Significance
α-Synuclein (α-Syn) aggregation underlies neurodegeneration in synucleinopathies. However, the nature of α-Syn aggregates and their toxic mechanisms in human pathology remains elusive. Here, we delineate a role of α-Syn oligomeric aggregates for axonal integrity in human neuronal models of synucleinopathies. α-Syn oligomers disrupt anterograde axonal transport of mitochondria by causing subcellular changes in transport-regulating proteins and energy deficits. An increase of α-Syn oligomers in human neurons finally results in synaptic degeneration. Together, our data provide mechanistic insights of α-Syn oligomeric toxicity in human neurons. Taking into account that α-Syn oligomers and axonal dysfunction are characteristic for early neurodegeneration in synucleinopathies, our data might deliver targets for therapeutic interference with early disease pathology.
Keywords: α-synuclein, oligomers, axonal transport, synucleinopathies, neurodegeneration
Abstract
α-Synuclein (α-Syn) aggregation, proceeding from oligomers to fibrils, is one central hallmark of neurodegeneration in synucleinopathies. α-Syn oligomers are toxic by triggering neurodegenerative processes in in vitro and in vivo models. However, the precise contribution of α-Syn oligomers to neurite pathology in human neurons and the underlying mechanisms remain unclear. Here, we demonstrate the formation of oligomeric α-Syn intermediates and reduced axonal mitochondrial transport in human neurons derived from induced pluripotent stem cells (iPSC) from a Parkinson’s disease patient carrying an α-Syn gene duplication. We further show that increased levels of α-Syn oligomers disrupt axonal integrity in human neurons. We apply an α-Syn oligomerization model by expressing α-Syn oligomer-forming mutants (E46K and E57K) and wild-type α-Syn in human iPSC-derived neurons. Pronounced α-Syn oligomerization led to impaired anterograde axonal transport of mitochondria, which can be restored by the inhibition of α-Syn oligomer formation. Furthermore, α-Syn oligomers were associated with a subcellular relocation of transport-regulating proteins Miro1, KLC1, and Tau as well as reduced ATP levels, underlying axonal transport deficits. Consequently, reduced axonal density and structural synaptic degeneration were observed in human neurons in the presence of high levels of α-Syn oligomers. Together, increased dosage of α-Syn resulting in α-Syn oligomerization causes axonal transport disruption and energy deficits, leading to synapse loss in human neurons. This study identifies α-Syn oligomers as the critical species triggering early axonal dysfunction in synucleinopathies.
Physiologically, α-synuclein (α-Syn) is a presynaptic protein, which, when multiplied or mutated, is causative for a group of neurodegenerative disorders termed “synucleinopathies.” The pathological feature of synucleinopathies is α-Syn aggregates that evolve from small oligomers to fibrillar aggregates and accumulate in intracytoplasmic and intraneuritic deposits called Lewy bodies (LB) and Lewy neurites (LN), respectively (1). The presence of LB and LN is the major hallmark of synucleinopathies. For example, a widespread cortical Lewy pathology is evident in the majority of Parkinson’s disease (PD) cases carrying an α-Syn gene duplication (Dupl) (2). Although fibrillar α-Syn is the main component of LB and LN, α-Syn oligomers have been recently reported to characterize early stage lesions of synucleinopathies in PD patients’ postmortem brains (3), emphasizing their role in disease onset by yet largely unknown mechanisms.
In rodents, α-Syn overexpression and oligomerization resulted in axonopathy, neuritic and synaptic degeneration preceding neuronal death (4–8). Considered to be early pathogenic events of synucleinopathies, they might be attributed to cytoskeletal defects. Indeed, α-Syn oligomers specifically inhibit tubulin polymerization (9, 10), and α-Syn accumulation accompanies disruption of microtubules in cell lines (11, 12). We previously revealed a critical role of α-Syn oligomers for kinesin-microtubule motility in a cell-free system (13). Furthermore, impaired microtubule-dependent trafficking was associated with α-Syn aggregates in zebrafish and rodent neuronal models (11, 14, 15). Notably, alterations of axonal transport proteins were reported in neurons with α-Syn inclusions in postmortem PD brains (16) and in rat striatum and substantia nigra upon overexpression of the human A53T mutant α-Syn (8). Of note, oligomers of amyloid-β, a crucial protein for neurodegeneration in Alzheimer’s disease, selectively impaired fast transport of mitochondria (17) and induced neuritic dystrophy (18), suggesting a particular toxicity of oligomeric protein assemblies to neurite function. However, the specific role of α-Syn oligomers in the regulation of axonal transport and involvement of axonal transport aberrations in human synucleinopathies remains elusive.
In the present study, we analyze the impact of α-Syn oligomers generated by an α-Syn dosage increase on axonal transport in human neurons. First, we demonstrate an impaired axonal transport of mitochondria accompanied by increased α-Syn aggregation and formation of α-Syn intermediates in neurons derived from induced pluripotent stem cells (iPSC) of a PD patient with α-Syn Dupl. To better understand the impact of α-Syn aggregation, we established a model that allows the investigation of distinct α-Syn species in human iPSC-derived neurons by introducing wild-type human α-Syn (WTS) or its oligomer-forming mutants (E46K and E57K). Specific impairment of anterograde mitochondrial transport was observed upon pronounced α-Syn oligomerization in E46K and E57K neurons and was partly rescued by inhibiting α-Syn oligomer formation. α-Syn oligomer-induced transport alterations were accompanied by reduced ATP levels, a pathologic subcellular relocation of proteins regulating axonal transport, and an apparent synaptic degeneration. In summary, we demonstrate a link between α-Syn oligomers and dysfunctional mitochondrial axonal transport with a consequent synaptic degeneration in human neurons as an early phenotype of synucleinopathies.
Results
Reduction of Mitochondrial Axonal Transport and Enhanced α-Syn Aggregation in Human Neurons Carrying an α-Syn Dupl.
