Abstract
Background
Hypertension is a complex condition and a common cardiovascular risk factor. Dietary docosahexaenoic acid (DHA) modulates atherosclerosis and hypertension, possibly via an inflammatory mechanism. IL‐1 (interleukin 1) has an established role in atherosclerosis and inflammation, although whether IL‐1 inhibition modulates blood pressure is unclear.
Methods and Results
Male apoE−/− (apolipoprotein E–null) mice were fed either a high fat diet or a high fat diet plus DHA (300 mg/kg per day) for 12 weeks. Blood pressure and cardiac function were assessed, and effects of DHA on wall shear stress and atherosclerosis were determined. DHA supplementation improved left ventricular function, reduced wall shear stress and oscillatory shear at ostia in the descending aorta, and significantly lowered blood pressure compared with controls (119.5±7 versus 159.7±3 mm Hg, P<0.001, n=4 per group). Analysis of atheroma following DHA feeding in mice demonstrated a 4‐fold reduction in lesion burden in distal aortas and in brachiocephalic arteries (P<0.001, n=12 per group). In addition, DHA treatment selectively decreased plaque endothelial IL‐1β (P<0.01).
Conclusions
Our findings revealed that raised blood pressure can be reduced by inhibiting IL‐1 indirectly by administration of DHA in the diet through a mechanism that involves a reduction in wall shear stress and local expression of the proinflammatory cytokine IL‐1β.
Keywords: docosahexaenoic acid, endothelium, hypertension, inflammation, interleukin 1, wall shear stress
Subject Categories: Atherosclerosis, Animal Models of Human Disease, Basic Science Research, Growth Factors/Cytokines, Hypertension
Clinical Perspective
What Is New?
In a mouse model of atherosclerosis, supplementation of a Western diet with docosahexaenoic acid improved left ventricular function, reduced aberrant hemodynamic flow patterns in the aorta, and lowered blood pressure.
Atheroma burden was reduced 4‐fold in the docosahexaenoic acid–supplemented group at 2 anatomic sites.
IL‐1 (interleukin 1) is plausibly implicated in the mechanism for these effects because docosahexaenoic acid selectively decreased plaque endothelial IL‐1β.
What Are the Clinical Implications?
Regular supplementation of the diet with docosahexaenoic acid alters blood‐flow patterns in the vasculature, reducing local IL‐1–induced inflammation and atherosclerosis and, consequently, lowering blood pressure.
Given the importance of the CANTOS (Canakinumab Antiinflammatory Thrombosis Outcome Study), our data suggest that strategies that control local IL‐1β may also be useful in hypertension.
Hypertension is a major healthcare concern, given its prevalence among the population and its devastating complications, namely, stroke and ischemic heart disease (IHD).1 Evidence suggests that hypertension and dyslipidemia are the underlying risk factors in IHD.2 In the search for a novel therapeutic intervention, the importance of inflammatory molecules and immune cell involvement in the disease is increasing.
Arterial inflammation has been shown in patients with systemic hypertension.3 Cytokines, including IL‐1 (interleukin 1), are key mediators in inflammation.4, 5 In pathological vascular disease, the main role of IL‐1 is to regulate leukocyte migration and accumulation.6, 7 IL‐1 has also been shown to promote endothelial cell dysfunction,8 a cardinal process in the pathogenesis of hypertension and atherosclerosis. Several studies, in animals9, 10 and humans,11, 12 have postulated a key role for IL‐1β in the pathogenesis of atherosclerosis and clinical sequelae such as hypertension.13, 14
Recent studies suggest that dietary fat, especially saturated fatty acids, are deleterious for atherosclerosis and hypertension.15 Although saturated fat is proatherogenic, ω‐3 fatty acids (n3FA), especially docosahexaenoic acid (DHA; 22:6n‐3), are known to be anti‐atherogenic,16 with multiple underlying mechanisms.17 Consequently, it has been suggested that patients at high risk of the disease need to increase their fish and fish‐derived food intake and decrease saturated fat intake.18, 19
Epidemiological, case–control, and prospective clinical studies have demonstrated positive effects of n3FAs in the reduction of cardiovascular mortality rates among IHD patients.20 These effects have been ascribed to the improvement of cardiovascular risk profiles, resulting in disease prevention21; however, the data on IHD and hypertension are less robust. Clinical trials in post–myocardial infarction patients have suggested that DHA, specifically, may exert a therapeutic effect, particularly on hypertension and the risk of reinfarction,22 although this has not been shown unequivocally or linked with atherosclerotic plaque stabilization/regression.23, 24 DHA supplementation decreased blood pressure in spontaneously hypertensive rats25, 26 and in a dog model of hypertension,27 but no data are available for mouse models with atherosclerosis.
Consequently, it is clear that DHA has broad anti‐inflammatory effects, but whether it has inhibitory effects on IL‐1–dependent inflammation in the setting of hypertension and the mechanisms involved needs further elucidation.
We aimed to investigate whether DHA supplementation in mice could alter vascular contractility via an IL‐1β–mediated mechanism, contributing to blood pressure reduction in experimental atherosclerosis. We showed that DHA reduces wall shear stress (WSS; calculated by computational flow dynamics) in the descending aorta, especially at ostia, and that this is associated with significantly decreased arterial blood pressure, enhanced left ventricular (LV) function, and a reduction in intraplaque inflammation directed by IL‐1β.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure; however, requests for specific elements of the these materials will be considered.
Detailed methods can be found in the supplemental information (Data S1).
Diet‐Induced Hypertension in Mice
Male ApoE−/− (apolipoprotein E–null) mice were bred in‐house at the University of Sheffield. Food and water were given ad libitum in a controlled environment (temperature 22–25°C, humidity 55±5%, and 12‐hour light cycle). Starting at 8 weeks of age, the mice were housed individually and randomly separated into 1 of 2 groups (n=12 per group). The control group was fed a Western‐type high‐fat diet (HFD) containing 21% (wt/wt) fat, 0.15% (wt/wt) cholesterol, and 0.296% (wt/wt) sodium, whereas the DHA‐treated group received the HFD and DHA (99% purified). In this model, mice have raised blood pressure as a result of HFD feeding.9
DHA feeding was achieved by mixing the DHA with jelly using a previously described “jelly‐delivery” protocol28 at a final concentration of 300 mg/kg per day. Briefly, DHA was placed into separate shallow molds (12 doses of DHA per mold). Flavored jelly was dissolved in hot water, according to the manufacturer's instructions and allowed to cool, then poured into each mold and mixed with the DHA to achieve an even suspension. Molds were placed immediately in a freezer and later cut into 12 equal cubes, measuring ≈1 cm3, containing a single dose of drug. The mice were monitored daily for their DHA intake. Body weights were measured weekly over 12 weeks. All animal care and procedures were closely conducted under ASPA 1986, UK and in accordance with Home Office guidelines under license 70/7992.
Biochemistry
To confirm absorption of DHA, red blood cell ω‐3 composition and indexes were measured by gas chromatography in pooled blood samples of the mice (4 mice per group).29
Blood Pressure Analysis
Systolic and diastolic blood pressure was measured in the mice using a Visitech tail‐cuff system, as described previously.9
Echocardiography
Echocardiograms were performed in the mice fed either a HFD alone or a HFD and DHA over 12 weeks, as described previously.30
Atherosclerosis Analysis
The extent of atherosclerosis was assessed in cross‐sectional aortic sinus and brachiocephalic sections, as described.31 Mean lesion size was calculated from measurements of 5 sinus sections starting from the 3 cusp area. Analysis was conducted blinded per individual animal and experimental conditions, and measurements from all sections averaged per mouse. A second assessment of atherosclerosis was conducted in the whole aortas using an en face method.32 Lesion areas were analyzed using a NIS‐Elements software system (Nikon).
Immunohistochemical Analysis
Paraffin‐embedded aortic sections were immunostained, as described previously.33 Heat‐mediated antigen retrieval was performed using citrate buffer (pH 6.0). Following blockage of nonspecific binding by incubation with either 1% (wt/vol) bovine serum albumin or 1% (wt/vol) nonfat milk, the sections were incubated with the relevant primary antibody overnight at 4°C (anti–IL‐1β, 1:100, or anti–IL‐1ra, 1:100). Positive staining was detected using a biotinylated secondary antibody followed by an avidin/biotin complex with horse radish peroxidase label (ABC‐HRP complex) and visualized using DAB. Cell nuclei were counterstained using hematoxylin. Images were taken and analyzed using NIS‐Elements software.
