Significance
Neurotrophin receptors are a class of receptor tyrosine kinases that couple to signaling pathways critical for neuronal survival and growth. One member, TrkB, is particularly interesting because it plays a role in many severe degenerative neurological diseases. The TrkB natural ligand brain-derived neurotrophic factor (BDNF) is not suitable to be developed as a drug or therapy as proved by previous unsuccessful clinical trials. Here we report a selection method that produced potent full agonist antibodies that mimic BDNF function, yet with better biophysical properties. This study paves the road for the development of agonist antibodies for other receptor tyrosine kinases.
Keywords: antibody, TrkB, agonist, combinatorial library, membrane tethered
Abstract
The diverse physiological roles of the neurotrophin family have long prompted exploration of their potential as therapeutic agents for nerve injury and neurodegenerative diseases. To date, clinical trials of one family member, brain-derived neurotrophic factor (BDNF), have disappointingly failed to meet desired endpoints. Contributing to these failures is the fact that BDNF is pharmaceutically a nonideal biologic drug candidate. It is a highly charged, yet is a net hydrophobic molecule with a low molecular weight that confers a short t1/2 in man. To circumvent these shortcomings of BDNF as a drug candidate, we have employed a function-based cellular screening assay to select activating antibodies of the BDNF receptor TrkB from a combinatorial human short-chain variable fragment antibody library. We report here the successful selection of several potent TrkB agonist antibodies and detailed biochemical and physiological characterization of one such antibody, ZEB85. By using a human TrkB reporter cell line and BDNF-responsive GABAergic neurons derived from human ES cells, we demonstrate that ZEB85 is a full agonist of TrkB, comparable in potency to BDNF toward human neurons in activation of TrkB phosphorylation, canonical signal transduction, and mRNA transcriptional regulation.
Extensive studies, including gene-KO analyses, have demonstrated a pivotal role of neurotrophic factors in sculpting the nervous system during mammalian development (1). In the adult nervous system, additional roles of these protein growth factors have been identified as regulators of synaptic plasticity and neurotransmitter phenotypes. Among such factors are the neurotrophins: nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF), and neurotrophin (NT)3 and NT4. Since the discovery that NGF and BDNF, in addition to actions upon peripheral sympathetic and sensory neurons, regulate the neurotransmitter phenotype of basal forebrain cholinergic neurons, there has been great interest in exploring the potential of neurotrophin family members as therapeutic agents in nerve injury and neurodegenerative diseases. As a comprehensive understanding of the biological role of BDNF has emerged, potential therapeutic utility has extended to neurological conditions such as Alzheimer’s, Parkinson’s, and Huntington’s diseases, as well as retinal diseases and hearing disorders (2). In addition to the knowledge accumulated by using animal models, genetic data strongly support the relevance of BDNF in humans. For example, an SNP in the BDNF gene has been linked to mild memory impairment (3), and loss-of-allele mutations in NTRK2 and BDNF have been linked to severe developmental defects and obesity (4, 5). Also, NT4 mutations have been associated with rare cases of glaucoma (6, 7).
Based on studies demonstrating survival-promoting effects of BDNF on cultured motor neurons (8), the ability of locally applied BDNF to prevent atrophy/loss of axotomized facial nerve motor neurons in neonatal mice and the ameliorating effects of s.c.-injected BDNF in genetic models of motor neuron atrophy (9), BDNF was advanced to randomized, double-blinded phase III clinical trials in amyotrophic lateral sclerosis (ALS). Disappointingly, s.c. administration of BDNF in patients with ALS failed to meet primary endpoints of improved lung function and overall survival (10), despite positive efficacy trends in smaller phase II studies. To circumvent possible limited access of s.c.-administered BDNF to the cell body of motor neurons within the spinal cord, small trials were also undertaken with direct intrathecal infusions of BDNF (11), but these studies also did not convincingly demonstrate any benefits to patients. These trials were conducted knowing that BDNF is not an ideal biologic drug candidate, given its high net charge (pI of ∼9.6) and propensity to adhere to glass and plastic surfaces, requiring complex drug formulation with excipients. Further, and most relevant for systemic routes of administration for diseases such as ALS, the relatively small protein molecular mass (27 kDa) of BDNF confers only a short serum t1/2 in man. Additionally, when delivered directly to the CNS by intraventricular or intrathecal routes, BDNF, unlike NGF, diffuses very poorly across the ependymal layer of the ventricular lining (12). Diffusion within the CNS is equally limited when BDNF is infused directly into brain parenchyma (13).
Possible approaches to circumventing the poor drug-like properties of the BDNF protein itself include (i) development of highly selective small-molecule or peptide agonists of TrkB, the tyrosine kinase receptor activated by BDNF and NT4 (14–17); or (ii) developing molecules that up-regulate the endogenous levels of BDNF expression at selective sites or in specific neuronal types within the CNS (18–20). Although a number of studies have reported the discovery of small-molecule mimetics of BDNF that act as TrkB agonists, two recent independent studies have cast doubt on the usefulness of such reagents (17, 21). Alternatively, several reports have demonstrated that it is feasible through conventional hybridoma techniques to select TrkB-activating antibodies from mice immunized with TrkB ectodomain protein (22, 23). Although a few studies have reported modest efficacy of TrkB-activating antibodies in animal models (24, 25), there has been only limited characterization of the actions of these TrkB agonist antibodies on highly enriched human neurons, and their clinical potential is uncertain.
With the intention of reinvestigating the clinical potential of neurotrophin receptor agonists in neural compartments such as the retina and the inner ear, we have taken advantage of a function-based screening technology to allow discovery of Trk receptor agonist antibodies from large combinatorial libraries of human short-chain variable fragment (scFv) antibodies (26, 27). We report here the successful selection of several TrkB-agonist antibodies, the first application of our function-based screening platform to a member of the large receptor tyrosine kinase family. The TrkB-activating agonist antibodies we have identified range from full to partial agonists in our reporter cell assay. We characterized the most potent of these antibodies (ZEB85) on highly enriched cultures of BDNF-responsive GABAergic neurons derived from human ES (hES) cells (28–30). We describe here the selection methodology and detailed characterization of ZEB85 in the format of an scFv–Fc fusion protein antibody. We show that ZEB85 is a full agonist of TrkB with a potency and activity similar to that of BDNF.
Results
Reporter Cell Line.