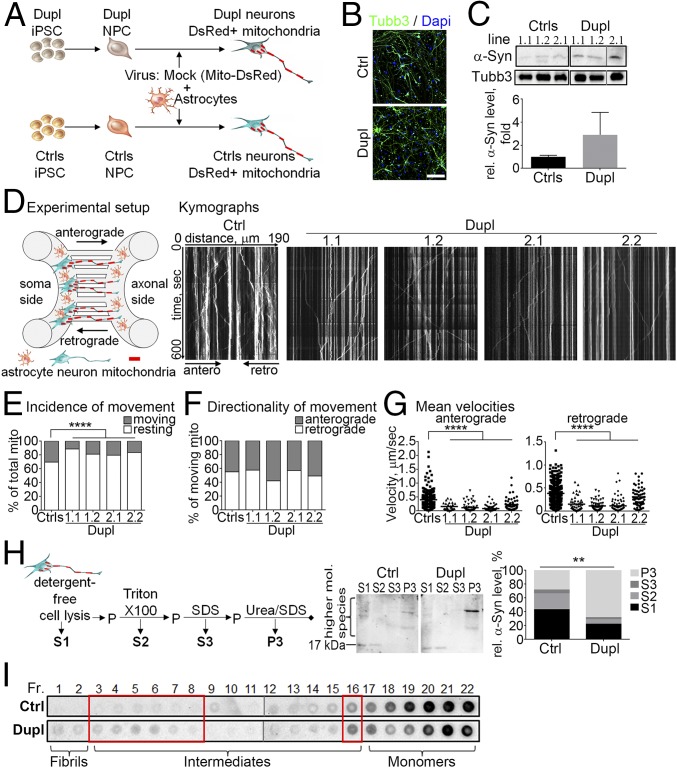
To investigate whether a cell-autonomously elevated intracellular level of α-Syn affects axonal transport in human neurons, we generated iPSC from fibroblasts of a PD patient carrying an α-Syn Dupl and from healthy individuals [controls (Ctrls); Fig. 1A]. Ctrls and Dupl iPSC differentiated equally well into neurons based on expression of the neuronal marker β3-tubulin (Tubb3; Fig. 1B). IPSC were differentiated to cortical forebrain glutamatergic neurons since dementia and widespread cortical Lewy pathology have been reported for the majority of α-Syn Dupl PD cases (2), including our patient. Increased protein levels of α-Syn were detected in Dupl neurons compared with Ctrl neurons (Fig. 1C).
Fig. 1.
Human iPSC-derived PD Dupl neurons reveal increased α-Syn aggregation and impaired axonal transport. (A) IPSC from a PD Dupl patient and Ctrls were differentiated into NPC and neurons and infected with a Mito-DsRed lentivirus. (B) Ctrls and Dupl neurons expressed neuronal marker β3-tubulin (Tubb3, green). (Scale bar: 100 µm.) (C) Elevated α-Syn protein expression in Dupl neurons (three NPC lines: 1.1 and 1.2 from the Dupl1 iPSC clone and 2.1 from the Dupl2 iPSC clone) compared with Ctrls (three iPSC clones from two individuals: 1.1 and 1.2 from Ctrl1 and 2.1 from Ctrl2) by Western blot (WB). (D) Neurons were differentiated in microfluidic devices for 20 d, and transport of Mito-DsRed–positive mitochondria was measured (Experimental setup). Representative kymographs of Ctrl and four Dupl lines: three NPC lines as in C and the additional NPC line 2.2 from the Dupl2 iPSC clone. (E) Less frequently moving mitochondria (mito) in Dupl neuronal lines compared with Ctrls. (F) No differences in movement directionality. (G) Significantly impaired velocities of anterograde and retrograde transport in Dupl neurons. At least 20 neurites per line were tracked. Results from three different iPSC clones from two control individuals are shown collectively as Ctrls in E–G. (H) Neurons were lysed sequentially, and the amount of α-Syn was determined in the detergent-free soluble fraction (S1), Triton X-100–soluble (S2), SDS-soluble (S3), and urea/SDS-soluble (P3) fractions (Left) by WB using an anti–α-Syn Ab Syn1 (Middle: representative Ctrl and Dupl lines). Increase of insoluble α-Syn (P3 fraction) in Dupl neurons (Middle and Right). mol., molecular; P, pellet. (I) Analysis of α-Syn species by sucrose density gradient centrifugation revealed the increased presence of α-Syn aggregation intermediates in Dupl neurons (representative Ctrl and Dupl lines; red boxes). Dot blots within one black box were from the same membrane. Fractions (Fr.) 1–11 and 12–22 of each sample (Ctrl or Dupl) were from different parts of the same membrane (separated by gray lines). Fr. 1–22 correspond to sucrose concentrations gradiently decreasing from 60 to 10%. **P ≤ 0.01; ****P ≤ 0.01.
To analyze axonal transport in human neurons, Ctrls and Dupl iPSC-derived neuronal precursor cells (NPC) were differentiated into neurons in microfluidic chambers (Fig. 1D). Mitochondria were visualized by an overexpression of Mito-DsRed (Fig. 1 A and D). Due to the unidirectional growth of axons, we were able to specifically evaluate anterograde (soma to axonal terminal) and retrograde (axonal terminal to soma) transport using kymographs (Fig. 1D). The incidence of mitochondrial movement was decreased in Dupl neurons (Fig. 1E), while the directionality of movement was unchanged with slight variations between NPC lines (Fig. 1F). A significant reduction of the mean velocity of mitochondrial movement was determined in Dupl neurons for both anterograde and retrograde transport (P < 0.0001, Fig. 1G), indicating a bidirectional disruption of axonal transport in neurons in the presence of increased α-Syn levels.
We next asked whether axonal transport alterations in Dupl neurons occur coincidently with α-Syn aggregation. We assessed α-Syn solubility in Dupl neurons by sequential extraction of proteins first with detergent-free buffer (soluble fraction S1) and subsequently with buffers containing detergents of increasing solubilization capacity, resulting in Triton X-100–soluble (S2), SDS-soluble (S3), and urea/SDS-soluble (P3) fractions (Fig. 1H). An increase of α-Syn in the P3 fraction and thus a decreased solubility of α-Syn in Dupl neurons was observed (P < 0.01 for the P3 fraction, Fig. 1H), indicating an ongoing α-Syn aggregation in these neurons. A further assessment of the composition of α-Syn aggregates by sucrose density gradient centrifugation using a calibration with preformed characterized α-Syn species (19) revealed an increased signal in several fractions enriched for α-Syn aggregation intermediates in Dupl neurons (red boxes, Fig. 1I). These results imply a tight link between axonal transport alterations of mitochondria and enhanced formation of early α-Syn intermediate species in human neurons.
