Significance
Recent research has identified human subjects who have highly superior autobiographical memory (HSAM). Here, we investigated, using fMRI, the neural activation induced by retrieval of autobiographical memories (AMs) and semantic memories (SMs) in subjects with HSAM and control subjects. While their brains were being scanned, subjects had to retrieve AMs as well as SMs (e.g., examples of animals). The subjects with HSAM displayed a superior ability to retrieve details of AMs, supported by enhanced activation of several brain regions, including the medial prefrontal cortex and temporoparietal junction, as well as increased connectivity of the prefrontal cortex with the hippocampus, a region well known to be involved in memory representation. These findings suggest that activation of these systems may play a critical role in enabling HSAM.
Keywords: long-term memory, prefrontal cortex, hippocampus, fMRI, functional connectivity
Abstract
Brain systems underlying human memory function have been classically investigated studying patients with selective memory impairments. The discovery of rare individuals who have highly superior autobiographical memory (HSAM) provides, instead, an opportunity to investigate the brain systems underlying enhanced memory. Here, we carried out an fMRI investigation of a group of subjects identified as having HSAM. During fMRI scanning, eight subjects with HSAM and 21 control subjects were asked to retrieve autobiographical memories (AMs) as well as non-AMs (e.g., examples of animals). Subjects were instructed to signal the “access” to an AM by a key press and to continue “reliving” it immediately after. Compared with controls, individuals with HSAM provided a richer AM recollection and were faster in accessing AMs. The access to AMs was associated with enhanced prefrontal/hippocampal functional connectivity. AM access also induced increased activity in the left temporoparietal junction and enhanced functional coupling with sensory cortices in subjects with HSAM compared with controls. In contrast, subjects with HSAM did not differ from controls in functional activity during the reliving phase. These findings, based on fMRI assessment, provide evidence of interaction of brain systems engaged in memory retrieval and suggest that enhanced activity of these systems is selectively involved in enabling more efficient access to past experiences in HSAM.
The ability to remember personal experiences [i.e., autobiographical memories (AMs)] is essential for survival (1). Brain systems underlying human AM function have been classically investigated studying patients with selective memory impairments (2). The discovery of extremely rare individuals who spontaneously show highly superior autobiographical memory (HSAM) (3, 4) provides, instead, an opportunity to investigate the brain processes underlying enhanced AMs. Individuals with HSAM demonstrate an extraordinary ability to recall vividly and accurately many remote autobiographical events, irrespective of their emotional saliency, and without the explicit use of mnemonic strategies. In contrast, their performance is generally comparable to that of control subjects in performance assessed by laboratory memory tests (3–5). Prior MRI assessment of HSAM revealed that several brain regions differ in size and shape [e.g., parahippocampal gyrus, posterior insula, intraparietal sulcus (IPS), putamen, caudate] as well as in coherence of fiber tracts (e.g., uncinate fasciculus) compared with those of control subjects (4). The present study investigated brain activity induced by AMs using fMRI.
Prior evidence of detailed reexperiencing in subjects with HSAM (3–5) suggests that subjects with HSAM may express increased neural activity underlying memory reliving. Previous fMRI investigations of normal (i.e., not superior) AM retrieval assessed memory access and memory reliving by asking participants to confirm elicitation of an AM through a response button (access phase) and then to continue to elaborate on the retrieved event (reliving phase) in as much detail as possible for the remaining part of the trial (6). Previous studies using this approach have reported activity in prefrontal/medial temporal regions related to access and activity in sensory cortex related to reliving (7). Retrieval by subjects with HSAM may therefore involve enhanced activity in sensory cortex associated with detailed reliving of reactivated experiences. Alternatively, HSAM might entail enhanced prefrontal/medial temporal resources devoted to AM access. This interpretation is consistent with recent findings showing a selective decrease of neural activity in the medial prefrontal cortex as well as a reduced hippocampal volume in individuals who have impaired AM retrieval (8). These findings may suggest that individuals with HSAM show hyperfunctioning of prefrontal/hippocampal regions. In the present study, we addressed this question by performing an fMRI examination of a group of subjects with HSAM.
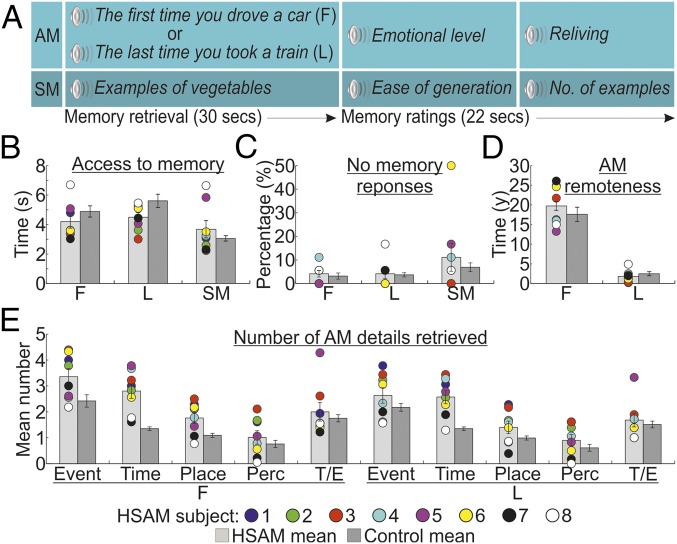
During fMRI, subjects with HSAM and controls were asked to mentally retrieve “easy” AMs, thus guaranteeing good performance in controls. Subjects were presented with memory cues pointing to specific spatiotemporal coordinates that emphasize the difference between very old and more recent AMs (e.g., “the first time you drove a car” or “the last time you took a train”). Participants confirmed the appearance of the AM through a response button (access phase) and then continued to relive the retrieved event in as much detail as possible (6) (reliving phase; Fig. 1A). The specificity of AM activations was controlled by subtracting neural activity induced by accessing and generating examples of specific semantic categories [e.g., “example of vegetables”; i.e., a semantic memory (SM) task]. In comparison to controls, the subjects with HSAM had faster access and more detailed retrieval of AMs. Memory access was associated with increased prefrontal/hippocampal functional connectivity and increased connectivity between the temporoparietal junction (TPJ) and sensory cortices. The prefrontal and hippocampal regions were found to be particularly involved with access to remote AMs. In contrast, subjects with HSAM did not differ from controls in brain activity during the reliving phase.
