Abstract
Background
Adipokines mediate cardiometabolic risk associated with obesity but their role in the pathogenesis of obesity‐associated heart failure remains uncertain. We investigated the associations between circulating adipokine concentrations and echocardiographic measures in a community‐based sample.
Methods and Results
We evaluated 3514 Framingham Heart Study participants (mean age 40 years, 53.8% women) who underwent routine echocardiography and had select circulating adipokines measured, ie, leptin, soluble leptin receptor, fatty acid–binding protein 4, retinol‐binding protein 4, fetuin‐A, and adiponectin. We used multivariable linear regression, adjusting for known correlates (including weight), to relate adipokine concentrations (independent variables) to the following echocardiographic measures (dependent variables): left ventricular mass index, left atrial diameter in end systole, fractional shortening, and E/e′. In multivariable‐adjusted analysis, left ventricular mass index was inversely related to circulating leptin and fatty acid–binding protein 4 concentrations but positively related to retinol‐binding protein 4 and leptin receptor levels (P≤0.002 for all). Left atrial end‐systolic dimension was inversely related to leptin but positively related to retinol‐binding protein 4 concentrations (P≤0.0001). E/e′ was inversely related to leptin receptor levels (P=0.0002). We observed effect modification by body weight for select associations (leptin receptor and fatty acid–binding protein 4 with left ventricular mass index, and leptin with left atrial diameter in end systole; P<0.05 for interactions). Fractional shortening was not associated with any of the adipokines. No echocardiographic trait was associated with fetuin‐A or adiponectin concentrations.
Conclusions
In our cross‐sectional study of a large, young to middle‐aged, relatively healthy community‐based sample, key indices of subclinical cardiac remodeling were associated with higher or lower circulating concentrations of prohypertrophic and antihypertrophic adipokines in a context‐specific manner. These observations may offer insights into the pathogenesis of the cardiomyopathy of obesity.
Keywords: echocardiography, fatty acid–binding protein 4, fetuin‐A, leptin, retinol‐binding protein 4
Subject Categories: Epidemiology, Echocardiography, Biomarkers, Obesity
Clinical Perspective
What Is New?
Data from the Framingham Heart Study indicate that circulating concentrations of adipokines, hormones secreted by the adipose tissue, are associated with echocardiographic markers of cardiac remodeling.
Higher leptin and fatty acid–binding protein 4 levels are associated with lower left ventricular mass index, and leptin is also associated with lower left atrial end‐systolic dimension, which suggests possible antihypertrophic effects of leptin and fatty acid–binding protein 4.
Higher retinol‐binding protein 4 was associated with both higher left ventricular mass index and higher left atrial end‐systolic dimension, suggesting possible prohypertrophic effects of retinol‐binding protein 4.
What Are the Clinical Implications?
The link between obesity and heart failure, so‐called obesity cardiomyopathy, might be mediated via the effect of several adipokines on cardiac remodeling.
The observation that serum leptin levels increase with higher body mass index but are associated with lower left ventricular mass index could be explained by leptin resistance in obesity and may be important when leptin‐activity blockers are considered as potential therapeutic targets in cachectic conditions.
Retinol‐binding protein 4 may be a potential therapeutic target in the cardiomyopathy of obesity.
Obesity is a key risk factor for heart failure (HF) in multiple studies.1, 2, 3, 4 The underlying mechanisms by which excess adiposity elevates HF risk are multifactorial, ranging from increased hemodynamic pressure and volume overload, adverse cardiac remodeling, neurohormonal alterations, concomitant cardiometabolic disturbances and small vessel disease, to coexistent comorbidities such as sleep apnea.5
Attention has recently focused on the potential existence of a distinctive cardiomyopathy related to obesity that is characterized by structural and functional cardiac remodeling.5 Excess adiposity has been associated with concentric and eccentric left ventricular (LV) hypertrophy (LVH), LV diastolic dysfunction, left atrial enlargement, pulmonary hypertension, and right ventricular hypertrophy.6 In this context, it is noteworthy that the adipose tissue is an endocrine tissue which secretes hormones (referred to as adipokines) that mediate several of the cardiometabolic consequences of obesity. Several adipokines have receptors on cardiac myocytes,7, 8, 9 exhibit prohypertrophic and antihypertrophic effects on the myocardium,10, 11, 12, 13 and have been associated with risk of overt HF.14 Prior investigations relating adipokines to cardiac structure and function have focused on single adipokines10, 11, 15, 16, 17, 18, 19, 20, 21 and related them to traditional measures of cardiac remodeling (such as LV mass [LVM]). These studies often focused on obese individuals with comorbidities16, 22, 23, 24, 25, 26, 27, 28 or the elderly20, 21, 29 and were limited by modest sample sizes. To our knowledge, no prior investigation has related a panel of adipokines of contemporary interest to a range of cardiac remodeling indices in a younger sample relatively free of comorbidities.
Accordingly, we investigated the cross‐sectional associations between a panel of adipokines and echocardiographic indices of cardiac remodeling in a community‐based sample of young to middle‐aged adults. We hypothesized that antihypertrophic adipokines would be associated with lower LVM, left atrial diameter in end systole (LAD), and better LV systolic and diastolic function, whereas prohypertrophic adipokines would demonstrate a reverse pattern of associations.
Methods
Study Sample
The design and selection criteria of the Framingham Heart Study (FHS) Third Generation and Omni 2 cohorts have been previously published.30 The study sample for this investigation was comprised of participants enrolled in these cohorts who attended their first examination cycle (2002–2005). The institutional review board of Boston University Medical Center approved the study protocol and all participants provided written informed consent. The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
We evaluated 3634 attendees at the first examination who had data on echocardiographic measures and circulating adipokine concentrations (see below). We excluded individuals with prevalent cardiovascular disease, previous heart valve disease, coronary bypass surgery, or missing covariates (n=120), leaving 3514 individuals eligible for the present investigation.
Measurements of Covariates and Adipokines
At their FHS examinations, participants provided a detailed medical history and underwent phlebotomy (after an overnight fast) for laboratory assessment of standard cardiovascular risk factors (including a lipid panel and renal function) and a cardiovascular‐targeted physical examination that included anthropometry and blood pressure measurements performed using standardized protocols. We defined hypertension as a systolic blood pressure ≥140 mm Hg or a diastolic blood pressure ≥90 mm Hg or the current use of antihypertensive medications. Participants were classified as having diabetes mellitus if they had fasting glucose concentrations ≥126 mg/dL, nonfasting glucose concentrations ≥200 mg/dL, or used any hypoglycemic medication. Current smoking was defined as smoking regularly during the year antedating the FHS examination.