Increased α-Syn Oligomerization Coincides with Impaired Anterograde Axonal Transport.
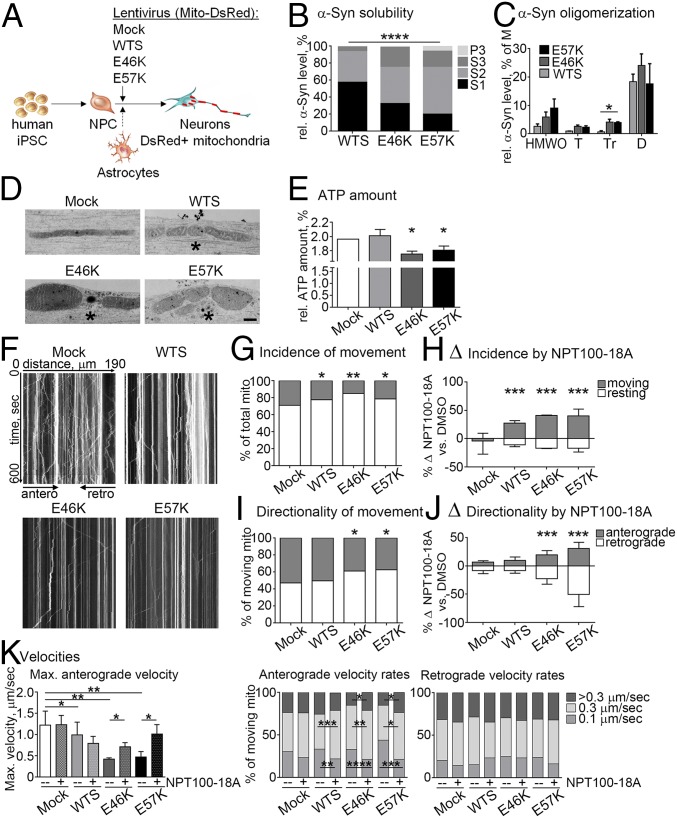
Aggregation of α-Syn is a multistep process involving different aggregation states with intermediate oligomeric species as initial steps. To understand the early stage pathology and to dissect the toxic nature of α-Syn intermediate species in human neurons, we aimed to decipher the effect of α-Syn oligomers on axonal transport. We thus modeled the process of α-Syn aggregation by introducing WTS, the familial α-Syn mutant E46K, and the nonnaturally occurring oligomer-prone E57K α-Syn mutant (6) into Ctrl iPSC-derived neurons by lentiviral (LV) infection (Fig. 2A). Mito-DsRed was encoded by each LV vector to visualize mitochondria (Fig. 2A). The efficiency of LV infection was similarly high under all conditions (97–100%, SI Appendix, Fig. S1A), resulting in comparable expression levels of WTS and α-Syn mutants in human iPSC-derived neurons (SI Appendix, Fig. S1B). Sequential protein extraction revealed a significant increase of α-Syn levels in the less- and insoluble fractions (S2, S3, and P3) in α-Syn mutants, in particular E57K, compared with WTS (P value of the interaction between α-Syn variants and relative fraction size ≤0.0001, Fig. 2B), pointing toward a strong aggregation process of α-Syn in E46K- and E57K-expressing neurons. To further verify the presence of α-Syn oligomers, we performed size exclusion chromatography (SEC) calibrated with preformed oligomeric species of recombinant α-Syn to assign α-Syn monomers and oligomers of different sizes to SEC fractions. This analysis showed significantly enhanced levels of α-Syn in the fraction enriched for trimers in E46K and E57K neurons compared with WTS (Fig. 2C). Thus, the expression of E46K and E57K in human neurons increases the proportion of small α-Syn oligomers.
Fig. 2.
Increased α-Syn oligomerization and impaired anterograde axonal transport in iPSC-derived neurons expressing α-Syn mutants. (A) Control iPSC-derived neurons (20 d of differentiation) were infected with WTS or α-Syn mutants (E46K or E57K) and Mito-DsRed lentivirus. (B) An increase of insoluble α-Syn [Triton X-100–soluble (S2), SDS-soluble (S3), and urea/SDS-soluble (P3) fractions] was observed in E57K and E46K neurons. S1, a detergent-free soluble fraction. (C) Soluble α-Syn multimers were analyzed by size exclusion chromatography. Soluble α-Syn trimers (Tr) were significantly increased in E46K and E57K lysates compared with WTS. D, dimers; HMWO, higher-molecular-weight oligomers; M, monomers; T, tetramers. (D) Clustering (asterisks) and shortening of mitochondria in neurites with α-Syn oligomers (E46K, E57K) compared with elongated mitochondria in control neurons (Mock). (Scale bar: 500 nm.) (E) ATP levels were significantly reduced in neurons expressing the α-Syn mutants E46K and E57K. (F) Representative kymographs. (G) Less frequently moving mitochondria were found in all α-Syn overexpressing neurons and (H) can be restored by NPT100-18A. Positive deltas indicate an increase and negative deltas indicate a decrease of a value in NPT100-18A compared with DMSO (diluent control in all NPT100-18A experiments). (I) Neurons expressing α-Syn mutants (E46K and E57K) revealed a lower incidence of anterograde axonal transport, which was significantly increased by NPT100-18A (J). (K) Decreased maximal (max.) anterograde velocities in WTS, E46K, and E57K neurons, which were improved by NPT100-18A in α-Syn mutant neurons (Left). NPT100-18A reduced slow-moving (0.1 µm/s) and increased middle-speed (0.3 µm/s) anterograde mitochondria frequencies in WTS, E46K, and E57K neurons (Middle). Significant increase of fast-moving (>0.3 µm/s) anterograde mitochondria by NPT100-18A was observed in E46K and E57K neurons (Middle). No changes of retrograde axonal transport were detected independently of NPT100-18A usage (Right). *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, ****P ≤ 0.0001. Data are presented as mean ± SD in H, J, and K and as mean ± SEM in C. Data from two independent iPSC clones from Ctrl1 are presented collectively in H, J, and K.