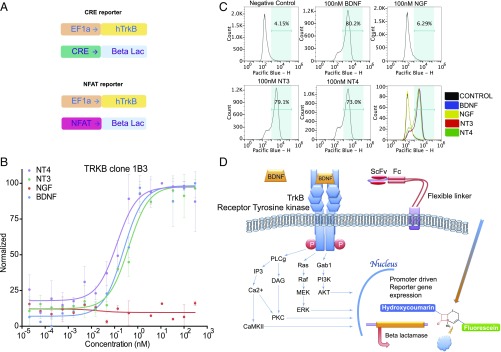
We have previously established technology that allows the direct function-based screening of large combinatorial antibody or peptide libraries to discover agonist antibodies or agonist peptides to cell-surface receptors, such as G protein-coupled receptors and cytokine receptors (26, 27, 31). In the present study, we have applied this technology to generate fully human agonist antibodies to the receptor tyrosine kinase TrkB. In its most effective form, the selection protocol is a two-step process: generation of a highly sensitive TrkB reporter cell line and phage scFv library panning of the TrkB ectodomain. Two independent TrkB reporter cell lines were constructed. An HEK293 and a CHO cell line were separately infected with a lentiviral vector encoding the full-length human NTRK2 gene under the EF1a promoter. The HEK293 cells were also infected with a lentiviral vector encoding the β-lactamase protein regulated by a CRE response element, whereas the CHO cells were infected with the β-lactamase gene expression regulated by an NFAT response element (Fig. 1A). From pilot experiments, we determined that only a narrow range of TrkB receptor expression permitted the generation of high signal-to-noise reporter cell lines. Overly high cell-surface expression resulted in TrkB kinase domain autophosphorylation and thus ligand independent activation of the reporter readout, somewhat typical when receptor tyrosine kinases are overexpressed. Conversely, underexpression of TrkB resulted in an inadequate fluorescent signal to enable cell sorting. As our screening system relies on FACS, high background gives way to a high false-positive rate with overall low selection efficiency. This is particularly problematic when selecting for rare agonist antibodies in the library. When expressed within the optimal range, ligand stimulation of TrkB in the reporter cell results in activation of canonical signals that induce expression of the β-lactamase reporter construct; in turn, addition of a β-lactamase FRET pair substrate results in its cleavage and emission of a fluorescent signal (Fig. 1D). When they had been grown up as stable cell lines, both the hTrkB–CRE–β-lactamase line and the hTrkB–NFAT–β-lactamase lines showed high signal-to-noise ratios when treated with 10 nM BDNF.
Fig. 1.
Reporter cell line construction and characterization. (A) Design of exogenously transduced lentiviral constructs for the reporter cell lines. (B) Concentration–response curves of different ligands on the TrkB reporter line (NFAT). (C) Flow cytometry analysis of the TrkB reporter cell line (NFAT) activation when treated with different ligands. (D) Illustration of the reporter mechanism when activated.
As assessed by flow cytometry, the TrkB (CHO-NFAT) reporter cell line was shown to bind BDNF but not NGF (Fig. 1C). This TrkB reporter cell line also bound NT3 and NT4, known ligands of this receptor (Fig. 1C). Similarly, as shown in Fig. 1B, the TrkB reporter cell line was activated by BDNF, NT3, and NT4, but not NGF, confirming that the cell line behaved as expected for binding and functional response to TrkB agonists. A TrkA reporter cell line generated in a similar fashion for the purpose of selectivity screening was shown to selectively bind NGF but not BDNF (SI Appendix, Fig. S1A). This TrkA cell line was activated by NGF but not by BDNF (SI Appendix, Fig. S1B). Nontransfected HEK293 or CHO cells did not respond to any of the neurotrophins (SI Appendix, Fig. S1C). Collectively, these results show that the TrkB reporter cell lines complied with the binding and activation properties expected for TrkB and demonstrate the high signal-to-noise ratio necessary for the high-capacity function-based antibody selection procedure used here.
Panning of Human TrkB Ectodomain.
In the second step of candidate agonist antibody selection, the ectodomain protein of hTrkB was “panned” with a large (>1010) combinatorial scFv antibody library expressed in phage, as described previously (27) and as outlined in the scheme in SI Appendix, Fig. S2. The binders or “hits” (∼106) were cloned into a lentiviral vector that encodes a transmembrane domain and an Ig Fc domain in tandem with each of the unique scFv panning hit sequences, such that, when expressed in individual cells, each scFv protein is tethered to the extracellular side of the plasma membrane as an Fc-dimer. The virus was titered and infected into a single plate (1–2 × 107 cells) of the HEK293-CRE or CHO-NFAT TrkB reporter cell lines. We targeted lentiviral infection to a multiplicity of infection of ∼2. In this manner, each cell in the plate becomes an independent autocrine assay point. The scheme in Fig. 1D illustrates the selection system in the reporter cell line: when a given surface-expressed (i.e., tethered) scFv fragment activates the TrkB receptor, a fluorescent signal is generated and those cells are selected by FACS. To enrich for resulting hits, antibody genes from positive-signal cells were subcloned back into the lentiviral vector and reinfected into fresh reporter cells. After three rounds of this enrichment, selected clones were prepared as scFv–Fc constructs and expressed as proteins in HEK293 cells. Following purification, these hits were assayed in the reporter cell line to verify agonist activity.
Antibody Selection and Characterization.
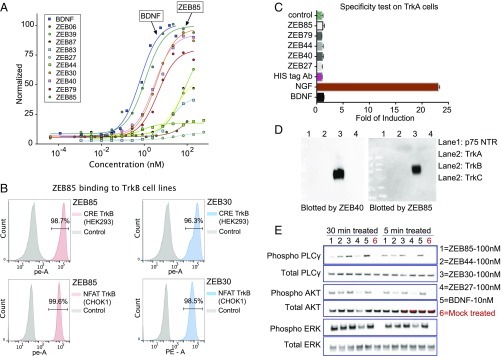
By using the HEK293-CRE and the CHO-NFAT TrkB reporter cell lines, we identified a total of ∼50 active clones from our function-based screening assay. DNA sequencing of these scFv antibody fragments revealed some antibodies that were closely related in sequence to each other, whereas others were quite disparate (SI Appendix, Fig. S3). We selected 10 of the more active clones with distinct sequences for further study. These were expressed as scFv–Fc fusion protein constructs for initial activity assessment in the CHO-NFAT TrkB reporter cell line. Fig. 2A shows that several potent scFv–Fc agonist antibodies were identified with EC50 values ranging from 0.5 nM to 20 nM, compared with BDNF with an EC50 of 0.3 nM. In addition, some antibodies appeared to be full agonists (ZEB85, ZEB30) compared with BDNF, whereas others (ZEB44, ZEB27) showed partial agonism with Emax (agonist maximum response) values 10–60% that of BDNF. By using flow cytometry, antibodies ZEB30 and ZEB85 were also shown to bind to the TrkB reporter cell line (Fig. 2B).
Fig. 2.
Characterization of the active antibody clones identified from the functional screening. (A) Concentration–response curve of 10 representative active clones on TrkB reporter cell line. (B) Purified ZEB85 antibody binding to the TrkB reporter cell surface. (C) Representative TrkB agonist clones are not active on TrkA reporter cell line. (D) Western blot of Trk family ectodomains with two TrkB agonist clones. (E) Time-dependent phosphorylation of TrkB receptor downstream signaling molecules when stimulated by agonist ligands.
The activity profile of ZEB85 in the human TrkB reporter cell line suggests that it possesses full agonist activity, raising the question whether this particular antibody binds to the native agonist site on TrkB. To test this, we used flow cytometry to determine whether neurotrophins could compete for binding of ZEB85 to TrkB reporter cells. ZEB85 efficiently bound TrkB cells over a range of concentrations, and 0.25 μg/mL (2.3 nM) was used for neurotrophin displacement studies. A 4- and 12-fold molar excess of BDNF reduced binding of ZEB85 by 80% and 89%, respectively (SI Appendix, Fig. S4). Binding was also reduced by NT4, and NT3 to a slightly lesser extent, whereas NGF had no effect (SI Appendix, Fig. S4), consistent with TrkB pharmacology. These results suggest that ZEB85 binds at, or very near, the BDNF agonist site on TrkB.
