Abstract
Background
We investigated the association between the content of linoleic acid in adipose tissue, a biomarker of long‐term intake of linoleic acid, and the risk of ischemic stroke and its subtypes.
Methods and Results
The Danish cohort study Diet, Cancer and Health included 57 053 patients aged 50 to 65 years at enrollment. All participants had an adipose tissue biopsy performed at enrollment, while information on ischemic stroke during follow‐up was obtained from the Danish National Patient Register. Stroke diagnoses were all validated and classified according to the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification. Cases and a randomly drawn subcohort of 3500 patients had their fatty acid composition in adipose tissue determined by gas chromatography. Hazard ratios with 95% confidence intervals were calculated using weighted Cox proportional hazard regression. During 13.5 years of follow‐up, 1879 ischemic stroke cases were identified, for which 1755 adipose biopsies were available, while adipose biopsies were available for 3203 participants in the subcohort. When comparing the highest and the lowest quartiles of adipose tissue content of linoleic acid there was a negative association with the rate of total ischemic stroke (hazard ratio, 0.78; 95% confidence interval, 0.65–0.93) and large artery atherosclerosis (hazard ratio, 0.61; 95% confidence interval, 0.43–0.88), while there was an indication of a negative association with small‐vessel occlusion (hazard ratio, 0.87; 95% confidence interval, 0.69–1.11). There was no clear association with the rate of cardioembolism.
Conclusions
The content of linoleic acid in adipose tissue was inversely associated with the risk of total ischemic stroke and stroke caused by large artery atherosclerosis.
Keywords: adipose tissue, fatty acid, ischemic, stroke
Subject Categories: Ischemic Stroke, Epidemiology
Clinical Perspective
What Is New?
This is the largest study to investigate the association between the content of linoleic acid in adipose tissue (a long‐term biomarker of intake) and ischemic stroke and its subtypes.
A high content of linoleic acid in adipose tissue was associated with a lower risk of total ischemic stroke and stroke caused by large artery atherosclerosis.
What Are the Clinical Implications?
A high intake of linoleic acid may be associated with a lower risk of ischemic stroke.
Future guidelines for the prevention of ischemic stroke should consider recommending linoleic acid as part of a healthy diet.
Introduction
Linoleic acid (LA) is an essential fatty acid, mainly derived from vegetable oils, seeds, nuts, meats, and eggs1 and is the most widely consumed polyunsaturated fatty acid in the human diet. Most previous long‐term follow‐up studies on stroke have been based on self‐reported dietary intake of LA and were consequently affected by measurement error. Interestingly, the content of LA in adipose tissue has been shown to be an appropriate biomarker of long‐term intake (6–18 months) of LA2 and may reflect dietary intake of LA more objectively.
Previous studies have reported an inverse association between biomarkers of LA exposure and the risk of coronary heart disease3, 4 but these findings may not seem to apply to ischemic stroke.5, 6, 7, 8 Furthermore, only few studies with biomarkers of LA exposure and the risk of ischemic stroke have been undertaken and results have been inconsistent. Moreover, LA has been suggested to play different roles in different cerebral arteries9, 10, 11 and the influence of LA could thus be different among subtypes of ischemic stroke. In this study, we examined the association between the content of LA in adipose tissue and incident ischemic stroke, as well as subtypes classified according to The Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification,12 a system for categorization mainly based on pathogenesis.
Methods
Study Population
The study was based on the Diet, Cancer and Health cohort, which was established to investigate diet and lifestyle factors as risk factors for incident cancer and health and has been described in detail elsewhere.13 Briefly, between 1993 and 1997, 160 725 men and women aged 50 to 64 years, living in and around Copenhagen and Aarhus in Denmark, were invited by mail to participate. The study recruited 57 053 participants. All participants gave written informed consent, and the study was approved by the relevant ethics committees and the Danish Data Protection Agency. Because of the sensitive nature of the data collected for this study, requests to access the data set from qualified researchers trained in human subject confidentiality protocols may be sent to the Diet, Cancer and Health Steering Committee at the Danish Cancer Society (contact information: Louise Hansen, louhan@cancer.dk). For the current study, we used a case‐cohort design, which included a subcohort of 3500 participants randomly drawn from the whole cohort and all incident cases. Participants with cancer or stroke before recruitment or missing information on potential confounders, risk factors, or the exposure under study were excluded.
Covariates
At enrollment, the participants completed a lifestyle questionnaire regarding information on education status, smoking habits, physical activity, history of hypercholesterolemia (self‐reported and/or use of lipid‐lowering medication), hypertension (self‐reported and/or use of antihypertensive medication), and diabetes mellitus (self‐reported and/or use of insulin). Anthropometric measurements (height, weight, and waist circumference) were obtained by a laboratory technician. Body mass index was calculated as weight (kg)/height (m)2. Information on alcohol intake and intake of foods was obtained from a validated 192‐item semiquantitative food frequency questionnaire.14, 15 Information on atrial fibrillation/atrial flutter before baseline was obtained by linkage with the National Patient Register. Discharge diagnoses from in‐hospital patients have been registered since 1977 using International Classification of Diseases, Revision 8 (ICD‐8), discharge codes 42793 or 42794 and from 1993 ICD‐10 discharge code I489.