In conditions of increased α-Syn oligomerization, we observed clustering of thickened and short mitochondria in E46K and E57K neurites compared with thin and elongated mitochondria within control neurites (asterisk, Fig. 2D). A mixture of short and long, partly clustered mitochondria was found in WTS neurons (Fig. 2D). The mitochondrial phenotype observed in E46K and E57K neurons coincided with significantly decreased amounts of ATP measured in these neurons, but not in WTS neurons (Fig. 2E).
Furthermore, α-Syn oligomerization propensities were translated into profound differences in mitochondrial axonal transport in human neurons (representative kymographs, Fig. 2F). Specifically, the movement incidence was significantly reduced in WTS, E46K, and E57K neurons (Fig. 2G), whereas the presence of α-Syn oligomers significantly changed the directionality of movement toward decreased frequency of anterograde transport in E46K and E57K neurons (Fig. 2I). Together, while increased dosage of α-Syn due to either genetic or LV overexpression results in an overall reduction of mitochondrial movement frequency, α-Syn oligomers specifically decrease the anterograde axonal transport accompanied by reduced ATP levels.
Interfering with α-Syn Oligomer Formation Improves Mitochondrial Axonal Transport.
To better link α-Syn oligomers and dysfunctional mitochondrial anterograde transport, we used the de novo-developed compound NPT100-18A that reduces α-Syn toxicity in PD models by interfering with α-Syn oligomer formation (20, 21). NPT100-18A restored mitochondrial movement incidence in WTS, E46K, and E57K neurons (Fig. 2H and SI Appendix, Fig. S2A) and improved movement directionality toward increased anterograde motility without influencing retrograde transport frequency in α-Syn mutant neurons (Fig. 2J and SI Appendix, Fig. S2B).
In line with impaired movement incidence, expression of WTS, E46K, and E57K led to significantly reduced numbers of mitochondria within axons compared with Mock, while the mitochondrial numbers within axons were increased by NPT100-18A in E46K and E57K, but not in WTS neurons (SI Appendix, Fig. S2C). Accordingly, impaired maximal anterograde velocities were increased by NPT100-18A in E46K and E57K, but not in WTS neurons (Fig. 2K, Left).
Furthermore, reduced mean velocities and decreased frequencies of fast moving (>0.3 µm/s) mitochondria in anterograde direction were detected in E46K and E57K neurons compared with Mock (Fig. 2K and SI Appendix, Fig. S2D, respectively). Whereas mean anterograde velocity was improved only in E57K neurons (SI Appendix, Fig. S2D), NPT100-18A significantly reduced the amount of slow-moving (0.1 µm/s), increased the amount of middle-speed (0.3 µm/s), and restored to a control level the amount of fast-moving (>0.3 µm/s) anterograde mitochondria in E46K and E57K neurons (Fig. 2K, Middle).
In WTS neurons, NPT100-18A inhibited the rate of slow-moving and increased the rate of middle-speed mitochondria, but did not influence the numbers of fast-moving anterograde mitochondria, which were not altered compared with Mock in the absence of the compound (Fig. 2K). Mean anterograde velocity revealed only a slight, not significant, reduction in WTS compared with Mock neurons and was not affected by NPT100-18A (SI Appendix, Fig. S2D). No changes of retrograde mitochondrial axonal transport and no influence of NPT100-18A on this transport were found (Fig. 2K and SI Appendix, Fig. S2D, Right). Of note, the highest efficiency of NPT100-18A in improving anterograde mitochondrial transport in α-Syn oligomer-prone mutant neurons was accompanied by its ability to reduce insoluble α-Syn levels in these neurons, indicative of reduced α-Syn aggregation (SI Appendix, Fig. S2E).
Thus, the highest effectiveness of NPT100-18A toward anterograde axonal transport in neurons with strong α-Syn aggregation and higher levels of small oligomers (as evident in E46K and E57K neurons) indicates that small α-Syn oligomers have a significant effect on mitochondrial axonal transport.
α-Syn Oligomerization Is Associated with Alterations in Adaptor Motor Proteins.
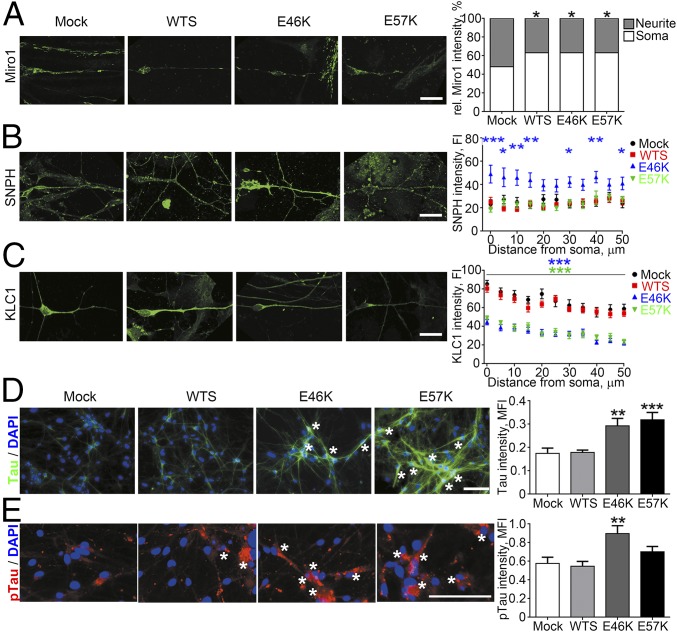
Axonal transport of mitochondria involves adaptor proteins that either facilitate transport by attaching mitochondria to the motor proteins via Mitochondrial Rho GTPase 1 (Miro1) and Kinesin Light Chain 1 (KLC1) or impair motility of axonal mitochondria by docking them to microtubules via Syntaphilin (SNPH) (22). In line with impaired movement incidence of mitochondria, neuritic levels of Miro1 were significantly lower compared with levels in the soma in WTS, E46K, and E57K neurons (Fig. 3A). Interestingly, SNPH levels in neurites, especially proximal at the soma, were significantly increased in E46K neurons (Fig. 3B). Notably, significant reduction of KLC1 in neurites over 50 µm in length was determined in α-Syn oligomer-containing E46K and E57K neurons (Fig. 3C), corresponding to a severe reduction of anterograde transport velocities in these neurons.
