Abstract
Encapsulating peritoneal sclerosis (EPS) is a debilitating condition characterized by a fibrocollagenous membrane encasing the small intestine, resulting in recurrent small bowel obstructions. EPS is most commonly associated with long-term peritoneal dialysis, though medications, peritoneal infection, and systemic inflammatory disorders have been implicated. Many cases remain idiopathic. Diagnosis is often delayed given the rarity of the disorder combined with non-specific symptoms and laboratory findings. Although cross-sectional imaging with computed tomography of the abdomen can be suggestive of the disorder, many patients undergo exploratory laparotomy for diagnosis. Mortality approaches 50% one year after diagnosis. Treatment for EPS involves treating the underlying condition or eliminating possible inciting agents (i.e. peritoneal dialysis, medications, infections) and nutritional support, frequently with total parenteral nutrition. EPS-specific treatment depends on the disease stage. In the inflammatory stage, corticosteroids are the treatment of choice, while in the fibrotic stage, tamoxifen may be beneficial. In practice, distinguishing between stages may be difficult and both may be used. Surgical intervention, consisting of peritonectomy and enterolysis, is time-consuming and high-risk and is reserved for situations in which conservative medical therapy fails in institutions with surgical expertise in this area. Herein we review the available literature of the etiology, pathogenesis, diagnosis, and treatment of this rare, but potentially devastating disease.
Keywords: Abdominal cocoon, Sclerosing encapsulating peritonitis, Peritoneal sclerosis, Peritonectomy, Enterolysis, Peritoneal dialysis, Tamoxifen, Corticosteroids
Core tip: Encapsulating peritoneal sclerosis (EPS) is a rare, but potentially devastating disorder. Most literature is derived from the nephrology literature surrounding peritoneal dialysis, however, the gastroenterologist is likely to encounter EPS from a variety of etiologies. We present a comprehensive review of EPS from all etiologies and a summary of treatments from a gastroenterologist’s perspective including the role of nutrition, surgery, immunosuppression, anti-fibrotic agents, and the novel use of μ-opioid antagonists and guanylate cyclase C agonists in the management of patients with EPS.
INTRODUCTION
Definition
Encapsulating peritoneal sclerosis (EPS) is a rare clinical syndrome characterized by an acquired, inflammatory fibrocollagenous membrane encasing the small intestine, resulting in symptoms of bowel obstruction. It is defined by the International Society for Peritoneal Dialysis as “a syndrome continuously, intermittently, or repeatedly presenting with symptoms of intestinal obstruction caused by adhesions of a diffusely thickened peritoneum”[1]. Owtschinnikow first described encasement of the intestines by a fibrocollagenous membrane in 1907 and coined the term peritonitis chronica fibrosa incapsulata[2]. EPS has also been known as abdominal cocoon and sclerosing encapsulating peritonitis. It should not be confused with peritoneal encapsulation, a congenital condition characterized by an accessory peritoneal membrane encapsulating the intestines, without adhesions to the encased intestine often incidentally recognized during unrelated surgery[3].
Etiology
EPS can be divided into primary (idiopathic) or secondary in which a trigger for the inflammatory process can be identified. Primary EPS was classically thought to afflict adolescent women in tropical and subtropical areas leading to theories of retrograde menstruation or gynecologic infection as the cause. Though the largest studies of primary EPS confirm its equatorial predilection, men are more commonly affected than women in a 2:1 ratio[4,5] and the etiology remains unclear.
In secondary EPS, a local or systemic factor can be identified as triggering peritoneal inflammation. Implicated triggers include medications[6-9], infection[10-16], mechanical or chemical intraperitoneal irritants[17-27], cirrhosis[28], organ transplantation[29-31], endometriosis[32], gynecologic neoplasms[33,34], dermoid cyst rupture[35], and systemic rheumatologic and inflammatory disorders[36-38] (Table 1).
Table 1.
Classification and etiologies of encapsulating peritoneal sclerosis
| Primary (idiopathic) | |
| 2:1 (male:female) Most commonly reported in tropical/subtropical regions | |
| Secondary | |
| Medications Practolol[6] Methotrexate[7,8] Antiepileptic drugs[9] Infection Tuberculosis[10] Non-tuberculous mycobacteria[11] Bacterial peritonitis[12] Cytomegalovirus[13] Fungus[14,15] Parasite[16] Cirrhosis[28] Organ transplantation Liver[29] Small intestine[30] Renal[31] Gynecologic neoplasms Luteinized thecoma[33] Luteinizing granulosa cell tumor[34] | Mechanical or chemical irritation Peritoneal dialysis[17] Intraperitoneal chemotherapy[18] Ventriculoperitoneal shunt[20] Peritoneovenous shunt[21] Intraperitoneal iodine[22] Abdominal trauma[23] Intraabdominal surgery[24] Foreign body[25] Talcum powder[19] Asbestos[26] Silica[27] Endometriosis[32] Dermoid cyst rupture[35] Rheumatologic/systemic inflammatory conditions Sarcoidosis[36] Systemic lupus erythematosis[37] Familial Mediterranean fever[38] |
Pathophysiology
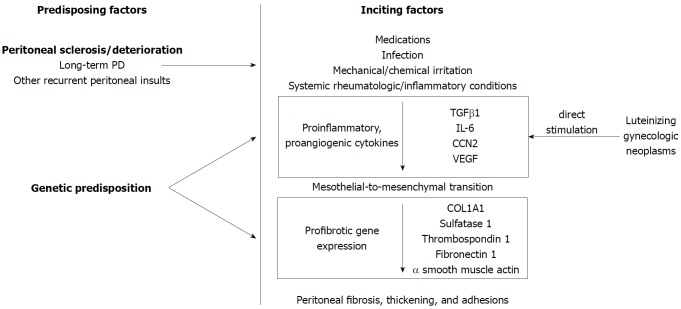
EPS is thought to occur when a peritoneal inflammatory process (inciting factor) occurs in patients with a predisposing condition (Figure 1). In peritoneal dialysis (PD) literature, this is referred to as the “two-hit” hypothesis[39] in which the “first hit” or predisposing condition is the non-inflammatory peritoneal sclerosis resulting from repeated dialysis sessions. In support of this, cumulative incidence of EPS increases dramatically with time on PD[40,41]. A proinflammatory “second hit”[42] precipitates a cascade of proinflammatory [transforming growth factor β1 (TGFβ1), interleukin-6 (IL-6), CCN2] and proangiogenic [vascular endothelial growth factor (VEGF)] cytokines[43,44]. TGFβ1 promotes transdifferentiation of peritoneal mesothelial to mesenchymal cells resulting in mesothelial cell depletion[45,46], increased production of extracellular matrix components [collagen type 1, alpha 1 (COL1A1)] and fibrogenesis resulting in a fibrocollagenous cocoon[47] (Figure 1).
Figure 1.
Pathophysiology of encapsulating peritoneal sclerosis. Certain predisposing factors may be present for encapsulating peritoneal sclerosis to form, such as genetic predisposition or being on long-term peritoneal dialysis. Inciting factors that may push the physiology towards a pro-inflammatory and pro-fibrotic state include medications, repeated chemical or mechanical peritoneal irritation, recurrent infections, and systemic rheumatologic or inflammatory conditions.
