Abstract
Background
There is limited information about the long‐term survival of older patients after myocardial infarction (MI).
Methods and Results
CRUSADE (Can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the ACC/AHA guidelines) was a registry of MI patients treated at 568 US hospitals from 2001 to 2006. We linked MI patients aged ≥65 years in CRUSADE to their Medicare data to ascertain long‐term mortality (defined as 8 years post index event). Long‐term unadjusted Kaplan–Meier mortality curves were examined among patients stratified by revascularization status. A landmark analysis conditioned on surviving the first year post‐MI was conducted. We used multivariable Cox regression to compare mortality risks between ST‐segment–elevation myocardial infarction and non–ST‐segment–elevation myocardial infarction patients. Among 22 295 MI patients ≥ age 65 years (median age 77 years), we observed high rates of evidence‐based medication use at discharge: aspirin 95%, β‐blockers 94%, and statins 81%. Despite this, mortality rates were high: 24% at 1 year, 51% at 5 years, and 65% at 8 years. Eight‐year mortality remained high among patients who underwent percutaneous coronary intervention (49%), coronary artery bypass graft (46%), and among patients who survived the first year post‐MI (59%). Median survival was 4.8 years (25th, 75th percentiles 1.1, 8.5); among patients aged 65–74 years it was 8.2 years (3.3, 8.9) while for patients aged ≥75 years it was 3.1 years (0.6, 7.6). Eight‐year mortality was lower among ST‐segment–elevation myocardial infarction than non–ST‐segment–elevation myocardial infarction patients (53% versus 67%); this difference was not significant after adjustment (hazard ratio 0.94, 95% confidence interval, 0.88–1.00).
Conclusions
Long‐term mortality remains high among patients with MI in routine clinical practice, even among revascularized patients and those who survived the first year.
Keywords: elderly, mortality, myocardial infarction, revascularization, survival
Subject Categories: Mortality/Survival, Cardiovascular Disease, Revascularization
Clinical Perspective
What Is New?
Long‐term mortality over an 8‐year follow‐up period among older patients with myocardial infarction aged ≥65 years is high at 65%.
Long‐term mortality remains higher than 45% even among relatively younger patients aged 65 to 74 years, patients who underwent revascularization, and patients who survived the first year post–myocardial infarction.
What Are the Clinical Implications?
These data describing the long‐term mortality rates enable clinicians and patients to engage in discussions about long‐term prognosis to help guide shared‐decision making.
Introduction
By 2030, 71 million adults will be age 65 years or older and will comprise 20% of the total population.1 Coronary disease disproportionately affects older adults; however, long‐term outcomes of older patients after myocardial infarction (MI) are not well studied, as these patients are often under‐represented in randomized clinical trials and prospective longitudinal studies rarely follow patients beyond the first few years after their index event.1, 2, 3, 4 Knowledge of long‐term prognosis can aid in treatment decisions for older patients with MI. The CRUSADE (Can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the ACC/AHA guidelines) study was a large national registry of patients with acute coronary syndrome treated between 2001 and 2006 at >500 hospitals across the United States. Its linkage to Centers for Medicare and Medicaid Services data afforded a unique opportunity to examine longitudinal outcomes out to 8 years after the initial MI event. Our study aims to epidemiologically describe the long‐term mortality of older patients with MI treated in routine clinical practice. We examined long‐term mortality stratified by age group, prior MI, sex, diabetes mellitus, MI type, and revascularization status during the index MI.
Methods
Reproducibility
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
CRUSADE was a national quality improvement initiative that collected data between 2001 and 2006 on patients with non–ST‐segment–elevation myocardial infarction (NSTEMI).5 Between September 2004 and December 2006, 235 of the participating hospitals began submitting data on patients with STEMI. The definition of MI in CRUSADE required patients to present with ischemic chest pain lasting ≥10 minutes within the 24 hours before hospitalization admission and positive cardiac markers (either creatine kinase‐MB or troponin levels above the upper limit of normal, which were designated NSTEMI) or ischemic ST‐segment elevation on ECG (which were designated STEMI).6 A total of 76 112 patients, treated for STEMI or NSTEMI at 514 hospitals between 2001 and 2006, were linked to Centers for Medicare and Medicaid Services claims data through the end of 2014 using 5 indirect identifiers: index MI hospital identifier, admission date, discharge date, age, and sex.7 To evaluate the outcomes of both NSTEMI and STEMI patients, we started with all patients in the linked database when STEMI data collection began in CRUSADE (n=23 083). We excluded linked patient records missing date of death (n=12) and nonindex admissions for patients who had repeat admissions in the CRUSADE (n=776). The final analysis population was 22 295 patients with MI treated at 344 hospitals, from October 2004 to December 2006 (Figure 1). Among these, 19 755 (88.6%) and 2540 (11.4%) were NSTEMI and STEMI, respectively.
Figure 1.