To determine selectivity toward TrkB, we assayed the antibodies in the TrkA reporter cell line. In contrast to NGF, none of the 10 TrkB-activating scFv–Fc antibodies showed any activation of the TrkA reporter cells (Fig. 2C). TrkB antibody selectivity was also demonstrated by examining ectodomain binding. Analyses of Western blots showed that our TrkB agonist antibodies recognized human TrkB ectodomain but not that of TrkA or TrkC, nor the low-affinity neurotrophin receptor p75 (Fig. 2D). As controls, commercial antibodies (SI Appendix, Methods S1) specific for TrkA, TrkC, and p75 recognized their respective ectodomains on these filters. To demonstrate that the TrkB agonist antibodies were capable of canonical signaling similar to that of BDNF, the CHO-NFAT TrkB reporter cell line was exposed to antibodies ZEB85, ZEB44, ZEB30, and ZEB27 for 5 or 30 min, and Western blots prepared from these cell lysates were probed for total and phosphorylated PLCγ, AKT, and MAPK. As with BDNF, all of the antibodies stimulated canonical signaling; however, subtle differences in magnitude and time course were evident, consistent with differences seen in quantitative activation (Fig. 2E). Taken together, the results from Fig. 2 demonstrate that our methodology of autocrine selection of agonist antibodies from large combinatorial libraries was able to identify an array of potent and selective TrkB agonist antibodies that behave similarly to BDNF in terms of binding and signal transduction. From a technical perspective, we have shown this to be a flexible system, in that TrkB activating antibodies were successfully selected by using an HEK293 cell with a CRE reporter construct or a CHO cell with an NFAT reporter construct as host cells for the hTrkB receptor. It is important to highlight that our function-based selection assay is greatly facilitated in terms of high capacity and ease of use in that all manipulations to generate hits are carried out at the DNA level; only at the endpoint of advancing candidates was protein production and purification necessary to further validate TrkB activating antibodies. Furthermore, this DNA-based, high-throughput selection capacity allows rapid generation and screening of millions of variants to optimize activity, affinity, stability, and expression of a given antibody.
Characterization of ZEB85 on Human Neurons.
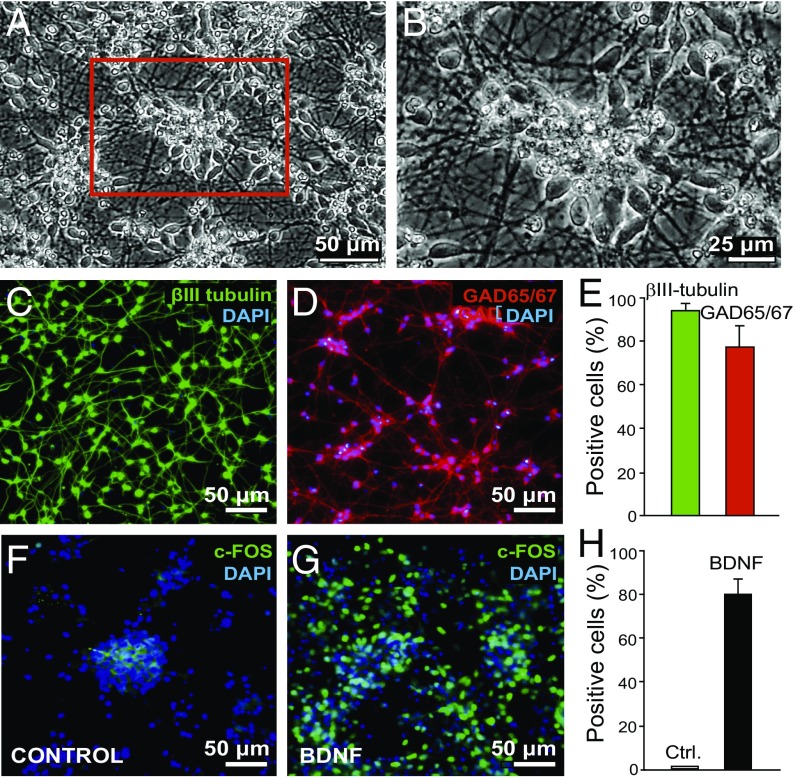
To examine the intrinsic effects of TrkB-activating antibodies on human neurons, H9 human embryonic stem cell-derived neural progenitors were differentiated into TrkB-expressing GABAergic inhibitory neurons as described previously (28–30). As reported, these neurons are electrically active and develop functional synaptic contacts (28). When differentiated, these neurons were phase-bright and extended neurites contacting their neighbors (Fig. 3 A and B). By using immunohistochemical markers, >90% of the cells expressed the neuronal marker β-III tubulin (Fig. 3 C and E) and >75% expressed the GABAergic phenotypic marker glutamate dehydrogenase (GAD65/67; Fig. 3 D and E). To show that the cells were responsive to BDNF, and thus expressed TrkB, we stimulated neurons with BDNF (50 ng/mL) and assessed c-Fos induction at 12 h. Compared with the untreated control (Fig. 3F), c-Fos immunoreactivity was detected in 75–80% of neurons treated with BDNF (Fig. 3 G and H). Based on these observations, the differentiated human neurons described herein represent a useful model system in which to examine signaling parameters trigged by ZEB85.
Fig. 3.
H9 hES cells differentiate as a monolayer of inhibitory neurons that are highly responsive to BDNF. (A and B) Phase-contrast microscopy of 30 DIV (days in vitro) neurons. (C) Immunocytochemistry of 30 DIV neurons stained with β-ΙΙΙ tubulin and DAPI. (D) Immunocytochemistry of 30 DIV neurons with GAD65/67 and DAPI. (E) Quantification of C and D. Values are mean ± SD of three independent experiments. (F and G) The 30 DIV neurons were untreated (F) or treated with 50 ng/mL BDNF (G) for 12 h and subsequently fixed and immunostained with DAPI or c-Fos antibody. Quantification is shown in H. Values are mean ± SD of two independent experiments.
ZEB85 Phosphorylates Human TrkB in a Time- and Concentration-Dependent Manner.
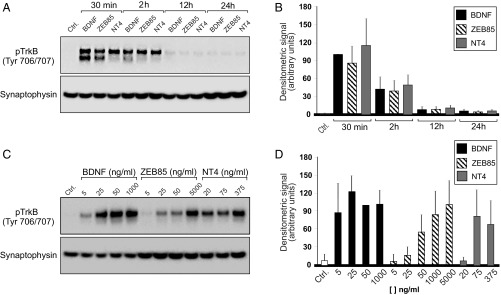
By using the characterized human neuronal cultures, we tested the ability of the agonist antibody ZEB85 to stimulate TrkB phosphorylation and compared its signaling pattern to that of BDNF and NT4 over 24 h. Agonist-stimulated TrkB phosphorylation was evident throughout the time course tested (Fig. 4A). Quantitatively, TrkB phosphorylation was robustly elevated at 30 min and 2 h post treatment with all three agonists, and declined to near baseline levels at 12 and 24 h (Fig. 4B). Importantly, ZEB85 induced TrkB phosphorylation to the same extent as BDNF and NT4. To determine the relative potency of each ligand, cultures were treated with a wide concentration range of ZEB85, BDNF, and NT4 for 30 min. Each agonist showed a concentration-dependent increase in TrkB phosphorylation (Fig. 4C). Quantification of six independent experiments yielded an EC50 for TrkB phosphorylation by ZEB85 of 470 pM (Fig. 4D), whereas that for BDNF was 190 pM, a value consistent with its reported EC50 on survival assays using cultured neurons (32). The EC50 of NT4 in these assays was 1.8 nM, considerably higher than for BDNF and ZEB85 (Fig. 4D). It is of note that ZEB30, a full agonist in the TrkB reporter cell assay (Fig. 2A), stimulated TrkB phosphorylation in hES-derived neurons to the same extent as ZEB85, BDNF, and NT4; two partial agonists, ZEB27 and ZEB44, elicited a weaker TrkB phosphorylation response, consistent with their partial agonist profile depicted in the reporter cell.