In PD, genetic variation in the receptor for advanced glycation end products may predispose an individual to peritoneal deterioration[48]. While no genetic studies exist outside the PD literature, genetic predisposition may explain why only a small proportion of patients with recurrent peritonitis develop EPS or why an individual may develop EPS even with a single, discrete exposure[24]. Among gynecologic neoplasms, reports of EPS are confined to luteinizing neoplasms[33,34] without other overt inflammatory trigger, leading to the hypothesis that they directly stimulate peritoneal inflammation and fibrosis, though the potential stimulatory cytokine has yet to be identified[49].
Epidemiology and natural history
Given its rarity and heterogeneity of etiologies, the incidence and prevalence of EPS as a whole is unknown. In a review of idiopathic EPS, cases were more commonly reported from tropical and subtropical countries [China (54%), India (18%), Turkey (9%), and Nigeria (3%)][6]. The mean age was 34.7 (range 7-87 years) with a 2:1 male predominance.
In peritoneal dialysis, the annual incidence of EPS varies from 0.14% to 2.5%[41,50] with decreasing incidence in more recent studies likely due to improved dialysis techniques[17,50]. The most significant risk factor for EPS development is duration of PD[17] with a low cumulative incidence at 3 years increasing after 5 years[17,40,41]. At 8 years, 10%-20% of PD patients will develop EPS[17,51-54]. The prevalence of EPS in PD has been observed between 0.4% and 8.9%[17]. Mortality in PD patients approaches 50% one year after diagnosis[17,50,55] though it is difficult to ascertain how much of this is related to EPS or the comorbidities that accompany end-stage renal disease.
DIAGNOSIS
Clinical presentation
The diagnosis of EPS is clinical, based on a constellation of clinical findings, and confirmed radiographically or by laparotomy[5,17,56,57]. No gold standard exists for diagnosis. However, a diagnostic algorithm is proposed in Figure 2. A majority of PD-associated EPS patients present after withdrawal of PD, with onset of symptoms occurring up to 5 years later[17]. In the largest case series of idiopathic EPS, the average duration of symptoms was 3.9 years prior to presentation with the vast majority presenting malnourished (75%, mean BMI 17.5 kg/m2)[4]. The most common presenting symptoms were abdominal pain (86%), abdominal distension (82%), and nausea and vomiting (54%). Twenty-nine percent of patients underwent “emergency surgery” on presentation implying that, despite the chronic, insidious nature of the disease, a significant percentage present with more acute obstruction, ischemia, or perforation[4].
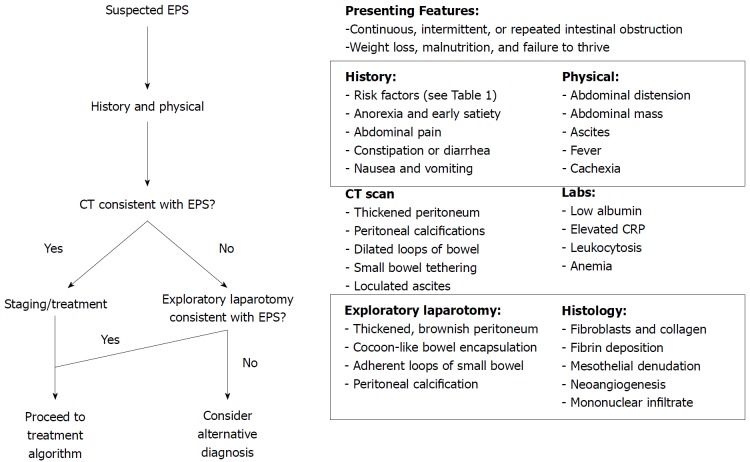
Figure 2.
Proposed algorithm for the diagnosis of encapsulating peritoneal sclerosis. The diagnosis of EPS is a clinical one that can be confirmed with imaging or at laparotomy. Various presenting features and symptoms, clues obtained in the history (risk factors) and physical exam can point towards the diagnosis of EPS. CT/MRI findings include thickened peritoneum, calcifications, and may even demonstrate cocooning of the bowel with proximal dilation, as seen in our case. EPS: Encapsulating peritoneal sclerosis
Imaging
Imaging is often helpful in differentiating EPS from other causes of intestinal obstruction. Abdominal plain films showing peritoneal calcification as well as dilated loops of bowel with air-fluid levels may suggest advanced EPS[56,58]. Small bowel follow-through may show delayed transit, distension proximal to small bowel adhesions, and have a “cauliflower” appearance from compression of tightly adherent loops of bowel by EPS[5]. On ultrasonography, dilated loops of bowel may be matted together and tethered posteriorly or encased by a dense fibrous membrane[5]. Intraperitoneal echogenic strands may cause the bowel wall to appear trilaminar[56,58].
CT scan is currently the best studied and most commonly used imaging technique in the diagnosis of EPS. Small-bowel loops are often tethered together in an enveloping, thickened peritoneum, typically accompanied with proximal bowel dilation[58]. Other radiographic features include loculated ascites, increased density of mesenteric fat, and localized or diffuse peritoneal calcification[56,59]. While the presence of complex loculations could be from intra-abdominal hemorrhage, it should raise suspicion for perforation or sepsis, especially if they contain gas[60]. Increased bowel wall enhancement or thickening indicates ongoing inflammation or transition to transmural fibrosis[58,60]. Magnetic resonance imaging has been used less frequently for diagnosis but likely has similar yields. Advantages include avoidance of ionizing radiation and better delineation of bowel encasement and peritoneal thickening[61].
Laboratory
Laboratory findings in EPS are non-specific and related to underlying infection, malnutrition, and inflammation[30,39,62]. Levels of inflammatory cytokines have been shown to be higher in dialysate in EPS patients compared to PD controls up to years in advance of clinical development of EPS[63-65]. However, no biomarker has been found to be useful in predicting EPS development[64].
Histopathology
Histologic findings in EPS are non-specific and may overlap with findings in simple peritoneal sclerosis or infectious peritonitis[17]. Microscopically, the mesothelial cell layer is denuded with fibroblast proliferation and fibrocollagenous deposition frequently with fibrin deposition[42]. An inflammatory mononuclear cell infiltrate may be present in active inflammation[42]. Podoplanin, a transmembrane glycoprotein found on peritoneal mesothelial cells that binds inflammatory cytokines, helps differentiate EPS from peritoneal sclerosis and peritonitis[45,66]. Generally, a thickened fibrocollagenous membrane in the setting of the previously described clinical syndrome is sufficient for diagnosis.
EPS staging
A staging system has been proposed in the PD-associated EPS literature, based on a combination of clinical, laboratory, and radiographic findings[53,56,57]. Nakamoto et al[56] categorized patients with EPS into Stage 1 (pre-EPS), Stage 2 (inflammatory), Stage 3 (encapsulating), and Stage 4 (chronic) based on abdominal symptoms, inflammation, encapsulation, and intestinal findings (Table 2). Different therapeutic approaches have been proposed depending on the stage of disease[53,56,57].
Table 2.