Computational Fluid Dynamics
Computational fluid dynamics of the unsteady flow in the aortic branch, including the ostia, was performed using the unsteady Navier‐Stokes code ANSYS Fluent 17.1, following a similar model to that used by Luong et al.34 The geometry of a single representative murine aortic geometry was derived from postmortem optical projection tomography.35 Briefly, the thoracic descending aorta was carefully dissected and embedded in 1.5% (wt/vol) low‐melting‐point agarose gel. The sample was dehydrated in pure ethanol (twice, 24 hours each) and optically cleared in BABB (benzyl alcohol and benzyl benzoate at a 1:2 ratio) for 24 hours. The aorta was scanned using a Bioptonics 3001 tomograph (Bioptonics). Projection images (×400) were obtained with a 0.9° rotation step, and these were reconstructed into a 3‐dimensional image that contained a stack of 1024 cross‐sections using NRecon software (Skyscan). The 3‐dimensional image was then triangulated into a high‐resolution STL (standard triangle language) surface, and an intermediate surface mesh comprising 2.2 million triangles was produced using image processing and reconstruction (ImageJ v1.49u36) and image segmentation (3‐dimensional slicer v4.5.037) software. Further mesh manipulation and cleaning were performed using Paraview v4.3.138 and the specialized vascular modeling software @neuFuse v7.3 to produce a high‐resolution mesh comprising 1.3 million triangles. This resolution was chosen as a compromise to accommodate the wide range of length scales from the aortic arch down to the ostia diameters required in this study.
The lumen was meshed using ANSYS ICEM computational fluid dynamics, generating a hybrid tetrahedral mesh with a prismatic boundary layer representation. Based on a mesh independence analysis,34 a final volume mesh size of 1 million cells was used to deliver converged WSS, with simulation times of 36 hours on 4 cores of an Intel i7 3.4 GHz processor. The geometry was assumed to be rigid. Blood was modeled as a Newtonian fluid with a dynamic viscosity of η=0.004 Pa/s and a density of ρ=1235 kg/m3. Calculated with a typical maximum flow speed of V=1 m/s and a hydraulic diameter at the aortic root of D=1.58 mm, the maximum Reynolds number is . The flow was thus modeled as laminar.
The inlet boundary condition was a time‐dependent prescribed velocity profile (Dirichlet condition), calculated as a third‐order Bezier spline approximation39 based on a digitized US Doppler measurement.
Outlet conditions at all exits were von Neumann outlet conditions with a split of 69.8%, 16%, 8%, and 6% for descending aorta, innominate, common carotid, and subclavian, respectively, and 0.14% for each of the 12 ostia. All outlet conditions were in phase with the inlet velocity profile.
Numerical solutions were obtained using cell‐based gradients with second‐order pressure, quadratic upwind momentum, and second‐order implicit time discretization. Time step size was chosen as 1/1000th of the cycle time. Three cycles were simulated to allow for fully developed periodicity, and the last cycle was evaluated.
Three‐dimensional time‐dependent WSS data were exported to calculate the time‐averaged WSS magnitude (TAWSS), the oscillatory shear index (OSI), and transverse WSS (transWSS):
Although OSI quantifies the oscillatory character of near wall flow, transWSS is a metric for instantaneous WSS vector components that are perpendicular to the mean WSS vector in the local endothelium plane, as proposed previously.40
Statistical Analysis
Data are expressed as mean±SEM and were analyzed using Prism software (v6; GraphPad). Paired blood pressure data were analyzed by paired 2‐way ANOVA followed by Sidak posttest. For 2‐group comparisons, data were analyzed by Student t test for normally distributed data. Statistical significance was achieved when P<0.05.
Results
DHA supplementation of the HFD was well tolerated by the mice, with no side effects, and absorption was confirmed by specific changes in free fatty acid composition (increased by 38 μmol/L in DHA‐fed mice) in red blood cell membranes (Figure 1B). The red blood cell ω‐3 index in the DHA‐fed mice increased by an average of 25% compared with the control mice (anecdotal observation under experimental conditions; Figure 1A).
Figure 1.
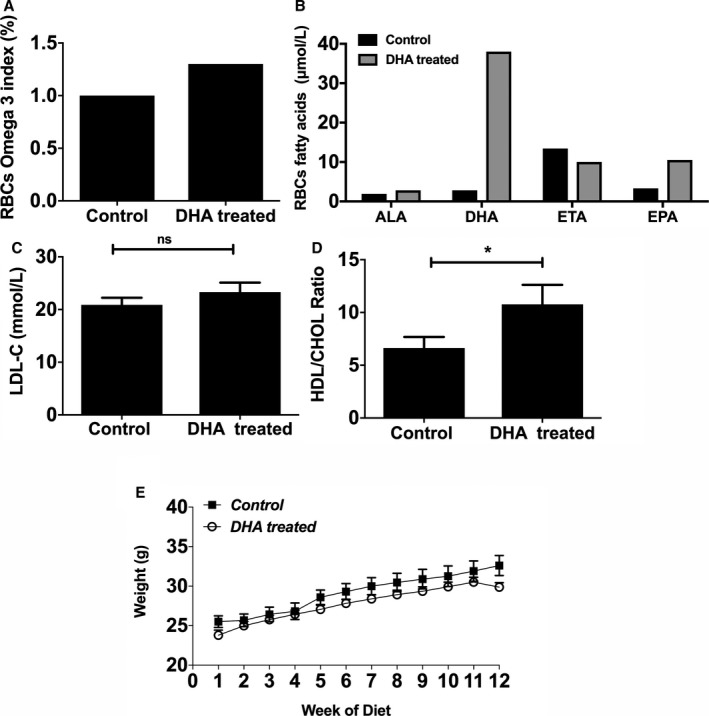
DHA feeding of apoE−/− (apolipoprotein E–null) mice induces changes in RBC ω‐3 fatty acid compositions without any significant effect on their body weights. A, The ω‐3 index (percentage) is enhanced in DHA‐fed mice compared with controls. The apoE−/− mice were fed an HFD alone (control) or an HFD and DHA (300 mg/kg per day) for 12 weeks. Data are from pooled blood of n=4 mice per group. Blood was sampled at the experimental end point. B, Fatty acid composition in RBCs of DHA‐fed mice compared with controls (μmol/L; pooled blood from 4 mice per group). C, Plasma LDL‐C (in mmol/L) and (D) HDL/CHOL ratio, measured enzymatically (n=10 per group). E, Body weights of the mice (in g) were recorded weekly. Data are shown as mean±SEM, analyzed by Student t test (C and D) or 2‐way ANOVA followed by Tukey test (E), *P<0.05. ALA indicates α‐linolenic acid (18:3n‐3); DHA, docosahexaenoic acid (22:6n‐3); EPA, eicosapentaenoic acid (20:5n‐3); ETA, eicosatetraenoic acid (20:4n‐3); HDL/CHOL, high‐density lipoprotein/total cholesterol; HFD, high‐fat diet; LDL‐C, low‐density lipoprotein cholesterol; RBC, red blood cell.
Plasma levels of LDL‐C (low‐density lipoprotein cholesterol) in DHA‐treated mice were not significantly different from those in the controls (Figure 1C), but the HDL (high‐density lipoprotein)/cholesterol ratio significantly increased in the DHA‐supplemented mice compared with controls (Figure 1D). There was no significant change in body weight between the 2 groups (Figure 1E).
We analyzed blood pressure data collected over the feeding period. At baseline, there was no difference in starting values between groups. Subsequently, with feeding, systolic blood pressure rose in both groups until week 5 and then declined in the DHA‐supplemented group only (132.3±9.18 versus 146.6±3.563 mm Hg, respectively, P<0.05, n=4). This fall in systolic blood pressure in the DHA‐supplemented group continued for a further 7 weeks (119.5±7.33 mm Hg in the DHA group compared with 159.7±2.482 mm Hg, P<0.001 at week 12; Figure 2A). Diastolic blood pressure in the DHA‐fed mice also significantly decreased compared with the control group (75±14.65 versus 100.5±7.549, respectively; P<0.01; Figure 2B).
Figure 2.
