Abstract
The silverleaf whitefly Bemisia tabaci is an important and invasive crop pest in many countries. Previous laboratory studies with Arabidopsis demonstrated that B. tabaci can suppress jasmonic acid (JA) defenses and thereby enhance B. tabaci performance. Whether B. tabaci can suppress JA-regulated host plant defenses in field is unknown. In the present study, we found that, relative to wild-type (WT) tomato plants, transgenic tomato mutants that activated JA defenses (35s::prosys) or impaired JA defenses (spr-2 and def-1) did not affect the survival or reproduction of B. tabaci adults in growth chamber experiments. In contrast, tomato mutants that activated JA defenses slowed B. tabaci nymphal development, while mutants that impaired JA defenses accelerated nymphal development. These effects of JA defenses on nymphal development were also documented under semi-field conditions. Changes in the expression of defense genes and in the production of phytohormones indicated that B. tabaci adults can suppress JA-dependent defenses after infestation for >72 h. The suppression of JA was correlated with the induction of salicylic acid (SA) in B. tabaci-infested leaves under laboratory and under semi-field conditions. If SA signaling was blocked, JA accumulation increased in infested leaves and B. tabaci nymphal development was delayed. These results indicate that, although JA signaling helps in mediating tomato responses against B. tabaci nymphs, B. tabaci can inhibit JA biosynthesis and its action in an SA-dependent manner.
Keywords: Bemisia tabaci, jasmonic acid, salicylic acid, plant defense, tomato
Introduction
Plants have various defenses against herbivores, and some defenses are induced. Induced plant defenses include the production of antibiotic or antixenotic metabolites that directly influence herbivore performance and behavior (Bleeker et al., 2009; Luan et al., 2013), and the release of volatiles that indirectly recruit parasitoids or predators to infested plants and that thereby indirectly affect herbivores (Turlings et al., 1990; Kessler and Baldwin, 2001; Dicke et al., 2009). The jasmonic acid (JA) signaling pathway is especially important in mediating induced plant defenses against herbivores (Ament et al., 2004; Kessler et al., 2004; Thaler et al., 2012), although salicylic acid (SA) and ethylene (ET) signaling pathways are also important in some cases (Penninckx et al., 1998; Zhang et al., 2013b). In tomato, for example, JA regulates the expression of defense-related genes (Farmer et al., 1992) and the emission of herbivore-induced volatiles that may attract natural enemies (Ament et al., 2004). Accordingly, the blocking of JA synthesis or action can increase the susceptibility of tomato plants to herbivores (Bosch et al., 2014), and can interfere with the pest control by natural enemies (Thaler et al., 2002).
Upon attack, plants perceive elicitors or herbivore-associate molecular patterns, and thereby trigger the JA signaling pathway and anti-herbivore defense responses (Schmelz et al., 2009). Growing evidence indicates that insect herbivores can exploit their elicitors or effectors to circumvent plant defenses for their own benefit (Kant et al., 2015). The butterfly Pieris brassicae, for example, utilizes egg-derived elicitors to inhibit the expression of JA-regulated defense genes in Arabidopsis (Bruessow et al., 2010). The honeydew secreted by the pea aphid Acyrthosiph pisum can suppress JA accumulation in broad bean plants (Schwartzberg and Tumlinson, 2014). Larvae of the Colorado potato beetle, Leptinotarsa decemlineata, exploit bacteria in their oral secretions to suppress JA-dependent defenses in tomato and to thereby promote larval growth (Chung et al., 2013). The suppression of JA-dependent defenses in above cases is mainly dependent on a functional SA-signaling pathway. However, some herbivores can suppress JA responses through other SA-independent mechanisms. For example, two spider mite species, Tetranychus urticae and T. evansi can suppress the defenses downstream of both JA and SA simultaneously in tomato (Sarmento et al., 2011; Alba et al., 2015). The leafhopper Macrosteles quadrilineatus suppresses JA defenses via an effector derived from a vectored phytoplasma, resulting in increased performance of its larvae (Sugio et al., 2011).
The silverleaf whitefly Bemisia tabaci is an important and invasive pest of crops worldwide. B. tabaci is a genetically diverse group, and more than 20 biotypes have been named from population of this species complex (Perring, 2001). The major global invasion event associate with B. tabaci is the invasion by the individuals of biotype B (Middle East–Asia Minor 1) which in the past 20 years has spread rapidly around the world (De Barro et al., 2011). B. tabaci has four larval stages. Except first instar larvae, other stage larvae are immobile and settle at a suitable feeding site until adult emerge. In general, the developmental duration of B. tabaci from egg to adult ranges from 19 to 24 days, which depends on host plant species (Zang et al., 2006). Studies with Arabidopsis showed that feeding by B. tabaci adults or nymphs can down-regulate JA-regulated defenses (Van de Ven et al., 2000; Kempema et al., 2007; Zarate et al., 2007) and that such down-regulation may be mediated by SA cross-talk (Walling, 2008). With the suppression of JA defenses, B. tabaci nymphal development was significantly accelerated on Arabidopsis (Zarate et al., 2007; Zhang et al., 2013a). Because the whitefly–Arabidopsis interaction rarely occurs in the field, however, the ecological relevance of the interaction is unclear. Remarkably, recent works have demonstrated that B. tabaci infestation can suppress the JA-regulated volatile emissions in Lima bean and tomato (Zhang et al., 2009; Su et al., 2018), as well as the expression of JA-dependent genes (Su et al., 2015). However, it still remains unknown whether B. tabaci feeding can suppress JA-regulated host plant defenses in field. In the present study, we compared the performance of B. tabaci adults and nymphs on wild-type (WT) tomato plants, on mutant/transgenic plants that constitutively activate or impair JA signaling pathways, and on WT plants treated with exogenous JA or SA. We then measured the changes in endogenous JA and SA and transcript levels of JA- and SA-regulated defense genes in response to B. tabaci infestation of tomato plants. Finally, we examined the effects of JA and SA defenses on B. tabaci development and determined the effects of SA signaling on the suppression of JA defenses by B. tabaci in semi-field experiment with transgenic NahG plants. Our results demonstrate that, although JA defenses in tomato are crucial for defending against B. tabaci nymphs, B. tabaci can suppress the JA defenses in an SA-dependent manner.
Materials and Methods
Plants and Insects
Wild-type tomato (Solanum lycopersicum) cv Moneymaker (MM) is the parental line for the SA-silenced NahG, which overexpresses the bacterial salicylate hydroxylase transgene (NahG gene). WT tomato cv Castlemart (CM) is the parental line for the JA-silenced spr-2 and def-1, and also for 35s::prosys, in which JA signaling is constitutive. The spr-2 mutant carries a point mutation that results in loss of function of FATTY ACID DESATURASE 7 (FAD7), which is required for the JA biosynthesis (Li et al., 2003). The def-1 mutant is deficient in the induction of both proteinase inhibitor I and proteinase inhibitor II following mechanical damage (Lightner et al., 1993). 35S::prosys seeds were collected from a 35S::prosys homozygote that had been backcrossed five times to its WT line cv CM (Howe and Ryan, 1999). Tomato plants were grown in 500 cm3 pots containing a commercial potting mix (Fafard Growing Mix 1, Agawam, MA, United States) and were kept in a climate-controlled chamber (25 ± 2°C, 60–70% RH, 10L: 14D photoperiod). Plants with four to five fully expanded leaves were used for experiments.
A colony of virus-free B. tabaci (Gennadius) MEAM1 (Hemiptera: Aleyrodidae) was maintained on WT MM plants in a separate climate-controlled chamber (25 ± 2°C, 50–60% RH, 10L: 14D). For all experiments, plants were infested with B. tabaci adults within 48 h after adult emergence.
Laboratory experiments 1–6 were performed in a climate-controlled chamber (25 ± 2°C, 60–70% RH, 10L: 14D photoperiod), and each tomato genotype or treatment was represented by 10–15 replicate plants, unless noted otherwise.