Fig. 3.
Spatial distribution and expression of kinesin adaptor proteins is profoundly altered in the presence of α-Syn oligomers. (A) Significantly decreased neurite-to-soma ratios of Miro1 were measured in WTS, E46K, and E57K neurons. (B) A significant increase of SNPH was found in E46K neurites. (C) Significantly decreased levels of KLC1 were found in neurites of oligomer-containing E46K and E57K neurons. (D) Significantly increased total Tau levels (green) especially in a perinuclear region (asterisks) and (E) pTau (red) accumulations (asterisks) in E46K and E57K neurons compared with Mock and WTS. Tau and pTau levels were normalized to the number of DAPI-positive viable cells. Data are presented as mean ± SEM. MFI, mean fluorescence intensity. *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. (Scale bars: 20 µm in A–C and 100 µm in D and E.)
The axonal microtubule-associated protein Tau is known to selectively reduce kinesin-dependent transport (23). Interestingly, Tau protein expression was significantly increased in E46K and E57K neurons compared with control and WTS neurons with the highest level in the perinuclear regions (asterisks, Fig. 3D). The perinuclear regions of E46K and E57K neurons were also enriched for phosphorylated Tau (pTau) clumps (asterisks, Fig. 3E), indicating Tau pathology.
Thus, KLC1 reduction in neurites in conjunction with increased Tau pathology is specifically associated with impaired anterograde axonal transport and elevated levels of α-Syn oligomers.
α-Syn Oligomers Affect the Axonal and Synaptic Integrity in Human Neurons.
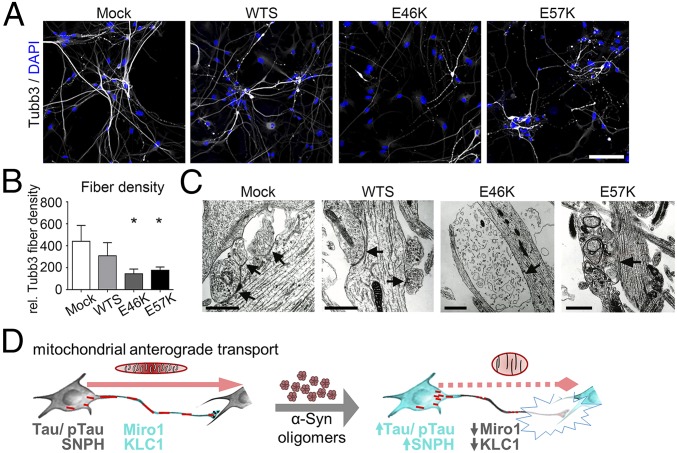
α-Syn oligomer-associated transport and energy deficits might affect the axonal compartment and synapses in human neurons. Axonal fiber densities, visualized by Tubb3 staining, were reduced in E46K and E57K neurons compared with controls (Mock) (Fig. 4 A and B).
Fig. 4.
Axonal and synaptic compartments are severely affected in the presence of increased levels of α-Syn oligomers. (A) β3-Tubulin (Tubb3) immunostaining reveals (B) impaired axonal fiber densities in neuronal cultures expressing mutant α-Syn (E46K and E57K) compared with Mock. (Scale bar: 100 µm.) Tubb3 fiber densities were normalized to numbers of neurons. *P ≤ 0.05. Data are shown as mean ± SD. (C) Ultrastructural analysis identifies irregular synaptic structures and lack of postsynaptic densities (arrows) in α-Syn mutant neuronal cultures (E46K and E57K). (Scale bars: 1 µm.) (D) A model of axonopathy induced by α-Syn oligomers in human neurons. α-Syn oligomers lead to mitochondrial morphological alterations and to impaired anterograde axonal transport, attributed to reduced abundancy of Miro1 and KLC1 within axons accompanied by increased levels of SNPH and Tau/pTau. These changes result in a profound degeneration of synapses.
Before analyses of the synaptic compartment, the maturity of differentiated neurons was confirmed by whole-cell patch clamp analysis showing similar firing behavior of the neurons for evoked action potentials (SI Appendix, Fig. S3A). Ultrastructural characterization of the synaptic compartment identified intact synapses containing presynaptic vesicles and postsynaptic densities (arrows) in Mock and WTS neuronal cultures (Fig. 4C). In contrast, barely detectable postsynaptic densities, irregular presynaptic membranous inclusions, and dystrophic synaptic structures were characteristic for E46K and E57K neuronal cultures (Fig. 4C). Consistently, the analysis of isolated synaptosomes (SI Appendix, Fig. S3B) determined reduced amounts of the presynaptic proteins: synapsin I, synaptosomal-associated protein 25 (SNAP25), and synaptophysin (SYN38) in synaptosomal preparations of E46K and E57K neuronal cultures compared with control (SI Appendix, Fig. S3 D and E). The synaptic nature of isolated synaptosomes was confirmed by their double positivity for pre- and postsynaptic markers, synapsin I, and postsynaptic density 95, respectively (SI Appendix, Fig. S3C). Interestingly, reduced levels of kinesin 1 were found in synaptosomal compartments of E46K and E57K cultures (SI Appendix, Fig. S3E). These data together reveal profound structural abnormalities of axonal and synaptic compartments in α-Syn oligomer-containing neurons that are in line with impaired anterograde axonal transport and ATP deficit in these neurons.
In conclusion, increased dosage of α-Syn impairs axonal transport due to the critical impact of α-Syn oligomers on axonal biology. Thus, we propose a model of neurodegeneration in synucleinopathies, where critical levels of α-Syn oligomers reduce anterograde axonal transport of mitochondria due to Tau pathology and redistribution of kinesin adaptor proteins. Transport deficits co-occur with morphological and functional alterations of mitochondria upon pronounced α-Syn oligomerization, finally leading to synaptic degeneration in human neurons (Fig. 4D).