Fig. 4.
Effect of ZEB85, BDNF, and NT4 on TrkB phosphorylation by 30 DIV H9 neurons. (A) Representative immunoblot of the time course of TrkB phosphorylation. Cultures were treated with 50 ng/mL BDNF, 5 μg/mL ZEB85, or 75 ng/mL NT4 at the indicated time points. Cell lysates were subjected to Western blot analyses for phospho-TrkB or synaptophysin (used as loading control) as indicated in Materials and Methods. (B) Densitometric values (mean ± SE) quantified from the blots of six independent differentiation experiments. (C) Representative blots of the concentration response of TrkB phosphorylation. Cultures were treated with the indicated concentrations of ZEB85, BDNF, or NT4 for 30 min and then subjected to Western blot analysis. (D) Densitometric values (mean ± SE) quantified from the blots of six independent differentiations experiments.
Effect of TrkB Activation on Transcriptional Regulation in Human Neurons: Comparison of BDNF, ZEB85, and NT4.
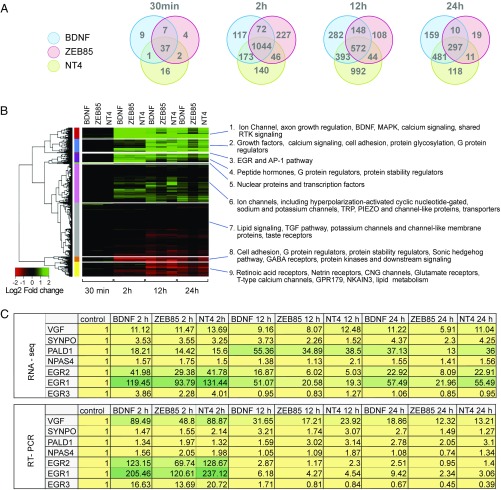
To further compare downstream signaling of hES cell-derived neurons when activated with the three TrkB agonists, we extracted RNA from neurons treated with ZEB85, BDNF, or NT4 for 30 min or 2, 12, or 24 h and performed RNA-sequencing (RNA-seq) analyses (SI Appendix, Methods S1). The top 1,000 shared genes whose expression was up- or down-regulated by ZEB85, BDNF, or NT4 across the time course was determined for the four different time points by using a threshold of an adjusted P value <0.01 and absolute fold change >2. We observed a striking concordance of altered levels of gene expression with all three agonist treatments compared with untreated neurons. At the first time point tested (30 min), the three TrkB agonists modified expression of a similar set of 37 genes (Fig. 5A). Eighteen of these genes were immediate-early response genes and included EGR1, EGR2, EGR3, EGR4, cFOS, NPAS4, and ARC. This pattern of concordance persisted throughout the time course for the three ligands. At 2 h posttreatment, the expression levels of 1,406, 1,389, and 1,403 genes were altered by BDNF, ZEB85, and NT4, respectively (Fig. 5A), and 1,044 were common to all three TrkB agonists. At the 12-h time point, the expression levels of 1,395, 872, and 2,001 genes were differentially regulated upon treatment with BDNF, ZEB85, and NT4, respectively (Fig. 5A), and 572 of these genes were common to treatment with the three agonists. Finally, at 24 h, 947, 337, and 907 genes were differentially expressed by the respective Trk ligands. It is interesting to note that >88% of the genes with altered expression upon exposure to ZEB85 were common to treatment with BDNF or NT4. However, there was only ∼50% overlap between the transcriptional changes common to BDNF and NT4.
Fig. 5.
RNA sequencing on 30 DIV H9 neurons treated with 50 ng/mL of BDNF, 100 ng/mL of NT4, and 5 μg/mL of ZEB85. (A) Venn diagrams illustrating the number of differentially expressed genes under the three treatment conditions (BDNF, ZEB85, NT4) and four time points (30 min, 2 h, 12 h, and 24 h). (B) Heat map of hierarchical cluster analysis with Gene Ontology annotation for the top 1,000 differentially expressed genes; absolute fold change >2 and adjusted P value <0.01. (C) Expression fold changes relative to the control of seven genes obtained with RNA-seq and RT-PCR. Green color indicates higher level of increase, and bright yellow indicates intermediate level of change.
Analysis of Differentially Expressed Genes.
When the differentially expressed genes were clustered by using a Gene Ontology analysis of expression in nine different functional categories, a high degree of similarity was observed for the 12 different experimental conditions in terms of genomewide expression (Fig. 5B). Whereas comparatively few changes were detected at 30 min, by 2 h, the changes observed were strikingly similar following treatment with any one of the three TrkB agonists. After 12- and 24-h treatments, the transcriptional changes induced by BDNF and NT4 were still robust, whereas the neurons treated with ZEB85 showed fewer genes differentially expressed compared with the baseline untreated control. Consistent with the proposed neurotrophic role of TrkB agonists, we observed a two- to fourfold down-regulation of the proapoptotic gene HRK/DP5 by all three agonists over the 24-h time course. Likewise, the antiapoptotic genes GADD45A and CDKN1A (p21) were up-regulated 10–13-fold at 2 h and remained elevated through the 24-h time course by the three ligands (SI Appendix, Table S2). Previous reports have shown that BDNF up-regulates genes important in synaptic function, such as ARC, SYT2 (synaptotagmin-2), and ACAN (aggrecan) (33, 34). Consistently, these genes were also up-regulated by ZEB85 and NT4 to similar levels as with BDNF throughout the 24-h time course (SI Appendix, Table S2).
To validate the results obtained with RNA-seq in Fig. 5A, the levels of transcriptional regulation exerted by ZEB85, BDNF, and NT4 on seven of these genes was determined by RT-PCR, and included the highly up-regulated transcription factors, EGR1, EGR2, EGR3, and NPAS4, as well as the nerve growth factor-inducible gene (VGF), synaptic protein (SYNPO), and a phosphatase domain protein (PALD1). After 2 h, all of the genes measured by RT-PCR except PALD1 were up-regulated to higher levels than observed in the RNA-seq analysis. At 12 and 24 h, regulation was quantitatively lower as assessed by RT-PCR compared with RNA-seq values (Fig. 5C). Overall, these results corroborate our RNA-seq data and validate the extensive translational regulation that TrkB agonists, including the antibody ZEB85, exert upon human neurons.
Characterization of ZEB85 on Rodent Neurons.