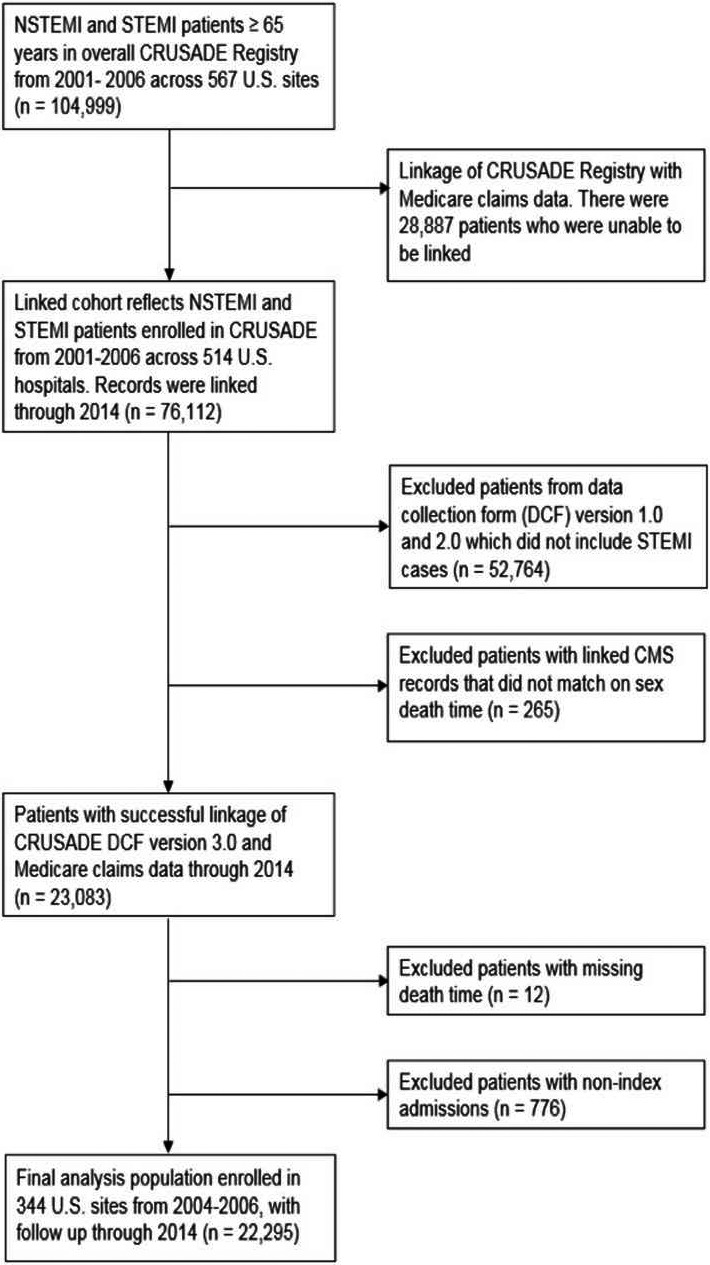
Patient flow diagram. CMS indicates Centers for Medicare and Medicaid Services; CRUSADE, Can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the ACC/AHA guidelines; NSTEMI, non–ST‐segment–elevation myocardial infarction; STEMI, ST‐segment–elevation myocardial infarction.
Data Collection and Definitions
Data in CRUSADE were abstracted by a trained data collector at each hospital after local institutional review board approval. The data were collected anonymously, thus informed consent was not required. Variables collected include demographics, prehospital data, medical history, pertinent signs and symptoms, laboratory values such as cardiac biomarkers, in‐hospital treatments, associated major contraindications to evidence‐based therapies, and in‐hospital outcomes. The outcome of interest in this analysis was all‐cause mortality. Time to all‐cause mortality was defined as the number of days between date of index MI admission and date of death. The death date was ascertained from Medicare denominator files.
Statistical Analysis Plan
Patient baseline demographics, clinical characteristics, discharge medications, and interventions were described for the overall population, then stratified by MI type (NSTEMI versus STEMI) and in‐hospital revascularization status (percutaneous coronary intervention [PCI] versus coronary artery bypass grafting [CABG] versus medical management).
The unadjusted Kaplan–Meier event rates for mortality were estimated at 1, 5, and 8 years after the index MI. Similarly, the unadjusted 8‐year mortality rates were examined in a landmark analysis conditioned on surviving the first year post‐MI. Median survival from date of index admission was calculated overall, then stratified by subgroups: age group at the index MI, prior MI, sex, diabetes mellitus, MI type, and revascularization status during index MI.
Kaplan–Meier curves were displayed to estimate the probability of mortality during the long‐term follow‐up stratified by MI type and revascularization status. The log‐rank test was used to assess whether the differences between the curves were statistically significant at P<0.05.
Cox proportional hazards models were performed to explore the association between 8‐year mortality and subgroups (ie, prior MI, sex, diabetes mellitus, MI type, and in‐hospital revascularization). Robust standard errors were used to account for clustering of patients within hospitals. Covariates included in these models are based on the validated and published CRUSADE long‐term mortality model: age, sex, race, weight, family history of coronary artery disease, hypertension, current/recent smoker, diabetes mellitus, dyslipidemia, prior PCI, prior MI, prior CABG, prior heart failure, prior stroke, peripheral artery disease, systolic blood pressure and heart rate on admission, signs of heart failure on admission, initial hematocrit, initial serum creatinine, and initial troponin ratio (ratio over institutional upper limit of normal).8 The urgency to revascularize differs between MI types, hence MI type was added to the model comparing mortality by revascularization status.
The percentage of missing data was low, <2% for most variables. For modeling, missing values of the continuous covariates were imputed to the MI type and sex‐specific median of the nonmissing values. For categorical variables, missing values were imputed to the most frequent group. All statistical analyses were performed at the Duke Clinical Research Institute using SAS software (version 9.4; SAS Institute).
Results
Clinical Characteristics and In‐Hospital Management
Among 22 295 patients with MI studied in this analysis, the median age was 77 years, 47.5% were female, and 13.3% were nonwhite race. Baseline characteristics are presented in Table 1. NSTEMI patients compared with STEMI patients were older (median: 78 versus 76 years), had a higher prevalence of prior cardiovascular disease (prior MI, prior heart failure, prior stroke, prior PCI, and prior CABG) and cardiac risk factors (hypertension, diabetes mellitus, and dyslipidemia).
Table 1.