Discussion
Aberrations of axonal transport have been speculated to contribute to neurodegeneration in PD and synucleinopathies (8, 16, 24). This study directly measures axonal transport in human PD-specific neurons in conjunction with elevated α-Syn levels and α-Syn aggregation intermediates. In iPSC-derived neurons of a PD patient with an α-Syn Dupl, mitochondrial axonal transport deficits were accompanied by increased formation of α-Syn aggregation intermediates. To define which of the α-Syn species has the deleterious effect on axonal transport, we compared WTS overexpression with specific α-Syn oligomer-forming mutants. We identified small α-Syn oligomers as the crucial species involved in functional impairment of axons in human neurons. Transport defects could be recovered by inhibiting α-Syn oligomer formation. α-Syn oligomers promoted a subcellular relocation of transport-regulating proteins and resulted in energy deficits. Finally, increased levels of α-Syn oligomers resulted in a profound degeneration of synaptic structures.
The analysis of α-Syn aggregation in α-Syn Dupl neurons confirms and expands previous studies, which showed increased α-Syn expression or α-Syn accumulation in iPSC-based PD neuronal models carrying a triplication of α-Syn gene (Tripl) (25–28) or a leucine-rich repeat kinase 2 (LRRK2) mutation (29, 30). α-Syn oligomers and amyloid aggregates in α-Syn Tripl neurons caused lysosomal dysfunction due to disrupted hydrolase trafficking (28). Our report shows an increased formation of α-Syn aggregation intermediates and defective axonal transport of mitochondria in iPSC-derived neurons of a PD patient carrying an α-Syn Dupl. Our data indicate that elevated amounts of α-Syn and accumulation of α-Syn aggregation intermediates are pathologically connected with alterations of mitochondrial axonal transport.
A recent gene expression study revealed that iPSC-derived neurons most resemble fetal brain tissue, thereby suggesting particular suitability of iPSC-based models to study disease predisposition (31). Interestingly, α-Syn oligomer-induced mitochondrial motility changes and the formation of oligomers in α-Syn Dupl neurons were observed at early neuronal differentiation stages in our study. This might reflect abnormalities in developing neurons characterized by increased α-Syn oligomer levels. The developmental abnormalities in turn may determine their lower resilience to stressors during aging. Earlier human iPSC-based studies found significant alterations in mitochondrial motility in iPSC-derived neurons from sporadic and LRRK2 mutant PD patients (32, 33). In line with this, in animal models, α-Syn aggregation, caused by either α-Syn mutant or WTS overexpression or by extracellular α-Syn preformed fibrils, affected the expression level of axonal transport proteins and disrupted axonal transport of mitochondria or autophagosomes (8, 14, 15). While extracellular spreading of α-Syn aggregates may be a prominent mechanism of pathology propagation, cell-autonomous α-Syn aggregation is an initiating event in synucleinopathies. Therefore, our data emphasize that a disruption of axonal mitochondrial transport by endogenously formed α-Syn aggregation intermediates represents an early cellular phenotype in synucleinopathies.
Impaired transport of mitochondria is associated with axonal degeneration in mouse dopamine neurons (34, 35) and synaptic dysfunction (22). Indeed, our study reveals reduced axonal fiber densities, impaired levels of presynaptic proteins (synapsin I, SNAP25, and SYN38) in synaptosomes, and ultrastructurally degenerated synaptic structures in neurons with the strongest disruption of mitochondrial axonal transport and pronounced α-Syn oligomerization. α-Syn overexpression and aggregation are known to lead to “vacant synapses” (36), and synaptic degeneration was found in a mouse model overexpressing the E57K mutant of α-Syn (5). Accordingly, the overexpression of human WTS or mutant α-Syn led to alterations of axonal transport proteins, axonopathy, and synaptic dysfunction preceding neuronal loss in rat brain in vivo (7, 8). In line with this, α-Syn oligomers were recently shown to cause synaptic impairment in rat primary neuronal cultures without inducing a prominent toxicity (37). Thus, α-Syn oligomers are the critical species promoting axonopathy and synaptic loss probably due to an impaired axonal transport.
Our study shows that impaired anterograde axonal transport of mitochondria was accompanied by reduced Miro1 and KLC1 levels in axons and a soma-proximal increase of Tau and pTau levels in α-Syn oligomer-containing neurons. Dysregulation of Miro1 caused mitochondrial axonal transport abnormalities in sporadic and LRRK2 mutant PD iPSC-derived neurons (32). In line with this, impaired KLC1 signals have been detected early in PD pathology in postmortem human brains (16). Increase of Tau selectively inhibits kinesin-dependent mitochondria trafficking in N2a cells (38). Importantly, our previous findings revealed that α-Syn oligomers, but not WTS seeds, inhibited kinesin-driven microtubule gliding in a cell-free system (13). Additionally, reduced ATP levels, emphasizing mitochondrial dysfunction, were detected in the presence of α-Syn oligomers. In line with this, mitochondrial morphology and function were compromised by α-Syn overexpression in zebrafish neurons (14) and in skin fibroblasts of an α-Syn Tripl PD patient (39). Notably, mitochondrial ATP is a principal source of energy for mitochondrial transport (40). Together, these results indicate that increased levels of α-Syn oligomers impair axonal transport by influencing a subcellular organization of transport-regulating proteins and mitochondrial function.
A recent study revealed profound α-Syn oligomeric pathology at early PD stages by analyzing postmortem brain tissues (3), supporting the notion that α-Syn oligomer-induced alterations represent an early step in synucleinopathies. The results of our study indicate that α-Syn oligomers critically impact axonal function. Since two independent iPSC-based models of increased α-Syn oligomerization in human neurons, applied in our study, might be extrapolated for both sporadic and inherited forms of PD, our data emphasize the hypothesis that axon involvement is an early and predominant feature in the course of synucleinopathies (41). Moreover, our study provides important mechanistic details of early axonopathy due to α-Syn oligomer formation resulting in axonal transport deficits, ATP deficiency, and severe synaptic degeneration. Taking into account that α-Syn oligomers and axonal dysfunction are characteristic in early neurodegeneration in synucleinopathies, our data might be particularly important for the development of new therapeutic approaches aiming at an early intervention.
Materials and Methods
Extended experimental procedures are described in SI Appendix, SI Materials and Methods. All vectors are listed in SI Appendix, Table S1, and antibodies are listed in SI Appendix, Table S2.