Stages of peritoneal dialysis-associated encapsulating peritoneal sclerosis with associated clinical, serologic, and radiographic profiles
|
Nakamoto 2005 |
Nakayama 2014 |
|||
| Terminology | Clinical findings | Terminology | Clinical findings | |
| Stage 1 | Pre-EPS stage | Loss of ultrafiltration capacity Development of a high transport Hypoproteinemia Bloody dialysate, ascites Calcifications in the peritoneum | Pre-stage | Abdominal symptoms: Mild Inflammation: Mild Encapsulation: None |
| Stage 2 | Inflammation stage | Increased CRP, leukocytosis Fever, chills, weight loss, anorexia Diarrhea, ascites | Inflammatory | Abdominal symptoms: Nausea, diarrhea Inflammation: Mild to severe Encapsulation: Partial |
| Stage 3 | Encapsulating stage | Decreased clinical signs of systemic inflammation Early signs of ileus (abdominal pain, nausea, vomiting) | Encapsulating | Abdominal symptoms: Periodic ileus Inflammation: Mild Encapsulation: Present |
| Stage 4 | Ileus stage | Anorexia Complete ileus Abdominal mass | Chronic | Abdominal symptoms: Persistent ileus Inflammation: None to mild Encapsulation: Present |
TREATMENT
Treatment of underlying condition
When possible, the underlying condition leading to EPS should be treated. In the case of PD, this involves cessation of peritoneal dialysis and transition to hemodialysis[57]. In non-PD EPS, potential offending medications should be withdrawn and underlying infection or inflammatory conditions should be treated. Despite withdrawal of the offending agent or treatment of the underlying condition, resolution of EPS is unlikely given its chronic, fibrotic nature and treatment of ongoing inflammation or the underlying fibrosis is frequently necessary[6,8,14,67].
Nutritional support
On diagnosis of EPS, nutritional status should be assessed. While bowel rest and TPN alone are not effective in treating EPS[68], ensuring adequate nutrition is essential. Given obstruction, enteral feeding is often not tolerated and TPN is required[69]. A case series in China, showed preoperative TPN reduced serious postoperative complications and hospital stay[4].
Immunosuppression
The effect of immunosuppression on EPS was first noted in kidney transplant patients who developed improvement in EPS symptoms after institution of immunosuppression[70]. Numerous medications have been tried targeting the inflammatory component of EPS, including corticosteroids, colchicine, azathioprine, cyclosporine, mycophenolate mofetil (MMF), and mammalian target of rapamycin (mTOR) inhibitors[71-76]. Of these, corticosteroids are the best studied and, while the mTOR inhibitors and MMF carry the theoretical advantage of improving fibrosis as well, evidence is anecdotal and largely confined to post-kidney transplant patients with additional indications for immunosuppression[73,75].
Evidence for corticosteroid use is confined to observational studies with inconsistent formulation, dosing, duration, and end points (Table 3). In the earliest study by Kuriyama et al[77], all patients who did not receive corticosteroids died within 8 mo, while those who received prednisolone survived at 1-3 years of follow-up with only one requiring surgical intervention. In a larger retrospective case series without a control comparison group, mortality was 25% for stage 4 EPS over the study period[71] which is lower than what is generally reported in natural history studies[17,50,55].
Table 3.
Summary of studies of interventions in encapsulating peritoneal sclerosis
| Design | Patient population | Treatment | Outcome | Comments | |
| Kuriyama 2001 | Retrospective case-control | n = 11 Japan PD patients Age - 49.1 yr 27% female | Steroids Prednisolone Dose - 0.5 mg/kg/d Duration - NS n = 5 | Steroids All remained alive at 1-3 yr after diagnosis. | All control patients were diagnosed prior to 1997 and all who received steroids were diagnosed after 1997. |
| Control TPN-alone n = 6 | Control All died of EPS-related complications within 8 mo of diagnosis. | ||||
| Kawanishi 2004 | Prospective cohort | n = 48 Japan PD patients Age - 54.7 yr 25% female | Steroids - Prednisolone Dose - 10-40 mg/d Methylprednisolone Dose - 0.5-1.0 g/d Duration – NS n = 39 | Steroids Recovery - 38.5% Surgery - 15.4% Mortality - 31% | Six steroid patients underwent surgery. Surgical treatment consisted of total enterolysis. |
| Surgery Total enterolysis n = 12 | Surgery Recovery - 58.3 % Mortality - 33% | ||||
| Control TPN-alone n = 3 | Control Recovery - 0% Mortality - 66% | ||||
| Maruyama 2008 | Retrospective case series | n = 79 Japan PD patients | Steroids Prednisolone Dose – 2.6-60 mg/d Duration - 1-36 mo n = 79 | Steroids Mortality – Stage 2 – 3.6% Stage 3 – 14.3% Stage 4 – 25% | Did not compare to a control group. |
| Balasubramanian 2009 | Retrospective case series | n = 111 United Kingdom PD patients Age - 52.0 yr 53% female | Steroids ± immunosuppression n = 7 | Steroids ± immunosuppression Median survival 7 mo | Dose and duration of medications not specified. Numerous patients received combinations of therapies. Immunosuppression consisted of azathioprine, cyclosporin, tacrolimus, mycophenolate mofetil, or sirolimus. Unable to analyze statistically due to heterogeneity in groups. |
| Tamoxifen n = 17 | Tamoxifen Median survival 15 mo | ||||
| Tamoxifen + steroids ± immunosuppression n = 8 | Tamoxifen + steroids ± immunosuppression Median survival 14 mo | ||||
| Surgery Adhesiolysis (n = 5) jejunostomy (n = 1) Small bowel resection (n = 1) Ileal-transverse colon bypass (n = 1) | Surgery Median survival 17 mo | ||||
| Control No specific drug therapy (n = 46) | Control Median survival 13 mo | ||||
| Korte 2011 | Retrospective survival analysis | n = 63 Netherlands PD patients Age 43.4 yr 50% female | Tamoxifen Dose - Dose 10 mg/d to 20 mg twice daily Duration – at least 4 wk n = 24 | Tamoxifen Mortality rate - 45.8% | None underwent surgery in either group. Patients in both groups received steroids, which was not analyzed separately other than noting a trend towards improved mortality in the tamoxifen group. |
| Control No tamoxifen n =39 | Control Mortality rate - 74.4% | ||||
| Kawanishi 2011 | Retrospective case series | n = 181 Japan PD patients | Surgery Total enterolysis n = 181 | Surgery Recurrence – 25.4% Surgical mortality – 7.7% Overall mortality – 35% 0/17 with Noble plication experienced recurrence at 8 mo | Heterogeneous operation types. Those after April 2007 received Noble plication. |
| Ulmer 2013 | Retrospective case series | n = 26 Netherlands PD patients Age 54 yr 11% female | Surgery Peritonectomy and enterolysis (PEEL) | Surgery Major morbidity - 31% Reoperation – 17% Recurrence – 10% Surgical mortality – 10% | 8 patients received steroids, 1 tamoxifen, and 1 tacrolimus pre-operatively. |
PD: Peritoneal dialysis; EPS: Encapsulating peritoneal sclerosis.
Despite this, one prospective cohort study found only 38.5% recovered on steroids, while the remainder died or required surgical intervention[68]. Similarly, the largest retrospective study involving steroids found no improvement in median survival, though treatment groups were too heterogeneous for meaningful analysis[55].