Fig. 1.
Task and behavioral results. (A) Sequence of events in an example trial, involving 30 s to access and elaborate [first (F) and last (L)] AMs or SMs and 22 s to provide memory ratings. (B) Time to access AMs and SMs in seconds. (C) Percentages of no memory trials. (D) Remoteness of reported AMs (in years). (E) Mean number of details according to the categories of Levine et al. (9) [event, time, place, perceptual (Perc), thought/emotion (T/E)] and AM remoteness. In all graphs, individual scores for subjects with HSAM are plotted. The error bars represent the SEM.
Results
Behavioral Data.
Behavioral results are illustrated in Fig. 1. First, we assessed whether subjects with HSAM were faster than controls to access their AMs. We performed a group (HSAM vs. control) by trial type (first AM, last AM, and SM) ANOVA on the response latencies, defining the time needed to access a specific AM or SM (Fig. 1B). This analysis revealed a main effect of trial type [F(2, 54) = 21.2, P < 0.001; η2 = 0.440], indicating faster response latencies to access SMs (mean = 3.368 s) than first (4.545 s) and last (5.059 s) AMs. This effect was further qualified by the significant group × trial type interaction [F(2, 54) = 5.8, P = 0.005; η2 = 0.177], indicating that subjects with HSAM had faster access to AMs than control subjects (first AM: 4.196 vs. 4.894 s, P = 0.029; last AM: 4.504 vs. 5.614 s, P = 0.013), but not to SMs (3.681 vs. 3.056 s, P = 0.074). The main effect of group was not significant [F(1, 27) < 1]. A similar 2 × 3 ANOVA on the “no memory response” data (Fig. 1C) revealed a main effect of trial type [F(2, 54) = 3.7, P = 0.032; η2 = 0.120], indicating that subjects failed more often to retrieve SMs (9%) than first (3.7%) and last (3.9%) AMs. This effect was not further modulated by the group factor, as indicated by the absence of both main effect of group and group × type of trial interaction (both F < 1). No differences were found between the groups in the self-evaluation ratings of the emotional level and reliving of AMs and the ease of generation and number of generated example for SMs during scanning, as assessed by two-tailed independent sample t tests (t values ranging between −1.4 and 0.9, P > 0.181; SI Appendix, Fig. S1).
After the fMRI scanning, participants were presented again with the memory cues and asked to provide a verbal account of their memories. We analyzed the temporal distribution (Fig. 1D) of the retrieved AMs using a group by remoteness of AM (first or last AM) ANOVA. This analysis revealed a main effect of remoteness [F(1, 27) = 188.4, P < 0.001; η2 = 0.875], indicating that first AMs were older than last AMs: 18.76 y and 2.16 y, respectively. The two groups did not differ in the remoteness of AMs reported, as indicated by the absence of both main effect of group and group × age of AM interaction (both F < 1.3, P > 0.257). Finally, we compared how detailed were the AMs reported by the two groups, using a group by remoteness of AM by type of detail [event, time, place, perceptual, thought/emotion (9)] ANOVA. Subjects with HSAM retrieved a greater number of details than controls (2.0 vs. 1.4), as evidenced by the main effect of group [F(1, 27) = 10.1, P = 0.004; η2 = 0.272]. This was particularly true for the remote events, as indicated by the three-way interaction [F(4, 108) = 2.5, P = 0.047; η2 = 0.085], showing more details reported in the event (P = 0.005), time (P < 0.001), and place (P = 0.048) categories relative to remote AMs, but only more details in the time category (P < 0.001) relative to recent AMs (Fig. 1E). Subjects with HSAM also provided higher vivid descriptions of their AMs when assessed qualitatively (t values ranging between 5.0 and 18.2, P < 0.001; SI Appendix, Fig. S2).
Overall, these findings indicate that, compared with controls, subjects with HSAM had faster and more vivid access to AMs, especially for the most remote AMs, but had normal SMs.
Assessment of Obsessive/Compulsive Traits in Individuals with HSAM.
The findings of previous studies suggest that subjects with HSAM tend to have symptoms of obsessiveness/compulsiveness (10). To assess whether individuals with HSAM participating in the current study experienced obsessive/compulsive symptomatology, they were administered the Personality Assessment Inventory, which included, among others, the “obsessive-compulsive” subscale. The average score at the obsessive-compulsive subscale for individuals with HSAM was 67, corresponding to the 92nd percentile in terms of expressing obsessive/compulsive-related symptoms. We assessed whether faster access to AMs (averaging across first and last AM types) and number of retrieved details correlated with obsessive/compulsive traits (averaging across the five categories). However, our analyses failed to reveal any significant correlation (r = 0.32, P = 0.444 and r = −0.69, P = 0.58, respectively).
fMRI Data.