Adipose Tissue Biopsies
Adipose tissue samples were taken at baseline from subcutaneous fat from the buttocks of all participants using a Luer lock system (Terumo, Terumo Corp) consisting of a needle, a venoject multisample Luer adaptor, and an evacuated blood tube, according to the method of Beynen and Katan.16 Samples were flushed with nitrogen and stored at −150°C until analysis. When analyzed, biopsies were thawed and adipose tissue was removed to a glass and preheated at 50°C for 10 minutes. According to International Union of Pure and Applied Chemistry standard methods for analysis of oils, fats, and derivatives, the fat was dissolved in heptane at 50°C and fatty acids were transesterified by 2 mol/L potassium hydroxide in methanol at 50°C for 2 minutes. The fatty acid composition was determined by gas chromatography on a CP‐sil 88 60 m×0.25 mm ID capillary column, consisting of a highly substituted, stabilized cyanopropyl stationary phase, using a Varian 3900 GC with a CP‐8400 autosampler (Varian) equipped with a flame ionization detector.17 Helium was used as carrier gas, and individual fatty acids were identified using commercially available standards (Nu‐Chek Prep, Inc.). LA content in adipose tissue was expressed as weight percentage of total fatty acids. The interassay coefficient of variation for determination of LA in adipose tissue was 1.0%.
Identification of Ischemic Stroke Cases
The outcome in this study was defined as incident nonfatal and fatal ischemic stroke as well as subtypes of ischemic stroke. Potential stroke cases were identified by linkage to the Danish National Patient Register where all hospital contacts and discharge diagnoses from in‐hospital patients have been registered since 1977 and from 1995 diagnoses from emergency departments and outpatient visits have been recorded. Until December 31, 1993, possible stroke cases included patients with an ICD‐8 discharge code of 430, 431, 433, 434, 436.01, or 436.90 and from then on an ICD‐10 discharge code of I60, I61, I63, or I64 as either a primary or secondary diagnosis. Stroke was defined as a disease with rapid onset of focal or global neurologic deficit of vascular origin that persisted beyond 24 hours. Ischemic strokes were divided into 5 subtypes according to the TOAST classification12 large artery atherosclerosis, cardioembolism, small‐vessel occlusion, stroke of other cause, and stroke of undetermined cause. Stroke of other cause included cases of stroke with rare causes. Stroke of undetermined cause included cases where the pathogenesis could not be determined with any degree of confidence and included cases with ≥2 potential causes identified, as well as those with incomplete evaluation. All case records were reviewed by a physician with neurological experience. Diagnoses were validated and characterized on the basis of clinical appearance, computed tomography, magnetic resonance imaging scan, autopsy records, spinal fluid examination, and other relevant information.18 A random sample of 100 patients was initially reviewed by a physician with neurological experience and a senior consultant neurologist, and disagreements were discussed at fixed intervals. Few disagreements were found and agreed upon for the first 100 cases and after that only the physician with neurological experience performed the TOAST classification.
Statistical Analyses
Hazard ratios for total ischemic stroke and ischemic stroke subtypes with 95% confidence intervals were calculated using sex‐stratified Cox proportional hazard regression models allowing for different baseline hazards between sexes. Age was the underlying time axis, and the analyses were performed as if the full cohort had been included. The observation time for each participant was the period from the date of enrollment until occurrence of ischemic stroke, death from another cause, emigration, or end of follow‐up (December 30, 2009). After proper exclusion, each patient was assigned weights, 1 for cases and N/n for noncases in the subcohort, with N being the number of noncases in the cohort and n being the number of noncases in the subcohort.19 Stata uses as a default robust variance estimation when applying weighted Cox regression, and hence the variance was properly adjusted. For each stroke subtype, only patients with a diagnosis of the subtype in question were included as cases. The cases of other subtypes that appeared in the subcohort were censored at the time of diagnosis as their risk of a subsequent stroke will change. The analyses were planned a priori and included predefined potential confounders and risk factors. We used 3 different models: model 1A representing an age‐ and sex‐adjusted model that included age at baseline (years, continuous) and sex allowing for separate baseline hazards. The second model (model 1B) representing an educational‐ and lifestyle‐adjusted model included the same covariates as model 1A plus the following potential confounders and risk factors: educational status (<7, 8–10, and >10 years), waist circumference adjusted for body mass index (continuous), smoking status (noncurrent, current <15, ≥15 g of tobacco per day), physical activity (h/wk, continuous), alcohol intake (continuous), and alcohol abstain (yes, no). In model 2, possible intermediate variables were added to model 1A including hypertension (yes, no, unknown), hypercholesterolemia (yes, no, unknown), diabetes mellitus (yes, no, unknown), and atrial fibrillation/atrial flutter (yes, no) before baseline. Continuous variables were included using restricted cubic splines with 3 knots. Analyses were performed with LA content in adipose tissue divided into quartiles using the lowest quartile as the reference. Furthermore, LA content in adipose tissue was included as a continuous variable and spline functions were plotted to visually assess the shape of the association with the 12.5 percentile (median in the lowest quartile) of adipose tissue content of LA as a reference. The spline functions were graphed for the 2.5 to 97.5 percentiles of the exposure distribution. The proportional hazards assumption in the Cox regression analyses was evaluated by a log‐rank test based on Schoenfeld residuals. All analyses were conducted using Stata 14 (StataCorp). Two‐sided P values <0.05 were considered statistically significant.
