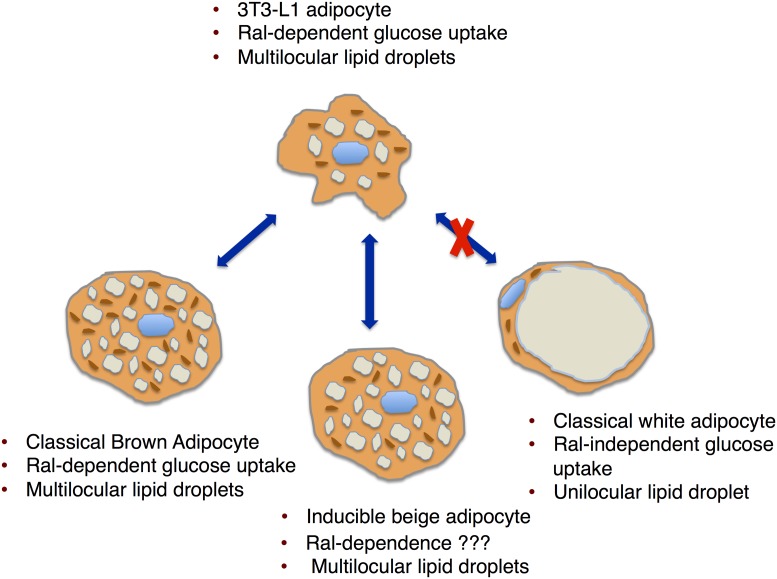
In PNAS, Skorobogatko et al. (1) report that RalA signaling regulates glucose homeostasis in mice by regulating GLUT4 translocation to the plasma membrane and glucose uptake in brown adipose tissue, but not in white adipose tissue. Interestingly, the foundational work leading to this paper was carried out in 3T3-L1 adipocytes. Quite to their surprise, manipulation of RalA signaling in mice impacted glucose transport in brown adipose tissue exactly as predicted from the 3T3-L1 model. However, RalA signaling in white adipose tissue did not affect glucose uptake (Fig. 1). This was unexpected given the fact that 3T3-L1 cells are frequently used as a model to predict the function of white adipose tissue, not brown adipose tissue.
Fig. 1.
What type of adipocyte is modeled by the 3T3-L1 adipocyte? Based on lipid droplet morphology and Ral-dependent glucose uptake, the 3T3-L1 adipocyte models a thermogenic adipocyte.
For decades, 3T3-L1 adipocytes have served as a workhorse for studying mechanisms of adipocyte differentiation, adipocyte gene expression, triglyceride synthesis, insulin and beta-adrenergic signal transduction, and insulin-dependent glucose uptake as a model for white adipose tissue. Howard Green established the 3T3-derived adipocyte lines (3T3-L1) in the mid-1970s using clonal selection of 3T3 mouse fibroblast lines derived from disaggregated Swiss mouse embryo (2). In that first report, Green and Meuth (2) speculated that the 3T3-L1 model most resembled a brown adipose cell, or possibly an immature white adipose cell. This was largely due to the fact that the differentiation cells displayed multilocular lipid droplets, rather than a unilocular lipid droplet characteristic of classic white adipocytes. Thirty-four years later, this question of what type of adipocyte is modeled by 3T3-L1 adipocytes has not been answered. The results of Skorobogatko et al. (1) provide compelling evidence that Howard Green’s initial speculation was correct: 3T3-L1 adipocytes are a model of thermogenic adipocytes.
Since the cloning and identification of the insulin-responsive glucose transporter, GLUT4, 3T3-L1 adipocytes have served as the primary model for mechanistic studies to unravel the connection between insulin signaling and translocation of GLUT4 to the plasma membrane. The fascination with unraveling the complicated itinerary of GLUT4 was sparked by the observation that insulin recruits a pool of intracellular glucose transporters to the cell surface to clear plasma glucose (3, 4). Detailed analysis of the GLUT4 translocation pathway in 3T3-L1 adipocytes has revealed that GLUT4 exocytosis is regulated by insulin signaling through the PI3K/Akt pathway, and that numerous ras-family GTPases are involved in converting the akt signal to membrane trafficking steps (reviewed in ref. 5).