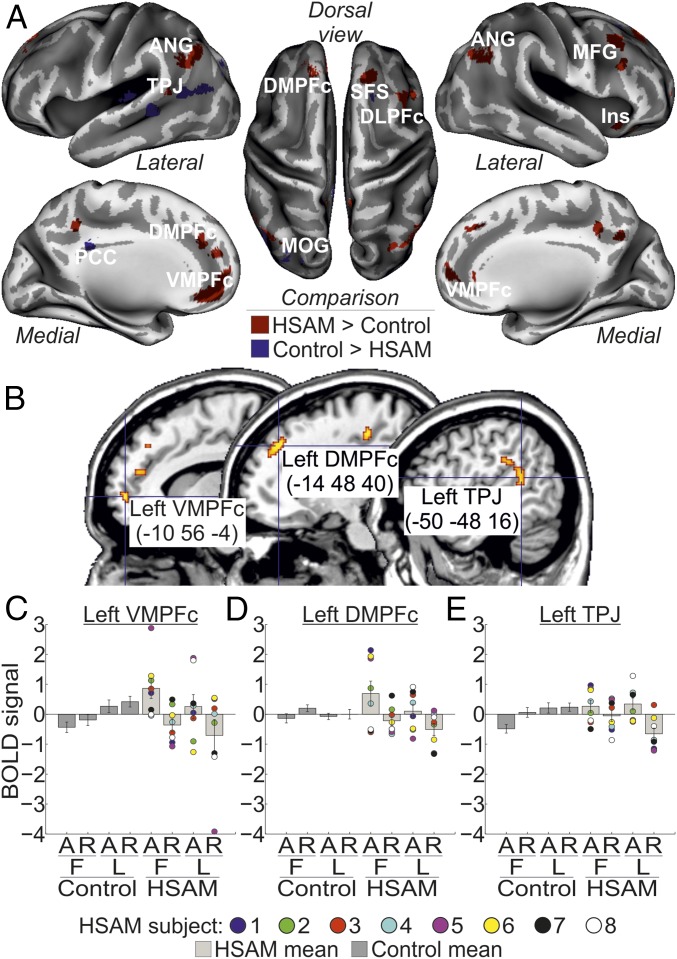
The main aim of the present study was to investigate neural activation associated with AM retrieval in subjects with HSAM compared with control subjects. In the HSAM group, AM retrieval recruited a large network of areas extending along the frontoparietal cortex (Fig. 2A and Table 1). This activation included dorsomedial regions, such as the left dorsomedial prefrontal cortex (DMPFc; the right DMPFc was marginally significant) and the left and right ventromedial prefrontal cortex (VMPFc), and lateral regions, such as the right dorsolateral prefrontal cortex, the left TPJ, and the left and right angular gyrus, plus the right insula. By contrast, we observed in the control group increased activity in a few areas involving the right superior frontal sulcus and the posterior cingulate cortex (PCC), plus activation of visual areas [i.e., the left middle occipital gyrus (MOG)].
Fig. 2.
(A) Regions activated by AM in the HSAM group (red map) and in the control group (blue map) overlaid on an inflated template. ANG, angular gyrus; DLPFc, dorsolateral prefrontal cortex; Ins, insula; MFG, middle frontal gyrus; SFS, superior frontal sulcus. (B) Sagittal sections on a standard Montreal Neurological Institute template showing the anatomical location of the regions selectively involved with the access phase to AM in the HSAM group (i.e., left VMPFc, left DMPFc, left TPJ). For display purposes, all maps are displayed at a threshold of P-uncorrected = 0.001. (C–E) Bar plots summarizing the activity (expressed in arbitrary units ± 90% confidence interval) of the left VMPFc, DMPFc, and TPJ, respectively, which showed increased activity during access to (A) [vs. reliving of (R)] AMs (compare bars 5 and 7 vs. bars 6 and 8), selectively in the HSAM group (compare bars 5–8 vs. bars 1–4). Individual scores are plotted for subjects with HSAM. BOLD, blood oxygen level-dependent.
Table 1.
MNI coordinates (x, y, z), Z-values, and P-FWE–corrected values for areas showing a main effect of group, HSAM vs. control group or vice versa
| Area | x, y, z | Z-value | P-FWE–corrected |
| HSAM > control | |||
| Left TPJ | −50, −48, 16 | 7.00 | <0.001 |
| Left ANG | −50, −64, 40 | 6.42 | <0.001 |
| Right ANG | 46, −68, 38 | 5.36 | 0.002 |
| Left VMPFc | −10, 56, −4 | 5.75 | <0.001 |
| Right VMPFc | 18, 62, 2 | 5.31 | 0.002 |
| Right DLPFc | 44, 22, 42 | 5.66 | <0.001 |
| Left DMPFc | −14, 48, 40 | 5.64 | <0.001 |
| Right Ins | 30, 24, −14 | 4.93 | 0.048 |
| Control > HSAM | |||
| Left MOG | −36, −66, 30 | 6.25 | <0.001 |
| Right SFS | 18, 20, 44 | 4.85 | 0.016 |
| Left PCC | −12, −38, 28 | 4.68 | 0.033 |
ANG, angular gyrus; DLPFc, dorsolateral prefrontal cortex; Ins, insula; SFS, superior frontal sulcus.
Next, we assessed the contribution of these group-specific activations in the access or reliving phase (i.e., phase × group interaction). Three of the regions activated by AM retrieval in subjects with HSAM were found to contribute selectively to memory access, namely, the left VMPFc (peaking at: x, y, z = −8, 58, 0; Z = 3.44; P = 0.011), the left DMPFc (x, y, z = −14, 42, 40; Z = 3.14; P = 0.027), and the left TPJ (x, y, z = −54, −44, 20; Z = 3.23; P = 0.021) (Fig. 2B). Activity in these regions selectively increased for the access vs. reliving phase (Fig. 2 C–E; compare bars 5 and 7 vs. bars 6 and 8) in the HSAM group only (compare bars 5–8 vs. bars 1–4). None of the selected regions of interest (ROIs) showed a selective involvement with the reliving phase in subjects with HSAM instead. Similarly, none of the regions activated by AM retrieval in the control group was found to contribute selectively to the access or reliving phase. Analogously, no ROIs were found to reveal any AM type × group interaction. However, the left VMPFc (peaking at: x, y, z = −12, 52, 2; Z = 3.51; P = 0.009) showed a more selective contribution during access to remote AMs (i.e., the three-way phase × AM type × group interaction). The left DMPFc showed a similar trend, despite not being statistically significant, peaking at: x, y, z = −10, 44, 44; Z = 2.75; P = 0.069. Activity in the left VMPFc selectively increased during access to first AMs in subjects with HSAM (Fig. 2C).
