Significance
Reactive oxygen species (ROS) cause oxidative stress and damage in many pathological conditions, but they can also function as signaling molecules in physiological processes. It is difficult, however, to decipher where ROS come from and which ROS are involved in these processes. In this article, we demonstrate that a NADPH oxidase (NOX) and an extracellular superoxide dismutase (SOD3) function in follicle cells of Drosophila egg chambers to produce hydrogen peroxide, which regulates follicle rupture and ovulation, a process essential for reproduction. NOX and SOD3 are expressed in human follicles and could potentially play similar roles in humans. Our work thus provides potential targets for treating ROS-related infertility or developing novel contraceptive approaches.
Keywords: NADPH oxidase, superoxide dismutase, hydrogen peroxide, ovulation, octopamine
Abstract
Ovarian reactive oxygen species (ROS) are believed to regulate ovulation in mammals, but the details of ROS production in follicles and the role of ROS in ovulation in other species remain underexplored. In Drosophila ovulation, matrix metalloproteinase 2 (MMP2) is required for follicle rupture by degradation of posterior follicle cells surrounding a mature oocyte. We recently demonstrated that MMP2 activation and follicle rupture are regulated by the neuronal hormone octopamine (OA) and the octopamine receptor in mushroom body (OAMB). In the current study, we investigated the role of the superoxide-generating enzyme NADPH oxidase (NOX) in Drosophila ovulation. We report that Nox is highly enriched in mature follicle cells and that Nox knockdown in these cells leads to a reduction in superoxide and to defective ovulation. Similar to MMP2 activation, NOX enzymatic activity is also controlled by the OA/OAMB-Ca2+ signaling pathway. In addition, we report that extracellular superoxide dismutase 3 (SOD3) is required to convert superoxide to hydrogen peroxide, which acts as the key signaling molecule for follicle rupture, independent of MMP2 activation. Given that Nox homologs are expressed in mammalian follicles, the NOX-dependent hydrogen peroxide signaling pathway that we describe could play a conserved role in regulating ovulation in other species.
Ovulation is a key step in animal reproduction and involves multiple endocrine, paracrine, and autocrine signaling molecules, such as progesterone, epidermal growth factors, and prostaglandins. These molecules ultimately activate proteinases that break down the ovarian follicle wall, releasing a fertilizable oocyte (1–3). Several lines of evidence indicate that reactive oxygen species (ROS) also play indispensable roles in mammalian ovulation (4–8). However, there is no genetic evidence to support an in vivo role of ROS in ovulation, and the enzymes responsible for ROS production during ovulation are still unknown.
ROS are oxygen-derived, chemically reactive small molecules and include superoxide anion (O2•−), hydrogen peroxide (H2O2), and hydroxyl radicals (OH•) (9). The physiological generation of ROS can occur as a byproduct of aerobic metabolism or as the primary function of the family of NADPH oxidases (NOXs). NOX enzymes transfer an electron across the cell membrane from NADPH in the cytosol to oxygen (O2) in the luminal or extracellular space. This movement of an electron generates O2•−, which can be rapidly converted into H2O2 by superoxide dismutases (SODs).
The mammalian NOX family comprises seven members (NOX1–5 and DUOX1–2), which have marked differences in tissue distribution and play a variety of physiological roles (10, 11). Members of this family are also expressed in mammalian ovaries. Nox4 and Nox5, for example, are expressed in human granulosa cells (12). NOX4 and its accessory proteins in human granulosa cells show age-dependent reductions in protein expression, which correlates with low fertility (13). Importantly, pharmacological inhibition of NOX enzymes blocks follicle-stimulating hormone-induced oocyte maturation in mouse cumulus–oocyte complex in vitro (14). Despite these observations, a role for NOX in mammalian ovulation has not been demonstrated.
The NOX family of enzymes is evolutionarily conserved across species (15). The Drosophila genome contains one Nox gene encoding NOX and one Duox gene encoding DUOX. DUOX has an additional peroxidase domain and has been well studied in gut–microbe interaction, wing formation, and wound healing (16–18). Much less is known about Nox. Earlier work reported that Nox regulates ovarian muscle contraction, which somehow influences ovulation (19). However, the mechanism of NOX regulation of ovulation and the cellular localization of NOX in Drosophila remain unclear.
Recent work challenges the concept that ovulation is controlled by ovarian muscle contraction in Drosophila. Instead, Drosophila ovulation involves active proteolytic degradation of the follicle wall and follicle rupture and shares much in common with mammalian ovulation. Like in mammals, each oocyte in Drosophila is encapsulated in a layer of somatic follicle cells to form an egg chamber, which develops through 14 distinct stages to become a mature follicle (stage-14 egg chamber) in ovarioles (20). In mature follicles, the zinc finger transcription factor Hindsight (HNT) induces the expression of matrix metalloproteinase 2 (MMP2) in posterior follicle cells and octopamine receptor in mushroom body (OAMB) in all follicle cells (21). During ovulation, octopamine (OA) is released from neuron terminals in the ovary and binds to its receptor OAMB in stage-14 follicle cells. OAMB receptor activation causes an increase in intracellular calcium that activates MMP2 enzymatic activity, which breaks down posterior follicle cells and induces follicle rupture (22, 23). Strikingly, the entire process of follicle rupture can be recapitulated ex vivo by culturing isolated mature follicles with OA in the absence of ovarian muscles and oviducts (23). This work casts doubt on the proposed involvement of ovarian muscles in follicle rupture/ovulation.