RalA is a small GTPase and is thought to serve as a signaling intermediate that can connect extracellular signals to cellular transformation (6, 7). Importantly, Ral proteins were found to be activated by PI3K in EGF-stimulated cells (8). Ral proteins attracted the attention of scientists studying insulin-dependent GLUT4 translocation after it was learned that activated RalA proteins associated with components of the exocyst, an evolutionarily conserved multiprotein complex that tethers exocytic vesicles to the sites of exocytosis on the plasma membrane (9, 10). Using the 3T3-L1 model, Ewart et al. (11) showed that exocyst components redistributed to the plasma membrane in response to insulin and that overexpression of exocyst components increased glucose uptake. These observations prompted them to assume that insulin-dependent GLUT4 was regulated by the exocyst, although the direct experiment was not carried out. Later, Chen et al. (12) built on these observations by showing that RalA was activated by insulin signaling in the 3T3-L1 adipocytes, and that RalA was required for GLUT4 translocation. This work and subsequent papers have convincingly demonstrated that insulin-dependent RalA activation and association with the exocyst complex is essential for GLUT4 translocation in 3T3-L1 adipocytes (13).
An early clue that 3T3-L1 cells may not model white adipose tissue went almost unnoticed. Manipulation of the exocyst component, Exo70, in 3T3-L1 adipocytes inhibited insulin-dependent GLUT4 translocation in 3T3-L1 cells (14). Lizunov et al. (15) probed primary cultured white adipocytes to determine if the exocyst played a role in insulin-dependent GLUT4 translocation. In contrast to 3T3-L1 cells, GLUT4 membrane fusion was not impacted by manipulation of Exo70 in the primary adipocytes. At the time, this discrepancy was explained by the difference in cellular architecture between 3T3-L1 adipocytes and primary cultured adipocytes. While this explanation makes some sense, it was not scientifically satisfying because there have been no tests of the hypothesis.
Support for the notion that differentiated 3T3-L1 cells model white adipose tissue comes from the fact that the patterns of gene expression are most similar to white adipose tissue (16). These measurements of gene expression may be misleading because they are made under conditions that do not provoke further differentiation to a thermogenic adipocyte. When 3T3-L1 cells are induced with norepinephrine or isoproterenol, the thermogenic gene expression profile is induced (16, 17). In this case, UCP1 expression is up-regulated and oxygen consumption is increased. Thus, 3T3-L1 adipocytes may serve as a model for immature brown adipocytes, or possibly so-called beige/brite inducible thermogenic adipocytes, a refinement of Howard Green’s original speculation. Interestingly, the protocol for differentiating 3T3-L1 adipocytes, using a mixture of dexamethasone, isobutylmethyl xanthine, and insulin, is the same protocol used to differentiate primary adipocytes from stromal vascular cells isolated from adipose tissue. Importantly, this protocol is successful only when the
The results of Skorobogatko et al. provide compelling evidence that Howard Green’s initial speculation was correct: 3T3-L1 adipocytes are a model of thermogenic adipocytes.
stromal vascular cells are isolated from s.c. fat, also the major site of thermogenic adipocytes (18).
We now see that RalA signaling may be an unexpected pathway that distinguishes thermogenic from nonthermogenic adipocytes. Skorobogatko et al. (1) show that RalA signaling can be activated in all adipocyte fat pads by inactivating its GAP, but signals to glucose uptake only in brown adipocytes. This work shines a spotlight on the role of thermogenic adipocytes as an attractive target for management of glucose homeostasis, but not necessarily through regulation of body mass. Since the discovery of inducible thermogenic adipocytes, intense research activity has focused on thermogenic cells and their potential role in energy balance (19). This new work suggests a different role for thermogenic adipocytes in the regulation of glucose homeostasis.
Mechanistically, we have no clues why RalA signaling leads to GLUT4 translocation and glucose uptake only in brown adipose tissue and not white adipose tissue. It is possible that a signaling intermediate that links RalA to GLUT4 translocation is missing in white adipose tissue, as speculated by the authors. It is also possible that RalA signaling to GLUT4 is not the major determinate of RalA-dependent glucose uptake. It is possible that RalA signaling is also targeting other glucose transporters in the brown adipose pad. Olsen et al. (20) reported that GLUT1 translocation occurs in primary cultured brown adipocytes under both anabolic activation (insulin signaling) and sympathetic activation (adrenergic signaling). It is possible that RalA is playing an important role in signaling to GLUT1 translocation as well as to GLUT4 translocation. All this would be testable using the 3T3-L1 model as well as the mouse model of enhanced RalA signaling described by Skorobogatko et al. (1).
Footnotes
The author declares no conflict of interest.
See companion article on page 7819.