Performance of B. tabaci on Wild-Type (CM) and Mutant Tomato Plants
In laboratory experiment 1, 10 B. tabaci adults (1:1 male-to-female sex ratio) were released into a clip cage attached to the abaxial surface of a leaf (third or fourth leaf from the top) of WT CM, spr-2, def-1, and 35s::prosys plants. The adults were allowed to feed and oviposit on the leaves. After 5 or 14 days, the fecundity was assessed by counting the eggs on the leaves with a binocular microscope.
In laboratory experiment 2, 20 B. tabaci adults were released into a clip cage (20.0 mm high, 45.0 mm diameter) attached to the abaxial surface of a leaf (third or fourth leaf from the top) of WT CM, spr-2, def-1, and 35s::prosys plants. At 7, 10, and 14 days after the adults had been introduced, adult survival was consistently assessed by counting the surviving adults.
In laboratory experiment 3, 100 B. tabaci adults (a mixture of females and males) were released and allowed to feed on WT CM, spr-2, def-1, and 35s::prosys plants in a ventilated cage (21.0 cm high, 13.5 cm diameter; one plant per cage). After 24 h of infestation and oviposition, the adults were removed from the plants by aspiration. At 21 days after introduction of adults, the number of nymphs and their developmental stages (first through fourth instars) were recorded. Developmental rate was estimated by calculating the proportion of nymphs represented by fourth instars (red-eye stage) on each plant.
B. tabaci Development as Affected by Exogenous JA or SA
In laboratory experiment 4, JA or SA (Sigma-Aldrich) was dissolved in 0.2 mL of acetone and dispersed in water (containing 0.1% Tween 20) to produce a 0.5 mM JA or SA solution. Each WT CM plant was sprayed with 5.0 mL of the JA or SA solution with a hand-sprayer. Plants that were sprayed with 5 mL of water containing 0.2 mL of acetone and 0.1% Tween 20 were used as the control. Twenty-four hours later, the plants were placed in cages and infested with B. tabaci adults as described for laboratory experiment 3. After 24 h of infestation and oviposition, the adults were removed from the plants by aspiration. Twenty-one days after adults were placed on the plants, the proportion of nymphs represented by each instar was determined on each plant.
Phytohormone and Gene-Expression Assay on Leaves Infested With B. tabaci Adults
In laboratory experiment 5, 100 B. tabaci adults were equally released into two clip cages that were secured to the abaxial surface of two leaves of one WT CM plant (about 1:1 male-to-female sex ratio). Plants clipped two cages without B. tabaci were used as the control. After 6, 12, 24, 48, 72, and 120 h postinfestation, leaves were collected from two infested or non-infested plants and were pooled as one biological sample respectively. These samples were frozen in liquid nitrogen and stored at -80°C until we extracted phytohormones and RNA. Each combination of time and treatment (with and without infestation) was represented by three biological replicates.
Gene-Expression Assay on Leaves Infested with B. tabaci Nymphs
In laboratory experiment 6, 100 adult whiteflies (≤48 h after emergence) were collected and averagely released into two clip cages that secured to the abaxial surface of two leaves of one plant (50 adults/cage/leaf). Whiteflies were allowed to feed and oviposit on the leaves for 24 h. After that, all adult whiteflies were removed from the plants by aspiration. At 5, 7, and 10 days after the adults had been removed, leave that carried with nymphs were collected from two plants, and pooled as one biological sample.
Quantification of Endogenous JA and SA
Endogenous JA and SA were extracted and quantified as described by Almeida Trapp et al. (2014). In brief, plant material (250–300 mg) was frozen and ground in liquid nitrogen. For quantification, [9, 10]-dihydro-JA (15 ng) and d6-SA (20 ng) were added as internal standards. Samples were analyzed using a GC/MS system (6890N/5973 MSD, Agilent Technologies, Inc., Palo Alto, CA, United States) equipped with an HP-5-MS column (30 m × 0.25 mm × 0.25 mm; J&W Scientific, Agilent Technologies). Endogenous JA, SA, and their internal standards were analyzed in full-scan mode.
Total RNA Isolation and cDNA Synthesis
To minimize wounding- and dehydration-induced gene expression, leaf samples were quickly harvested and immediately frozen in liquid nitrogen. Each combination of time and treatment (with and without infestation) was represented by three biological replicates. Frozen samples were ground to a fine powder in liquid nitrogen with a pestle and mortar. Total RNA was extracted from 100 mg of each leaf sample using a plant RNA isolation kit (Axygen, Hangzhou, China) according to the manufacturer’s instructions. RNA concentration and purity were determined using a NanoDrop TM ND-2000 spectrophotometer (Thermo Scientific, Wilmington, DE, United States), and the integrity of RNA was also assessed by 1% agarose gel electrophoresis and ethidium bromide staining. First-strand cDNA was synthesized from 200 ng of RNA using a First-Strand cDNA Synthesis Kit (Bio-Rad, Hangzhou, China) according to the manufacturer’s instructions.
Quantitative Real-Time PCR
The transcript levels of the LoxD, PI-I, PI-II, and Pr-1b were quantified by real-time quantitative RT-PCR (qRT-PCR). LoxD encodes a key enzyme in the octadecanoid pathway, which is important for JA biosynthesis in tomato (Heitz et al., 1997). Proteinase inhibitors I and II (PI-I and PI-II) are regulated by JA signaling and confer insect resistance in many Solanaceous plants, including tomato (Johnson et al., 1989; Li et al., 2002). Pr-1b is an SA-regulated pathogenesis-related (PR) gene in tomato (Jacobs et al., 2013). qRT-PCR was carried out on an ABI 7500 Real Time PCR System with a 96-well rotor. The amplification reactions were performed in a final volume of 20 μL that contained 10 μL of iQTM SYBR® supermix (BioRad, Hangzhou, China), 0.8 μL of forward primer (5 μM) and reverse primer (5 μM) pairs, and 1 μL of cDNA first-strand template. Thermal cycling conditions were 5 min at 95°C, followed by 40 cycles of 15 s at 95°C, 15 s at 58–62°C, and 30 s at 72°C. Primers used for quantitative RT-PCR are listed in Supplementary Table S1. All reactions were run in duplicate, and average values were used in the analysis.
Semi-Field Experiments
The effects of JA- or SA-dependent defenses on the performance of B. tabaci were assessed under semi-field conditions at China Jiliang University from June to September of 2016. In semi-field experiment 1, plants of each of the following six genotypes were planted in the field: WT MM, WT CM, spr-2, def-1, 35s, and NahG. Each genotype was represented by 30 plants, which were planted in five rows with 40 cm between adjacent plants. The plants of each genotype were grouped together, and adjacent genotypes were 2 m apart. Six weeks after planting, 15 plants were randomly selected from each genotype and were subjected to bioassay. Each plant was infested with 50 B. tabaci adults that were placed in a clip cage attached to the abaxial surface of one leaf. After 24 h, all adults were removed from the plants by aspiration. During the period of field experiments the average temperature was 31.5 ± 2.6°C, in which condition nymphal development will be significantly accelerated. Therefore, we observed the number of nymphs and their developmental stages (first through fourth instars) at 16 after introduction of adults, which is earlier than we observed in laboratory.
Semi-field experiment 2 was conducted to determine the effects of SA signaling on the suppression of JA defenses by B. tabaci. Thirty plants each of WT MM or SA-silenced NahG were planted in the field. Each of 12 plants per genotype was infested with 150 B. tabaci adults that were placed in three clip cages (50 adults/cage) attached to the third, fourth, and fifth leaf from the top. Twelve plants per genotype that had clip cages without B. tabaci were used as controls. At 3 and 5 days after infestation, leaves were collected from two randomly selected infested and non-infested plants per genotype and were pooled as one biological sample. At each timepoint, three biological samples were collected from B. tabaci-infested or uninfested plants. The samples were subjected to phytohormone analysis as described for laboratory experiment 5.