Whereas the protein sequence of mature BDNF is identical in all mammals sequenced thus far, small but significant sequence differences have been identified in the ectodomain of TrkB among mammalian species. Although such receptor sequence differences have been shown to be irrelevant to the cross-species potency of BDNF, these differences may result in cross-species differences in potency of TrkB agonist antibodies. To enable appropriate efficacy studies of ZEB85 in animal models of neurodegenerative disorders, we were interested to determine the relative potency of ZEB85 on rodent neurons compared with BDNF. We thus examined the effect of ZEB85 on TrkB phosphorylation in cultures of mouse cortical neurons and its effect on axotomized adult mouse retinal ganglion cells (RGCs) in explant cultures. ZEB85 stimulated TrkB phosphorylation in cultured mouse cortical neurons, albeit with lower potency compared with BDNF (Fig. 6A). To compare the ability of TrkB agonist antibodies to stimulate dendritic outgrowth in an intact neuronal network, adult mouse retinal explants were cultured in the presence of BDNF, ZEB85, or ZEB44. After 3 d, RGCs in explants were labeled with DiI/DiO, counterstained with DAPI, and imaged by confocal microscopy. Fig. 6B demonstrates the typical dendritic arbor pattern of RGCs in retinae treated with vehicle, TrkB agonist antibodies, or BDNF. Sholl analysis (Fig. 6 C–E) revealed a significant increase in dendrite intersections, area under the curve (AUC), and total dendrite length for BDNF as previously reported (35). ZEB85 (50 μg/mL) also significantly improved Sholl analysis parameters, albeit with less efficacy and potency than 100 ng/mL of BDNF. ZEB44 (50 μg/mL) resulted in a modest but significant increase in Sholl AUC and dendritic length, consistent with its partial agonist profile in the reporter cell assay.
Fig. 6.
TrkB phosphorylation in cultured mouse cortical neurons and dendritic outgrowth in mouse retinal explants by TrkB agonists. (A) Phospho-TrkB Western blot of cultured mouse cortical neurons after addition of BDNF (Left) and ZEB85 (Right). Embryonic day 21 mouse cortical neurons were cultured for 7 d. BDNF (100 ng/mL) or ZEB85 (50 μg/mL) was added, and cultures were harvested at 15–240 min and assayed for phospho-TrkB (arrow) by Western blot. Molecular weight markers are on the right. (B) Dendritic field analysis of RGCs in retinal explants after 3 d of treatment with ZEB44, ZEB85, BDNF, or vehicle (control). Neurons were labeled by DiI/DiO DiOlistics. Arrows indicate RGC axons. (Scale bars: 100 μm.) (C) Sholl analysis of dendritic fields of RGCs after TrkB agonist antibody or BDNF treatment. Numbers of RGCs are indicated. +P < 0.05, ZEB44 vs. vehicle (control); #P < 0.05 and ###P < 0.001, ZEB85 vs. vehicle; *P < 0.05, **P < 0.01, and ***P < 0.001, BDNF vs. vehicle, Kruskal–Wallis test. (D) AUC analysis of Sholl plots and (E) quantification of total dendrite length after various agonist treatments. *P < 0.05, **P < 0.01, and ***P < 0.005 vs. vehicle, ANOVA, Tukey post hoc test.
Discussion
The therapeutic potential of neurotrophic factors to treat neurological disorders has been assessed in more than 20 clinical trials targeting motor neuron disorders, Parkinson’s disease, Alzheimer’s disease, and peripheral neuropathies, disappointingly for the most part, with little or no clinical benefit (36). Among several obstacles that may have stymied these trials were poorly understood pharmacokinetics, limited pharmacodynamic markers, suboptimal biophysical properties, and unexpected clinical side effects of some of these biologic drug candidates. Whereas a clinical trial testing efficacy of systemically delivered BDNF in ∼1,000 patients with ALS over 3 y raised no safety concerns, the unfavorable physiochemical properties and short t1/2 of recombinant BDNF were most likely the major causes of failure to show clinically meaningful efficacy. As a highly charged homodimer, BDNF does not readily penetrate the blood–brain barrier (37), and therefore several alternative strategies have been undertaken to activate TrkB signaling within the CNS. These strategies include the search for small molecules that up-regulate BDNF expression (18) or those that activate TrkB directly. Although there have been multiple reports on the discovery of small-molecule TrkB agonists, recent comprehensive surveys using a wide range of assay systems failed to confirm that any of these agents activate TrkB in a manner consistent with TrkB phosphorylation and canonical signal transduction characteristics of BDNF (17, 21). Although not a direct resolution of the possible issue of limited blood–brain barrier penetration, TrkB agonist antibodies may be an attractive alternative to BDNF for certain therapeutic indications. Several such antibodies have already been reported and convincingly shown to activate TrkB in reporter systems or in neurons derived from induced pluripotent cells (16, 22, 38). By using human neurons derived from embryonic stem cells grown under conditions favoring the development of TrkB-responsive neurons, we report here on the detailed characterization of a TrkB activating antibody. We propose that the fully human TrkB agonist antibodies described here may help circumvent the poor drug-like qualities encountered with BDNF and allow us to revisit the therapeutic potential of TrkB agonists in certain neurological diseases.
The majority of clinically approved antibody drugs are antagonists that block specific receptors, such as the case for HER-2/EGF receptors (39, 40), or sequester specific proteins, as in the case of anti-TNF or anti-VEGF antibodies (41, 42). Conversely, among the vast chemical diversity represented by the human antibody repertoire, it is possible to identify antibodies that activate cell-surface receptors or ion channels. Such agonist antibodies are rare and thus harder to identify than blocking or neutralizing antibodies. Conventional mouse immunization/hybridoma technology or in vitro phage display combinatorial antibody technologies are effectively designed to select high-affinity binders to a target protein. Although activating antibodies may be among those binders, in practice, it has required technically demanding, low-throughput, low-yield secondary assays to identify and optimize any such agonists. To greatly increase the probability of finding such rare agonist antibodies, we have employed a previously described high-capacity function-based target receptor reporter cell system that facilitates the selection of agonist antibodies from combinatorial human antibody libraries with a large diversity (26, 27). Here, we have described an application of this technology to select agonist antibodies of a receptor tyrosine kinase, the BDNF receptor TrkB. In this study, we identified ∼50 agonist antibodies among a library of ∼10 billion scFv antibody fragments, illustrating the power of the technology to select rare events. Among these, we found a series of molecules with full and partial agonist activities toward TrkB and potencies in the picomolar to nanomolar range. Thus, in addition to the discovery of candidate therapeutic antibodies, our repertoire of TrkB agonist antibodies are likely to provide interesting tools to further dissect signal transduction pathways and biological responses affected by TrkB stimulation (43).
We chose to perform an in-depth characterization of one antibody, ZEB85, to compare its agonist properties to the cognate TrkB ligands, BDNF and NT4. Several lines of evidence suggest that ZEB85 is a potent, full agonist of human TrkB. First, concentration–response experiments in our sensitive TrkB reporter cell line (Fig. 2A) show that the maximal response to ZEB85 is approximately equal to that of BDNF and NT4 (see also Fig. 1B). Also, in the hES cell-derived neuronal cells, the Emax for TrkB phosphorylation was the same for ZEB85 compared with BDNF and NT4 (Fig. 4 B and D). As with Emax values, the potency of ZEB85 was similar to that of BDNF in the reporter cell line and neuronal cells, with EC50 values for BDNF in the 190–300 pM range and ∼500 pM in both cell types for ZEB85. These intrinsic potency values for BDNF are consistent with its reported EC50 on survival assays in cultured neurons (32). Second, agonist-induced levels of PLCγ, AKT, and ERK phosphorylation by ZEB85 in the reporter cell line are nearly identical to those of BDNF across time (Fig. 2E). Conversely, ZEB27 elicited submaximal canonical phosphorylation signaling, commensurate with its partial activity (∼20% of BDNF) observed in the reporter cell line assay. Third, and perhaps most striking, RNA-seq analyses on human neuron-like cells showed that the numbers and subsets of genes transcriptionally regulated by all three TrkB ligands were qualitatively similar over a 24-h time course when each agonist was applied as a single pulse. Quantitative algorithms and statistical analyses of gene expression changes greater than twofold revealed that the changes observed in gene expression were common to all three ligands at 30 min. This overlap was even more evident at 2 and 12 h post ligand addition, corroborating the like nature of ZEB85 and BDNF as TrkB agonists. It remains possible that the rates of ZEB85 application may reveal differences in TrkB signaling compared with BDNF (44), and this will be an important consideration for dosing regimens in future rodent efficacy studies.