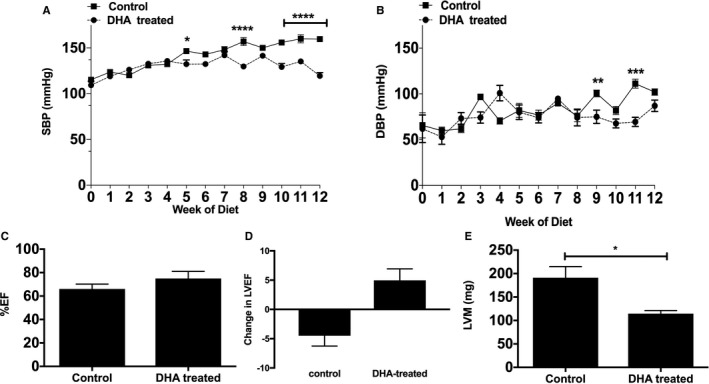
DHA significantly reduces HFD induced hypertension in apoE−/− (apolipoprotein E–null) mice. A, SBP and (B) DBP were measured in freely moving apoE−/− mice fed either HFD alone (control) or HFD and DHA (DHA treated) for 12 weeks (n=4 per group) using a tail‐cuff system (see Methods for details). All data are expressed as mean±SEM, analyzed by 2‐way ANOVA and Tukey posttest, *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001. Effects of DHA feeding on (C) left ventricular EF (%EF) and change in (D) EF and (E) LVM (in mg). Echocardiographic data were measured in anesthetized mice and are presented as mean±SEM (n=4 per group), analyzed by unpaired Student t test, *P<0.05. DBP, diastolic blood pressure; DHA, docosahexaenoic acid (22:6n‐3); EF, ejection fraction; HFD, high‐fat diet; LVM, left ventricular mass; SBP, systolic blood pressure.
Changes in LV wall thickness and function were recorded using echocardiography and were analyzed at week 0 (baseline) and week 12. In the DHA‐supplemented group, the percentage of LV ejection fraction increased after 12 weeks, although this was not statistically significant compared with control mice (Figure 2C). However, the change in ejection fraction between time 0 and 12 weeks showed the control group experienced a significant decrease, whereas the DHA group had an increase in ejection fraction (Figure 2D). LV mass was significantly decreased in the DHA group after 12 weeks (Figure 2E) compared with controls. Collectively, these data suggest that DHA supplementation may protect against LV hypertrophy induced by HFD feeding in apoE−/− mice, secondary to high blood pressure.
Atherosclerotic lesion analysis showed no difference in lesion size in the aortic root (Figure 3A and 3B) but a significant reduction in lesion size in brachiocephalic artery in DHA‐supplemented HFD mice versus controls (Figure 3C and 3D).
Figure 3.
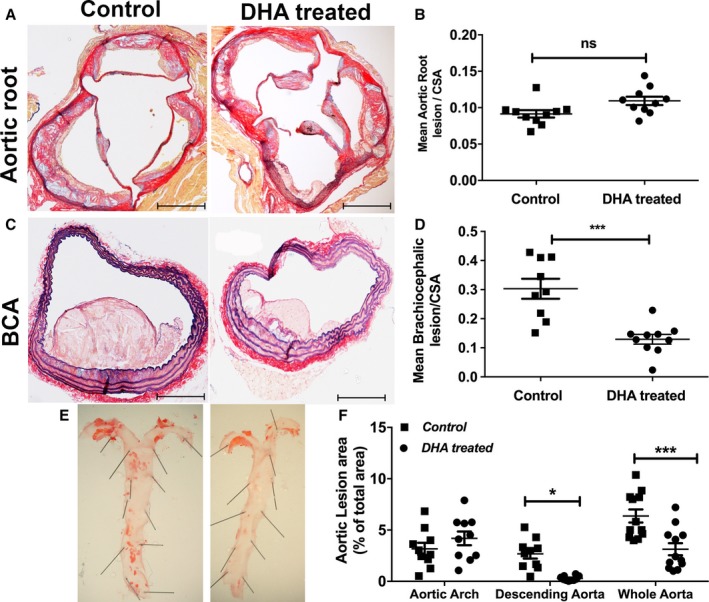
Differential effects of DHA feeding on lesion development in different areas of the vascular beds. Male apoE−/− (apolipoprotein E–null) mice aged 8 weeks were fed a Western‐type HFD alone (control) or an HFD and jelly containing DHA (DHA treated; 300 mg/kg per day) daily over 12 weeks. A, Representative images of aortic roots stained with AB/EVG after 12 weeks of feeding (n=12 per group). Scale bars=100 μm. B, Mean lesion area of aortic root sections, normalized to CSA (n=12). Data are mean±SEM, analyzed by unpaired Student t test, P=ns. C, Representative images of brachiocephalic sections stained with AB/EVG (scale bars=100 μm). D, Mean lesion area of brachiocephalic arteries, normalized to CSA. Data are mean±SEM, analyzed by unpaired Student t test, 9 or 10 per group, ***P<0.001. E, Representative en face morphometric images of the total aortic lesion area and (F) whole aortic, aortic arch, and descending aortic lesion area calculated as a percentage of the total surface area of the whole aorta, showing significant reduction in the total lesion formation in the distal aorta in the DHA group compared with control. Data are mean±SEM, analyzed by 2‐way ANOVA and Tukey post‐test, 11 or 12 per group, *P<0.05, ***P<0.001. AB/EVG indicates alcian blue and elastic van Gieson; CSA, cross‐sectional area; DHA, docosahexaenoic acid (22:6n‐3); HFD, high‐fat diet.
The distribution of plaques in the whole thoracic aorta was significantly altered with DHA feeding, leading to a 45±12% decrease in the total aortic Oil Red O–stained atherosclerotic area (P<0.001) compared with controls (Figure 3E and 3F). This effect was localized in the descending aortic plaques only (a reduction of 30±5%, P<0.05; Figure 3F), with no effect of DHA supplementation observed in the aortic arch.
Optical projection tomography imaging of the descending aorta and its intercostal vessels and reconstruction of slices to make a 3‐dimensional macrogeometry were used to build a computational fluid dynamic model of blood flow at intercostal branches. Using flow parameters from our echocardiography study and estimated boundary conditions from ourselves34 and others,41 simulations of flow showed a reduction in time‐averaged WSS (23.63±1.9 versus 18.53±1.5 Pa, P<0.001) and, more important, both OSI (0.004±0.001 versus 0.003±0.0002, P<0.01) and transWSS (0.355±0.035 versus 0.285±0.03 Pa, P<0.001) in mice supplemented with DHA (Figure 4).
Figure 4.
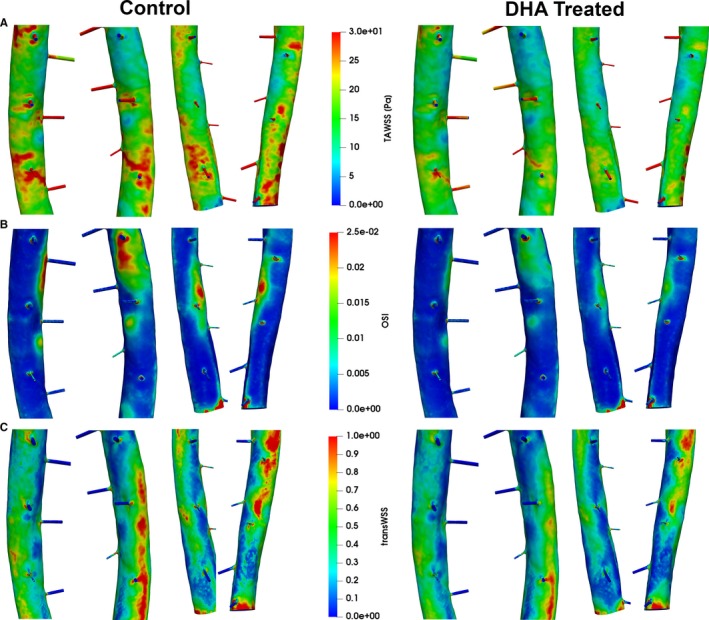
Flow simulations for descending aortas with intercostal branches dissected from DHA‐treated and non–DHA‐treated pro‐atherosclerotic mice. Shown are (A) TAWSS magnitude (TAWSS), (B) OSI, and (C) transWSS for left upper, right upper, left lower, and right lower 3 pairs of the ostia (from left to right) for the control group vs the DHA‐treated group. Flow characteristics remain similar between control and DHA‐treated vessels but show elevated wall shear stress levels throughout that correlate with the higher maximum flow speed. OSI shows only a small decrease in DHA‐treated animals, located mainly below and between ostia branches. TransWSS is again higher in the control group. DHA indicates docosahexaenoic acid (22:6n‐3); OSI, oscillatory shear index; TAWSS, time‐average wall shear stress; transWSS, transverse wall shear stress.