References
- 1.Skorobogatko Y, et al. RalA controls glucose homeostasis by regulating glucose uptake in brown fat. Proc Natl Acad Sci USA. 2018;115:7819–7824. doi: 10.1073/pnas.1801050115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Green H, Meuth M. An established pre-adipose cell line and its differentiation in culture. Cell. 1974;3:127–133. doi: 10.1016/0092-8674(74)90116-0. [DOI] [PubMed] [Google Scholar]
- 3.Cushman SW, Wardzala LJ. Potential mechanism of insulin action on glucose transport in the isolated rat adipose cell. Apparent translocation of intracellular transport systems to the plasma membrane. J Biol Chem. 1980;255:4758–4762. [PubMed] [Google Scholar]
- 4.Suzuki K, Kono T. Evidence that insulin causes translocation of glucose transport activity to the plasma membrane from an intracellular storage site. Proc Natl Acad Sci USA. 1980;77:2542–2545. doi: 10.1073/pnas.77.5.2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stöckli J, Fazakerley DJ, James DE. GLUT4 exocytosis. J Cell Sci. 2011;124:4147–4159. doi: 10.1242/jcs.097063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chardin P, Tavitian A. The ral gene: A new ras related gene isolated by the use of a synthetic probe. EMBO J. 1986;5:2203–2208. doi: 10.1002/j.1460-2075.1986.tb04485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.White MA, Vale T, Camonis JH, Schaefer E, Wigler MH. A role for the Ral guanine nucleotide dissociation stimulator in mediating Ras-induced transformation. J Biol Chem. 1996;271:16439–16442. doi: 10.1074/jbc.271.28.16439. [DOI] [PubMed] [Google Scholar]
- 8.Tian X, Rusanescu G, Hou W, Schaffhausen B, Feig LA. PDK1 mediates growth factor-induced Ral-GEF activation by a kinase-independent mechanism. EMBO J. 2002;21:1327–1338. doi: 10.1093/emboj/21.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moskalenko S, et al. The exocyst is a Ral effector complex. Nat Cell Biol. 2002;4:66–72. doi: 10.1038/ncb728. [DOI] [PubMed] [Google Scholar]
- 10.Sugihara K, et al. The exocyst complex binds the small GTPase RalA to mediate filopodia formation. Nat Cell Biol. 2002;4:73–78. doi: 10.1038/ncb720. [DOI] [PubMed] [Google Scholar]
- 11.Ewart MA, Clarke M, Kane S, Chamberlain LH, Gould GW. Evidence for a role of the exocyst in insulin-stimulated Glut4 trafficking in 3T3-L1 adipocytes. J Biol Chem. 2005;280:3812–3816. doi: 10.1074/jbc.M409928200. [DOI] [PubMed] [Google Scholar]
- 12.Chen XW, Leto D, Chiang SH, Wang Q, Saltiel AR. Activation of RalA is required for insulin-stimulated Glut4 trafficking to the plasma membrane via the exocyst and the motor protein Myo1c. Dev Cell. 2007;13:391–404. doi: 10.1016/j.devcel.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 13.Chen XW, et al. A Ral GAP complex links PI 3-kinase/Akt signaling to RalA activation in insulin action. Mol Biol Cell. 2011;22:141–152. doi: 10.1091/mbc.E10-08-0665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bao Y, Lopez JA, James DE, Hunziker W. Snapin interacts with the Exo70 subunit of the exocyst and modulates GLUT4 trafficking. J Biol Chem. 2008;283:324–331. doi: 10.1074/jbc.M706873200. [DOI] [PubMed] [Google Scholar]
- 15.Lizunov VA, Lisinski I, Stenkula K, Zimmerberg J, Cushman SW. Insulin regulates fusion of GLUT4 vesicles independent of Exo70-mediated tethering. J Biol Chem. 2009;284:7914–7919. doi: 10.1074/jbc.M806460200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrison S, McGee SL. 3T3-L1 adipocytes display phenotypic characteristics of multiple adipocyte lineages. Adipocyte. 2015;4:295–302. doi: 10.1080/21623945.2015.1040612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller CN, et al. Isopreterenol increases uncoupling, glycolysis, and markers of beiging in mature 3T3-L1 adipocytes. PLoS One. 2015;10:e0138344. doi: 10.1371/journal.pone.0138344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Church CD, Berry R, Rodeheffer MS. Isolation and study of adipocyte precursors. Methods Enzymol. 2014;537:31–46. doi: 10.1016/B978-0-12-411619-1.00003-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olsen JM, et al. Glucose uptake in brown fat cells is dependent on mTOR complex 2-promoted GLUT1 translocation. J Cell Biol. 2014;207:365–374. doi: 10.1083/jcb.201403080. [DOI] [PMC free article] [PubMed] [Google Scholar]