Results
A total of 57 053 men and women agreed to participate (Figure 1). Of those, 569 participants had cancer and 582 participants had a stroke before baseline and were subsequently excluded. A further 564 participants were excluded as a result of missing information on potential confounders and risk factors. The subcohort included 3410 participants for whom adipose tissue biopsies were available for 3203. During a median follow‐up of 13.5 years, a total of 1879 ischemic stroke cases were identified. Adipose tissue biopsies were available for 1755 ischemic stroke cases (113 appeared in the subcohort). Cases included 300 strokes caused by large artery atherosclerosis, 781 strokes caused by small‐vessel occlusion, 99 strokes caused by cardioembolism, 91 strokes of other causes, and 484 strokes of undetermined causes. Baseline characteristics of ischemic stroke cases and the subcohort are given in Table 1. Case participants were older and more likely men compared with the subcohort participants. Furthermore, compared with the subcohort, case participants had less education, higher body mass index, larger waist circumference, and were more likely to be smokers and have higher alcohol intake and were less physically active. Finally, case participants more often had hypercholesterolemia, hypertension, diabetes mellitus, and atrial fibrillation/atrial flutter compared with the subcohort participants. Table S1 shows baseline characteristics according to quartiles of LA content in adipose tissue for participants in the subcohort and for case participants with ischemic stroke.
Figure 1.
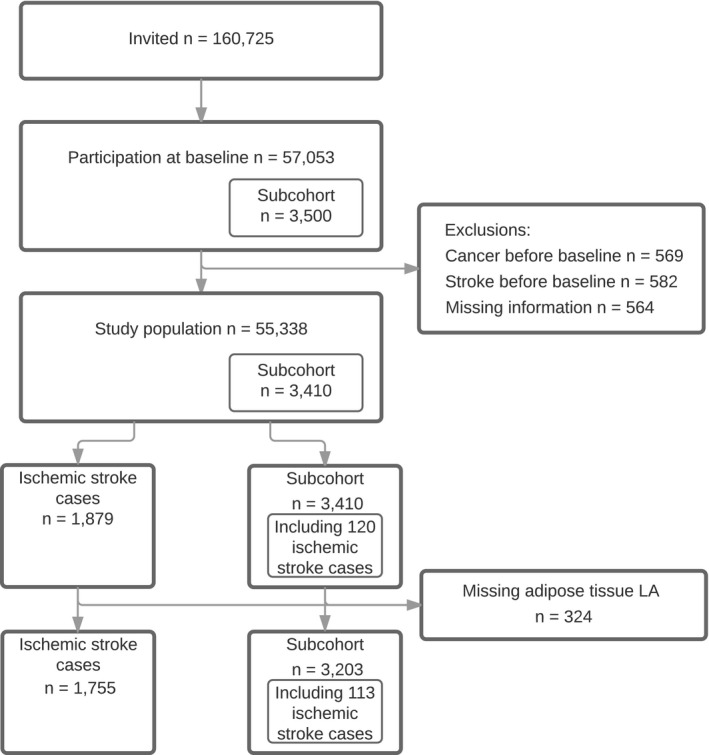
Flowchart illustrating the subcohort and ischemic stroke cases from the Diet, Cancer and Health Cohort. LA indicates linoleic acid.
Table 1.