Compared with activity on human TrkB in our reporter cell or in cultures of human neurons derived from hES cells, ZEB85 showed weaker effects toward rodent neurons in terms of TrkB phosphorylation on cultures of embryonic rat cortical neurons (Fig. 6A) or in preservation of the dendritic arbors of RGCs in explant cultures of adult mouse retina (Fig. 6 B–E). This is not surprising, given that the human TrkB receptor was used for phage panning and reporter cell selection and there are sequence differences in the extracellular domains of human vs. rat TrkB. With the ultimate objective of evaluating a TrkB agonist antibody for future clinical development, our primary focus has been to determine the extent of equivalency of binding potency, TrkB phosphorylation, downstream signaling cascades, and transcriptional activation of ZEB85 compared with BDNF in human neurons.
Quantitative PCR analyses of the most highly up-regulated genes showed that the well-annotated ERG gene family, transcription factors known to be regulated by the neurotrophins, was coordinately regulated by all three TrkB agonists with a similar time course and magnitude. Similarly, TrkB agonists increased the expression of ARC at 30 min (15-fold), peaking at 2 h (25-fold). BDNF has been shown to regulate ERG3 through activation of the protein kinase C pathway (45), and ERG3 transcriptional activity induces the expression of ARC (46), a protein involved in synaptic plasticity and long-term potentiation (47). In terms of synaptic function, ZEB85, BDNF, and NT4 treatment of neurons also induced expression of SYT2 (synaptotagmin-2), a Ca2+-sensing synaptic protein important for neurotransmitter release in striatal neurons (48). All three TrkB agonists tested also induced expression of ACAN (aggrecan; 60-fold at 2 h), an ECM protein critical in formation of perineural nets linked to maintenance of synapse integrity and neuroprotection (49). Additionally, ZEB85, BDNF, and NT4 down-regulated the proapoptotic gene HRK/DP5, which is known to be induced in neurons following neurotrophin deprivation (50), and up-regulated GADD45A and CDKN1A (p21), genes in pathways with known antiapoptotic, neuroprotective, and neurogenesis-related functions (51–54). These observations are consistent with the proposed neurotrophic role (55) and synaptic function of TrkB agonists and set the stage to examine the neuroprotective and neurorestorative effects of the full agonist ZEB85 in conditions of neuronal stress and neurodegeneration.
Derivation of mouse monoclonal TrkB agonist antibodies by conventional immunization techniques has previously been reported, demonstrating that it is possible to identify such agonists by standard hybridoma approaches. Qian et al. (23) identified mouse monoclonal antibody 29D7 and showed that it cross-reacted with human TrkB in stimulating receptor phosphorylation in a human TrkB reporter cell line and exhibited typical canonical signaling in these cells, with activities in the 100-pM range. The antibody also increased neurite outgrowth and survival of differentiated SH-SY5Y human neuroblastoma cells. A humanized version of this antibody, TAM-163, was derived and shown to be a partial agonist in human TrkB reporter cells with an EC50 of ∼1 nM. This antibody decreased body weight in mice but possessed the opposite effect in nonhuman primates (56). As with our antibody ZEB85, neither of these antibodies bound to p75.
In addition, a further mouse monoclonal TrkB agonist antibody, 38B8, has been reported to show agonist activity toward mouse and human TrkB in reporter cell lines and efficacy in models of obesity and metabolic disease (57). Traub et al. (38) have reported the generation of two humanized TrkB agonist antibodies (AB2 and AB20) by grafting the complementary determining regions of two mouse monoclonal antibodies to separate human IgG backbones and characterized these antibodies on TrkB reporter cells and iPS-derived neurons (GRINCH neurons). The EC50 values for BDNF were 540 pM in their reporter cell line and 25 pM in GRINCH neurons. Corresponding values for AB2 were 390 pM and 67 pM, whereas antibody AB20 showed EC50 values of 404 pM in the reporter cell line and 80 pM in GRINCH neurons. In these studies, RNA-seq was also performed in the GRINCH neurons treated with BDNF and agonist antibodies for 6 h. In contrast to our observation of extensive transcriptional regulation of many hundreds of genes in hES cell-derived neurons treated with ZEB85, the Traub et al. study reported changes in mRNA levels (greater than the twofold threshold) in less than 50 genes. Possible explanations for the quantitative discrepancy may lie in the different cell types used in the two studies—iPSCs vs. ES cells—and differences in the differentiation protocols to generate neurons. Further, the hES cell neurons derived in the present study were shown to be highly enriched for the GABAergic phenotype (>75%) and also expressed functional TrkB as shown by c-Fos induction with BDNF or ZEB85. Conversely, the GRINCH neuron-like cultures derived by Traub et al. (58) have been shown to express a variety of different neurotransmitter phenotypes. The percentage of cells responding to BDNF in these cultures was not reported (59, 60).
In summary, we have demonstrated that a function-based reporter cell system can be employed effectively to screen combinatorial antibody libraries to select agonist (i.e., activating) antibodies for the receptor tyrosine kinase, TrkB, the cognate receptor for the neurotrophic factor BDNF. Although we have presented detailed characterization of one such antibody, ZEB85, the methodology yields an interesting array of antibodies over the range of full and partial agonists. Our approach may have several benefits in the search for potentially therapeutic biologic drugs. Most notably, a TrkB agonist antibody circumvents the biophysical and pharmacokinetic pitfalls of BDNF. Also, antibodies offer flexibility of format in terms of delivery to different compartments, including systemic or localized injections to improve t1/2. Finally, an agonist antibody has the potential to overcome immunogenicity that has been problematic with several recombinant human growth factors. Although, initially, almost all human recombinant proteins generate a transient immune response when administered to humans, the majority of such initial responses do not lead to the generation of neutralizing antibodies. However, in the case of ciliary neurotrophic factor and thrombopoietin, s.c. injections of recombinant human versions of these small proteins induced neutralizing antibodies in patients, resulting in the termination of clinical trials. Thus, a fully human agonist antibody may circumvent this issue. We conclude that the use of our function-based screening approach successfully selected multiple rare TrkB agonist antibodies and may serve as a template to aid the selection of agonist antibodies to other receptor tyrosine kinases.
Materials and Methods
Detailed descriptions of study materials and methods are provided in SI Appendix, Methods S1, including all reagents and protocols for phage panning and lentivirus preparation; reporter-based functional selection, expression, purification, and characterization of scFv–Fc fusion proteins; molecular biology reagents; hES cell neural differentiation; construction of cDNA libraries and RNA-seq, quantitative RT-PCR, cell lysis, and protein extraction; Western blotting; immunofluorescence; mouse cortical neuron cultures; and mouse retinal explants.
Supplementary Material
Acknowledgments
We thank Angela Marchbank for assistance with RNA-seq experiments. This work was supported by Zebra Biologics and by the Sêr Cymru programme of the Welsh Government.