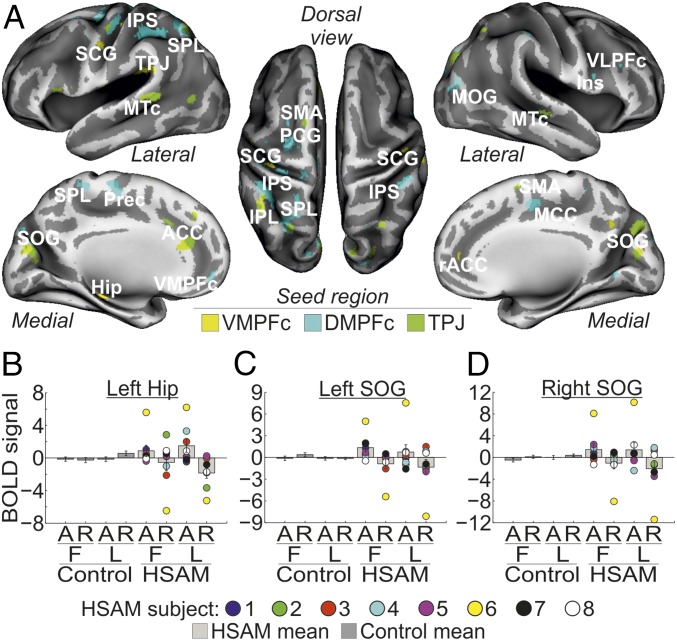
We then examined the functional coupling of the regions showing a selective involvement with AM access in subjects with HSAM (left VMPFc, left DMPFc, and left TPJ) with the rest of the brain. Analyses of interregional connectivity revealed large networks of areas functionally connected with the seed regions during AM access in subjects with HSAM (Fig. 3A and Table 2). Specifically, the left VMPFc connected with the medial temporal lobe and, in particular, with the left hippocampus and rostral portion of the anterior cingulate cortex (ACC). The VMPFc was also found to be connected with another seed region, namely, the left TPJ, and with the left and right subcentral gyrus. Activity in the left DMPFc was found instead to be synchronized during AM access in subjects with HSAM with the left and right IPS along the dorsal frontoparietal network and with prefrontal regions, such as the right ventrolateral prefrontal cortex (VLPFc) extending anteriorly and medially to the left and right VMPFc. The left DMPFc was also functionally connected with the medial portion of the cingulate cortex, the precentral gyrus, and the MOG. Finally, the left TPJ showed increased coupling during AM access in subjects with HSAM with adjacent regions of the left parietal cortex, such as the superior and inferior parietal lobe, plus other posterior regions in the occipital and temporal lobes, such as the left and right superior occipital gyrus and the left and right medial temporal cortex. The left TPJ also showed increased coupling with the ACC and the supplementary motor area bilaterally. Despite a large variability across the individual values (some representative areas are shown in signal plots in Fig. 3 B–D), the general pattern of activity of all of these regions showed increased coupling with the respective seed region during access to (vs. reliving of) AMs (compare bars 5 and 7 vs. bars 6 and 8), selectively in the HSAM group (compare bars 5–8 vs. bars 1–4).
Fig. 3.
(A) Regions showing functional connectivity with the left VMPFc (yellow map), left DMPFc (cyan map), and left TPJ (green map) during access to AM in the HSAM group overlaid on an inflated template. For display purposes, all maps are displayed at a threshold of P-uncorrected = 0.005. Hip, hippocampus; Ins, insula; MCC, medial cingulate cortex; MTc, medial temporal cortex; PCG, precentral gyrus; Prec, precuneus; rACC, rostral anterior cingulate cortex; SCG, subcentral gyrus; SMA, supplementary motor area; SOG, superior occipital gyrus; SPL, superior parietal lobe. Signal plots show the pattern of functional connectivity for some representative seed regions, specifically, the left Hip (B) and the left and right SOG (C and D). All signal plots revealed increased coupling (expressed in arbitrary units ± 90% confidence interval) with the respective seed region during access to (A) [vs. reliving of (R)] AMs (compare bars 5 and 7 vs. bars 6 and 8), selectively in the HSAM group (compare bars 5–8 vs. bars 1–4). Individual scores are plotted for subjects with HSAM. BOLD, blood oxygen level-dependent.
Table 2.