Baseline Characteristics of Participants in the Subcohort and Ischemic Stroke Cases
| Characteristics | Subcohort (n=3203) | Ischemic Stroke Cases (n=1755) |
|---|---|---|
| Age at enrollment, y | 56.3 (50.7–64.2) | 58.9 (51.0–64.6) |
| Sex, % (No.) | ||
| Male | 54.0 (1731) | 61.9 (1087) |
| Female | 46.0 (1472) | 38.1 (668) |
| Education, % (No.) | ||
| <7 y | 32.9 (1053) | 41.0 (719) |
| 8 to 10 y | 45.0 (1440) | 42.6 (747) |
| >10 y | 22.2 (710) | 16.5 (289) |
| Body mass index, kg/m2 | 25.7 (20.6–33.4) | 26.3 (21.0–34.9) |
| Waist circumference, cm | 90.0 (69.5–111.0) | 93.0 (72.0–116.0) |
| Smoking status, % (No.) | ||
| Noncurrent smoker | 63.9 (2047) | 50.5 (887) |
| Current <15 g/d | 13.6 (436) | 15.4 (271) |
| Current ≥15 g/d | 22.5 (720) | 34.0 (597) |
| Physical activity, h/wk | 2.5 (0.0–10.5) | 2.0 (0.0–11.0) |
| Alcohol intake, g/d | 13.7 (0.8–65.4) | 15.0 (0.5–79.4) |
| Abstains from alcohol, % (No.) | ||
| Yes | 2.1 (68) | 3.0 (52) |
| No | 97.9 (3135) | 97.0 (1703) |
| Hypercholesterolemia, % (No.) | ||
| Yes | 7.9 (253) | 10.8 (190) |
| No | 49.4 (1581) | 48.4 (850) |
| Unknown | 42.7 (1369) | 40.8 (715) |
| Hypertension, % (No.) | ||
| Yes | 15.7 (502) | 28.8 (505) |
| No | 71.7 (2297) | 57.7 (1012) |
| Unknown | 12.6 (404) | 13.6 (238) |
| Diabetes mellitus, % (No.) | ||
| Yes | 2.0 (65) | 4.3 (76) |
| No | 92.9 (2974) | 89.6 (1572) |
| Unknown | 5.1 (164) | 6.1 (107) |
| Atrial fibrillation/atrial flutter, % (No.) | ||
| Yes | 0.9 (30) | 1.4 (24) |
| No | 99.1 (3173) | 98.6 (1731) |
Values are expressed as medians (5th–95th percentile) unless otherwise indicated.
A higher adipose tissue content of LA was associated with a lower rate of total ischemic stroke (hazard ratio, 0.78; 95% confidence interval, 0.65–0.93) when comparing the highest with the lowest quartile of adipose tissue content of LA (Table 2 and Figure 2). A test for a linear trend across quartiles was statistically significant (P=0.004). We also observed a dose‐dependent negative association between adipose tissue content of LA and the rate of large artery atherosclerosis (hazard ratio, 0.61; 95% confidence interval, 0.43–0.88) (Table 2 and Figure 2). When testing for a linear trend across quartiles, a statistically significant negative association was found (P=0.021). For cardioembolism, no consistent associations were found with the content of LA in adipose tissue (Table 2 and Figure 2). There was an indication of a negative association between the content in adipose tissue of LA and the risk of small‐vessel occlusion (hazard ratio, 0.87; 95% confidence interval, 0.69–1.11 for the highest quartile compared with the lowest quartile) (Table 2 and Figure 2).
Table 2.
Adipose Tissue Content of LA in Quartiles and Association With Ischemic Stroke and Ischemic Stroke Subtypes
| Type of Ischemic Stroke and Quartile Content of LA | No. | Model 1Aa | Model 1Bb | Model 2c |
|---|---|---|---|---|
| HR (95% CI) | HR (95% CI) | HR (95% CI) | ||
| Total ischemic stroke | 1755 | |||
| Q1d | 555 | 1 | 1 | 1 |
| Q2e | 439 | 0.84 (0.71–0.99) | 0.92 (0.77–1.09) | 0.84 (0.71–0.99) |
| Q3f | 399 | 0.76 (0.65–0.90) | 0.85 (0.71–1.02) | 0.77 (0.65–0.92) |
| Q4g | 362 | 0.66 (0.56–0.79) | 0.78 (0.65–0.93) | 0.65 (0.54–0.77) |
| P trend | P<0.001 | P=0.004 | P<0.001 | |