Cells and Cell Culture.
Human iPSC from a PD patient with α-Syn Dupl were provided by Douglas Galasko, University of California, San Diego [clones SDi1-R-C3 (Dupl1) and SDi1-R-C11 (Dupl2)]. This sample was from a female PD patient with a disease onset at the age of 58 with a progressive disease course who developed dementia. iPSC from two healthy Caucasian individuals with no history of neurologic disease served as controls (Ctrl1.1, clone UKERi33Q-R1-016; Ctrl1.2, clone UKERi33Q-R1-06; and Ctrl2.1, clone UKERi1E4-R1-016, previously reported in ref. 42).
All experiments with human iPSC-derived cells were reviewed and approved by the Institutional Ethics Review Board (Nr. 4120: Generation of human neuronal models of neurodegenerative diseases). Written informed consent was received from the participants before inclusion in the study at the movement disorder clinics at the Department of Molecular Neurology, Universitätsklinikum Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg and at the Department of Neurosciences, University of California, San Diego.
Materials and Data Availability.
All antibodies and materials are commercially available. The source of vectors is provided in SI Appendix, Table S1. All experimental procedures are described in detail in SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Naime Denguir, Daniela Graef, Elke Meyer, Holger Wend, and Petra Wenzeler for excellent technical support; Angelika Lampert for help with interpretation of the results; Edward Rockenstein for fruitful discussion; Zacharias Kohl and Sonja Plötz for providing the control fibroblasts; Dieter Chichung Lie for the pCAG-MCS_IRES_Mito-DsRed plasmid; and Fred H. Gage for lentivirus packaging plasmids. Financial support for this work was provided by the Interdisciplinary Center for Clinical Research (IZKF N3, E11, and E25, University Hospital Erlangen); the Bavarian Ministry of Education and Culture, Science and the Arts in the framework of the Bavarian Molecular Biosystems Reseach Network (BioSysNet) and the Bavarian Research Network Induced Pluripotent Stem Cells (ForIPS); the German Federal Ministry of Education and Research (BMBF: 01GQ113, 01GM1520A, 01EK1609B); the Johannes and Frieda Marohn Foundation (W.X. and I.P.); the Deutsche Forschungsgemeinschaft (DFG) funded research training group GRK2162 (to B.W., J.W., A.R., R.-M.B., and K.S.); and DFG Grant INST 410/45-1 FUGG. The present work was performed by R.-M.B. in partial fulfillment of the requirements for obtaining the degree “doctor of medicine.”
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1713129115/-/DCSupplemental.
References
- 1.Lashuel HA, Overk CR, Oueslati A, Masliah E. The many faces of α-synuclein: From structure and toxicity to therapeutic target. Nat Rev Neurosci. 2013;14:38–48. doi: 10.1038/nrn3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konno T, Ross OA, Puschmann A, Dickson DW, Wszolek ZK. Autosomal dominant Parkinson’s disease caused by SNCA duplications. Parkinsonism Relat Disord. 2016;22(Suppl 1):S1–S6. doi: 10.1016/j.parkreldis.2015.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roberts RF, Wade-Martins R, Alegre-Abarrategui J. Direct visualization of alpha-synuclein oligomers reveals previously undetected pathology in Parkinson’s disease brain. Brain. 2015;138:1642–1657. doi: 10.1093/brain/awv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winner B, et al. Role of α-synuclein in adult neurogenesis and neuronal maturation in the dentate gyrus. J Neurosci. 2012;32:16906–16916. doi: 10.1523/JNEUROSCI.2723-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rockenstein E, et al. Accumulation of oligomer-prone α-synuclein exacerbates synaptic and neuronal degeneration in vivo. Brain. 2014;137:1496–1513. doi: 10.1093/brain/awu057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winner B, et al. In vivo demonstration that alpha-synuclein oligomers are toxic. Proc Natl Acad Sci USA. 2011;108:4194–4199. doi: 10.1073/pnas.1100976108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decressac M, Mattsson B, Lundblad M, Weikop P, Björklund A. Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of α-synuclein in midbrain dopamine neurons. Neurobiol Dis. 2012;45:939–953. doi: 10.1016/j.nbd.2011.12.013. [DOI] [PubMed] [Google Scholar]
- 8.Chung CY, Koprich JB, Siddiqi H, Isacson O. Dynamic changes in presynaptic and axonal transport proteins combined with striatal neuroinflammation precede dopaminergic neuronal loss in a rat model of AAV alpha-synucleinopathy. J Neurosci. 2009;29:3365–3373. doi: 10.1523/JNEUROSCI.5427-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, et al. Oligomeric alpha-synuclein inhibits tubulin polymerization. Biochem Biophys Res Commun. 2007;356:548–553. doi: 10.1016/j.bbrc.2007.02.163. [DOI] [PubMed] [Google Scholar]
- 10.Esteves AR, Arduíno DM, Swerdlow RH, Oliveira CR, Cardoso SM. Microtubule depolymerization potentiates alpha-synuclein oligomerization. Front Aging Neurosci. 2010;1:5. doi: 10.3389/neuro.24.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee HJ, Khoshaghideh F, Lee S, Lee SJ. Impairment of microtubule-dependent trafficking by overexpression of alpha-synuclein. Eur J Neurosci. 2006;24:3153–3162. doi: 10.1111/j.1460-9568.2006.05210.x. [DOI] [PubMed] [Google Scholar]
- 12.Zhou RM, et al. Molecular interaction of α-synuclein with tubulin influences on the polymerization of microtubule in vitro and structure of microtubule in cells. Mol Biol Rep. 2010;37:3183–3192. doi: 10.1007/s11033-009-9899-2. [DOI] [PubMed] [Google Scholar]