MNI coordinates (x, y, z), Z-values, and P-FWE values for areas showing increased functional connectivity with the seed regions (left VMPFc, left DMPFc, left TPJ)
| Area | x, y, z | Z-value | P-FWE–corrected |
| Left VMPFc functional connectivity | |||
| Left Hip | −32, −30, −12 | 6.82 | <0.001 |
| Left TPJ | −50, −32, 24 | 6.35 | <0.001 |
| Left SCG | −44, −6, 14 | 6.93 | <0.001 |
| Right SCG | 46, −6, 22 | 6.02 | <0.001 |
| Right PostCG | 50, −16, 34 | 6.44 | <0.001 |
| Left SMG | −52, −46, 30 | 6.04 | <0.001 |
| rACC | 16, 46, 4 | 5.96 | <0.001 |
| Left STc | −56, −12, 14 | 5.84 | <0.001 |
| Left DMPFc functional connectivity | |||
| MCC | 12, −8, 50 | Inf. | <0.001 |
| Left PCG | −12, −30, 70 | 7.83 | <0.001 |
| Right IPS | 44, −38, 56 | 7.56 | <0.001 |
| Left IPS | −40, −38, 52 | 7.44 | <0.001 |
| Right Ins | 48, 2, 16 | 7.32 | <0.001 |
| Left SFG | −24, −8, 60 | 7.27 | <0.001 |
| Left Prec | −14, −42, 70 | 7.16 | <0.001 |
| Left SPL | −26, −62, 56 | 6.74 | <0.001 |
| Right VLPFc | 38, 34, 22 | 6.98 | <0.001 |
| Right VMPFc | 14, 56, −14 | 6.51 | <0.001 |
| Left VMPFc | −8, 54, −12 | 5.91 | <0.001 |
| Right MOG | 42, −78, 14 | 7.32 | <0.001 |
| Left TPJ functional connectivity | |||
| Left SPL | −16, −66, 42 | 6.95 | <0.001 |
| Left IPL | −40, −52, 50 | 5.90 | <0.001 |
| ACC | −6, 38, 16 | 6.08 | <0.001 |
| Left SOG | −22, −74, 26 | 6.68 | <0.001 |
| Right SOG | 22, −80, 36 | 6.50 | <0.001 |
| Left MTc | 60, −16, −12 | 6.53 | <0.001 |
| Right MTc | −60, −40, 6 | 6.05 | <0.001 |
| Right SMA | 14, −8, 52 | 6.86 | <0.001 |
| Left SMA | −12, −8, 68 | 6.02 | <0.001 |
Hip, hippocampus; Inf., infinite; Ins, insula; IPL, inferior parietal lobe; MCC, middle cingulate cortex; MTc, medial temporal cortex; PCG, precentral gyrus; PostCG, postcentral gyrus; Prec, precuneus; rACC, rostral anterior cingulate cortex; SCG, subcentral gyrus; SFG, superior frontal gyrus; SMA, supplementary motor area; SMG, supramarginal gyrus; SOG, superior occipital gyrus; SPL, superior parietal lobe; STc, superior temporal cortex.
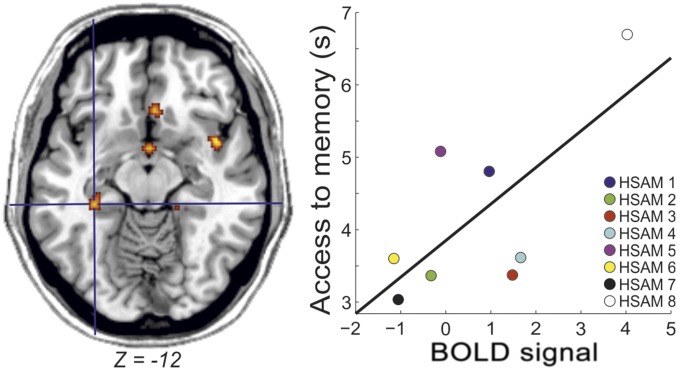
Finally, we investigated whether the brain activity related to access remote or recent memories covaried with access latencies or as a function of obsessive/compulsive tendencies in subjects with HSAM. We found that in subjects with HSAM (P = 0.026), but not in control subjects, the activity of the left hippocampus increased as a function of individual latencies to access remote memories (Fig. 4). No other effects were observed in the other ROIs or as a function of the individual scores of obsessiveness.
Fig. 4.
Increased activity in the left hippocampus as a function of increased latency to memory access in subjects with HSAM. BOLD, blood oxygen level-dependent.
Discussion
The subjects with HSAM were faster and more efficient in retrieving AMs. In contrast, they did not differ from control subjects in retrieving semantic information. The findings strongly suggest that the shorter latencies in providing AMs reflect superior access to details of past experiences on the part of subjects with HSAM. Additionally, the findings indicate that, in comparison to controls, the subjects with HSAM remembered more autobiographical details of their past experiences, consistent with extensive prior investigations of HSAM (11, 12), especially for the most remote AMs. The findings also confirmed those of previous research indicating that subjects with HSAM tend to express obsessive/compulsive symptoms. However, we did not find evidence in our HSAM sample that the individual level of obsessiveness is related to the memory access, in either response latency or underlying brain activity. While we estimated a reliable statistical power for the current experiment (Methods), we cannot exclude that smaller effect sizes may be detected with larger sample sizes. Therefore, future experiments will determine whether the null effects reported in this study reflect a lack of difference or limited statistical power.
The major aim of the present investigation was to determine whether HSAM is associated with enhanced activation of brain systems as assessed by fMRI. The findings provide supporting evidence. Cortical activity increased in several areas, selectively in association with autobiographical remembering, and the increase was greater in subjects with HSAM than in controls. During AM retrieval (irrespective to the access or reliving phase), compared with controls, twice as many brain areas were activated in subjects with HSAM. However, while it might be expected that the increased brain activity in HSAM is specifically devoted to memory reliving, given the richness of details provided by subjects with HSAM (3–5), we did not observe any neural difference between subjects with HSAM and control subjects during the reliving phase. In contrast, the findings suggest that the increase in neural activity was specifically involved in accessing AMs, recruiting a left-lateralized frontoparietal network (VMPFc, DMPFc, and TPJ) in subjects with HSAM only during memory access. Additionally, these HSAM-related regions showed enhanced functional coupling with brain areas crucial for memory retrieval selectively during memory access vs. reliving. These results suggest that HSAM may involve enhanced activation of specific brain areas involved in accessing representations of autobiographical experiences. One may argue that the quicker memory access of subjects with HSAM than control subjects might confound the fMRI analysis, requiring a comparison between different amounts of blood oxygen level-dependent signals (i.e., less signal for the access phase of HSAM than control subjects). Although the finding of greater brain activity in subjects with HSAM during memory access goes against this potential confound, future research will have to solve this possible limitation.
The enhanced AM access in individuals with HSAM involved increased brain activity within core regions of the frontoparietal cortex, namely, the medial prefrontal cortex and TPJ. These areas have been associated with the retrieval of autobiographical material (7, 13). AM retrieval is thought to be supported by an extensive network of brain regions, most pronounced in the left hemisphere (a meta-analysis is provided in ref. 14), that has typically been interpreted as reflecting the variety of cognitive processes engaged during AM retrieval (15, 16): executive control and retrieval monitoring (dorsolateral prefrontal cortex/DMFCc), episodic remembering (hippocampus), emotion-related processes (VLPFc and amygdala), self-processing (DMPFc/VMPFc, PCC), and visuospatial processing (retrosplenial cortex, precuneus, and parietal regions). Whereas the recruitment of the medial prefrontal cortex and TPJ reflects normal functioning of AM retrieval, the current findings provide evidence of increased activation of these regions associated with enhanced functioning of AM retrieval in individuals with HSAM.