| Large artery atherosclerosis | 300 | |||
| Q1d | 100 | 1 | 1 | 1 |
| Q2e | 67 | 0.71 (0.51–0.98) | 0.72 (0.51–1.01) | 0.71 (0.51–0.99) |
| Q3f | 77 | 0.82 (0.60–1.12) | 0.84 (0.61–1.17) | 0.84 (0.61–1.17) |
| Q4g | 56 | 0.57 (0.40–0.80) | 0.61 (0.43–0.88) | 0.58 (0.41–0.83) |
| P trend | P=0.004 | P=0.021 | P=0.010 | |
| Cardioembolism | 99 | |||
| Q1d | 31 | 1 | 1 | 1 |
| Q2e | 31 | 1.08 (0.65–1.81) | 1.28 (0.75–2.19) | 1.03 (0.61–1.74) |
| Q3f | 17 | 0.59 (0.32–1.09) | 0.71 (0.37–1.37) | 0.57 (0.30–1.06) |
| Q4g | 20 | 0.67 (0.38–1.18) | 0.86 (0.46–1.59) | 0.57 (0.31–1.07) |
| P trend | P=0.052 | P=0.311 | P=0.024 | |
| Small‐vessel occlusion | 781 | |||
| Q1d | 250 | 1 | 1 | 1 |
| Q2e | 184 | 0.77 (0.62–0.96) | 0.90 (0.72–1.13) | 0.78 (0.62–0.97) |
| Q3f | 174 | 0.73 (0.59–0.91) | 0.87 (0.69–1.03) | 0.74 (0.59–0.92) |
| Q4g | 173 | 0.70 (0.56–0.88) | 0.87 (0.69–1.11) | 0.69 (0.55–0.87) |
| P trend | P=0.002 | P=0.236 | P=0.001 | |
| Stroke of other causes | 91 | |||
| Q1d | 31 | 1 | 1 | 1 |
| Q2e | 25 | 0.82 (0.48–1.42) | 0.89 (0.51–1.55) | 0.85 (0.50–1.45) |
| Q3f | 13 | 0.44 (0.23–0.85) | 0.45 (0.22–0.92) | 0.45 (0.23–0.87) |
| Q4g | 22 | 0.72 (0.41–1.26) | 0.76 (0.42–1.39) | 0.73 (0.42–1.31) |
| P trend | P=0.107 | P=0.173 | P=0.127 | |
| Stroke of undetermined cause | 484 | |||
| Q1d | 143 | 1 | 1 | 1 |
| Q2e | 132 | 0.98 (0.76–1.28) | 1.04 (0.79–1.37) | 0.98 (0.75–1.28) |
| Q3f | 118 | 0.89 (0.68–1.17) | 0.93 (0.70–1.23) | 0.90 (0.68–1.18) |
| Q4g | 91 | 0.66 (0.50–0.88) | 0.74 (0.54–1.00) | 0.63 (0.46–0.84) |
| P trend | P=0.003 | P=0.036 | P=0.002 | |
CI indicates confidence interval; HR, hazard ratio; LA, linoleic acid.
Model 1A included age at inclusion. Sex was included by allowing for different baseline hazards.
Model 1B includes model 1A plus alcohol intake, alcohol abstain, waistline adjusted for body mass index, education status, smoking, and physical activity.
Model 2 includes model 1A plus hypertension, hypercholesterolemia, diabetes mellitus, and atrial fibrillation/atrial flutter.
Q1<9.55.
Q2=9.55–10.59.
Q3=10.6–11.68.
Q4>11.68.
Figure 2.
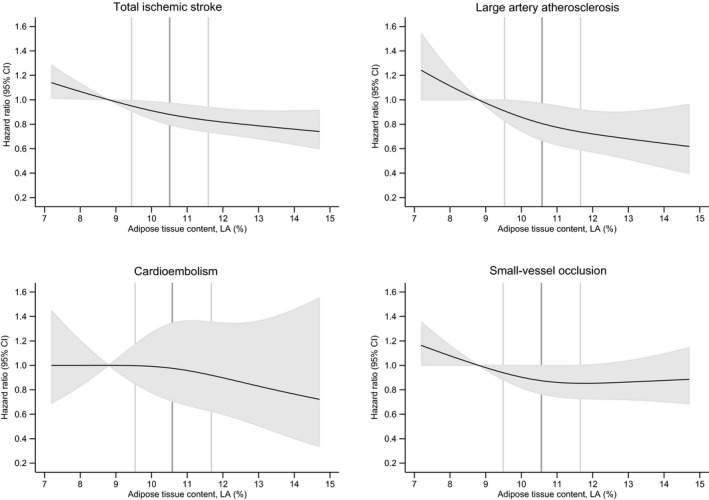
Spline curves for adipose tissue content of linoleic acid (LA) in percentage of total fatty acids and the risk of incident total ischemic stroke, large artery atherosclerosis, cardioembolism, and small‐vessel occlusion. Data presented for model 1B, adjusting for educational and lifestyle factors. The 2.5 to 97.5 percentile of LA is shown, with the 12.5 percentile (median in the lowest quartile) as reference. Black lines show the hazard ratios and the shaded grey areas show the 95% confidence intervals. The 25, 50, and 75 percentiles of LA are shown by vertical lines.
Figure 3 illustrates the underlying dietary patterns of different food and beverage groups for participants according to the content of LA in adipose tissue in the subcohort. Results indicate that compared with participants in the lowest quartile of adipose tissue LA content, participants with a high content of adipose tissue LA tended to consume less alcohol, fatty dairy products, butter and other animal fat, eggs, red meat, snacks and fatty potatoes, and soft drinks and juices, and less fruits, vegetables, whole grain cereals, vegetable oils and mayonnaises, margarines, lean dairy products, and poultry.