We assayed (on stored blood samples frozen at −80°C) plasma concentrations of fetuin‐A and fatty acid–binding protein 4 (FABP4) using sandwich ELISA (BioVendor Research and Diagnostic Products), and leptin, its soluble leptin receptor (LR), retinol‐binding protein 4 (RBP4) and adiponectin concentrations using ELISA (R&D Systems).31 The average interassay coefficients of variation for the adipokines were <5%.31 The ratio of leptin to LR concentrations yielded the free leptin index (FLI), which is a measure of bioavailable leptin that is not bound to its soluble receptor.
Echocardiographic Indices
All participants underwent routine transthoracic echocardiography with Doppler color flow and Tissue Doppler imaging during their examination using a Hewlett‐Packard 5500 machine (Philips Healthcare). We investigated the following echocardiographic indices: primary measures (see below): LVM index (LVMI), LAD, LV fractional shortening (FS), and diastolic function (E/e′); and secondary measures: LV end‐diastolic dimension (LVDD), LV end‐systolic dimension (LVSD), and LV wall thickness (LVWT).
We used 2‐dimensionally guided M‐mode digital images and a leading edge‐to‐leading edge technique (recommended by the American Society of Echocardiography)32 to measure end‐diastolic thicknesses of the LV septum and posterior wall (summed to calculate wall thickness [LVWT]), LVDD, LVSD, and LAD. LVM was calculated using the method by Devereux et al as equal to (0.8×1.04 ((LVDD+septal wall thickness+posterior wall thickness)3−LVDD3)+0.6).33 LVM was indexed to height (as an obesity‐independent measure) in meters to create LVMI.
To assess LV systolic function, we used FS defined as [(LVDD−LVSD)/LVDD]×100. E′ was measured using tissue Doppler imaging at the lateral mitral annulus and E was measured using transmitral Doppler flow velocities.34 The ratio of early transmitral flow velocity (E) and early systolic mitral annulus velocity (e′) was used as an indicator of LV diastolic function. The reproducibility of echocardiographic measurements at FHS has been previously reported.34, 35
Statistical Analysis
We generated baseline characteristics separately for men and women and compared the respective means, medians, or proportions using 2‐sided t test, Wilcoxon rank sum test, or chi‐square test, as appropriate. All adipokine levels were standardized within sex, and subsequent analyses were performed for pooled sexes. LVMI and E/e′ were natural‐logarithmically transformed to normalize their skewed distributions. In our primary analysis, we examined the association between the adipokines (independent variables, modeled individually as continuous variables to maximize statistical power) and LVMI, LAD, FS, and E/e′ as the dependent variables (separate models for each trait) using multivariable linear regression models. We chose these specific echocardiographic measures as our primary traits because they reflect complementary aspects of cardiac structure and LV function, and have been associated with obesity in prior reports.36, 37, 38 Generalized estimating equations were used in all regression analyses to account for relatedness among individuals in the Gen 3 cohort. Multivariable models adjusted for cohort type (Third Generation versus Omni 2), age, sex, height, weight, systolic and diastolic blood pressure, antihypertensive medications, heart rate, diabetes mellitus, smoking status, blood lipids (high‐density lipoprotein, low‐density lipoprotein, and triglycerides), and serum creatinine. Additional models were evaluated where body mass index was incorporated as a covariate instead of height and weight.
For the adipokines that demonstrated a statistically significant association with any echocardiographic trait, we fitted additional models incorporating the adipokines conjointly. As leptin, LR, and FLI are correlated markers (the latter being derived as the ratio of the concentrations of the 2 former biomarkers), we did not include all of them in the same model. We tested for effect modification by body weight of any potential association between adipokines and echocardiographic measures by incorporating appropriate interaction terms into the models (adipokines×weight, where the primary effects for the adipokines were statistically significant). If the interaction term was statistically significant (P<0.05), we stratified analyses by the sex‐specific median weight.
To gain insights into potential associations of adipokines with LVMI or FS, we performed secondary analyses relating adipokines to LVDD and LVWT (components used to estimate LVMI) and LVSD (used with LVDD to calculate FS). To better interpret the effect sizes from the regression analyses, ie, the quantitative changes in echocardiographic measures with increments in circulating adipokine concentrations, we estimated additional multivariable models that fitted sex‐specific tertiles of those adipokines that were associated with echocardiographic traits, and pictorially displayed the adjusted least square means and their standard errors for the corresponding echocardiographic traits. Log‐LVMI and log‐E/e′ were exponentiated back to their native units for ease of interpretation.
To account for multiple statistical testing, we used a Bonferroni‐corrected P value of ≤0.002 (0.05/24, the denominator being 6 [number of adipokines tested]×4 [number of primary echocardiographic traits analyzed]). All analyses were performed using SAS version 9.4 (SAS Institute).
Results
Clinical Characteristics
The clinical characteristics of our young to middle‐aged (mean age 40 years, 53.8% women) study sample are presented in Table 1. The sample was relatively healthy, with a low prevalence of hypertension, diabetes mellitus, smoking, and dyslipidemia. Approximately 20% of the participants were obese.
Table 1.
Baseline Characteristics of the Study Sample
| Men (n=1625) | Women (n=1889) | |
|---|---|---|
| Clinical characteristics | ||
| Age, y | 40±9 | 40±9 |
| Nonwhite, % | 5.9 | 6.8 |
| Height, cma | 178±7 | 164±6 |
| Weight, kga | 87±15 | 68±15 |
| Body mass index, kg/m2 a | 27.4±4.3 | 25.5±5.4 |
| Obesity, %a | 22.4 | 17.3 |
| Heart rate, beats per mina | 58±9 | 61±9 |
| Systolic blood pressure, mm Hga | 120±13 | 113±14 |
| Diastolic blood pressure, mm Hga | 78±9 | 72±9 |
| Hypertension, %a | 19.8 | 11.5 |
| Current smoking, % | 15.0 | 13.6 |
| Fasting blood glucose, mg/dLa | 96 (91, 101) | 89 (85, 95) |
| Diabetes mellitus, %a | 3.4 | 2.0 |
| Serum creatinine, mg/dLa | 0.91±0.13 | 0.70±0.11 |
| Low‐density lipoprotein, mg/dLa | 120±31 | 105±30 |
| High‐density lipoprotein, mg/dLa | 47±13 | 62±16 |
| Triglycerides, mg/dLa | 105 (71, 154) | 80 (59, 112) |
| Lipid‐lowering treatment, %a | 8.7 | 3.3 |
| Circulating adipokine concentrations | ||
| Leptin, pg/mLa | 4027 (2277, 6940) | 11 912 (6401, 22 215) |
| Leptin receptor, ng/mLa | 18.5 (13.1, 23.5) | 19.4 (13.7, 25.2) |
| Free leptin indexa | 237 (113, 478) | 632 (302, 1356) |
| FABP4, ng/mLa | 14.8 (10.7, 19.4) | 17.9 (13.2, 25.4) |
| RBP4, μg/mLa | 42.6 (36.4, 49.1) | 36.5 (30.3, 44.6) |
| Fetuin‐A, μg/mLa | 422 (332, 531) | 447 (346, 575) |
| Adiponectin, ng/mLa | 5119 (3276, 7897) | 10 047 (6653, 14 656) |
| Echocardiographic characteristics | ||
| LV wall thickness, cma | 2.0±0.2 | 1.7±0.2 |
| LV systolic dimension, cma | 3.3±0.3 | 3.0±0.3 |
| LV diastolic dimension, cma | 5.2±0.4 | 4.7±0.3 |
| LV mass index, g/ma | 104 (93, 117) | 77 (69, 88) |
| LAD, cma | 3.9±0.4 | 3.5±0.4 |
| Fractional shortening, %a | 35±3 | 36±3 |
| E/e′a | 5.4 (4.7, 6.3) | 5.6 (4.9, 6.7) |
Data are shown as means±SD or median (quartile 1, quartile 3) for continuous variables and as frequency (percentage) for categorical variables. Left ventricular (LV) mass is indexed to height. FABP4 indicates fatty acid–binding protein 4; LAD, left atrial diameter in end systole; RBP4, retinol‐binding protein‐4.