The increased activation of medial prefrontal regions might be related to enhanced self-reference processes in individuals with HSAM. Recent literature reported consistent activations of both the ventral and dorsal prefrontal cortex during the engagement of self-referential processes in AM retrieval (17). Moscovitch and Winocur (18) suggested that the VMPFc is selectively involved with monitoring the “truthfulness” of AMs during retrieval, providing the feeling of having recollected the correct AM. The enhanced production of confabulation and false memories found in patients with an impaired VMPFc provides support for this suggestion (19). In contrast, increased activity in the DMPFc may reflect recall of experienced events (20). The enhanced activation of the medial prefrontal cortex found in subjects with HSAM during AM retrieval is in line with evidence of an increased propensity of individuals with HSAM to express self-referential processes as well as mental rumination of their prior experiences (10–12).
The present design allowed differentiating brain activity related to retrieval of remote vs. more recent AMs. We found a selective increased activity in the VMPFc during access to remote AMs in subjects with HSAM. Bonnici and Maguire (21) reported evidence that the VMPFc in normal subjects is implicated in memory representation of events up to 2 y of remoteness, but not for more remote events. Here, we found instead an extended temporal window up to 20 y (Fig. 1D) in which the VMPFc contributes to access AMs (and, specifically, to the most remote AM details, in line with the behavioral data; Fig. 1E) in subjects with HSAM. The current findings also indicate that the VMPFc is functionally connected with the right hippocampus during AM access of individuals with HSAM. Extensive findings of both human and animal subjects have suggested that functional coupling between these two regions is essential for episodic/long-term memory retrieval (22, recently reviewed in refs. 23, 24). Consistently, the present findings suggest that enhanced prefrontal/hippocampal coupling sustains enhanced memory performance in individuals with HSAM. This evidence is consistent with the findings of a single HSAM case study, indicating greater than usual connectivity of the left hippocampus with prefrontal, but also premotor, and retrosplenial cingulate cortex (25).
Importantly, an opposite pattern of results (i.e., decreased neural activity of the VMPFc and hippocampus) was found in subjects with severely deficient AMs (SDAMs). Palombo et al. (8) reported that three subjects with SDAMs, who had otherwise preserved cognitive functions, expressed decreased neural activity in the left VMPFc and reduced hippocampal volume. The evidence that the VMPFc and hippocampus play a key role in both subjects with impaired AM (8) and normal subjects (7, 13–15), as well as in subjects with HSAM, suggests that the current level of prefrontal/hippocampal activity may play a critical role in determining the hypofunctioning (i.e., SDAM) vs. hyperfunctioning (i.e., HSAM) of AM retrieval. Although the increased hippocampal activity in subjects with HSAM might potentially reflect task-related encoding activity (26), the fact that the hippocampal activity increased as a function of longer latencies (indicating increased difficulty) to access the most remote (first-time) events appears to indicate a selective role of the hippocampus in AM retrieval. This latter finding is in agreement with the hypothesis that AMs might permanently depend on the hippocampal activity (27). Together with the VMPFc, the hippocampus therefore appears to enable subjects with HSAM to have faster and more detailed remote memory access (21).
In addition to enhanced prefrontal/hippocampal functional connectivity, memory access in subjects with HSAM was further supported by increased activity of the ventral parietal cortex (left TPJ) during AM retrieval. A growing body of evidence indicates that TPJ lesions entail dysfunctions related to self vs. other distinctions (28). The increased activation of TPJ in HSAM might therefore be linked to an increased capability of subjects with HSAM to select the correct AMs, better distinguishing between facts experienced by self or others. However, the findings also suggest a more parsimonious interpretation (29, 30). TPJ activity during AM access in subjects with HSAM might reflect internal attentional capture driven by information reactivated from long-term memory by the search mechanisms (i.e., the prefrontal/hippocampal cortex). The functional coupling between the left TPJ and the visual and auditory sensory cortex is consistent with this “attentional” account. Recent findings revealed the causal role played by the TPJ in the modulation of sensory representations (31, 32), as well as in mental imagery (33). Accordingly, after internal focusing on reactivated memories, the TPJ might contribute to activate and maintain sensory representations in visual and auditory cortex, triggering visual/auditory imagery (34, 35). These TPJ-centered mechanisms might contribute to the enhanced memory performance of individuals with HSAM, allowing these subjects to check, as early as during AM access, the validity of recollected AMs through visual/auditory imagery. This possibility is consistent with the prevailing view that episodic memories are based (at least in part) on the reactivation of the sensory representation developed at encoding (36, 37). However, previous findings suggest that imagery-related activity in sensory cortex occurs after full access to the memory trace in normal subjects, progressively increasing during explicit reliving of memory details (7, 38). In contrast, the present findings indicate that in individuals with HSAM, the recruitment of neural resources possibly devoted to visual or auditory imagery [i.e., the visual and auditory sensory cortices (34, 35)] is anticipated in the access phase, thus contributing to their enhanced memory performance.
These findings have identified brain activation that differs in subjects with HSAM and control subjects, and they suggest that the differential activation may play a role in enabling more efficient access, with subsequent enhanced retrieval, to autobiographical information. These findings provide targets for brain stimulation and/or therapeutic interventions to enhance memory retrieval in conditions related to altered memory functioning.
Methods
Participants.