In this study, we investigated the role of Nox in Drosophila ovulation. To our surprise, we found that ovarian muscle Nox does not play a major role in ovulation but rather that Nox is enriched in mature follicle cells and is essential for follicle rupture/ovulation. OA/OAMB-Ca2+ signaling activates NOX enzymatic activity to produce extracellular O2•−, which is converted into H2O2 by an extracellular SOD3. Our results suggest that NOX-produced ROS in mature follicles play a conserved role in regulating follicle rupture/ovulation across species.
Results
NOX Functions in Mature Follicle Cells for Drosophila Ovulation.
Previous work indicated that NOX functions in ovarian muscles to control muscle contraction and ovulation (19). However, a careful examination of the Gal4 drivers used previously (SI Appendix, Fig. S1) and our observation of almost-normal egg laying by females with Nox knockdown in muscles (SI Appendix, Table S1) indicated that ovarian muscle NOX does not likely play a major role in ovulation. Microarray and RNA-sequencing analysis (24, 25) showed that Nox is enriched in stage-13/14 egg chambers but not in activated oocytes (SI Appendix, Fig. S2A). RT-PCR analysis of isolated follicle cells and oocytes from mature follicles (SI Appendix, Fig. S2B) further supports that Nox is enriched in follicle cells.
To probe the function of follicular NOX in late oogenesis and ovulation, we knocked down Nox in mature follicle cells. We used two independent RNA interference (RNAi) lines driven by two well-characterized Gal4 drivers, 47A04-Gal4 and 44E10-Gal4 (21–23, 26). 44E10-Gal4 is specifically expressed in follicle cells of all stage-14 egg chambers, whereas 47A04-Gal4 is only expressed in follicle cells of late-stage-14 egg chambers (21). Both Nox-RNAi lines significantly reduced Nox mRNA levels in mature follicles, with Nox-RNAi1 showing a more potent reduction (Fig. 1A). Females expressing Nox-RNAi were subjected to an egg-laying assay and showed a significant reduction in their ability to lay eggs, indicating that Nox in mature follicle cells is required for efficient egg laying (Fig. 1B). This egg-laying defect in Nox-knockdown females is not likely to be due to an oogenesis problem, as ovaries from these females contained normal or even higher numbers of mature follicles (Fig. 1C).
Fig. 1.
NOX functions in mature follicle cells for ovulation. (A) qRT-PCR quantification of Nox mRNA in mature follicles from females of control and Nox-i driven by 47A04-Gal4 or 44E10-Gal4. (B) Quantification of egg laying from control and Nox-i females. Also see SI Appendix, Table S2 for the number of females analyzed. (C) Quantification of mature follicles in each female’s ovaries after egg laying. The numbers of females used in each genotype are 44, 24, 43, 73, 48, and 42. (D) The egg-laying time in control or Nox-i females driven by 47A04-Gal4 (Left) or 44E10-Gal4 (Right). Also see SI Appendix, Table S2. (E) Quantification of follicle rupture after 3-h culture with 20 μM OA. The numbers of mature follicles used in each genotype are 816, 601, 275, 569, 330, and 387. *P < 0.05, **P < 0.01, ***P < 0.001. Nox-i, Nox-RNAi.
Next, we examined whether Nox-knockdown females are defective in ovulation and/or oviposition (the process of laying down eggs). Females with Nox knockdown (particularly with Nox-RNAi1) took a much longer time to ovulate than control females, indicating an ovulation defect (Fig. 1D and SI Appendix, Table S2). Together, these data suggest that Nox in mature follicle cells is required for normal ovulation.
To determine whether Nox regulates follicle rupture, a process induced by follicular OA/OAMB signaling during ovulation (23), we cultured Nox-knockdown follicles ex vivo by OA stimulation. Consistent with previous results (21), control follicles isolated based on 47A04 and 44E10 expression showed 76% and 39% rupture, respectively, after a 3-h culture with OA (Fig. 1E). The difference in rupture rate is due to the fact that 47A04 is expressed only in fully matured follicles (21). By contrast, Nox-knockdown follicles showed a significant reduction in OA-induced follicle rupture (Fig. 1E and SI Appendix, Fig. S2 C–H), indicating that Nox is required for normal follicle rupture. Consistent with this conclusion, pretreatment of mature follicles with diphenyleneiodonium (DPI) or VAS2870, potent NOX enzymatic inhibitors (27), was sufficient to inhibit OA-induced follicle rupture in a dose-dependent manner (SI Appendix, Fig. S2 I and J). Furthermore, the addition of butylated hydroxyanisole (BHA), a broad-spectrum ROS scavenger, in the culture medium also inhibited OA-induced follicle rupture (SI Appendix, Fig. S2K). Together, these data suggest that NOX functions in mature follicle cells to promote OA-induced follicle rupture and ovulation.
NOX Does Not Interfere with the OA/OAMB-Ca2+–MMP2 Pathway.
OA/OAMB signaling in mature follicle cells leads to an intracellular Ca2+ rise and MMP2 activation (23). To determine whether NOX functions upstream of the Ca2+ rise in the OA/OAMB-Ca2+–MMP2 pathway, we used ionomycin, a potent Ca2+ ionophore, to stimulate follicle rupture directly. More than 90% of control follicles ruptured after a 3-h ionomycin stimulation, in contrast to 70% (in the case of 47A04) and 40–60% (in the case of 44E10) of Nox-knockdown follicles (Fig. 2A and SI Appendix, Fig. S3 A–F). This defect was more obvious when examined before the end of the 3-h culture (Fig. 2B). These data suggest that Nox regulates molecules downstream of Ca2+ in the OA/OAMB-Ca2+–MMP2 pathway, or alternatively that Nox regulates a different pathway for follicle rupture that is independent from MMP2.
Fig. 2.