Statistical Analysis
Data of female fecundity, adult survival, gene-expression, and phytohormone levels were analyzed by two-way ANOVA. Normalized gene expression was calculated using the 2-ΔCt method with GAPDH as a reference gene, and values were subsequently log2 transformed for data analysis. The total number of eggs recorded after 5 or 14 days was divided by the number of females that were still alive at the end of the experiments. The percentage of fourth instars of B. tabaci on different plants were analyzed using the general linear model (GLM) for univariate analysis. If treatments were significant (P < 0.05), Tukey’s multiple-comparison tests were used to analyze significant differences between pairs.
Results
Adult Performance
The number of eggs laid per female per day did not differ on CM, spr-2, def-1, and 35s::prosys plants (plant genotype: P = 0.95; time: P = 0.54; interaction: P = 0.91; Figure 1A). Adult survival rate did not differ on CM, spr-2, def-1, and 35s::prosys plants (plant genotype: P = 0.13; time: P < 0.001; interaction: P = 0.89; Figure 1B). These data indicate that JA-dependent defenses do not affect the performance of B. tabaci adults.
FIGURE 1.
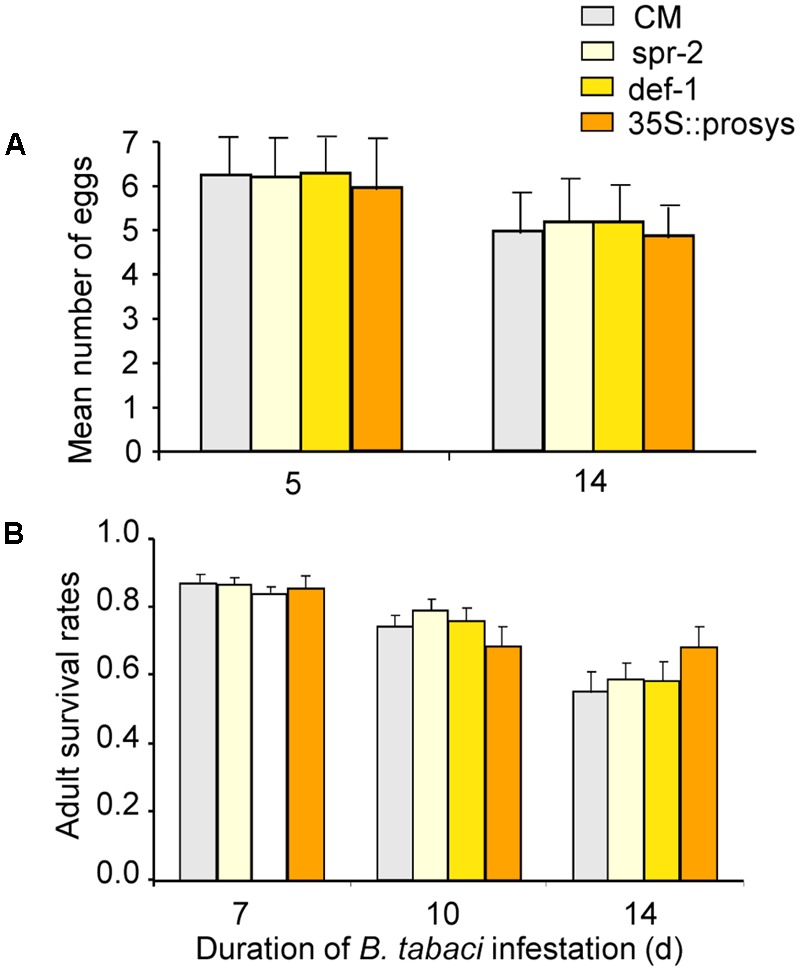
Performance of B. tabaci adults on wild-type (CM) and JA-signaling mutants of tomato. (A) Number of eggs laid per female per day on plants after 5 days of infestation. (B) The proportion of adults that had survived at 7, 10, and 14 days after introduction. Values are means (± SE) of 10–15 biological replicates.
Nymph Performance
At 21 days postinfestation, the total number of nymphs survived did not differ on CM, spr-2, def-1, and 35s::prosys plants (Supplementary Table S2). The proportion of fourth instars was significantly higher on spr-2 and def-1 plants than on CM plants (def-1: P < 0.001; spr-2: P < 0.001; Figure 2A). In contrast, the proportion of fourth instars was significantly lower on 35s::prosys plants than on CM plants (P < 0.001; Figure 2A).
FIGURE 2.
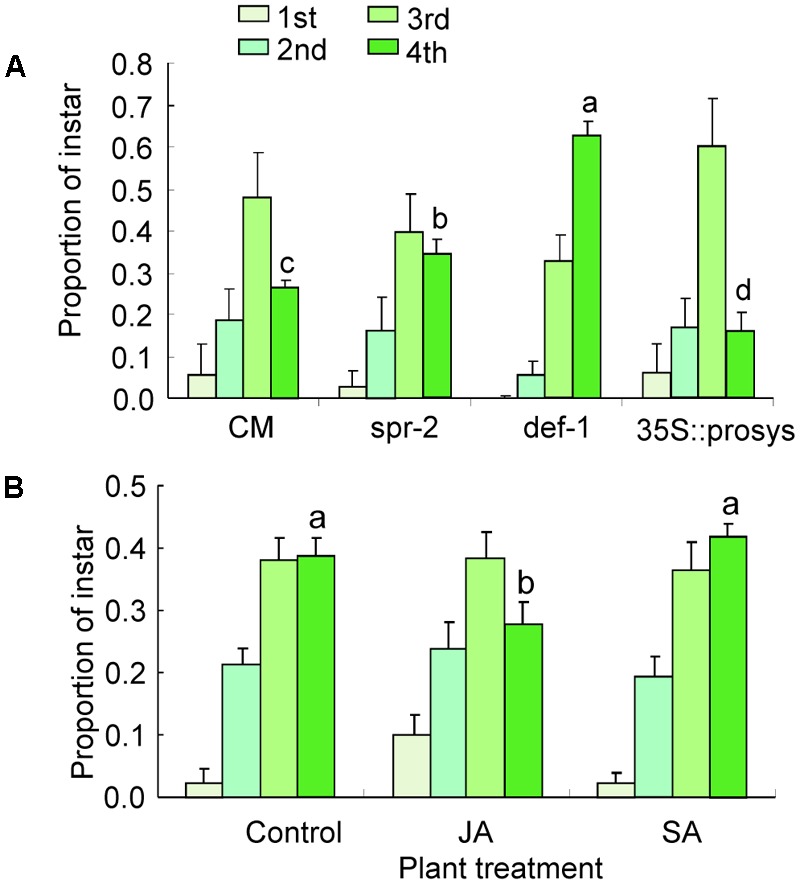
Performance of B. tabaci nymphs on tomato plants. (A) The proportion of nymphs represented by each instar at 21 days after adults were placed on CM and JA-signaling mutants of tomato; (B) The proportion of nymphs represented by each instar at 21 days after adults were placed on untreated (Control) and JA- or SA-treated CM plants. Values are means (±SE) of 10–15 biological replicates. Means for the fourth-instar with different letters are significantly different (univariate analysis with GLM, P < 0.05).
Likewise, exogenous JA or SA treatments have no effects on the total number of nymphs survived at 21 days postinfestation (Supplementary Table S2). When CM plants were sprayed with JA, the proportion of fourth instars was significantly reduced relative to the non-sprayed control (P < 0.001; Figure 2B). In contrast, the proportion of fourth instars was unaffected by exogenous SA treatment (Figure 2B). These data indicate that JA signaling pathway is important for defense against B. tabaci nymphs.