Statistically significant differences between men and women (using 2‐sided t test, Wilcoxon rank sum test, or chi‐square test, as appropriate).
Association Between Adipokines and Echocardiographic Indices
In multivariable‐adjusted analyses, LVMI was inversely associated with circulating leptin, FABP4 concentrations and FLI, but positively related to LR and RBP4 concentrations. LAD was inversely related to plasma leptin levels and FLI, but positively associated with blood RBP4 concentrations. Blood concentrations of LR were inversely associated with E/e′. These results remained essentially unchanged in secondary analyses adjusting for body mass index instead of height and weight (Table S1). Analyses of components of LVMI and LVSD yielded findings concordant with our primary analyses: leptin, FLI, and FABP4 were inversely associated with both LVDD and LVWT, whereas LR was positively associated with LVWT, and RBP4 with LVDD (Table S2). Associations of these adipokines with LVSD mirrored their associations with LVDD. Figures 1 and 2 and Figure S1 display the relationship of sex‐specific tertiles of the adipokines significantly associated with LVMI, LAD, and E/e′ in Table 2 to values of these traits in their native units, respectively.
Figure 1.
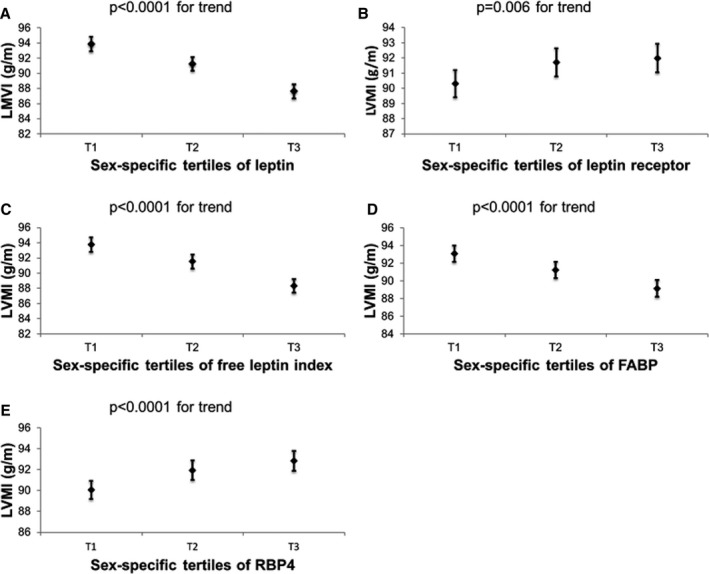
Least squares means and their standard errors of left ventricular mass index (LVMI) according to sex‐specific tertiles of (A) leptin, (B) leptin receptor, (C) free leptin index, (D) fatty acid–binding protein 4 (FABP), and (E) retinol‐binding protein 4 (RBP4). All models were adjusted for cohort, age, sex, height, weight, systolic blood pressure, diastolic blood pressure, heart rate, diabetes mellitus, antihypertensive treatment, smoking, creatinine, high‐density lipoprotein, low‐density lipoprotein, and triglycerides.
Figure 2.
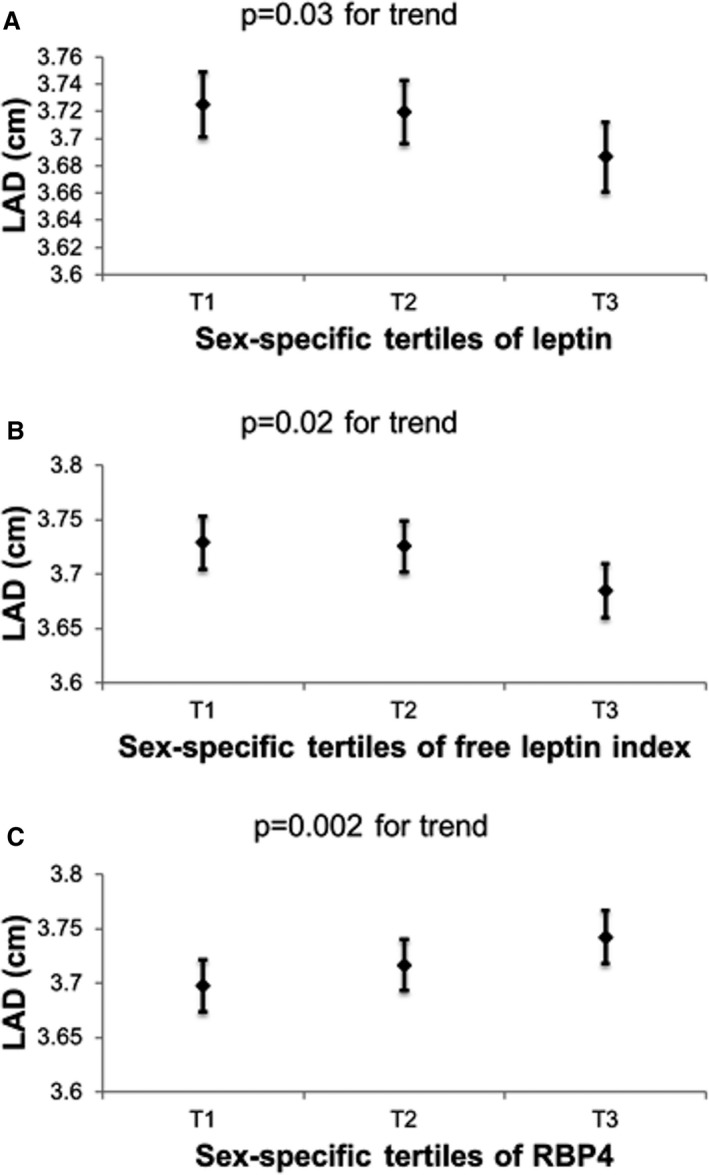
Least squares means and their standard errors of left atrial diameter in end systole (LAD) according to sex‐specific tertiles of (A) leptin, (B) free leptin index, and (C) retinol‐binding protein 4 (RBP4). All models were adjusted for cohort, age, sex, height, weight, systolic blood pressure, diastolic blood pressure, heart rate, diabetes mellitus, antihypertensive treatment, smoking, serum creatinine, high‐density lipoprotein, low‐density lipoprotein, and triglycerides.