- 13.Prots I, et al. α-Synuclein oligomers impair neuronal microtubule-kinesin interplay. J Biol Chem. 2013;288:21742–21754. doi: 10.1074/jbc.M113.451815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Donnell KC, et al. Axon degeneration and PGC-1α-mediated protection in a zebrafish model of α-synuclein toxicity. Dis Model Mech. 2014;7:571–582. doi: 10.1242/dmm.013185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volpicelli-Daley LA, et al. Formation of α-synuclein Lewy neurite-like aggregates in axons impedes the transport of distinct endosomes. Mol Biol Cell. 2014;25:4010–4023. doi: 10.1091/mbc.E14-02-0741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chu Y, et al. Alterations in axonal transport motor proteins in sporadic and experimental Parkinson’s disease. Brain. 2012;135:2058–2073. doi: 10.1093/brain/aws133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rui Y, Zheng JQ. Amyloid β oligomers elicit mitochondrial transport defects and fragmentation in a time-dependent and pathway-specific manner. Mol Brain. 2016;9:79. doi: 10.1186/s13041-016-0261-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin M, Selkoe DJ. Systematic analysis of time-dependent neural effects of soluble amyloid β oligomers in culture and in vivo: Prevention by scyllo-inositol. Neurobiol Dis. 2015;82:152–163. doi: 10.1016/j.nbd.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiang W, et al. Posttranslational modification and mutation of histidine 50 trigger alpha synuclein aggregation and toxicity. Mol Neurodegener. 2015;10:8. doi: 10.1186/s13024-015-0004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kouroupi G, et al. Defective synaptic connectivity and axonal neuropathology in a human iPSC-based model of familial Parkinson’s disease. Proc Natl Acad Sci USA. 2017;114:E3679–E3688. doi: 10.1073/pnas.1617259114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wrasidlo W, et al. A de novo compound targeting α-synuclein improves deficits in models of Parkinson’s disease. Brain. 2016;139:3217–3236. doi: 10.1093/brain/aww238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sheng ZH, Cai Q. Mitochondrial transport in neurons: Impact on synaptic homeostasis and neurodegeneration. Nat Rev Neurosci. 2012;13:77–93. doi: 10.1038/nrn3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dixit R, Ross JL, Goldman YE, Holzbaur EL. Differential regulation of dynein and kinesin motor proteins by tau. Science. 2008;319:1086–1089. doi: 10.1126/science.1152993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lamberts JT, Hildebrandt EN, Brundin P. Spreading of α-synuclein in the face of axonal transport deficits in Parkinson’s disease: A speculative synthesis. Neurobiol Dis. 2015;77:276–283. doi: 10.1016/j.nbd.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Byers B, et al. SNCA triplication Parkinson’s patient’s iPSC-derived DA neurons accumulate α-synuclein and are susceptible to oxidative stress. PLoS One. 2011;6:e26159. doi: 10.1371/journal.pone.0026159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devine MJ, et al. Parkinson’s disease induced pluripotent stem cells with triplication of the α-synuclein locus. Nat Commun. 2011;2:440. doi: 10.1038/ncomms1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oliveira LM, et al. Elevated α-synuclein caused by SNCA gene triplication impairs neuronal differentiation and maturation in Parkinson’s patient-derived induced pluripotent stem cells. Cell Death Dis. 2015;6:e1994. doi: 10.1038/cddis.2015.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mazzulli JR, Zunke F, Isacson O, Studer L, Krainc D. α-Synuclein-induced lysosomal dysfunction occurs through disruptions in protein trafficking in human midbrain synucleinopathy models. Proc Natl Acad Sci USA. 2016;113:1931–1936. doi: 10.1073/pnas.1520335113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen HN, et al. LRRK2 mutant iPSC-derived DA neurons demonstrate increased susceptibility to oxidative stress. Cell Stem Cell. 2011;8:267–280. doi: 10.1016/j.stem.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sánchez-Danés A, et al. Disease-specific phenotypes in dopamine neurons from human iPS-based models of genetic and sporadic Parkinson’s disease. EMBO Mol Med. 2012;4:380–395. doi: 10.1002/emmm.201200215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brennand K, et al. Phenotypic differences in hiPSC NPCs derived from patients with schizophrenia. Mol Psychiatry. 2015;20:361–368. doi: 10.1038/mp.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsieh CH, et al. Functional impairment in miro degradation and mitophagy is a shared feature in familial and sporadic Parkinson’s disease. Cell Stem Cell. 2016;19:709–724. doi: 10.1016/j.stem.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooper O, et al. Pharmacological rescue of mitochondrial deficits in iPSC-derived neural cells from patients with familial Parkinson’s disease. Sci Transl Med. 2012;4:141ra90. doi: 10.1126/scitranslmed.3003985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim-Han JS, Antenor-Dorsey JA, O’Malley KL. The Parkinsonian mimetic, MPP+, specifically impairs mitochondrial transport in dopamine axons. J Neurosci. 2011;31:7212–7221. doi: 10.1523/JNEUROSCI.0711-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sterky FH, Lee S, Wibom R, Olson L, Larsson NG. Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proc Natl Acad Sci USA. 2011;108:12937–12942. doi: 10.1073/pnas.1103295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott DA, et al. A pathologic cascade leading to synaptic dysfunction in alpha-synuclein-induced neurodegeneration. J Neurosci. 2010;30:8083–8095. doi: 10.1523/JNEUROSCI.1091-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peelaerts W, et al. α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature. 2015;522:340–344. doi: 10.1038/nature14547. [DOI] [PubMed] [Google Scholar]
- 38.Ebneth A, et al. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: Implications for Alzheimer’s disease. J Cell Biol. 1998;143:777–794. doi: 10.1083/jcb.143.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mak SK, Tewari D, Tetrud JW, Langston JW, Schüle B. Mitochondrial dysfunction in skin fibroblasts from a Parkinson’s disease patient with an alpha-synuclein triplication. J Parkinsons Dis. 2011;1:175–183. doi: 10.3233/JPD-2011-11025. [DOI] [PubMed] [Google Scholar]
- 40.Zala D, et al. Vesicular glycolysis provides on-board energy for fast axonal transport. Cell. 2013;152:479–491. doi: 10.1016/j.cell.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 41.Tagliaferro P, Burke RE. Retrograde axonal degeneration in Parkinson disease. J Parkinsons Dis. 2016;6:1–15. doi: 10.3233/JPD-150769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Havlicek S, et al. Gene dosage-dependent rescue of HSP neurite defects in SPG4 patients’ neurons. Hum Mol Genet. 2014;23:2527–2541. doi: 10.1093/hmg/ddt644. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.