Endogenous JA and SA Levels in Response to B. tabaci Feeding
Whitefly infestation had a significant effect on JA accumulation (whitefly: P = 0.04; time: P = 0.24; interaction: P = 0.51). JA amount was significantly higher in B. tabaci-infested leaves than in non-infested leaves of CM plants at 6–48 h after infestation (P < 0.001) and peaked at 12 h (Figure 3A). After 48 h, JA amount in B. tabaci-infested leaves dropped and did not significantly differ from the amount in non-infested leaves (Figure 3A).
FIGURE 3.
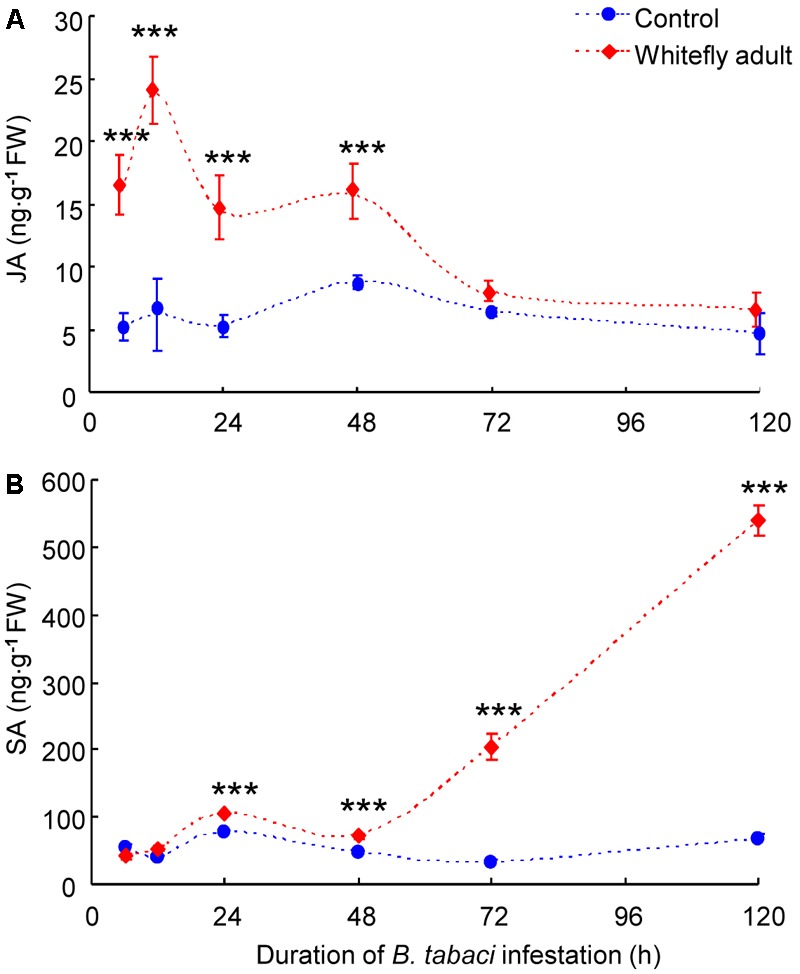
Levels of the phytohormones JA (A) and SA (B) in B. tabaci-infested and in non-infested leaves of WT CM plants. Values are means (±SE) of three biological replicates. Asterisks above bars indicate significant differences (two-way ANOVA; ∗∗∗P < 0.001). FW, fresh weight.
Whitefly infestation had a significant effect on SA accumulation (whitefly: P = 0.03; time: P = 0.43; interaction: P = 0.73). SA amount did not differ in B. tabaci-infested leaves vs. non-infested leaves of CM plants at 6 h or 12 h after infestation but was greater in the infested than in the non-infested leaves at 24–120 h after infestation (P < 0.001; Figure 3B).
Defense-Related Gene Expression Induced by B. tabaci Adults and Nymphs
We next quantified the transcript levels of the following defense-related genes in infested and non-infested leaves of CM plants: LoxD, PI-I, PI-II, and Pr-1b. Whitefly adult infestation had significant effects on the expression of LoxD (whitefly: P = 0.31; time: P = 0.03; interaction: P < 0.001), PI-I (whitefly: P < 0.001; time: P = 0.06; interaction: P = 0.02), PI-II (whitefly: P < 0.001; time: P = 0.07; interaction: P = 0.03), and Pr-1b (whitefly: P = 0.01; time: P = 0.09; interaction: P = 0.01). For LoxD, transcript levels were increased by infestation at 6 and 12 h (6 h: P = 0.047; 12 h: P = 0.045), were unaffected by infestation at 24 and 48 h, and were reduced by infestation at 72 and 120 h (72 h: P = 0.008; 120 h: P = 0.01; Figure 4A). Infestation increased PI-I transcript levels at 6–48 h (P = 0.002–0.04; Figure 4B) and increased PI-II transcript levels at 6–24 h (P = 0.022–0.05; Figure 4C); thereafter, PI-I and PI-II transcript levels were similar in B. tabaci-infested and in non-infested leaves (Figures 4B,C). For Pr-1b, transcript levels were unaffected by infestation at 6 and 12 h but were increased at longer infestation times (24–120 h: P = 0.003–0.042; Figure 4D).
FIGURE 4.
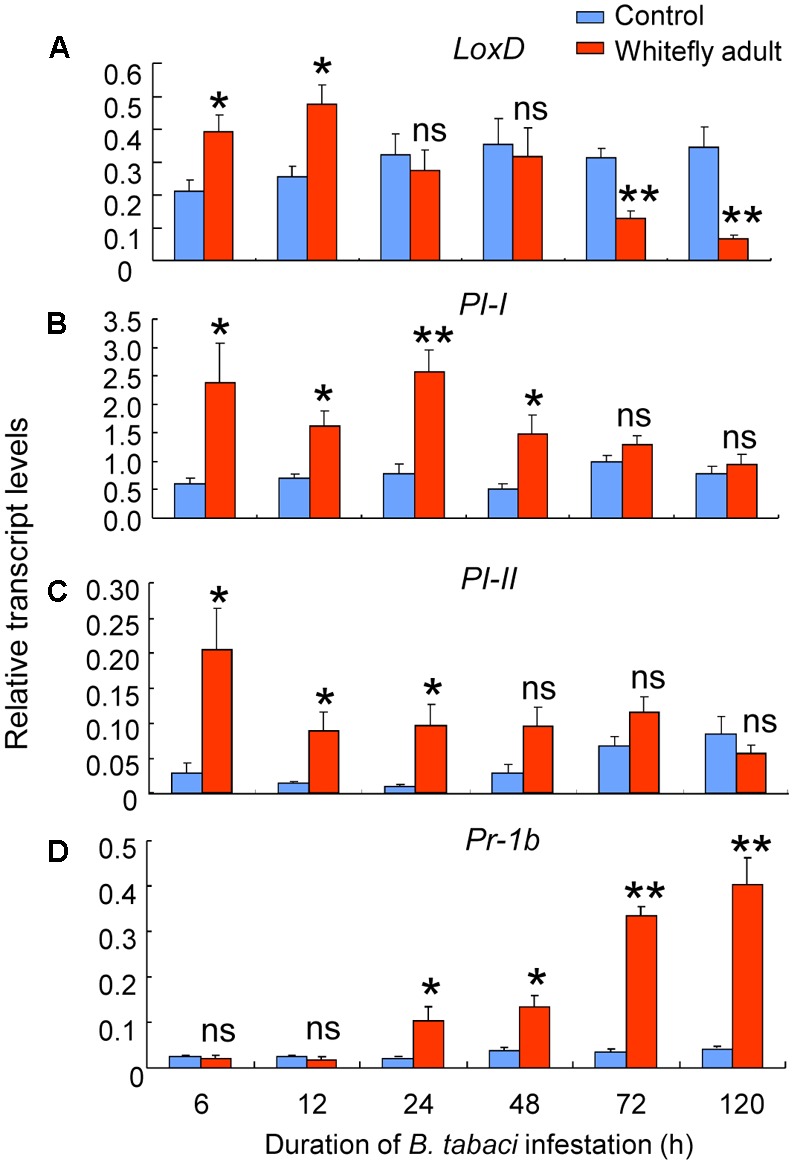
Expression of defense-related genes induced by B. tabaci adult infestation of wild-type CM plants. The transcript levels of LoxD (A), PI-I (B), PI-II (C), and Pr-1b (D) were quantified by qRT-PCR and were normalized to the amount of GAPDH transcripts in each sample. Values are means (± SE) of three biological replicates. Asterisks above bars indicated significant differences compared to the non-infested control (two-way ANOVA; ∗P < 0.05, ∗∗P < 0.01). ns, not significant.