Eight individuals with HSAM (five male, mean age = 32.5 y, age range: 24–37 y) recruited in accordance with the previous literature (4) (SI Appendix) and 21 control subjects (10 male, mean age = 32.5 y, age range: 24–39 y) participated in the study. All participants gave written consent to participate in the study, which was approved by the independent Ethics Committee of the Santa Lucia Foundation (CE/PROG.540). Before conducting the experiment, we performed a power analysis that estimated a reliable statistical power of 84% for our sample size (eight subjects with HSAM plus a minimum of 20 controls) based on an effect size of 0.5, in line with those reported by the previous literature on HSAM (10), and a significance level of 0.05.
Task and Stimuli.
During scanning, participants were asked to retrieve AMs and non-AMs (SMs) (Fig. 1A and SI Appendix). The experiment included three functional runs, each including 18 memory cues: 12 AM trials (six first-time and six last-time events) and six SM trials, and a variable intertrial interval (2–3 s, uniformly distributed). After scanning, participants were asked to provide details about memories retrieved during the experiment (SI Appendix).
MRI and fMRI Data Analysis.
A Siemens Allegra (Siemens Medical Systems) operating at 3 T and equipped for echoplanar imaging was used to acquire the fMRI scans (SI Appendix). We used SPM12 (Wellcome Department of Cognitive Neurology) implemented in MATLAB 7.4 (The MathWorks, Inc.) for data preprocessing (SI Appendix) and statistical analyses. Each participant underwent three fMRI runs, each comprising 477 volumes. Statistical inference was based on a two-step random effects approach (SI Appendix). Briefly, the first-level models included separated access and reliving regressors (6) for each of the three trial types: first AM, last AM, and SM. For each subject, we estimated contrast images that removed the activity associated with access to and reliving of SMs (control condition) to the main AM conditions. For the second-level group analysis, the single-subject contrast images of parameter estimates were entered into a mixed-design ANOVA with group (HSAM vs. control) as a between-subjects variable and phase (access vs. reliving) and AM type (first vs. last) as within-subjects variables. First of all, we highlighted the regions involved with AMs in the HSAM vs. control group (and vice versa), irrespective of phase and AM type. As an additional constraint, we considered only voxels showing an overall activation across all conditions and groups (T-contrast, P-uncorrected = 0.001), ensuring that we selected only regions activated by AM retrieval (e.g., ref. 39). The statistical threshold was set to P-family-wise error (FWE)–corrected < 0.05 at the voxel level, considering the whole brain as the volume of interest. This comparison allowed us to highlight different circuits recruited by AM retrieval in the HSAM and control groups (Fig. 2A and Table 1). The resulting activations were used to define ROIs that were then used to test for condition-specific effects in interaction with the group variable (i.e., the two-way phase × group and AM type × group interactions and the three-way phase × AM type × group interaction). For this, we considered spheres (8-mm radius, matching the FWHM of the smoothing filter) centered on the regions activated by AM retrieval in the two groups (Table 1) as the volume of interest (small volume correction) (40).
Functional connectivity analysis.
The procedure described above allowed us to identify three regions selectively involved in AM access in subjects with HSAM (i.e., the significant group × phase interaction), namely, the left VMPFc, left DMPFc, and left TPJ (Fig. 2B). Given that these seed regions are related to HSAM, we did not expect any increased functional connectivity in the control group. The main goal of this analysis was to understand whether additional neural resources supported access to memory in individuals with HSAM (SI Appendix).
ROI correlations with memory access latencies and obsessiveness scores in subjects with HSAM.
Finally, we conducted exploratory analyses using multiple regression models to investigate whether the brain activity related to the access to remote or recent memories covaried as a function of the individual latency to access memories or as a function of obsessive/compulsive traits in subjects with HSAM (SI Appendix).
Supplementary Material
Acknowledgments
We thank Bruna Rubino for helping with telephone interviews and Dr. Flavia Chiarotti for helping with power analysis. Supported by the Italian Ministry of Education, University and Research, MIUR Grants PNRA16_00047 (to V.S. and S.M.) and PRIN_2015SKN9YT_002 (to P. Campolongo).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1802730115/-/DCSupplemental.
References
- 1.Allen TA, Fortin NJ. The evolution of episodic memory. Proc Natl Acad Sci USA. 2013;110:10379–10386. doi: 10.1073/pnas.1301199110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenberg DL, Rubin DC. The neuropsychology of autobiographical memory. Cortex. 2003;39:687–728. doi: 10.1016/s0010-9452(08)70860-8. [DOI] [PubMed] [Google Scholar]
- 3.Parker ES, Cahill L, McGaugh JL. A case of unusual autobiographical remembering. Neurocase. 2006;12:35–49. doi: 10.1080/13554790500473680. [DOI] [PubMed] [Google Scholar]
- 4.LePort AK, et al. Behavioral and neuroanatomical investigation of highly superior autobiographical memory (HSAM) Neurobiol Learn Mem. 2012;98:78–92. doi: 10.1016/j.nlm.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LePort AK, Stark SM, McGaugh JL, Stark CE. A cognitive assessment of highly superior autobiographical memory. Memory. 2017;25:276–288. doi: 10.1080/09658211.2016.1160126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Daselaar SM, et al. The spatiotemporal dynamics of autobiographical memory: Neural correlates of recall, emotional intensity, and reliving. Cereb Cortex. 2008;18:217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- 7.Cabeza R, St Jacques P. Functional neuroimaging of autobiographical memory. Trends Cogn Sci. 2007;11:219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Palombo DJ, Alain C, Söderlund H, Khuu W, Levine B. Severely deficient autobiographical memory (SDAM) in healthy adults: A new mnemonic syndrome. Neuropsychologia. 2015;72:105–118. doi: 10.1016/j.neuropsychologia.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Levine B, Svoboda E, Hay JF, Winocur G, Moscovitch M. Aging and autobiographical memory: Dissociating episodic from semantic retrieval. Psychol Aging. 2002;17:677–689. [PubMed] [Google Scholar]
