar]
- 23. Yuki I, Kan I, Vinters HV, Kim RH, Golshan A, Vinuela FA, Sayre JW, Murayama Y, Vinuela F. The impact of thromboemboli histology on the performance of a mechanical thrombectomy device. AJNR Am J Neuroradiol. 2012;33:643–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Surface vs Interaction Using SEM. Adhesive interaction was more frequently observed at interaction sites with a dense surface (38 of 58), as compared with adhesive interaction at interaction sites with a porous filamentous surface (6 of 21) (P=0.011)
Table S2. Thrombus Characteristics. The presence of Zahn lines, cholesterol crystals, nuclear fragmentation is given per thrombus and determines thrombus age. In our study most thrombi were classified as lytic according to the criteria by Rittersma et al1
Figure S1. Stent 1. Thrombus entrapped in stent photographed (A) and visualized using micro‐CT (B). Both mechanical (C, D) and adhesive interactions (G) are observed using SEM. Details of the SEM images reveal both porous filamentous and dense surfaces (E and F). Histological staining using hematoxylin‐eosin (H), Okajima (I), and RF (J) show thrombus containing both fibrin and erythrocyte‐rich areas. The fibrin‐like surface is pink in (H), red in (I), and purple in (J).
Figure S2. Stent 2. Thrombus entrapped in stent photographed (A) and visualized using micro‐CT (B). Predominantly mechanical interaction is observed using SEM (C, D). Details of the SEM images reveal that the thrombus surface is mainly dense (E), but spots of porous filamentous surfaces also were observed (F). Histological staining using HE (G), Okajima (H), and RF (I) show thrombus containing mostly erythrocytes. The fibrin‐like surface is pink in (G), red in (H), and purple in (I).
Figure S3. Stent 3. Thrombus entrapped in stent photographed (A) and visualized using micro‐CT (B). Both adhesive (C) and mechanical interactions (D) are observed using SEM. Details of the SEM images reveal that the thrombus surface is mainly dense (E, F, G). Histological staining using hematoxylin‐eosin (H), Okajima (I), and RF (J) show thrombus containing mostly erythrocytes and little fibrin. The fibrin‐like surface is pink in (H), red in (I), and purple in (J).
Figure S4. Stent 4. Thrombus entrapped in stent photographed (A) and visualized using micro‐CT (B). Both mechanical (C) and adhesive interactions (D) are observed using SEM. Details of the SEM images reveal that the thrombus surface is mainly porous filamentous (E, F). Histological staining using hematoxylin‐eosin (G), Okajima (H), and RF (I) show thrombus containing mostly erythrocytes. The fibrin‐like surface is pink in (G), red in (H), and purple in (I).
Figure S5. Stent 5. Thrombus entrapped in stent photographed (A) and visualized using micro‐CT (B). Predominantly adhesive interaction is observed using SEM (C, E). Details of the SEM images reveal that the thrombus surface is mainly dense (D), but spots of porous filamentous surfaces also were observed (F). Histological staining using hematoxylin‐eosin (G), Okajima (H), and RF (I) show thrombus containing mostly erythrocytes. The fibrin‐like surface is pink in (G), red in (H), and purple in (I).
Figure S6. Stent 6. Thrombus entrapped in stent photographed (A) and visualized using micro‐CT (B). Predominantly adhesive interaction is observed using SEM (C and D). Details of the SEM images reveal that the thrombus surface is mainly dense (F), but spots of porous filamentous surfaces also were observed (E). Histological staining using hematoxylin‐eosin (G), Okajima (H), and RF (I) shows thrombus containing both fibrin and erythrocyte‐rich areas. The fibrin‐like surface is pink in (G), red in (H), and purple in (I).
Figure S7. Stent 7. Thrombus entrapped in stent photographed (A) and visualized using micro‐CT (B). Predominantly mechanical interaction is observed using SEM (C and D). Details of the SEM images reveal both porous filamentous and dense surfaces (E and F). Histological staining using hematoxylin‐eosin (G), Okajima (H), and RF (I) show thrombus containing both fibrin and erythrocyte‐rich areas. The fibrin‐like surface is pink in (G), red in (H), and purple in (I).