NOX does not interfere with the OA/OAMB-Ca2+–MMP2 pathway. (A) Quantification of follicle rupture after 3-h culture with 5 μM ionomycin. The numbers of follicles used in each genotype are 199, 134, 111, 446, 357, and 268. (B) Cumulative follicle rupture in 3 h in response to ionomycin stimulation. Mature follicles were isolated according to 47A04-Gal4 and three groups of each genotype (∼90 follicles) were used. (C and D) Representative images show MMP2::GFP expression (green in C and D and white in Insets) in mature follicles of control (C) and Nox-i1 (D) driven by 44E10-Gal4. The mature follicle cells are marked by 44E10-Gal4 driving UAS-RFP (44E10>RFP; red in C and D). Only the posterior portions of the follicles are shown. DAPI (blue in C and D) is used to mark nuclei. (E) Quantification of posterior MMP activity in control and Nox-i mature follicles with 47A04-Gal4 or 44E10-Gal4 after 3-h culture with OA using in situ zymography. The numbers of mature follicles used in each genotype are 556, 539, 322, 478, 466, and 259. (F) Representative images show three categories of BM configurations (according to Vkg::GFP expression in green) in isolated mature follicles. (G) Quantification of BM configuration of isolated mature follicles from control or Nox-i1 females with 44E10-Gal4. (H) Quantification of follicle rupture after treatment with or without 20 nM 20E for 30 min followed by a 6-h OA culture. The numbers of mature follicles used in each genotype are 327, 355, 324, 367, 169, and 226. **P < 0.01, ***P < 0.001. BM, basement membrane; Iono, ionomycin; Nox-i, Nox-RNAi.
To differentiate between these two hypotheses, we measured MMP2 expression and activation in Nox-knockdown follicles. MMP2 protein is properly expressed in posterior follicle cells of Nox-knockdown egg chambers (Fig. 2 C and D). In situ zymography showed that Nox-knockdown follicles had slightly reduced MMP activation following OA stimulation (Fig. 2E and SI Appendix, Fig. S3 G–L); however, there were no differences in collagen IV [a target of MMP2 (21), encoded by Viking (Vkg)] between control and Nox-knockdown follicles (Fig. 2 F and G). These data suggest that MMP2 is unlikely to be a major downstream effector of NOX in the follicle rupture process. Consistent with this, Mmp2 mRNA and genes regulating Mmp2 expression and activation, including Oamb and Hnt (21), were not down-regulated in Nox-knockdown follicles (SI Appendix, Fig. S3M).
ROS regulate steroid progesterone production during mammalian ovulation (7). In addition, parallel ecdysteroid signaling is required for Drosophila ovulation (26). To determine whether NOX interferes with ecdysteroid production in mature follicle cells, we attempted to rescue the rupture defect of Nox-knockdown follicles with 20-hydroxyecdysone (20E). As previously reported, the addition of 20E partially rescues the defect of shd-knockdown follicles (26), which lack the ability to convert E to 20E. By contrast, the addition of 20E had no effect on the ability of Nox-knockdown follicles to respond to OA-induced rupture (Fig. 2H). It is thus unlikely that NOX affects 20E production. In addition, receptors for ecdysteroid signaling were not affected in Nox-knockdown follicles (SI Appendix, Fig. S3N). Given that ecdysteroid signaling strongly interferes with OA-induced MMP2 activation, we believe that NOX does not interfere with ecdysteroid signaling. Together, these data suggest that NOX regulates an unidentified target/pathway for follicle rupture.
OA Activates NOX in Mature Follicle Cells to Produce Superoxide.
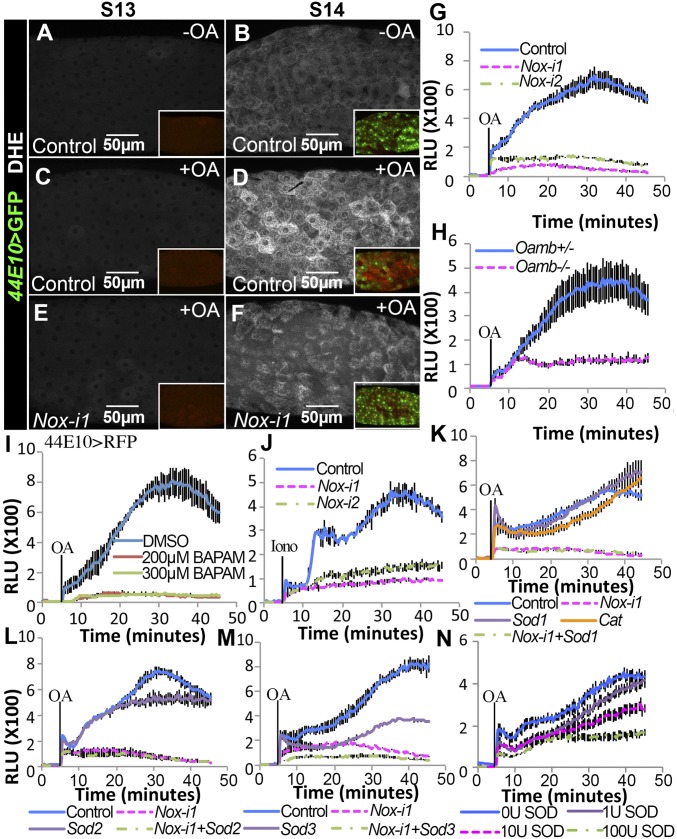
Although NOX does not interfere with the OA/OAMB-Ca2+–MMP2 pathway, OA/OAMB signaling may still regulate the enzymatic activity of NOX, as its N-terminal region contains EF-hand domains for Ca2+ binding. To test this hypothesis, we examined O2•− production in follicle cells upon OA stimulation. The fluorescent signal of dihydroethidium (DHE), a specific O2•− indicator (28, 29), was dramatically increased in stage-14 follicle cells throughout the entire egg chamber after OA stimulation, but not in stage-13 follicle cells (Fig. 3 A–D). This increase was blocked in Nox-knockdown follicle cells (Fig. 3 E and F). To quantify O2•− production in mature follicles, we developed a luminescence assay based on the dye l-012, which has been used to detect O2•− in ovaries previously (19). Consistent with DHE staining, OA induced a sharp increase in O2•− production in control follicles, which peaked at ∼30–40 min (Fig. 3G). In contrast, the increase in O2•− production was significantly dampened in Nox-knockdown follicles (Fig. 3G) or follicles treated with the NOX inhibitor DPI or the ROS scavenger BHA (SI Appendix, Fig. S4A). In addition, when we used entire ovaries to measure OA-induced O2•− production, Nox knockdown in mature follicle cells almost completely blocked the OA-induced O2•− production (SI Appendix, Fig. S4B). This finding indicates that OA-induced O2•− production is mainly restricted to mature follicle cells and depends on NOX. Thus, these data suggest that OA activates NOX in mature follicle cells to generate O2•−. Not surprisingly, OA-induced O2•− production required OAMB (Fig. 3H). In addition, chelating the intracellular Ca2+ with 1,2-Bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester) (BAPTA-AM) blocked OA-induced O2•− production (Fig. 3I), and ionomycin was sufficient to induce O2•− production in a NOX-dependent manner (Fig. 3J). These results suggest that follicular adrenergic signaling induces an intracellular Ca2+ rise, which activates NOX enzymatic activity in all mature follicle cells, in addition to MMP2 enzymatic activity in posterior follicle cells, during Drosophila ovulation.