Baseline Characteristics
| Variables | Overall (n=22 295) | MI Type | Revascularization Statusa | |||
|---|---|---|---|---|---|---|
| NSTEMI (n=19 755) | STEMI (n=2540) | PCI (n=8053) | CABG (n=1924) | Medical Management (n=12 057) | ||
| Demographics | ||||||
| Age, yb | 77 (71, 83) | 78 (72, 84) | 76 (70, 82) | 75 (70, 81) | 74 (69, 79) | 80 (73, 86) |
| Female (%) | 47.5 | 47.9 | 44.9 | 43.3 | 33.4 | 52.6 |
| White race (%) | 86.7 | 86.4 | 89.1 | 88.1 | 89.2 | 85.3 |
| Medical history | ||||||
| Family history of CAD, % | 26.9 | 27.0 | 26.1 | 29.1 | 31.9 | 24.5 |
| Hypertension, % | 76.6 | 77.6 | 68.4 | 74.4 | 74.6 | 78.5 |
| Diabetes mellitus, % | 34.9 | 36.0 | 26.3 | 30.9 | 34.3 | 37.6 |
| Current/recent smoker, % | 13.0 | 12.5 | 16.3 | 15.0 | 16.0 | 11.1 |
| Dyslipidemia, %c | 53.7 | 54.4 | 47.7 | 56.8 | 56.0 | 51.6 |
| Prior MI, % | 28.9 | 30.3 | 18.7 | 24.9 | 19.9 | 33.1 |
| Prior PCI, % | 20.8 | 21.4 | 16.4 | 24.7 | 15.4 | 19.0 |
| Prior CABG, % | 21.5 | 22.9 | 11.0 | 20.8 | 5.8 | 24.6 |
| Prior HF, % | 21.1 | 22.5 | 9.6 | 12.0 | 8.7 | 29.0 |
| Prior stroke, % | 13.0 | 13.4 | 9.7 | 9.0 | 7.5 | 16.5 |
| Peripheral artery disease, % | 14.2 | 14.9 | 9.2 | 11.7 | 11.6 | 16.4 |
| Hospitalization features | ||||||
| Weight, kgb | 76.1 (64, 88.5) | 76.0 (64.0, 88.9) | 76.3 (65.0, 87.7) | 79.0 (68.0, 91.0) | 80.3 (71.0, 91.6) | 73.0 (61.2, 85.7) |
| Heart rate (per min)b , d | 83.0 (70.0, 100.0) | 84.0 (70.0, 101.0) | 78.0 (66.0, 93.0) | 78 (66, 93) | 83 (70, 99) | 87 (73, 105) |
| Systolic BP, mm Hgb , d | 142 (121, 164) | 143 (121, 164) | 137 (116, 160) | 145 (124, 165) | 146 (126, 166) | 140 (118, 162) |
| Troponin (×ULN)b , d | 2.2 (0.5, 10.3) | 2.3 (0.6, 10.0) | 1.8 (0.3, 16.9) | 2.0 (0.4, 10.0) | 2.5 (0.6, 11.7) | 2.3 (0.6, 10.6) |
| Creatinine, mg/dLb , d , e | 1.2 (1.0, 1.5) | 1.2 (1.0, 1.5) | 1.1 (0.9, 1.4) | 1.1 (0.9, 1.3) | 1.1 (0.9, 1.3) | 1.3 (1.0, 1.7) |
| Hematocrit, %b , d | 39.2 (35.2, 42.8) | 39.0 (35.0, 42.6) | 40.4 (36.9, 44.0) | 40.4 (36.8, 43.7) | 40.8 (37.4, 44.0) | 38.0 (33.8, 41.8) |
| Total cholesterol, mg/dLb , d | 156.0 (131.0, 185.0) | 156.0 (130.0, 185.0) | 157.0 (132.0, 186.0) | 159.0 (133.0, 187.0) | 163.0 (135.0, 195.0) | 153.0 (128.0, 183.0) |
| HDL, mg/dLb , d | 40.0 (32.1, 50) | 40.0 (33.0, 50.0) | 39.5 (32.0, 49.0) | 40.0 (33.0, 49.0) | 39.0 (31.0, 49.0) | 41.0 (33.0, 51.0) |
| LDL, mg/dLb , d | 90.0 (68.0, 116.0) | 89.0 (68.0, 115.0) | 92.0 (71.0, 116.0) | 92.0 (70.0, 116.0) | 96.0 (72.0, 124.0) | 87.0 (66.0, 112.0) |
| Triglycerides, mg/dLb , d | 106.0 (74.0, 154.0) | 106.0 (74.0, 154.0) | 106.0 (75.0, 150.0) | 111.0 (78.0, 158.5) | 114.0 (79.0, 164.0) | 100.0 (71.0, 147.0) |
| Signs of HFd | 29.2 | 30.6 | 18.3 | 17.2 | 23.2 | 38.2 |
| Diagnostic catheterization, % | 63.9 | 61.3 | 83.8 | N/A | N/A | 35.8 |
| Number of diseased vesselsf | ||||||
| None, % | 8.6 | 9.3 | 5.2 | 2.4 | 1.6 | 23.1 |
| 1, % | 27.1 | 25.7 | 35.0 | 36.3 | 4.8 | 19.7 |
| 2, % | 27.8 | 27.6 | 29.2 | 33.4 | 21.5 | 20.4 |
| 3, % | 36.4 | 37.4 | 30.6 | 27.9 | 72.1 | 36.9 |
| Discharge medications | ||||||
| Aspirin | 95.3 | 95.0 | 97.5 | 97.9 | 96.5 | 92.8 |
| β‐Blocker | 93.9 | 93.7 | 95.2 | 94.8 | 93.0 | 93.3 |
| Statin | 81.3 | 80.3 | 88.4 | 88.6 | 83.7 | 74.5 |
| Clopidogrel | 72.9 | 71.0 | 86.7 | 97.3 | 32.3 | 55.5 |
| ACE inhibitor or ARBg | 70.3 | 69.5 | 76.9 | 75.4 | 55.7 | 69.1 |
| Discharge interventions | ||||||
| Smoking cessation counseling | 83.2 | 82.5 | 87.1 | 89.4 | 90.8 | 74.1 |
| Cardiac rehabilitation referral | 65.2 | 63.6 | 77.1 | 74.0 | 87.4 | 50.5 |
| Diet modification counseling | 83.5 | 82.8 | 89.2 | 89.8 | 93.2 | 76.5 |
ACE indicates angiotensin converting enzyme; ARB, angiotensin receptor blocker; BP, blood pressure; CABG, coronary artery bypass grafting; CAD, coronary artery disease; HF, heart failure; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MI, myocardial infarction; N/A, not applicable; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; ULN, upper limit of normal.
There were 261 patients with missing in‐hospital revascularization status.
Values presented as median (25th, 75th percentiles).
Dyslipidemia defined as known total cholesterol >200 mg/dL or chronic treatment with lipid‐lowering agent.
This reflects initial admission value.
Among nondialysis patients.
Among patients who underwent a diagnostic catheterization.
Among patients with guideline‐recommended indications for therapy: hypertension, diabetes mellitus, heart failure, and ejection fraction <40%.
Among patients with STEMI, 65.5% of patients were treated with primary PCI, 8.0% were treated with CABG, and 25.9% were treated with medical management. Among patients with NSTEMI, 32.3% underwent PCI, 8.7% underwent CABG, and 57.7% underwent medical management. In comparison with patients who underwent PCI or CABG, medically managed patients were older, more often females, and had a higher rate of the following comorbidities: hypertension, diabetes mellitus, prior MI, prior CABG, prior heart failure, prior stroke, and peripheral artery disease.