In contrast, whitefly nymph feeding did not affect the expression of LoxD (whitefly: P = 0.31; time: P = 0.23; interaction: P = 0.84), PI-I (whitefly: P = 0.38; time: P = 0.19; interaction: P = 0.73), PI-II (whitefly: P = 0.22; time: P = 0.14; interaction: P = 0.94), and Pr-1b (whitefly: P = 0.11; time: P = 0.33; interaction: P = 0.48; Figure 5).
FIGURE 5.
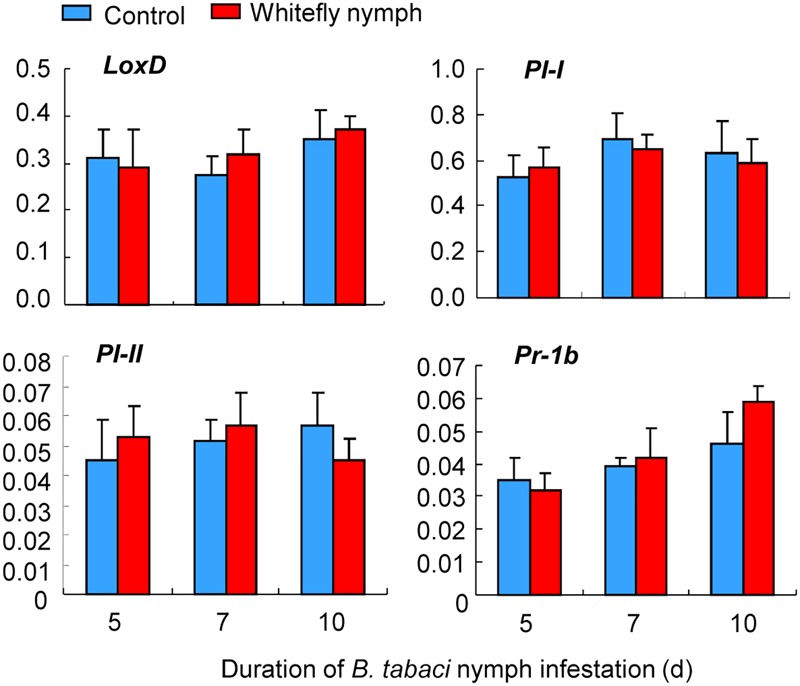
Expression of defense-related genes induced by B. tabaci nymph infestation of wild-type CM plants. The transcript levels of LoxD, PI-I, PI-II, and Pr-1b were quantified by qRT-PCR and were normalized to the amount of GAPDH transcripts in each sample. Values are means (±SE) of three biological replicates.
Nymph Performance Under Semi-Field Conditions
At 16 days postinfestation, the total number of nymphs survived did not differ on MM and NahG plants (Supplementary Table S3). The proportion of fourth instars was significantly lower on NahG plants, in which SA is silenced, than on MM plants (P < 0.001; Figure 6A). These results indicate that impairment of SA pathway slows B. tabaci nymphal development under semi-field conditions.
FIGURE 6.
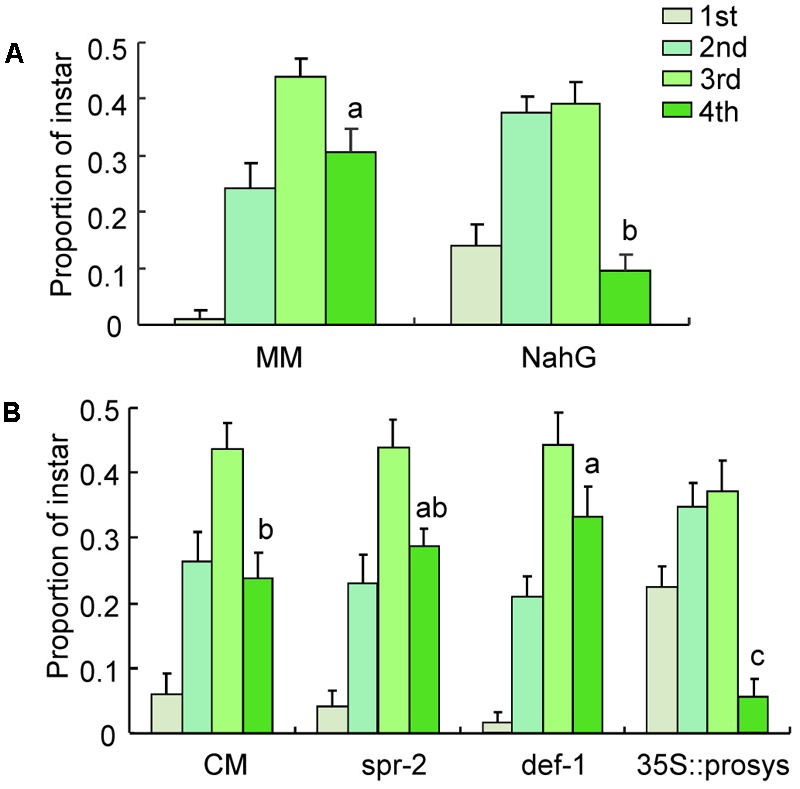
Performance of B. tabaci nymphs on different tomato genotypes under semi-field conditions. (A) The proportions of nymphs represented by each instar at 16 days after adults were placed on wild-type (MM) tomato plants and SA-silenced mutant (NahG) tomato plants; (B) The proportion of nymphs represented by each instar at 16 days after adults were placed on wild-type (CM) tomato plants and JA-signaling mutant tomato plants. Values are means (±SE) of 10 biological replicates. Means for the fourth instar with different letters are significantly different (univariate analysis with GLM, P < 0.05).
At 16 days postinfestation, the total number of nymphs survived did not differ on CM, spr-2, def-1, and 35s::prosys plants (Supplementary Table S3). The proportion of fourth instars on def-1 plants but not on spr-2 plants was significantly higher than the proportion on CM plants (def-1: P < 0.001; spr-2: P = 0.69; Figure 6B). In contrast, the proportion of fourth instars was significantly lower on 35s::prosys plants than on CM plants (P < 0.001; Figure 6B). These results indicate that JA signaling pathway is also important in defending against B. tabaci nymphs under semi-field conditions.
Endogenous JA and SA Levels Induced by B. tabaci Under Semi-Field Conditions
For MM plants in semi-field experiment 1, whitefly infestation had significant effects on JA (whitefly: P < 0.001; time: P = 0.23; interaction: P = 0.73) and SA (whitefly: P < 0.001; time: P = 0.48; interaction: P = 0.53) accumulation. The JA amount at 3 and 5 days was significantly lower in B. tabaci-infested plants than in non-infested plants (at 3 days: P < 0.001; at 5 days: P < 0.001; Figure 7A). In contrast, the SA amount of MM plants at 3 and 5 days was significantly higher in B. tabaci-infested plants than in non-infested plants (at 3 days: P < 0.01; at 5 days: P < 0.001; Figure 7A).
FIGURE 7.