Fig. 3.
OA activates NOX to produce superoxide extracellularly. (A–F) Representative images show DHE staining (white in A–F) in control (A–D) and Nox-i1 (E and F) follicles after 30-min culture without (A and B) or with (C–F) OA stimulation. The Insets are low-magnification images with 44E10>GFP expression (green, marking stage-14 follicles) and DHE staining (red). (G–N) l-012 Luminescence-dependent O2•− quantification in mature follicles stimulated with OA (G–I and K–N) or ionomycin (J) at the 5-min time point. Mature follicles with different genotypes were isolated according to 44E10>RFP expression. Mature follicles in I were pretreated with BAPTA-AM for 30 min before l-012 detection. Mature follicles in N were supplemented with SOD extract from bovine erythrocytes in the culture medium. Iono, ionomycin; Nox-i, Nox-RNAi; RLU, relative luminometer unit.
NOX Functions to Produce Superoxide Extracellularly.
It is unknown where NOX is localized subcellularly in mature follicle cells, as a NOX antibody is not available. To probe where NOX is localized to produce O2•− for follicle rupture, we overexpressed three distinct Sods—cytoplasmic Sod1 (30), mitochondrial Sod2 (31), and extracellular Sod3 (32, 33)—in mature follicle cells to dismutate O2•− into H2O2. Superoxide can hardly diffuse through cell membranes; thus, subcellularly localized SOD is required to dismutate O2•−. Overexpression of Sod1 in mature follicle cells did not reduce the amount of O2•− generated by OA stimulation (Fig. 3K), nor did overexpression of Sod2 (Fig. 3L). In contrast, overexpression of Sod3 significantly reduced the amount of OA-induced O2•− in mature follicles (Fig. 3M). We also confirmed that ectopic SOD3 is indeed secreted into the extracellular space (SI Appendix, Fig. S5 A–C). Furthermore, the addition of SOD extract from bovine erythrocytes in the culture medium was sufficient to reduce OA-induced O2•− in a dose-dependent manner (Fig. 3N). These data not only confirm the specificity of l-012 for O2•− detection but also suggest that NOX produces extracellular O2•−, which can be dismutated by extracellular SOD3 but not cytoplasmic SOD1 or mitochondrial SOD2.
H2O2, but Not Superoxide, Is the Key Signaling Molecule for Follicle Rupture.
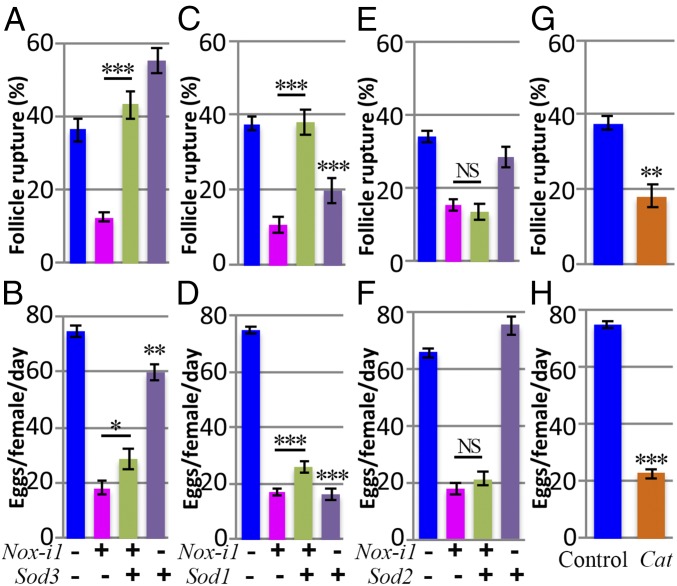
Despite the fact that NOX regulates follicle rupture by generating O2•−, which can be quickly converted to H2O2 by SOD3, it is still unknown whether O2•− or its derivative H2O2 is the signaling molecule responsible for follicle rupture. We reasoned that if O2•− is the signaling molecule for follicle rupture, overexpression of Sod3 in WT or Nox-knockdown follicles, which reduces or further reduces the O2•− level (Fig. 3M), would lead to defective rupture or an enhanced rupture defect, respectively. By contrast, overexpression of Sod1 or Sod2, which did not affect the O2•− level, would have a minimal effect. To our surprise, mature follicles with Sod3 overexpression alone had normal or even better follicle rupture in response to OA stimulation, and Sod3 overexpression in the Nox-knockdown follicles fully rescued the defect of OA-induced follicle rupture (Fig. 4A). This result indicates that H2O2, but not O2•−, is likely the signaling molecule for follicle rupture. Unfortunately, Sod3 overexpression only partially rescued the egg-laying defect of Nox-knockdown females (Fig. 4B). This could be due to an insufficient amount of O2•− converted to H2O2 to execute normal physiology or because O2•− plays other roles in the egg-laying process in addition to being converted to H2O2 for follicle rupture/ovulation.