Analysis of inflammatory cytokines showed mice fed a diet supplemented with DHA had reduced plasma IL‐8, regulated on activation, normal T cell expressed and secreted; CCL5 (RANTES), and MCP‐1 (monocyte chemoattractant protein 1), but there was no change in TNF‐α (tumor necrosis factor α) or IL‐1 versus controls (Figure 5A–5F); however, we were able to measure the apical cytokine IL‐1β directly in atherosclerotic lesions by immunostaining. Although we saw no difference in IL‐1β levels in the lesion as a whole (Figure 6A), we did observe a significant reduction in endothelial IL‐1β expression in the DHA‐supplemented mice compared with controls (Figure 6B). IL‐1α levels did not differ between groups (Figure 6C). Levels of IL‐1ra, however, were increased in DHA‐fed mice (Figure 6D). Concomitantly, Western blot analysis of aortas showed levels of TLR‐4 (Toll‐like receptor 4) were reduced following DHA feeding (Figure 6F). Analysis of aortic sections for macrophages showed a significant reduction in DHA‐treated animals compared with controls (Figure 6E). There were no differences in collagen levels (Figure 7) between groups; however, there was a reduction in smooth muscle cells, analyzed by Western blotting for smooth muscle actin, in DHA‐fed mice (Figure 6G).
Figure 5.
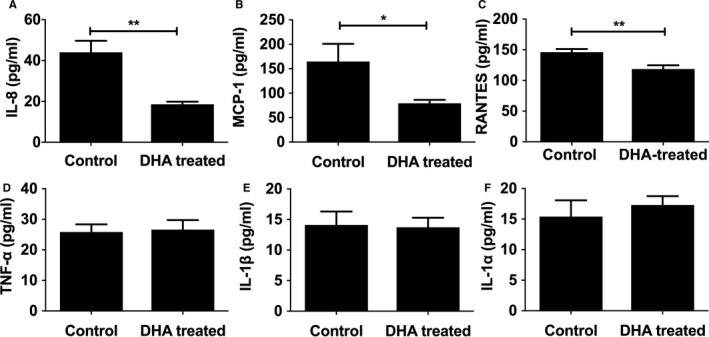
Plasma pro‐inflammatory profiles in response to DHA feeding. Male apoE−/− (apolipoprotein E–null) mice, from 8 weeks of age, were fed either an HFD alone or an HFD and DHA (300 mg/kg per day) daily. Following 12 weeks of diet, freshly isolated plasma was analyzed using cytometric bead arrays for (A) IL‐8, (B) MCP‐1, (C) RANTES, (D) TNF‐α, (E) IL‐1β, and (F) IL‐1α (in pg/mL; 8–10 per group). Data are expressed as mean±SEM, analyzed by unpaired Student t test, *P<0.05, **P<0.01. DHA indicates docosahexaenoic acid (22:6n‐3); HFD, high‐fat diet; IL, interleukin; MCP‐1, monocyte chemoattractant protein 1; TNF‐α, tumor necrosis factor α.
Figure 6.
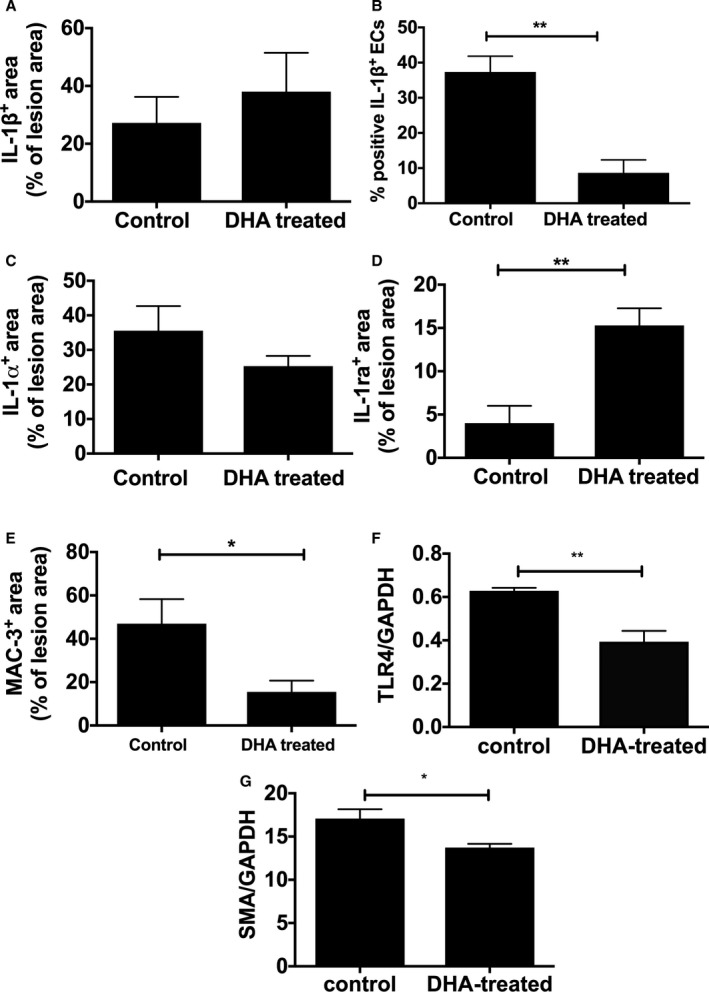
IL‐1 distribution in aortic atherosclerosis and lesion characteristics in response to DHA feeding. Male apoE−/− (apolipoprotein E–null) mice, from 8 weeks of age, were fed either an HFD alone or an HFD and DHA (300 mg/kg per day) daily. Following 12 weeks of diet, immunostaining, measured semiquantitatively as a percentage of total lesions, showed no difference in (A) IL‐1β or (B) IL‐1α. The number of IL‐1β–positive ECs, measured semiquantitatively as a percentage of total number of ECs, is lower in DHA‐treated mice compared with control (B). D, IL‐1ra is increased and (E) Mac‐3 (CD107b) is decreased in DHA‐treated animals. Image analysis was performed using NIS‐Elements software, and data are represented as mean±SEM, 6 to 8 per group. Student t tests indicate a significant difference with *P<0.05; **P<0.01. Western blot analysis of mouse aortas showed (F) TLR‐4 and (G) SMA levels are significantly decreased following DHA feeding. DHA indicates docosahexaenoic acid (22:6n‐3); EC, endothelial cell; HFD, high‐fat diet; IL, interleukin; SMA, smooth muscle actin; TLR‐4, Toll‐like receptor 4.
Figure 7.
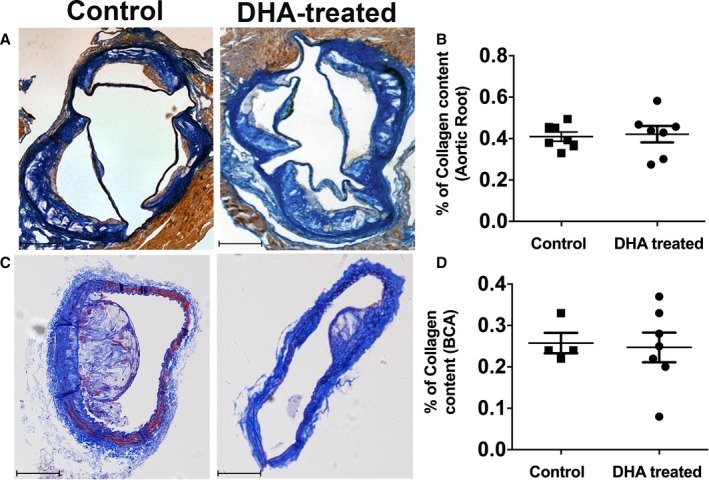
Collagen content in the different vasculature is not affected by DHA feeding. A, Collagen content in the aortic root of studied groups. Representative images of aortic roots of apoE−/− (apolipoprotein E–null) mice fed with an HFD alone or an HFD and DHA for 12 weeks, stained for martius scarlet blue. Collagen stains bright blue. Scale bars=100 μm. B, Quantification of the collagen content within the aortic roots of the 2 studied groups, measured as a percentage of the total lesion area. C, Collagen content in BCAs. Representative images of BCA of apoE−/− mice fed with an HFD alone or an HFD and DHA for 12 weeks, stained for martius scarlet blue. Collagen stains bright blue. Scale bars=100 μm. D, Quantification of the collagen content within the BCAs of the 2 studied groups measured as a percentage of the total lesion area. Data are shown as mean±SEM, n=7 per group, analyzed by unpaired Student t test, P=ns. BCA indicates brachiocephalic artery; DHA, docosahexaenoic acid (22:6n‐3); HFD, high‐fat diet.