- 10.LePort AK, Stark SM, McGaugh JL, Stark CE. Highly superior autobiographical memory: Quality and quantity of retention over time. Front Psychol. 2016;6:2017. doi: 10.3389/fpsyg.2015.02017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McGaugh JL. Making lasting memories: Remembering the significant. Proc Natl Acad Sci USA. 2013;110:10402–10407. doi: 10.1073/pnas.1301209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGaugh JL. Highly superior autobiographical memory. In: Byrne JH, editor. Learning and Memory: A Comprehensive Reference. 2nd Ed. Academic; Oxford: 2017. pp. 137–145. [Google Scholar]
- 13.Maguire EA. Neuroimaging studies of autobiographical event memory. Philos Trans R Soc Lond B Biol Sci. 2001;356:1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fossati P. Imaging autobiographical memory. Dialogues Clin Neurosci. 2013;15:487–490. doi: 10.31887/DCNS.2013.15.4/pfossati. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrews-Hanna JR, Saxe R, Yarkoni T. Contributions of episodic retrieval and mentalizing to autobiographical thought: Evidence from functional neuroimaging, resting-state connectivity, and fMRI meta-analyses. Neuroimage. 2014;91:324–335. doi: 10.1016/j.neuroimage.2014.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martinelli P, Sperduti M, Piolino P. Neural substrates of the self-memory system: New insights from a meta-analysis. Hum Brain Mapp. 2013;34:1515–1529. doi: 10.1002/hbm.22008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moscovitch M, Winocur G. The frontal cortex and working with memory. In: Stuss DTE, Knight RTE, editors. Principles of Frontal Lobe Function. Oxford Univ Press; New York: 2002. pp. 188–209. [Google Scholar]
- 19.Turner MS, Cipolotti L, Yousry TA, Shallice T. Confabulation: Damage to a specific inferior medial prefrontal system. Cortex. 2008;44:637–648. doi: 10.1016/j.cortex.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Summerfield JJ, Hassabis D, Maguire EA. Cortical midline involvement in autobiographical memory. Neuroimage. 2009;44:1188–1200. doi: 10.1016/j.neuroimage.2008.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnici HM, Maguire EA. Two years later–Revisiting autobiographical memory representations in vmPFC and hippocampus. Neuropsychologia. 2018;110:159–169. doi: 10.1016/j.neuropsychologia.2017.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheldon S, Levine B. The medial temporal lobe functional connectivity patterns associated with forming different mental representations. Hippocampus. 2018;28:269–280. doi: 10.1002/hipo.22829. [DOI] [PubMed] [Google Scholar]
- 23.Jin J, Maren S. Prefrontal-hippocampal interactions in memory and emotion. Front Syst Neurosci. 2015;9:170. doi: 10.3389/fnsys.2015.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sheldon S, Levine B. The role of the hippocampus in memory and mental construction. Ann N Y Acad Sci. 2016;1369:76–92. doi: 10.1111/nyas.13006. [DOI] [PubMed] [Google Scholar]
- 25.Brandt J, Bakker A. Neuropsychological investigation of “the amazing memory man”. Neuropsychology. 2018;32:304–316. doi: 10.1037/neu0000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wixted JT, et al. Coding of episodic memory in the human hippocampus. Proc Natl Acad Sci USA. 2018;115:1093–1098. doi: 10.1073/pnas.1716443115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- 28.Eddy CM. The junction between self and other? Temporo-parietal dysfunction in neuropsychiatry. Neuropsychologia. 2016;89:465–477. doi: 10.1016/j.neuropsychologia.2016.07.030. [DOI] [PubMed] [Google Scholar]
- 29.Cabeza R, Ciaramelli E, Moscovitch M. Cognitive contributions of the ventral parietal cortex: An integrative theoretical account. Trends Cogn Sci. 2012;16:338–352. doi: 10.1016/j.tics.2012.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cabeza R, Ciaramelli E, Olson IR, Moscovitch M. The parietal cortex and episodic memory: An attentional account. Nat Rev Neurosci. 2008;9:613–625. doi: 10.1038/nrn2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beauchamp MS, Sun P, Baum SH, Tolias AS, Yoshor D. Electrocorticography links human temporoparietal junction to visual perception. Nat Neurosci. 2012;15:957–959. doi: 10.1038/nn.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fiori F, Candidi M, Acciarino A, David N, Aglioti SM. The right temporoparietal junction plays a causal role in maintaining the internal representation of verticality. J Neurophysiol. 2015;114:2983–2990. doi: 10.1152/jn.00289.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grol M, Vingerhoets G, De Raedt R. Mental imagery of positive and neutral memories: A fMRI study comparing field perspective imagery to observer perspective imagery. Brain Cogn. 2017;111:13–24. doi: 10.1016/j.bandc.2016.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Huijbers W, Pennartz CM, Rubin DC, Daselaar SM. Imagery and retrieval of auditory and visual information: Neural correlates of successful and unsuccessful performance. Neuropsychologia. 2011;49:1730–1740. doi: 10.1016/j.neuropsychologia.2011.02.051. [DOI] [PubMed] [Google Scholar]
- 35.Kraemer DJ, Macrae CN, Green AE, Kelley WM. Musical imagery: Sound of silence activates auditory cortex. Nature. 2005;434:158. doi: 10.1038/434158a. [DOI] [PubMed] [Google Scholar]
- 36.Folkerts S, Rutishauser U, Howard MW. Human episodic memory retrieval is accompanied by a neural contiguity effect. J Neurosci. 2018;38:4200–4211. doi: 10.1523/JNEUROSCI.2312-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xue G. The neural representations underlying human episodic memory. Trends Cogn Sci. 2018;22:544–561. doi: 10.1016/j.tics.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Rubin DC. The basic-systems model of episodic memory. Perspect Psychol Sci. 2006;1:277–311. doi: 10.1111/j.1745-6916.2006.00017.x. [DOI] [PubMed] [Google Scholar]
- 39.Büchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. Neuroimage. 1998;8:140–148. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- 40.Worsley KJ, et al. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.