Footnotes
Conflict of interest statement: This work was supported by Zebra Biologics. P.S.D. and R.M.L. are affiliated with Zebra Biologics.
Data deposition: Next-generation sequencing data related to this study have been uploaded in ArrayExpress (accession no. E-MTAB-6975).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1806660115/-/DCSupplemental.
References
- 1.Mitre M, Mariga A, Chao MV. Neurotrophin signalling: Novel insights into mechanisms and pathophysiology. Clin Sci (Lond) 2017;131:13–23. doi: 10.1042/CS20160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagahara AH, Tuszynski MH. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat Rev Drug Discov. 2011;10:209–219. doi: 10.1038/nrd3366. [DOI] [PubMed] [Google Scholar]
- 3.Egan MF, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell. 2003;112:257–269. doi: 10.1016/s0092-8674(03)00035-7. [DOI] [PubMed] [Google Scholar]
- 4.Yeo GS, et al. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat Neurosci. 2004;7:1187–1189. doi: 10.1038/nn1336. [DOI] [PubMed] [Google Scholar]
- 5.Friedel S, et al. Mutation screen of the brain derived neurotrophic factor gene (BDNF): Identification of several genetic variants and association studies in patients with obesity, eating disorders, and attention-deficit/hyperactivity disorder. Am J Med Genet B Neuropsychiatr Genet. 2005;132B:96–99. doi: 10.1002/ajmg.b.30090. [DOI] [PubMed] [Google Scholar]
- 6.Pasutto F, et al. Heterozygous NTF4 mutations impairing neurotrophin-4 signaling in patients with primary open-angle glaucoma. Am J Hum Genet. 2009;85:447–456. doi: 10.1016/j.ajhg.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vithana EN, et al. Identification of a novel mutation in the NTF4 gene that causes primary open-angle glaucoma in a Chinese population. Mol Vis. 2010;16:1640–1645. [PMC free article] [PubMed] [Google Scholar]
- 8.Thoenen H, Hughes RA, Sendtner M. Trophic support of motoneurons: Physiological, pathophysiological, and therapeutic implications. Exp Neurol. 1993;124:47–55. doi: 10.1006/exnr.1993.1173. [DOI] [PubMed] [Google Scholar]
- 9.Ikeda K, et al. Effects of brain-derived neurotrophic factor on motor dysfunction in wobbler mouse motor neuron disease. Ann Neurol. 1995;37:505–511. doi: 10.1002/ana.410370413. [DOI] [PubMed] [Google Scholar]
- 10.Anonymous A controlled trial of recombinant methionyl human BDNF in ALS: The BDNF Study Group (phase III) Neurology. 1999;52:1427–1433. doi: 10.1212/wnl.52.7.1427. [DOI] [PubMed] [Google Scholar]
- 11.Ochs G, et al. A phase I/II trial of recombinant methionyl human brain derived neurotrophic factor administered by intrathecal infusion to patients with amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:201–206. doi: 10.1080/14660820050515197. [DOI] [PubMed] [Google Scholar]
- 12.Morse JK, et al. Brain-derived neurotrophic factor (BDNF) prevents the degeneration of medial septal cholinergic neurons following fimbria transection. J Neurosci. 1993;13:4146–4156. doi: 10.1523/JNEUROSCI.13-10-04146.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Croll SD, et al. Co-infusion with a TrkB-Fc receptor body carrier enhances BDNF distribution in the adult rat brain. Exp Neurol. 1998;152:20–33. doi: 10.1006/exnr.1998.6836. [DOI] [PubMed] [Google Scholar]
- 14.Wilkie N, et al. The non-peptidyl fungal metabolite L-783,281 activates TRK neurotrophin receptors. J Neurochem. 2001;78:1135–1145. doi: 10.1046/j.1471-4159.2001.00504.x. [DOI] [PubMed] [Google Scholar]
- 15.Jang SW, et al. A selective TrkB agonist with potent neurotrophic activities by 7,8-dihydroxyflavone. Proc Natl Acad Sci USA. 2010;107:2687–2692. doi: 10.1073/pnas.0913572107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cazorla M, Arrang JM, Prémont J. Pharmacological characterization of six trkB antibodies reveals a novel class of functional agents for the study of the BDNF receptor. Br J Pharmacol. 2011;162:947–960. doi: 10.1111/j.1476-5381.2010.01094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Todd D, et al. A monoclonal antibody TrkB receptor agonist as a potential therapeutic for Huntington’s disease. PLoS One. 2014;9:e87923. doi: 10.1371/journal.pone.0087923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deogracias R, et al. Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proc Natl Acad Sci USA. 2012;109:14230–14235. doi: 10.1073/pnas.1206093109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simmons DA, et al. Up-regulating BDNF with an ampakine rescues synaptic plasticity and memory in Huntington’s disease knockin mice. Proc Natl Acad Sci USA. 2009;106:4906–4911. doi: 10.1073/pnas.0811228106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blanco RE, Soto I, Duprey-Díaz M, Blagburn JM. Up-regulation of brain-derived neurotrophic factor by application of fibroblast growth factor-2 to the cut optic nerve is important for long-term survival of retinal ganglion cells. J Neurosci Res. 2008;86:3382–3392. doi: 10.1002/jnr.21793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boltaev U, et al. Multiplex quantitative assays indicate a need for reevaluating reported small-molecule TrkB agonists. Sci Signal. 2017;10:eaal1670. doi: 10.1126/scisignal.aal1670. [DOI] [PubMed] [Google Scholar]
- 22.Bai Y, et al. An agonistic TrkB mAb causes sustained TrkB activation, delays RGC death, and protects the retinal structure in optic nerve axotomy and in glaucoma. Invest Ophthalmol Vis Sci. 2010;51:4722–4731. doi: 10.1167/iovs.09-5032. [DOI] [PubMed] [Google Scholar]
- 23.Qian MD, et al. Novel agonist monoclonal antibodies activate TrkB receptors and demonstrate potent neurotrophic activities. J Neurosci. 2006;26:9394–9403. doi: 10.1523/JNEUROSCI.1118-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simmons DA, et al. A small molecule TrkB ligand reduces motor impairment and neuropathology in R6/2 and BACHD mouse models of Huntington’s disease. J Neurosci. 2013;33:18712–18727. doi: 10.1523/JNEUROSCI.1310-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmid DA, et al. A TrkB small molecule partial agonist rescues TrkB phosphorylation deficits and improves respiratory function in a mouse model of Rett syndrome. J Neurosci. 2012;32:1803–1810. doi: 10.1523/JNEUROSCI.0865-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang H, et al. Selecting agonists from single cells infected with combinatorial antibody libraries. Chem Biol. 2013;20:734–741. doi: 10.1016/j.chembiol.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Xie J, Zhang H, Yea K, Lerner RA. Autocrine signaling based selection of combinatorial antibodies that transdifferentiate human stem cells. Proc Natl Acad Sci USA. 2013;110:8099–8104. doi: 10.1073/pnas.1306263110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Telezhkin V, et al. Forced cell cycle exit and modulation of GABAA, CREB, and GSK3β signaling promote functional maturation of induced pluripotent stem cell-derived neurons. Am J Physiol Cell Physiol. 2016;310:C520–C541. doi: 10.1152/ajpcell.00166.2015. [DOI] [PubMed] [Google Scholar]