Figure 3.
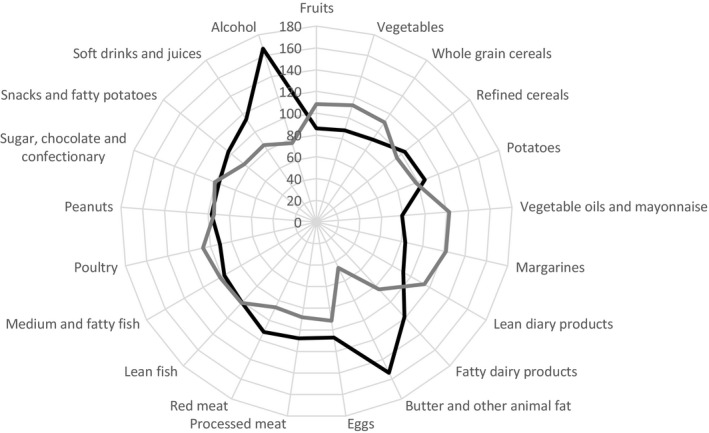
Radar chart illustrating the percentage‐wise difference in energy‐adjusted median intake of 21 groups of foods and beverages for participants in the subcohort with the lowest and the highest adipose tissue content of linoleic acid. First quartile is illustrated with a black line and fourth quartile with a gray line.
Discussion
We found a statistically significant lower rate of total ischemic stroke and large artery atherosclerosis associated with the content of LA in adipose tissue, whereas there was an indication of a negative association for small‐vessel occlusion. For cardioembolism, no clear association was found.
We used adipose tissue biopsies to investigate LA in relation to the risk of ischemic stroke and subtypes. Because of a slow turnover time, the fatty acid content in adipose tissue may serve as an objective, long‐term measure of dietary intake of fatty acids in the previous 6 to 18 months, and is considered the gold standard for long‐term dietary intake of fatty acids.2, 20 Also, the content of LA in adipose tissue is known to be strongly correlated with dietary LA intake.21, 22 Another strength of the study is that ischemic stroke diagnoses were validated and subclassified according to the TOAST classifications.12 Only few participants were lost to follow‐up, which limits the risk of selection bias. Furthermore, information bias is unlikely to have affected the results, as information on ischemic stroke cases was obtained by linkage with the Danish National Patient Register and thus case ascertainment was independent of LA measurement. Detailed information on potential confounders and risk factors in the study limits but does not eliminate the risk of residual confounding. Only baseline information on adipose tissue content of LA and potential confounders were included, which is a limitation, because changes in dietary habits and lifestyle risk factors may have occurred over time. Information on atrial fibrillation/atrial flutter was ascertained by linkage with the Danish National Patient Register, but participants with asymptomatic atrial fibrillation/atrial flutter might not have been admitted for hospital evaluation and, therefore, atrial fibrillation/atrial flutter might have been undercaptured. In addition, the study cohort included only Danish, middle‐aged, white participants and the study results may not be generalizable to other ethnicities or age groups. When we adjusted for educational and lifestyle factors (model 1B), the pattern of association remained the same as in the age‐ and sex‐adjusted model (model 1A), although the associations were weakened, which could indicate confounding. Hypertension, hypercholesterolemia, diabetes mellitus, and atrial fibrillation/atrial flutter are known risk factors for ischemic stroke but they are also possible intermediate factors in the causal pathway between LA and ischemic stroke; hence, adjustment may introduce selection stratification bias. We considered model 1B our main analysis since the results have a clear and unbiased interpretation. The radar chart (Figure 3) shows that a high content of LA in adipose tissue was associated with overall healthier eating habits. The study results can therefore not directly be interpreted as associations for LA independently of the diet contributing to LA intake. Since there is no clear indication of a different risk of developing ischemic stroke between the 2 sexes, the analyses were not performed separately. Instead, we analyzed data assuming equal association of the exposure for both sexes but allowing for different baseline hazards. We used a case‐cohort design, which is a good way to analyze various exposures and outcomes. Analysis of adipose tissue biopsy for fatty acid composition is expensive and time consuming and would for these reasons have been impossible to perform in the full cohort of >57 000 patients. By using weighed Cox proportional hazard regression, the statistical analyses were performed as if the full cohort had been included.