The proportion of patients discharged on evidence‐based medications was high: aspirin 95.3%, β‐blocker 93.9%, statin 81.3%, and clopidogrel 72.9%. Furthermore, 83.2% of smokers received smoking cessation counseling and 83.5% of all patients received dietary counseling.
Mortality
The unadjusted in‐hospital mortality rate was 7%. As illustrated in Figure 2, the unadjusted mortality rate was 24% at 1 year and continued to rise steadily beyond the first year. Mortality rates were 51% at 5 years, and 65% at 8 years. Among patients who survived the first year post‐MI, observed mortality rates were lower but approximated those of the overall population over time. The 8‐year mortality was 59% in the landmark population.
Figure 2.
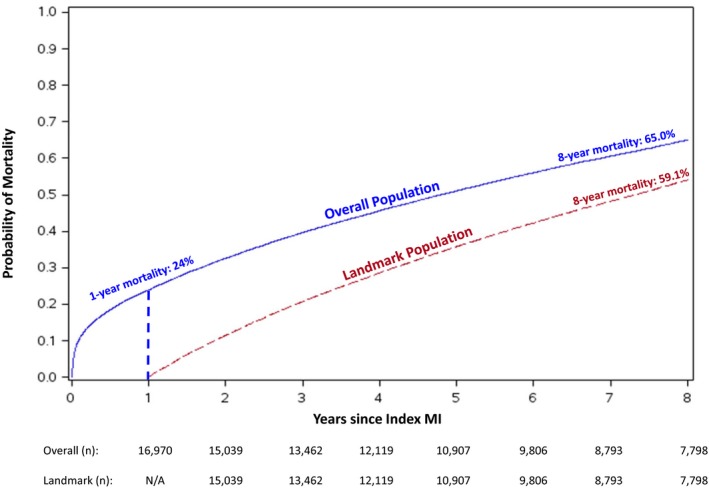
Cumulative Kaplan–Meier mortality estimates during an 8‐year follow‐up period in the overall population (blue line) and landmark population, conditional on surviving 1 year from the index MI hospitalization (dashed red line). n is the number of patients at risk. MI indicates myocardial infarction; N/A, not applicable.
The median survival was 4.8 years (25th, 75th percentiles: 1.1, 8.5 years). The observed 8‐year mortality rate was 45% among patients aged 65 to 74 years and 77% among patients ≥75 years. In contrast to actuarial life expectancy data from the United States National Vital Statistics Reports,9 post‐MI patients had substantially lower life expectancy. Median actuarial and post‐MI survival, stratified by age group, are shown in Figure 3. Table S1 provides the sample sizes and interquartile range of the age groups depicted in Figure 3. The differences are especially marked in the relatively younger cohort of patients aged 65 to 74 years. Note, actuarial data are presented only to provide context; there was no statistical testing conducted to determine statistical significance.
Figure 3.
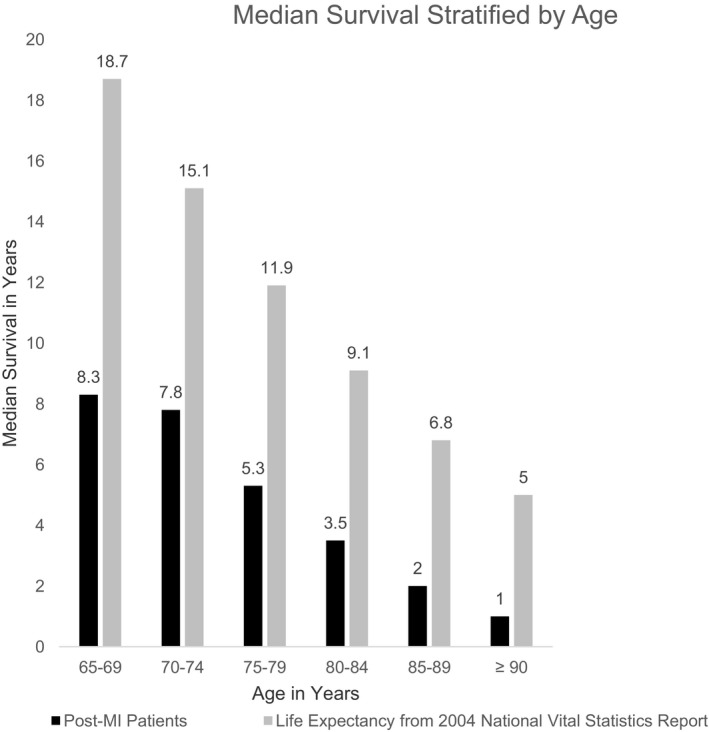
Median survival in years, stratified by age group at presentation for the index MI. The black bars reflect post‐MI patients in the CRUSADE‐CMS‐linked data set. The gray bars represent expected lifespan from the United States National Vital Statistics Report.9 The largest difference in survival is noted among the relatively younger patients. Data from the 2004 National Vital Statistics Report are presented to provide context. The gray bars reflect expected lifespan of adults by ascending order of age: 65, 70, 75, 80, 85, and 90 years. Direct comparisons cannot be made between the median survival among our cohort and the expected lifespan because of the differences in age categorization. The sample sizes for the post‐MI patients depicted in the black bars are provided in Table S1. CMS indicates Centers for Medicare and Medicaid Services; MI, myocardial infarction.
Mortality Stratified by Subgroups
Probability of observed mortality, stratified by MI type and then by revascularization status, separately, are shown in Figure 4. The Kaplan–Meier curves for mortality among patients with NSTEMI demonstrate a steeper curve over an 8‐year follow‐up period in comparison with patients with STEMI (Figure 4A). However, after adjustment for differences in demographic and clinical characteristics, the differences in 8‐year probability of mortality compared among patients with NSTEMI and STEMI attenuated with an adjusted hazard ratio of 0.94 (95% confidence interval, 0.88–1.00, Table 2). The discordance between the steeper NSTEMI curve and the statistically insignificant differences in risk of mortality between NSTEMI and STEMI after adjustment may reflect the higher comorbidity burden within the NSTEMI cohort. The early (first 1–2‐year) unadjusted mortality rate among patients undergoing CABG was higher than those undergoing PCI, after which the curves begin to overlap. Alternatively, the mortality curves for medically managed patients revealed higher rates of mortality early and consistently higher rates of mortality over the long‐term follow‐up. These associations between treatment strategy and long‐term mortality persisted after multivariable adjustment (Table 2). Figure 5 shows Kaplan–Meier mortality curves stratified by both MI type and revascularization. Among these groups, 8‐year mortality was lowest in patients with STEMI treated with revascularization and highest in unrevascularized patients with NSTEMI.