Table 2.
Associations Between Circulating Adipokine Concentrations and Echocardiographic Indices
| Cardiac Structural Measures | LV Function Measures | |||||||
|---|---|---|---|---|---|---|---|---|
| Log‐LVMI | LAD | FS | Log‐E/e′ | |||||
| Estimate | P Value | Estimate | P Value | Estimate | P Value | Estimate | P Value | |
| Leptin | −0.037 | <0.0001a | −0.039 | <0.0001a | 0.009 | 0.92 | 0.009 | 0.14 |
| LR | 0.009 | 0.002a | 0.008 | 0.21 | −0.107 | 0.08 | −0.016 | 0.0002a |
| FLI | −0.024 | <0.0001a | −0.028 | 0.001a | 0.050 | 0.52 | 0.014 | 0.0055 |
| FABP4 | −0.019 | <0.0001a | −0.010 | 0.18 | 0.047 | 0.52 | 0.002 | 0.61 |
| RBP4 | 0.012 | <0.0001a | 0.025 | 0.0001a | −0.068 | 0.29 | −0.007 | 0.09 |
| Fetuin‐A | −0.002 | 0.45 | −0.006 | 0.24 | 0.11 | 0.04 | 0.005 | 0.13 |
| Adiponectin | −0.002 | 0.57 | 0.008 | 0.20 | −0.061 | 0.34 | 0.004 | 0.26 |
Estimates are β coefficients per 1‐SD increase for each within‐sex standardized adipokine. Models are adjusted for cohort, age, sex, height, weight, systolic blood pressure, diastolic blood pressure, heart rate, diabetes mellitus, antihypertensive treatment, smoking, serum creatinine, high‐density lipoprotein, low‐density lipoprotein, and triglycerides. FABP4 indicates fatty acid–binding protein 4; FLI, free leptin index; FS, fractional shortening; LAD, left atrial diameter in end systole; LR, leptin receptor; LV, left ventricular; LVMI, LV mass index; RBP4, retinol‐binding protein 4.
Bonferroni‐corrected P≤0.002.
FS was not associated with any of the adipokines. Circulating concentrations of fetuin‐A and adiponectin were not associated with any of the echocardiographic traits.
Modeling Adipokines Conjointly in Relation to LV Traits
When modeled conjointly, LVMI was associated inversely with circulating leptin (and FLI) and FABP4 concentrations, but positively with RBP4 and LR levels. In these analyses, the adipokines recapitulated the results of the main analyses in terms of the statistical significance, effect size, and directionality of associations (Table 3).
Table 3.
Association Between Log‐LVMI and Adipokines: Conjoint Modeling of Circulating Leptin, LR, FLI, FABP4, and RBP4 Concentrations
| Estimate | P Value | |
|---|---|---|
| (A) Model including leptin, FABP4, and RBP4 | ||
| Leptin | −0.034 | <0.0001a |
| FABP4 | −0.014 | <0.0001a |
| RBP4 | 0.012 | <0.0001a |
| (B) Model including LR, FABP4, and RBP4 | ||
| LR | 0.009 | 0.0013a |
| FABP4 | −0.020 | <0.0001a |
| RBP4 | 0.012 | 0.0001a |
| (C) Model including FLI, FABP4, and RBP4 | ||
| FLI | −0.022 | <0.0001a |
| FABP4 | −0.017 | <0.0001a |
| RBP4 | 0.012 | <0.0001a |
Estimates are β coefficients per 1‐SD increase for each within‐sex standardized adipokine. Left ventricular mass index (LVMI) was log‐transformed. Models are adjusted for cohort, age, sex, height and weight, systolic blood pressure, diastolic blood pressure, heart rate, antihypertensive medication, smoking, serum creatinine, high‐density lipoprotein, low‐density lipoprotein, and triglycerides. FABP4 indicates fatty acid–binding protein 4; FLI, free leptin index; LR, leptin receptor; RBP4, retinol‐binding protein 4.
Bonferroni‐corrected P≤0.002.
Effect Modification by Weight and Stratified Analyses
We observed several statistically significant interactions: between LR and weight for LVMI (P=0.007), FABP4 and weight for LVMI (P=0.03), and leptin and weight for LAD (P=0.01). Accordingly, we stratified individuals into 2 groups based on the sex‐specific median weight (65.3 kg in women and 84.8 kg in men). In these analyses, directionality of associations for the adipokines were similar to the main analyses and statistical significance was maintained in both weight strata (using a P<0.05 threshold for these secondary analyses). Effect sizes for leptin (in relation to LAD) and FABP4 (with LVMI) were of a greater magnitude in the stratum with weight below the sex‐specific median, ie, standardized regression coefficients were 2‐ to 3‐fold compared with that for the group at or above the median weight. For LR, the association with LVMI was larger (effect size) in the group with weight above the median, but the quantitative estimates were close (Table S3).
Discussion
Obesity has been consistently associated with LVH and subtle myocardial dysfunction in numerous studies.36, 37, 38 Recent reports5 have proposed the existence of a distinct condition termed the cardiomyopathy of obesity, which has been hypothesized to be an adipocyte‐mediated “paracrine” disorder.39 Accordingly, investigators have related circulating concentrations of adipokines to imaging indices of cardiac remodeling. These prior studies have typically been conducted in small samples, or samples with an overrepresentation of obese individuals or elderly people, and usually focused on a single adipokine.10, 11, 13, 21, 22, 23, 24, 25, 26
Principal Findings
We related a panel of established and novel adipokines to a group of echocardiographic traits that reflect distinct aspects of cardiac structural and functional remodeling in a young to middle‐aged cohort relatively free of comorbidities.