Only a few studies relating biomarkers of LA to ischemic stroke have been published. However, in a follow‐up study with 168 ischemic stroke cases, an inverse association was found between serum LA and the risk of ischemic stroke.5 In contrast, no association between serum LA and risk of ischemic stroke was found in a case‐control study with 93 ischemic stroke cases.6 In another case‐control study7 with 122 cases of ischemic stroke, statistically significant negative associations were found between serum LA and total ischemic stroke and small‐vessel occlusion, whereas negative but not statistically significant associations for large artery atherosclerosis and cardioembolism were observed. Furthermore, a case‐control study8 with 34 ischemic stroke cases found that serum LA content was significantly lower in patients with small‐vessel occlusions and large artery atherosclerosis than among controls. Our findings of an inverse association for large artery atherosclerosis and indication of an inverse association for small‐vessel occlusion with a higher content of LA in adipose tissue are in agreement with these findings. The effect of LA on ischemic stroke may act through several mechanisms. Numerous studies have thus reported a beneficial effect of LA on blood lipids and blood pressure.23, 24 Since hypercholesterolemia and hypertension have a different impact on the large precerebral arteries and the small cerebral arteries,9, 25, 26 this could partly explain the observed differences in risk between subtypes of ischemic stroke. We found a lower risk of large artery atherosclerosis and indications of a negative association between LA content in adipose tissue and small‐vessel occlusion. Although there are more cases with small‐vessel occlusion, the negative association between LA and total ischemic stroke might be driven by cases of large artery atherosclerosis.
Stroke of other causes and stroke of undetermined causes belong to the TOAST classification and results were accordingly reported. The subtype stroke of other causes includes cases with rare causes of stroke of different origin, while the subtype stroke of undetermined causes includes cases of stroke with ≥2 potential causes identified, as well as those with incomplete evaluation.12 We conducted analyses on these outcomes but decided not to discuss them further because the results were without clear interpretation.
Conclusions
The content of LA in adipose tissue was inversely associated with the risk of total ischemic stroke, and stroke caused by large artery atherosclerosis, while there was an indication of a negative association between adipose tissue LA and small‐vessel occlusion. There was no clear association between the content of LA in adipose tissue and cardioembolism.
Sources of Funding
This work was supported by a research grant from the Danish Heart Foundation. The primary data collection for the Diet, Cancer and Health study was funded by the Danish Cancer Society.
Disclosures
None.
Supporting information
Table S1. Baseline Characteristics According to Quartiles of LA in Adipose Tissue for Participants in the Subcohort and Cases With Ischemic Stroke
(J Am Heart Assoc. 2018;7:e009820 DOI: 10.1161/JAHA.118.009820.)
Some of the results on total ischemic stroke have been presented at the European Society of Cardiology Congress, August 26–30, 2017, in Barcelona, Spain. An abstract has been published in the European Heart Journal.
References
- 1. Whelan J, Fritsche K. Linoleic acid. Adv Nutr. 2013;4:311–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hodson L, Skeaff CM, Fielding BA. Fatty acid composition of adipose tissue and blood in humans and its use as a biomarker of dietary intake. Prog Lipid Res. 2008;47:348–380. [DOI] [PubMed] [Google Scholar]
- 3. Block RC, Harris WS, Reid KJ, Spertus JA. Omega‐6 and trans fatty acids in blood cell membranes: a risk factor for acute coronary syndromes? Am Heart J. 2008;156:1117–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lopes C, Aro A, Azevedo A, Ramos E, Barros H. Intake and adipose tissue composition of fatty acids and risk of myocardial infarction in a male Portuguese community sample. J Am Diet Assoc. 2007;107:276–286. [DOI] [PubMed] [Google Scholar]
- 5. Yamagishi K, Folsom AR, Steffen LM; ARIC Study Investigators . Plasma fatty acid composition and incident ischemic stroke in middle‐aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Cerebrovasc Dis. 2013;36:38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Goede J, Verschuren WM, Boer JM, Kromhout D, Geleijnse JM. N‐6 and n‐3 fatty acid cholesteryl esters in relation to incident stroke in a Dutch adult population: a nested case‐control study. Nutr Metab Cardiovasc Dis. 2013;23:737–743. [DOI] [PubMed] [Google Scholar]
- 7. Iso H, Sato S, Umemura U, Kudo M, Koike K, Kitamura A, Imano H, Okamura T, Naito Y, Shimamoto T. Linoleic acid, other fatty acids, and the risk of stroke. Stroke. 2002;33:2086–2093. [DOI] [PubMed] [Google Scholar]