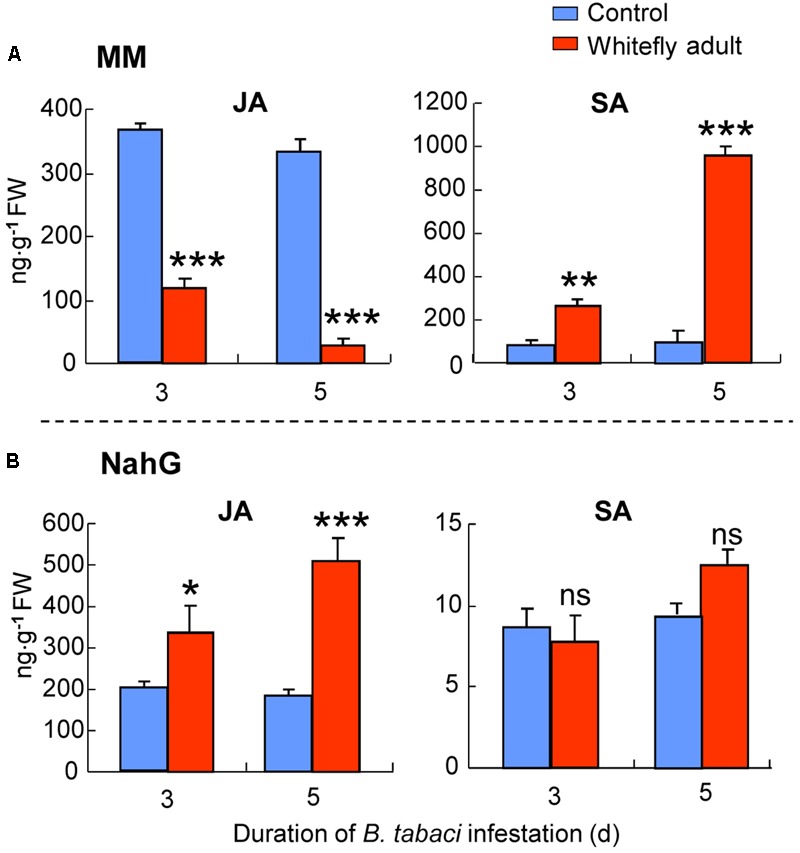
JA and SA levels in MM (A) or NahG plants (B) as affected by B. tabaci infestation under semi-field conditions. Values are means (±SE) of three biological replicates. Asterisks above bars indicated significant differences compared to the non-infested control (two-way ANOVA; ∗P < 0.05; ∗∗P < 0.01; ∗∗∗P < 0.001). ns, not significant. FW means fresh weight.
For NahG plants in semi-field experiment 1, whitefly infestation had significant effect on JA (whitefly: P = 0.003; time: P = 0.15; interaction: P = 0.40), but not SA (whitefly: P = 0.18; time: P = 0.26; interaction: P = 0.83) accumulation. The JA amount at 3 and 5 days was significantly higher in B. tabaci-infested plants than in non-infested plants (at 3 days: P = 0.04; at 5 days: P < 0.001; Figure 7B). In contrast, the SA amount of NahG plants was not significantly affected by B. tabaci infestation (Figure 7B).
Discussion
Our data show that the reproduction and survival of B. tabaci adults were unaffected by impairment of JA defenses (spr-2 and def-1) or constitutive activation of JA defenses (35s::prosys; Figure 1). The development of B. tabaci nymphs, however, was significantly accelerated by impairment of JA defenses and was substantially slowed by constitutive activation or artificial induction of JA defenses (Figure 2). These results are consistent with previous findings that impairment of JA defenses in Arabidopsis accelerates the development of B. tabaci nymphs (Zarate et al., 2007; Zhang et al., 2013b), and that activation of JA defenses in tomato slows nymphal development (Sánchez-Hernández et al., 2006). Our data also confirmed that JA defenses affect the development of B. tabaci nymphs under semi-field conditions (Figure 6B). Taken together, these results indicate that JA-regulated defenses are important in protecting tomato against B. tabaci nymphs but not adults.
It has been well-documented that the JA signaling pathway helps mediate plant defenses against herbivores in both laboratory and field (Ament et al., 2004; Kessler et al., 2004; Bosch et al., 2014). An appropriate JA-regulated defense response to herbivory depends on whether plants can recognize the elicitors or compounds released by herbivores during feeding or oviposition (Erb et al., 2012). Previous findings showed that whiteflies rarely puncture mesophyll cells, i.e., they avoid eliciting wound responses and contacting the potential defensive compounds that are stored within the vacuoles and apoplasts of these cells (Walling, 2000; Kempema et al., 2007). In the current study, however, feeding by B. tabaci adults induced the expression of JA-dependent genes (Figures 4A–C) and the accumulation of endogenous JA (Figure 3A) in the early of the infestation (6–48 h). Our data are partially consistent with previous findings that infestation by B. tabaci adults or application of their saliva activates the expression of the JA-dependent genes LOX, AOS, and Chi9 in tomato at 24 h after infestation/application (Su et al., 2015). The induction of JA defenses by B. tabaci during initial feeding indicates that tomato plants recognize the elicitors derived from the insect’s oral secretions. Additional research is needed to identify B. tabaci elicitors and their mechanism(s) of action.
As the time of B. tabaci infestation increased in the current study, JA levels and the expression levels of two JA-dependent genes (PI-I and PI-II) declined to levels similar to those in non-infested leaves, and the expression level of another JA-dependent gene, LoxD, declined to levels that were even lower than those in non-infested leaves (Figures 3A, 4). This indicated that B. tabaci adults can suppress JA defenses after long periods of infestation. Regarding the underlying mechanisms, recent studies showed that the presence of the bacterial symbiont, Hamiltonella defensa, helps regulate the suppression of JA defenses by B. tabaci Mediterranean (MED), which is another cryptic species of B. tabaci (Su et al., 2015). Given that the MEAM1 species used in the current study also harbors H. defensa (Su et al., 2014), further experiments are needed to determine whether the suppression of JA defenses by the MEAM1 species of B. tabaci is mediated by H. defensa or other symbionts.
In contrast to the JA induction at initial period of adults feeding, B. tabaci nymphs did not activate the expression of JA-regulated genes (LoxD, PI-I, and PI-II) after 5, 7, or 10 days of infestation on tomato (Figure 5). This implied that B. tabaci nymphs induce different transcriptional responses than adults. Such transcriptional difference was also found in whitefly–squash interaction that B. tabaci nymph feeding induces the expression of SLW1 and SLW2, whereas adult feeding does not (Van de Ven et al., 2000). In addition, the influence of insect developmental stage on plant responses to herbivores has been noted for volatile production in the maize–Pseudaletia separata interaction (Takabayashi et al., 1995).
Substantial evidence indicates that SA can reduce plant defenses against herbivory by interfering with steps in JA biosynthesis. In Lima bean, for example, SA blocked steps downstream of 12-oxo-phytodienoic acid (OPDA), an early intermediate of the JA-signaling cascade, and consequently suppressed the accumulation of endogenous JA (Engelberth et al., 2001). In Arabidopsis, SA inhibited the export of OPDA from the plastid to the cytosol and thereby prevented the further processing of OPDA to JA (Laudert and Weiler, 1998). In view of the induction of SA-dependent responses by whiteflies in many plant species (Zarate et al., 2007; Zhang et al., 2009; Rodríguez-Álvarez et al., 2015), it seems possible that the suppression of JA by whiteflies might be mediated in an SA-dependent manner, as proposed by Walling (2008). In the present study, the biosynthesis of JA was apparently not inhibited in B. tabaci-infested leaves, and JA content was higher in infested than in non-infested leaves during the first 48 h of infestation (Figure 3A). In the case of SA, however, the endogenous SA content during the first 48 h of infestation was too low to interfere with JA production (Figure 3B; Engelberth et al., 2001). The higher levels of SA that had accumulated at 72 h and thereafter apparently blocked the biosynthesis of JA in B. tabaci-infested leaves (Figure 3B). That JA suppression associated with SA induction was also observed in B. tabaci-infested leaves under semi-field conditions (Figure 7A). Moreover, if SA signaling was blocked, JA content increased in B. tabaci-infested leaves (Figure 7B), and nymphal development was delayed (Figure 6A). These data indicate the potential key role of SA in mediating the suppression of JA by B. tabaci. Our previous report indicated that the potential site of the JA and SA antagonism induced by B. tabaci might be located in the downstream of the JA signaling pathway (Zhang et al., 2013a). This speculation was also supported by recent findings that SA antagonizes JA signaling downstream of COI1, possibly by interfering with JA-regulated transcription factor ORA59, which was demonstrated to be degraded by SA (Van der Does et al., 2013).