Fig. 4.
H2O2 but not superoxide is the key signaling molecule for follicle rupture. (A and B) Quantification of OA-induced follicle rupture (A) and egg laying (B) using females with 44E10-Gal4 driving Nox-i1 and/or Sod3::3xHA expression. The numbers of follicles used in A are 349, 354, 325, and 283, while the numbers of females used in B are 45, 50, 40, and 20. (C and D) Quantification of OA-induced follicle rupture (C) and egg laying (D) using females with 44E10-Gal4 driving Nox-i1 and/or Sod1 expression. The numbers of follicles in C are 445, 355, 357, and 341, while the numbers of females in D are 75, 50, 50, and 50. (E and F) Quantification of OA-induced follicle rupture (E) and egg laying (F) using females with 44E10-Gal4 driving Nox-i1 and/or Sod2 expression. The numbers of follicles used in E are 174, 182, 178, and 162, while the number of females used in F is 25 for each genotype. (G and H) Quantification of OA-induced follicle rupture (G) and egg laying (H) using females with 44E10-Gal4 driving Cat expression. The numbers of mature follicles used in each genotype in G are 445 and 256. The number of females used in H is 50 for each genotype. *P < 0.05, **P < 0.01, ***P < 0.001. Nox-i, Nox-RNAi.
Consistent with the idea that H2O2 is the key signaling molecule for follicle rupture, overexpression of Sod1, which could produce intracellular H2O2 to compensate for the loss of NOX/SOD3-generated extracellular H2O2, exerted a similar rescue effect as Sod3 (Fig. 4 C and D). In contrast, overexpression of Sod2 in mitochondria did not show any rescue effect (Fig. 4 E and F), indicating that subcellular production of H2O2 is essential for follicle rupture. Consistent with this, overexpression of Catalase (Cat), an enzyme converting H2O2 to H2O and O2 (34), in mature follicle cells led to a strong reduction in OA-induced follicle rupture and egg-laying number (Fig. 4 G and H), but did not affect O2•− production (Fig. 3K). Notably, Sod1 overexpression alone caused a severe defect in OA-induced follicle rupture and egg laying (Fig. 4 C and D), indicating that too much intracellular H2O2 may be toxic for follicle rupture. Not surprisingly, the addition of H2O2 in the culture medium did not rescue the rupture defect of Nox-knockdown follicles (SI Appendix, Fig. S5D). Taken together, we favor the idea that a spatiotemporal burst of H2O2 production in the extracellular environment of mature follicle cells is critical for OA-induced follicle rupture.
SOD3 Is Required to Convert Superoxide to H2O2 for Follicle Rupture.
The above studies indicate that SOD3 likely functions outside the mature follicle cells to convert NOX-produced O2•− to H2O2 to regulate follicle rupture/ovulation. To test this hypothesis, we specifically knocked down Sod3 in mature follicle cells. Females with Sod3 knockdown laid <20 eggs/female per day, similar to Nox-knockdown females (Fig. 5A). In addition, Sod3-knockdown mature follicles were defective in OA-induced follicle rupture (Fig. 5B). Furthermore, the defective follicle rupture/ovulation in Sod3-knockdown females could be significantly rescued by overexpression of Sod3 (Fig. 5 A and B). These data suggest that follicular SOD3 is indeed required for follicle rupture/ovulation. As predicted, O2•− accumulated fivefold in Sod3-knockdown follicles in comparison with control follicles and this accumulation could be partially reduced by overexpression of Sod3 (Fig. 5C). These results further demonstrate that H2O2, not O2•−, is responsible for regulating follicle rupture. However, it is unclear whether H2O2 acts extracellularly or diffuses through the cell membrane to reach its targets for follicle rupture. In conclusion, we identified an OA/OAMB-Ca2+–NOX-SOD3 pathway that regulates H2O2 production and follicle rupture in all mature follicle cells in addition to the previously identified OA/OAMB-Ca2+–MMP2 pathway in posterior follicle cells (Fig. 5D).
Fig. 5.
SOD3 in mature follicle cells is required for ovulation. (A and B) Quantification of egg laying (A) and OA-induced follicle rupture (B) using females with 44E10-Gal4 driving Sod3-i and/or Sod3::3xHA expression. Forty to 50 females were used in A, and the numbers of follicles used in B are 219, 240, 227, and 237. (C) l-012 Luminescence-dependent O2•− quantification in mature follicles with 44E10-Gal4 driving Sod3-i and/or Sod3::3xHA expression. Note the y axis is different from those in Fig. 3. Mature follicles were stimulated with 20 μM OA at the 5-min point. (D) A schematic diagram shows the signaling pathways downstream of the OA/OAMB in mature follicle cells to regulate follicle rupture. ***P < 0.001. RLU, relative luminometer unit.