Figure 4.
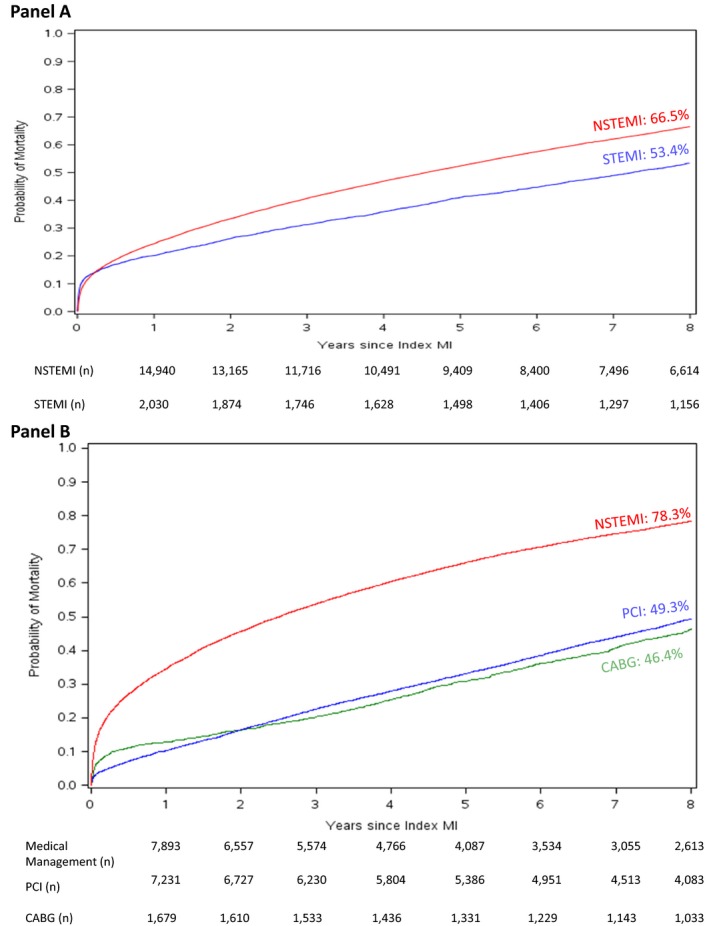
Cumulative Kaplan–Meier mortality estimates stratified by subgroup. A, MI type, NSTEMI (red) vs STEMI (blue). B, Revascularization strategy, Medical Management (red), PCI (blue), CABG (green); n is the number of patients at risk. CABG indicates coronary artery bypass grafting; MI, myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction.
Table 2.
Long‐Term Observed Mortality Rates and Hazard Ratios Stratified by Subgroups
| Subgroup | Subcategory | Observed Event Rate % (95% CI) | Median Follow‐Up Times During the Entire Study Period in Years (25th to 75th Percentiles) | Adjusted HR (95% CI) |
|---|---|---|---|---|
| Prior MI | Presence of prior MI | 74.5 (73.4–75.5) | 3.4 (0.8–8.1) | 1.12 (1.07–1.16) |
| Absence of prior MI | 61.1 (60.3–61.8) | 5.6 (1.3–8.6) | Reference | |
| Sex | Male | 62.6 (61.7–63.5) | 5.2 (1.3–8.6) | 1.18 (1.14–1.23) |
| Female | 67.8 (66.9–68.7) | 4.3 (0.9–8.4) | Reference | |
| DM | Presence of DM | 73.7 (72.7–74.7) | 3.5 (0.8–8.1) | 1.28 (1.23–1.33) |
| Absence of DM | 60.3 (59.5–61.1) | 5.7 (1.4–8.6) | Reference | |
| MI type | STEMI | 53.4 (51.5–55.3) | 7.2 (1.8–8.7) | 0.94 (0.88–1.00) |
| NSTEMI | 66.5 (65.9–67.2) | 4.6 (1.1–8.5) | Reference | |
| Revascularization Status | PCI | 49.3 (48.2–50.4) | 8.0 (3.4–8.8) | 0.64 (0.61–0.67) |
| CABG | 46.4 (44.2–48.6) | 8.2 (3.9–9.0) | 0.61 (0.56–0.66) | |
| Medical management | 78.3 (77.6–79.1) | 2.5 (0.4–7.1) | Reference |
CABG indicates coronary artery bypass grafting; CI, confidence interval; DM, diabetes mellitus; HR, hazard ratio; MI, myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction.
Figure 5.
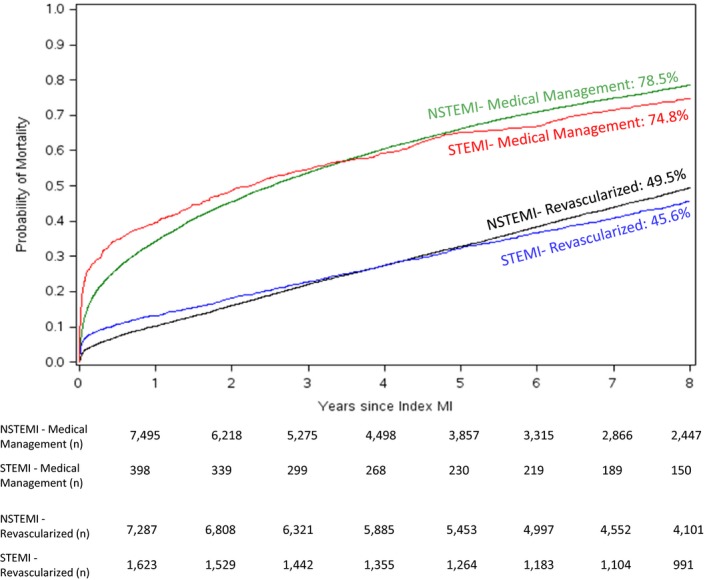
Probability of mortality as a function of time since index MI in years stratified by medically managed STEMI (red line), revascularized (PCI or CABG) STEMI (blue line), medically managed NSTEMI (green line), and revascularized NSTEMI (black line). n is the number of patients at risk. CABG indicates coronary artery bypass grafting; MI, myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction.