Discussion
Contemporary primary and secondary therapies for cardiovascular disease prevent the progression of atherosclerosis and stabilize the plaque, providing significant clinical benefit42; however, not all patients respond to risk factor–modifying therapy, especially patients with hypertension.43, 44, 45 The Ω‐3 fatty acids, particularly DHA (a major type of n3FA in fish oil) and α‐linolenic acid (ALA; the most common land‐based type of n3FAs), have been shown to play an important role in preventive medicine.46, 47 A diet supplemented with n3FAs leads to increased incorporation of the n3FAs (ALA, DHA, eicosapentaenoic acid) in plasma lipoproteins48 associated with lower rates of plaque vulnerability.49, 50 In clinical studies, however, DHA has been mostly mixed with eicosapentaenoic acid or given as fish oil, and thus its individual effects remain obscured. ALA may have a cardioprotective role; however, the overall evidence is mixed and remains inconclusive.51, 52 Similarly, several animal models of atherosclerosis have investigated the anti‐atherogenic effects of n3FAs,53 but these studies also did not directly define the differential effects of individual n3FAs on atherosclerosis. To our knowledge, this study is the first to define a common anti‐atherogenic and anti‐hypertensive mechanism of DHA. It is also the first to perform fluid dynamics analysis of blood flow around the intercostal branches in a mouse and to suggest a potential mechanism by which DHA alters flow patterns in the distal aortas, leading to modulation of inflammation.
It is published that n3FAs have a blood pressure–lowering effect in both normotensive54, 55 and hypertensive56 individuals. In addition, studies using animal models have shown that fish oil has a generalized blood pressure–lowering effect.57 Nevertheless, as with atherosclerosis, the individual effects of n3FAs in hypertension are less studied. DHA is more commonly investigated for its anti‐hypertensive effects,27 and there is an endogenous conversion for DHA to eicosapentaenoic acid occurring mainly in the liver.58 Consequently, this study focused on the specific effects of DHA on atherosclerosis‐related hypertension.
Our data show that DHA significantly decreases blood pressure in our experimental model, reinforcing previous interventional studies in humans59, 60 and consistent with a report that DHA supplementation significantly reduces 24‐hour ambulatory blood pressure in hypercholesterolemic patients.61 In animal studies, DHA reduces systolic blood pressure and vascular wall thickness in spontaneously hypertensive rats25, 62 and aldosterone‐induced hypertension in dogs.27 Our study, however, is the first to elucidate the hypotensive effects of DHA in experimental atherosclerosis induced in mice by feeding a Western diet.
In contrast, ALA supplementation did not show any significant impact on blood pressure in atherosclerotic mice (Mabruka A Alfaidi, PhD, 2017, unpublished data). ALA as an antihypertensive agent has been studied in a limited number of studies,63 and the lack of effect on blood pressure with ALA is at odds with previous clinical studies that demonstrate the hypotensive effects of ALA in hypertensive individuals.56 Mice, however, are deficient in the enzyme that is responsible for the endogenous metabolism of ALA64 and thus may respond differently to ALA than humans. As such, ALA investigations were not pursued in this study.
An independent association exists between LV mass and atherosclerotic coronary disease in patients with IHD,65 and we observed that supplementation with DHA significantly attenuated the increase in LV mass after 12 weeks of HFD feeding in mice. Our finding of a significant reduction of total aortic tree lesions by DHA is in agreement with another recent study.66 However, we go further than this and show that DHA supplementation of a Western diet in mice significantly and selectively decreases atherosclerosis in the distal parts of the aorta and brachiocephalic arteries. Such differential atherosclerotic responses have been reported before but have not been explained.67 Our novel computational flow analyses indicate that flow effects are part of the explanation for this, and we postulate that the effect of DHA is more pronounced in areas with a high oscillatory or a high transverse component of the WSS compared with areas where there is high average WSS. In some locations along the aorta (especially in the arch), the plaque formation in the control group correlates with high OSI, whereas in regions with generally low OSI, there seems to be a correlation with high transWSS. The exact relationship between these WSS derivatives and atherosclerosis is not yet fully understood, although hypertensive remodeling of resistance vessels is postulated to be due to a combination of genetic factors, maladaptation of walls of vessels in the microcirculation to adverse mechanical conditions, and the influence of neurohumoral and local trophic factors.68, 69, 70
The local anti‐inflammatory effect of DHA has been previously associated with a thickening in the fibrous cap and more stable plaque formation.45 In our study, however, smooth muscle cell content was decreased and collagen content was not altered. DHA is known to suppress smooth muscle cell proliferation in cell culture,20, 71 and our data suggest that the mechanism of atheroprotection mediated by DHA may be complex, linked to blood flow patterns and WSS affecting inflammation locally and at distal atheroprone sites.
We suggest that DHA, through modulation of complex blood flow potentially via changes in viscosity,72 transduces and inhibits apical inflammatory signals that orchestrate inflammation in atherosclerosis. Human studies suggest that supplementing the diet of young males with DHA in fish oil significantly reduces IL‐1β production in LPS (lipopolysaccharide)‐stimulated monocytes.73 In addition, Vijay‐Kumar et al have reported that monocytes harvested from mice fed fish oil containing DHA produced less IL‐1β compared with controls.74 We found, however, that DHA supplementation in mice had no effect on the plasma levels of IL‐1. The discrepancy between our data and these published results could occur because measurement of cytokines in plasma is notoriously difficult or because, in our model, DHA may decrease local production of cytokines such as IL‐1β in cells inside lesions themselves. Indeed, we observed a decrease in endothelial IL‐1β expression in atherosclerotic lesions of DHA‐fed animals versus controls. The primary cellular origin of IL‐1β in atherosclerosis is unclear, but we have previously shown in coronary atherosclerotic plaques of patients with IHD that IL‐1β is predominantly expressed in relatively large amounts in the endothelium and vasa vasorum.75 We did show robust reductions in IL‐8, RANTES, and MCP‐1 in plasma, although previous animal studies have reported no significant correlation between DHA feeding and these plasma cytokines.76 Most of these studies, however, were small and used fish oil, with little attention given to specific DHA effects. Our findings do agree with data from in vitro studies, particularly from cultured cells, including monocytes and endothelial cells.77, 78, 79, 80 We thus conclude that the atheroprotective effect of DHA is transduced by alterations in flow leading to an arterial environment in which levels of these plasma chemoattractants are reduced.
Our previous studies have shown that atherosclerotic mice treated with anakinra or the murine version of canakinumab, as well as IL‐1R1 knockout mice, have reduced blood pressure and atherosclerosis formation compared with controls9, 81 and that this finding is replicated in our DHA‐fed mice. The reduction in IL‐1‐induced biomarkers (IL‐8, MCP‐1, RANTES) in mice fed DHA is also replicated in these mice when IL‐1 signaling is inhibited. This combined with a reduction in TLR‐4 levels and an increase in IL‐1ra levels in DHA‐fed mice all suggest DHA is acting via an IL‐1 mechanism (Figure 8). Furthermore, and of potential clinical relevance, unpublished data from the IL‐A HEART study82, 83 in our group show that in a post hoc secondary analysis of blood pressure data, systolic and diastolic blood pressure and mean arterial pressure decreased in the group receiving IL‐1ra (baseline versus day 14: 131.4±2.4 versus 124.7±1.6 mm Hg, systolic, P<0.01; 75.2±1.5 versus 71.1±1.2 mm Hg, diastolic, P=ns; 93.9±1.7 versus 88.6±1.2 mm Hg, mean arterial pressure, P<0.05; n=76 patients [IL‐1ra] and n=71 [placebo], t test). Given other recent data,84 we speculate that strategies to block IL‐1 signaling directly could modulate hypertension.
Figure 8.
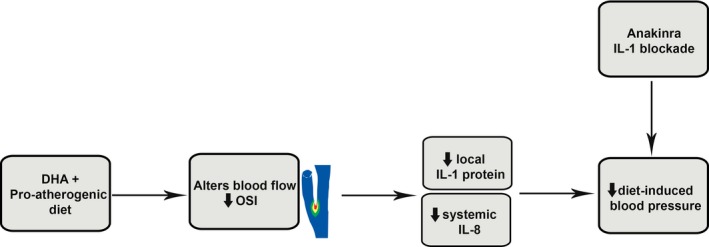
Schematic showing the potential mechanism whereby DHA selectively leads to alterations in oscillatory shear in distal vessels, reduced inflammation, and lower blood pressure. DHA indicates docosahexaenoic acid (22:6n‐3); IL, interleukin; OSI, oscillatory shear index.
Our DHA study does have limitations. It is not clear, for example, whether the effect on atherosclerosis is related to a direct effect of DHA on blood pressure or whether the opposite applies. The same limitation applies to our previous studies that lowered blood pressure by inhibiting IL‐1 signaling directly, without using DHA. To fully address this question, analysis of atherosclerosis in a study group whereby blood pressure is lowered by a non‐DHA and non–IL‐1 mechanism would need to be performed.
In conclusion, our study provides the first evidence that regular supplementation of DHA in the proatherogenic diet of mice influences arterial walls in such a way as to alter blood flow patterns distally in the vascular tree. We suggest that this may lead to reduced local inflammation (IL‐1 production) in vessel walls; reduced lesion burden in distal vessels; and, consequently, lowered blood pressure. Further studies targeting IL‐1 for lowering hypertension directly in mice or humans are warranted.