Discussion
Ovarian ROS are indispensable for ovulation in mice (7). However, the site of production of ROS is unknown and it is unclear whether ROS play a conserved role in ovulation across species. In this study, we provide genetic evidence that follicular ROS are required for ovulation in Drosophila. We demonstrate that NOX, whose activity is regulated by follicular adrenergic signaling, regulates follicle rupture and ovulation by producing O2•− in the extracellular space of mature follicle cells (Fig. 5D). In addition, our data suggest that an extracellular SOD3 converts this O2•− into H2O2, which is the key signaling molecule responsible for regulating follicle rupture (Fig. 5D). H2O2 can partially mimic LH in regulating cumulus expansion and gene expression in mammalian follicles (7). It is thus plausible that H2O2 plays a conserved role in regulating follicle rupture/ovulation from insects to mammals.
Members of the NOX family are also expressed in mouse and human granulosa cells and are functional in producing ROS (12–14). Norepinephrine, the mammalian counterpart of OA, is highly enriched in human follicular fluid and causes ROS generation in human granulosa cells (35). It will be interesting to determine whether norepinephrine plays a similar role as OA in generating ROS through regulating NOX activity during follicle rupture/ovulation in mammals.
Why would Drosophila mature follicles use NOX to generate ROS during follicle rupture? ROS can be generated through the mitochondrial respiratory chain and membrane-bound NOX family enzymes, as well as by a host of intracellular enzymes, such as xanthine oxidase, cyclooxygenases, cytochrome p450 enzymes, and lipoxygenases that produce ROS as part of their normal enzymatic function (36). As high-level cytoplasmic ROS are detrimental to cell function and viability, limiting O2•−/H2O2 production in the extracellular environment may be essential for cell viability and function. This is consistent with our finding that overexpression of Sod1, which presumably produces extra-cytoplasmic H2O2, led to a disruption in follicle rupture and egg laying (Fig. 4 C and D). Interestingly, Nox-knockdown follicles overexpressing Sod1 had normal follicle rupture (Fig. 4C), likely due to compensation of NOX-generated H2O2 by intracellularly produced H2O2, whereas bathing Nox-knockdown follicles in H2O2 did not rescue the defect in OA-induced follicle rupture (SI Appendix, Fig. S5D). These findings suggest that local ROS production is essential for cellular physiology, while global ROS may be detrimental.
Interestingly, Sod3 knockdown alone was sufficient to cause follicle rupture defects in Drosophila (Fig. 5 A and B), yet mice lacking SOD3 are healthy and fertile (37). It is possible that SOD1 can compensate for the loss of SOD3 in mouse follicles, as mice lacking SOD1 or both SOD1 and SOD3 are subfertile or infertile, respectively (38–40).
This study solved a conundrum in Drosophila ovulation. Previous work demonstrated that follicle rupture requires OA/OAMB induction of MMP2 activity in posterior follicle cells. However, OA/OAMB induces a rise in intracellular Ca2+ in all mature follicle cells (22, 23). What is the role of OA/OAMB-Ca2+ in nonposterior follicle cells? Our work demonstrated that OA/OAMB-Ca2+ signaling activates NOX in all follicle cells to produce O2•− and H2O2, which are important for follicle rupture (Fig. 3 A–F). NOX-generated ROS had a minimal effect on MMP2 activity, implying that these ROS regulate an independent pathway that is required for follicle rupture (Fig. 5D). Further studies should test whether region-specific Nox knockdown, such as only in nonposterior follicle cells, causes a follicle rupture defect.
The targets of H2O2 in regulating follicle rupture are still unknown. Biological redox reactions catalyzed by H2O2 typically affect protein function by promoting the oxidation of cysteine residues (41). The best-characterized examples of H2O2-mediated signal transduction include several protein tyrosine phosphatases in growth factor signaling pathways, such as platelet-derived growth factor, epidermal growth factor (EGF), insulin, and B cell receptor signaling (36, 41, 42). Oxidation of the cysteine residue in the active-site motif of these phosphatases reversibly inactivates phosphatase activity and promotes growth factor signaling. The timing of H2O2 production and follicle rupture makes it unlikely that H2O2 promotes follicle rupture in Drosophila follicle cells by regulating growth factor signaling. The peak production of O2•− (and presumably of H2O2) is ∼30–40 min after OA stimulation (Fig. 3), which coincides with the beginning of follicle rupture (23). There is not enough time to allow growth factor signaling-mediated transcription and translation to occur before rupture happens. Alternatively, H2O2 is also involved in the activation of the ADAM (a disintegrin and metalloprotease) family of metalloproteases, possibly through direct oxidation of a cysteine residue that prevents the inhibition of catalytic domain by the prodomain of the enzyme (14, 43, 44). We favor the idea that NOX-generated H2O2 activates ADAM or other proteinases to regulate follicle rupture in addition to MMP2 activation. Microarray and RNA-sequencing analysis identified multiple proteinases that are up-regulated in Drosophila follicle cells during ovulation (24, 25), and at least six different proteinases have been suggested to be involved in mammalian ovulation (45). Recent bioinformatics and large-scale proteomic analyses have predicted >500 proteins containing redox-active cysteine residues (46, 47), some of which could serve as the downstream effectors of H2O2 for follicle rupture.
Materials and Methods
Details are described in SI Appendix, SI Materials and Methods. This includes information on Drosophila genetics, egg laying and ovulation time, ex vivo follicle rupture, in situ zymography, qRT-PCR, ROS detection, immunostaining, and microscopy.