- 8. Park Y, Park S, Yi H, Kim HY, Kang SJ, Kim J, Ahn H. Low level of n‐3 polyunsaturated fatty acids in erythrocytes is a risk factor for both acute ischemic and hemorrhagic stroke in Koreans. Nutr Res. 2009;29:825–830. [DOI] [PubMed] [Google Scholar]
- 9. Jackson C, Sudlow C. Are lacunar strokes really different? A systematic review of differences in risk factor profiles between lacunar and nonlacunar infarcts. Stroke. 2005;36:891–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reed DM, Resch JA, Hayashi T, MacLean C, Yano K. A prospective study of cerebral artery atherosclerosis. Stroke. 1988;19:820–825. [DOI] [PubMed] [Google Scholar]
- 11. Aquil N, Begum I, Ahmed A, Vohra EA, Soomro BA. Risk factors in various subtypes of ischemic stroke according to TOAST criteria. J Coll Physicians Surg Pak. 2011;21:280–283. [PubMed] [Google Scholar]
- 12. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 13. Tjonneland A, Olsen A, Boll K, Stripp C, Christensen J, Engholm G, Overvad K. Study design, exposure variables, and socioeconomic determinants of participation in Diet, Cancer and Health: a population‐based prospective cohort study of 57,053 men and women in Denmark. Scand J Public Health. 2007;35:432–441. [DOI] [PubMed] [Google Scholar]
- 14. Overvad K, Tjonneland A, Haraldsdottir J, Ewertz M, Jensen OM. Development of a semiquantitative food frequency questionnaire to assess food, energy and nutrient intake in Denmark. Int J Epidemiol. 1991;20:900–905. [DOI] [PubMed] [Google Scholar]
- 15. Tjonneland A, Overvad K, Haraldsdottir J, Bang S, Ewertz M, Jensen OM. Validation of a semiquantitative food frequency questionnaire developed in Denmark. Int J Epidemiol. 1991;20:906–912. [DOI] [PubMed] [Google Scholar]
- 16. Beynen AC, Katan MB. Rapid sampling and long‐term storage of subcutaneous adipose‐tissue biopsies for determination of fatty acid composition. Am J Clin Nutr. 1985;42:317–322. [DOI] [PubMed] [Google Scholar]
- 17. Rix TA, Joensen AM, Riahi S, Lundbye‐Christensen S, Overvad K, Schmidt EB. Marine n‐3 fatty acids in adipose tissue and development of atrial fibrillation: a Danish cohort study. Heart. 2013;99:1519–1524. [DOI] [PubMed] [Google Scholar]
- 18. Luhdorf P, Overvad K, Schmidt EB, Johnsen SP, Bach FW. Predictive value of stroke discharge diagnoses in the Danish National Patient Register. Scand J Public Health. 2017;45:630–636. DOI: 10.1177/1403494817716582. [DOI] [PubMed] [Google Scholar]
- 19. Kalbfleisch JD, Lawless JF. Likelihood analysis of multi‐state models for disease incidence and mortality. Stat Med. 1988;7:149–160. [DOI] [PubMed] [Google Scholar]
- 20. Seidelin KN. Fatty acid composition of adipose tissue in humans. Implications for the dietary fat‐serum cholesterol‐CHD issue. Prog Lipid Res. 1995;34:199–217. [DOI] [PubMed] [Google Scholar]
- 21. van Staveren WA, Deurenberg P, Katan MB, Burema J, de Groot LC, Hoffmans MD. Validity of the fatty acid composition of subcutaneous fat tissue microbiopsies as an estimate of the long‐term average fatty acid composition of the diet of separate individuals. Am J Epidemiol. 1986;123:455–463. [DOI] [PubMed] [Google Scholar]
- 22. Knutsen SF, Fraser GE, Beeson WL, Lindsted KD, Shavlik DJ. Comparison of adipose tissue fatty acids with dietary fatty acids as measured by 24‐hour recall and food frequency questionnaire in Black and White Adventists: the Adventist Health Study. Ann Epidemiol. 2003;13:119–127. [DOI] [PubMed] [Google Scholar]
- 23. Mensink RP, Zock PL, Kester AD, Katan MB. Effects of dietary fatty acids and carbohydrates on the ratio of serum total to HDL cholesterol and on serum lipids and apolipoproteins: a meta‐analysis of 60 controlled trials. Am J Clin Nutr. 2003;77:1146–1155. [DOI] [PubMed] [Google Scholar]
- 24. Bjermo H, Iggman D, Kullberg J, Dahlman I, Johansson L, Persson L, Berglund J, Pulkki K, Basu S, Uusitupa M, Rudling M, Arner P, Cederholm T, Ahlstrom H, Riserus U. Effects of n‐6 PUFAs compared with SFAs on liver fat, lipoproteins, and inflammation in abdominal obesity: a randomized controlled trial. Am J Clin Nutr. 2012;95:1003–1012. [DOI] [PubMed] [Google Scholar]
- 25. Lv P, Jin H, Liu Y, Cui W, Peng Q, Liu R, Sun W, Fan C, Teng Y, Sun W, Huang Y. Comparison of risk factor between lacunar stroke and large artery atherosclerosis stroke: a cross‐sectional study in China. PLoS One. 2016;11:e0149605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tirschwell DL, Smith NL, Heckbert SR, Lemaitre RN, Longstreth WT Jr, Psaty BM. Association of cholesterol with stroke risk varies in stroke subtypes and patient subgroups. Neurology. 2004;63:1868–1875. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics According to Quartiles of LA in Adipose Tissue for Participants in the Subcohort and Cases With Ischemic Stroke