While EPS is thought to develop out of an inflammatory insult, active inflammation may not be ongoing at the time of presentation. It is difficult to ascertain which patients are actively inflamed using non-specific markers such as CRP and clinical observation. We recommend targeting immunosuppressive therapy to those thought to have an active inflammatory component after excluding infection. The appropriate dosing and duration of corticosteroids are not established. However the Dutch EPS Registry, in 2011 guidelines, suggests IV methylprednisolone (500-1000 mg/d) for 2-3 d in those who present with acute obstructive symptoms without infection[78]. In those who present with more subacute symptoms without infection, it is reasonable to treat with prednisolone at 0.5-1.0 mg/kg/d for 1 mo and taper over the course of a year[78]. Absence of improvement after 1 mo may be considered a treatment failure and we recommend stopping steroids and considering alternative therapy such as tamoxifen or surgery. In the absence of data, other immunosuppressive medications should be reserved for patients with additional indications such as organ transplantation or strong contraindications to surgery.
Anti-fibrotics
Immunosuppression alone may not be effective in those patients who have already developed signficant fibrosis (Stage 3). Tamoxifen is a selective estrogen receptor modulator (SERM) with strong anti-fibrotic properties related to inhibition of TGF-β, an important cytokine in the fibrosis process[79]. Its successful use in EPS was first described by Allaria et al[80] in 1999. Similar to corticosteroids, evidence for tamoxifen is limited to observational studies in PD patients (Table 3).
A retrospective Dutch study reported a significant difference in mortality (45.8% vs 74.4%, P < 0.05) with tamoxifen at 130 mo of follow-up with a trend towards improved survival on survival analysis after adjusting for calendar time, use of corticosteroids, and use of parental nutrition[81]. In contrast, a retrospective study of PD patients from the United Kingdom (UK), did not find any survival benefit with tamoxifen (median 15 mo) compared to no therapy (12 mo) although it was limited by significant treatment heterogeneity[55].
The dose and duration of tamoxifen treatment is not well-defined. Most studies use between 10-40 mg daily. The Dutch EPS Registry suggests starting with 20mg BID[78]. A clinical response is typically seen within 1-6 mo[11,14,82]. Treatment should be continued for at least a year and tapered off thereafter as long as the underlying condition is controlled and the patient has had an adequate clinical and radiologic response. Resolution may be seen in form of clinical improvement and/or evidenced by resolution of peritoneal thickening on follow-up imaging. Potential side effects of tamoxifen including deep venous thrombosis, stroke, hot flashes and endometrial carcinoma should be discussed prior to initiating therapy[83].
Surgical treatment of EPS
Given the time-consuming, hazardous, and technical nature of surgical techniques for EPS, surgery is recommended only in patients who have failed conservative, medical therapy and, if possible, in centers with experience in such operations. Surgical techniques vary from those with curative intent, such as enterolysis (ablation of fibrotic tissue and lysis of adhesions), to those aimed at addressing a specific complication such as limited lysis of adhesions or resection of perforated or ischemic bowel. The former techniques are preferred. They have lower frequency of symptom recurrence, but are time-consuming, often technically difficult, and carry a risk of bowel injury[84-86].
In a 17-year review of 239 cases in one center in Japan, in which enterolysis alone was primarily employed, mortality was 35.4% (7.7% attributed to post-operative complications and 18.2% to persistent EPS-related complications)[87]. During initial experience, 25.4% required a second operation for persistent obstructive symptoms[85], but after institution of Noble plication (suturing of the intestines to each other) in addition to enterolysis, this improved to 12.3%[88]. More recent experience in other institutions is also encouraging with only 10% requiring reoperation after peritonectomy and enterolysis (PEEL) and 10% mortality at 1 year[86]. Continuation of steroids or tamoxifen post-operatively may reduce recurrence[89].
Dysmotility
While the primary driver of obstructive symptoms in EPS is mechanical and related to adhesions and constriction within the encapsulating peritoneum, we hypothesize dysmotility may also play a role both through disruption of the myenteric plexus by fibrosis and increased endogenous opioids from activated lymphocytes inhibiting both propulsive motor and secretory activity in the gut[66,90,91]. Successful use of methylnaltrexone to combat inflammation-associated dysmotility has been described in anti-Hu-associated intestinal pseudo-obstruction[92]. We suggest use of a μ-opioid antagonist and a guanylate cyclase C agonist in patients who remain symptomatic, but have already been started on appropriate anti-fibrotic therapy (Figure 3).
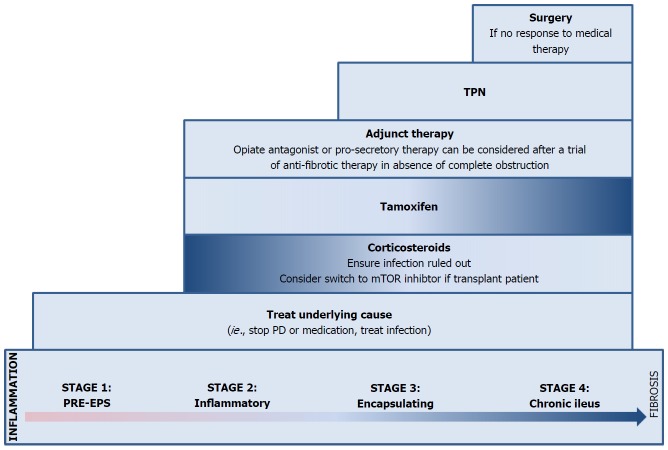
Figure 3.
Therapeutic and management approach for encapsulating peritoneal sclerosis. Treatment strategies should be tailored to each patient, depending on the extent and stage of EPS. In all stages, especially during the early stages, the underlying cause should be identified and treated or removed. During the earlier stages (Stage 1-2), the pathophysiology tends to be more inflammatory and the degree of sclerosis tends to be minimal. Thus, after infection has been ruled out, corticosteroids may be of benefit. In the later stages (Stage 3-4), more advanced sclerosis may be present, and patients may start exhibiting signs and symptoms of partial or complete bowel obstruction. In treating abdominal pain, opioids should be avoided. However, this may not always be possible and thus opiate antagonists are recommended in this setting. Tamoxifen plays an increasing rule in the later stages. If poor oral intake or malnutrition is present, total parenteral nutrition may be required. If symptoms are severe and there has been no response to medical therapy, surgical intervention may be considered. EPS: Encapsulating peritoneal sclerosis.
CONCLUSION
Encapsulating peritoneal sclerosis is a rare, but devastating condition associated with high morbidity and mortality. A high index of suspicion is warranted in patients with unexplained recurrent symptoms of bowel obstruction. A careful history should be obtained to identify patients with known risk factors. EPS can be divided into primary (idiopathic) or secondary. Implicated triggers may include medications, infections, chronic mechanical or chemical irritation, especially in patients on peritoneal dialysis, or in various other chronic, inflammatory diseases (cirrhosis, history of organ transplant, gynecologic or rheumatologic conditions). Diagnosis is clinical, and can be confirmed by x-ray or laparotomy. Treatment should be directed at the underlying condition, optimizing nutrition, and corticosteroids or tamoxifen alone-or in combination-depending on disease state and contraindications. In patients who have failed conservative medical therapy, surgical enterolysis should be considered.
Prior studies have been limited by inconsistent definitions and staging of EPS, and all treatment studies have been observational with variable dosage, duration of treatment, and outcomes. Since treatment may vary based on disease stage, future studies should appropriately document disease stage when assessing treatment. Long-term longitudinal data from past case series may shed more light on the natural history of EPS and mortality benefits of certain treatments. A gap in knowledge still exists in our understanding of the underlying pathogenesis of EPS and fibrosis, and will need to be bridged in order to develop effective therapies.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: There are no financial disclosures or conflicts of interest in the production of this manuscript.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 17, 2018
First decision: May 30, 2018
Article in press: June 25, 2018
P- Reviewer: Larentzakis A, Sonoda H S- Editor: Gong ZM L- Editor: A E- Editor: Yin SY
Contributor Information
Christopher J Danford, Division of Gastroenterology and Hepatology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02115, United States.