Supplementary Material
Acknowledgments
We thank Drs. Lynn Cooley, Paul Salvaterra, Konrad Basler, and Michael O’Connor for sharing reagents and fly lines; Bloomington Drosophila Stock Center and Vienna Drosophila Resource Center for fly stocks; and Developmental Studies Hybridoma Bank for antibodies. We thank Drs. Kyle Hadden, Rahul Kanadia, Joseph LoTurco, and Li Wang for sharing reagents and equipment. We also thank Lylah Deady, Elizabeth Knapp, and Wei Shen in J.S.’s laboratory for technical support and discussion. The Leica SP8 confocal microscope is supported by an NIH Award (S10OD016435) to Akiko Nishiyama. J.S. is supported by the University of Connecticut Start-Up Fund, NIH/National Institute of Child Health and Human Development Grant R01-HD086175, and the Bill & Melinda Gates Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1800115115/-/DCSupplemental.
References
- 1.Espey LL, Richards JS. Ovulation. In: Neill JD, editor. Physiology of reproduction. 3rd Ed. Academic; Amsterdam: 2006. pp. 425–474. [Google Scholar]
- 2.Fan H-Y, Liu Z, Mullany LK, Richards JS. Consequences of RAS and MAPK activation in the ovary: The good, the bad and the ugly. Mol Cell Endocrinol. 2012;356:74–79. doi: 10.1016/j.mce.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takahashi T, Fujimori C, Hagiwara A, Ogiwara K. Recent advances in the understanding of teleost medaka ovulation: The roles of proteases and prostaglandins. Zool Sci. 2013;30:239–247. doi: 10.2108/zsj.30.239. [DOI] [PubMed] [Google Scholar]
- 4.Rizzo A, Roscino MT, Binetti F, Sciorsci RL. Roles of reactive oxygen species in female reproduction. Reprod Domest Anim. 2012;47:344–352. doi: 10.1111/j.1439-0531.2011.01891.x. [DOI] [PubMed] [Google Scholar]
- 5.Jain S, Saxena D, Kumar GP, Laloraya M. NADPH dependent superoxide generation in the ovary and uterus of mice during estrous cycle and early pregnancy. Life Sci. 2000;66:1139–1146. doi: 10.1016/s0024-3205(00)00417-3. [DOI] [PubMed] [Google Scholar]
- 6.Kodaman PH, Behrman HR. Endocrine-regulated and protein kinase C-dependent generation of superoxide by rat preovulatory follicles. Endocrinology. 2001;142:687–693. doi: 10.1210/endo.142.2.7961. [DOI] [PubMed] [Google Scholar]
- 7.Shkolnik K, et al. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci USA. 2011;108:1462–1467. doi: 10.1073/pnas.1017213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yacobi K, Tsafriri A, Gross A. Luteinizing hormone-induced caspase activation in rat preovulatory follicles is coupled to mitochondrial steroidogenesis. Endocrinology. 2007;148:1717–1726. doi: 10.1210/en.2006-1533. [DOI] [PubMed] [Google Scholar]
- 9.Schieber M, Chandel NS. ROS function in redox signaling and oxidative stress. Curr Biol. 2014;24:R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 11.Brown DI, Griendling KK. Nox proteins in signal transduction. Free Radic Biol Med. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kampfer C, et al. Pigment-epithelium derived factor (PEDF) and the human ovary: A role in the generation of ROS in granulosa cells. Life Sci. 2014;97:129–136. doi: 10.1016/j.lfs.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Maraldi T, et al. NADPH oxidase-4 and MATER expressions in granulosa cells: Relationships with ovarian aging. Life Sci. 2016;162:108–114. doi: 10.1016/j.lfs.2016.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Chen Q, et al. PKCδ and θ possibly mediate FSH-induced mouse oocyte maturation via NOX-ROS-TACE cascade signaling pathway. PLoS One. 2014;9:e111423. doi: 10.1371/journal.pone.0111423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguirre J, Lambeth JD. Nox enzymes from fungus to fly to fish and what they tell us about Nox function in mammals. Free Radic Biol Med. 2010;49:1342–1353. doi: 10.1016/j.freeradbiomed.2010.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee K-A, et al. Inflammation-modulated metabolic reprogramming is required for DUOX-dependent gut immunity in Drosophila. Cell Host Microbe. 2018;23:338–352, e5. doi: 10.1016/j.chom.2018.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Hurd TR, Liang F-X, Lehmann R. Curly encodes dual oxidase, which acts with heme peroxidase curly Su to shape the adult Drosophila wing. PLoS Genet. 2015;11:e1005625. doi: 10.1371/journal.pgen.1005625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Razzell W, Evans IR, Martin P, Wood W. Calcium flashes orchestrate the wound inflammatory response through DUOX activation and hydrogen peroxide release. Curr Biol. 2013;23:424–429. doi: 10.1016/j.cub.2013.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritsick DR, Edens WA, Finnerty V, Lambeth JD. Nox regulation of smooth muscle contraction. Free Radic Biol Med. 2007;43:31–38. doi: 10.1016/j.freeradbiomed.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spradling AC. Developmental genetics of oogenesis. In: Bate M, Martinez-Arias A, editors. The Development of Drosophila Melanogaster. Cold Spring Harbor Lab Press; Cold Spring Harbor, NY: 1993. pp. 1–70. [Google Scholar]