In addition to suppressing JA defenses of tomato in the current study, recent findings show that B. tabaci adults prefer to oviposit on tomato plants that had been pre-infested with conspecifics (Su et al., 2018) or tomato plants in which JA signaling is blocked (Sánchez-Hernández et al., 2006). Such oviposition preference for infested tomato is highly related with the suppression of flavonoids by B. tabaci infestation (Su et al., 2018). Considering that flavonoids are known to have anti-herbivore function (Onkokesung et al., 2014), we believe that B. tabaci adults have acquired the ability to select the best food sources for their offspring. Given the high mobility of whiteflies in field, such host selection behavior of B. tabaci would facilitate its dispersion, establishment, and population growth in field.
Author Contributions
P-JZ and X-PY designed the experiments. Y-CH and CZ conducted the experiments. P-JZ and Z-HY analyzed and interpreted the data. P-JZ and X-PY drafted and revised the paper. All authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This work was financially supported by the National Key R&D Program of China (2017YFD0200400) and the National Natural Science Foundation of China (31471779 and 31301676).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2018.01065/full#supplementary-material
References
- Alba J. M., Schimmel B. C. J., Glas J. J., Ataide L. M. S., Pappas M. L., Villarroel C. A., et al. (2015). Spider mites suppress tomato defences downstream of jasmonate and salicylate independently of hormonal crosstalk. New Phytol. 205 828–840. 10.1111/nph.13075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida Trapp M., De Souza G. D., Rodrigues-Filho E., Boland W., Mithöfer A. (2014). Validated method for phytohormone quantification in plants. Front. Plant Sci. 5:417. 10.3389/fpls.2014.00417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ament K., Kant M. R., Sabelis M. W., Haring M. A., Schuurink R. C. (2004). Jasmonic acid is a key regulator of spider mite-induced volatile terpenoid and methyl salicylate emission in tomato. Plant Physiol. 135 2025–2037. 10.1104/pp.104.048694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleeker P. M., Diergaarde P. J., Ament K., Guerra J., Weidner M., Schütz S., et al. (2009). The role of specific tomato volatiles in tomato-whitefly interaction. Plant Physiol. 151 925–935. 10.1104/pp.109.142661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M., Wright L. P., Gershenzon J., Wasternack C., Hause B., Schaller A., et al. (2014). Jasmonic acid and its precursor 12-oxophytodienoic acid control different aspects of constitutive and induced herbivore defenses in tomato. Plant Physiol. 166 396–410. 10.1104/pp.114.237388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruessow F., Gouhier-Darimont C., Buchala A., Metraux J. P., Reymond P. (2010). Insect eggs suppress plant defense against chewing herbivores. Plant J. 62 876–885. 10.1111/j.1365-313X.2010.04200.x [DOI] [PubMed] [Google Scholar]
- Chung S. H., Rosa C., Scully E. D., Peiffer M., Tooker J. F., Hoover K., et al. (2013). Herbivore exploits orally secreted bacteria to suppress plant defenses. Proc. Natl. Acad. Sci. U.S.A. 110 15728–15733. 10.1073/pnas.1308867110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Barro P. J., Liu S. S., Boykin L. M., Dinsdale A. B. (2011). Bemisia tabaci: a statement of species status. Annu. Rev. Entomol. 56 1–19. 10.1146/annurev-ento-112408-085504 [DOI] [PubMed] [Google Scholar]
- Dicke M., van Loon J. J. A., Solar R. (2009). Chemical complexity of volatiles from plants induced by multiple attack. Nat. Chem. Biol. 5 317–324. 10.1038/nchembio.169 [DOI] [PubMed] [Google Scholar]
- Engelberth J., Koch T., Schüler G., Bachmann N., Rechtenbach J., Boland W. (2001). Ion channel-forming alamethicin is a potent elicitor of volatile biosynthesis and tendril coiling. Cross talk between jasmonate and salicylate signaling in lima bean. Plant Physiol. 125 369–377. 10.1104/pp.125.1.369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb M., Meldau S., Howe G. A. (2012). Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 17 250–259. 10.1016/j.tplants.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E. E., Johnson R. R., Ryan C. A. (1992). Regulation of expression of proteinase-inhibitor genes by methyl jasmonate and jasmonic acid. Plant Physiol. 98 995–1002. 10.1104/pp.98.3.995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitz T., Bergey D. R., Ryan C. A. (1997). A gene encoding a chloroplast-targeted lipoxygenase in tomato leaves is transiently induced by wounding, systemin, and methyl jasmonate. Plant Physiol. 114 1085–1093. 10.1104/pp.114.3.1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe G. A., Ryan C. A. (1999). Suppressors of systemin signaling identify genes in the tomato wound response pathway. Genetics 153 1411–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J. M., Milling A., Mitra R. M., Hogan C. S., Ailloud F., Prior P., et al. (2013). Ralstonia solanacearum requires PopS, an ancient AvrE-family effector, for virulence and to overcome salicylic acid-mediated defenses during tomato pathogenesis. MBio 4:e00875-13. 10.1128/mBio.00875-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., Narvaez J., An G., Ryan C. (1989). Expression of proteinase inhibitors I and II in transgenic tobacco plants: effects on natural defense against Manduca sexta larvae. Proc. Natl. Acad. Sci. U.S.A. 86 9871–9875. 10.1073/pnas.86.24.9871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant M., Jonckheere W., Knegt B., Lemos F., Liu J., Schimmel B. C. J., et al. (2015). Mechanisms and ecological consequences of plant defence induction and suppression in herbivore communities. Ann. Bot. 115 1015–1051. 10.1093/aob/mcv054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempema L. A., Cui X., Holzer F. M., Walling L. L. (2007). Arabidopsis transcriptome changes in response to phloem-feeding silverleaf whitefly nymphs. Similarities and distinctions in responses to aphids. Plant Physiol. 143 849–865. 10.1104/pp.106.090662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler A., Baldwin I. T. (2001). Defensive function of herbivore-induced plant volatile emissions in nature. Science 291 2141–2144. 10.1126/science.291.5511.2141 [DOI] [PubMed] [Google Scholar]
- Kessler A., Halitschke R., Baldwin I. T. (2004). Silencing the jasmonate cascade: induced plant defenses and insect populations. Science 305 665–668. 10.1126/science.1096931 [DOI] [PubMed] [Google Scholar]
- Laudert D., Weiler E. W. (1998). Allene oxide synthase: a major control point in Arabidopsis thaliana octadecanoid signaling. Plant J. 15 675–684. 10.1046/j.1365-313x.1998.00245.x [DOI] [PubMed] [Google Scholar]