Steven C Lin, Division of Gastroenterology and Hepatology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02115, United States.
Martin P Smith, Department of Radiology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02115, United States.
Jacqueline L Wolf, Division of Gastroenterology and Hepatology, Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA 02115, United States.
References
- 1.Kawaguchi Y, Kawanishi H, Mujais S, Topley N, Oreopoulos DG. Encapsulating peritoneal sclerosis: definition, etiology, diagnosis, and treatment. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Perit Dial Int. 2000;20 Suppl 4:S43–S55. [PubMed] [Google Scholar]
- 2.Owtschinnikow PJ. Peritonitis chronica fibrosa incapsulata. Arch für Klin Chir. 1907;83:623–634. [Google Scholar]
- 3.Casas JD, Mariscal A, Martínez N. Peritoneal encapsulation: CT appearance. AJR Am J Roentgenol. 1998;171:1017–1019. doi: 10.2214/ajr.171.4.9762988. [DOI] [PubMed] [Google Scholar]
- 4.Li N, Zhu W, Li Y, Gong J, Gu L, Li M, Cao L, Li J. Surgical treatment and perioperative management of idiopathic abdominal cocoon: single-center review of 65 cases. World J Surg. 2014;38:1860–1867. doi: 10.1007/s00268-014-2458-6. [DOI] [PubMed] [Google Scholar]
- 5.Akbulut S. Accurate definition and management of idiopathic sclerosing encapsulating peritonitis. World J Gastroenterol. 2015;21:675–687. doi: 10.3748/wjg.v21.i2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eltringham WK, Espiner HJ, Windsor CW, Griffiths DA, Davies JD, Baddeley H, Read AE, Blunt RJ. Sclerosing peritonitis due to practolol: a report on 9 cases and their surgical management. Br J Surg. 1977;64:229–235. doi: 10.1002/bjs.1800640402. [DOI] [PubMed] [Google Scholar]
- 7.Sarker S, Kodali S, Weber F. A new meaning to butterflies in the stomach. Gastroenterology. 2015;148:e12–e13. doi: 10.1053/j.gastro.2014.07.037. [DOI] [PubMed] [Google Scholar]
- 8.Sachdev A, Usatoff V, Thaow C. Sclerosing encapsulating peritonitis and methotrexate. Aust N Z J Obstet Gynaecol. 2006;46:58–59. doi: 10.1111/j.1479-828X.2006.00517.x. [DOI] [PubMed] [Google Scholar]
- 9.Nauen DW, Martin A, Katz A, Cohen D, Ranganathan S. A case of luteinizing thecoma with sclerosing peritonitis: revisiting a link with anti-epileptic drugs. Pediatr Blood Cancer. 2010;54:470–472. doi: 10.1002/pbc.22325. [DOI] [PubMed] [Google Scholar]
- 10.Sharma V, Mandavdhare HS, Rana SS, Singh H, Kumar A, Gupta R. Role of conservative management in tubercular abdominal cocoon: a case series. Infection. 2017;45:601–606. doi: 10.1007/s15010-017-1012-5. [DOI] [PubMed] [Google Scholar]
- 11.Simbli MA, Niaz FA, Al-Wakeel JS. Encapsulating peritoneal sclerosis in a peritoneal dialysis patient presenting with complicated Mycobacterium fortuitum peritonitis. Saudi J Kidney Dis Transpl. 2012;23:635–641. [PubMed] [Google Scholar]
- 12.Hsu YH, Hsia CC, Tsai DM, Tu HY, Hung KY, Huang JW. Development of encapsulating peritoneal sclerosis following bacterial peritonitis in a peritoneal dialysis patient. Am J Kidney Dis. 2010;55:198–202. doi: 10.1053/j.ajkd.2009.06.043. [DOI] [PubMed] [Google Scholar]
- 13.Browne LP, Patel J, Guillerman RP, Hanson IC, Cass DL. Abdominal cocoon: a unique presentation in an immunodeficient infant. Pediatr Radiol. 2012;42:263–266. doi: 10.1007/s00247-011-2135-y. [DOI] [PubMed] [Google Scholar]
- 14.Gupta S, Woodrow G. Successful treatment of fulminant encapsulating peritoneal sclerosis following fungal peritonitis with tamoxifen. Clin Nephrol. 2007;68:125–129. doi: 10.5414/CNP68125. [DOI] [PubMed] [Google Scholar]
- 15.Tan J, Manickam R, Pisharam J, Telisinghe P, Chong VH. Mucormycosis--a possible trigger pathogen for encapsulating peritoneal sclerosis. Perit Dial Int. 2012;32:479–481. doi: 10.3747/pdi.2011.00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salamone G, Atzeni J, Agrusa A, Gulotta G. A rare case of abdominal cocoon. Ann Ital Chir. 2013:84. [PubMed] [Google Scholar]
- 17.Brown EA, Bargman J, van Biesen W, Chang MY, Finkelstein FO, Hurst H, Johnson DW, Kawanishi H, Lambie M, de Moraes TP, et al. Length of Time on Peritoneal Dialysis and Encapsulating Peritoneal Sclerosis - Position Paper for ISPD: 2017 Update. Perit Dial Int. 2017;37:362–374. doi: 10.3747/pdi.2017.00018. [DOI] [PubMed] [Google Scholar]
- 18.Takebayashi K, Sonoda H, Shimizu T, Ohta H, Ishida M, Mekata E, Endo Y, Tani T, Tani M. Successful surgical approach for a patient with encapsulating peritoneal sclerosis after hyperthermic intraperitoneal chemotherapy: a case report and literature review. BMC Surg. 2014;14:57. doi: 10.1186/1471-2482-14-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wirnsberger GH, Ganser K, Domej W, Sauseng G, Moore D, Moczygemba M, Krejs GJ. Sclerosing encapsulating peritonitis: differential diagnosis to peritoneal encapsulation and abdominal cocoon--a case report. Z Gastroenterol. 1992;30:534–537. [PubMed] [Google Scholar]
- 20.Sigaroudinia MO, Baillie C, Ahmed S, Mallucci C. Sclerosing encapsulating peritonitis--a rare complication of ventriculoperitoneal shunts. J Pediatr Surg. 2008;43:E31–E33. doi: 10.1016/j.jpedsurg.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Lin CH, Yu JC, Chen TW, Chan DC, Chen CJ, Hsieh CB. Sclerosing encapsulating peritonitis in a liver transplant patient: a case report. World J Gastroenterol. 2005;11:5412–5413. doi: 10.3748/wjg.v11.i34.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Keating JP, Neill M, Hill GL. Sclerosing encapsulating peritonitis after intraperitoneal use of povidone iodine. Aust N Z J Surg. 1997;67:742–744. doi: 10.1111/j.1445-2197.1997.tb07126.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaur S, Doley RP, Chabbhra M, Kapoor R, Wig J. Post trauma abdominal cocoon. Int J Surg Case Rep. 2015;7C:64–65. doi: 10.1016/j.ijscr.2014.10.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaman L, Iqbal J, Thenozhi S. Sclerosing encapsulating peritonitis: complication of laparoscopic cholecystectomy. J Laparoendosc Adv Surg Tech A. 2010;20:253–255. doi: 10.1089/lap.2010.0024. [DOI] [PubMed] [Google Scholar]