Sources of Funding
This work was supported by a PhD studentship grant to Dr Alfaidi, from Medical School, Omar Almokhtar University, Al‐Bayda, Libya, and by British Heart Foundation grant PG/13/8/29989, UK Medical Research Council Experimental Medicine Grant (G0502131), the National Institute for Health Research, and a UK Medical Research Council Clinical Research Training Fellowship (AR‐MR/K002406/1). Rothman is supported by a Wellcome Trust Clinical Research Career Development Fellowship (206632/Z/17/Z).
Disclosures
None.
Supporting information
Data S1. Supplemental methods.
(J Am Heart Assoc. 2018;7:e008757 DOI: 10.1161/JAHA.118.008757.)
References
- 1. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R; Prospective Studies Collaboration . Age‐specific relevance of usual blood pressure to vascular mortality: a meta‐analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 2. Fauci AS, Braunwald E, Kasper DL, Hauser SL, Longo DL, Jameson JL, Loscalzo J. Harrison's Principles of Internal Medicine. New York: The McGraw‐ Hill Companies; 2008. [Google Scholar]
- 3. Payne RA, Wilkinson IB, Webb DJ. Arterial stiffness and hypertension emerging concepts. Hypertension. 2010;55:9–14. [DOI] [PubMed] [Google Scholar]
- 4. Fearon WF, Fearon DT. Inflammation and cardiovascular disease—role of the interleukin‐1 receptor antagonist. Circulation. 2008;117:2577–2579. [DOI] [PubMed] [Google Scholar]
- 5. Kleemann R, Zadelaar S, Kooistra T. Cytokines and atherosclerosis: a comprehensive review of studies in mice. Cardiovasc Res. 2008;79:360–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rogus J, Beck JD, Offenbacher S, Huttner K, Iacoviello L, Latella MC, de Gaetano M, Wang H‐Y, Kornman KS, Duff GW. IL1B gene promoter haplotype pairs predict clinical levels of interleukin‐1 beta and C‐reactive protein. Hum Genet. 2008;123:387–398. [DOI] [PubMed] [Google Scholar]
- 7. Chi HH, Messas E, Levine RA, Graves DT, Amar S. Interleukin‐1 receptor signaling mediates atherosclerosis associated with bacterial exposure and/or a high‐fat diet in a murine apolipoprotein E heterozygote model—pharmacotherapeutic implications. Circulation. 2004;110:1678–1685. [DOI] [PubMed] [Google Scholar]
- 8. Aird WC. Endothelium in health and disease. Pharmacol Rep. 2008;60:139–143. [PubMed] [Google Scholar]
- 9. Chamberlain J, Francis S, Brookes Z, Shaw G, Graham D, Alp NJ, Dower S, Crossman DC. Interleukin‐1 regulates multiple atherogenic mechanisms in response to fat feeding. PLoS One. 2009;4:e5073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boesen EI, Sasser JM, Saleh MA, Potter WA, Woods M, Warner TD, Pollock JS, Pollock DM. Interleukin‐1beta, but not interleukin‐6, enhances renal and systemic endothelin production in vivo. Am J Physiol Renal Physiol. 2008;295:F446–F453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Abbate A, Kontos MC, Grizzard JD, Biondi‐Zoccai GG, Van Tassell BW, Robati R, Roach LM, Arena RA, Roberts CS, Varma A, Gelwix CC, Salloum FN, Hastillo A, Dinarello CA, Vetrovec GW; VCU‐ART Investigators . Interleukin‐1 blockade with anakinra to prevent adverse cardiac remodeling after acute myocardial infarction (Virginia Commonwealth University Anakinra Remodeling Trial [VCU‐ART] Pilot study). Am J Cardiol. 2010;105:1371–1377.e1371. [DOI] [PubMed] [Google Scholar]
- 12. Mauno V, Hannu K, Esko K. Proinflammation and hypertension: a population‐based study. Mediators Inflamm. 2008;2008:619704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ling YH, Krishnan SM, Chan CT, Diep H, Ferens D, Chin‐Dusting J, Kemp‐Harper BK, Samuel CS, Hewitson TD, Latz E, Mansell A, Sobey CG, Drummond GR. Anakinra reduces blood pressure and renal fibrosis in one kidney/DOCA/salt‐induced hypertension. Pharmacol Res. 2017;116:77–86. [DOI] [PubMed] [Google Scholar]
- 14. Dalekos GN, Elisaf M, Bairaktari E, Tsolas O, Siamopoulos KC. Increased serum levels of interleukin‐1beta in the systemic circulation of patients with essential hypertension: additional risk factor for atherogenesis in hypertensive patients? J Lab Clin Med. 1997;129:300–308. [DOI] [PubMed] [Google Scholar]
- 15. Grimsgaard S, Bonaa KH, Jacobsen BK, Bjerve KS. Plasma saturated and linoleic fatty acids are independently associated with blood pressure. Hypertension. 1999;34:478–483. [DOI] [PubMed] [Google Scholar]
- 16. Hall WL. Dietary saturated and unsaturated fats as determinants of blood pressure and vascular function. Nutr Res Rev. 2009;22:18–38. [DOI] [PubMed] [Google Scholar]
- 17. Calder PC. Long‐chain fatty acids and inflammation. Proc Nutr Soc. 2012;71:284–289. [DOI] [PubMed] [Google Scholar]
- 18. Krauss RM, Eckel RH, Howard B, Appel LJ, Daniels SR, Deckelbaum RJ, Erdman JW, Kris‐Etherton P, Goldberg IJ, Kotchen TA, Lichtenstein AH, Mitch WE, Mullis R, Robinson K, Wylie‐Rosett J, St Jeor S, Suttie J, Tribble DL, Bazzarre TL. AHA Dietary Guidelines—revision 2000: a statement for healthcare professionals from the Nutrition Committee of the American Heart Association. Stroke. 2000;31:2751–2766. [DOI] [PubMed] [Google Scholar]
- 19. Deckelbaum RJ. N‐6 and n‐3 fatty acids and atherosclerosis ratios or amounts? Arterioscler Thromb Vasc Biol. 2010;30:2325–2326. [DOI] [PubMed] [Google Scholar]
- 20. Yates CM, Calder PC, Rainger GE. Pharmacology and therapeutics of omega‐3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther. 2014;141:272–282. [DOI] [PubMed] [Google Scholar]
- 21. Marchioli R, Barzi F, Bomba E, Chieffo C, Di Gregorio D, Di Mascio R, Franzosi MG, Geraci E, Levantesi G, Maggioni AP, Mantini L, Marfisi RM, Mastrogiuseppe G, Mininni N, Nicolosi GL, Santini M, Schweiger C, Tavazzi L, Tognoni G, Tucci C, Valagussa F; GISSI‐Prevenzione Investigators . Early protection against sudden death by n‐3 polyunsaturated fatty acids after myocardial infarction—time‐course analysis of the results of the Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico (GISSI)‐Prevenzione. Circulation. 2002;105:1897–1903. [DOI] [PubMed] [Google Scholar]
- 22. Vedin I, Cederholm T, Levi YF, Basun H, Garlind A, Irving GF, Joenhagen ME, Vessby B, Wahlund L‐O, Palmblad J. Effects of docosahexaenoic acid‐rich n‐3 fatty acid supplementation on cytokine release from blood mononuclear leukocytes: the OmegAD study. Am J Clin Nutr. 2008;87:1616–1622. [DOI] [PubMed] [Google Scholar]
- 23. Rizos EC, Ntzani EE, Bika E, Kostapanos MS, Elisaf MS. Association between omega‐3 fatty acid supplementation and risk of major cardiovascular disease events a systematic review and meta‐analysis. JAMA. 2012;308:1024–1033. [DOI] [PubMed] [Google Scholar]
- 24. Kwak SM, Myung S‐K, Lee YJ, Seo HG; Korean Meta‐analysis Study Group . Efficacy of omega‐3 fatty acid supplements (eicosapentaenoic acid and docosahexaenoic acid) in the secondary prevention of cardiovascular disease a meta‐analysis of randomized, double‐blind, placebo‐controlled trials. Arch Intern Med. 2012;172:686–694. [DOI] [PubMed] [Google Scholar]
- 25. Engler MM, Engler MB, Pierson DM, Molteni LB, Molteni A. Effects of docosahexaenoic acid on vascular pathology and reactivity in hypertension. Exp Biol Med (Maywood). 2003;228:299–307. [DOI] [PubMed] [Google Scholar]
- 26. Morin C, Rousseau E, Blier PU, Fortin S. Effect of docosahexaenoic acid monoacylglyceride on systemic hypertension and cardiovascular dysfunction. Am J Physiol Heart Circ Physiol. 2015;309:H93–H102. [DOI] [PubMed] [Google Scholar]