- Li C. Y., Liu G. H., Xu C. C., Lee G. I., Bauer P., Ling H. Q., et al. (2003). The tomato suppressor of prosystemin-mediated responses 2 gene encodes a fatty acid desaturase required for the biosynthesis of jasmonic acid and the production of a systemic wound signal for defense gene expression. Plant Cell 15 1646–1661. 10.1105/tpc.012237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C. Y., Williams M. M., Loh Y. T., Lee G. I., Howe G. A. (2002). Resistance of cultivated tomato to cell content-feeding herbivores is regulated by the octadecanoid-signaling pathway. Plant Physiol. 130 494–503. 10.1104/pp.005314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightner J., Pearce G., Ryan C. A., Browse J. (1993). Isolation of signaling mutants of tomato (Lycopersicon esculentum). Mol. Gen. Genet. 241 595–601. 10.1007/BF00279902 [DOI] [PubMed] [Google Scholar]
- Luan J. B., Yao D. M., Zhang T., Walling L. L., Yang M., Wang Y. J., et al. (2013). Suppression of terpenoid synthesis in plants by a virus promotes its mutualism with vectors. Ecol. Lett. 16 390–398. 10.1111/ele.12055 [DOI] [PubMed] [Google Scholar]
- Onkokesung N., Reichelt M., van Doorn A., Schuurink R. C., van Loon J. J. A., Dicke M. (2014). Modulation of flavonoid metabolites in Arabidopsis thaliana through overexpression of the MYB75 transcription factor: role of kaempferol-3,7-dirhamnoside in resistance to the specialist insect herbivore Pieris brassicae. J. Exp. Bot. 65 2203–2217. 10.1093/jxb/eru096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penninckx I., Thomma B. P. H. J., Buchala A., Métraux J. P., Broekaert W. F. (1998). Concomitant activation of jasmonate and ethylene response pathways is required for induction of a plant defensin gene in Arabidopsis. Plant Cell 10 2103–2113. 10.1105/tpc.10.12.2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perring T. M. (2001). The Bemisia tabaci species complex. Crop Prot. 20 725–737. [Google Scholar]
- Rodríguez-Álvarez C. I., López-Climent M. F., Gómez-Cadenas A., Kaloshian I., Nombela G. (2015). Salicylic acid is required for Mi-1-mediated resistance of tomato to whitefly Bemisia tabaci, but not for basal defense to this insect pest. Bull. Entomol. Res. 105 574–582. 10.1017/S0007485315000449 [DOI] [PubMed] [Google Scholar]
- Sánchez-Hernández C., López M. G., Délano-Frier J. P. (2006). Reduced levels of volatile emissions in jasmonate-deficient spr2 tomato mutants favour oviposition by insect herbivores. Plant Cell Environ. 29 546–557. 10.1111/j.1365-3040.2005.01431.x [DOI] [PubMed] [Google Scholar]
- Sarmento R. A., Lemos F., Bleeker P. M., Schuurink R. C., Oliveira M. G. A., Lima E. R., et al. (2011). A herbivore that manipulates plant defence. Ecol. Lett. 14 229–236. 10.1111/j.1461-0248.2010.01575.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelz E. A., Engelberth J., Alborn H. T., Tumlinson J. H., Teal P. E. A. (2009). Phytohormone based activity mapping of insect herbivore-produced elicitors. Proc. Natl. Acad. Sci. U.S.A. 106 653–657. 10.1073/pnas.0811861106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzberg E. G., Tumlinson J. H. (2014). Aphid honeydew alters plant defence responses. Funct. Ecol. 28 386–394. 10.1111/1365-2435.12182 [DOI] [Google Scholar]
- Su Q., Chen G., Mescher M. C., Peng Z., Xie W., Wang S., Wu Q., et al. (2018). Whitefly aggregation on tomato is mediated by feeding-induced changes in plant metabolites that influence the behavior and performance of conspecifics. Funct. Ecol. 32 1180–1193. 10.1111/1365-2435.13055 [DOI] [Google Scholar]
- Su Q., Oliver K. M., Xie W., Wu Q. J., Wang S. L., Zhang Y. J. (2015). The whitefly-associated facultative symbiont Hamiltonella defensa suppresses induced plant defences in tomato. Funct. Ecol. 29 1007–1018. 10.1111/1365-2435.12405 [DOI] [Google Scholar]
- Su Q., Xie W., Wang S., Wu Q., Ghanim M., Zhang Y. (2014). Location of symbionts in the whitefly Bemisia tabaci affects their densities during host development and environmental stress. PLoS One 9:e91802. 10.1371/journal.pone.0091802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio A., Kingdom H. N., MacLean A. M., Grieve V. M., Hogenhout S. A. (2011). Phytoplasma protein effector SAP11 enhances insect vector reproduction by manipulating plant development and defense hormone biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 108 E1254–E1263. 10.1073/pnas.1105664108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabayashi J., Takahashi S., Dicke M., Posthumus M. A. (1995). Developmental stage of herbivore Pseudaletia separata affects production of herbivore-induced synomone by corn plants. J. Chem. Ecol. 21 273–287. [DOI] [PubMed] [Google Scholar]
- Thaler J. S., Farag M. A., Paré P. W., Dicke M. (2002). Jasmonate-deficient plants have reduced direct and indirect defences against herbivores. Ecol. Lett. 5:764–774. 10.1046/j.1461-0248.2002.00388.x [DOI] [Google Scholar]
- Thaler J. S., Humphrey P. T., Whiteman N. K. (2012). Evolution of jasmonate and salicylate signal crosstalk. Trends Plant Sci. 17 260–270. 10.1016/j.tplants.2012.02.010 [DOI] [PubMed] [Google Scholar]
- Turlings T. C. J., Tumlinson J. H., Lewis W. J. (1990). Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 250 1251–1253. 10.1126/science.250.4985.1251 [DOI] [PubMed] [Google Scholar]
- Van de Ven W. T. G., LeVesque C. S., Perring T. M., Walling L. L. (2000). Local and systemic changes in squash gene expression in response to silverleaf whitefly feeding. Plant Cell 12 1409–1423. 10.1105/tpc.12.8.1409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Does D., Leon-Reyes A., Koornneef A., Van Verk M. C., Rodenburg N., Pauwels L., et al. (2013). Salicylic acid suppresses jasmonic acid signaling downstream of SCFCOI1-JAZ by targeting GCC promoter motifs via transcription factor ORA59. Plant Cell 25 744–761. 10.1105/tpc.112.108548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling L. L. (2000). The myriad plant responses to herbivores. J. Plant Growth Regul. 19 195–216. 10.1007/s003440000026 [DOI] [PubMed] [Google Scholar]
- Walling L. L. (2008). Avoiding effective defenses: strategies employed by phloem-feeding insects. Plant Physiol. 146 859–866. 10.1104/pp.107.113142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang L. S., Chen W. Q., Liu S. S. (2006). Comparison of performance on different host plants between the B biotype and a non-B biotype of Bemisia tabaci from Zhejiang, China. Entomol. Exp. Appl. 121 221–227. 10.1111/j.1570-7458.2006.00482.x [DOI] [Google Scholar]
- Zarate S. I., Kempema L. A., Walling L. L. (2007). Silverleaf whitefly induces salicylic acid defenses and suppresses effectual jasmonic acid defenses. Plant Physiol. 143 866–875. 10.1104/pp.106.090035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P. J., Li W. D., Huang F., Zhang J. M., Xu F. C., Lu Y. B. (2013a). Feeding by whiteflies suppresses downstream jasmonic acid signaling by eliciting salicylic acid signaling. J. Chem. Ecol. 39 612–619. 10.1007/s10886-013-0283-2 [DOI] [PubMed] [Google Scholar]
- Zhang P. J., Xu C. X., Zhang J. M., Lu Y. B., Wei J. N., Liu Y. Q., et al. (2013b). Phloem-feeding whiteflies can fool their host plants, but not their parasitoids. Funct. Ecol. 27 1304–1312. 10.1111/1365-2435.12132 [DOI] [Google Scholar]
- Zhang P. J., Zheng S. J., van Loon J. J. A., Boland W., David A., Mumm R., et al. (2009). Whiteflies interfere with indirect plant defense against spider mites in Lima bean. Proc. Natl. Acad. Sci. U.S.A. 106 21202–21207. 10.1073/pnas.0907890106 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