Our principal findings are 4‐fold. First, we observed that leptin and FABP4 concentrations were inversely related to LVMI, whereas LR and RBP4 concentrations were positively associated. The standardized regression coefficients for leptin levels (for LVMI) were almost twice that for FABP4 and RBP4 concentrations. Figure 1 suggests that the absolute increment in LVMI from the first to the top tertile was modest (2–5 g/m). Evaluation of components of LVMI revealed associations of these adipokines with LVDD and LVWT that were directionally concordant with those observed for LVMI. These associations for LVMI were maintained when the adipokines were conjointly modeled, consistent with the potential independent influences they may have on cardiac remodeling traits. Overall, these findings are consistent with the concept that some adipokines are antihypertrophic, whereas others are prohypertrophic (see below). They are also in line with putative influences of the adipokines on both LV cavity size and wall thickness. We speculate, therefore, that LV geometry in obese individuals may be determined by the relative balance of the antihypertrophic and prohypertrophic mediators because levels of both types of adipokines are elevated in obesity. This premise warrants further evaluation in future studies of larger samples of elderly individuals and in individuals with a greater burden of comorbidities.
Second, associations of LAD with circulating leptin, FLI, and RBP4 concentrations were directionally concordant with the relationship of these adipokines with LVMI, ie, adipokines inversely associated with LVMI were associated inversely with LAD, and vice versa. The concordant directionality of associations makes physiological sense as a greater LVMI is typically accompanied by an increased LAD, and both LVH and left atrial enlargement are common echocardiographic features associated with excess adiposity.40
Third, we observed effect modification of associations of select adipokines with LVMI and LAD by body weight. Associations in the 2 weight strata were directionally concordant with those in the main analyses, yet effect sizes were modestly different, quantitatively speaking. For instance, the magnitude of association for leptin with LAD was twice as large in the group with weight below the sex‐specific median (consistent with the concept of leptin resistance in obesity [see below]). These observations are consistent with the concept that the effect of adipokines on cardiac remodeling may be context‐specific, with the degree of obesity being a distinctive modifier.
Fourth, fetuin‐A and adiponectin were not associated with any of the echocardiographic measures. On a parallel note, FS was not associated with any of the adipokines. It is conceivable that the range of values for echocardiographic measures was too narrow in our relatively healthy young to middle‐aged sample to permit elucidation of putative associations with adipokines. As noted above, associations for select adipokines may be more evident given a background with greater obesity and/or comorbidity.
Comparisons With the Published Literature
Associations of leptin, its soluble receptor, and FLI with cardiac remodeling
We observed an inverse association of leptin concentrations with LVMI and LAD, and of FLI with LVMI, whereas LR was positively associated with LVMI and inversely associated with E/e′. These results are consistent with an antihypertrophic effect of leptin on cardiomyocytes.10, 15
Previous publications reporting the associations between leptin and cardiac mass and function have yielded varying results. Similar to our observations, several population‐based studies, including MESA (Multi‐Ethnic Study of Atherosclerosis)10 and a previous study of elderly participants in the original cohort of FHS,29 have reported an inverse association between circulating leptin concentrations and LVM but no association with measures of LV systolic function.29 In contrast, other observational studies of men with hypertension,22 of severely obese patients23 and of patients with myocardial infarction24 have reported a positive association between leptin and cardiac hypertrophy. Furthermore, in patients with CAD16 and acute myocardial infarction,24 a positive association has been reported between circulating leptin levels and markers of LV diastolic dysfunction. This inconsistency in the published literature might be potentially explained by the possible development of leptin end‐organ resistance in the heart with increasing obesity or in the context of ischemic injury. Leptin resistance at the hypothalamic level is well described in obesity,41 yet it is unclear whether such end‐organ resistance extends to other tissues with LRs.
The negative association between circulating LR concentrations and E/e′ might indicate a favorable effect of LR and, therefore, an adverse effect of leptin on LV diastolic function (as suggested by some reports noted above16, 24). This finding is challenging to reconcile with the inverse relationship of leptin and LVMI because typically an increase in LVMI is a frequent concomitant finding of LV diastolic dysfunction. However, circulating leptin levels have been associated with increased risk of HF,42, 43 an observation that may be consistent with an adverse effect of leptin on cardiac remodeling and diastolic function.
In summary, the putative effects of leptin on the cardiovascular system are likely complex44 and need further investigation and careful consideration, especially as leptin‐activity blockers are viewed as potential therapeutic targets in cachectic conditions.45
Associations of circulating FABP 4 with echocardiographic measures
FABP4 binds hydrophobic molecules including fatty acids and facilitates their transport across membranes. It is highly expressed in and secreted by the adipose tissue46 but is also expressed in several other tissues including the heart.8 We observed an inverse association between circulating FABP4 levels and LVMI. Prior studies analyzing associations between FABP4 levels and LVMI have been inconsistent with reports of both no associations11 and of positive associations.25, 26, 27
The previous publications are not directly comparable to our investigation because of their smaller sample sizes11 (N<200), referral bias,25, 26, 27 and focus on obese individuals,25, 26, 27 whereas our study sample comprised relatively healthy individuals. In this context, it is noteworthy that experimental studies have also yielded variable results. A recent investigation reported that FABP4 overexpression in mice was associated with cardiac hypertrophy only in the presence of aorta constriction,8 whereas another study noted a dose‐dependent negative inotropic effect of FABP4 on rat cardiomyocytes.47 It is therefore possible that FABP4 has different effects on the heart based on underlying hemodynamic conditions. A recent population‐based study linked higher FABP4 levels to an increased risk of HF.14 Thus, the effects of FABP4 on the heart remain unclear and additional studies of larger samples are warranted to replicate (or refute) our observations.
Associations of circulating RBP4 with cardiac remodeling
RBP4 is secreted by the liver and the adipose tissue (it is both a hepatokine and an adipokine), and higher blood levels have been linked to insulin resistance, diabetes mellitus, and coronary artery disease in some reports.48, 49, 50, 51 We observed that circulating RBP4 levels were positively associated with LVMI and LAD. These findings are in line with 2 previous experimental studies that reported an association between RBP4 and cardiac hypertrophy.9, 12 However, our observations are in contrast to another report that noted lack of any association of circulating RBP4 with echocardiographic traits (including LVM) in a moderately sized elderly cohort of men.20 These differences across reports may be related to differences in the study sample characteristics (such as age) and also sample size. In summary, the putative association between circulating RBP4 levels and cardiac hypertrophy needs further investigation in larger samples with a wider age range.
Associations of circulating fetuin‐A with echocardiographic traits
Fetuin‐A is a phosphorylated glycoprotein mainly secreted by the liver but is expressed in adipose tissue as well (it is also a hepatokine/adipokine). Circulating fetuin‐A levels increase with increasing body mass index. As a tyrosine kinase inhibitor, fetuin‐A is linked to insulin resistance and diabetes mellitus but it is also an inhibitor of calcification by building complexes with calcium and phosphorus.52, 53 Circulating fetuin‐A concentrations were not associated with any of the echocardiographic traits we evaluated in our sample. It is challenging to compare our reports with the published literature because prior studies of fetuin‐A and LV remodeling have largely focused on patients with varying degrees of CKD, in which context inverse associations with LV diastolic dysfunction28 and LVH54 have been described. Future investigations of blood fetuin‐A concentrations and cardiac remodeling in community‐based samples are warranted to replicate our findings.