Furthermore, we examined the observed 8‐year mortality rates and present the adjusted hazard ratios of several clinically relevant subgroups in Table 2. Patients with a prior MI (74.5%) had a higher mortality rate compared with patients without a prior MI (61.1%); the adjusted hazard ratio was 1.12 (95% confidence interval, 1.07–1.16). The observed 8‐year mortality was lower among males (62.6%) compared with females (67.8%). However, after adjustment for baseline variables, there was a higher hazard of mortality; the adjusted hazard ratio was 1.18 (95% confidence interval, 1.14–1.23). The 8‐year mortality rate was higher for patients with diabetes mellitus (73.7%) versus without diabetes mellitus (60.3%), and this risk remained higher after adjustment: hazard ratio 1.28 (95% confidence interval, 1.23–1.33). Factors associated with 8‐year mortality are depicted in Table S2.
Discussion
This analysis of the CRUSADE registry linked to Centers for Medicare and Medicaid Services claims data provides a unique long‐term perspective on mortality among older patients with MI treated in US clinical practice. Despite high rates of guideline‐directed medical therapy prescribed at discharge, 8‐year mortality for an older cohort (median age 77 years) was high at 65% among MI patients ≥65 years. The median survival of post‐MI patients is markedly lower than actuarial survival of similarly aged adults in the US population. Eight‐year mortality rates exceeded 45% even among the cohort aged 65 to 74 years, patients who underwent in‐hospital revascularization, and patients who survived 1 year post‐index MI.
This study represents one of the largest cohorts of patients with MI treated in routine US clinical practice. While there was a difference in the long‐term mortality curves between patients presenting with NSTEMI versus STEMI, this difference did not remain significant after multivariable adjustment. These data echo prior studies that revealed a difference in both short‐ and long‐term prognosis between NSTEMI and STEMI patients.10, 11 A prior publication of the CRUSADE registry that followed patients aged ≥65 years up to 5 years post‐MI showed lower unadjusted mortality rates for patients revascularized surgically (24.2%) versus percutaneously (33.5%) compared with unrevascularized patients (50.0%).12 Our analysis extended the follow‐up to 8 years, now showing similar mortality between patients treated with CABG versus PCI; however, both groups still had 8‐year mortality rates exceeding 45%. By depicting the mean survival of patients with MI alongside actuarial data (Figure 3), we underscore the difference in natural history among older patients with and without MIs and emphasize the severity of this disease process. We also performed a landmark analysis of patients who have favorable early outcomes by surviving the first year post‐index MI. Early survival may predict better long‐term outcomes. A British study evaluated long‐term survival of patients with MI who survived at least 30 days post‐index event. The median age of that study population was 71 years, and the 7‐year survival was 69% among men and 53% among women.13 In our landmark analysis conditioned upon surviving 1 year post‐MI, we observed an 8‐year mortality rate of 59%. These results emphasize a high mortality risk even among patients who survived up to 1 year after their initial MI and underscore the importance of secondary prevention strategies for patients with MI both early and late after the index MI.
Several factors potentially explain the high mortality rates appreciated in our study. Older patients with MI often present late to medical care because of atypical symptoms, cognitive impairments, or difficulty accessing care.14 The MITRA (maximal individual therapy in acute myocardial infarction) study showed a prehospital delay of 210 minutes in MI patients aged >75 years compared with 155 minutes in patients ≤75 years.15 Rates of coronary revascularization, either PCI or CABG, were low in both STEMI and NSTEMI patients. This may reflect less invasive practice patterns during our study period 2004 and 2006. However, revascularization rates for stable NSTEMI patients aged 65 years and older did not increase substantially when observed between 2007 and 2012, as the rates of PCI were 36% and CABG 8% then.16 Underutilization of evidence‐based therapies has been well described among older patients with MI.14 While evidence‐based medications were prescribed at high rates at the time of discharge, our data could not examine medication dosing, persistence, and adherence over the study period. Prior studies demonstrated suboptimal adherence to statins among older patients with MI, and low medication adherence was associated with a higher risk of major cardiovascular events.17, 18 The high observed death rate in older patients may reflect the influence of multiple comorbidities and frailty on post‐MI recovery.1 Our study underscores the large comorbidity burden among older MI patients; 77% had hypertension, 35% had diabetes mellitus, 17% had renal insufficiency, and 13% had prior stroke. Older patients with MI also contend with competing risks from other illnesses. While our study could not distinguish between cardiovascular and noncardiovascular causes of death, in the TRILOGY ACS (Targeted Platelet Inhibition to Clarify the Optimal Strategy to Medically Manage Acute Coronary Syndromes) trial, which studied NSTE ACS (Non ST elevation acute coronary syndrome) patients ≥75 years, 292 of 368 deaths (79%) were adjudicated as death from a cardiovascular cause.19