- 25.Árnadóttir M, Jónasson JG, Indridason ÓS. Encapsulating peritoneal sclerosis following a peritoneal foreign body reaction to Dacron fibres-a case report. NDT Plus. 2011;4:107–109. doi: 10.1093/ndtplus/sfq202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrion A, Pira E, Mollo F. Peritoneal plaques and asbestos exposure. Arch Pathol Lab Med. 1983;107:609–610. [PubMed] [Google Scholar]
- 27.Castelli MJ, Armin AR, Husain A, Orfei E. Fibrosing peritonitis in a drug abuser. Arch Pathol Lab Med. 1985;109:767–769. [PubMed] [Google Scholar]
- 28.Wakabayashi H, Okano K, Suzuki Y. Clinical challenges and images in GI. Image 2. Perforative peritonitis on sclerosing encapsulating peritonitis (abdominal cocoon) in a patient with alcoholic liver cirrhosis. Gastroenterology. 2007;132:854, 1210. doi: 10.1053/j.gastro.2007.01.060. [DOI] [PubMed] [Google Scholar]
- 29.Lee KW, Cho CW, Lee N, Lee S, Kim JM, Choi GS, Kwon CH, Joh JW, Lee SK. Encapsulating peritoneal sclerosis in liver transplant recipients: a report of 2 cases. Ann Surg Treat Res. 2017;92:164–167. doi: 10.4174/astr.2017.92.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rumbo C, Zambernardi A, Cabanne A, Rumbo M, Gondolesi G. Sclerosing peritonitis, a rare complication after intestinal transplant. Report of one case successfully treated with adjustment of immunosuppression. Pediatr Transplant. 2013;17:E125–E129. doi: 10.1111/petr.12110. [DOI] [PubMed] [Google Scholar]
- 31.Morrow EH, Gallo AE, Melcher ML. Sclerosing peritonitis after kidney transplantation: a not-so-silky cocoon. Dig Dis Sci. 2011;56:307–310. doi: 10.1007/s10620-010-1471-3. [DOI] [PubMed] [Google Scholar]
- 32.Frigerio L, Taccagni GL, Mariani A, Mangili G, Ferrari A. Idiopathic sclerosing peritonitis associated with florid mesothelial hyperplasia, ovarian fibromatosis, and endometriosis: a new disorder of abdominal mass. Am J Obstet Gynecol. 1997;176:721–722. doi: 10.1016/S0002-9378(97)70581-7. [DOI] [PubMed] [Google Scholar]
- 33.Bahar B, Hu Z, Szpaderska A, Liotta M, Potkul RK, Smith D, Erşahin Ç. Fatal case of luteinized thecoma with sclerosing peritonitis in a 40-year-old woman. Int J Gynecol Pathol. 2014;33:30–34. doi: 10.1097/PGP.0b013e31827d1a65. [DOI] [PubMed] [Google Scholar]
- 34.Walker J, Moss EL, Ganesan R, Hirschowitz L. Sclerosing peritonitis associated with a luteinized adult granulosa cell tumor. Int J Gynecol Pathol. 2012;31:141–144. doi: 10.1097/PGP.0b013e3182307b28. [DOI] [PubMed] [Google Scholar]
- 35.Fossey SJ, Simson JN. Sclerosing encapsulating peritonitis secondary to dermoid cyst rupture: a case report. Ann R Coll Surg Engl. 2011;93:e39–e40. doi: 10.1308/147870811X582495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ngô Y, Messing B, Marteau P, Nouël O, Pasquiou A, Lavergne A, Rambaud JC. Peritoneal sarcoidosis. An unrecognized cause of sclerosing peritonitis. Dig Dis Sci. 1992;37:1776–1780. doi: 10.1007/BF01299875. [DOI] [PubMed] [Google Scholar]
- 37.Pepels MJ, Peters FP, Mebis JJ, Ceelen TL, Hoofwijk AG, Erdkamp FL. Sclerosing peritonitis: an unusual cause of ascites in a patient with systemic lupus erythematosus. Neth J Med. 2006;64:346–349. [PubMed] [Google Scholar]
- 38.Dabak R, Uygur-Bayramiçli O, Aydin DK, Dolapçioglu C, Gemici C, Erginel T, Turan C, Karadayi N. Encapsulating peritonitis and familial Mediterranean fever. World J Gastroenterol. 2005;11:2844–2846. doi: 10.3748/wjg.v11.i18.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alston H, Fan S, Nakayama M. Encapsulating Peritoneal Sclerosis. Semin Nephrol. 2017;37:93–102. doi: 10.1016/j.semnephrol.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 40.Johnson DW, Cho Y, Livingston BE, Hawley CM, McDonald SP, Brown FG, Rosman JB, Bannister KM, Wiggins KJ. Encapsulating peritoneal sclerosis: incidence, predictors, and outcomes. Kidney Int. 2010;77:904–912. doi: 10.1038/ki.2010.16. [DOI] [PubMed] [Google Scholar]
- 41.Kawanishi H, Moriishi M. Epidemiology of encapsulating peritoneal sclerosis in Japan. Perit Dial Int. 2005;25 Suppl 4:S14–S18. [PubMed] [Google Scholar]
- 42.Honda K, Oda H. Pathology of encapsulating peritoneal sclerosis. Perit Dial Int. 2005;25 Suppl 4:S19–S29. [PubMed] [Google Scholar]
- 43.Abrahams AC, Habib SM, Dendooven A, Riser BL, van der Veer JW, Toorop RJ, Betjes MG, Verhaar MC, Watson CJ, Nguyen TQ, et al. Patients with encapsulating peritoneal sclerosis have increased peritoneal expression of connective tissue growth factor (CCN2), transforming growth factor-β1, and vascular endothelial growth factor. PLoS One. 2014;9:e112050. doi: 10.1371/journal.pone.0112050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lambie MR, Chess J, Summers AM, Williams PF, Topley N, Davies SJ; GLOBAL Fluid Study Investigators. Peritoneal inflammation precedes encapsulating peritoneal sclerosis: results from the GLOBAL Fluid Study. Nephrol Dial Transplant. 2016;31:480–486. doi: 10.1093/ndt/gfv440. [DOI] [PubMed] [Google Scholar]
- 45.Braun N, Alscher DM, Fritz P, Edenhofer I, Kimmel M, Gaspert A, Reimold F, Bode-Lesniewska B, Ziegler U, Biegger D, et al. Podoplanin-positive cells are a hallmark of encapsulating peritoneal sclerosis. Nephrol Dial Transplant. 2011;26:1033–1041. doi: 10.1093/ndt/gfq488. [DOI] [PubMed] [Google Scholar]