- 21.Deady LD, Li W, Sun J. The zinc-finger transcription factor Hindsight regulates ovulation competency of Drosophila follicles. eLife. 2017;6:e29887. doi: 10.7554/eLife.29887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deady LD, Shen W, Mosure SA, Spradling AC, Sun J. Matrix metalloproteinase 2 is required for ovulation and corpus luteum formation in Drosophila. PLoS Genet. 2015;11:e1004989. doi: 10.1371/journal.pgen.1004989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deady LD, Sun J. A follicle rupture assay reveals an essential role for follicular adrenergic signaling in Drosophila ovulation. PLoS Genet. 2015;11:e1005604. doi: 10.1371/journal.pgen.1005604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tootle TL, Williams D, Hubb A, Frederick R, Spradling A. Drosophila eggshell production: Identification of new genes and coordination by Pxt. PLoS One. 2011;6:e19943. doi: 10.1371/journal.pone.0019943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eichhorn SW, et al. mRNA poly(A)-tail changes specified by deadenylation broadly reshape translation in Drosophila oocytes and early embryos. eLife. 2016;5:e16955. doi: 10.7554/eLife.16955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knapp E, Sun J. Steroid signaling in mature follicles is important for Drosophila ovulation. Proc Natl Acad Sci USA. 2017;114:699–704. doi: 10.1073/pnas.1614383114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cifuentes-Pagano E, Meijles DN, Pagano PJ. The quest for selective Nox inhibitors and therapeutics: Challenges, triumphs and pitfalls. Antioxid Redox Signal. 2014;20:2741–2754. doi: 10.1089/ars.2013.5620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peshavariya HM, Dusting GJ, Selemidis S. Analysis of dihydroethidium fluorescence for the detection of intracellular and extracellular superoxide produced by NADPH oxidase. Free Radic Res. 2007;41:699–712. doi: 10.1080/10715760701297354. [DOI] [PubMed] [Google Scholar]
- 29.Owusu-Ansah E, Yavari A, Banerjee U. A protocol for in vivo detection of reactive oxygen species. Protoc Exch. 2008 doi: 10.1038/nprot.2008.23. [DOI] [Google Scholar]
- 30.Phillips JP, Campbell SD, Michaud D, Charbonneau M, Hilliker AJ. Null mutation of copper/zinc superoxide dismutase in Drosophila confers hypersensitivity to paraquat and reduced longevity. Proc Natl Acad Sci USA. 1989;86:2761–2765. doi: 10.1073/pnas.86.8.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirby K, Hu J, Hilliker AJ, Phillips JP. RNA interference-mediated silencing of Sod2 in Drosophila leads to early adult-onset mortality and elevated endogenous oxidative stress. Proc Natl Acad Sci USA. 2002;99:16162–16167. doi: 10.1073/pnas.252342899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jung I, Kim T-Y, Kim-Ha J. Identification of Drosophila SOD3 and its protective role against phototoxic damage to cells. FEBS Lett. 2011;585:1973–1978. doi: 10.1016/j.febslet.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 33.Blackney MJ, Cox R, Shepherd D, Parker JD. Cloning and expression analysis of Drosophila extracellular Cu Zn superoxide dismutase. Biosci Rep. 2014;34:e00164. doi: 10.1042/BSR20140133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Missirlis F, Phillips JP, Jäckle H. Cooperative action of antioxidant defense systems in Drosophila. Curr Biol. 2001;11:1272–1277. doi: 10.1016/s0960-9822(01)00393-1. [DOI] [PubMed] [Google Scholar]
- 35.Saller S, et al. Norepinephrine, active norepinephrine transporter, and norepinephrine-metabolism are involved in the generation of reactive oxygen species in human ovarian granulosa cells. Endocrinology. 2012;153:1472–1483. doi: 10.1210/en.2011-1769. [DOI] [PubMed] [Google Scholar]
- 36.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol. 2011;194:7–15. doi: 10.1083/jcb.201102095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. Proc Natl Acad Sci USA. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ho Y-S, et al. Reduced fertility in female mice lacking copper-zinc superoxide dismutase. J Biol Chem. 1998;273:7765–7769. doi: 10.1074/jbc.273.13.7765. [DOI] [PubMed] [Google Scholar]
- 39.Matzuk MM, Dionne L, Guo Q, Kumar TR, Lebovitz RM. Ovarian function in superoxide dismutase 1 and 2 knockout mice. Endocrinology. 1998;139:4008–4011. doi: 10.1210/endo.139.9.6289. [DOI] [PubMed] [Google Scholar]
- 40.Sentman M-L, et al. Phenotypes of mice lacking extracellular superoxide dismutase and copper- and zinc-containing superoxide dismutase. J Biol Chem. 2006;281:6904–6909. doi: 10.1074/jbc.M510764200. [DOI] [PubMed] [Google Scholar]
- 41.Rhee SG. Cell signaling: H2O2, a necessary evil for cell signaling. Science. 2006;312:1882–1883. doi: 10.1126/science.1130481. [DOI] [PubMed] [Google Scholar]
- 42.Tonks NK. Redox redux: Revisiting PTPs and the control of cell signaling. Cell. 2005;121:667–670. doi: 10.1016/j.cell.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 43.Zhang Z, et al. Reactive oxygen species mediate tumor necrosis factor alpha-converting, enzyme-dependent ectodomain shedding induced by phorbol myristate acetate. FASEB J. 2001;15:303–305. doi: 10.1096/fj.00-0371fje. [DOI] [PubMed] [Google Scholar]
- 44.Myers TJ, et al. Mitochondrial reactive oxygen species mediate GPCR-induced TACE/ADAM17-dependent transforming growth factor-α shedding. Mol Biol Cell. 2009;20:5236–5249. doi: 10.1091/mbc.E08-12-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ohnishi J, Ohnishi E, Shibuya H, Takahashi T. Functions for proteinases in the ovulatory process. Biochim Biophys Acta. 2005;1751:95–109. doi: 10.1016/j.bbapap.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 46.Fomenko DE, Xing W, Adair BM, Thomas DJ, Gladyshev VN. High-throughput identification of catalytic redox-active cysteine residues. Science. 2007;315:387–389. doi: 10.1126/science.1133114. [DOI] [PubMed] [Google Scholar]
- 47.Weerapana E, et al. Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature. 2010;468:790–795. doi: 10.1038/nature09472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.