Associations of circulating adiponectin with cardiac remodeling
In the present investigation, we did not observe any associations between adiponectin and the echocardiographic indices evaluated. These observations are consistent with some prior reports55 but not with others that have reported both inverse13 and positive18 relationships of circulating adiponectin concentrations with LVM. One potential reason for the inconsistent results in the literature may be because adiponectin associations are context‐specific, with an inverse association being observed in individuals at low risk for LVH but a positive association in patients at higher risk.18, 56
Experimental results are more consistent in this regard. In adiponectin‐deficient mice, pressure overload leads to an increase in LVH and cardiomyocyte size compared with wild‐type mice. These findings are consistent with increased extracellular signal‐regulated kinase and diminished AMP‐activated protein kinase signaling in the myocardium in adiponectin‐deficient mice.57 Additional studies of larger samples that encompass a wider distribution of cardiovascular disease risk factors and comorbidities may yield additional insights into context‐specific effects of adiponectin on cardiac remodeling.
Limitations and Strengths
Our study sample was comprised of young to middle‐aged relatively healthy participants with a low prevalence of cardiovascular disease risk factors and comorbidities. As such, our results may not be generalizable to other age groups or to samples of individuals at higher risk with a greater prevalence of comorbidities. In addition, our study design was cross‐sectional, which precludes any causal inferences.
Despite these limitations, our investigation is strengthened by its large community‐based sample, assays of an array of adipokines (including several of contemporary interest), and our evaluation of a range of echocardiographic traits reflecting distinctive aspects of cardiac structural and functional remodeling.
Conclusions
In our investigation of a large, young to middle‐aged, relatively healthy community‐based sample, we observed a distinctive pattern of association of adipokines with cardiac remodeling. Higher circulating leptin and FABP4 levels were associated with lower LVMI, whereas higher LR and RBP4 concentrations were positively associated with LVMI. Associations with LAD were directionally consistent (with inverse association with leptin levels and positive association with RBP4 concentrations). We observed effect modification of these associations by weight consistent with a context‐dependent influence of adipokines on cardiac remodeling that may vary with the degree of adiposity and presence/absence of comorbidities. These observations suggest that the potential role of adipokines as paracrine mediators of obesity‐induced cardiomyopathy39 is likely complex and warrants further investigation against a varying background of cardiac stressors (including age, obesity, insulin resistance, and other comorbidities, as our study sample was relatively healthy).
Sources of Funding
This work was supported by the National Heart, Lung, and Blood Institute contracts N01‐HC‐25195 and HHSN268201500001I (Vasan) and the following grants: R01HL131532 (Cheng), R01HL134168 (Cheng), R01 DK080739, R01HL126136, R01 HL 080124, R01 HL 077477 (Vasan), and R01 HL 70100. von Jeinsen was supported by the German Heart Foundation/German Foundation of Heart Research.
Disclosures
Mitchell is the owner of Cardiovascular Engineering, Inc—a company that develops and manufactures devices to measure vascular stiffness—and serves as a consultant to and receives honoraria from Novartis, Merck, Servier, and Philips. The remaining authors have no disclosures to report.
Supporting information
Table S1. Associations Between Circulating Adipokine Concentrations and Echocardiographic Indices: Adjusted for BMI Instead of Height and Weight
Table S2. Secondary Analysis of Associations Between Adipokine Levels and the Components of LVMI and LV End‐Systolic Dimensions
Table S3. Associations of Circulating Adipokine Concentrations and Echocardiographic Indices Stratified by Sex‐Specific Median Weight
Figure S1. Least square means of E/e′ according to sex‐specific tertile of circulating concentrations of soluble leptin receptor.
(J Am Heart Assoc. 2018;7:e008997 DOI: 10.1161/JAHA.118.008997.)
References
- 1. Schmidt M, Bøtker HE, Pedersen L, Sørensen HT. Young adulthood obesity and risk of acute coronary syndromes, stable angina pectoris, and congestive heart failure: a 36‐year cohort study. Ann Epidemiol. 2014;24:356–361. [DOI] [PubMed] [Google Scholar]
- 2. Levitan EB, Yang AZ, Wolk A, Mittleman MA. Adiposity and incidence of heart failure hospitalization and mortality: a population‐based prospective study. Circ Heart Fail. 2009;2:202–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kenchaiah S, Sesso HD, Gaziano JM. Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation. 2009;119:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kenchaiah S, Evans J, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. [DOI] [PubMed] [Google Scholar]
- 5. Wong C, Marwick TH. Obesity cardiomyopathy: pathogenesis and pathophysiology. Nat Clin Pract Cardiovasc Med. 2007;4:436–443. [DOI] [PubMed] [Google Scholar]
- 6. Wong C, Marwick TH. Alterations in myocardial characteristics associated with obesity: detection, mechanisms, and implications. Trends Cardiovasc Med. 2007;17:1–5. [DOI] [PubMed] [Google Scholar]