- 29.Arber C, et al. Activin A directs striatal projection neuron differentiation of human pluripotent stem cells. Development. 2015;142:1375–1386. doi: 10.1242/dev.117093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cambray S, et al. Activin induces cortical interneuron identity and differentiation in embryonic stem cell-derived telencephalic neural precursors. Nat Commun. 2012;3:841. doi: 10.1038/ncomms1817. [DOI] [PubMed] [Google Scholar]
- 31.Zhang H, et al. Autocrine selection of a GLP-1R G-protein biased agonist with potent antidiabetic effects. Nat Commun. 2015;6:8918. doi: 10.1038/ncomms9918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Tébar A, Barde YA. Binding characteristics of brain-derived neurotrophic factor to its receptors on neurons from the chick embryo. J Neurosci. 1988;8:3337–3342. doi: 10.1523/JNEUROSCI.08-09-03337.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mariga A, Zavadil J, Ginsberg SD, Chao MV. Withdrawal of BDNF from hippocampal cultures leads to changes in genes involved in synaptic function. Dev Neurobiol. 2015;75:173–192. doi: 10.1002/dneu.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gokce O, Runne H, Kuhn A, Luthi-Carter R. Short-term striatal gene expression responses to brain-derived neurotrophic factor are dependent on MEK and ERK activation. PLoS One. 2009;4:e5292. doi: 10.1371/journal.pone.0005292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Binley KE, Ng WS, Barde YA, Song B, Morgan JE. Brain-derived neurotrophic factor prevents dendritic retraction of adult mouse retinal ganglion cells. Eur J Neurosci. 2016;44:2028–2039. doi: 10.1111/ejn.13295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartus RT, Johnson EM., Jr Clinical tests of neurotrophic factors for human neurodegenerative diseases, part 2: Where do we stand and where must we go next? Neurobiol Dis. 2017;97:169–178. doi: 10.1016/j.nbd.2016.03.026. [DOI] [PubMed] [Google Scholar]
- 37.Pardridge WM, Kang YS, Buciak JL. Transport of human recombinant brain-derived neurotrophic factor (BDNF) through the rat blood-brain barrier in vivo using vector-mediated peptide drug delivery. Pharm Res. 1994;11:738–746. doi: 10.1023/a:1018940732550. [DOI] [PubMed] [Google Scholar]
- 38.Traub S, et al. Pharmaceutical characterization of tropomyosin receptor kinase B-agonistic antibodies on human induced pluripotent stem (hiPS) cell-derived neurons. J Pharmacol Exp Ther. 2017;361:355–365. doi: 10.1124/jpet.117.240184. [DOI] [PubMed] [Google Scholar]
- 39.Giusti RM, Shastri KA, Cohen MH, Keegan P, Pazdur R. FDA drug approval summary: Panitumumab (Vectibix) Oncologist. 2007;12:577–583. doi: 10.1634/theoncologist.12-5-577. [DOI] [PubMed] [Google Scholar]
- 40.Wong DJ, Hurvitz SA. Recent advances in the development of anti-HER2 antibodies and antibody-drug conjugates. Ann Transl Med. 2014;2:122. doi: 10.3978/j.issn.2305-5839.2014.08.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen DL, et al. Optimizing the use of anti-tumor necrosis factor in the management of patients with Crohn’s disease. Ther Adv Chronic Dis. 2015;6:147–154. doi: 10.1177/2040622315579621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun. 2005;333:328–335. doi: 10.1016/j.bbrc.2005.05.132. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 44.Ji Y, et al. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat Neurosci. 2010;13:302–309. doi: 10.1038/nn.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts DS, Hu Y, Lund IV, Brooks-Kayal AR, Russek SJ. Brain-derived neurotrophic factor (BDNF)-induced synthesis of early growth response factor 3 (Egr3) controls the levels of type A GABA receptor alpha 4 subunits in hippocampal neurons. J Biol Chem. 2006;281:29431–29435. doi: 10.1074/jbc.C600167200. [DOI] [PubMed] [Google Scholar]
- 46.Yin Y, Edelman GM, Vanderklish PW. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci USA. 2002;99:2368–2373. doi: 10.1073/pnas.042693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li L, Carter J, Gao X, Whitehead J, Tourtellotte WG. The neuroplasticity-associated arc gene is a direct transcriptional target of early growth response (Egr) transcription factors. Mol Cell Biol. 2005;25:10286–10300. doi: 10.1128/MCB.25.23.10286-10300.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pang ZP, et al. Synaptotagmin-2 is essential for survival and contributes to Ca2+ triggering of neurotransmitter release in central and neuromuscular synapses. J Neurosci. 2006;26:13493–13504. doi: 10.1523/JNEUROSCI.3519-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Morawski M, Brückner G, Jäger C, Seeger G, Arendt T. Neurons associated with aggrecan-based perineuronal nets are protected against tau pathology in subcortical regions in Alzheimer’s disease. Neuroscience. 2010;169:1347–1363. doi: 10.1016/j.neuroscience.2010.05.022. [DOI] [PubMed] [Google Scholar]
- 50.Imaizumi K, et al. Critical role for DP5/Harakiri, a Bcl-2 homology domain 3-only Bcl-2 family member, in axotomy-induced neuronal cell death. J Neurosci. 2004;24:3721–3725, and erratum (2004) 24:4488. doi: 10.1523/JNEUROSCI.5101-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ma DK, et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–1077. doi: 10.1126/science.1166859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu B, et al. Gadd45b is a novel mediator of neuronal apoptosis in ischemic stroke. Int J Biol Sci. 2015;11:353–360. doi: 10.7150/ijbs.9813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Langley B, et al. Pulse inhibition of histone deacetylases induces complete resistance to oxidative death in cortical neurons without toxicity and reveals a role for cytoplasmic p21(waf1/cip1) in cell cycle-independent neuroprotection. J Neurosci. 2008;28:163–176. doi: 10.1523/JNEUROSCI.3200-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Harms C, et al. Phosphatidylinositol 3-Akt-kinase-dependent phosphorylation of p21(Waf1/Cip1) as a novel mechanism of neuroprotection by glucocorticoids. J Neurosci. 2007;27:4562–4571. doi: 10.1523/JNEUROSCI.5110-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen SD, Wu CL, Hwang WC, Yang DI. More insight into BDNF against neurodegeneration: Anti-apoptosis, anti-oxidation, and suppression of autophagy. Int J Mol Sci. 2017;18:E545. doi: 10.3390/ijms18030545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perreault M, et al. Activation of TrkB with TAM-163 results in opposite effects on body weight in rodents and non-human primates. PLoS One. 2013;8:e62616. doi: 10.1371/journal.pone.0062616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tsao D, et al. TrkB agonists ameliorate obesity and associated metabolic conditions in mice. Endocrinology. 2008;149:1038–1048. doi: 10.1210/en.2007-1166. [DOI] [PubMed] [Google Scholar]
- 58.Traub S, Stahl H, Rosenbrock H, Simon E, Heilker R. Upscaling of hiPS cell-derived neurons for high-throughput screening. SLAS Discov. 2017;22:274–286. doi: 10.1177/1087057116678161. [DOI] [PubMed] [Google Scholar]
- 59.Chambers SM, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280, and erratum (2009) 27:485. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Goslin K, Banker G. Experimental observations on the development of polarity by hippocampal neurons in culture. J Cell Biol. 1989;108:1507–1516. doi: 10.1083/jcb.108.4.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.