Our study was not intended to replace results from randomized clinical trials studying revascularization strategies. However, these data help address a common question posed by patients and caregivers; clinicians can educate patients about their anticipated long‐term prognosis. The mortality of patients treated in routine community practice is likely worse than those of patients typically included in randomized trial populations, regardless of revascularization status. Armed with these data, patients and clinicians can have more meaningful conversations about post‐MI prognosis and more optimally engage in shared decision making, especially as observed survival in post‐MI patients was markedly lower than actuarial survival among similarly aged US adults (Figure 3).9 Clinicians should acknowledge that these long‐term data, calculated based on data from the CRUSADE study in 2001 to 2006, may be different from contemporary prognoses, although we have not observed a substantial increase in revascularization rates among older patients with MI treated in the contemporary era. From a healthcare perspective, these statistics are sobering. These findings should lead to reflections on appropriate utilization and optimal dosing of secondary prevention therapies, along with revascularization when indicated for older patients with MI. Novel medication approaches to secondary prevention may be of particular interest to older patients with MI. Furthermore, older patients with MI are often excluded or underrepresented in clinical trials.1 The high observed mortality underscores the need for older patients with MI to be represented in these trials, and also that trials involving this high‐risk group can be informative with relatively small sample sizes. An example of a study focused on the older population is the SENIOR‐RITA (The British Heart Foundation older patients with non‐ST Segment elevation Myocardial Infarction Randomised Interventional Treatment) trial, which randomizes patients ≥75 years with type 1 NSTEMI to invasive versus conservative treatment strategies.20
Limitations
This study has several limitations. First, hospitals voluntarily participated in the CRUSADE registry, which selected for centers interested in quality improvement, and the generalizability of these findings may be limited to the studied hospitals. Notably, the CRUSADE study included a variety of hospital types: low and high bed volume, teaching and nonteaching, and both urban and rural hospitals. Second, while hospitals were instructed to retrospectively capture consecutive NSTEMI patients, STEMI patients were only collected after October 2004 in a subset of hospitals that volunteered to participate in the STEMI data collection initiative.21 Third, although a broad range of patient‐level clinical factors were used in adjustment, the possibility of confounding by unmeasured covariates remains. For example, we lack data on coronary lesion complexity as well as patient and provider preferences that play an important role in the management decisions regarding revascularization versus medical management. Comparisons of unadjusted, observed mortality between revascularized and nonrevascularized patients were conducted to understand prognosis, but causality should not be implied. We avoided conducting head‐to‐head comparisons of revascularization modalities (PCI versus CABG) as such comparative effectiveness analyses would be severely limited by confounding. Fourth, cause of death was not ascertained. Fifth, our data do not permit comparisons of treatment or outcomes between older and younger (<65 years) patients. Finally, as the primary goal of our study was to examine 8‐year mortality, the index MI in our study preceded the introduction of more potent antiplatelet agents, such as ticagrelor and prasugrel, or newer stent technologies that may improve the prognosis of patients with MI.16
Conclusions
Patients with MI enrolled in the CRUSADE registry aged 65 years and older (median age 77 years) have a 65% 8‐year mortality rate. Long‐term mortality rates exceed 45% even among patients who underwent coronary revascularization, and patients who survived the first year after their index MI event. The 4.8‐year median survival is substantially lower than actuarial estimates for this age group. These findings better inform clinicians and patients regarding the long‐term prognosis following a MI, and demonstrate an opportunity for improving long‐term outcomes in older patients with MI.
Sources of Funding
This project was funded by a research grant to the Duke Clinical Research Institute from Novartis.
Disclosures
Sharma is an employee of Novartis Pharmaceutical Corporation. Pagidipati: ownership in: Freedom Health, Inc.; Physician Partners, LLC; RXAdvance, LLC; Florida Medical Associates, LLC. Cowper reports research support from GE Healthcare, Bristol‐Myers Squibb, Pfizer, Eli Lilly, Tenax Therapeutics, Gilead Sciences, AGA Medical Corporation, AstraZeneca, Bayer, and Novartis. Roe reports research funding from Eli Lilly, Sanofi‐Aventis, Daiichi‐Sanko, Janssen Pharmaceuticals, Ferring Pharmaceuticals, Myokardia, AstraZeneca, American College of Cardiology, American Heart Association, Familial Hypercholesterolemia Foundation; consulting or honoraria from PriMed, AstraZeneca, Boehringer‐Ingelheim, Merck, Actelion, Amgen, Myokardia, Eli Lilly, Novartis, Daiichi‐Sanyko, Quest Diagnostics, and Elsevier Publishers. Peterson reports research funding for projects from Abiomed, Amgen, Inc, AstraZeneca, Daiichi Sankyo, Genetech, Janssen Pharmaceuticals, Merck & Co, Regeneron Pharmaceuticals, and Sanofi‐Aventis; consulting or personal fees from AstraZeneca, Bayer Corporation, Janssen Pharmaceuticals, Merck & Co, Sanofi‐Aventis, and SignalPath. Wang reports research grants from the Duke Clinical Research Institute from AstraZeneca, Boston Scientific, Bristol Myers Squibb, Daiichi Sankyo, Eli Lilly, Gilead Sciences, Novartis, Pfizer, and Regeneron, as well as consulting or honoraria from Merck, Gilead, and Pfizer, Inc. The remaining authors have no disclosures to report.
Supporting information
Table S1. Sample Size of Post‐MI Patients Depicted in the Black Bars in Figure 3
Table S2. Factors Associated With 8‐Year Mortality
(J Am Heart Assoc. 2018;7:e007230 DOI: 10.1161/JAHA.117.007230.)
This analysis was presented as an oral presentation at the Scientific Session of the American College of Cardiology, March 19, 2017 in Washington, DC.