- 7. Ghantous CM, Azrak Z, Hanache S, Abou‐Kheir W, Zeidan A. Differential role of leptin and adiponectin in cardiovascular system. Int J Endocrinol. 2015;2015:534320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang J, Qiao C, Chang L, Guo Y, Fan Y, Villacorta L, Chen YE, Zhang J. Cardiomyocyte overexpression of FABP4 aggravates pressure overload‐induced heart hypertrophy. PLoS One. 2016;11:e0157372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gao W, Wang H, Zhang L, Cao Y, Bao JZ, Liu ZX, Wang LS, Yang Q, Lu X. Retinol‐binding protein 4 induces cardiomyocyte hypertrophy by activating TLR4/MyD88 pathway. Endocrinology. 2016;157:2282–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sharma S, Colangelo LA, Allison MA, Lima JAC, Ambale‐Venkatesh B, Kishi S, Liu K, Greenland P. Association of serum leptin with future left ventricular structure and function: the Multi‐Ethnic Study of Atherosclerosis (MESA). Int J Cardiol. 2015;193:64–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fuseya T, Furuhashi M, Yuda S, Muranaka A, Kawamukai M, Mita T, Ishimura S, Watanabe Y, Hoshina K, Tanaka M, Ohno K, Akasaka H, Ohnishi H, Yoshida H, Saitoh S, Shimamoto K, Miura T. Elevation of circulating fatty acid‐binding protein 4 is independently associated with left ventricular diastolic dysfunction in a general population. Cardiovasc Diabetol. 2014;13:126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kraus BJ, Sartoretto JL, Polak P, Hosooka T, Shiroto T, Eskurza I, Lee SA, Jiang H, Michel T, Kahn BB. Novel role for retinol‐binding protein 4 in the regulation of blood pressure. FASEB J. 2015;29:3133–3140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. McManus DD, Lyass A, Ingelsson E, Massaro JM, Meigs JB, Aragam J, Benjamin EJ, Vasan RS. Relations of circulating resistin and adiponectin and cardiac structure and function: the Framingham Offspring Study. Obesity. 2012;20:1882–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Djoussé L, Bartz TM, Ix JH, Kochar J, Kizer JR, Gottdiener JS, Tracy RP, Mozaffarian D, Siscovick DS, Mukamal KJ, Zieman SJ. Fatty acid‐binding protein 4 and incident heart failure: the Cardiovascular Health Study. Eur J Heart Fail. 2013;15:394–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Allison MA, Bluemke DA, McClelland R, Cushman M, Criqui MH, Polak JF, Lima JA. Relation of leptin to left ventricular hypertrophy (from the Multi‐Ethnic Study of Atherosclerosis). Am J Cardiol. 2013;112:726–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Puurunen VP, Lepojärvi ES, Piira OP, Hedberg P, Junttila MJ, Ukkola O, Huikuri HV. High plasma leptin levels are associated with impaired diastolic function in patients with coronary artery disease. Peptides. 2016;84:17–21. [DOI] [PubMed] [Google Scholar]
- 17. Norvik JV, Schirmer H, Ytrehus K, Jenssen TG, Zykova SN, Eggen AE, Eriksen BO, Solbu MD. Low adiponectin is associated with diastolic dysfunction in women: a cross‐sectional study from the Tromsø Study. BMC Cardiovasc Disord. 2017;17:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bidulescu A, Liu J, Musani SK, Fox ER, Samdarshi TE, Sarpong DF, Vaccarino V, Wilson PW, Arnett DK, Din‐Dzietham R, Taylor HA, Gibbons GH. Association of adiponectin with left ventricular mass in blacks: the Jackson Heart Study. Circ Heart Fail. 2011;4:747–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pääkkö T, Ukkola O, Ikäheimo M, Kesäniemi YA. Plasma adiponectin levels are associated with left ventricular hypertrophy in a random sample of middle‐aged subjects. Ann Med. 2010;42:143–149. [DOI] [PubMed] [Google Scholar]
- 20. Ingelsson E, Lind L. Circulating retinol‐binding protein 4 and subclinical cardiovascular disease in the elderly. Diabetes Care. 2009;32:733–735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gustafsson S, Lind L, Zethelius B, Venge P, Flyvbjerg A, Söderberg S, Ingelsson E. Adiponectin and cardiac geometry and function in elderly: results from two community‐based cohort studies. Eur J Endocrinol. 2010;162:543–550. [DOI] [PubMed] [Google Scholar]
- 22. Paolisso G, Tagliamonte MR, Galderisi M, Zito GA, Petrocelli A, Carella C, de Divitiis O, Varricchio M. Plasma leptin level is associated with myocardial wall thickness in hypertensive insulin‐resistant men. Hypertension. 1999;34:1047–1052. [DOI] [PubMed] [Google Scholar]
- 23. Perego L, Pizzocri P, Corradi D, Maisano F, Paganelli M, Fiorina P, Barbieri M, Morabito A, Paolisso G, Folli F, Pontiroli AE. Circulating leptin correlates with left ventricular mass in morbid (grade III) obesity before and after weight loss induced by bariatric surgery: a potential role for leptin in mediating human left ventricular hypertrophy. J Clin Endocrinol Metab. 2005;90:4087–4093. [DOI] [PubMed] [Google Scholar]
- 24. Piestrzeniewicz K, Luczak K, Maciejewski K, Goch JH. Impact of obesity and adipokines on cardiac structure and function in men with first myocardial infarction. Arch Med Sci. 2008;4:152–160. [Google Scholar]
- 25. Baessler A, Lamounier‐Zepter V, Fenk S, Strack C, Lahmann C, Loew T, Schmitz G, Blüher M, Bornstein SR, Fischer M. Adipocyte fatty acid‐binding protein levels are associated with left ventricular diastolic dysfunction in morbidly obese subjects. Nutr Diabetes. 2014;4:e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Engeli S, Utz W, Haufe S, Lamounier‐Zepter V, Pofahl M, Traber J, Janke J, Luft FC, Boschmann M, Schulz‐Menger J, Jordan J. Fatty acid binding protein 4 predicts left ventricular mass and longitudinal function in overweight and obese women. Heart. 2013;99:944–948. [DOI] [PubMed] [Google Scholar]
- 27. Balci MM, Arslan U, Firat H, Kocaoğlu I, Vural MG, Balci KG, Maden O, Gürbüz OA, Ardiç S, Yeter E. Serum levels of adipocyte fatty acid‐binding protein are independently associated with left ventricular mass and myocardial performance index in obstructive sleep apnea syndrome. J Investig Med. 2012;60:1020–1026. [DOI] [PubMed] [Google Scholar]
- 28. Talib A, Nakagawa N, Saito E, Matsuki M, Kobayashi M, Akasaka K, Hirayama T, Ishida H, Sato N, Hasebe N. The balance of fetuin‐A and osteoprotegerin is independently associated with diastolic dysfunction in hemodialysis patients. Hypertens Res. 2012;35:426–433. [DOI] [PubMed] [Google Scholar]
- 29. Lieb W, Sullivan LM, Aragam J, Harris TB, Roubenoff R, Benjamin EJ, Vasan RS. Relation of serum leptin with cardiac mass and left atrial dimension in individuals >70 years of age. Am J Cardiol. 2009;104:602–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Splansky GL, Corey D, Yang Q, Atwood LD, Cupples LA, Benjamin EJ, D'Agostino RB, Fox CS, Larson MG, Murabito JM, O'Donnell CJ, Vasan RS, Wolf PA, Levy D. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol. 2007;165:1328–1335. [DOI] [PubMed] [Google Scholar]
- 31. Zachariah JP, Quiroz R, Nelson KP, Teng Z, Keaney JF, Sullivan LM, Vasan RS. Prospective relation of circulating adipokines to incident metabolic syndrome: the Framingham Heart Study. J Am Heart Assoc. 2017;6:e004974 DOI: 10.1161/JAHA.116.004974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M‐mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. [DOI] [PubMed] [Google Scholar]
- 33. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. [DOI] [PubMed] [Google Scholar]