- 27. Stanley WC, Cox JW, Asemu G, O'Connell KA, Dabkowski ER, Xu W, Ribeiro RF Jr, Shekar KC, Hoag SW, Rastogi S, Sabbah HN, Daneault C, des Rosiers C. Evaluation of docosahexaenoic acid in a dog model of hypertension induced left ventricular hypertrophy. J Cardiovasc Transl Res. 2013;6:1000–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. West LE, Steiner T, Judge HM, Francis SE, Storey RF. Vessel wall, not platelet, P2Y(12) potentiates early atherogenesis. Cardiovasc Res. 2014;102:429–435. [DOI] [PubMed] [Google Scholar]
- 29. Klem S, Klingler M, Demmelmair H, Koletzko B. Efficient and specific analysis of red blood cell glycerophospholipid fatty acid composition. PLoS One. 2012;7:e33874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hameed A, Bennett E, Ciani B, Hoebers LPC, Milner R, Lawrie A, Francis SE, Grierson AJ. No evidence for cardiac dysfunction in Kif6 mutant mice. PLoS One. 2013;8:e54636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Daugherty A, Whitman SC. Quantification of atherosclerosis in mice. Methods Mol Biol. 2003;209:293–309. [DOI] [PubMed] [Google Scholar]
- 32. Freigang S, Ampenberger F, Spohn G, Heer S, Shamshiev AT, Kisielow J, Hersberger M, Yamamoto M, Bachmann MF, Kopf M. Nrf2 is essential for cholesterol crystal‐induced inflammasome activation and exacerbation of atherosclerosis. Eur J Immunol. 2011;41:2040–2051. [DOI] [PubMed] [Google Scholar]
- 33. Chamberlain J, Evans D, King A, Dewberry R, Dower S, Crossman D, Francis S. Interleukin‐1 beta and signaling of interleukin‐1 in vascular wail and circulating cells modulates the extent of neointima formation in mice. Am J Pathol. 2006;168:1396–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Luong L, Duckles H, Schenkel T, Mahmoud M, Tremoleda JL, Wylezinska‐Arridge M, Ali M, Bowden NP, Villa‐Uriol MC, van der Heiden K, Xing R, Gijsen FJ, Wentzel J, Lawrie A, Feng S, Arnold N, Gsell W, Lungu A, Hose R, Spencer T, Halliday I, Ridger V, Evans PC. Heart rate reduction with ivabradine promotes shear stress‐dependent anti‐inflammatory mechanisms in arteries. Thromb Haemost. 2016;116:181–190. [DOI] [PubMed] [Google Scholar]
- 35. Kirkby NS, Low L, Wu J, Miller E, Seckl JR, Walker BR, Webb DJ, Hadoke PW. Generation and 3‐dimensional quantitation of arterial lesions in mice using optical projection tomography. J Vis Exp. 2015;99:e50627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fedorov A, Beichel R, Kalpathy‐Cramer J, Finet J, Fillion‐Robin JC, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M, Buatti J, Aylward S, Miller JV, Pieper S, Kikinis R. 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging. 2012;30:1323–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ahrens J, Geveci B, Law C. An End‐User Tool for Large Data Visualisation, Visualisation Handbook. Academic Press: Elsevier; 2005. ISBN‐13:978‐0123875822. [Google Scholar]
- 39. Schenkel T, Malve M, Reik M, Markl M, Jung B, Oertel H. MRI‐based CFD analysis of flow in a human left ventricle: methodology and application to a healthy heart. Ann Biomed Eng. 2009;37:503–515. [DOI] [PubMed] [Google Scholar]
- 40. Mohamied Y, Rowland EM, Bailey EL, Sherwin SJ, Schwartz MA, Weinberg PD. Change of direction in the biomechanics of atherosclerosis. Ann Biomed Eng. 2015;43:16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Suo J, Oshinski JN, Giddens DP. Blood flow patterns in the proximal human coronary arteries: relationship to atherosclerotic plaque occurrence. Mol Cell Biomech. 2008;5:9–18. [PubMed] [Google Scholar]
- 42. Aikawa M, Rabkin E, Sugiyama S, Voglic SJ, Fukumoto Y, Furukawa Y, Shiomi M, Schoen FJ, Libby P. An HMG‐CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001;103:276–283. [DOI] [PubMed] [Google Scholar]
- 43. Fuster V, Kovacic JC. Acute coronary syndromes: pathology, diagnosis, genetics, prevention and treatment. Circ Res. 2014;114:1847–1851. DOI: 10.1161/CIRCRESAHA.114.302806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Seropian IM, Toldo S, Van Tassell BW, Abbate A. Anti‐inflammatory strategies for ventricular remodeling following ST‐segment elevation acute myocardial infarction. J Am Coll Cardiol. 2014;63:1593–1603. [DOI] [PubMed] [Google Scholar]
- 45. Libby P, Tabas I, Fredman G, Fisher EA. Inflammation and its resolution as determinants of acute coronary syndromes. Circ Res. 2014;114:1867–1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Winnik S, Lohmann C, Richter EK, Schafer N, Song WL, Leiber F, Mocharla P, Hofmann J, Klingenberg R, Boren J, Becher B, Fitzgerald GA, Luscher TF, Matter CM, Beer JH. Dietary alpha‐linolenic acid diminishes experimental atherogenesis and restricts T cell‐driven inflammation. Eur Heart J. 2011;32:2573–2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vedtofte MS, Jakobsen MU, Lauritzen L, Heitmann BL. Dietary alpha‐linolenic acid, linoleic acid, and n‐3 long‐chain PUFA and risk of ischemic heart disease. Am J Clin Nutr. 2011;94:1097–1103. [DOI] [PubMed] [Google Scholar]
- 48. Leeson CP, Mann A, Kattenhorn M, Deanfield JE, Lucas A, Muller DP. Relationship between circulating n‐3 fatty acid concentrations and endothelial function in early adulthood. Eur Heart J. 2002;23:216–222. [DOI] [PubMed] [Google Scholar]
- 49. Kashiyama T, Ueda Y, Nemoto T, Wada M, Masumura Y, Matsuo K, Nishio M, Hirata A, Asai M, Kashiwase K, Kodama K. Relationship between coronary plaque vulnerability and serum n‐3/n‐6 polyunsaturated fatty acid ratio. Circ J. 2011;75:2432–2438. [DOI] [PubMed] [Google Scholar]
- 50. Cawood AL, Ding R, Napper FL, Young RH, Williams JA, Ward MJ, Gudmundsen O, Vige R, Payne SP, Ye S, Shearman CP, Gallagher PJ, Grimble RF, Calder PC. Eicosapentaenoic acid (EPA) from highly concentrated n‐3 fatty acid ethyl esters is incorporated into advanced atherosclerotic plaques and higher plaque EPA is associated with decreased plaque inflammation and increased stability. Atherosclerosis. 2010;212:252–259. [DOI] [PubMed] [Google Scholar]
- 51. Brouwer IA, Katan MB, Zock PL. Dietary alpha‐linolenic acid is associated with reduced risk of fatal coronary heart disease, but increased prostate cancer risk: a meta‐analysis. J Nutr. 2004;134:919–922. [DOI] [PubMed] [Google Scholar]
- 52. Kromhout D, Giltay EJ, Geleijnse JM; Alpha Omega Trial G . N‐3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363:2015–2026. [DOI] [PubMed] [Google Scholar]
- 53. Brown AL, Zhu X, Rong S, Shewale S, Seo J, Boudyguina E, Gebre AK, Alexander‐Miller MA, Parks JS. Omega‐3 fatty acids ameliorate atherosclerosis by favorably altering monocyte subsets and limiting monocyte recruitment to aortic lesions. Arterioscler Thromb Vasc Biol. 2012;32:2122–2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Sanders TAB, Vickers M, Haines AP. Effect on blood‐lipids and hemostasis of a supplement of cod‐liver oil, rich in eicosapentaenoic and docosahexaenoic acids, in healthy‐young men. Clin Sci. 1981;61:317–324. [DOI] [PubMed] [Google Scholar]
- 55. Ayer JG, Harmer JA, Xuan W, Toelle B, Webb K, Almqvist C, Marks GB, Celermajer DS. Dietary supplementation with n‐3 polyunsaturated fatty acids in early childhood: effects on blood pressure and arterial structure and function at age 8 y. Am J Clin Nutr. 2009;90:438–446. [DOI] [PubMed] [Google Scholar]
- 56. Wang L, Manson JE, Forman JP, Gaziano JM, Buring JE, Sesso HD. Dietary fatty acids and the risk of hypertension in middle‐aged and older women. Hypertension. 2010;56:598–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen HW, Lii CK, Chen WT, Wang ML, Ou CC. Blood pressure‐lowering effect of fish oil is independent of thromboxane A(2) level in spontaneously hypertensive rats. Prostaglandins Leukot Essent Fatty Acids. 1996;54:147–154. [DOI] [PubMed] [Google Scholar]