- 46.Lopez-Anton M, Lambie M, Lopez-Cabrera M, Schmitt CP, Ruiz-Carpio V, Bartosova M, Schaefer B, Davies S, Stone T, Jenkins R, et al. miR-21 Promotes Fibrogenesis in Peritoneal Dialysis. Am J Pathol. 2017;187:1537–1550. doi: 10.1016/j.ajpath.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 47.Reimold FR, Braun N, Zsengellér ZK, Stillman IE, Karumanchi SA, Toka HR, Latus J, Fritz P, Biegger D, Segerer S, et al. Transcriptional patterns in peritoneal tissue of encapsulating peritoneal sclerosis, a complication of chronic peritoneal dialysis. PLoS One. 2013;8:e56389. doi: 10.1371/journal.pone.0056389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Maruyama Y, Numata M, Nakayama M, Matsuo N, Nordfors L, Hosoya T, Lindholm B. Relationship between the -374T/A receptor of advanced glycation end products gene polymorphism and peritoneal solute transport status at the initiation of peritoneal dialysis. Ther Apher Dial. 2007;11:301–305. doi: 10.1111/j.1744-9987.2007.00473.x. [DOI] [PubMed] [Google Scholar]
- 49.Clement PB, Young RH, Hanna W, Scully RE. Sclerosing peritonitis associated with luteinized thecomas of the ovary. A clinicopathological analysis of six cases. Am J Surg Pathol. 1994;18:1–13. doi: 10.1097/00000478-199401000-00001. [DOI] [PubMed] [Google Scholar]
- 50.Betjes MG, Habib SM, Boeschoten EW, Hemke AC, Struijk DG, Westerhuis R, Abrahams AC, Korte MR. Significant Decreasing Incidence of Encapsulating Peritoneal Sclerosis in the Dutch Population of Peritoneal Dialysis Patients. Perit Dial Int. 2017;37:230–234. doi: 10.3747/pdi.2016.00109. [DOI] [PubMed] [Google Scholar]
- 51.Hoshii S, Honda M, Itami N, Oh S, Matsumura C, Moriya S, Mori M, Hatae K, Ito Y, Karashima S. Sclerosing encapsulating peritonitis in pediatric peritoneal dialysis patients. Pediatr Nephrol. 2000;14:275–279. doi: 10.1007/s004670050758. [DOI] [PubMed] [Google Scholar]
- 52.Phelan PJ, Walshe JJ, Al-Aradi A, Garvey JP, Finnegan K, O’Kelly P, McWilliams J, Ti JP, Morrin MM, Morgan N, et al. Encapsulating peritoneal sclerosis: experience of a tertiary referral center. Ren Fail. 2010;32:459–463. doi: 10.3109/08860221003658274. [DOI] [PubMed] [Google Scholar]
- 53.Nakayama M, Miyazaki M, Honda K, Kasai K, Tomo T, Nakamoto H, Kawanishi H. Encapsulating peritoneal sclerosis in the era of a multi-disciplinary approach based on biocompatible solutions: the NEXT-PD study. Perit Dial Int. 2014;34:766–774. doi: 10.3747/pdi.2013.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rigby RJ, Hawley CM. Sclerosing peritonitis: the experience in Australia. Nephrol Dial Transplant. 1998;13:154–159. doi: 10.1093/ndt/13.1.154. [DOI] [PubMed] [Google Scholar]
- 55.Balasubramaniam G, Brown EA, Davenport A, Cairns H, Cooper B, Fan SL, Farrington K, Gallagher H, Harnett P, Krausze S, et al. The Pan-Thames EPS study: treatment and outcomes of encapsulating peritoneal sclerosis. Nephrol Dial Transplant. 2009;24:3209–3215. doi: 10.1093/ndt/gfp008. [DOI] [PubMed] [Google Scholar]
- 56.Nakamoto H. Encapsulating peritoneal sclerosis -- a clinician’s approach to diagnosis and medical treatment. Perit Dial Int. 2005;25 Suppl 4:S30–S38. [PubMed] [Google Scholar]
- 57.Moinuddin Z, Summers A, Van Dellen D, Augustine T, Herrick SE. Encapsulating peritoneal sclerosis-a rare but devastating peritoneal disease. Front Physiol. 2015;5:470. doi: 10.3389/fphys.2014.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ti JP, Al-Aradi A, Conlon PJ, Lee MJ, Morrin MM. Imaging features of encapsulating peritoneal sclerosis in continuous ambulatory peritoneal dialysis patients. AJR Am J Roentgenol. 2010;195:W50–W54. doi: 10.2214/AJR.09.3175. [DOI] [PubMed] [Google Scholar]
- 59.Stuart S, Stott D, Goode A, Cash CJ, Davenport A. Can radiological assessment of abdominal computerized scans diagnose encapsulating peritoneal sclerosis in long-term peritoneal dialysis patients? Nephrology (Carlton) 2017;22:19–24. doi: 10.1111/nep.12718. [DOI] [PubMed] [Google Scholar]
- 60.Upponi S, Butler AJ, Watson CJ, Shaw AS. Encapsulating peritoneal sclerosis--correlation of radiological findings at CT with underlying pathogenesis. Clin Radiol. 2014;69:103–109. doi: 10.1016/j.crad.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Jovani M, Baticci F, Bonifacio C, Omodei PD, Malesci A. Abdominal cocoon or idiopathic encapsulating peritoneal sclerosis: magnetic resonance imaging. Dig Liver Dis. 2014;46:192–193. doi: 10.1016/j.dld.2013.08.136. [DOI] [PubMed] [Google Scholar]
- 62.Cho R, Ghag D, Karim MA, Lo C. Encapsulating peritoneal sclerosis: surgery, sustained drug therapy and treatment of recurrence at 1 year. BMJ Case Rep. 2015;2015 doi: 10.1136/bcr-2015-211490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lopes Barreto D, Struijk DG, Krediet RT. Peritoneal effluent MMP-2 and PAI-1 in encapsulating peritoneal sclerosis. Am J Kidney Dis. 2015;65:748–753. doi: 10.1053/j.ajkd.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 64.Goodlad C, Tam FW, Ahmad S, Bhangal G, North BV, Brown EA. Dialysate cytokine levels do not predict encapsulating peritoneal sclerosis. Perit Dial Int. 2014;34:594–604. doi: 10.3747/pdi.2012.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ahmad S, North BV, Qureshi A, Malik A, Bhangal G, Tarzi RM, Brown EA, Tam FW. CCL18 in peritoneal dialysis patients and encapsulating peritoneal sclerosis. Eur J Clin Invest. 2010;40:1067–1073. doi: 10.1111/j.1365-2362.2010.02353.x. [DOI] [PubMed] [Google Scholar]
- 66.Braun N, Fritz P, Ulmer C, Latus J, Kimmel M, Biegger D, Ott G, Reimold F, Thon KP, Dippon J, et al. Histological criteria for encapsulating peritoneal sclerosis - a standardized approach. PLoS One. 2012;7:e48647. doi: 10.1371/journal.pone.0048647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lalloo S, Krishna D, Maharajh J. Case report: abdominal cocoon associated with tuberculous pelvic inflammatory disease. Br J Radiol. 2002;75:174–176. doi: 10.1259/bjr.75.890.750174. [DOI] [PubMed] [Google Scholar]
- 68.Kawanishi H, Kawaguchi Y, Fukui H, Hara S, Imada A, Kubo H, Kin M, Nakamoto M, Ohira S, Shoji T. Encapsulating peritoneal sclerosis in Japan: a prospective, controlled, multicenter study. Am J Kidney Dis. 2004;44:729–737. doi: 10.1053/j.ajkd.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 69.de Freitas D, Jordaan A, Williams R, Alderdice J, Curwell J, Hurst H, Hutchison A, Brenchley PE, Augustine T, Summers AM. Nutritional management of patients undergoing surgery following diagnosis with encapsulating peritoneal sclerosis. Perit Dial Int. 2008;28:271–276. [PubMed] [Google Scholar]