- 34. Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Cheng S, Aragam J, Levy D, Benjamin EJ, Vasan RS, Mitchell GF. Relations of central hemodynamics and aortic stiffness with left ventricular structure and function: the Framingham Heart Study. J Am Heart Assoc. 2016;5:e002693 DOI: 10.1161/JAHA.115.002693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sundström J, Sullivan L, Selhub J, Benjamin EJ, D'Agostino RB, Jacques PF, Rosenberg IH, Levy D, Wilson PWF, Vasan RS. Relations of plasma homocysteine to left ventricular structure and function: the Framingham Heart Study. Eur Heart J. 2004;25:523–530. [DOI] [PubMed] [Google Scholar]
- 36. Turkbey EB, McClelland RL, Kronmal RA, Burke GL, Bild DE, Tracy RP, Arai AE, Lima JAC, Bluemke DA. The impact of obesity on the left ventricle. The Multi‐Ethnic Study of Atherosclerosis (MESA). JACC Cardiovasc Imaging. 2010;3:266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kishi S, Armstrong AC, Gidding SS, Colangelo LA, Venkatesh BA, Jacobs DR Jr, Carr JJ, Terry JG, Liu K, Goff DC Jr, Lima JA. Association of obesity in early adulthood and middle age with incipient left ventricular dysfunction and structural remodeling: the Coronary Artery Risk Development in Young Adults (CARDIA) study. JACC Heart Fail. 2014;2:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ, Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation. 2010;122:570–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leopold JA. Obesity‐related cardiomyopathy is an adipocyte‐mediated paracrine disease. Trends Cardiovasc Med. 2015;25:127–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Oliver W, Matthews G, Ayers CR, Garg S, Gupta S, Neeland IJ, Drazner MH, Berry JD, Matulevicius S, de Lemos JA. Factors associated with left atrial remodeling in the general population. Circ Cardiovasc Imaging. 2017;10:e005047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pan H, Guo J, Su Z. Advances in understanding the interrelations between leptin resistance and obesity. Physiol Behav. 2014;130:157–169. [DOI] [PubMed] [Google Scholar]
- 42. Lieb W, Sullivan LM, Harris TB, Roubenoff R, Benjamin EJ, Levy D, Fox CS, Wang TJ, Wilson PW, Kannel WB, Vasan RS. Plasma leptin levels and incidence of heart failure, cardiovascular disease, and total mortality in elderly individuals. Diabetes Care. 2009;32:612–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wannamethee SG, Shaper AG, Whincup PH, Lennon L, Sattar N. Obesity and risk of incident heart failure in older men with and without pre‐existing coronary heart disease: does leptin have a role? J Am Coll Cardiol. 2011;58:1870–1877. [DOI] [PubMed] [Google Scholar]
- 44. Sweeney G. Cardiovascular effects of leptin. Nat Rev Cardiol. 2010;7:22–29. [DOI] [PubMed] [Google Scholar]
- 45. Gertler A, Solomon G. Leptin‐activity blockers: development and potential use in experimental biology and medicine. Can J Physiol Pharmacol. 2013;91:873–882. [DOI] [PubMed] [Google Scholar]
- 46. Rodriguez‐Calvo R, Girona J, Alegret JM, Bosquet A, Ibarretxe D, Masana L. Role of the fatty acid binding protein 4 in heart failure and cardiovascular disease. J Endocrinol. 2017;233:R173–R184. [DOI] [PubMed] [Google Scholar]
- 47. Lamounier‐Zepter V, Look C, Alvarez J, Christ T, Ravens U, Schunck WH, Ehrhart‐Bornstein M, Bornstein SR, Morano I. Adipocyte fatty acid‐binding protein suppresses cardiomyocyte contraction: a new link between obesity and heart disease. Circ Res. 2009;105:326–334. [DOI] [PubMed] [Google Scholar]
- 48. Won JC, Park CY, Oh SW, Park SW. Increased plasma levels of retinol‐binding protein 4 with visceral obesity is associated with cardiovascular risk factors. J Diabetes Investig. 2012;3:457–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mallat Z, Simon T, Benessiano J, Clément K, Taleb S, Wareham NJ, Luben R, Khaw KT, Tedgui A, Boekholdt SM. Retinol‐binding protein 4 and prediction of incident coronary events in healthy men and women. J Clin Endocrinol Metab. 2009;94:255–260. [DOI] [PubMed] [Google Scholar]
- 50. Zabetian‐Targhi F, Mahmoudi MJ, Rezaei N, Mahmoudi M. Retinol binding protein 4 in relation to diet, inflammation, immunity, and cardiovascular diseases. Adv Nutr. 2015;6:748–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kotnik P, Fischer‐Posovszky P, Wabitsch M. RBP4: a controversial adipokine. Eur J Endocrinol. 2011;165:703–711. [DOI] [PubMed] [Google Scholar]
- 52. Trepanowski JF, Mey J, Varady KA. Fetuin‐A: a novel link between obesity and related complications. Int J Obes. 2015;39:734–741. [DOI] [PubMed] [Google Scholar]
- 53. Dabrowska AM, Tarach JS, Wojtysiak‐Duma B, Duma D. Fetuin‐A (AHSG) and its usefulness in clinical practice. Review of the literature. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2015;159:352–359. [DOI] [PubMed] [Google Scholar]
- 54. Cottone S, Nardi E, Mulè G, Vadalà A, Lorito MC, Riccobene R, Palermo A, Arsena R, Guarneri M, Cerasola G. Association between biomarkers of inflammation and left ventricular hypertrophy in moderate chronic kidney disease. Clin Nephrol. 2007;67:209–216. [DOI] [PubMed] [Google Scholar]
- 55. Fontes‐Carvalho R, Pimenta J, Bettencourt P, Leite‐Moreira A, Azevedo A. Association between plasma leptin and adiponectin levels and diastolic function in the general population. Expert Opin Ther Targets. 2015;19:1283–1291. [DOI] [PubMed] [Google Scholar]
- 56. Lee Y, Kim BK, Lim YH, Kim MK, Choi BY, Shin J. The relationship between adiponectin and left ventricular mass index varies with the risk of left ventricular hypertrophy. PLoS One. 2013;8:e70246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Shibata R, Ouchi N, Ito M, Kihara S, Shiojima I, Pimentel DR, Kumada M, Sato K, Schiekofer S, Ohashi K, Funahashi T, Colucci WS, Walsh K. Adiponectin‐mediated modulation of hypertrophic signals in the heart. Nat Med. 2004;10:1384–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Associations Between Circulating Adipokine Concentrations and Echocardiographic Indices: Adjusted for BMI Instead of Height and Weight
Table S2. Secondary Analysis of Associations Between Adipokine Levels and the Components of LVMI and LV End‐Systolic Dimensions
Table S3. Associations of Circulating Adipokine Concentrations and Echocardiographic Indices Stratified by Sex‐Specific Median Weight
Figure S1. Least square means of E/e′ according to sex‐specific tertile of circulating concentrations of soluble leptin receptor.