- 58. Mozaffarian D, Wu JH. Omega‐3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol. 2011;58:2047–2067. [DOI] [PubMed] [Google Scholar]
- 59. Sagara M, Njelekela M, Teramoto T, Taguchi T, Mori M, Armitage L, Birt N, Birt C, Yamori Y. Effects of docosahexaenoic acid supplementation on blood pressure, heart rate, and serum lipids in Scottish men with hypertension and hypercholesterolemia. Int J Hypertens. 2011;2011:809198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Miller PE, Van Elswyk M, Alexander DD. Long‐chain omega‐3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta‐analysis of randomized controlled trials. Am J Hypertens. 2014;27:885–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mori TABD, Burke V, Puddey IB, Beilin LJ. Docosahexaenoic acid but not eicosapentaenoic acid lowers ambulatory blood pressure and heart rate in humans. Hypertension. 1999;34:253–260. [DOI] [PubMed] [Google Scholar]
- 62. Encarnacion MMD, Warner GM, Gray CE, Cheng J, Keryakos HKH, Nath KA, Grande JP. Signaling pathways modulated by fish oil in salt‐sensitive hypertension. Am J Physiol Renal Physiol. 2008;294:F1323–F1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Paschos GK, Magkos F, Panagiotakos DB, Votteas V, Zampelas A. Dietary supplementation with flaxseed oil lowers blood pressure in dyslipidaemic patients. Eur J Clin Nutr. 2007;61:1201–1206. [DOI] [PubMed] [Google Scholar]
- 64. Barcelo‐Coblijn G, Murphy EJ. Alpha‐linolenic acid and its conversion to longer chain n‐3 fatty acids: benefits for human health and a role in maintaining tissue n‐3 fatty acid levels. Prog Lipid Res. 2009;48:355–374. [DOI] [PubMed] [Google Scholar]
- 65. Kishi S, Magalhaes TA, George RT, Dewey M, Laham RJ, Niinuma H, Friedman LA, Cox C, Tanami Y, Schuijf JD, Vavere AL, Kitagawa K, Chen MY, Nomura CH, Brinker JA, Rybicki FJ, Di Carli MF, Arbab‐Zadeh A, Lima JA. Relationship of left ventricular mass to coronary atherosclerosis and myocardial ischaemia: the CORE320 multicenter study. Eur Heart J Cardiovasc Imaging. 2015;16:166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wan J‐B, Huang L‐L, Rong R, Tan R, Wang J, Kang JX. Endogenously decreasing tissue n‐6/n‐3 fatty acid ratio reduces atherosclerotic lesions in apolipoprotein E‐deficient mice by inhibiting systemic and vascular inflammation. Arterioscler Thromb Vasc Biol. 2010;30:2487–2494. [DOI] [PubMed] [Google Scholar]
- 67. Peng D, Hiipakka RA, Reardon CA, Getz GS, Liao S. Differential anti‐atherosclerotic effects in the innominate artery and aortic sinus by the liver X receptor agonist T0901317. Atherosclerosis. 2009;203:59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Folkow B, Grimby G, Thulesius O. Adaptive structural changes of the vascular walls in hypertension and their relation to the control of the peripheral resistance. Acta Physiol Scand. 1958;44:255–272. [DOI] [PubMed] [Google Scholar]
- 69. Jacobsen JC, Gustafsson F, Holstein‐Rathlou NH. A model of physical factors in the structural adaptation of microvascular networks in normotension and hypertension. Physiol Meas. 2003;24:891–912. [DOI] [PubMed] [Google Scholar]
- 70. Augst AD, Ariff B, Mc GTSA, Xu XY, Hughes AD. Analysis of complex flow and the relationship between blood pressure, wall shear stress, and intima‐media thickness in the human carotid artery. Am J Physiol Heart Circ Physiol. 2007;293:H1031–H1037. [DOI] [PubMed] [Google Scholar]
- 71. Pakala R, Sheng WL, Benedict CR. Eicosapentaenoic acid and docosahexaenoic acid block serotonin‐induced smooth muscle cell proliferation. Arterioscler Thromb Vasc Biol. 1999;19:2316–2322. [DOI] [PubMed] [Google Scholar]
- 72. Terano T, Hirai A, Hamazaki T, Kobayashi S, Fujita T, Tamura Y, Kumagai A. Effect of oral administration of highly purified eicosapentaenoic acid on platelet function, blood viscosity and red cell deformability in healthy human subjects. Atherosclerosis. 1983;46:321–331. [DOI] [PubMed] [Google Scholar]
- 73. Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, Vandermeer JWM, Cannon JG, Rogers TS, Klempner MS, Weber PC, Schaefer EJ, Wolff SM, Dinarello CA. The effect of dietary supplementation with n‐3 poly‐unsaturated fatty‐acids on the synthesis of interleukin‐1 and tumor necrosis factor by mononuclear‐cells. N Engl J Med. 1989;320:265–271. [DOI] [PubMed] [Google Scholar]
- 74. Vijay‐Kumar M, Vanegas SM, Patel N, Aitken JD, Ziegler TR, Ganji V. Fish oil rich diet in comparison to saturated fat rich diet offered protection against lipopolysaccharide‐induced inflammation and insulin resistance in mice. Nutr Metab (Lond). 2011;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Galea J, Armstrong J, Gadsdon P, Holden H, Francis SE, Holt CM. Interleukin‐1 beta in coronary arteries of patients with ischemic heart disease. Arterioscler Thromb Vasc Biol. 1996;16:1000–1006. [DOI] [PubMed] [Google Scholar]
- 76. Calder PC. N‐3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83:1505S–1519S. [DOI] [PubMed] [Google Scholar]
- 77. Kelley DS, Taylor PC, Nelson GJ, Schmidt PC, Ferretti A, Erickson KL, Yu R, Chandra RK. Docosahexaenoic acid ingestion inhibits natural killer cell activity and production of inflammatory mediators in young healthy men. Lipids. 1999;34:317–324. [DOI] [PubMed] [Google Scholar]
- 78. Wann AKT, Mistry J, Blain EJ, Michael‐Titus A, Knight MM. Eicosapenaenoic acid (EPA) and docasahexaenoic acid (DHA) reduce IL‐1 beta mediated cartilage degradation. Int J Exp Pathol. 2011;92:A11–A12. [Google Scholar]
- 79. Mullen A, Loscher CE, Roche HM. Anti‐inflammatory effects of EPA and DHA are dependent upon time and dose‐response elements associated with LPS stimulation in THP‐1‐derived macrophages. J Nutr Biochem. 2010;21:444–450. [DOI] [PubMed] [Google Scholar]
- 80. De Caterina RCM, Clinton S, Gimbrone M, Libby P. The omega‐3 fatty acid docosahexaenoate reduces cytokine‐induced expression of proatherogenic and proinflammatory proteins in human endothelial cells. Arterioscler Thromb. 1994;14:1829–1836. [DOI] [PubMed] [Google Scholar]
- 81. Denes A, Drake C, Stordy J, Chamberlain J, McColl BW, Gram H, Crossman D, Francis S, Allan SM, Rothwell NJ. Interleukin‐1 mediates neuroinflammatory changes associated with diet‐induced atherosclerosis. J Am Heart Assoc. 2012;1:e002006 DOI: 10.1161/JAHA.112.002006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Morton AC, Rothman AM, Greenwood JP, Gunn J, Chase A, Clarke B, Hall AS, Fox K, Foley C, Banya W, Wang D, Flather MD, Crossman DC. The effect of interleukin‐1 receptor antagonist therapy on markers of inflammation in non‐ST elevation acute coronary syndromes: the MRC‐ILA Heart Study. Eur Heart J. 2015;36:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Rothman AM, Morton AC, Crossman DC; Investigators M‐IH . Canakinumab for atherosclerotic disease. N Engl J Med. 2018;378:197–198. [DOI] [PubMed] [Google Scholar]
- 84. Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, Fonseca F, Nicolau J, Koenig W, Anker SD, Kastelein JJP, Cornel JH, Pais P, Pella D, Genest J, Cifkova R, Lorenzatti A, Forster T, Kobalava Z, Vida‐Simiti L, Flather M, Shimokawa H, Ogawa H, Dellborg M, Rossi PRF, Troquay RPT, Libby P, Glynn RJ; Group CT . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supplemental methods.