- 70.Junor BJ, McMillan MA. Immunosuppression in sclerosing peritonitis. Adv Perit Dial. 1993;9:187–189. [PubMed] [Google Scholar]
- 71.Maruyama Y, Nakayama M. Encapsulating peritoneal sclerosis in Japan. Perit Dial Int. 2008;28 Suppl 3:S201–S204. [PubMed] [Google Scholar]
- 72.Ceri M, Unverdi S, Dogan M, Unverdi H, Karaca G, Kocak G, Kurultak I, Akbal E, Can M, Duranay M. Effect of sirolimus on the regression of peritoneal sclerosis in an experimental rat model. Int Urol Nephrol. 2012;44:977–982. doi: 10.1007/s11255-012-0167-3. [DOI] [PubMed] [Google Scholar]
- 73.Sud R, Garry L, Spicer ST, Allen RD, Eris JM, Wyburn K, Verran D, Cooper CL, Chadban S. A role for everolimus in post-transplant encapsulating peritoneal sclerosis: first case report. Nephrology (Carlton) 2014;19 Suppl 1:27–30. doi: 10.1111/nep.12196. [DOI] [PubMed] [Google Scholar]
- 74.Romagnoli J, Pedroso JA, Salerno MP, Favi E, Spagnoletti G, Citterio F. Posttransplant encapsulating peritoneal sclerosis, long-term success with everolimus and low-dose CNI: a case report. Transplant Proc. 2014;46:2368–2370. doi: 10.1016/j.transproceed.2014.07.060. [DOI] [PubMed] [Google Scholar]
- 75.Huddam B, Başaran M, Koçak G, Azak A, Yalçın F, Reyhan NH, Duranay M. The use of mycophenolate mofetil in experimental encapsulating peritoneal sclerosis. Int Urol Nephrol. 2015;47:1423–1428. doi: 10.1007/s11255-015-1015-z. [DOI] [PubMed] [Google Scholar]
- 76.Bozkurt D, Bicak S, Sipahi S, Taskin H, Hur E, Ertilav M, Sen S, Duman S. The effects of colchicine on the progression and regression of encapsulating peritoneal sclerosis. Perit Dial Int. 2008;28 Suppl 5:S53–S57. [PubMed] [Google Scholar]
- 77.Kuriyama S, Tomonari H. Corticosteroid therapy in encapsulating peritoneal sclerosis. Nephrol Dial Transplant. 2001;16:1304–1305. doi: 10.1093/ndt/16.6.1304. [DOI] [PubMed] [Google Scholar]
- 78.Habib SM, Betjes MG, Fieren MW, Boeschoten EW, Abrahams AC, Boer WH, Struijk DG, Ruger W, Krikke C, Westerhuis R, et al. Management of encapsulating peritoneal sclerosis: a guideline on optimal and uniform treatment. Neth J Med. 2011;69:500–507. [PubMed] [Google Scholar]
- 79.Loureiro J, Aguilera A, Selgas R, Sandoval P, Albar-Vizcaíno P, Pérez-Lozano ML, Ruiz-Carpio V, Majano PL, Lamas S, Rodríguez-Pascual F, et al. Blocking TGF-β1 protects the peritoneal membrane from dialysate-induced damage. J Am Soc Nephrol. 2011;22:1682–1695. doi: 10.1681/ASN.2010111197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allaria PM, Giangrande A, Gandini E, Pisoni IB. Continuous ambulatory peritoneal dialysis and sclerosing encapsulating peritonitis: tamoxifen as a new therapeutic agent? J Nephrol. 1999;12:395–397. [PubMed] [Google Scholar]
- 81.Korte MR, Fieren MW, Sampimon DE, Lingsma HF, Weimar W, Betjes MG; investigators of the Dutch Multicentre EPS Study. Tamoxifen is associated with lower mortality of encapsulating peritoneal sclerosis: results of the Dutch Multicentre EPS Study. Nephrol Dial Transplant. 2011;26:691–697. doi: 10.1093/ndt/gfq362. [DOI] [PubMed] [Google Scholar]
- 82.Moustafellos P, Hadjianastassiou V, Roy D, Velzeboer NE, Maniakyn N, Vaidya A, Friend PJ. Tamoxifen therapy in encapsulating sclerosing peritonitis in patients after kidney transplantation. Transplant Proc. 2006;38:2913–2914. doi: 10.1016/j.transproceed.2006.08.179. [DOI] [PubMed] [Google Scholar]
- 83.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, Bevers TB, Kavanah MT, Atkins JN, Margolese RG, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 84.Célicout B, Levard H, Hay J, Msika S, Fingerhut A, Pelissier E. Sclerosing encapsulating peritonitis: early and late results of surgical management in 32 cases. French Associations for Surgical Research. Dig Surg. 1998;15:697–702. doi: 10.1159/000018681. [DOI] [PubMed] [Google Scholar]
- 85.Kawanishi H, Ide K, Yamashita M, Shimomura M, Moriishi M, Tsuchiya S, Dohi K. Surgical techniques for prevention of recurrence after total enterolysis in encapsulating peritoneal sclerosis. Adv Perit Dial. 2008;24:51–55. [PubMed] [Google Scholar]
- 86.Ulmer C, Braun N, Rieber F, Latus J, Hirschburger S, Emmel J, Alscher MD, Steurer W, Thon KP. Efficacy and morbidity of surgical therapy in late-stage encapsulating peritoneal sclerosis. Surgery. 2013;153:219–224. doi: 10.1016/j.surg.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 87.Kawanishi H, Shintaku S, Moriishi M, Dohi K, Tsuchiya S. Seventeen years’ experience of surgical options for encapsulating peritoneal sclerosis. Adv Perit Dial. 2011;27:53–58. [PubMed] [Google Scholar]
- 88.Kawanishi H. Surgical and medical treatments of encapsulation peritoneal sclerosis. Contrib Nephrol. 2012;177:38–47. doi: 10.1159/000336934. [DOI] [PubMed] [Google Scholar]
- 89.Lo WK, Kawanishi H. Encapsulating peritoneal sclerosis--medical and surgical treatment. Perit Dial Int. 2009;29 Suppl 2:S211–S214. [PubMed] [Google Scholar]
- 90.Kurz A, Sessler DI. Opioid-induced bowel dysfunction: pathophysiology and potential new therapies. Drugs. 2003;63:649–671. doi: 10.2165/00003495-200363070-00003. [DOI] [PubMed] [Google Scholar]
- 91.Ketwaroo GA, Cheng V, Lembo A. Opioid-induced bowel dysfunction. Curr Gastroenterol Rep. 2013;15:344. doi: 10.1007/s11894-013-0344-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang C, Patel NJ, Jacobs WC, Ullman S, Berzin TM, Chuttani R, Lembo AJ, Wolf JL. Successful Treatment with Methylnaltrexone and IVIG for Paraneoplastic Syndrome-Associated Intestinal Pseudo-Obstruction. Gastroenterol Hepatol (NY) 2013;9:48–51. [PMC free article] [PubMed] [Google Scholar]