References
- 1. Alexander KP, Newby LK, Cannon CP, Armstrong PW, Gibler WB, Rich MW, Van de Werf F, White HD, Weaver WD, Naylor MD, Gore JM, Krumholz HM, Ohman EM; American Heart Association Council on Clinical Cardiology; Society of Geriatric Cardiology . Acute coronary care in the elderly, part I: non‐ST‐segment‐elevation acute coronary syndromes: a scientific statement for healthcare professionals from the American Heart Association Council on Clinical Cardiology: in collaboration with the Society of Geriatric Cardiology. Circulation. 2007;115:2549–2569. [DOI] [PubMed] [Google Scholar]
- 2. Tegn N, Abdelnoor M, Aaberge L, Endresen K, Smith P, Aakhus S, Gjertsen E, Dahl‐Hofseth O, Ranhoff AH, Gullestad L, Bendz B; After Eighty study Investigators . Invasive versus conservative strategy in patients aged 80 years or older with non‐ST‐elevation myocardial infarction or unstable angina pectoris (After Eighty study): an open‐label randomised controlled trial. Lancet. 2016;387:1057–1065. [DOI] [PubMed] [Google Scholar]
- 3. Velders MA, James SK, Libungan B, Sarno G, Frobert O, Carlsson J, Schalij MJ, Albertsson P, Lagerqvist B. Prognosis of elderly patients with ST‐elevation myocardial infarction treated with primary percutaneous coronary intervention in 2001 to 2011: a report from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR) registry. Am Heart J. 2014;167:666–673. [DOI] [PubMed] [Google Scholar]
- 4. Viana‐Tejedor A, Loughlin G, Fernandez‐Aviles F, Bueno H. Temporal trends in the use of reperfusion therapy and outcomes in elderly patients with first ST elevation myocardial infarction. Eur Heart J‐Acute Ca. 2015;4:461–467. [DOI] [PubMed] [Google Scholar]
- 5. Hoekstra JW, Pollack CV Jr, Roe MT, Peterson ED, Brindis R, Harrington RA, Christenson RH, Smith SC, Ohman EM, Gibler WB. Improving the care of patients with non‐ST‐elevation acute coronary syndromes in the emergency department: the CRUSADE initiative. Acad Emerg Med. 2002;9:1146–1155. [DOI] [PubMed] [Google Scholar]
- 6. Alexander KP, Chen AY, Wang TY, Rao SV, Newby LK, LaPointe NM, Ohman EM, Roe MT, Boden WE, Harrington RA, Peterson ED; CRUSADE Investigators . Transfusion practice and outcomes in non‐ST‐segment elevation acute coronary syndromes. Am Heart J. 2008;155:1047–1053. [DOI] [PubMed] [Google Scholar]
- 7. Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roe MT, Chen AY, Thomas L, Wang TY, Alexander KP, Hammill BG, Gibler WB, Ohman EM, Peterson ED. Predicting long‐term mortality in older patients after non‐ST‐segment elevation myocardial infarction: the CRUSADE long‐term mortality model and risk score. Am Heart J. 2011;162:875–883.e1. [DOI] [PubMed] [Google Scholar]
- 9. Arias E. United States life tables, 2004. Natl Vital Stat Rep. 2007;56:1–39. [PubMed] [Google Scholar]
- 10. McManus DD, Gore J, Yarzebski J, Spencer F, Lessard D, Goldberg RJ. Recent trends in the incidence, treatment, and outcomes of patients with STEMI and NSTEMI. Am J Med. 2011;124:40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia‐Garcia C, Subirana I, Sala J, Bruguera J, Sanz G, Valle V, Aros F, Fiol M, Molina L, Serra J, Marrugat J, Elosua R. Long‐term prognosis of first myocardial infarction according to the electrocardiographic pattern (ST elevation myocardial infarction, non‐ST elevation myocardial infarction and non‐classified myocardial infarction) and revascularization procedures. Am J Cardiol. 2011;108:1061–1067. [DOI] [PubMed] [Google Scholar]
- 12. Roe MT, Li S, Thomas L, Wang TY, Alexander KP, Ohman EM, Peterson ED. Long‐term outcomes after invasive management for older patients with non‐ST‐segment elevation myocardial infarction. Circ Cardiovasc Qual Outcomes. 2013;6:323–332. [DOI] [PubMed] [Google Scholar]
- 13. Smolina K, Wright FL, Rayner M, Goldacre MJ. Long‐term survival and recurrence after acute myocardial infarction in England, 2004 to 2010. Circ Cardiovasc Qual Outcomes. 2012;5:532–540. [DOI] [PubMed] [Google Scholar]
- 14. Avezum A, Makdisse M, Spencer F, Gore JM, Fox KA, Montalescot G, Eagle KA, White K, Mehta RH, Knobel E, Collet JP; GRACE Investigators . Impact of age on management and outcome of acute coronary syndrome: observations from the Global Registry of Acute Coronary Events (GRACE). Am Heart J. 2005;149:67–73. [DOI] [PubMed] [Google Scholar]
- 15. Haase KK, Schiele R, Wagner S, Fischer F, Burczyk U, Zahn R, Schuster S, Senges J. In‐hospital mortality of elderly patients with acute myocardial infarction: data from the MITRA (Maximal Individual Therapy in Acute Myocardial Infarction) registry. Clin Cardiol. 2000;23:831–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fanaroff AC, Peterson ED, Chen AY, Thomas L, Doll JA, Fordyce CB, Newby LK, Amsterdam EA, Kosiborod MN, de Lemos JA, Wang TY. Intensive care unit utilization and mortality among Medicare patients hospitalized with non‐ST‐segment elevation myocardial infarction. JAMA Cardiol. 2017;2:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bansilal S, Castellano JM, Garrido E, Wei HG, Freeman A, Spettell C, Garcia‐Alonso F, Lizano I, Arnold RJ, Rajda J, Steinberg G, Fuster V. Assessing the impact of medication adherence on long‐term cardiovascular outcomes. J Am Coll Cardiol. 2016;68:789–801. [DOI] [PubMed] [Google Scholar]
- 18. Choudhry NK, Setoguchi S, Levin R, Winkelmayer WC, Shrank WH. Trends in adherence to secondary prevention medications in elderly post‐myocardial infarction patients. Pharmacoepidemiol Drug Saf. 2008;17:1189–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roe MT, Goodman SG, Ohman EM, Stevens SR, Hochman JS, Gottlieb S, Martinez F, Dalby AJ, Boden WE, White HD, Prabhakaran D, Winters KJ, Aylward PE, Bassand JP, McGuire DK, Ardissino D, Fox KA, Armstrong PW. Elderly patients with acute coronary syndromes managed without revascularization: insights into the safety of long‐term dual antiplatelet therapy with reduced‐dose prasugrel versus standard‐dose clopidogrel. Circulation. 2013;128:823–833. [DOI] [PubMed] [Google Scholar]
- 20. Kunadian V. The British Heart Foundation SENIOR‐RITA Trial. ClinicalTrials.gov. Bethesda (MD): National Library of Medicine (US). 2000–2017. Available from: https://clinicaltrials.gov/ct2/show/NCT03052036?term=SENIOR+RITA&rank=1. Accessed June 6, 2017.
- 21. Gharacholou SM, Alexander KP, Chen AY, Wang TY, Melloni C, Gibler WB, Pollack CV Jr, Ohman EM, Peterson ED, Roe MT. Implications and reasons for the lack of use of reperfusion therapy in patients with ST‐segment elevation myocardial infarction: findings from the CRUSADE initiative. Am Heart J. 2010;159:757–763. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Sample Size of Post‐MI Patients Depicted in the Black Bars in Figure 3
Table S2. Factors Associated With 8‐Year Mortality


